Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic inflammatory disease characterized by persistent airflow
limitation, as well as extrapulmonary dysfunction such as skeletal
muscle dysfunction, increased risk of cardiovascular disease,
osteoporosis and depression (1,2).
Diagnosis and assessment of the severity of COPD are based on the
degree of airflow limitation by spirometry. However, the forced
expiratory volume in 1 sec (FEV1) does not directly
reflect systemic manifestations in patients with COPD (3). Although both chronic inflammation in
the airway and systemic inflammation have been attributed to the
pathogenesis of COPD, the origin of systemic inflammation in COPD
is not well understood (4–6).
microRNAs (miRNAs) are small noncoding RNAs, ranging
from 18 to 25 nucleotides in length, that post-transcriptionally
regulate gene expression. Recent studies have shown that miRNAs
control a wide range of biological functions such as cellular
proliferation, differentiation and apoptosis (7,8).
Dysregulation of miRNAs has been implicated in the pathogenesis of
several diseases, and their expression patterns in tumor tissues
and body fluid serve as biomarkers (9–11).
Several studies have examined the role of miRNAs in lung tissue of
COPD patients and in airway epithelial cells from smokers versus
nonsmokers (12,13). miRNAs exist stably in serum and
plasma (11,14), and one recent study revealed the
presence of circulating miRNAs within microvesicles (14). Since routine examination using
lung epithelium or sputum is sometimes difficult in COPD patients,
we sought for altered plasma miRNA expression levels in COPD
patients in order to develop a screening protocol for the
disease.
In the present study, we profiled levels of plasma
miRNAs in COPD patients and identified those that were
differentially expressed in COPD. We also assessed clinical
characteristics such as smoking history, duration of disease since
diagnosis, and the Global Initiative for Obstructive Lung Disease
(GOLD) stages of the COPD patients.
Materials and methods
Patients and samples
A total of 70 consecutive subjects who did not meet
any exclusion criteria were invited to participate, and 60 agreed
to take part in the study. Participants were classified into 4
groups: those who had never smoked (hereafter ‘nonsmokers’) without
COPD (n=10), current smokers without COPD (n=10), ex-smokers with
COPD (n=20), and current smokers with COPD (n=20). Age and
gender-matched healthy subjects were also enrolled in this study
(Table I). Current smokers were
defined as those who still smoked at the time of participation in
the study. Subjects were classified in the COPD group (n=40) if
they had a post-bronchodilator ratio of FEV1 to forced
vital capacity (FVC) of <0.70. All patients with COPD had stable
disease; patients with symptoms or clinical signs of COPD
exacerbation within 2 months prior to the study were excluded.
Other exclusion criteria included a diagnosis of asthma,
bronchiectasis, lung cancer, or upper or lower respiratory tract
infection in the preceding 4 weeks. Among the 40 COPD patients,
there were 21 patients in GOLD stage I, 9 in stage II, 8 in stage
III, and 2 in stage IV. This study was approved by our
institutional review board (no. 930, 24 June, 2008), and written
informed consent was obtained from all patients prior to collection
of specimens, according to the Declaration of Helsinki.
 | Table ICharacteristics of controls and COPD
patients. |
Table I
Characteristics of controls and COPD
patients.
| Non-smoker without
COPD | Smoker without
COPD | COPD ex-smoker | COPD current
smoker | P-value |
|---|
| No. of subjects | 10 | 10 | 20 | 20 | |
| Age (years) | 65.0±11.5 | 62.8±14.6 | 64.6±7.4 | 64.7±7.5 | 0.95 |
| Male (%) | 80 | 100 | 85 | 95 | 0.42 |
| Pack-years of
smoking | 0 | 47.6±34.8 | 69.3±42.8 | 77.4±72.7 | 0.39 |
RNA isolation
Isolation of plasma miRNAs for miRNA profiling or
quantification of individual miRNAs was performed using the mirVana
PARIS kit (Ambion, Austin, TX, USA), diluting 500 μl of
plasma with 500 μl of binding solution. After a 5-min
incubation, 1 μl of 1 nM ath-miR-159 (Hokkaido System
Science, Hokkaido, Japan) was added to each aliquot as a spike
control for losses in preparation, and the solution was then
vortexed for 30 sec and incubated on ice for 10 min. The subsequent
phenol extraction and filter cartridge steps were carried out
according to the manufacturer’s instructions (15,16).
miRNA expression profile
To assess the levels of specific miRNAs in plasma
samples, a fixed volume of 3 μl of RNA solution from the
50-μl elute was used as input in each reverse transcription
(RT) reaction. The RT reaction and pre-amplification step were set
up according to the manufacturer’s recommendations. miRNAs were
reverse transcribed using the Megaplex™ Primer Pools (Human Pools A
v2.1). RT reaction products from the plasma sample were further
amplified with Megaplex™ PreAmp Primers (Primers A v2.1). The
expression profile of miRNAs was determined using the Human TaqMan
miRNA Array card A (all were from Applied Biosystems, Bedford, MA,
USA). This array enables quantification of 377 human miRNAs and 3
endogenous controls (RNU6B, RNU44 and RNU48). Ath-miR-159 was also
included as an external reference. qRT-PCR was carried out on an
Applied Biosystems 7900HT thermal cycler using the manufacturer’s
recommended program (GeneSifter; VizX Labs, Seattle, WA, USA).
Quantification of individual miRNAs
To confirm the results obtained from the TaqMan
miRNA arrays, we measured expression levels by TaqMan miRNA assays
(hsa-miR-106b; 000442; Applied Biosystems). The input of each RT
reaction consisted of 10 ng of the total RNA. Using SDS2.2 software
(Applied Biosystems), plasma samples were run in duplicates
(15,16). Since we could not detect RNU6B in
plasma, which is commonly used as an internal standard for miRNA
expression analysis in cells, the plasma miRNA expression was
calculated based on the Ct values normalized by those of
ath-miR-159, which was spiked in each qRT-PCR aliquot.
Transforming growth factor-β1
(TGF-β1) measurement
Plasma collected from the 40 COPD patients (20
ex-smokers and 20 current smokers) and 20 healthy controls (10
nonsmokers and 10 current smokers) was used for the measurement of
TGF-β1. The biologically active TGF-β1
concentration was determined using a commercially available ELISA
kit (R&D Systems, Inc., Minneapolis, MN, USA).
Statistical analysis
GraphPad 5.0 software (GraphPad Software, Inc., San
Diego, CA, USA) was used for statistical analysis. The Mann-Whitney
test was used to determine statistical significance between 2
groups, and one-way analysis of variance was used to compare 3 or
more groups. A P-value of <0.05 was considered to indicate a
statistically significant difference. The receiver operating
characteristic (ROC) curve and the area under the ROC curve (AUC)
were used to assess the feasibility of using plasma miR-106b levels
for the diagnosis of COPD. We used the Youden index for
identification of the optimal cut-off point.
Results
COPD patient characteristics
There were no significant differences in age, body
mass index (BMI), FEV1 (% predicted),
FEV1/FVC, duration of smoking and pack-years between
COPD patients who were ex-smokers or current smokers. The duration
of disease since diagnosis in ex-smokers with COPD was
significantly longer than that in current smokers with COPD
(Table II).
 | Table IICharacteristics of COPD patients. |
Table II
Characteristics of COPD patients.
| COPD
| P-value |
|---|
| Ex-smoker | Current smoker |
|---|
| No. of
patients | 20 | 20 | |
| BMI | 22.2±3.5 | 22.5±3.6 | 0.76 |
| FEV1/FVC
(%) | 52.8±14.2 | 58.8±10.6 | 0.14 |
| FEV1 (%
of predicted) | 71.5±25.4 | 79.7±24.1 | 0.30 |
| GOLD stages | | | |
| Stage I (n) | 9 | 12 | 0.40 |
| Stage II (n) | 5 | 4 | |
| Stage III
(n) | 5 | 3 | |
| Stage IV (n) | 1 | 1 | |
| Duration of smoking
(years) | 37.50±7.78 | 41.95±6.24 | 0.053 |
| Duration of smoking
cessation (months) | 59.64±52.17 | 0 | |
| Duration of disease
since diagnosis (months) | 36.80±25.60 | 25.60±27.29 | 0.028a |
| Inhaled
corticosteroid use, yes/no | 3/17 | 1/19 | 0.61 |
| No. of peripheral
neutrophils (/μl) | 3761±1322 | 3987±1232 | 0.58 |
| Plasma
TGF-β1 level (ng/ml) | 36.44±6.53 | 35.58±9.23 | 0.74 |
Identification of differentially
expressed plasma miRNAs in COPD patients and healthy controls
We first compared miRNA expression levels in plasma
obtained from 3 randomly selected nonsmokers and 3 randomly
selected current smokers without COPD. Using the TaqMan low-density
array, we found that 6 miRNAs (miR-499-5p, miR-486-5p, miR-19a,
miR-92a, miR486-3p and miR-133b) appeared to be downregulated in
current smokers without COPD. However, the fold decrease was not
significant; those miRNAs were downregulated <1.5-fold (data not
shown). We next compared miRNA expression in plasma from randomly
chosen current smokers without COPD (n=3) with that of current
smokers with COPD (n=3). According to TaqMan low-density array
screening (card A), 214 of the 381 miRNAs were expressed in all
plasma samples. Of these, 205 miRNAs showed no particular
difference in expression level between these 2 groups. Although we
did not find any miRNA that was preferentially upregulated in COPD
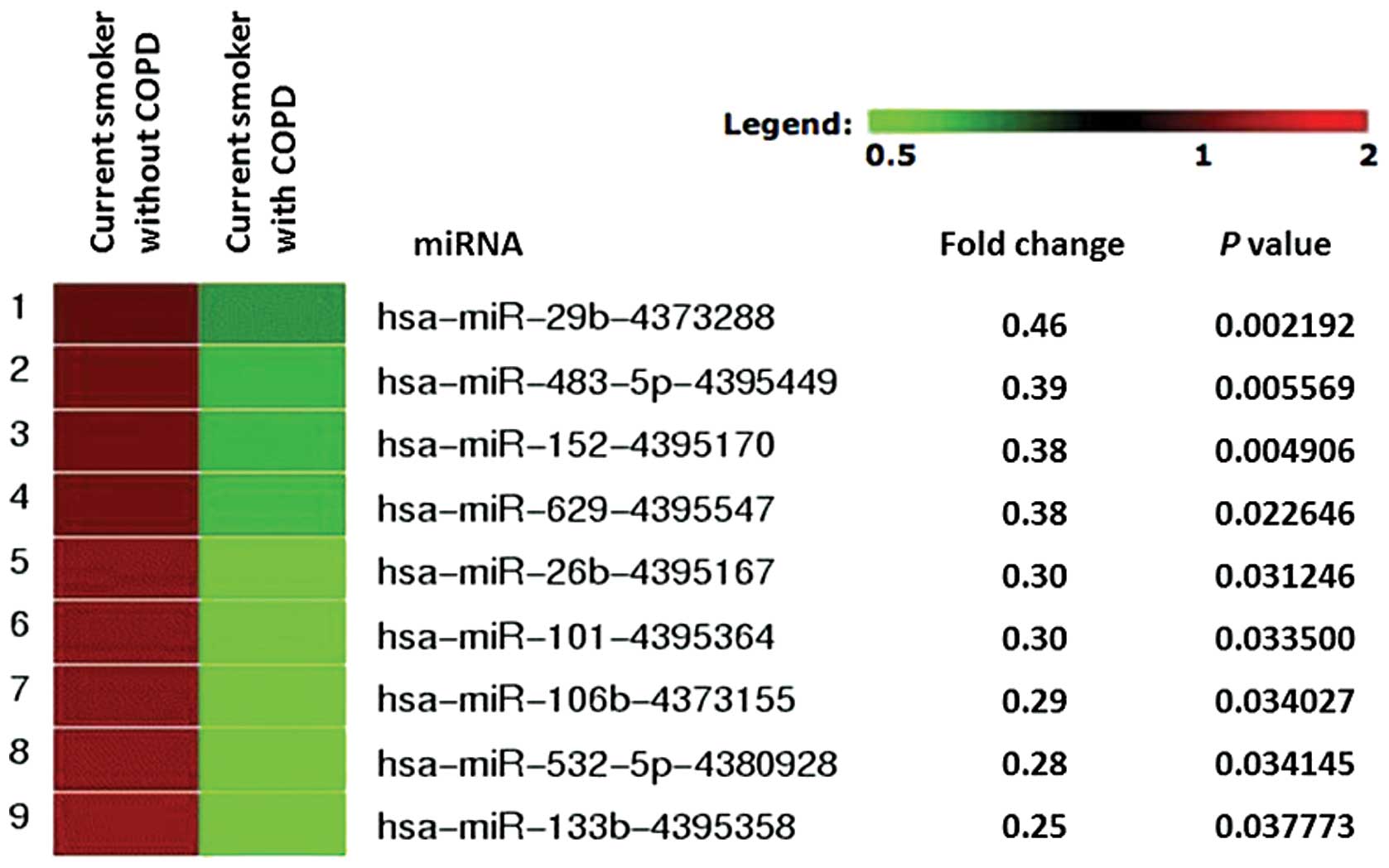
samples, we did find 9 miRNAs (miR-29b, miR-483-5p, miR-152,
miR-629, miR-26b, miR-101, miR-106b, miR-532-5p and miR-133b) that
were significantly downregulated in plasma from COPD patients, with
a fold change threshold of 2.0 or more, using GeneSifter software
(Fig. 1).
Plasma miR-106b levels are significantly
downregulated in patients with COPD
Based on array fold change, P-value, and the
biological relevance of the predicted target by database, such as
TargetScan (Table III), the TGF-β
receptor was thought to be a possible predictive target for
miR-106b; therefore, we chose miR-106b for further analysis.
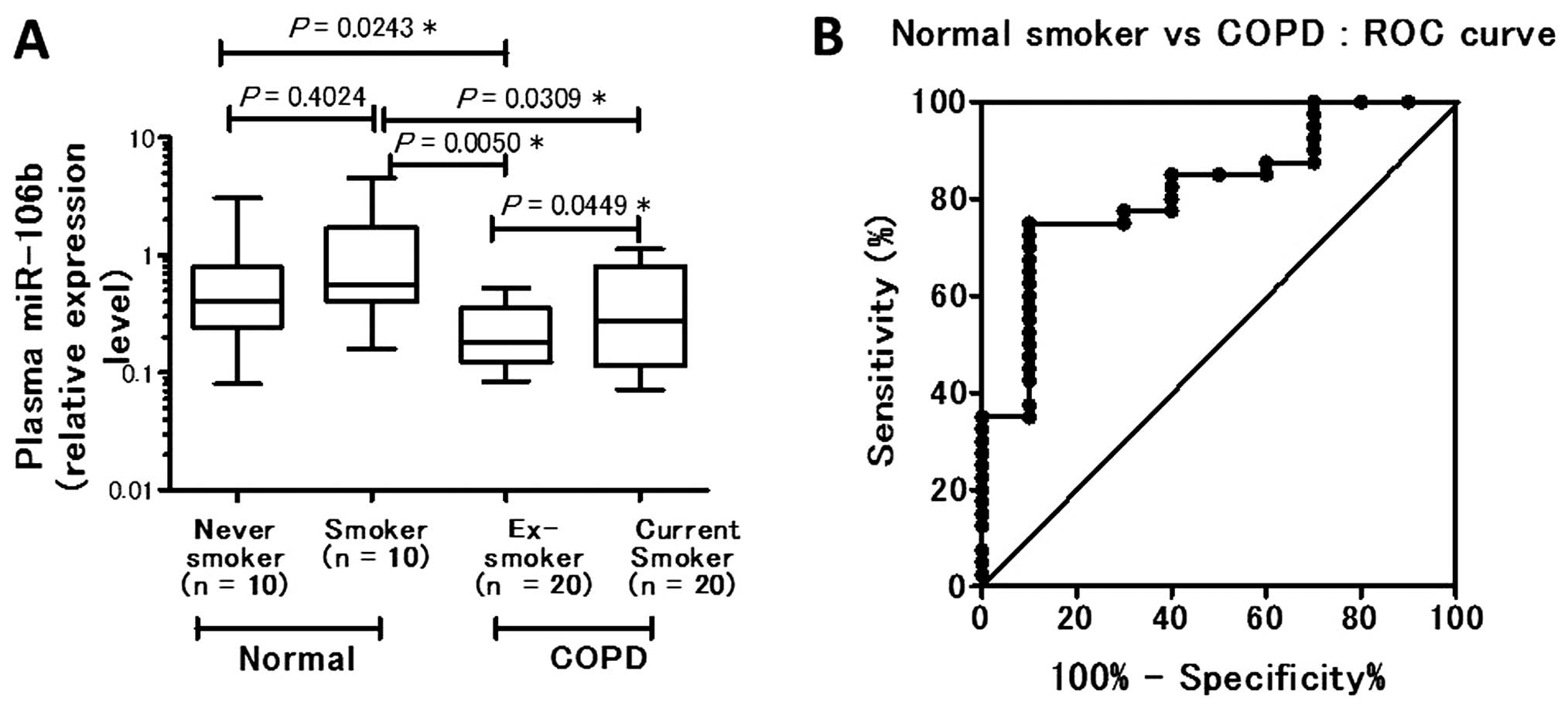
Moreover, the level of plasma miR-106b in COPD patients (n=40) was
found to be significantly lower than that in normal controls (n=20;
P=0.00243) (data not shown). Among the control subjects, no
significant difference in plasma miR-106b level was evident between
nonsmokers and smokers (P= 0.4024). The plasma miR-106b level was
significantly downregulated in COPD ex-smokers (P= 0.0050) and in
COPD current smokers (P= 0.0309) compared with the smokers without
COPD. Among the COPD patients, COPD ex-smokers had a significantly
lower plasma miR-106b level than COPD current smokers (P=0.0449)
(Fig. 2A). Based on the results
obtained from individual qRT-PCRs of miR-106b, we performed further
statistical analysis while combining the data of nonsmokers and
current smokers without COPD as controls.
 | Table IIIPredicted targest for miR-106b. |
Table III
Predicted targest for miR-106b.
| Ankyrin repeat
domain | ANKRD |
| Bone morphogenetic
protein receptor, type 2 | BMPR2 |
| Fibrinogen-like
2 | FGL2 |
| Integrin β8 | ITGB8 |
| Protocadherin
1-protocadherin 13 | PCDHA1–13 |
| Transforming growth
factor, β receptor 2 | TGFBR2 |
Receiver operating characteristic
curve
A receiver operating characteristic curve was
generated using the relative expression level compared with normal
smoker subjects. The AUC was 0.8200, indicating 75.00% sensitivity
(95% CI, 58.80–87.31) and 90.00% specificity (95% CI, 55.50–99.75)
when the cut-off level of plasma miR-106b in COPD patients at
diagnosis was 0.4005 (Fig.
2B).
There were significant differences in age and
duration of smoking between COPD patients with plasma miR-106b
<0.4005 (cut-off level) and those with plasma miR-106b
>0.4005 (Table IV). COPD
patients with plasma miR-106b <0.4005 were older (66.3±6.0 vs.
59.7±9.2 years; P=0.0121) and had smoked for a longer duration
(41.2±7.2 vs. 35.2±6.0 years; P=0.0219).
 | Table IVBackground characteristics of
patients with COPD when subgrouped according to the miR-106b
cut-off level. |
Table IV
Background characteristics of
patients with COPD when subgrouped according to the miR-106b
cut-off level.
| Plasma miR-106b
<0.4005 | Plasma miR-106b
>0.4005 | P-value |
|---|
| No. of
patients | 30 | 10 | |
| Age (years) | 66.3±5.99 | 59.7±9.23 | 0.0121a |
| BMI | 22.0±3.25 | 23.4±4.35 | 0.298 |
| Pack-years of
smoking | 76.3±63.9 | 64.6±42.6 | 0.595 |
| FEV1/FVC
(%) | 54.8±14.0 | 58.6±7.62 | 0.421 |
| FEV1 (%
of predicted) | 74.5±26.3 | 78.9±20.5 | 0.635 |
| Duration of smoking
(years) | 41.2±7.16 | 35.2±6.03 | 0.0219a |
| Duration of smoking
cessation (months) | 36.6±52.2 | 9.60±17.7 | 0.120 |
| Duration of disease
since diagnosis (months) | 30.7±27.9 | 17.20±26.2 | 0.186 |
| No. of peripheral
neutrophils (/μl) | 3866±1172 | 3884±1582 | 0.969 |
| Plasma
TGF-β1 level (ng/ml) | 35.7±8.19 | 37.0±7.29 | 0.660 |
Inverse correlations between plasma
miR-106b levels and duration of disease since diagnosis and
duration of smoking
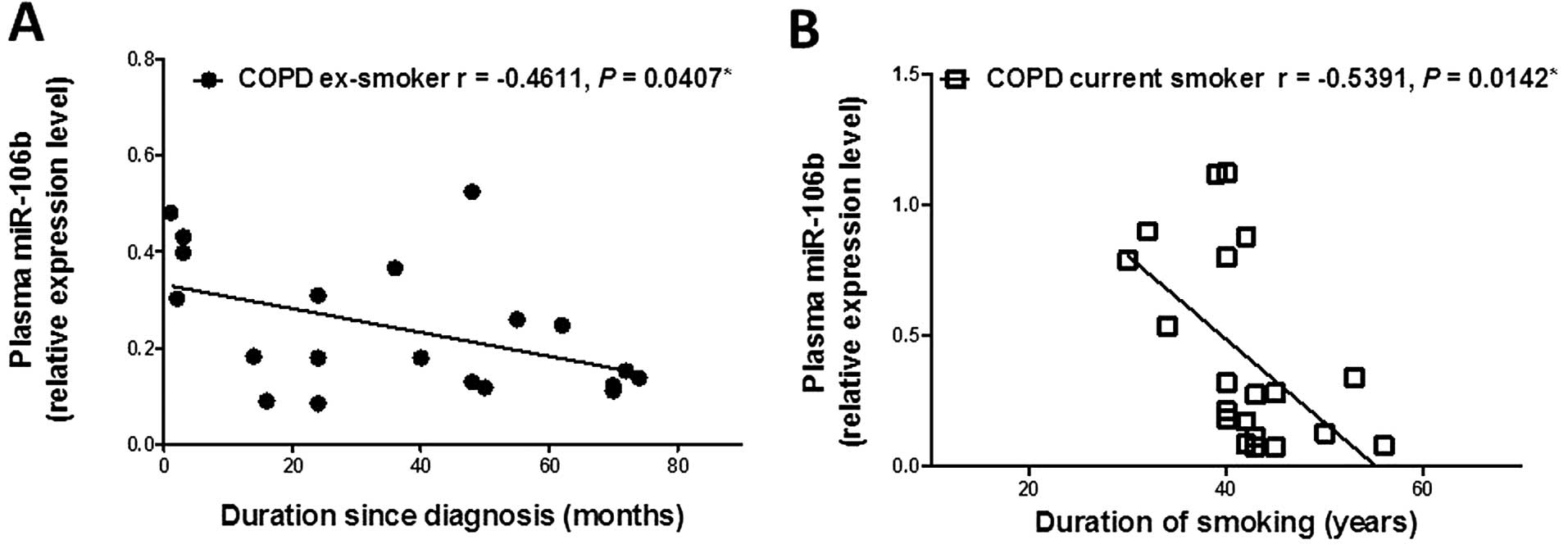
The plasma miR-106b level was inversely correlated
with duration of disease since diagnosis in ex-smokers with COPD
(r=−0.4611, P=0.0407; Fig. 3A),
although there was no relationship between the plasma miR-106b
level and duration of smoking or duration of smoking cessation in
COPD ex-smokers (Table V). The
plasma miR-106b level in COPD current smokers was inversely
correlated with duration of smoking (r=−0.5391, P=0.0142; Fig. 3B), while there was no relationship
between the plasma miR-106b level and duration of disease since
diagnosis in COPD current smokers. Plasma miR-106b levels showed no
relationship with FEV1 (% of predicted),
FEV1/FVC, or GOLD classification in patients with COPD,
suggesting that no relationship existed between plasma miR-106b and
the severity of airflow limitation (Table V).
 | Table VPearson correlation of miR-106b and
clinical characteristics in patients with COPD. |
Table V
Pearson correlation of miR-106b and
clinical characteristics in patients with COPD.
| COPD
|
|---|
Ex-smoker
| Current smoker
|
|---|
| Pearson
r | P-value | Pearson
r | P-value |
|---|
| Age (years) | −0.4031 | 0.0781 | −0.2845 | 0.2241 |
| BMI | 0.1359 | 0.5679 | −0.0716 | 0.7643 |
| Pack-years of
smoking | −0.0262 | 0.9126 | −0.1772 | 0.4547 |
| FEV1/FVC
(%) | 0.2260 | 0.3379 | 0.0842 | 0.7241 |
| FEV1 (%
of predicted) | 0.1009 | 0.6721 | −0.0911 | 0.7025 |
| Duration of smoking
(years) | −0.3147 | 0.1766 | −0.5391 | 0.0142a |
| Duration of smoking
cessation (months) | −0.2133 | 0.3665 | - | - |
| Duration of disease
since diagnosis (months) | −0.4611 | 0.0407a | 0.0911 | 0.7026 |
| No. of peripheral
neutrophils (/μl) | −0.3182 | 0.1716 | 0.3157 | 0.1879 |
| Plasma
TGF-β1 level (ng/ml) | 0.2794 | 0.2329 | −0.0423 | 0.8595 |
Plasma miR-106b and plasma
TGF-β1
The plasma TGF-β1 level was not
significantly elevated in COPD patients compared with healthy
controls. However, the plasma TGF-β1 level was inversely
correlated with duration of smoking cessation in ex-smokers with
COPD (r=−0.5019, P=0.0241) and with FEV1 (% of
predicted) in current smokers with COPD (r=−0.6333, P= 0.0027). The
plasma TGF-β1 level was not correlated with the plasma
miR-106 level or other clinical parameters (Table VI).
 | Table VIPearson correlation of TGF-β1 and
clinical characteristics in patients with COPD. |
Table VI
Pearson correlation of TGF-β1 and
clinical characteristics in patients with COPD.
| COPD
|
|---|
Ex-smoker
| Current smoker
|
|---|
| Pearson
r | P-value | Pearson
r | P-value |
|---|
| Age (years) | −0.3781 | 0.1003 | −0.1938 | 0.4128 |
| BMI | 0.3943 | 0.0854 | 0.1399 | 0.5562 |
| Pack-years of
smoking | 0.2225 | 0.3458 | 0.2640 | 0.2606 |
| FEV1/FVC
(%) | 0.0674 | 0.7778 | −0.3686 | 0.1098 |
| FEV1 (%
of predicted) | −0.0469 | 0.8444 | −0.6333 | 0.0027a |
| Duration of smoking
(years) | −0.2431 | 0.3018 | 0.0468 | 0.8447 |
| Duration of smoking
cessation (months) | −0.5019 | 0.0241a | - | - |
| Duration of disease
since diagnosis (months) | −0.1316 | 0.5803 | −0.2445 | 0.2988 |
| No. of peripheral
neutrophils (/μl) | 0.1447 | 0.5428 | 0.4229 | 0.0712 |
Discussion
To the best of our knowledge, this is the first
report to substantiate the clinical relevance of plasma miRNAs in
patients with COPD. There is an urgent need to clarify the
molecular pathogenesis of COPD to improve our understanding of the
heterogeneity of COPD patients and the therapeutic efficacy of
various treatments (17–19).
This study focused on plasma miRNAs, samples of
which can be easily collected, thereby providing a less invasive
systematic assessment. Plasma miRNAs were profiled using TaqMan
low-density array screening, and miR-106b was selected as a
candidate miRNA. We found that the level of plasma miR-106b in COPD
subjects was lower than that in smokers without COPD. Our findings
indicate that the plasma miR-106b level is related to duration
since diagnosis of COPD and duration of smoking.
In a previous study, airway epithelium was used for
miRNA profiling between smokers and nonsmokers (13). miRNA expression in COPD patients
was also extensively studied in various samples, including sputum
(20), fibroblasts (21), muscle (22) and lung tissue (12). In addition, miRNA profiling of
lung tissues has been performed using cigarette smoke-exposed rats
and a mouse model of lung fibrosis (23,24). Akbas et al (25) currently reported alteration of
serum miRNAs in COPD patients; their results were different from
our data, possibly due to the different technology, sample
materials and race of patients.
The most significant finding of this study was that
the plasma miR-106b levels in the current smoker and ex-smoker COPD
groups were decreased significantly compared with that of normal
smokers. Furthermore, the miR-106b level in the COPD ex-smokers
decreased significantly compared with the level in the COPD current
smokers. This clearly indicates that miR-106b was progressively
downregulated after discontinuation of smoking. This suggests that
this alteration could be linked to a systemic reaction even after
the cessation of smoking, which is characteristic of COPD patients
(26,27). Although it may be difficult to
estimate the exact onset of COPD, the plasma miR-106b level was
inversely correlated with duration of disease since diagnosis, but
not with smoking history or duration of smoking cessation. These
findings suggest a relationship between the progressive reduction
in plasma miR-106b levels and the deterioration of the COPD
condition, even after the discontinuation of smoking.
In silico analysis by microRNA.org
(targets and expression), Targetscan 5.2, and PicTar revealed
several predicted targets of miR-106b, including ankyrin repeat
domain, bone morphogenetic protein receptor type 2, fibrinogen-like
2, inte-grin β8, protocadherins 1–13 and TGF-β receptor 2 (Table III). The crosstalk between
integrins and TGF-β1 signaling has been proposed to
induce the differentiation of airway fibroblasts to myofibroblasts,
resulting in the thickening of small airways in COPD patients
(28). Another study found that
the TGF-β1 level of airway epithelial cells was elevated
in COPD patients (29), although
there were conflicting findings concerning plasma TGF-β1
levels in patients with COPD (30,31). Although we did not perform a
functional analysis to prove a relationship between miR-106b and
target genes, miR-106b may be involved in TGF-β1
signaling, since miR-106b regulates the cyclin-dependent kinase
inhibitor p21/CDKN1A, which is downstream of TGF-β1
(32,33).
In this study, we found a significant improvement in
the plasma TGF-β1 level in relation to the length of the
period of smoking cessation in COPD patients. In other words, an
elevated TGF-β1 level was associated with the
progressive decline of FEV1 (i.e., airway limitation),
and the TGF-β1 level decreased depending on the duration
of smoking cessation. In contrast, the plasma miR-106b level
progressively decreased, even after the COPD patients stopped
smoking. These observations suggest that the plasma
TGF-β1 level was linked to airway limitation due to
current smoking, whereas the plasma miR-106b level was linked to
the mechanism underlying persistent and systemic changes in COPD
patients. Therefore, the plasma miR-106b level could be an
important clinical indicator for COPD.
Although the number of patients studied was quite
small to draw a definitive conclusion, our findings suggest that
progressive reduction in the plasma miR-106b level may reflect
persistent and systemic changes even after the discontinuation of
smoking in COPD patients. Although the biological implications of
molecules regulated by miR-106b need to be clarified in future
research, the measurement of the plasma miR-106b level could
provide important information concerning COPD patients in clinical
practice.
Acknowledgements
We thank Mrs. C. Kobayashi for her
technical assistance. We are grateful to Associate Professor Edward
F. Barroga and Professor J. Patrick Barron, chairman of the
Department of International Medical Communications of Tokyo Medical
University, for their editorial review of this manuscript. This
study was supported by the Private University Strategic Research
Based Support Project ‘Epigenetics Research Project Aimed at
General Cancer Cure Using Epigenetic Targets’, from the Ministry of
Education, Culture, Sports, Science and Technology, Tokyo,
Japan.
References
|
1
|
Agusti A and Soriano JB: COPD as a
systemic disease. COPD. 5:133–138. 2008. View Article : Google Scholar
|
|
2
|
Fabbri LM, Luppi F, Beghe B and Rabe KF:
Complex chronic comorbidities of COPD. Eur Respir J. 31:204–212.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Celli BR, Cote CG, Marin JM, et al: The
body-mass index, airflow obstruction, dyspnea, and exercise
capacity index in chronic obstructive pulmonary disease. N Engl J
Med. 350:1005–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barnes PJ and Celli BR: Systemic
manifestations and comorbidities of COPD. Eur Respir J.
33:1165–1185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sinden NJ and Stockley RA: Systemic
inflammation and comorbidity in COPD: a result of ‘overspill’ of
inflammatory mediators from the lungs? Review of the evidence.
Thorax. 65:930–936. 2010.
|
|
6
|
Decramer M, Janssens W and Miravitlles M:
Chronic obstructive pulmonary disease. Lancet. 379:1341–1351. 2012.
View Article : Google Scholar
|
|
7
|
Chen HY, Yu SL, Li KC and Yang PC:
Biomarkers and transcriptome profiling of lung cancer. Respirology.
17:620–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katoh Y and Katoh M: Hedgehog signaling,
epithelial-tomesenchymal transition and miRNA (Review). Int J Mol
Med. 22:271–275. 2008.PubMed/NCBI
|
|
9
|
Lee EM, Shin S, Cha HJ, et al:
Suberoylanilide hydroxamic acid (SAHA) changes microRNA expression
profiles in A549 human non-small cell lung cancer cells. Int J Mol
Med. 24:45–50. 2009.PubMed/NCBI
|
|
10
|
Mitchell PS, Parkin RK, Kroh EM, et al:
Circulating microRNAs as stable blood-based markers for cancer
detection. Proc Natl Acad Sci USA. 105:10513–10518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Etheridge A, Lee I, Hood L, Galas D and
Wang K: Extracellular microRNA: a new source of biomarkers. Mutat
Res. 717:85–90. 2011.PubMed/NCBI
|
|
12
|
Ezzie ME, Crawford M, Cho JH, et al: Gene
expression networks in COPD: microRNA and mRNA regulation. Thorax.
67:122–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schembri F, Sridhar S, Perdomo C, et al:
MicroRNAs as modulators of smoking-induced gene expression changes
in human airway epithelium. Proc Natl Acad Sci USA. 106:2319–2324.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hunter MP, Ismail N, Zhang X, et al:
Detection of microRNA expression in human peripheral blood
microvesicles. PloS One. 3:e36942008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohyashiki JH, Umezu T, Kobayashi C, et al:
Impact on cell to plasma ratio of miR-92a in patients with acute
leukemia: in vivo assessment of cell to plasma ratio of miR-92a.
BMC Res Notes. 3:3472010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshizawa S, Ohyashiki JH, Ohyashiki M, et
al: Downregulated plasma miR-92a levels have clinical impact on
multiple myeloma and related disorders. Blood Cancer J. 2:e532012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vestbo J and Rennard S: Chronic
obstructive pulmonary disease biomarker(s) for disease activity
needed urgently. Am J Respir Crit Care Med. 182:863–864. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han MK: Update in chronic obstructive
pulmonary disease in 2010. Am J Respir Crit Care Med.
183:1311–1315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakao S and Tatsumi K: The importance of
epigenetics in the development of chronic obstructive pulmonary
disease. Respirology. 16:1056–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pottelberge GR, Mestdagh P, Bracke KR, et
al: MicroRNA expression in induced sputum of smokers and patients
with chronic obstructive pulmonary disease. Am J Respir Crit Care
Med. 183:898–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato T, Liu X, Nelson A, et al: Reduced
miR-146a increases prostaglandin E in chronic obstructive pulmonary
disease fibroblasts. Am J Respir Crit Care Med. 182:1020–1029.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewis A, Riddoch-Contreras J, Natanek SA,
et al: Downregulation of the serum response factor/miR-1 axis in
the quadriceps of patients with COPD. Thorax. 67:26–34. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Izzotti A, Calin GA, Arrigo P, Steele VE,
Croce CM and De Flora S: Downregulation of microRNA expression in
the lungs of rats exposed to cigarette smoke. FASEB J. 23:806–812.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cushing L, Kuang PP, Qian J, et al: miR-29
is a major regulator of genes associated with pulmonary fibrosis.
Am J Respir Cell Mol Biol. 45:287–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akbas F, Coskunpinar E, Aynaci E, Oltulu
YM and Yildiz P: Analysis of serum micro-RNAs as potential
biomarker in chronic obstructive pulmonary disease. Exp Lung Res.
38:286–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rutgers SR, Postma DS, ten Hacken NH, et
al: Ongoing airway inflammation in patients with COPD who do not
currently smoke. Thorax. 55:12–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lapperre TS, Postma DS, Gosman MM, et al:
Relation between duration of smoking cessation and bronchial
inflammation in COPD. Thorax. 61:115–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Margadant C and Sonnenberg A:
Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing.
EMBO Rep. 11:97–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takizawa H, Tanaka M, Takami K, et al:
Increased expression of transforming growth factor-beta1 in small
airway epithelium from tobacco smokers and patients with chronic
obstructive pulmonary disease (COPD). Am J Respir Crit Care Med.
163:1476–1483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mak JC, Chan-Yeung MM, Ho SP, et al:
Elevated plasma TGF-beta1 levels in patients with chronic
obstructive pulmonary disease. Respir Med. 103:1083–1089. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gong Y, Fan L, Wan H, et al: Lack of
association between the TGF-β(1) gene and development of COPD in
Asians: a case-control study and meta-analysis. Lung. 189:213–223.
2011.
|
|
32
|
Marwick JA, Kirkham P, Gilmour PS,
Donaldson K, Mac NW and Rahman I: Cigarette smoke-induced oxidative
stress and TGF-beta1 increase p21waf1/cip1 expression in alveolar
epithelial cells. Ann NY Acad Sci. 973:278–283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ivanovska I, Ball AS, Diaz RL, et al:
MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote
cell cycle progression. Mol Cell Biol. 28:2167–2174. 2008.
View Article : Google Scholar : PubMed/NCBI
|