Introduction
Accumulating evidence suggests that adipose
tissue-derived mesenchymal stem cells (AT-MSCs) possess the ability
to transdifferentiate into other cell types including hepatocytes,
similar to bone marrow-derived stem cells (BMSCs). Previous studies
have shown that AT-MSCs differentiate into hepatocyte-like cells by
culture with appropriate culture conditions, such as various
cytokine mixtures (1–5). However, the efficacy of the hepatic
differentiation of AT-MSCs is insufficient for therapeutic
application and the role of extrahepatic stem cells in liver
regeneration remains poorly understood. A successful
differentiation is generally achieved by the step-by-step addition
of growth factors, cytokines, and hormones, which emulate the
sequence of events occurring during in vivo hepatogenesis
(6). Therefore, studies have been
conducted to explore novel means of facilitating differentiation of
MSCs into hepatocytes. At present, no induction method can
completely represent or mimic the pathological environment of the
various types of liver diseases, as there is a marked regulation
and complicated interaction in vivo under certain
pathological environments. For example, liver regeneration after
partial hepatectomy (PH) injury is an extremely complex and
well-orchestrated phenomenon (7).
PH triggers a sequence of events that proceed in an orderly manner
and are observed from the first 5 min to 5–7 days, and induces
rapid induction of >100 genes not expressed in normal liver
(8). A number of cytokines are
upregulated during acute liver injury, including tumor necrosis
factor-α (TNF-α), interleukin-6 (IL-6), hepatocyte growth factor
(HGF), transforming growth factor-α (TGF-α), macrophage
inflammatory protein-2 (MIP-2), stem cell factor (SCF), and various
other cytokines (9–11).
Changes in the concentrations of the above signaling
molecules in the plasma likely account for the fact that the
signals for liver regeneration are transmitted by blood in pairs of
parabiotic rats (12) or isolated
hepatocytes in the adipose tissue when the orthotopic liver is
subjected to partial hepatectomy (13). By mimicking the injured liver
microenvironment, Wang et al (14) isolated BMSCs to induce
differentiation of these cells into hepatocyte-like cells with HGF
in vitro. Yang et al also reported that serum from
liver radiofrequency ablation (RFA)-treated rats induced the
differentiation of BMSCs into hepatic progenitor cells more
efficiently compared with HGF (15), suggesting that serum under certain
pathological environments may provide the ideal induction
conditions to study unknown events that BMSCs may be involved in
in vivo. Therefore, the present study aimed to determine
whether serum from hepatectomized rats is able to induce the
differentiation of AT-MSCs into hepatocyte-like cells, and to
explore the possible role of AT-MSCs in vivo during liver
regeneration.
Materials and methods
Materials
Low-glucose Dulbecco’s modified Eagle’s medium
(L-DMEM), fetal bovine serum (FBS) (both were from Invitrogen Life
Technologies, Carlsbad, CA, USA), and 0.25%
trypsin-ethylenediaminetetraacetic acid (EDTA; Sino-American
Company, Shanghai, China) were used for cell cultures. All other
chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA),
unless otherwise specified.
Experimental animals
Sixty-five male Sprague-Dawley (SD) rats weighing
140–160 g were obtained from the Laboratory Animals Center of Wuhan
University (Wuhan, Hubei, China). Experiment procedures were
carried out in accordance with the Code of Ethics of the World
Medical Association.
Isolation and culture of AT-MSCs
AT-MSCs were isolated from the groin adipose tissue
of SD rats. The capillaries of the adipose tissue were removed and
washed three times with phosphate-buffered solution (PBS) under
aseptic conditions. The adipose tissue was then minced into 3–5 mm
sections using scissors and scalpels prior to digestion using 0.1%
collagenase solution for 50 min in a water bath at 37°C. The
mixture was centrifuged at 1,500 rpm for 8 min at room temperature
(25°C), and the supernatant containing adipose tissue was
discarded. After the collected cells were washed three times with
PBS (1,500 rpm, 8 min), they were resuspended in L-DMEM containing
15% FBS, 1% L-glutamine, and 1% penicillin and streptomycin. Cells
(1×106 cells/ml)were seeded in culture flasks (T25;
Corning Inc., Corning, NY, USA) and maintained in a humidified
atmosphere of 95% air and 5% CO2 at 37°C. When the cells
reached 80–90% confluence, they were collected with 0.25%
Trypsin-EDTA and diluted 1:2 or 1:3 at each passage.
Flow cytometry (FACS) analysis
AT-MSCs of passage 3 (P3) were analyzed using a
FACSCalibur (FC500) Flow Cytometer (Becton-Dickinson, San Jose, CA,
USA). PE-conjugated CD34 (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), and fluorescein isothiocyanate (FITC)-conjugated
CD44, CD45 and CD90 (AbD Serotec) antibodies were employed.
Differentiation potential of AT-MSCs
AT-MSCs from P3 to passage 5 (P5) were seeded into
6-well plates at 3×104 cells/cm2 and grown to
90% confluence for the differentiation studies mentioned above. The
cells were treated with each induction medium for at least two
weeks, changing the medium twice a week.
Adipogenic differentiation protocol
The adipogenic medium consisted of L-DMEM
supplemented with 10% FBS, 0.5 mM 3-isobutyl-1-methylxanthine, 1
μM dexamethasone, 200 μM indomethacin, 10
μg/ml bovine insulin, and 1% penicillin and
streptomycin.
Osteogenic differentiation protocol
Osteogenic medium consisted of L-DMEM supplemented
with 10% FBS, 0.1 M dexamethasone, 0.3 mM ascorbic acid, and 10 mM
β-glycerol phosphate.
Hepatic differentiation protocol
Hepatic differentiation was induced over a period of
two weeks based on the published differentiation protocols
(16) with minor modifications.
The hepatic differentiation medium consisted of L-DMEM supplemented
with 10% FBS, 10 μg/ml transferrin, 10 μg/ml insulin,
0.1 mM ascorbic acid, 0.1 μM dexamethasone, 10 ng/ml HGF
(PeproTech; Rocky Hill, NJ, USA), 10 ng/ml bFGF (PeproTech), and 10
ng/ml OSM (PeproTech).
Immunofluorescence analysis
On days 7 and 14, samples of cultured cells were
rinsed and fixed with 4% paraformaldehyde at 4°C for 30 min. The
cells were permeabilized with 0.1% Triton X-100 for 15 min. After
blocking the fixed cells for 30 min at 37°C with 1% bovine serum
albumin (ALB), the cells were incubated overnight at 4°C in a
humidity chamber with the respective primary antibodies: rabbit
polyclonal anti-ALB (1:100) and goat polyclonal anti-α-fetoprotein
(AFP; 1:100) (both from Santa Cruz Biotechnology, Inc.). The cells
were then incubated with the corresponding secondary antibody:
rhodamine (TRITC)-conjugated donkey anti-rabbit IgG (1:100) and
FITC-conjugated Affinipure donkey anti-goat IgG (1:100) (both from
Proteintech Group, Inc., Chicago, IL, USA) for 30 min at room
temperature. After rinsing, the nuclei were counterstained with 5
μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Beyotime, China).
The cells were then visualized with a fluorescence microscope
(Olympus BX51, Japan), and the images were analyzed using Image-Pro
Plus 6.0.
Hepatic basal function evaluation of the
differentiation cells
Cells differentiated on days 0, 3, 6, 9, 12, 15, 18,
21 and 24 were extensively washed with PBS, and then incubated with
L-DMEM containing 5 mM NH4Cl overnight (12 h) in 5%
CO2 at 37°C prior to analysis. The supernatant was
collected and the urea concentration was measured using a
colorimetric assay kit (BioVision, USA) according to the
manufacturer’s instructions.
ALB production was evaluated on days 0, 6, 12, 18
and 24 using a quantitative enzyme-linked immunoassay kit (rat
albumin ELISA; ALPCO Diagnostics, Salem, NH, USA) according to the
manufacturer’s instructions.
Cytochrome P450 type 3A4 (CYP3A4) enzyme activity
was assessed by measurement of luciferase activity with the
P450-Glo™ CYP3A4 Assay (Luciferin-PFBE; Promega, Madison, WI, USA)
according to the manufacturer’s instructions. Briefly, the cells
were cultured in L-DMEM supplemented with 1 mmol/l phenobarbital
(Sigma) for 48 h with a daily medium change. The cells were
incubated at 37°C in L-DMEM supplemented with 50 μmol/l
luciferin PFBE (150 μl/well). After 3 h of incubation, 50
μl of medium was transferred in a opaque white 96-well plate
and mixed with 50 μl of luciferin detection reagent to
initiate luminescent reaction. After 20-min incubation at room
temperature, luminescence was measured with a Victor 3 luminometer
(Perkin-Elmer, Boston, MA, USA).
Establishment of 70% PH and collection of
post-hepatectomy serum
The 70% PH procedure was performed through a midline
abdominal incision (17) under
anesthesia with sodium pentobarbital (35–40 mg/kg, i.m. n=5). For
the sham operation (n=5), a similar surgical approach was used but
only a portion of the xiphoid was excised. Approximately 24 h after
surgery, blood samples were collected from the ventricle. The blood
samples were placed at 4°C for 2 h and centrifuged at 3,000 rpm for
10 min (4°C). The upper layer of each blood sample was aliquoted
into microcentrifuge tubes and stored at −80°C for future use.
Surgery was performed using sterile surgical techniques. Surgical
procedures were performed between 8 and 11 a.m. to minimize the
influence of diurnal rhythms on subsequent hepatocyte
proliferation.
AT-MSCs treated with post-hepatectomy
serum
P3–P5 AT-MSCs were induced to differentiate under
the following conditions: i) FBS group, L-DMEM supplemented with
10% FBS; ii) serum group, L-DMEM supplemented with 70% PH serum to
a final concentration of 10%; iii) HGF group, induction composition
is the same as the hepatic differentiation medium; iv) Sham group,
L-DMEM supplemented with serum from rats that had undergone the
sham operation to a final concentration of 10%. On days 7 and 14,
the cell type was identified. The abovementioned induction
experiments were repeated using three sets of serum (the serum
collected from one rat representing one set).
AT-MSC transplantation following PH
Approximately 24 h after 70% PH, ∼1×106
cells/rat in 200 μl of PBS were transfused into the tail
vein with a 30-gauge needle for >1 min (AT-MSC group, n=12).
Rats treated with PBS alone (PBS group, n=12) and untreated rats
(untreated group, n=12) served as the control groups. The animals
were sacrificed at 48 and 72 h after PH (n=6 for each time-point in
all treatment groups). Blood samples were collected from the
ventricle and the residual livers were harvested and weighed, and
then properly preserved for the subsequent procedures.
IL-6 and HGF concentration analysis by
ELISA
On days 3 and 6 of the cell culture, the culture
medium was removed and the cells were washed twice with PBS and
replenished with serum-free L-DMEM. After 24 h, the culture
supernatant was harvested and centrifuged at 3,000 rpm for 10 min
at 25°C. The concentrations of IL-6 and HGF were measured following
the manufacturer’s instructions using commercial ELISA kits
(Quantikine Mouse IL-6 ELISA kit and Mouse HGF Duoset ELISA
Development kit; Biotechnology Systems). The cells in the plate
were harvested by 0.25% trypsin-EDTA digestion, and the number of
cells was determined by hematocytometer counts. The total RNA was
then isolated.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from AT-MSCs cultured in each
group for mRNA expression analysis. cDNA synthesis and the
polymerase chain reaction (PCR) were performed using the ReverTra
Ace Reverse Transcriptase kit (Toyobo Co., Ltd., Tokyo, Japan) and
the Hot Start PCR kit (Takara), respectively, following the
manufacturer’s instructions. The PCR primer sequences and
conditions are shown in Table I.
The PCR reaction was 30 cycles.
 | Table IPrimer sequences and product
sizes. |
Table I
Primer sequences and product
sizes.
| Gene name | Primer
sequences | Product size
(bp) | T(°C)
annealing |
|---|
| IL-6 | Sense
5′-GCCTTCTTGGGACTGATGT-3′ | 541 | 61 |
| Antisense
5′-TGAGTTGGATGGTCTTGGT-3′ | | |
| HGF | Sense
5′-ACATCACTCCCGAGAACTT-3′ | 520 | 61 |
| Antisense
5′-AAACTAACCATCCACCCTAC-3′ | | |
| GAPDH | Sense
5′-CAGTGCCAGCCTCGTCTCAT-3′ | 595 | 65 |
| Antisense
5′-AGGGGCCATCCACAGTCTTC-3′ | | |
Assessment of liver functions
The serum alanine aminotransferase (ALT), aspartate
aminotransferase (AST), and ALB, were measured using a multiple
serum biochemical analyzer (Hitachi, Japan).
Liver regeneration rate
The total body weight of each rat that fasted was
weighed before it was sacrificed. The residual liver was then
excised and weighed. The ratio of the residual liver weight to the
whole body weight was utilized instead of the weight of the
residual liver alone.
Histology and immunohistochemistry
For conventional morphological evaluation, liver
tissue was fixed with 10% formalin, embedded with paraffin,
sectioned, and stained with hematoxylin and eosin using standard
histological techniques. The sections were also immunostained for
Ki-67 according to the manufacturer’s instructions. The samples
were counterstained with hematoxylin. The Ki-67-positive cells were
counted in at least five randomly selected high-power fields (×400)
per slide.
Statistical analysis
Data are presented as the mean ± standard
deviations. Statistical significance was calculated using the SPSS
19.0 program. The Student’s t-test was used for the pairwise
comparisons and one-way ANOVA was used for the multiple
comparisons. P<0.05 was considered statistically
significant.
Results
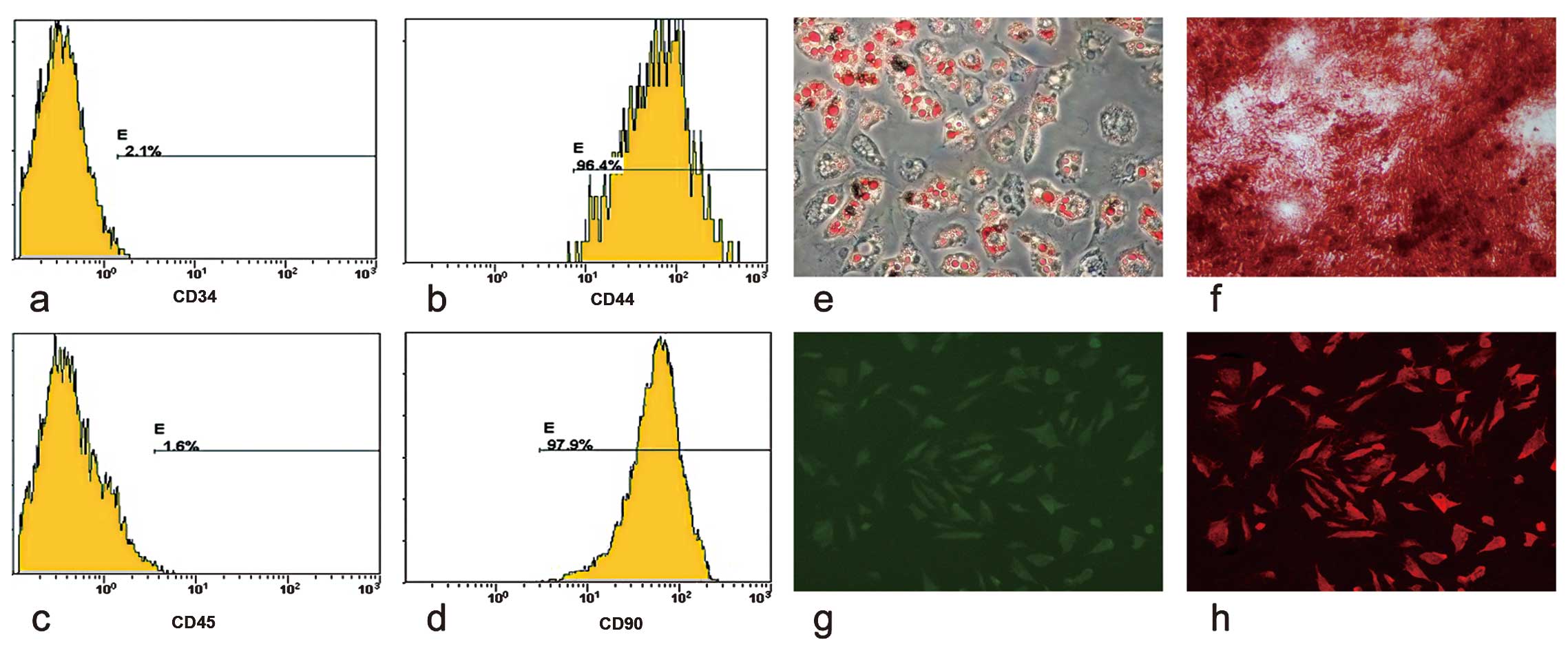
Characterization of AT-MSCs
The FACS analysis demonstrated that AT-MSCs
expressed CD44 and CD90 (Fig. 1b and
d), but not CD34 and CD45 (Fig.
1a and c). Two weeks after their exposure to adipogenic and
four weeks after exposure to osteogenic induction medium,
intracellular lipid droplets (Fig.
1e) and extracellular calcium phosphate precipitates (Fig. 1f) were observed using Oil Red O
and Alizarin-Red staining. Two weeks after the induction of hepatic
differentiation, AFP (green) and ALB (red) were observed using
immunofluorescence (Fig. 1g and
h).
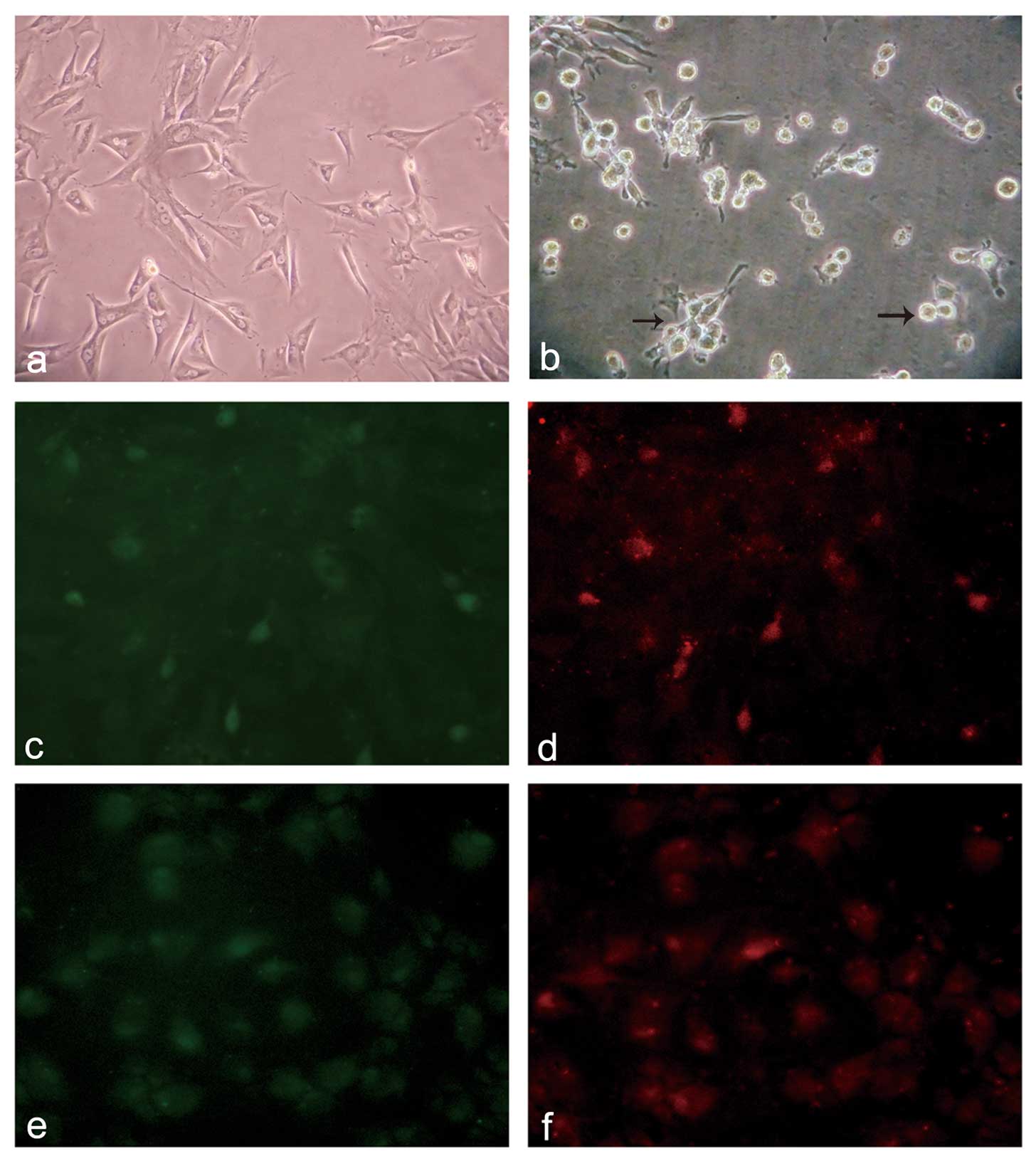
Morphological observations and
immunofluorescence staining of AT-MSCs cultured with
post-hepatectomy serum
After culturing the cells with the serum for one
week, some spindle-shaped cells transformed into round or polygonal
hepatocyte-like cells gradually, the ratio of the nucleoplasm
decreased, and adhesion ability weakened and was associated with a
reduced number of cells in the serum group at the time of the
medium change (Fig. 2a and b). By
contrast, the cells in the FBS and Sham groups maintained the
spindle-shaped phenotype of AT-MSCs. Following the two-week
induction, the green fluorescence of the FITC-labeled AFP and the
red fluorescence of the TRITC-labeled ALB were observed both in the
serum and HGF groups (Fig. 2c–f).
However, more positive cells were observed in the HGF group
compared with the serum group (positive cell ratio, 53.12±0.76 vs.
24.35±2.0, P<0.001).
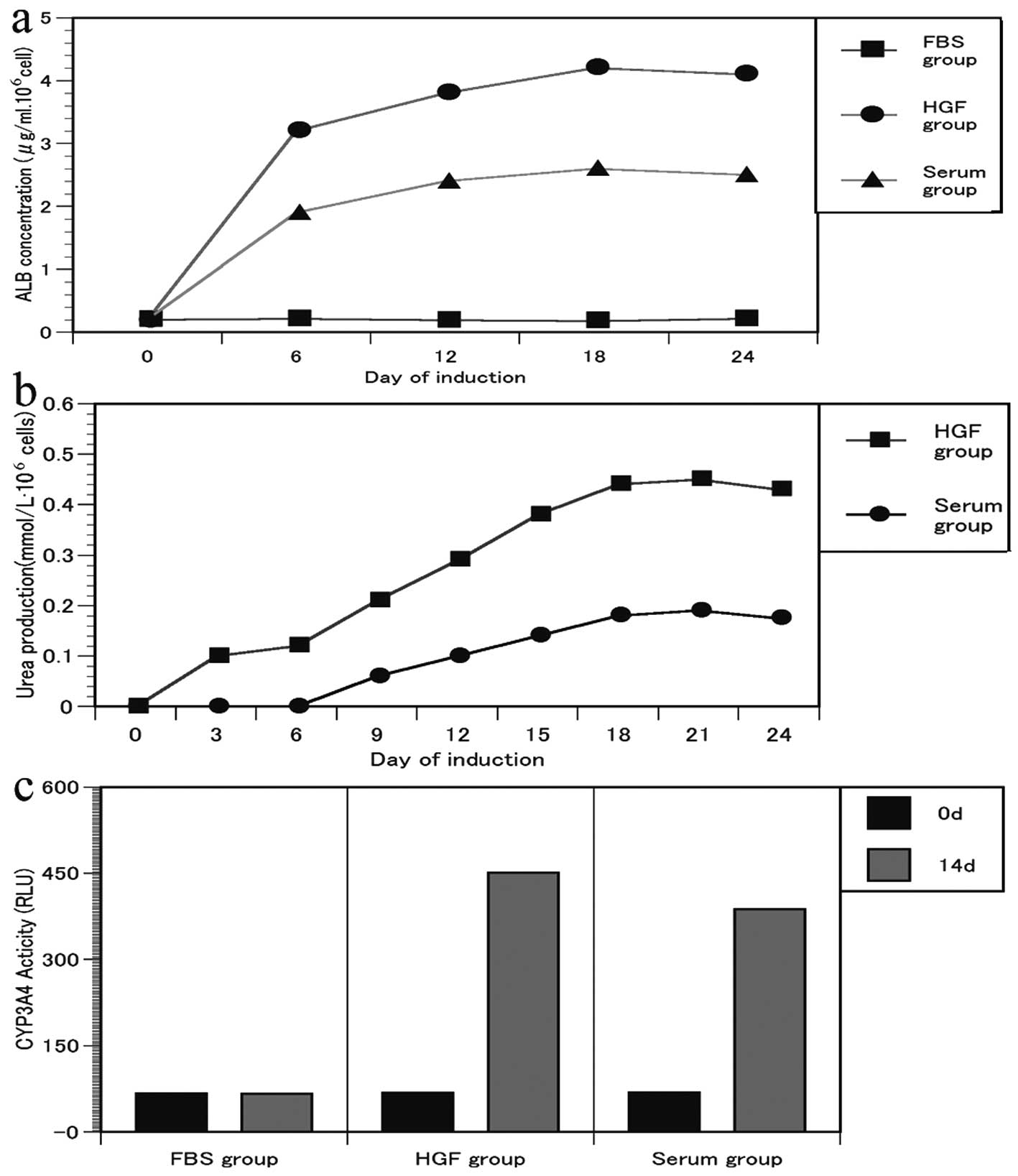
ALB secretion, urea synthesis and CYP3A4
enzyme activity
The cells produced ALB during differentiation and
the secreted ALB increased 2-fold during the 24 days of
differentiation (Fig. 3a). ALB
was almost not detected in supernatants of the FBS group.
Urea production was present and the urea levels
gradually increased until day 21 in a time-dependent manner in the
HGF and serum groups (Fig. 3b).
Cells in the FBS and Sham groups did not produce urea (data not
shown).
Activity of the CYP3A4 enzyme was detected in
differentiated AT-MSCs and was significantly enhanced when cells
were cultured with 1 mmol/l phenobarbital compared with
undifferentiated AT-MSCs (Fig.
3c). These changes suggest that the AT-MSCs had undergone
hepatocyte maturation.
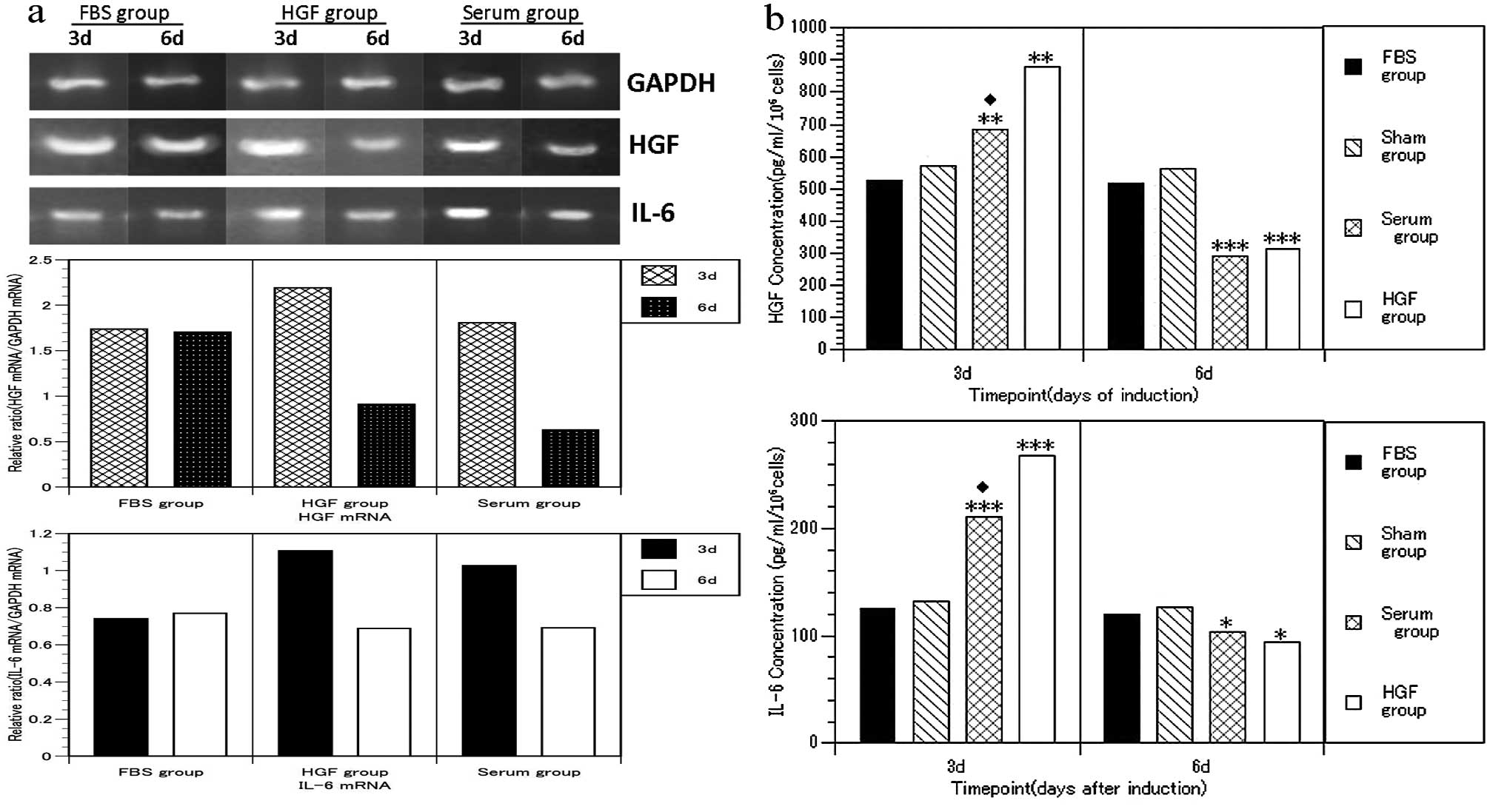
mRNA expression and cytokine
concentration in culture supernatant
An increase in IL-6 and HGF levels was detected (day
3) in the culture supernatant in the HGF and serum groups (Fig. 4b) (P<0.01) compared with the
FBS and Sham groups. However, these levels significantly decreased
on day 6 (P<0.05), coinciding with the changes in mRNA
expression (Fig. 4a).
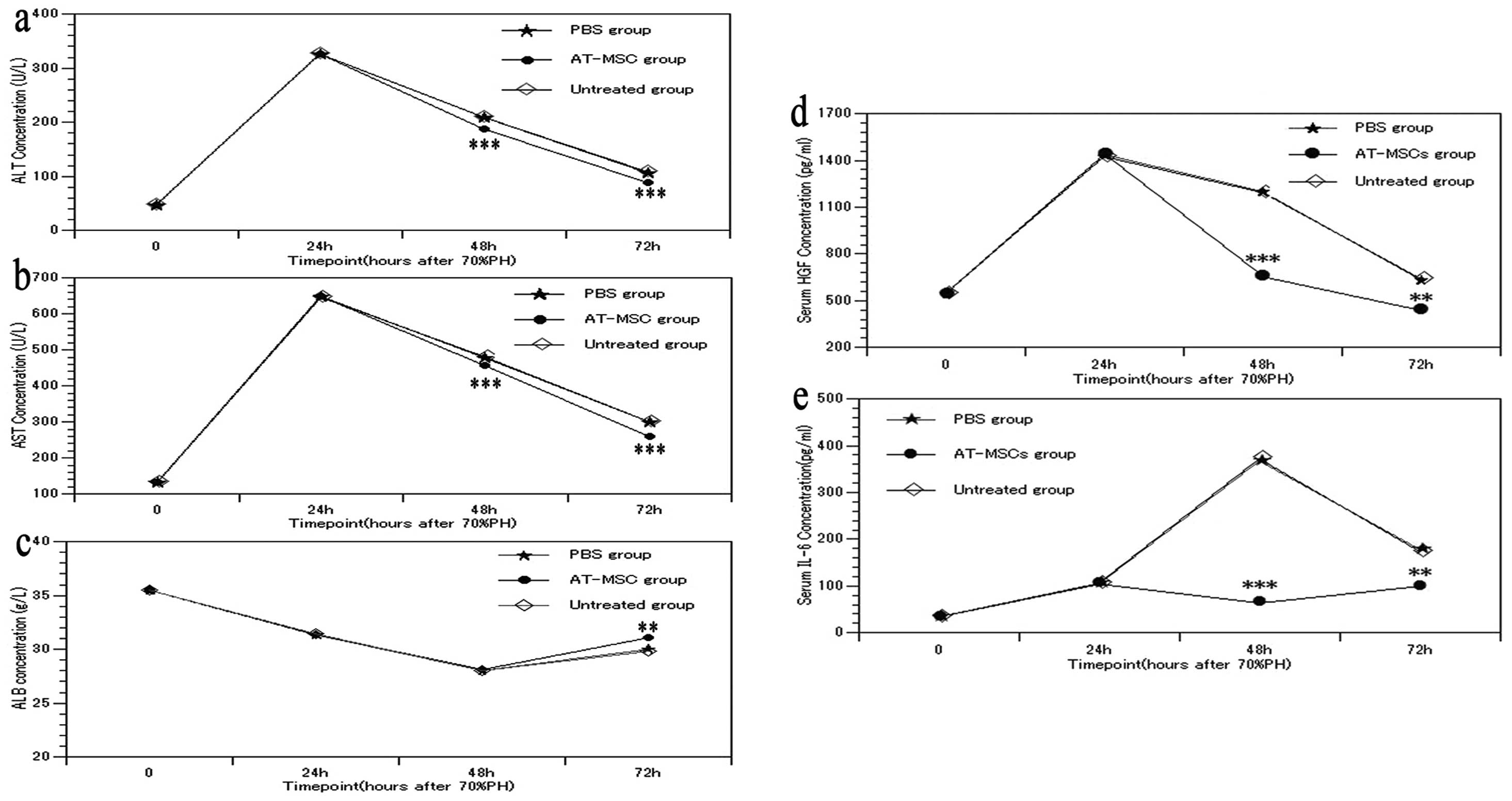
Effect of AT-MSC transplantation on liver
function after 70% PH
Approximately 24 h after AT-MSC transplantation, the
ALT and AST levels decreased to a level lower than that of the PBS
and untreated groups, and ∼48 h after the transplantation, this
change became more pronounced (Fig.
5a and b) (P<0.001). Simultaneously, ∼48 h after AT-MSC
transplantation, the ALB level in the AT-MSC group significantly
increased compared with that in the PBS and untreated groups
(Fig. 5c) (P<0.01). Thus,
AT-MSC transplantation attenuated hepatocyte injury and improved
the protein synthesis ability of the hepatocytes.
IL-6 and HGF concentration in serum after
AT-MSC transplantation
After 70% PH, the serum levels of HGF and IL-6 in
the PBS and untreated groups peaked within 24 h, gradually
decreased, and then reached normal levels after 72 h (Fig. 5d and e). However, the HGF and IL-6
levels in the AT-MSC group were significantly decreased after 24 h
of the transplantation (P<0.01) compared with the PBS and
untreated groups. This decrease was even closer to that of their
normal levels, showing that AT-MSC transplantation altered HGF and
IL-6 expression in vivo.
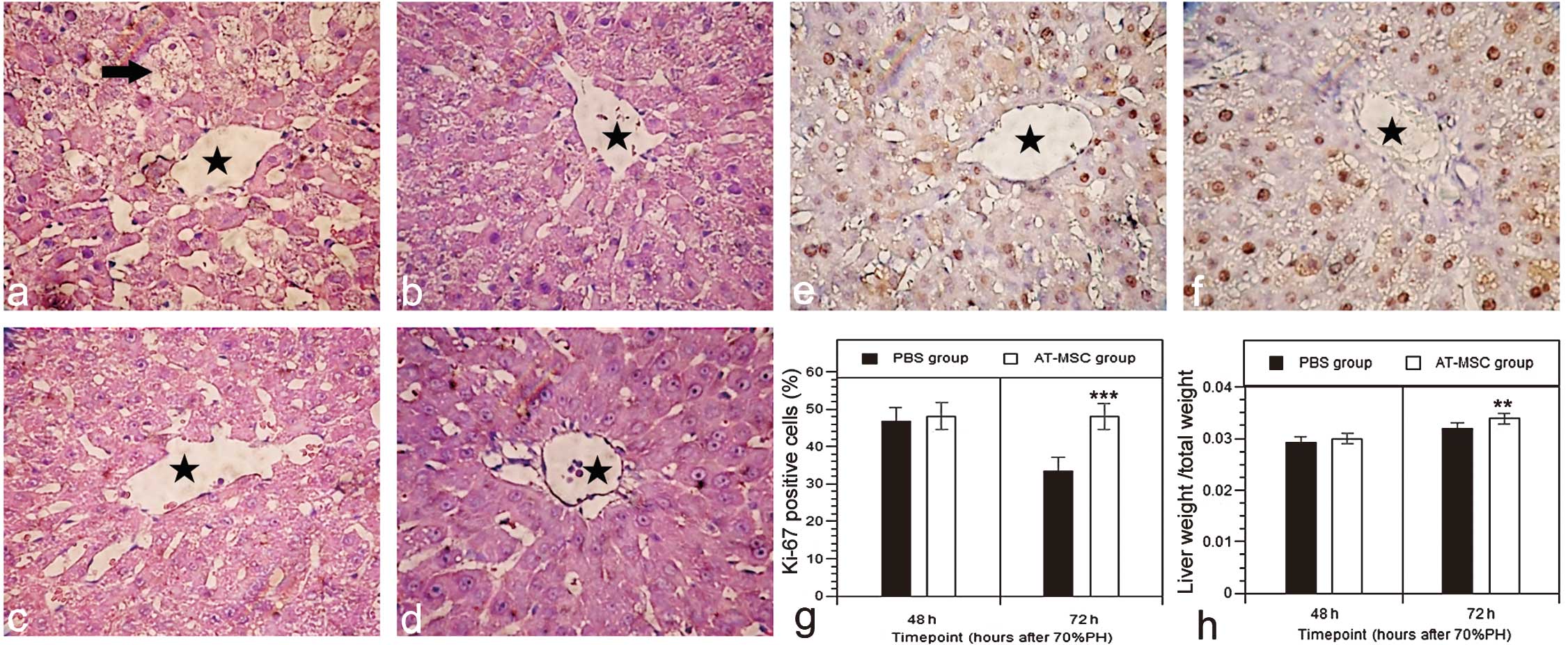
Histological examination of livers
Approximately 24 h after 70% PH, the hepatic
sinusoidal structure was well preserved in all the samples, and
inflammation or necrosis was not present. Histopathologic
examination showed vacuolar degeneration (Fig. 6a, arrows) and mild central vein
dilatation (Fig. 6a and c,
asterisks). These features are commonly observed after liver
resection. Approximately 24 h after AT-MSC transplantation, the
injured livers were found to have less vacuolar degeneration than
those in the PBS group (Fig. 6b),
and the changes were more apparent 48 h after the transplantation
(Fig. 6d). Approximately 48 h
after 70% PH, all the groups exhibited a similar proliferation
rate, although the proliferation rate 72 h after 70% PH was
significantly increased (Fig. 6e and
f) in the AT-MSC group compared with the PBS group (Fig. 6g; Ki-67-positive ratio, 48.07±0.88
vs. 35.33±0.81, P<0.001), and the residual liver weight was also
increased in the AT-MSC group (Fig.
6h) (P<0.01).
Discussion
The 70% PH model is a classical model for studying
liver regeneration, in which many cytokines and growth factors that
favor liver regeneration are released into the blood via the
injured liver tissue. Different studies have suggested that various
paracrine factors secreted by injured liver tissue have an
important role in stem cell recruitment and engraftment. Those
chemokines allow MSCs to enter the circulation prior to returning
to the injured liver (18–20).
In the present study, serum from PH-induced acute liver injury was
used as an agent to induce differentiation of AT-MSCs for the first
time. As a result, these cells resembled hepatocyte-like cells with
round or polygonal shape, expressed α-fetoprotein, secreted ALB,
synthesized urea, and acquired CYP450 3A4 enzyme activity, showing
that these cells partially function as mature hepatocytes. However,
the hepatic differentiation efficiency of AT-MSCs in the PH serum
group was significantly lower than that in the HGF group. This
result was different to that obtained by Yang et al
(15) who showed that the serum
collected after 24 h of 70% PH did not exhibit appropriate culture
conditions to promote the hepatic differentiation of AT-MSCs. It is
known that in a typical wound healing scenario, the injury to the
tissue results in disruption of capillary vascular networks and
extravasation of blood, accompanied by local release of coagulation
factors, platelets and growth factors (21). This does not occur after 70% PH.
Various changes in hepatic blood flow patterns and oxygen partial
pressure occur following the surgical removal of three liver lobes
without damage to the two residual lobes. The two types of
pathological processes of liver injury (PH and RFA injury) render
the composition in the serum markedly different, leading to
significantly different induction results, although the detailed
mechanism of action remains to be elucidated.
The therapeutic effect of MSC transplantation is
promising. However, studies have demonstrated that the rate of MSCs
differentiating into liver cells in vivo was low (22,23). Previous studies have suggested
that <3% of transplanted MSCs are attributed to the total organ
mass (24–26). Moreover, even induced
hepatocyte-like cells expressing a certain number of markers do not
necessarily indicate that these cells possess similar functions to
liver cells. MSCs act as ‘trophic mediators’ (27–32), which by secretion of bioactive
factors act as either immunosuppressors or promoters of
regeneration (29,33,34). A previous study has also shown
that AT-MSCs produce significantly more HGF, granulocyte
colony-stimulating factor (G-CSF), granulocyte-macrophage
colony-stimulating factor (GM-CSF), and IL-6 compared with BMSCs
and normal human dermal fibroblasts (NHDFs) (35). HGF is an important factor in the
process of differentiation from BMSCs to hepatocytes and
osteoblasts (36,37), IL-6 is necessary and sufficient
for enhanced MSC proliferation, and inhibits adipogenic and
chondrogenic differentiation (38). Moreover, an increase in the mRNA
expression levels of IL-6 was observed when BMSCs were co-cultured
with hepatocytes from GaIN-induced injured liver (39). Therefore, we examined the
expression of HGF and IL-6 during the induction process.
In this study, we found that AT-MSCs briefly
upregulated the mRNA expression of HGF and IL-6 when cultured with
the post-hepatectomy serum. The concentration of HGF and IL-6 in
the supernatant, as detected by ELISA, also conformed with the
change of the mRNA expression. Previous studies have shown that HGF
is a potent mitogen in vivo, and that an increasing HGF
expression benefits liver injury attenuation (40,41). It has also been reported that
implantation of the human HGF-expressing MSCs, improves liver
regeneration, reduces mortality in rats, and has a potent
antifibrotic effect on the small-for-size liver transplantation
model (42). Thus, the
upregulated expression of HGF and IL-6 may be the effector
molecules involved in the promotion of liver regeneration in
vivo. To validate our hypothesis, we transplanted AT-MSCs into
the rat tail vein at 24 h after 70% PH to observe the concentration
changes of HGF and IL-6 in vivo. We found that AT-MSC
transplantation significantly improved liver function and
facilitated liver regeneration, but did not increase the serum
levels of HGF and IL-6 as expected. By contrast, these results
showed that a reduced concentration of HGF and IL-6 may be more
beneficial for liver regeneration and function recovery, which is a
notewothy finding to explain the reduction in HGF and IL-6
concentrations following AT-MSC transplantation.
However, the reason for the concentration of HGF and
IL-6 transiently increasing from 24 to 48 h after the
transplantation remains to be verified, as well as whether other
cytokines/growth factors are involved in the regeneration process.
To clarify the aforementioned issues, more detailed studies are
required. Recently, a new study (43) has demonstrated that the acutely
transplanted neural stem/progenitor cells (NSPCs) had beneficial
effects on spinal cord injury, particularly neuroprotection and
neurohumoral secretion, whereas their in situ secretory
activity differed significantly from that predicted in
vitro. Authors of that study found that in the pathological
environment, overall transcriptional activity, external signal
transduction and neural differentiation of engrafted NSPCs were
significantly suppressed. Those results emphasize the vulnerability
of engrafted stem cells to environmental force, while emphasizing
the importance of in situ analysis in advancing the efficacy
and safety of stem cell-based therapies (43).
In conclusion, we have shown that serum from
hepatectomized rats after 24 h of surgery triggered the
differentiation of AT-MSCs into hepatocyte-like cells, although the
hepatic differentiation efficacy was extremely low. AT-MSCs
upregulated the expression of HGF and IL-6 when cultured with the
post-hepatectomy serum. However, they did not increase the serum
levels of HGF and IL-6 when transplanted in vivo as we
expected. This finding should therefore be studied further.
However, there were limitations to our study. One was that we did
not observe whether AT-MSCs are able to migrate into the injury
liver and differentiate into hepatocytes. Another one was the
concentration of the growth factors and cytokines in the blood
markedly altered during the liver regeneration process, thus, serum
from hepatectomized rats after 24 h of their operation did not
exhibit the entire change in blood after 70% PH. Thus, AT-MSCs may
act through multiple mechanisms to coordinate a marked integrated
response to liver regeneration after 70% PH in vivo.
Therefore, the processes by which these cells act to decrease the
serum levels of HGF and IL-6, as well as the mechanisms by which
they act to reverse hepatocyte damage and promote liver
regeneration in vivo remains to be investigated.
Abbreviations:
|
PH
|
partial hepatectomy;
|
|
AFP
|
α-fetoprotein;
|
|
ALB
|
albumin;
|
|
ALT
|
alanine aminotransferase;
|
|
AST
|
aspartate aminotransferase;
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium;
|
|
FBS
|
fetal bovine serum;
|
|
DAPI
|
4′,6-diamidino-2-phenylindole;
|
|
FITC
|
fluorescein isothiocyanate;
|
|
GFP
|
green fluorescent protein;
|
|
IL
|
interleukin;
|
|
mRNA
|
messenger RNA;
|
|
MSC
|
mesenchymal stem cell;
|
|
PBS
|
phosphate-buffered saline;
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction;
|
|
CYP3A4
|
cytochrome P450 type 3A4
|
Acknowledgements
This study was supported by the
Fundamental Research Funds for the Central Universities.
References
|
1
|
Banas A, Teratani T, Yamamoto Y, et al:
Rapid hepatic fate specification of adipose-derived stem cells and
their therapeutic potential for liver failure. J Gastroenterol
Hepatol. 24:70–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banas A, Teratani T, Yamamoto Y, et al:
Adipose tissue-derived mesenchymal stem cells as a source of human
hepatocytes. Hepatology. 46:219–228. 2007.PubMed/NCBI
|
|
3
|
Sgodda M, Aurich H, Kleist S, et al:
Hepatocyte differentiation of mesenchymal stem cells from rat
peritoneal adipose tissue in vitro and in vivo. Exp Cell Res.
313:2875–2886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto Y, Banas A, Murata S, et al: A
comparative analysis of the transcriptome and signal pathways in
hepatic differentiation of human adipose mesenchymal stem cells.
FEBS J. 275:1260–1273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Talens-Visconti R, Bonora A, Jover R, et
al: Hepatogenic differentiation of human mesenchymal stem cells
from adipose tissue in comparison with bone marrow mesenchymal stem
cells. World J Gastroenterol. 12:5834–5845. 2006.PubMed/NCBI
|
|
6
|
Snykers S, Vanhaecke T, Papeleu P, et al:
Sequential exposure to cytokines reflecting embryogenesis: the key
for in vitro differentiation of adult bone marrow stem cells into
functional hepatocyte-like cells. Toxicol Sci. 94:330–341. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michalopoulos GK: Liver regeneration. J
Cell Physiol. 213:286–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taub R: Liver regeneration: from myth to
mechanism. Nat Rev Mol Cell Biol. 5:836–847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brand S, Beigel F, Olszak T, et al: IL-22
is increased in active Crohn’s disease and promotes proinflammatory
gene expression and intestinal epithelial cell migration. Am J
Physiol Gastrointest Liver Physiol. 290:G827–G838. 2006.
|
|
10
|
Cressman DE, Greenbaum LE, DeAngelis RA,
et al: Liver failure and defective hepatocyte regeneration in
interleukin-6-deficient mice. Science. 274:1379–1383. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren X, Hogaboam C, Carpenter A and
Colletti L: Stem cell factor restores hepatocyte proliferation in
IL-6 knockout mice following 70% hepatectomy. J Clin Invest.
112:1407–1418. 2003.PubMed/NCBI
|
|
12
|
Moolten FL and Bucher NL: Regeneration of
rat liver: transfer of humoral agent by cross circulation. Science.
158:272–274. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jirtle RL and Michalopoulos G: Effects of
partial hepatectomy on transplanted hepatocytes. Cancer Res.
42:3000–3004. 1982.PubMed/NCBI
|
|
14
|
Wang PP, Wang JH, Yan ZP, et al:
Expression of hepatocyte-like phenotypes in bone marrow stromal
cells after HGF induction. Biochem Biophys Res Commun. 320:712–716.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Qu B, Huo JH, Wu SL, Zhang MY and
Wang ZR: Serum from radiofrequency-injured livers induces
differentiation of bone marrow stem cells into hepatocyte-like
cells. J Surg Res. 155:18–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seo MJ, Suh SY, Bae YC and Jung JS:
Differentiation of human adipose stromal cells into hepatic lineage
in vitro and in vivo. Biochem Biophys Res Commun. 328:258–264.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greene AK and Puder M: Partial hepatectomy
in the mouse: technique and perioperative management. J Invest
Surg. 16:99–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hatch HM, Zheng D, Jorgensen ML and
Petersen BE: SDF-1alpha/CXCR4: a mechanism for hepatic oval cell
activation and bone marrow stem cell recruitment to the injured
liver of rats. Cloning Stem Cells. 4:339–351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dalakas E, Newsome PN, Harrison DJ and
Plevris JN: Hematopoietic stem cell trafficking in liver injury.
FASEB J. 19:1225–1231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gomez-Aristizabal A, Keating A and Davies
JE: Mesenchymal stromal cells as supportive cells for hepatocytes.
Mol Ther. 17:1504–1508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schafer M and Werner S: Transcriptional
control of wound repair. Annu Rev Cell Dev Biol. 23:69–92. 2007.
View Article : Google Scholar
|
|
22
|
Mitchell C and Fausto N: Bone
marrow-derived hepatocytes: rare but promising. Am J Pathol.
161:349–350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fausto N: Liver regeneration and repair:
hepatocytes, progenitor cells, and stem cells. Hepatology.
39:1477–1487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malhi H and Gupta S: Hepatocyte
transplantation: new horizons and challenges. J Hepatobiliary
Pancreat Surg. 8:40–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Menthena A, Deb N, Oertel M, et al: Bone
marrow progenitors are not the source of expanding oval cells in
injured liver. Stem Cells. 22:1049–1061. 2004.PubMed/NCBI
|
|
26
|
Wang J, Clark JB, Rhee GS, Fair JH, Reid
LM and Gerber DA: Proliferation and hepatic differentiation of
adult-derived progenitor cells. Cells Tissues Organs. 173:193–203.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parekkadan B, van Poll D, Suganuma K, et
al: Mesenchymal stem cell-derived molecules reverse fulminant
hepatic failure. PLoS One. 2:e9412007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parekkadan B, van Poll D, Megeed Z, et al:
Immunomodulation of activated hepatic stellate cells by mesenchymal
stem cells. Biochem Biophys Res Commun. 363:247–252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sadat S, Gehmert S, Song YH, et al: The
cardioprotective effect of mesenchymal stem cells is mediated by
IGF-I and VEGF. Biochem Biophys Res Commun. 363:674–679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gnecchi M, He H, Noiseux N, et al:
Evidence supporting paracrine hypothesis for Akt-modified
mesenchymal stem cell-mediated cardiac protection and functional
improvement. FASEB J. 20:661–669. 2006. View Article : Google Scholar
|
|
32
|
Shito M, Balis UJ, Tompkins RG, Yarmush ML
and Toner M: A fulminant hepatic failure model in the rat:
involvement of interleukin-1beta and tumor necrosis factor-alpha.
Dig Dis Sci. 46:1700–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gnecchi M, Zhang Z, Ni A and Dzau VJ:
Paracrine mechanisms in adult stem cell signaling and therapy. Circ
Res. 103:1204–1219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Poll D, Parekkadan B, Cho CH, et al:
Mesenchymal stem cell-derived molecules directly modulate
hepatocellular death and regeneration in vitro and in vivo.
Hepatology. 47:1634–1643. 2008.PubMed/NCBI
|
|
35
|
Banas A, Teratani T, Yamamoto Y, et al:
IFATS collection: in vivo therapeutic potential of human adipose
tissue mesenchymal stem cells after transplantation into mice with
liver injury. Stem Cells. 26:2705–2712. 2008. View Article : Google Scholar
|
|
36
|
Miyazaki M, Akiyama I, Sakaguchi M, et al:
Improved conditions to induce hepatocytes from rat bone marrow
cells in culture. Biochem Biophys Res Commun. 298:24–30. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wen Q, Zhou L, Zhou C, Zhou M, Luo W and
Ma L: Change in hepatocyte growth factor concentration promote
mesenchymal stem cell-mediated osteogenic regeneration. J Cell Mol
Med. 16:1260–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pricola KL, Kuhn NZ, Haleem-Smith H, Song
Y and Tuan RS: Interleukin-6 maintains bone marrow-derived
mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J
Cell Biochem. 108:577–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lam SP, Luk JM, Man K, et al: Activation
of interleukin-6-induced glycoprotein 130/signal transducer and
activator of transcription 3 pathway in mesenchymal stem cells
enhances hepatic differentiation, proliferation, and liver
regeneration. Liver Transpl. 16:1195–1206. 2010. View Article : Google Scholar
|
|
40
|
Shiota G and Kawasaki H: Hepatocyte growth
factor in transgenic mice. Int J Exp Pathol. 79:267–277. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ueki T, Kaneda Y, Tsutsui H, et al:
Hepatocyte growth factor gene therapy of liver cirrhosis in rats.
Nat Med. 5:226–230. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu Y, Lu L, Qian X, et al: Antifibrotic
effect of hepatocyte growth factor-expressing mesenchymal stem
cells in small-for-size liver transplant rats. Stem Cells Dev.
19:903–914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumamaru H, Ohkawa Y, Saiwai H, et al:
Direct isolation and RNA-seq reveal environment-dependent
properties of engrafted neural stem/progenitor cells. Nat Commun.
3:11402012. View Article : Google Scholar : PubMed/NCBI
|