Introduction
It is well recognized that atherosclerosis is a
chronic inflammatory disease (1).
Vascular inflammatory response plays an important role in the
onset, development and evolution of atherosclerosis (2). Many reports have demonstrated that
vascular inflammatory response is closely associated with
endothelial dysfunction (3,4).
Endothelial cells can release several inflammatory factors in
response to a variety of harmful stimulations including increased
lysophosphatidylcholine (LPC) (5). LPC is the major phospholipid
component of oxidized low-density lipoprotein (ox-LDL) (6), and it is well known for its ability
to mimic the effects of ox-LDL (7). Increased plasma ox-LDL is an
independent risk factor in atherosclerosis and plays a key role in
initiation of the inflammatory response (8,9).
Recently, it has been reported that ox-LDL-induced inflammation in
endothelial cells is related to stimulation of high mobility group
box-1 (HMGB1) release (10).
HMGB1 is a non-histone DNA-binding nuclear protein
that can be positively released from immune-activated cells or
passively released from necrotic cells (11). There is growing evidence that
extracellular HMGB1 is a very potent pro-inflammatory mediator
(12,13). HMGB1 can stimulate the expression
and release of numerous inflammatory factors, such as tumor
necrosis factor-α (TNF-α), interleukins, intercellular adhesion
molecule-1 (ICAM-1) and selectins (14,15). It has been reported that the
pro-inflammatory effect of HMGB1 is mediated mainly through binding
to its specific receptors, including receptor for advanced
glycation endproduct (RAGE), Toll-like receptor (TLR)-2 and TLR-4
(16). The binding of HMGB1 to
its specific receptors ultimately results in the activation of
nuclear factor-κB (NF-κB), and then upregulation of inflammatory
factor expression and release (17,18). Therefore, the
HMGB1-RAGE/TLR-2/TLR-4-NF-κB pathway may be an important
inflammatory signaling pathway.
Paeoniflorin (PEF), a monoterpene glucoside, is the
primary active ingredient extracted from the dry root of
Paeonia, which is a traditional Chinese herbal medicine
extensively used in China for more than 1000 years to treat
numerous diseases, such as virus hepatitis, anemia, systemic lupus
erythematosus and gynecological diseases (19). In recent years, it has been
reported that PEF exhibits anti-inflammatory properties. In
Sprague-Dawley rats, both pretreatment and post-treatment with PEF
alleviated ischemia/reperfusion-induced cerebral injury, at least
in part, through inhibition of inflammatory factor production
(20). In addition, PEF was able
to protect against lipopolysaccharide-induced acute lung injury in
mice by alleviating inflammatory cell infiltration and
microvascular permeability (21).
However, the exact anti-inflammatory mechanisms of PEF remain
unclear.
In the present study, we investigated whether PEF
has an inhibitory effect on LPC-induced inflammatory factor
production using cultured human umbilical vein endothelial cells
(HUVECs), and whether the anti-inflammatory effect is related to
the inhibition of the HMGB1-RAGE/TLR-2/TLR-4-NF-κB pathway.
Materials and methods
Materials
HUVECs were obtained from the Tumor Research
Institute of Peking University (Peking, China). PEF (purity ≥98%)
was purchased from the Yangling Dongke Pharmaceutical Division
(Shanxi, China). LPC was purchased from Sigma-Aldrich (St. Louis,
MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were provided by Gibco-BRL (Grand Island, NY,
USA). TRIzol reagent was a product of Invitrogen Corp. (Carlsbad,
CA, USA). The First Strand cDNA Synthesis kit was purchased from
MBI Fermentas Inc. (Vilnius, Lithuania). RAGE, TLR-2, TLR-4 and
β-actin antibodies were from Abcam (Cambridge, UK).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was from Beyotime Biotechnology (Jiangsu, China). ICAM-1, MCP-1,
IL-6, TNF-α, HMGB1 and NF-κBp65 ELISA kits were obtained from the
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Cell culture and treatment
HUVECs were cultured in DMEM containing 10% (v/v)
FBS, 100 U/ml penicillin and 100 μg/ml streptomycin in a
humidified atmosphere of 5% CO2 at 37°C. Cells were
passaged by trypsinization and seeded at ∼105 cells/ml.
When cells reached 80% confluence, the culture medium was replaced
with serum-free medium for 24 h, and then cells were pretreated
with various concentrations (1, 10 or 100 μmol/l) of PEF 2 h
prior to exposure to LPC (10 mg/l) for 24 h. LPC was dissolved in
ethanol. The final concentration of ethanol was less than 0.1%
(v/v). Cells used in the experiments were from 5 to 8 passages.
Cell viability assay
MTT was used to determine cell viability. Briefly,
HUVECs were seeded at a density of 1×104 cells/well in a
96-well culture plate. After drug treatment, the cells were washed
twice with PBS to remove the medium, and 10 μl of MTT (0.5
mg/ml) was added to each well and incubated for an additional 4 h
at 37°C. Subsequently, 100 μl of dimethy sulfoxide (DMSO)
was added to dissolve the MTT, and the absorbance at 490 nm was
read on a microplate reader. Data are expressed as a percentage of
the control, which was considered to be 100% viable.
ELISA
After drug treatment, cell culture supernatants were
collected and centrifuged at 3,000 × g for 10 min to remove debris.
The nuclear lysates were prepared as described by Wu et al
(22). Briefly, cells were washed
with cold PBS and then scraped from the well. Cells were treated
with hypotonic buffer and centrifuged. The pellet was collected and
treated with cell extraction buffer, vortexed, centrifuged and the
supernatants (nuclear lysates) were stored at −70°C. The
concentrations of ICAM-1, MCP-1, IL-6, TNF-α and HMGB1 in cell
culture supernatants and NF-κBp65 in nuclear lysates were
determined by commercially available ELISA kits according to the
manufacturer’s instructions.
Quantitative real-time PCR
Total RNA was isolated from HUVECs using TRIzol
reagent and quantified by measuring the optical density at 260 nm.
cDNA was synthesized from 1 μg of total RNA, which was the
used for quantitative real-time PCR. Quantitative analysis of mRNA
expression was performed using the ABI 7300 real-time PCR system
with the Power SYBR-Green PCR Master Mix kit. PCR primers were as
follows: HMGB1 (forward, 5′-ATGTTGCGAAGAAACTGG-3′ and reverse,
5′-TTCAGCCTTGACAACTCC-3′); RAGE (forward,
5′-AAGCCCCTGGTGCCTAATGAG-3′ and reverse,
5′-CACCAATTGGACCTCCTCCA-3′); TLR-2 (forward,
5′-ATCCTCCAATCAGGCTTCTCT-3′ and reverse,
5′-ACACCTCTGTAGGTCACTGTTG-3′); TLR-4 (forward,
5′-ATATTGACAGGAAACCCCATCCA-3′ and reverse, 5′-AGAGAGATTGAGTAGGGGCAT
TT-3′); GAPDH (forward, 5′-CAATGACCCCTTCATTGA-3′ and reverse,
5′-GACAAGCTTCCCGTTCTCAG-3′). The PCR amplification profiles
consisted of denaturation at 95°C for 10 min, followed by 40 cycles
of denaturation at 95°C for 15 sec and annealing at 60°C for 60
sec. All amplification reactions for each sample were carried out
in triplicates, and the relative expression values were normalized
to the expression value of GAPDH.
Western blot analysis
The protein expression of RAGE, TLR-2 and TLR-4 was
determined by western blotting. Briefly, after drug treatment,
cells were lysed with ice-cold lysis buffer [0.33 mol/l Tris/HCl,
10% SDS (wt/vol), 40% glycerol (vol/vol) and 50 mmol/l DTT
containing bromophenol blue], and the protein content of the
lysates was measured using the bicinchoninic acid (BCA) method.
Equal amounts of protein were separated by 10% SDS-PAGE and
transferred to a nitrocellulose membrane. After being blocked with
TBST containing 5% bovine serum albumin, the membranes were
incubated with the primary antibody for RAGE, TLR-2, TLR-4 and
β-actin overnight at 4°C, and then incubated with the corresponding
horseradish peroxidase-conjugated secondary antibody at room
temperature for 1 h. The protein bands were quantitated by video
densitometry, and the results were normalized to β-actin
expression.
Statistical analysis
Data are expressed as means ± SEM. All values were
analyzed by analysis of variance followed by the
Student-Newman-Keuls test. P<0.05 was regarded as indicative of
statistical significance.
Results
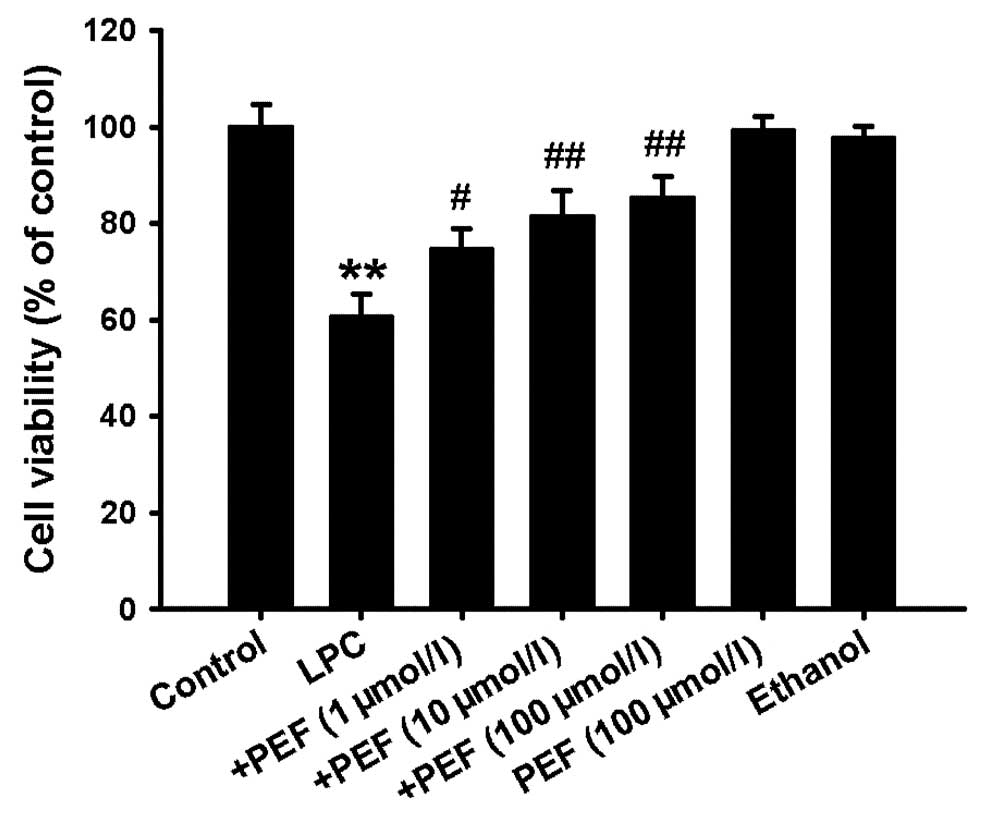
Effect of PEF on cell viability induced
by LPC
MTT assay showed that treatment of HUVECs with LPC
(10 mg/l) for 24 h significantly decreased the cell viability.
Pretreatment with PEF 2 h prior to exposure to LPC concentration
dependently inhibited the decrease in cell viability induced by
LPC. However, PEF alone had no effect on cell viability (Fig. 1).
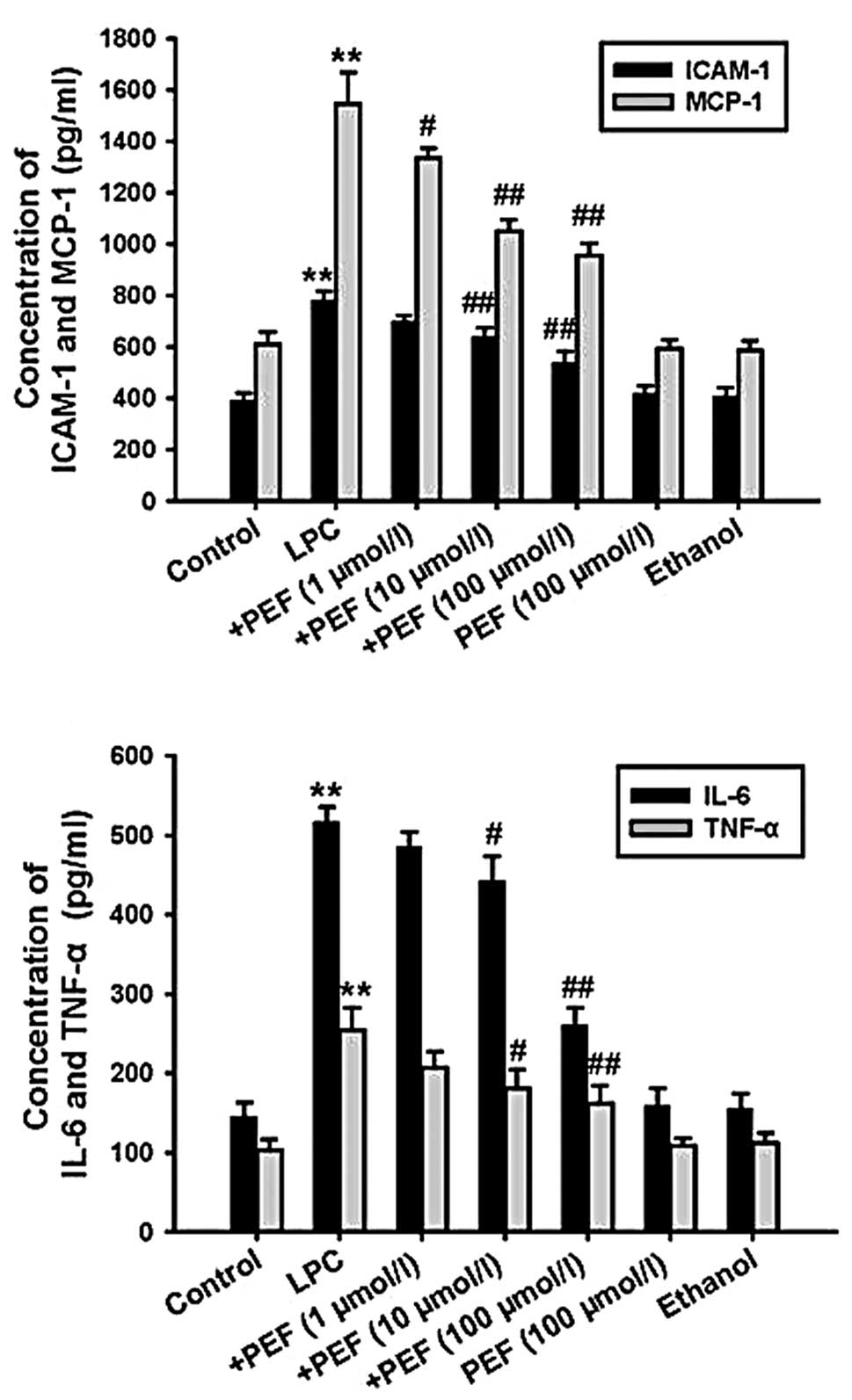
Effect of PEF on inflammatory factor
production induced by LPC
ELISA showed that treatment with LPC significantly
increased the concentration of ICAM-1, MCP-1, IL-6 and TNF-α in
cell culture supernatants. This effect of LPC was markedly
attenuated by pretreatment with PEF in a concentration-dependent
manner. However, PEF alone had no effect on inflammatory factor
production (Fig. 2).
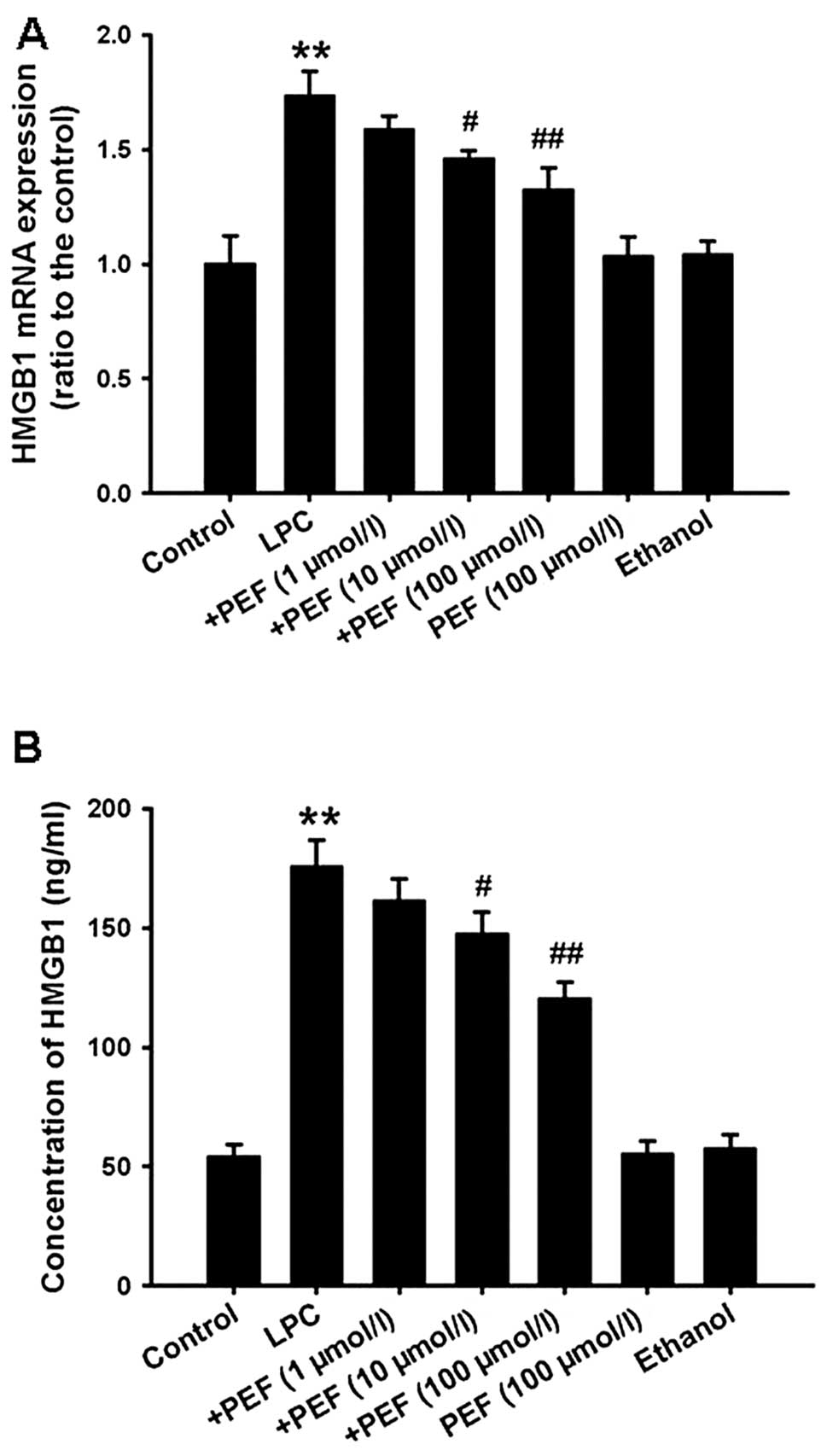
Effect of PEF on HMGB1 expression and
release induced by LPC
Real-time PCR analysis showed that HMGB1 mRNA
expression was significantly upregulated in LPC-treated HUVECs.
Consistent with this result, treatment with LPC markedly increased
the concentration of HMGB1 in cell culture supernatants. However,
these effects of LPC were reversed by pretreatment with PEF in a
concentration-dependent manner. PEF alone had no effect on HMGB1
mRNA expression and release (Fig.
3).
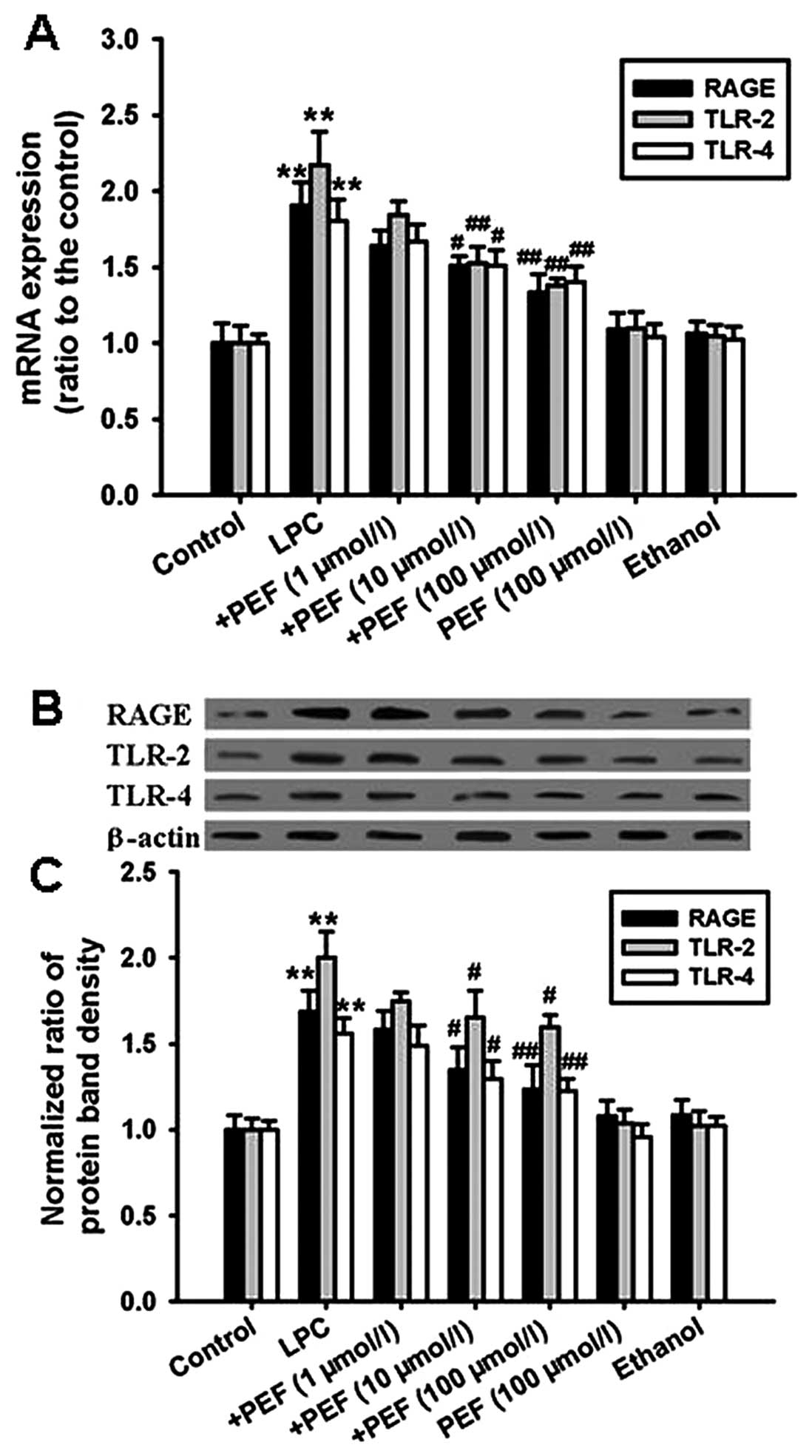
Effect of PEF on the expression of HMGB1
receptors induced by LPC
The mRNA expression of RAGE, TLR-2 and TLR-4 was
significantly upregulated in HUVECs treated with LPC compared with
the controls (Fig. 4A).
Consistent with the mRNA expression, treatment with LPC
significantly increased the protein expression of RAGE, TLR-2 and
TLR-4 (Fig. 4B and C). These
effects were attenuated by pretreatment with different
concentrations of PEF.
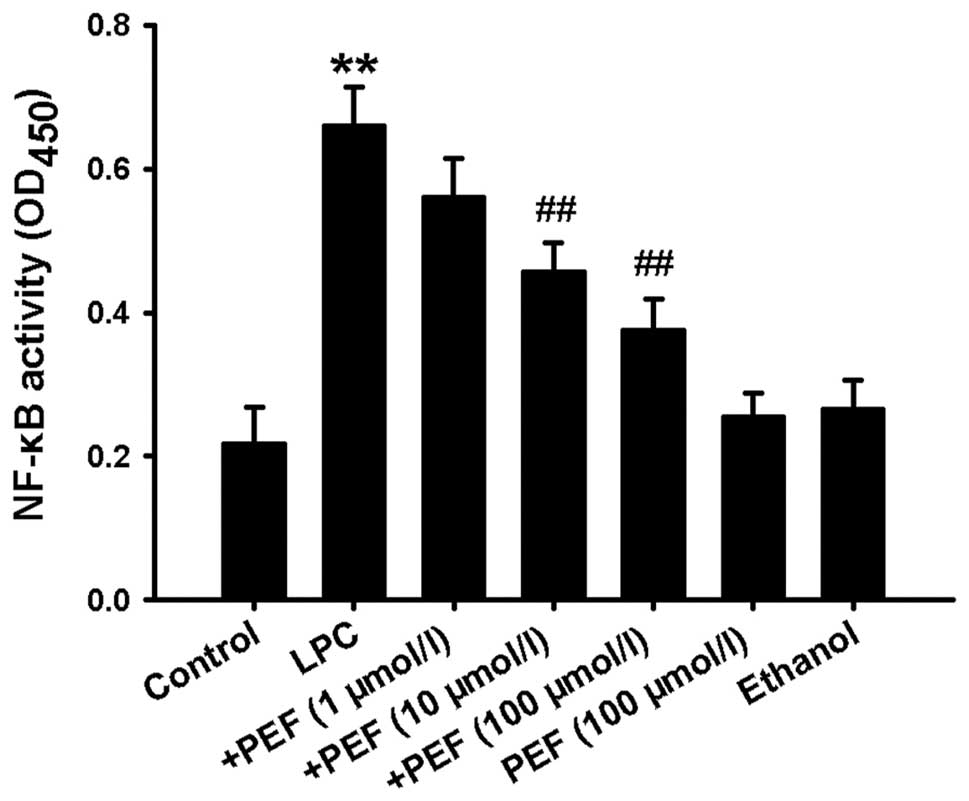
Effect of PEF on NF-κB activation induced
by LPC
Since activation of NF-κB is essential to
inflammatory factor production, we therefore detected NF-κB
activity. Treatment of HUVECs with LPC significantly increased
NF-κB activity, which was indicated by the increased optical
density of NF-κBp65 in the nuclear lysates (Fig. 5). This effect was markedly
inhibited by pretreatment with different concentrations of PEF.
Discussion
In the present study, we investigated the inhibitory
effect of PEF on LPC-induced inflammatory factor production and the
underlying mechanisms in HUVECs. The results showed that
pretreatment with PEF was able to inhibit LPC-induced inflammatory
factor production in a concentration-dependent manner, which was
related to inhibition of HMGB1 expression and release,
downregulation of RAGE, TLR-2 and TLR-4 expression, and decrease in
NF-κB activity.
Vascular inflammatory response is well known to play
a crucial role in all stages of atherosclerosis from the nascent
lesion to acute coronary syndromes (23). Inhibition of the inflammatory
response is a potential strategy to prevent the development of
atherosclerosis (8,9,24).
LPC is the major bioactive lipid component of ox-LDL and is
implicated as an important factor in the atherogenic activity of
ox-LDL (25). Many studies have
indicated that the atherogenic effect of LPC is closely related to
the inflammatory response. Gonçalves et al (26) reported that an increased level of
LPC plays a key role in human atherosclerotic plaque inflammation.
In addition, LPC was found to significantly increase the adherence
of monocytes to endothelial cells and upregulate the expression of
ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1), which are
both biomarkers in inflammatory progression (5). Therefore, inhibition of LPC-induced
inflammation may be a promising approach by which to prevent the
development of atherosclerosis.
HMGB1, also named amphoterin, is a highly conserved
chromatin binding protein that is expressed in multiple cell types
including endothelial cells (27). HMGB1 overexpression and release
can initiate and amplify the inflammatory response (28). Recently, extracellular HMGB1 was
identified as a potent pro-inflammatory cytokine (16) and plays a decisive role in the
mediation of inflammatory responses (29). Therefore, HMGB1 is recognized as a
potential therapeutic target in inflammatory diseases (28). As previously mentioned, HMGB1
interacts with RAGE, TLR-2 and TLR-4, the cell surface receptors of
HMGB1, to amplify inflammatory responses by activating NF-κB and
then inducing inflammatory factor expression and release (30). Therefore, the
HMGB1-RAGE/TLR-2/TLR-4-NF-κB pathway may be an important
inflammatory signaling pathway.
PEF, a monoterpene glucoside isolated from the dry
root of Paeonia, has been reported to have multiple
beneficial effects, such as the lowering of cholesterol levels,
anti-platelet agglutination and neuroprotective effects (31–33). Recently, there is growing evidence
that PEF exerts an anti-inflammatory effect in animal models of
collagen-induced arthritis (34),
ischemia/reperfusion-induced cerebral injury (20), lipopolysaccharide-induced acute
lung injury (21) and liver
inflammatory reactions (35).
However, the exact anti-inflammatory mechanisms of PEF are still
unclear. Since PEF has anti-inflammatory properties and the
HMGB1-RAGE/TLR-2/TLR-4-NF-κB pathway is an important inflammatory
signaling pathway, we therefore hypothesized that PEF may be able
to inhibit LPC-induced inflammatory factor production in HUVECs,
and the mechanism may be associated with inhibition of the
HMGB1-RAGE/TLR-2/TLR-4-NF-κB signaling pathway. In the present
study, we found that pretreatment of PEF significantly inhibited
LPC-induced inflammatory factor production concomitantly with
decreased expression and release of HMGB1, down-regulated mRNA and
protein expression of RAGE, TLR-2 and TLR-4, and decreased NF-κB
activity. These results confirmed our hypothesis.
In summary, the present study demonstrated that PEF
was able to inhibit LPC-induced inflammatory factor production,
which was related to inhibition of the HMGB1-RAGE/TLR-2/TLR-4-NF-κB
signaling pathway.
Acknowledgements
This study was supported by the
National Nature Science Foundation of China (81101476 to
S.Y.Y.).
References
|
1.
|
Ross R: Atherosclerosis is an inflammatory
disease. Am Heart J. 138:S419–S420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lin R, Liu J, Gan W and Yang G: C-reactive
protein-induced expression of CD40-CD40L and the effect of
lovastatin and fenofibrate on it in human vascular endothelial
cells. Biol Pharm Bull. 27:1537–1543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Loppnow H, Buerke M, Werdan K and
Rose-John S: Contribution of vascular cell-derived cytokines to
innate and inflammatory pathways in atherogenesis. J Cell Mol Med.
15:484–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kang H, Yang PY and Rui YC: Adenoviral
gene transfer of viral interleukin-10 protects cerebrovascular
impairment induced by lysophosphatidylcholine. Eur J Pharmacol.
580:175–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Matsumoto T, Kobayashi T and Kamata K:
Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med
Chem. 14:3209–3220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schaefer CA, Kuhlmann CR, Gast C, et al:
Statins prevent oxidized low-density lipoprotein- and
lysophosphatidylcholine-induced proliferation of human endothelial
cells. Vascul Pharmacol. 41:67–73. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Toshima S, Hasegawa A, Kurabayashi M, et
al: Circulating oxidized low density lipoprotein levels. A
biochemical risk marker for coronary heart disease. Arterioscler
Thromb Vasc Biol. 20:2243–2247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mazière C and Mazière JC: Activation of
transcription factors and gene expression by oxidized low-density
lipoprotein. Free Radic Biol Med. 46:127–137. 2009.PubMed/NCBI
|
|
10.
|
Yu X, Xing C, Pan Y, Ma H, Zhang J and Li
W: IGF-1 alleviates ox-LDL-induced inflammation via reducing HMGB1
release in HAECs. Acta Biochim Biophys Sin. 44:746–751. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zhang J, Takahashi HK, Liu K, et al:
Anti-high mobility group box-1 monoclonal antibody protects the
blood-brain barrier from ischemia-induced disruption in rats.
Stroke. 42:1420–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Naglova H and Bucova M: HMGB1 and its
physiological and pathological roles. Bratisl Lek Listy.
113:163–171. 2012.PubMed/NCBI
|
|
13.
|
Chen G, Ward MF, Sama AE and Wang H:
Extracellular HMGB1 as a proinflammatory cytokine. J Interferon
Cytokine Res. 24:329–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nadatani Y, Watanabe T, Tanigawa T, et al:
High mobility group box 1 promotes small intestinal damage induced
by nonsteroidal anti-inflammatory drugs through Toll-like receptor
4. Am J Pathol. 181:98–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lee W, Kim TH, Ku SK, Min KJ, Lee HS, Kwon
TK and Bae JS: Barrier protective effects of withaferin A in
HMGB1-induced inflammatory responses in both cellular and animal
models. Toxicol Appl Pharmacol. 262:91–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lee W, Ku SK, Bae JW and Bae JS:
Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory
responses in both cellular and animal models. Food Chem Toxicol.
50:1826–1833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Park JS, Svetkauskaite D, He Q, Kim JY,
Strassheim D, Ishizaka A and Abraham E: Involvement of toll-like
receptors 2 and 4 in cellular activation by high mobility group box
1 protein. J Biol Chem. 279:7370–7377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C
and Guo RX: HMGB1 activates nuclear factor-κB signaling by RAGE and
increases the production of TNF-α in human umbilical vein
endothelial cells. Immunobiology. 215:956–962. 2010.
|
|
19.
|
He DY and Dai SM: Anti-inflammatory and
immunomodulatory effects of Paeonia lactiflora pall., a
traditional Chinese herbal medicine. Front Pharmacol.
2:102011.PubMed/NCBI
|
|
20.
|
Tang NY, Liu CH, Hsieh CT and Hsieh CL:
The anti-inflammatory effect of paeoniflorin on cerebral infarction
induced by ischemia-reperfusion injury in Sprague-Dawley rats. Am J
Chin Med. 38:51–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhou H, Bian D, Jiao X, Wei Z, Zhang H,
Xia Y, He Y and Dai Y: Paeoniflorin protects against
lipopolysaccharide-induced acute lung injury in mice by alleviating
inflammatory cell infiltration and microvascular permeability.
Inflamm Res. 60:981–990. 2011. View Article : Google Scholar
|
|
22.
|
Wu WC, Hu DN, Gao HX, Chen M, Wang D,
Rosen R and McCormick SA: Subtoxic levels hydrogen peroxide-induced
production of interleukin-6 by retinal pigment epithelial cells.
Mol Vis. 16:1864–1873. 2010.PubMed/NCBI
|
|
23.
|
Wang HR, Li JJ, Huang CX and Jiang H:
Fluvastatin inhibits the expression of tumor necrosis factor-a and
activation of nuclear factor-kappaB in human endothelial cells
stimulated by C-reactive protein. Clin Chim Acta. 353:53–60. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Barish GD, Yu RT, Karunasiri MS, et al:
The Bcl6-SMRT/NCoR cistrome represses inflammation to attenuate
atherosclerosis. Cell Metab. 15:554–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Schmitz G and Ruebsaamen K: Metabolism and
atherogenic disease association of lysophosphatidylcholine.
Atherosclerosis. 208:10–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Gonçalves I, Edsfeldt A, Ko NY, et al:
Evidence supporting a key role of Lp-PLA2-generated
lysophosphatidylcholine in human atherosclerotic plaque
inflammation. Arterioscler Thromb Vasc Biol. 32:1505–1512.
2012.PubMed/NCBI
|
|
27.
|
Orlova VV, Choi EY, Xie C, et al: A novel
pathway of HMGB1-mediated inflammatory cell recruitment that
requires Mac-1-integrin. EMBO J. 26:1129–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Gao M, Hu Z, Zheng Y, Zeng Y, Shen X,
Zhong D and He F: Peroxisome proliferator-activated receptor γ
agonist troglitazone inhibits high mobility group box 1 expression
in endothelial cells via suppressing transcriptional activity of
nuclear factor κB and activator protein 1. Shock. 36:228–234.
2011.
|
|
29.
|
Kim TH, Ku SK, Lee T and Bae JS: Vascular
barrier protective effects of phlorotannins on HMGB1-mediated
proinflammatory responses in vitro and in vivo. Food Chem Toxicol.
50:2188–2195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yang EJ, Lee W, Ku SK, Song KS and Bae JS:
Anti-inflammatory activities of oleanolic acid on HMGB1 activated
HUVECs. Food Chem Toxicol. 50:1288–1294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Yang HO, Ko WK, Kim JY and Ro HS:
Paeoniflorin: an anti-hyperlipidemic agent from Paeonia
lactiflora. Fitoterapia. 75:45–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Koo YK, Kim JM, Koo JY, Kang SS, Bae K,
Kim YS, Chung JH and Yun-Choi HS: Platelet anti-aggregatory and
blood anticoagulant effects of compounds isolated from Paeonia
lactiflora and Paeonia suffruticosa. Pharmazie.
65:624–628. 2010.PubMed/NCBI
|
|
33.
|
Mao QQ, Zhong XM, Qiu FM, Li ZY and Huang
Z: Protective effects of paeoniflorin against
corticosterone-induced neurotoxicity in PC12 cells. Phytother Res.
26:969–973. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Zhang LL, Wei W, Wang NP, Wang QT, Chen
JY, Chen Y, Wu H and Hu XY: Paeoniflorin suppresses inflammatory
mediator production and regulates G protein-coupled signaling in
fibroblast-like synoviocytes of collagen induced arthritic rats.
Inflamm Res. 57:388–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Kim ID and Ha BJ: The effects of
paeoniflorin on LPS-induced liver inflammatory reactions. Arch
Pharm Res. 33:959–966. 2010. View Article : Google Scholar : PubMed/NCBI
|