Introduction
Pure titanium (CP-Ti; α-type titanium alloy) and
Ti6Al4V (α+β-type titanium alloy), compared with other metallic
biomaterials, have attracted considerable attention for biomedical
applications due to their excellent specific corrosion resistance
and high biocompatibility with bone (1). However, although the elastic modulus
of the Ti6Al4V alloy (110 GPa) is substantially lower than that of
the Co-Cr-Mo alloy (230 GPa) and stainless steel (205 GPa), which
are used as biomaterials, it is still much higher than that of
human cortical bone (10–30 GPa) (2). A lower elastic modulus is important
for minimizing the undesirable complication of stress
shielding-bone resorption caused by unbalanced stress distribution
between the bone and implant. In addition, vanadium has been
previously found to be toxic to the human body (3,4).
Therefore, to resolve the drawbacks of the Ti6Al4V alloy, β-type Ti
alloys are increasingly being considered for use in medical
implants due to their lower elastic modulus and lack of toxic
elements.
In particular, a review of 15 titanium alloys,
including Ti6Al4V, CP-Ti, and β-type titanium alloys, showed that
the Young’s modulus of all β-type titanium alloys was lower than
that of α or α+β-type titanium alloys, and that the Ti35Nb5Ta7Zr
alloy showed the lowest Young’s modulus (55 GPa). With regard to
toxicity, β-type Ti alloys consist of β-stabilizing elements such
as Nb, Mn, Sn, Ta and Zr, which are considered safe for the human
body (5,6). Moreover, the biocompatibility of the
β-type Ti alloy has been extensively investigated. Miura et
al (7) reported that
osteoblasts cultured on the surface of the TiNbSn alloy and on
CP-Ti surface showed no significant differences in the relative
growth ratio and relative absorbance ratio, indicating that the
bone compatibility of the TiNbSn alloy is similar to that of
CP-Ti.
A new β-type alloy composed of nontoxic elements,
Ti35Nb2Ta3Zr, has been fabricated by vacuum consumable arc
remelting. Our preliminary experiments focused on the
microstructure and mechanical properties of Ti35Nb2Ta3Zr, and
showed that the new alloy exhibited a considerably low Young’s
modulus (approximately 48 GPa) and a high ultimate tensile strength
(approximately 880 MPa) after cold rolling (8,9);
therefore, it could be a suitable candidate for orthopedic
applications. For clinical applications, biological compatibility
must be evaluated in vitro and in vivo. Therefore,
the present study evaluated the bone tissue compatibility of the
new Ti35Nb2Ta3Zr alloy as a first step toward its use for
biomedical applications.
Materials and methods
Specimen preparation
The Ti35Nb2Ta3Zr alloy (wt %) was prepared by arc
melting a mixture of high-purity sponge Ti, Ti-Nb interalloy, and
high-purity Ta and Zr. The ingot was re-melted 3 times at 1,223 K
for 1 h under vacuum to ensure compositional homogeneity and then
forged into a quadrate cast with a width of 70 mm, thickness of 30
mm, and length of 100 mm. Following solid solution at 1,053 K for
0.5 h, the alloy was rolled to reduce its thickness by 99%.
Pure Ti, Ti6Al4V and Ti35Nb2Ta3Zr were machined to
disks with a diameter of 13 mm and a thickness of 1 mm to fit the
24-well plates. The surfaces of the disks were ground using a
series of water-resistant emery papers with various degrees of
coarseness up to 1,200 grit. All the disks were cleaned in an
ultrasonic bath with ethanol and deionized (Milli-Q) water,
followed by sterilization in an autoclave prior to cell
culture.
Surface characterization
The surface topography was imaged using a
field-emission scanning electron microscope (FESEM, FEI NOVA
NanoSEM). The surface roughness (Ra) was determined using atomic
force microscopy (AFM, Nanoscope 3; Bruker, Germany).
Energy-dispersive X-ray detection (EDX, X-Max 80; Oxford
Instruments, UK) was used to determine the elemental composition.
The contact angle was measured using a video contact-angle
measurement system.
Cell culture
MG63 cells (Cell Bank of the Chinese Science
Academy, China) were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% (v/v) fetal bovine serum (Gibco) and
1% penicillin/streptomycin at 37°C in an atmosphere of 5%
CO2 and 95% air. The cells were cultured in osteogenic
media [the regular media described earlier, supplemented with 10 mM
β-glycerophosphate, 0.2 mM ascorbic acid, and 10−8 M
dexamethasone (Sigma)] to study cell differentiation. The culture
medium was replaced every 2 or 3 days. The cells were passaged by
lifting with trypsin/EDTA until they reached 80% confluence.
Cell adhesion
Osteoblasts were seeded on the samples at a density
of 1×104 cells/cm2. Following culture for 12
h, the non-adherent cells were removed by rinsing with
phosphate-buffered saline (PBS). The cells were fixed with 95%
alcohol and stained with 4′,6′-diamidino-2-phenylindole (DAPI). The
cells in 5 random fields were counted at low magnification under a
fluorescence microscope (Leica DM400).
Cell viability
The Cell Counting Kit-8 (CCK-8; Dojindo, Japan) was
used to determine the number of viable cells. In this assay,
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt (WST-8) is reduced by dehydrogenases in the cells
to provide an orange-colored product (formazan) that is soluble in
the tissue culture medium. The amount of the formazan dye generated
by the dehydrogenases in the cells is directly proportional to the
number of living cells. One milliliter of the cell suspension was
seeded onto each specimen with a density of 2×104
cells/ml. At 1, 4, 8 and 16 days after seeding, the samples were
gently rinsed 3 times with PBS, and 20 μl of prewarmed CCK-8
solution was added in 180 μl of culture medium. After 1 h of
further incubation, 100 μl of the working solution was
transferred to a new 96-well culture plate for measurement of the
absorbance using an ELISA reader at 450 nm.
Cell morphology
MG63 cells were seeded on the samples for 1 and 4
days at a density of 1×104 cells/cm2. At the
prescribed time-points, the cells were fixed with 4%
paraformaldehyde (Sigma) and washed with PBS. They were then
permeabilized with 0.1% Triton X-100 and rinsed 3 times with PBS.
Nonspecific binding sites were blocked with 1% BSA in PBS.
Subsequently, the samples were incubated for 1 h with 200 μl
of TRITC-conjugated phalloidin (Molecular Probes, USA) in a
humidified chamber at room temperature in the dark. Finally, cell
nuclei were stained by incubation with 5 μg/ml DAPI
(Dojindo) followed by 2 PBS rinses. The morphology of the cells was
examined by confocal laser scanning microscopy (LSM-510; Carl
Zeiss, Germany).
Alkaline phosphatase (ALP) activity
MG63 cells were seeded on the samples at a density
of 1×104 cells/cm2. The ALP activity was
examined after the cells were cultured for 4, 8 and 16 days by
measuring the transformation of p-nitrophenyl-phosphate (pNPP) into
p-nitrophenol (pNP). Following the selected incubation periods, the
cells were rinsed 3 times with PBS and lysed in 0.1% Triton X-100
(Sigma) by using standard freeze-thaw cycling. Fifty microliters of
the sample were mixed with 50 μl of freshly prepared pNPP
substrate (1 mg/ml) and incubated at 37°C for 30 min. The reaction
was stopped by the addition of 50 μl of 0.2 M NaOH. The
amount of pNP produced was quantified by measuring the absorbance
at 405 nm in an ELISA reader. The total protein content was
measured using a BCA protein assay kit and calculated with respect
to a series of albumin (BSA) standards. Finally, the ALP activity
was normalized to the total protein content.
Calcium deposition
Mineralization was detected and quantified by
Alizarin Red S (ARS) staining at 8 and 16 days. Cells were seeded
on the samples at a density of 1×104
cells/cm2. At the indicated time-points, the samples
were washed 3 times in PBS and fixed in 4% paraformaldehyde for 30
min. The cells were then rinsed with ddH2O and stained
with ARS (40 mM) for 20 min at room temperature. After the
nonspecific staining was removed by rinsing with ddH2O,
the stain was desorbed with 10% cetylpyridinium chloride (Sigma).
Finally, the dye was transferred to a 96-well plate and quantified
using an ELISA reader at an absorbance of 590 nm.
Gene expression analysis by real-time
polymerase chain reaction (RT-PCR)
MG63 cells were seeded on the samples at a density
of 1×104 cells/cm2 and cultured for 16 days.
At the prescribed time-point, the adherent cells were lysed by
TRIzol (Invitrogen) and the total RNA was isolated according to the
TRIzol protocol. Subsequently, the total RNA was
reverse-transcribed to cDNA by using the RevertAid™ First Strand
cDNA Synthesis kit (Fermentas) following the manufacturer’s
instructions. Specific primers for the genes encoding
osteoblast-related proteins, ALP, osteocalcin (OC), and osteopontin
(OP), and the housekeeping gene GAPDH were synthesized commercially
(Generay, Shanghai, China). The specific primers are listed in
Table I. Quantitative RT-PCR was
then performed using SYBR®-Green Premix Ex Taq™ II
(Takara). Threshold cycle (Ct) values were obtained and the
amplification was determined. The GAPDH gene was used as a
reference control gene to normalize the expression values of the
target genes. The results are reported as the relative gene
expression calculated using the 2−ΔΔCt method.
 | Table IPrimer sequences for polymerase chain
reaction. |
Table I
Primer sequences for polymerase chain
reaction.
| Gene | Primer sequences
(5′-3′) |
|---|
| ALP | F:
ATCGCCTACCAGCTCATG |
| R:
GTTCAGCTCGTACTGCATGTC |
| OC | F:
CTCACACTCCTCGCCCTATT |
| R:
GGTCAGCCAACTCGTCCAG |
| OP | F:
ACCTCACACATGGAAAGCGA |
| R:
CTGTGGAATTCACGGCTGAC |
| GAPDH | F:
TGACATCAAGAAGGTGGTGA |
| R:
TCCACCACCCTGTTGCTGTA |
Animal experiment
Twenty-four adult male New Zealand white rabbits
weighing 3.0–3.5 kg were used for the implantation study. This
animal experiment was approved by the Animal Care Committee of
Shanghai Sixth Hospital. The animals were anesthetized with
pentobarbital (25 mg/kg) by intravenous injection, and were
administered cefazolin sodium (20 mg/kg) by intravenous injection
prior to surgery. The distal medullary canal of the femur was used
as the implantation site. Prior to the surgical procedure, the area
surrounding the incision site was shaved and the skin was
sterilized using iodophor disinfectant. A longitudinal incision was
performed medially at the patella, and a 2.0-mm drill bit was used
to open the medullary canal through the intercondylar notch of the
distal femur. The distal medullary canal was then gently reamed
through a series of increasing drill bits (3, 4 and 4.5 mm). After
the medullary canal was rinsed with normal saline, the implants
were implanted into the medullary canal until the distal portion of
the implant was at the same level as the entry hole. The implants
were inserted into both femurs of each rabbit; the left femurs were
used for the push-out test and the right femurs were used for the
histological study. All rabbits were allowed to move freely using
the operated limbs without any limitation after the operation, and
the rabbits were observed daily for activity and weight-bearing on
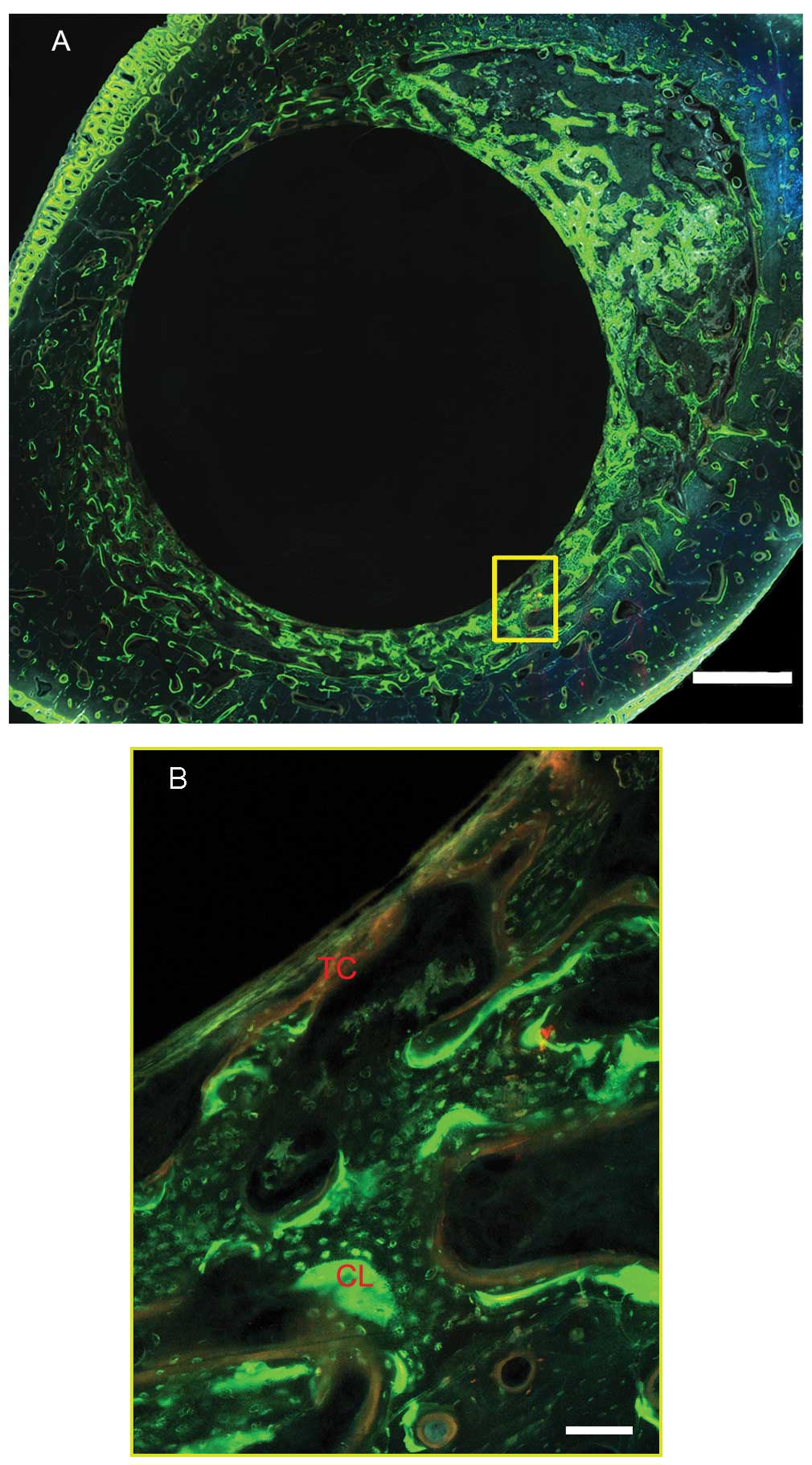
the operated limbs. The new bone formed was double labeled by
tetracycline (30 mg/kg) and calcein (5 mg/kg) (Sigma), which were
intraperitoneally injected 14 and 3 days, respectively, before the
animals were sacrificed. The animals were sacrificed by an overdose
of intravenous pentobarbital sodium 4, 8 and 12 weeks following rod
implantation, with 4 animals in each group.
Radiographic evaluation
X-ray radiography was performed after the rabbits
were sacrificed at 12 weeks to evaluate the osseointegration
between the implants and surrounding bone tissue.
Pull-out test
The pull-out test was performed within 12 h after
the animals were sacrificed. The femur implanted with the rod was
excised, and the protruding portion of the implant was exposed for
attachment to the testing machine (Instron 5569; Instron, USA). The
maximum pull-out force was recorded at 4, 8 and 12 weeks to
indicate the quality of the attachment to the bone tissue.
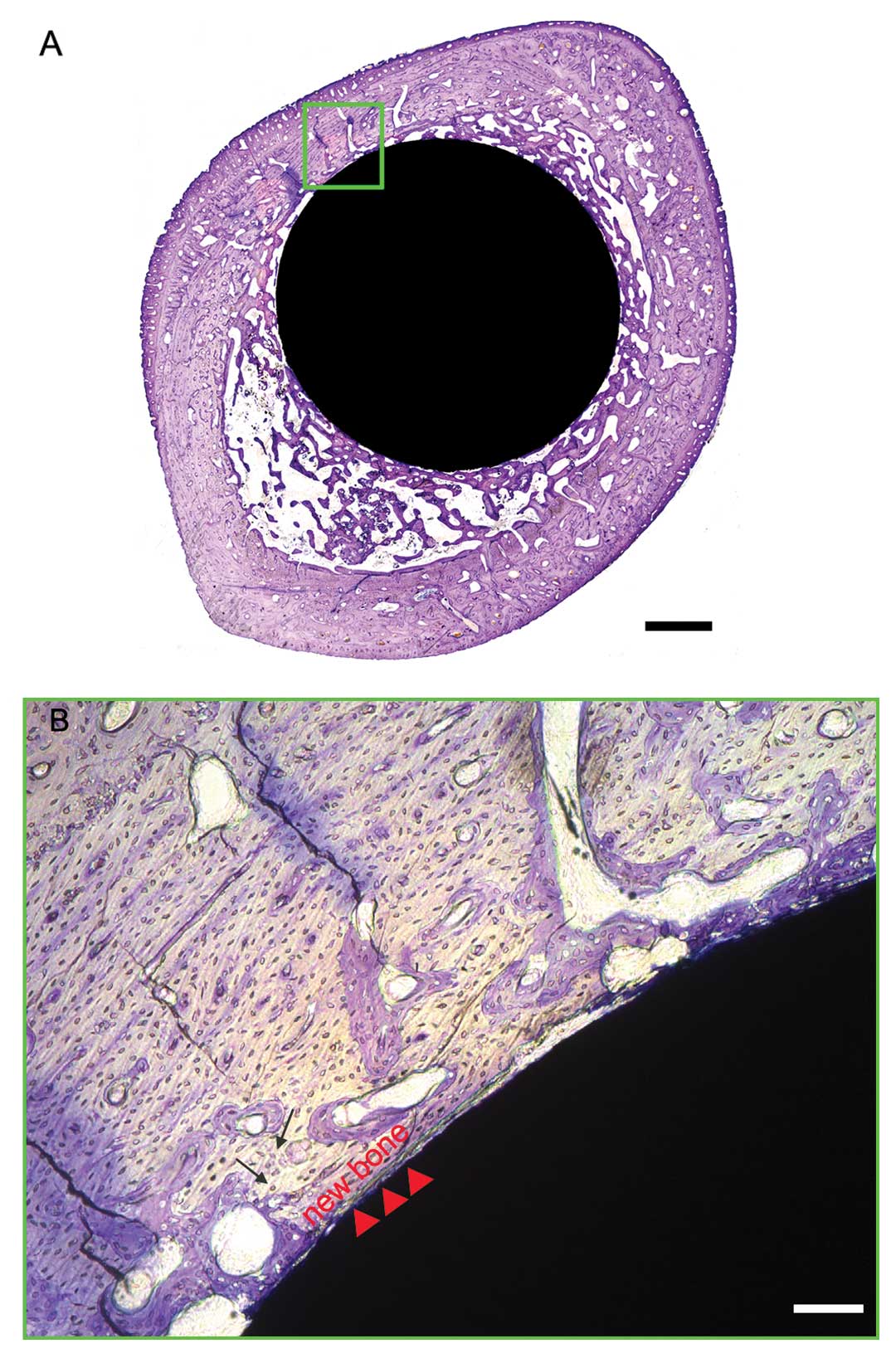
Histological evaluation
After the animals were sacrificed at the prescribed
time-points, the bone with the implant was fixed in acetone for 7
days, dehydrated in ascending grades of ethanol for 3 days each,
and embedded in methyl methacrylate without decalcification.
Sections (150 μm) were cut from each sample using a bone saw
(Leica SP1600) with the blade placed perpendicular to the long axis
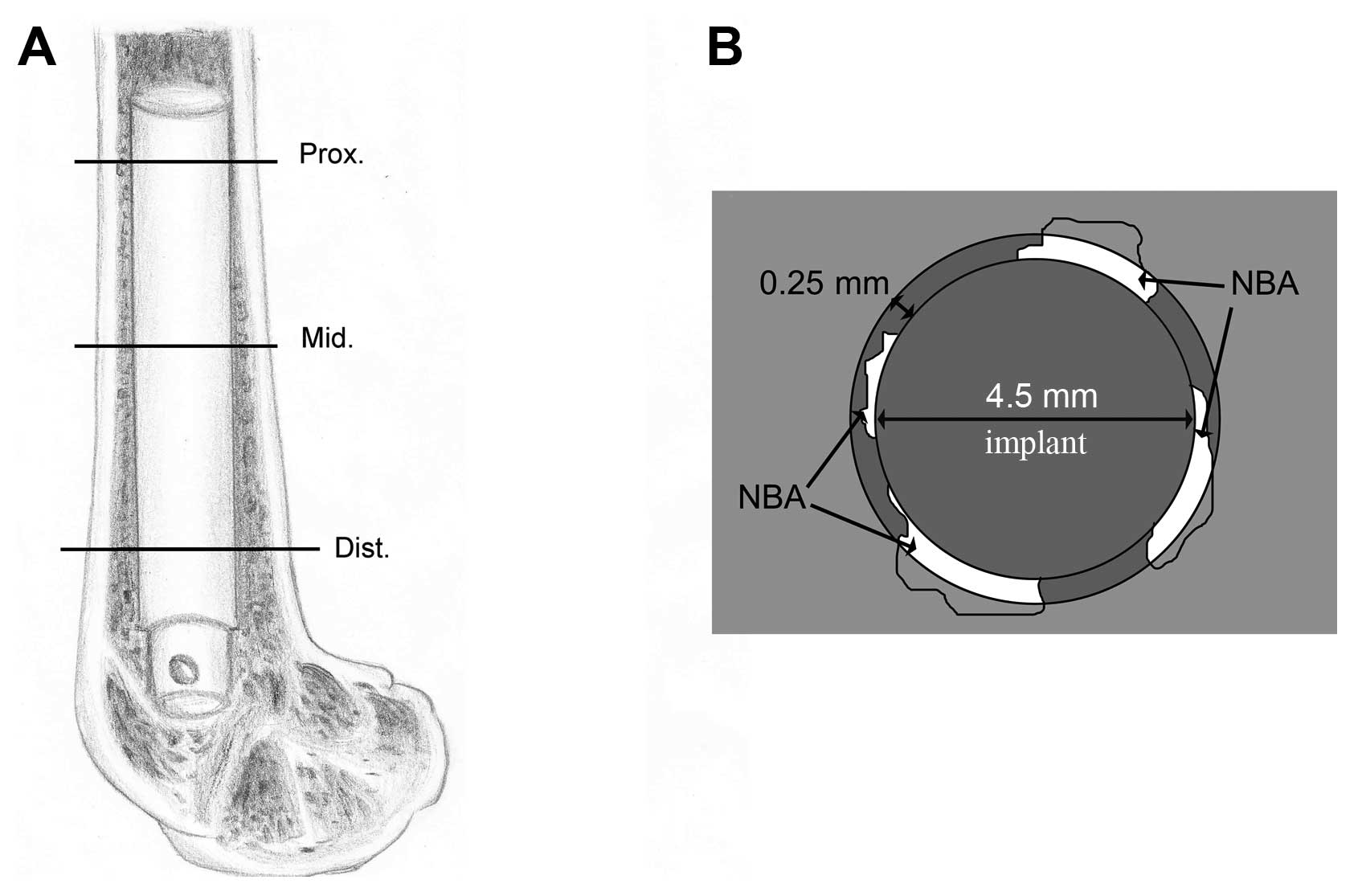
of the implant at 3 levels (proximal, middle and distal) of the
femur (Fig. 1A). Subsequently,
the sections were mounted on plastic slides and manually ground to
a thickness of 50–100 μm. Finally, the specimens were
stained with 1% methylene blue. The stained sections were observed
using a Leica microscope (Leica DM400), and the fluorochrome
markers were evaluated using a fluorescent microscope (Leica
DMI6000B). The surface bone apposition ratio [BAR (%)] and the new
bone area [NBA (mm2)] surrounding the implant were
measured to evaluate the osseointegration. The circumference of the
implant was recorded to calculate the percentage of the surface
that was in direct contact with the bone in each section, and the
BAR was defined as the percentage of the implant surface in direct
contact with the bone. The area of the new bone tissue was measured
in a circumferential area within 0.25 mm from the rod surface
(Fig. 1B), and the NBA was
determined by observing the methylene blue staining and labeling
with tetracycline and calcein.
Statistical analysis
Statistical analyses were conducted using SPSS 16.0.
The data were obtained from 3 independent experiments and expressed
as the means ± standard deviation. Differences among groups for the
in vitro experiments were analyzed by one-way analysis
(ANOVA). Two-way factorial ANOVA and Bonferroni post-hoc tests were
used for the in vivo experiments. A P-value of <0.05 was
considered to indicate statistically significant differences.
Results
Surface characterization
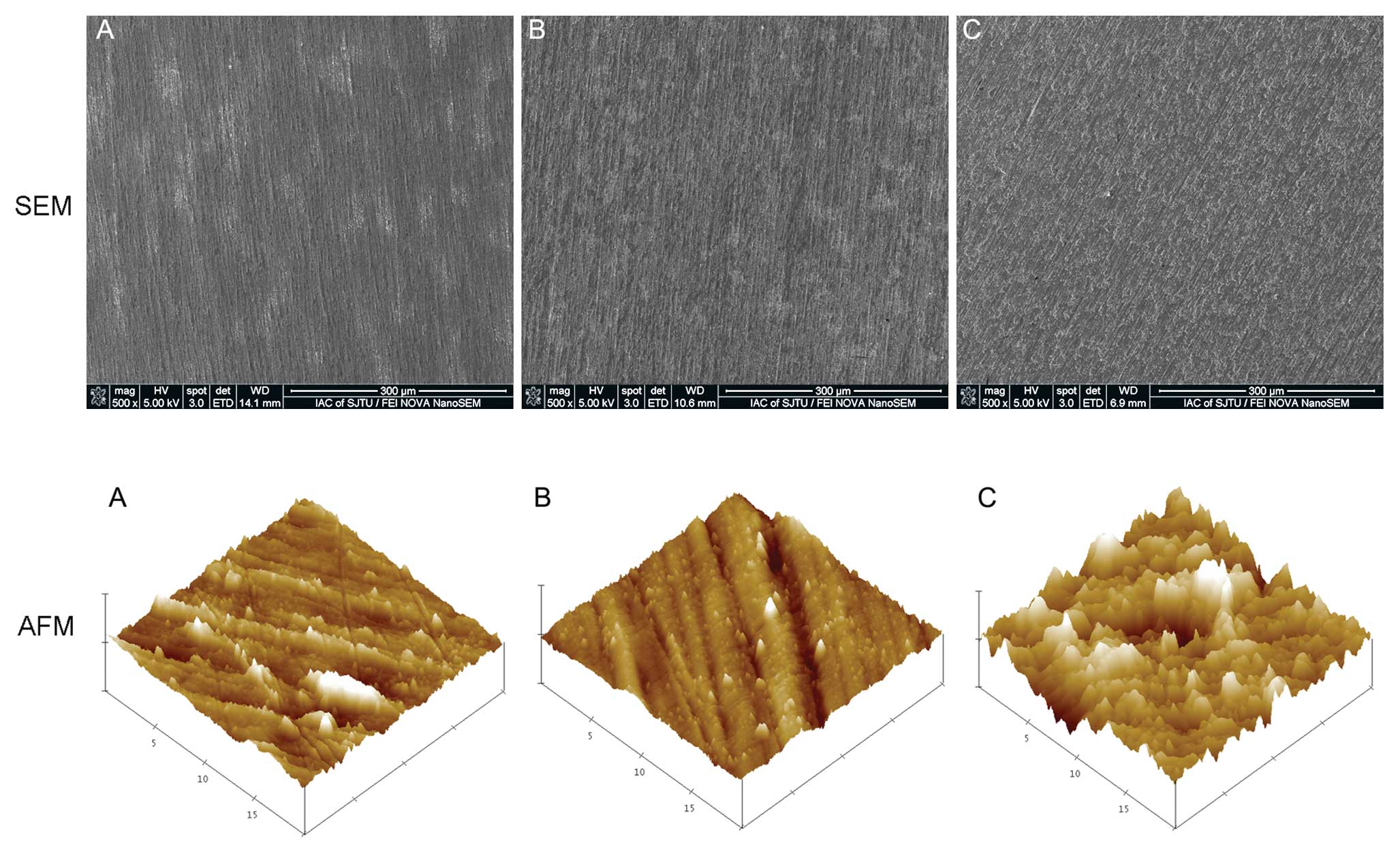
The SEM micrographs of Ti, Ti6Al4V, and Ti35Nb2Ta3Zr
are presented in Fig. 2. The
element composition measured by EDX was as follows: Ti, 59.55%; Nb,
35.44%; Ta, 2.14%; and Zr, 2.86%. The EDX results show that the
Ti35Nb2Ta3Zr alloy consisted of Ti, Nb, Ta and Zr; Al and V were
not observed. The Ra of the Ti, Ti6Al4V and Ti35Nb2Ta3Zr samples
determined by AFM (Fig. 2) is
summarized in Table II. The Ra of
Ti35Nb2Ta3Zr was the highest among the 3 samples. The surface
wettability measured as the water contact angle is also listed in
Table II; the surface of
Ti35Nb2Ta3Zr was found to be more hydrophilic than that of Ti and
Ti6Al4V.
 | Table IIComparison among Ti, Ti6Al4V and
Ti35Nb2Ta3Zr in terms of surface properties. |
Table II
Comparison among Ti, Ti6Al4V and
Ti35Nb2Ta3Zr in terms of surface properties.
| Ra (nm) | Contact angle
(°) |
|---|
| Ti | 31.933±3.61 | 39.8±2.6 |
| Ti6Al4V | 24.235±4.28 | 41.3±1.9 |
| Ti35Nb2Ta3Zr | 49.667±8.02 | 32.1±2.0 |
Cell adhesion and viability
The fluorescence micrographs of cells stained with
DAPI indicate cell attachment (Figs.
3 and 4). No significant
differences were observed among the substrates after 12 h of
culture. Fig. 4 shows the
viability of MG63 cells as measured by CCK8 following culture for
1, 4, 8 and 16 days on the 3 different alloys. The cell viability
increased with time on all substrates. On Day 1, the cell
proliferation was higher on Ti and Ti35Nb2Ta3Zr than on Ti6Al4V but
it did not differ between Ti and Ti35Nb2Ta3Zr. From Day 4, cells
cultured on Ti35Nb2Ta3Zr proliferated more rapidly than those
cultured on Ti, and significant differences were observed between
Ti and Ti35Nb2Ta3Zr on Days 4, 8 and 16.
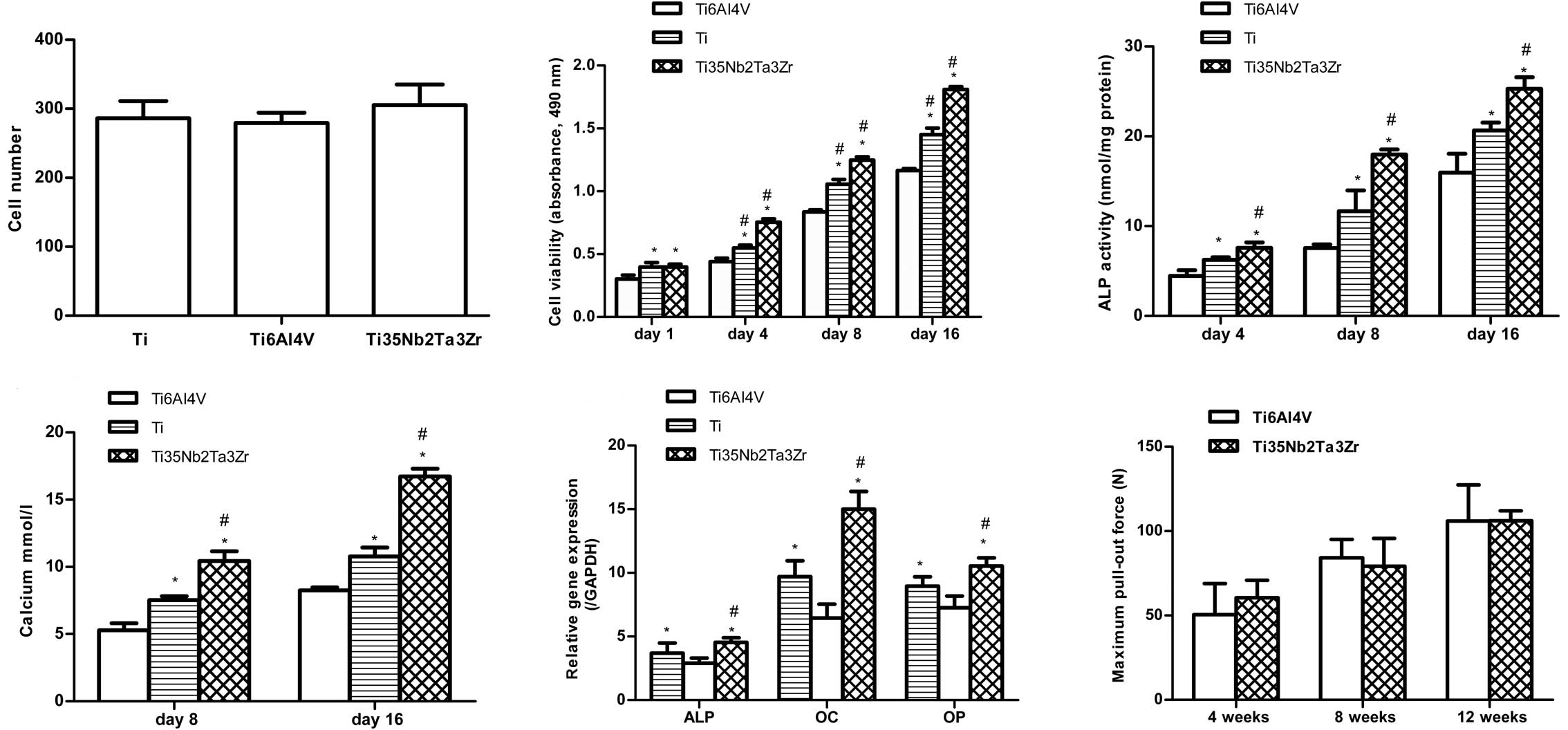 | Figure 4Osteoblast adhesion measured by cell
counting at 12 h, cell viability of MG63 cells after 1, 4, 8 or 16
days of culture, ALP activity (normalized to total protein amounts)
at 4, 8 and 16 days, calcium deposition after culture for 8 and 16
days and osteogenic mRNA expression (ALP, OC and OP) after culture
for 16 days on Ti, Ti6Al4V and Ti35Nb2Ta3Zr surfaces. The
failure-load values of Ti6Al4V and Ti35Nb2Ta3Zr alloys at 4, 8 and
12 weeks following implantation measured by the pull-out test. Data
are shown as the means ± SD. *P<0.05 compared with
Ti6Al4V; #P<0.05 compared with Ti. ALP, alkaline
phosphatase; OC, osteocalcin; OP, osteopontin. |
Cell morphology
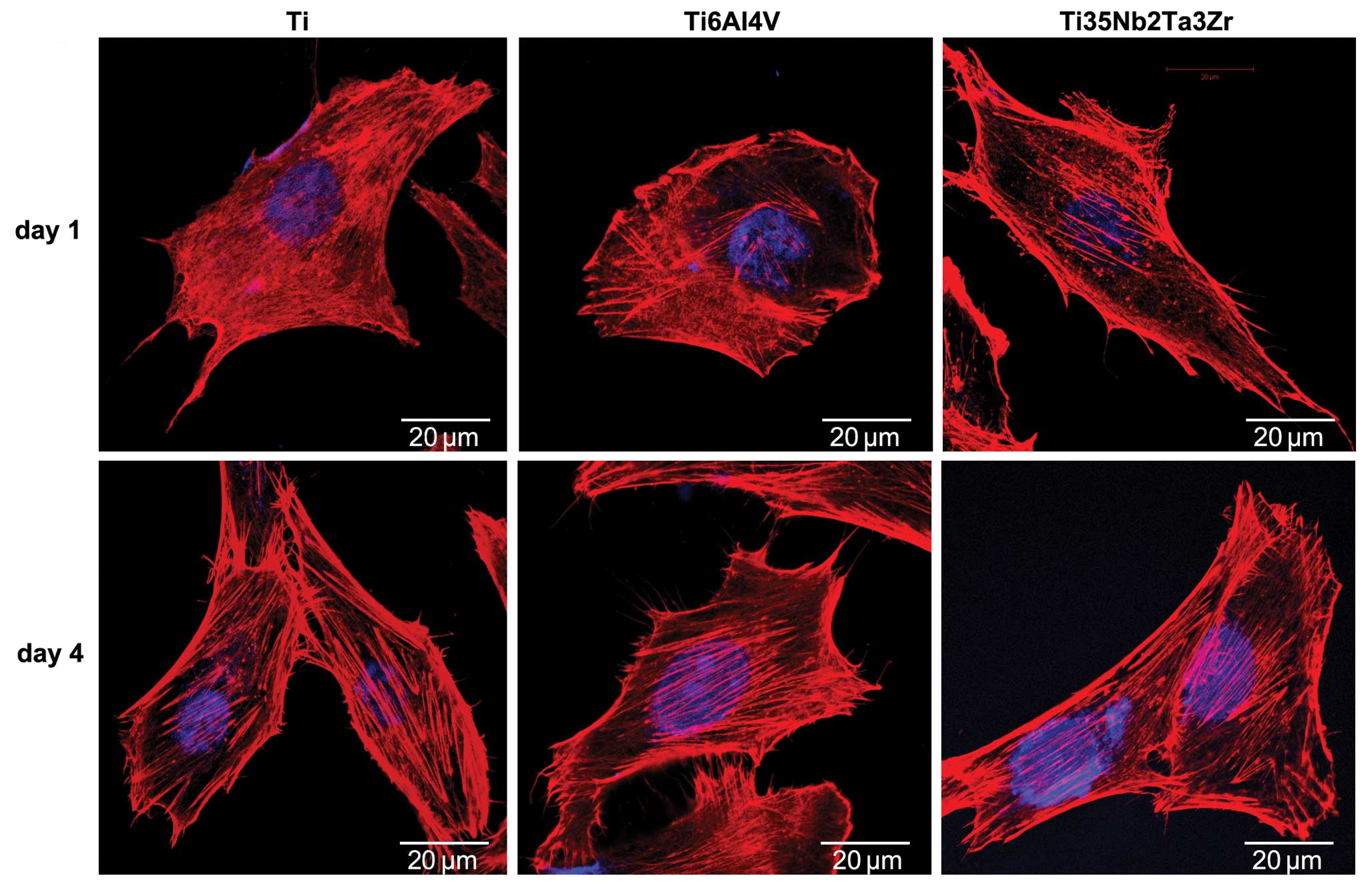
Fig. 5 shows the
morphology of osteoblasts with dual staining of actin filaments and
nuclei following culture on the surfaces of the Ti, Ti6Al4V and
Ti35Nb2Ta3Zr samples. On Day 1, round or fusiform cells were
observed on the samples, and several pseudopodia of the osteoblasts
were clearly observed on the surface of Ti35Nb2Ta3Zr. On Day 4, the
osteoblasts had spread well and showed a polygonal shape, and actin
filaments were clearly observed on all the samples.
ALP activity and calcium deposition
Fig. 4 shows the
ALP activity of osteoblasts cultured on Ti, Ti6Al4V and
Ti35Nb2Ta3Zr samples on 4, 8 and 16 days. The findings for ALP
activity were similar to those for cell viability. Among the 3
samples, Ti35Nb2Ta3Zr exhibited the highest ALP activity at all
time-points (P<0.05). The Alizarin Red assay was used to
determine the extent of calcium mineralization. Fig. 4 shows that the amount of calcium
on all 3 different samples increased in a time-dependent manner,
and the Ti35Nb2Ta3Zr surfaces, compared to Ti and Ti6Al4V, had
significantly high calcium levels after 8 and 16 days of culture
(P<0.05).
Real-time polymerase chain reaction
analysis
The gene expression of MG63 cells grown on Ti,
Ti6Al4V and Ti35Nb2Ta3Zr surfaces for 16 days was analyzed using
RT-PCR (Fig. 4). Significantly
higher mRNA levels of ALP, OC and OP were observed on the
Ti35Nb2Ta3Zr surfaces on Day 16 than on the Ti and Ti6Al4V surfaces
(P<0.05), which indicates that the osteoblast differentiation
was enhanced on Ti35Nb2Ta3Zr surfaces compared with Ti and Ti6Al4V
surfaces.
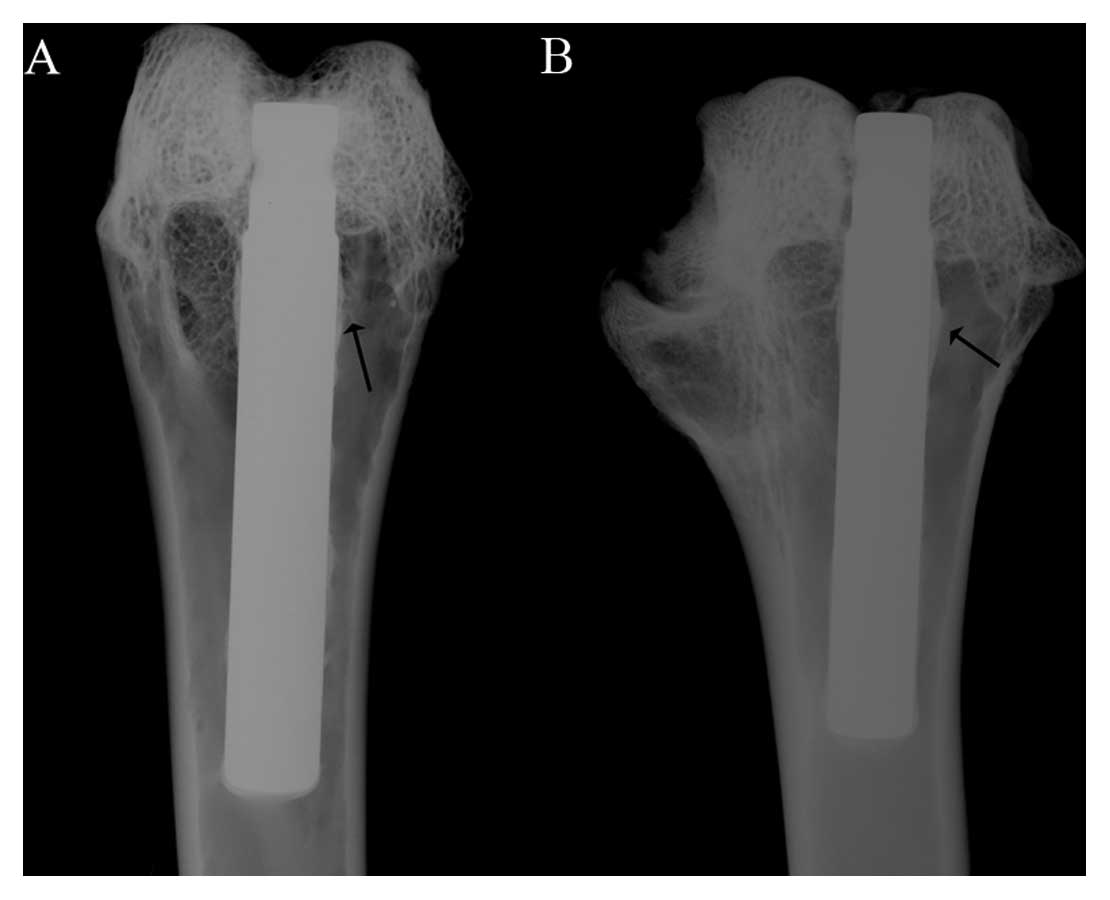
Radiographic evaluation
Fig. 6 shows
typical radiographs of rabbit femurs 12 weeks following
implantation with Ti6Al4V and Ti35Nb2Ta3Zr. The implants were
located in the medullary canal, and no fractures were observed in
the operated bone. New bone tissue was observed around the implants
as indicated by the arrow in Fig.
6. No significant differences were observed among the
radiographs for the different conditions.
Pull-out test
Fig. 4 shows the
failure loads. The failure-load values gradually increased with
time in both groups. However, there were no significant differences
between the 2 alloys at any time.
Histological evaluation
Figs. 7 and
8 show representative
histological images of the middle section of the Ti35Nb2Ta3Zr alloy
at 12 weeks after implantation. Newly formed bone tissue was
clearly observed around the surface of both the alloys. The dynamic
process of new bone formation was observed by light microscopy and
fluorescence microscopy (Fig. 8).
As shown in Fig. 7, good contact
was observed between the bone tissue and the Ti35Nb2Ta3Zr alloy.
Under fluorescence microscopy, a double line of tetracycline and
calcein was clearly observed, indicating new bone formation. The
formation of osteocytes (indicated by the arrows in Fig. 7) in the lamellar bone matrix was
irregular, indicating that the bone tissue grew well on the surface
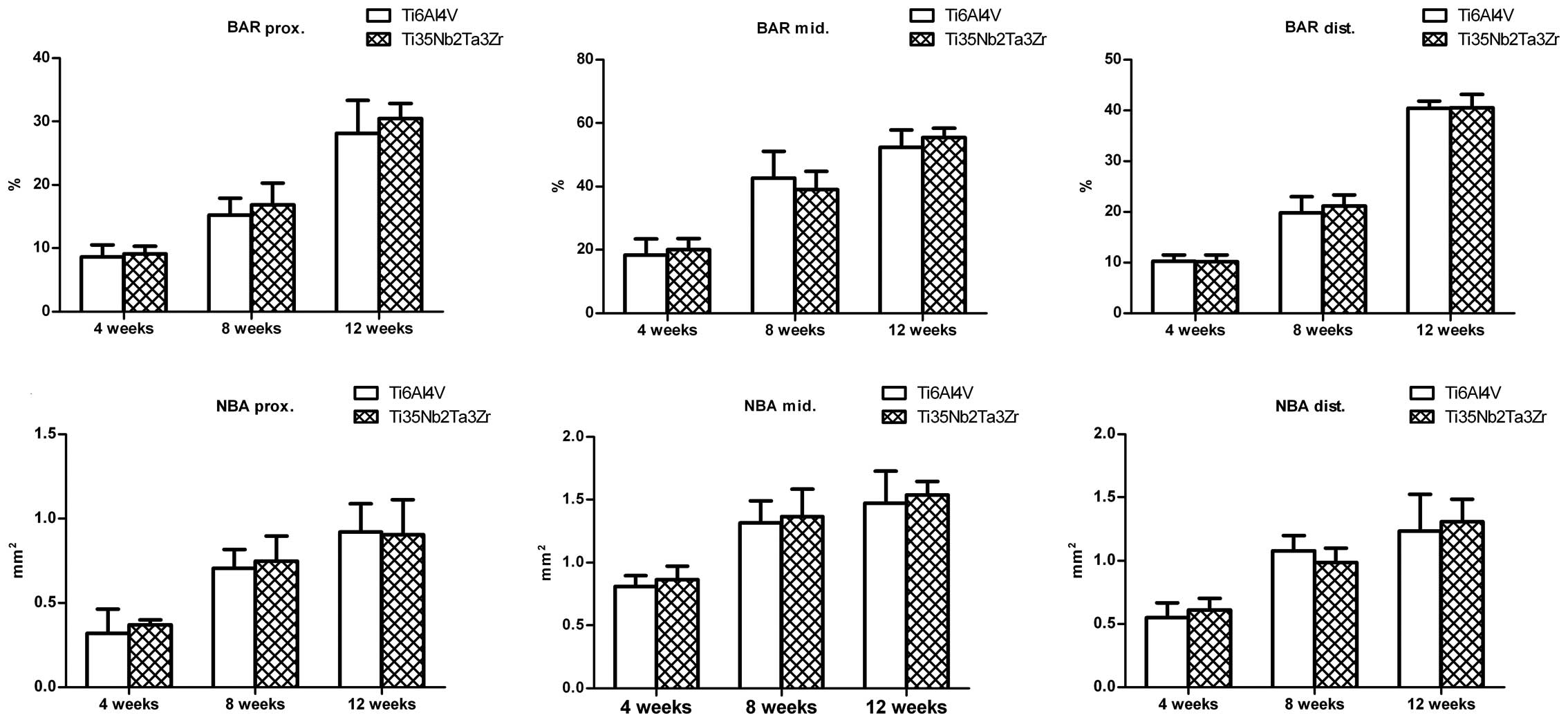
of the alloys. The BAR and NBA at the surface of the Ti6Al4V and
Ti35Nb2Ta3Zr alloys gradually increased in the 3 sections of the
femur. The BAR and NBA did not differ between the 2 groups in all
sections of the femur at 4, 8 and 12 weeks (Fig. 9).
Discussion
In the present study, the in vitro and in
vivo biocompatibility of a β-type Ti35Nb2Ta3Zr alloy with a low
elastic modulus of approximately 48 GPa was evaluated.
In vitro biological properties
A direct-contact culture test was performed to
assess the in vitro biocompatibility of Ti35Nb2Ta3Zr
(10–13). Ti6Al4V and Ti were used as
controls as they are known to be biocompatible. The proliferation
of MG63 cells was higher on Ti35Nb2Ta3Zr than on Ti6Al4V and Ti
after culture for 4 days and remained high until Day 16. The dual
staining of the actin filaments and nuclei of osteoblasts indicated
that the cells had spread well on the 3 samples. Significant
increases in the ALP activity and calcium deposition were observed
at all time-points, consistent with the mRNA expression of the
genes encoding the osteogenic proteins ALP, OC and OP.
Roughness and hydrophilicity are key factors in the
response of cells to biomaterials. Rough, hydrophilic surfaces on
Ti implants may increase the viability, ALP activity, and calcium
deposition of osteoblasts in vitro (14–17). The molecular mechanisms
responsible for the enhancement of osteogenic properties by surface
topography have been evaluated in several studies. Zhuang et
al (18) demonstrated that
cells cultured on smooth surfaces treated with PD98095 (an ERK1/2
inhibitor) or rough SLA surfaces (sand-blasted acid-etched
surfaces) without PD98095 treatment showed reduced extracellular
signal-regulated kinase 1/2 (ERK1/2) phosphorylation and increased
expression levels of osteogenic genes (i.e., Runx2, OSX, OPN and
OCN). Thus, the effect of surface roughness on osteoblastic
differentiation may be mediated through the inhibition of the
ERK1/2 pathway, which is consistent with the findings of other
studies (19,20). Olivares-Navarrete et al
(21) reported that the mRNA
level of non-canonical pathway ligands, receptors, and
intracellular signaling molecules was higher in cells cultured on
SLA surfaces than in those cultured on smooth surfaces. In
particular, increased ALP activity and osteocalcin production were
observed after osteoblasts were treated with Wnt5a (a non-canonical
pathway activator), suggesting that the non-canonical pathway was
involved in the differentiation of osteoblasts on microstructured
titanium surfaces. Similarly, Vlacic-Zischke et al (22) showed, using a whole-genome
analysis, that the TGF-β/BMP signaling pathway members BMP2, BMP6
and ACVR1 were upregulated in osteoblasts cultured on SLA and
modSLA (hydrophilic SLA). The expression of miRNAs has also been
investigated in relation to the modulation of osteogenic gene
expression by SLA and modSLA surfaces. Target predictions for the
differentially regulated miRNAs indicated that 35 and 10 miRNAs
were downregulated and upregulated, respectively, on modSLA
surfaces, and 32 and 9 miRNAs were downregulated and upregulated,
respectively, on SLA surfaces. Additionally, several genes involved
in the TGF-β/BMP and non-canonical Wnt/Ca2+ pathways
were identified as potential targets for the miRNAs during
differentiation on modSLA and SLA surfaces. These mechanisms may
also be involved in the enhanced proliferation and differentiation
of osteoblasts that we observed on Ti35Nb2Ta3Zr surfaces, which, as
described above, had rougher and more hydrophilic surfaces.
Tantalum and zirconium may have also contributed to
the observed effects on osteoblast cells, since the positive
effects of Ta and Zr on cell attachment, proliferation, and
differentiation have been well documented (23–28). Stiehler et al (23) reported that mesenchymal stem cells
cultured on Ta surfaces had a significantly increased mean cellular
area and cell-specific ALP activity compared with those cultured on
Ti and chromium surfaces. Additionally, Sista et al
(26) showed that the adhesion,
proliferation, and differentiation of MC3T3-E1 osteoblast cells
were better on TiZr than on TiNb or Ti. A key factor for this
difference was the presence of Zr in the TiZr alloy (26). A microRNA microarray analysis
showed that NOG, SHOX, IGF1, BMP1 and FGFR1 are differentially
regulated in osteoblasts exposed to zirconium (29). In the present study, Ta and Zr
were confirmed to be present on the surface of Ti35Nb2Ta3Zr by EDX.
Therefore, it is plausible that the presence of surface Ta and Zr
may have contributed to the enhanced osteoblast function observed
on Ti35Nb2Ta3Zr.
In vivo biomechanical and biological
properties
In an in vivo study, intramedullary rods were
inserted into rabbit femurs, and the bone compatibility of the
Ti35Nb2Ta3Zr alloy was assessed in comparison to Ti6Al4V, which is
considered bioinert material and has been widely used in medical
fields. The failure load gradually increased with time following
implantation in both groups, but there were no significant
differences between the failure loads of the Ti6Al4V and
Ti35Nb2Ta3Zr alloys at any time-point. These results are in
agreement with those of previous studies. Miura et al
(7) evaluated the pull-out
strength of Ti6Al4V and TiNbSn alloys at 3, 6 and 12 weeks
following implantation and reported that the failure loads of the 2
alloys increased with time, with no differences observed between
the alloys.
The methylene blue staining and double labeling of
tetracycline and calcein were used to evaluate new bone formation
at the bone-implant interface. The BAR and NBA of the Ti6Al4V and
Ti35Nb2Ta3Zr alloys gradually increased with time, and the values
did not differ between the 2 alloys. These results are similar to
those of previous studies. Miura et al (7) reported that the BAR and NBA of
Ti6Al4V and TiNbSn beneath cortical bone increased with time, but
the NBA of TiNbSn rapidly increased up to 6 weeks and reached a
peak. Lin et al (30)
evaluated Ti6Al4V and Ti7.5Mo alloys and obtained similar results.
However, the NBA of Ti6Al4V surrounded by cancellous bone decreased
with time after a peak at 12 weeks, and the NBA of Ti7.5Mo
continually increased after 12 weeks. No peak was observed in our
study. Although there was no significant difference in the BAR and
NBA between Ti6Al4V and Ti35Nb2Ta3Zr, the values of BAR and NBA
were higher for Ti35Nb2Ta3Zr, indicating that the in vivo
biocompatibility of Ti35Nb2Ta3Zr is equal to that of Ti6Al4V, which
has been widely used in biomedical fields. The lesser stiffness of
Ti35Nb2Ta3Zr may facilitate new bone formation.
In conclusion, the in vitro assay showed that
the adhesion, proliferation, and differentiation of osteoblasts
were better on Ti35Nb2Ta3Zr than on Ti and Ti6Al4V. The in
vivo study showed that the extent of new bone formation around
the Ti35Nb2Ta3Zr implants was equal to that around the Ti6Al4V
implants. In summary, the Ti35Nb2Ta3Zr alloy with a lower elastic
modulus has notable bone tissue compatibility in vitro and
in vivo, and may therefore be useful biomedical material in
the future.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant no. 81171688),
the Interdisciplinary (Engineering-Medical) Research Fund of
Shanghai Jiao Tong University (grant no. YG2011MS30), and the
Shanghai Municipal Health Bureau Science Fund for Young Scholars
(grant no. 2010QJ036A).
References
|
1
|
Rack H and Qazi J: Titanium alloys for
biomedical applications. Mater Sci Eng C. 26:1269–1277. 2006.
View Article : Google Scholar
|
|
2
|
Snyder SM and Schneider E: Estimation of
mechanical properties of cortical bone by computed tomography. J
Orthop Res. 9:422–431. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rae T: The toxicity of metals used in
orthopaedic prostheses. An experimental study using cultured human
synovial fibroblasts. J Bone Joint Surg Br. 63-B:435–440.
1981.PubMed/NCBI
|
|
4
|
Hallab NJ, Vermes C, Messina C, Roebuck
KA, Glant TT and Jacobs JJ: Concentration- and
composition-dependent effects of metal ions on human MG-63
osteoblasts. J Biomed Mater Res. 60:420–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guillemot F: Recent advances in the design
of titanium alloys for orthopedic applications. Expert Rev Med
Devices. 2:741–748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Long M and Rack HJ: Titanium alloys in
total joint replacement -a materials science perspective.
Biomaterials. 19:1621–1639. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miura K, Yamada N, Hanada S, Jung TK and
Itoi E: The bone tissue compatibility of a new Ti-Nb-Sn alloy with
a low Young’s modulus. Acta Biomater. 7:2320–2326. 2011.PubMed/NCBI
|
|
8
|
Wang LQ, Lu WJ, Qin JN, Zhang F and Zhang
D: Change in microstructures and mechanical properties of
biomedical Ti-Nb-Ta-Zr system alloy through cross-rolling. Mater
Trans. 49:1791–1795. 2008. View Article : Google Scholar
|
|
9
|
Wang LQ, Lu WJ, Qin JN, Zhang F and Zhang
D: Microstructure and mechanical properties of cold-rolled TiNbTaZr
biomedical beta titanium alloy. Mater Sci Eng A Struct Mater.
490:421–426. 2008. View Article : Google Scholar
|
|
10
|
Zhu L, Wang H, Xu J, Lin J and Wang X:
Effects of nacre-coated titanium surfaces on cell proliferation and
osteocalcin expression in MG-63 osteoblast-like cells. Afr J
Biotechnol. 10:15387–15393. 2011.
|
|
11
|
Lee MH, Kang JH and Lee SW: The
significance of differential expression of genes and proteins in
human primary cells caused by microgrooved biomaterial substrata.
Biomaterials. 33:3216–3234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Cai K, Luo Z, et al: Regulation of
the differentiation of mesenchymal stem cells in vitro and
osteogenesis in vivo by microenvironmental modification of titanium
alloy surfaces. Biomaterials. 33:3515–3528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schliephake H, Boetel C, Foerster A,
Schwenzer B, Reichert J and Scharnweber D: Effect of
oligonucleotide mediated immobilization of bone morphogenic
proteins on titanium surfaces. Biomaterials. 33:1315–1322. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu Z, Rausch-Fan X, Wieland M, Matejka M
and Schedle A: The initial attachment and subsequent behavior
regulation of osteoblasts by dental implant surface modification. J
Biomed Mater Res A. 82:658–668. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rausch-fan X, Qu Z, Wieland M, Matejka M
and Schedle A: Differentiation and cytokine synthesis of human
alveolar osteoblasts compared to osteoblast-like cells (MG63) in
response to titanium surfaces. Dent Mater. 24:102–110. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao G, Raines A, Wieland M, Schwartz Z
and Boyan B: Requirement for both micron-and submicron scale
structure for synergistic responses of osteoblasts to substrate
surface energy and topography. Biomaterials. 28:2821–2829. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Padial-Molina M, Galindo-Moreno P,
Fernandez-Barbero JE, et al: Role of wettability and nanoroughness
on interactions between osteoblast and modified silicon surfaces.
Acta Biomater. 7:771–778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhuang LF, Jiang HH, Qiao SC, et al: The
roles of extracellular signal-regulated kinase 1/2 pathway in
regulating osteogenic differentiation of murine preosteoblasts
MC3T3-E1 cells on roughened titanium surfaces. J Biomed Mater Res
A. 100:125–133. 2012. View Article : Google Scholar
|
|
19
|
Higuchi C, Myoui A, Hashimoto N, et al:
Continuous inhibition of MAPK signaling promotes the early
osteoblastic differentiation and mineralization of the
extracellular matrix. J Bone Miner Res. 17:1785–1794. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kono S, Oshima Y, Hoshi K, et al: Erk
pathways negatively regulate matrix mineralization. Bone. 40:68–74.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olivares-Navarrete R, Hyzy SL, Hutton DL,
et al: Role of non-canonical Wnt signaling in osteoblast maturation
on microstructured titanium surfaces. Acta Biomater. 7:2740–2750.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vlacic-Zischke J, Hamlet SM, Frus T,
Tonetti MS and Ivanovski S: The influence of surface microroughness
and hydrophilicity of titanium on the up-regulation of TGFβ/BMP
signalling in osteoblasts. Biomaterials. 32:665–671.
2011.PubMed/NCBI
|
|
23
|
Stiehler M, Lind M, Mygind T, et al:
Morphology, proliferation, and osteogenic differentiation of
mesenchymal stem cells cultured on titanium, tantalum, and chromium
surfaces. J Biomed Mater Res A. 86:448–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balla VK, Bodhak S, Bose S and
Bandyopadhyay A: Porous tantalum structures for bone implants:
fabrication, mechanical and in vitro biological properties. Acta
Biomater. 6:3349–3359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balla VK, Banerjee S, Bose S and
Bandyopadhyay A: Direct laser processing of a tantalum coating on
titanium for bone replacement structures. Acta Biomater.
6:2329–2334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sista S, Wen C, Hodgson PD and Pande G:
The influence of surface energy of titanium-zirconium alloy on
osteoblast cell functions in vitro. J Biomed Mater Res A. 97:27–36.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hempel U, Hefti T, Kalbacova M,
Wolf-Brandstetter C, Dieter P and Schlottig F: Response of
osteoblast-like SAOS-2 cells to zirconia ceramics with different
surface topographies. Clin Oral Implants Res. 21:174–181. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramaswamy Y, Wu C, Van Hummel A, Combes V,
Grau G and Zreiqat H: The responses of osteoblasts, osteoclasts and
endothelial cells to zirconium modified calcium-silicate-based
ceramic. Biomaterials. 29:4392–4402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palmieri A, Pezzetti F, Brunelli G, et al:
Zirconium oxide regulates RNA interfering of osteoblast-like cells.
J Mater Sci Mater Med. 19:2471–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin DJ, Chuang CC, Chern Lin JH, Lee JW,
Ju CP and Yin HS: Bone formation at the surface of low modulus
Ti-7.5Mo implants in rabbit femur. Biomaterials. 28:2582–2589.
2007. View Article : Google Scholar : PubMed/NCBI
|