Introduction
Radiation therapy is an important and effective
method for the treatment of tumors. However, this widely used
treatment increases the occurrence of long-term side effects caused
by radiation damage to normal tissues near the tumor. Sequelae in
the skeleton and bone marrow can occur as late consequences for
survivors following radiation therapy of cancer (1). Bones within the irradiated volume
can absorb radiation doses, resulting in an increased fracture risk
(2–6). In addition to bone fracture,
demineralization of bone, thinning of bone, sclerosis, and loss of
trabecular connections have been characterized as consequences of
radiotherapy (7–10). Damage to osteoblasts and
osteocytes is conceived to be a primary contributor to the reduced
bone mineral density that is observed following irradiation
(7,8,10,11). Previous studies suggest that
radiation can impair bone formation by inducing a decrease in
osteoblast proliferation and differentiation, inducing cell-cycle
arrest, reducing collagen production, and increasing sensitivity to
apoptotic agents in vitro and in vivo (12–15).
During the radiation therapy of tumors, the absorbed
dose in surrounding tissues can be substantial. Cancer patients
receive daily doses (fractions) of 1.8–2.0 Gy targeted locally to
the tumor and delivered over a period of a few minutes, with the
total dose to the tumor ranging from 50 to 80 Gy or more. For large
solar particle events (16)
lasting 8–24 h, the whole body cumulative dose for protons could
reach the 1–2 Gy level (1.0–2.0 Sv) depending upon the tissue site
in the space radiation environment (17). It remains unknown what the effects
of radiation on signaling pathways are, which is the topic of the
present study.
Bone remodeling is a temporally and spatially
regulated process that results in the coordinated resorption and
formation of skeletal tissue (18). Bone remodeling occurs in basic
multicellular units in which osteoclasts resorb bone and
osteoblasts form newly synthesized matrix in a coordinated process
that takes ∼4 months. The number and function of osteoclasts and
osteoblasts are regulated by extracellular and intracellular
signals acting in a coordinated fashion that maintains skeletal
homeostasis. The fate of mesenchymal cells and their
differentiation into cells of the osteoblast lineage is tightly
controlled by signaling molecules such as bone morphogenetic
proteins (BMPs) and Wnt, which favor osteoblastogenesis, and by
others such as Notch, which impairs osteoblast differentiation
(19–21). In addition to bone construction,
osteoblasts are involved in the regulation of bone resorption
through receptor activator of nuclear factor-κB (22) ligand (RANKL) and secretion of a
soluble decoy receptor (osteoprotegerin, OPG). RANKL is a ligand
for its receptor, RANK, and is a key factor in osteoclast
differentiation and activation. OPG prevents osteoclast
differentiation and activation and protects the skeleton from bone
resorption by blocking RANK/RANKL interaction. In short, the
balance between RANKL and OPG determines the formation and activity
of osteoclasts (23).
Notch was first identified in Drosophila
melanogaster, where its inactivation causes notches in the wing
blade. The Notch signaling pathway is an evolutionarily conserved
communication system. It mediates cell-cell interactions that are
crucial for cell-fate determination, differentiation and many other
biological processes. In mammals there are four receptors termed
Notch1–4, which are single-pass transmembrane receptors. The
ligands Delta/Serrate/Lag-2 (DSL) and Jagged1 and 2, and Delta1, 3,
and 4 are single-pass transmembrane proteins expressed on the
neighboring cells (24). Notch
signaling relies on the surface interaction between the Notch
receptor and membrane bound ligands in adjacent cells (25). Following ligand receptor
interactions, Notch is cleaved and the Notch intracellular domain
(NICD) is released (26). Hairy
enhancer of split (Hes) and Hes related with YRPW motif (Hey)
transcription factors are established targets of Notch signaling.
Seven Hes proteins (Hes1–7) and three Hey proteins (Hey1, Hey2 and
HeyL) have been identified, and all but Hes2 and Hes3 are targets
of Notch canonical signaling (27). Although Notch may act either as a
suppressor or inducer of osteoblast differentiation in
vitro, studies in transgenic murine models revealed that
activation of Notch signaling arrests commitment of pluripotent
precursors to the osteoblastic lineage and suppresses osteoblast
differentiation (28).
Notch signaling has been shown to be important in
the regulation of osteoblast differentiation, and therapeutic
radiation has been shown to induce changes to the skeletal system.
However, little information is available on the changes in Notch
signaling in irradiated osteoblasts. The purpose of this study was
thus to analyze the effect of radiation (2 and 4 Gy) on the Notch
pathway in vitro in differentiating osteoblasts. In order to
assess the radiation damage on osteoblast differentiation, total
RNA and protein were collected three days after radiation exposure.
Furthermore, the effects of radiation on the Notch signaling
pathway at the early and terminal stages of differentiation were
analyzed by qRT-PCR and western blotting.
Materials and methods
Cell culture
MC3T3-E1 cells were obtained from the Cell Bank of
the Institute of Basic Medicine at the Chinese Academy of Medical
Science (Beijing, China) and maintained in α-MEM supplemented with
1% L-glutamine and 10% heat-inactivated FBS and 100 μg/ml of
penicillin/streptomycin, in a humidified atmosphere of 5%
CO2 at 37°C. The cells were split when the culture
reached 80% confluency. The medium was changed every 3 days.
Induction of osteoblastogenic
differentiation and radiation exposure
MC3T3-E1 cells were cultured in differentiation
medium consisting of α-MEM supplemented with 10% FBS, 50
μg/ml ascorbic acid (Sigma, St. Louis, MO, USA), and 10 mM
β-glycerophosphate (Sigma) for 8 days to differentiate into
osteoblast precursor cells (early stages of osteoblast
differentiation) and 18 days to differentiate into osteoblast cells
(terminal stage of osteoblast differentiation) (29). This supplemented medium is
referred to as osteoblast differentiation medium or OBDM. It was
refreshed every 3 days. The cells were exposed to 0, 2, or 4 Gy of
γ-radiation on the fifth day (osteoblast precursor) or the
fifteenth day (osteoblast). 137Cs was used as a
radiation source.
Alkaline phosphatase staining
To detect osteoblasts, alkaline phosphatase (ALP)
staining was performed using the alkaline phosphatase kit (Nanjing
Jiancheng Bioengineering Institute). For ALP staining, MC3T3-E1
cells were cultured with OBDM in 24-well plates. After 8 days and
18 days, ALP staining was performed according to the manufacturer’s
instructions.
RNA extraction, reverse transcription and
qRT-PCR analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). First-strand cDNA was synthesized
using the reverse transcription kit (Invitrogen) with total RNA (1
μg). qRT-PCR analyses for Notch1, Notch2, Notch3, Notch4,
Jagged1, Jagged2, Delta1, Runx2, Hes1, ALP, RANKL, OPG and M-CSF
were performed using the CFX96 Touch™ Real-time PCR detection
system (Bio-Rad, Hercules, CA, USA). The reaction conditions were:
50°C for 2 min and 94°C for 3 min, followed by 40 cycles of 94°C
for 15 sec, 60°C for 20 sec and 72°C for 20 sec. The levels of
β-actin mRNA were used as the internal control, and the
gene-specific mRNA expression was normalized against β-actin
expression. The sequences of the primers used in these analyses are
listed in Table I.
 | Table ISequences of primers for quantitative
real-time polymerase chain reaction. |
Table I
Sequences of primers for quantitative
real-time polymerase chain reaction.
| Gene | Sequence |
|---|
| Notch1 | Forward:
AGCCTCTCCACCAATACCTT |
| Reverse:
GGCTGGAGCTGTAAGTTCTG |
| Notch2 | Forward:
TTCACTCTCGAATGCAACTG |
| Reverse:
AGCGGCAATTGTAGGTATTG |
| Notch3 | Forward:
TTGGGAAATCTGCCTTACAC |
| Reverse:
GTCTCTTCCTTGCTGTCCTG |
| Notch4 | Forward:
TCCGGACTTTTAAGGCCAAA |
| Reverse:
TTCCCATTGCTGTGCATACTCT |
| Delta1 | Forward:
CTGAGGTGTAAGATGGAAGCG |
| Reverse:
CAACTGTCCATAGTGCAATGG |
| Jagged-1 | Forward:
CTCTGGAAACCTCTGTCAGC |
| Reverse:
TCAGGTGTGAGCAGTTCTTG |
| Jagged-2 | Forward:
AAGGACATACTCTACCAGTGC |
| Reverse:
ACGTCCTTGGTACTTCTGACG |
| Runx2 | Forward:
CGGCCCTCCCTGAACTCT |
| Reverse:
TGCCTGCCTGGGATCTGTA |
| Hes1 | Forward:
CCTCTGAGCACAGAAAGTCA |
| Reverse:
GCCGGGAGCTATCTTTCTTA |
| OPG | Forward:
ACAGAGACCAGGAAATGGTG |
| Reverse:
CTCTCCATCAAGGCAAGAAG |
| RANKL | Forward:
CCAAGATCTCTAACATGACG |
| Reverse:
CACCATCAGCTGAAGATAGT |
| M-CSF | Forward:
CATCGAGACCCTCAGACATT |
| Reverse:
GCTGCTTCTTTCATCCAGTC |
| ALP | Forward:
TCAGGGCAATGAGGTCACATC |
| Reverse:
CACAATGCCCACGGACTTC |
| β-actin | Forward:
GAGACCTTCAACACCCCAGCC |
| Reverse:
AATGTCACGCACGATTTCCC |
Western blotting
Cells were lysed in M-PER Mammalian Protein
Extraction Reagent (Thermo Scientific) containing protease
inhibitors (Complete Ultra Mini EDTA-free protease inhibitor
cocktail tablets, Roche). Protein samples were separated by
electrophoresis in a 10% SDS-polyacrylamide gel and transferred
onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). The
membrane was blocked with 5% skimmed milk (BD). The following
primary antibodies were used: mouse monoclonal antibody to β-actin
(1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse
monoclonal antibody to Notch1 (1:1000; AbD Serotec), goat
polyclonal antibody to Notch2 (1:500; Santa Cruz Biotechnology),
goat polyclonal antibody to Jagged1 (1:500; Santa Cruz
Biotechnology), goat polyclonal antibody to Runx2 (1:500; Santa
Cruz Biotechnology), rabbit polyclonal antibody to Hes1 (1:1000;
Santa Cruz Biotechnology), goat polyclonal antibody to M-CSF
(1:500; Santa Cruz Biotechnology), goat polyclonal antibody to ALP
(1:1000; Santa Cruz Biotechnology), rabbit polyclonal antibody to
RANKL (1:500; Santa Cruz Biotechnology), mouse polyclonal antibody
to OPG (1:500; Abcam) followed by corresponding
peroxidase-conjugated antibodies (1:5000) according to the
manufacturer’s instructions (ZSGB-BIO). Infrared fluorescence of
the secondary antibodies was read on a Bio-Rad ChemiDoc™ XRS+
system.
Statistical analysis
Data are presented as means ± SD. The data were
compared using two-tailed unpaired Student’s t-test (SPSS 17.0 for
Windows). P<0.05 was indicative of a statistically significant
difference.
Results
In this study, we applied a previously established
method to induce MC3T3-E1 cell differentiation into osteoblasts and
osteoblast precursors. ALP is required for bone matrix
mineralization and is used as an osteoblast differentiation marker
(30). MC3T3-E1 cells exhibit
ALP-positive staining (3) after
18 days of culture in OBDM, indicating that MC3T3-E1 cells
successfully differentiated into osteoblasts (Fig. 1).
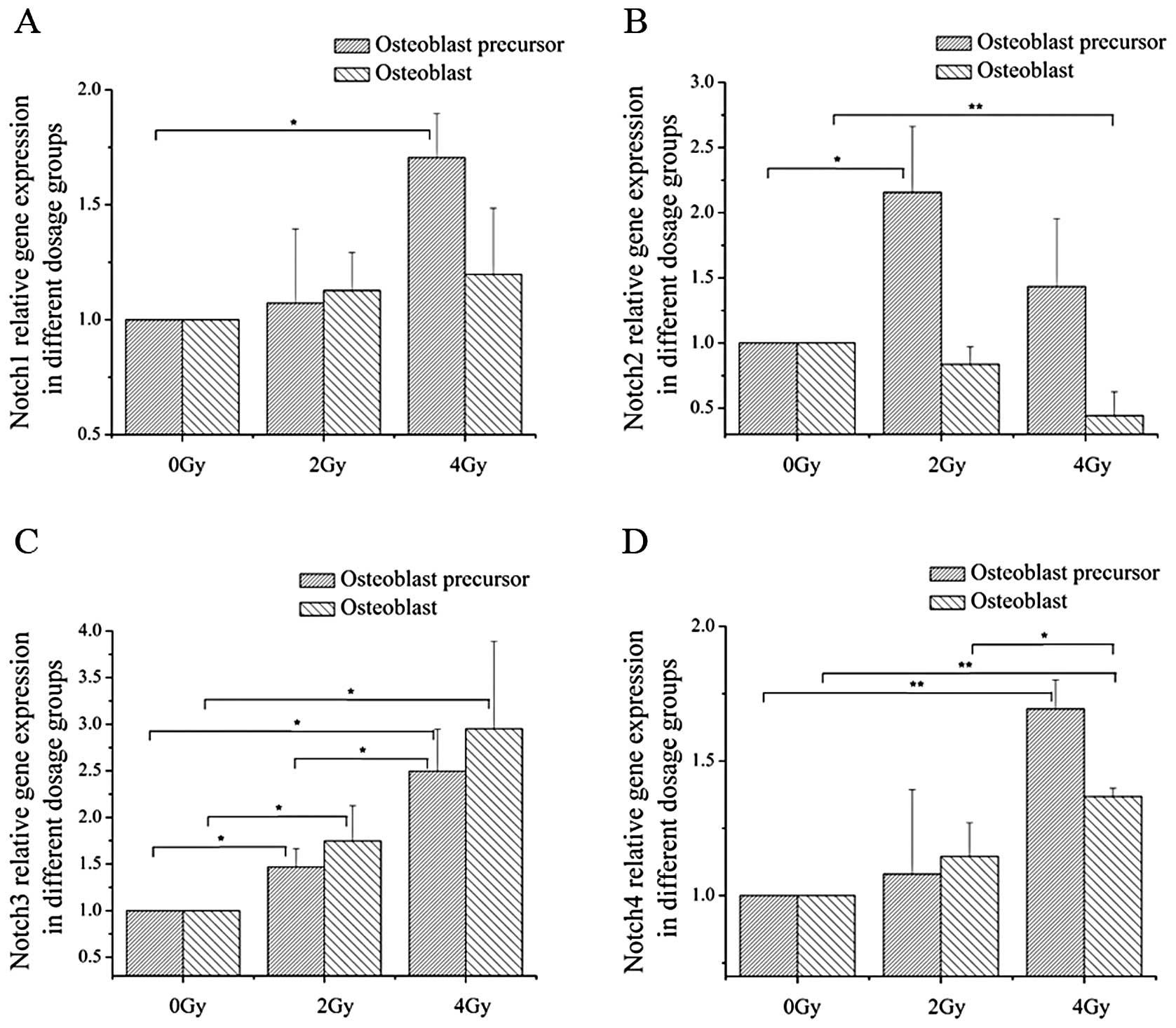
In order to determine the effect of radiation on the
expression of Notch signaling receptors, mRNA expression of
Notch1–4 was determined by qPCR, after 2 and 4 Gy of irradiation at
the different differentiation stages. At the early stages of
osteoblast differentiation, the mRNA expression level of Notch2 and
Notch3 increased after exposure to 2 Gy of irradiation, and Notch1,
Notch3 and Notch4 mRNA expression was upregulated after treatment
with 4 Gy of irradiation. In addition, at the terminal stages of
differentiation, 4 Gy of irradiation decreased the Notch2 mRNA
expression level and increased the mRNA expression level of Notch4;
both 2- and 4-Gy irradiation increased Notch3 expression (Fig. 2). The protein expression levels of
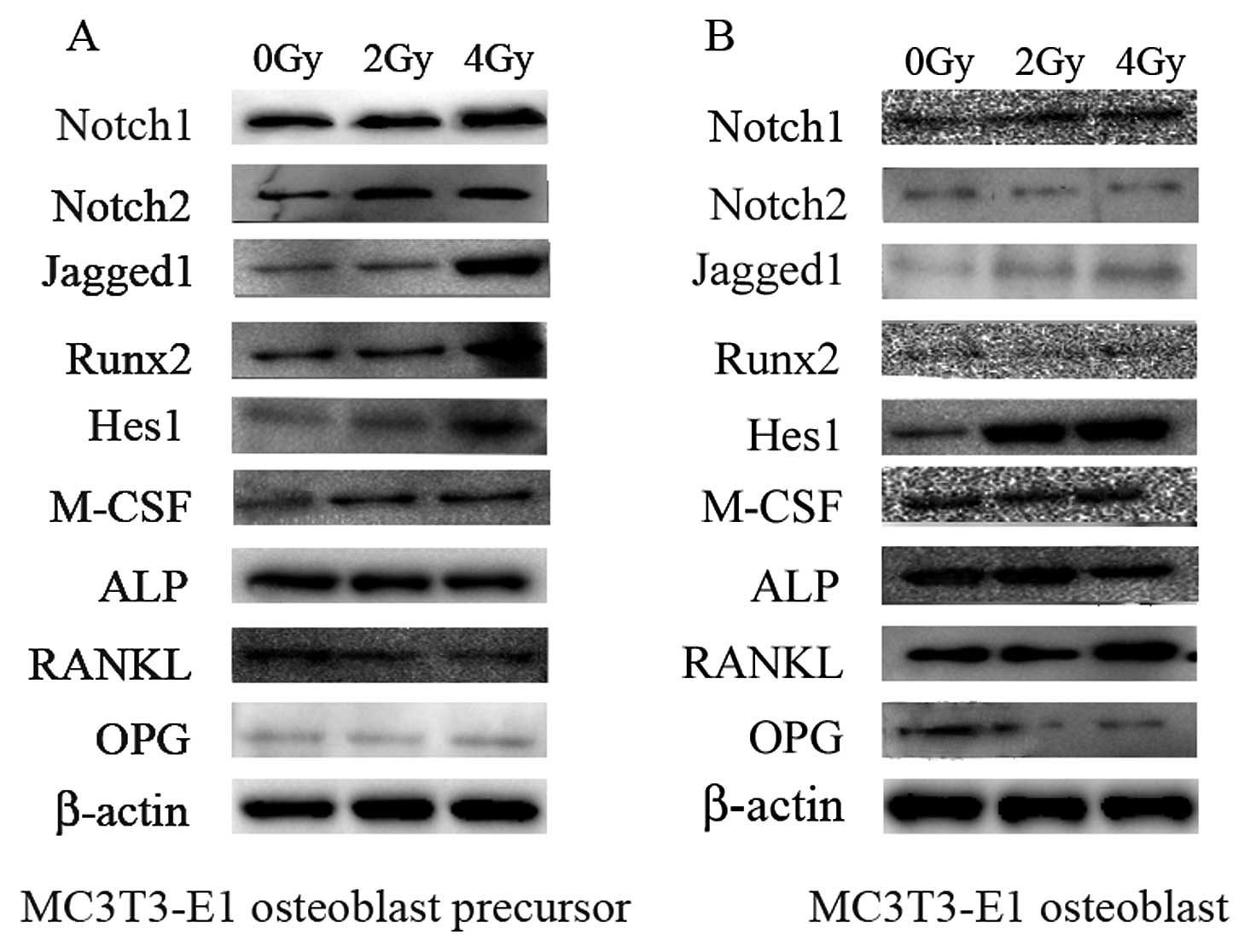
Notch1 and Notch2 were verified by western blotting (Fig. 5).
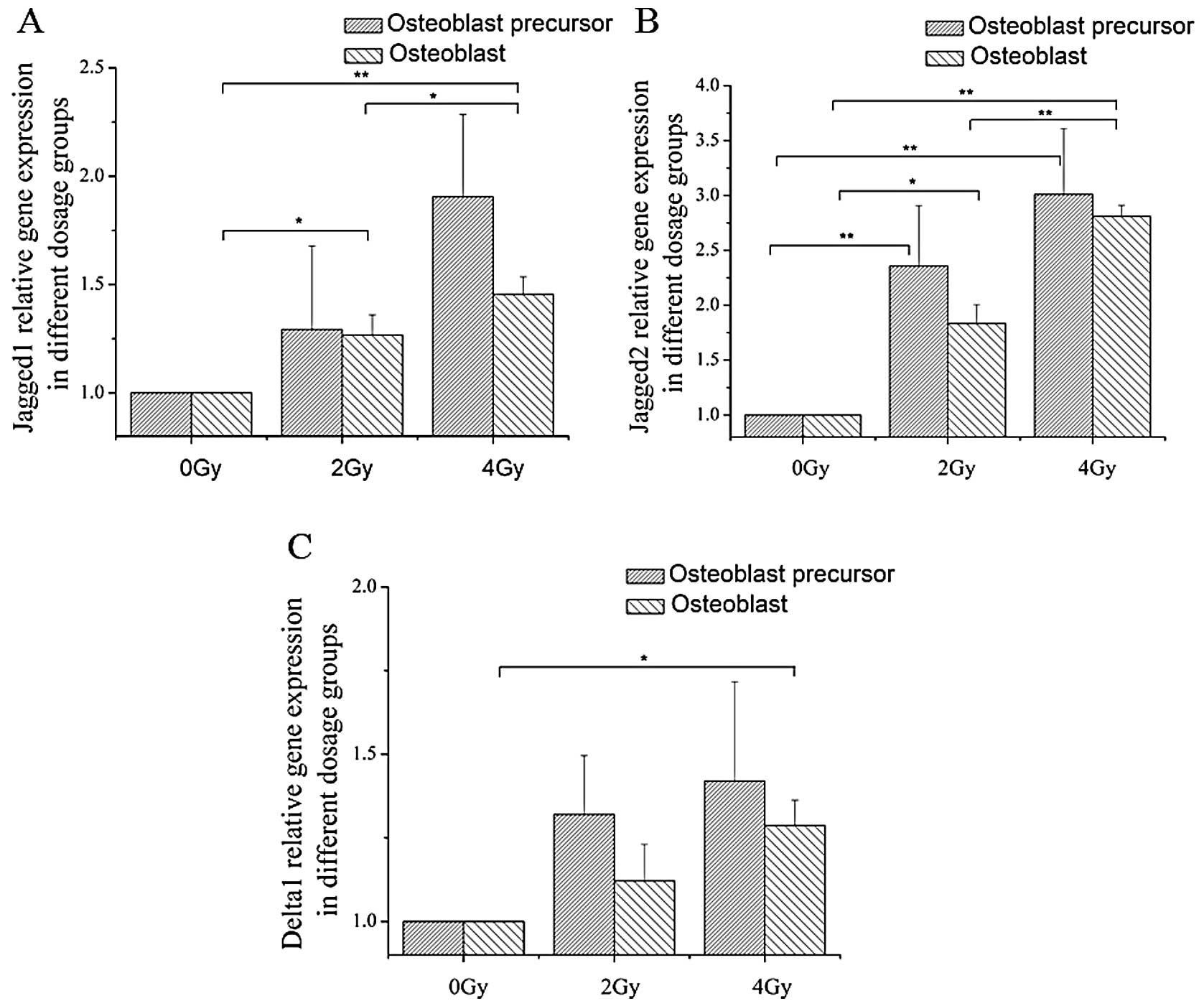
Three Notch ligands, Jagged1, Jagged2 and Delta1,
were tested. Jagged1 mRNA and protein expression levels were found
to be upregulated at the early stage of differentiation in cells
receiving 4 Gy of irradiation and at later stages when cells were
treated with both 2 and 4 Gy of irradiation (Figs. 3 and 5). The mRNA expression of Jagged2
increased at both the early and late stages following exposure to 2
and 4 Gy of irradiation. Additionally, for Delta1, the mRNA
expression level only significantly increased at the terminal stage
of osteoblast differentiation after 4 Gy of irradiation (Fig. 3).
The expression of Hes1, a known target of Notch
signaling, was significantly upregulated at the terminal stages of
MC3T3-E1 cells that received both 2 and 4 Gy of irradiation at the
mRNA (Fig. 4) and protein levels
(Fig. 5). This indicates that
Notch signaling was increased in radiated cells. Runx2
(Runt-related transcription factor, also called Cbfa1 or Pebp2aA),
an important transactivator of Notch signaling, was repressed at
the mRNA and protein levels after 2 and 4 Gy of irradiation
following exposure during the terminal stage of differentiation
(Figs. 4 and 5).
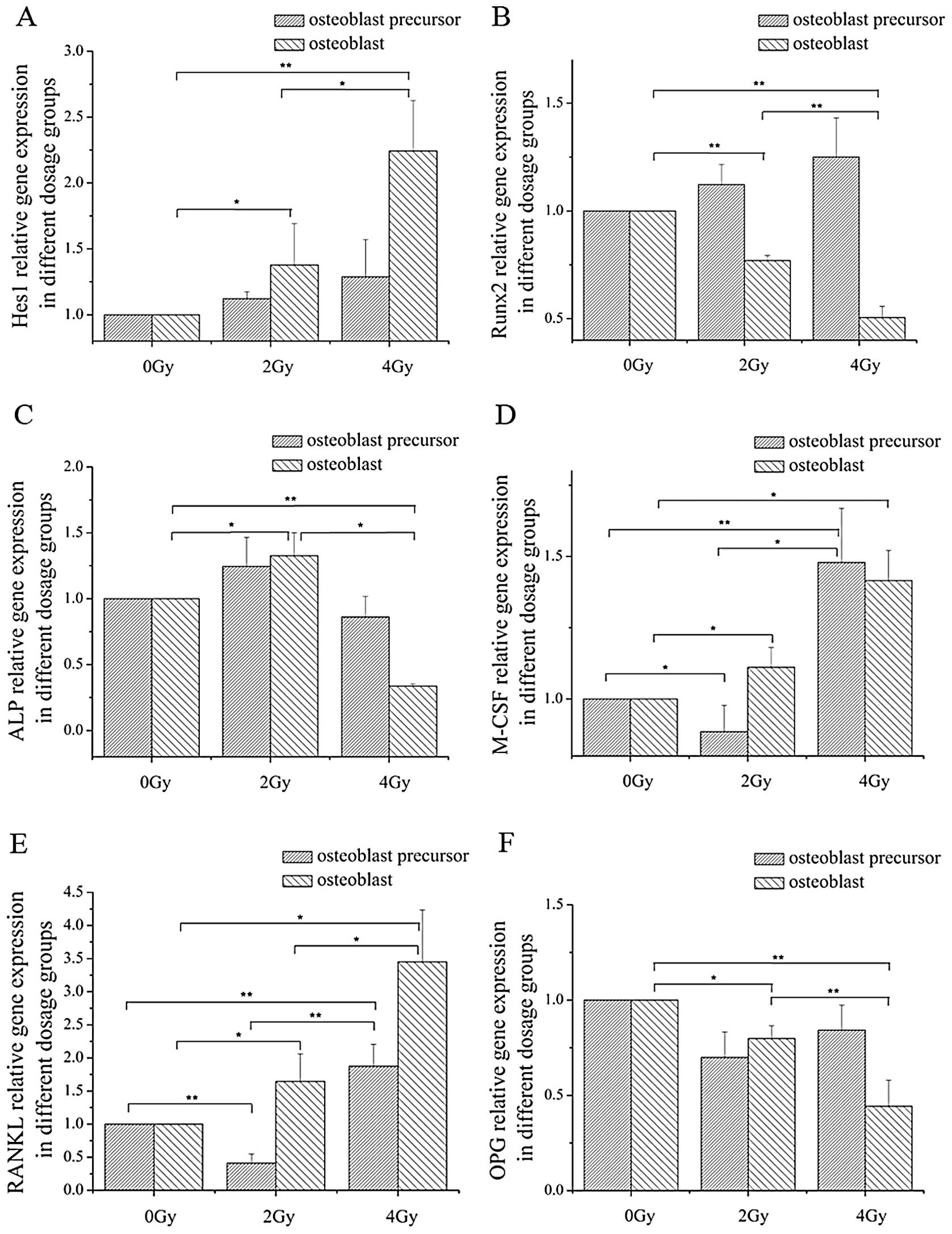 | Figure 4Relative expression of Hes1, Runx2,
ALP, M-CSF, RANKL and OPG. The expression profiles of (A) Hes1, (B)
Runx2, (C) ALP, (D) M-CSF, (E) RANKL and (F) OPG were determined
using the 2−ΔΔCt method. Each value represents the fold
changes of each gene, respectively (mean ± SD) of triplicate
cultures. *P<0.05, **P<0.01. |
The expression of ALP, a marker for osteoblasts, was
investigated. The ALP mRNA expression in the osteoblast precursors
was not affected by 2 or 4 Gy of irradiation. However, 2 Gy of
irradiation slightly induced ALP levels, and 4 Gy of radiation
exposure repressed ALP mRNA expression at the terminal stage of
osteoblast differentiation (Fig.
4). Radiation exposure of 4 Gy also decreased ALP protein
expression at the terminal stage of osteoblast differentiation
(Fig. 5). Although 2 Gy of
irradiation decreased M-CSF mRNA expression at the early stages of
differentiation, 2 Gy of irradiation increased M-CSF levels at the
late differentiation stages. Moreover, M-CSF expression was induced
by 4 Gy of irradiation at both early and terminal stage (Fig. 4). However, the protein level of
M-CSF increased at the early stages of differentiation after 2 and
4 Gy of radiation exposure. At the terminal stage of
differentiation, the protein level of M-CSF increased following 4
Gy of radiation exposure. However, there was no increase in the
protein level of M-CSF at the terminal stage of osteoblast
differentiation following 2 Gy of radiation (Fig. 5).
RANKL and OPG expression, key regulators of
osteoclast differentiation and function, was determined at
different differentiation stages with 0, 2 and 4 Gy of radiation
exposure. The RANKL mRNA expression level in osteoblast precursors
was repressed by 2 Gy of irradiation, but induced by 4 Gy of
irradiation. In osteoblasts differentiated from MC3T3-E1 cells, 2
and 4 Gy of irradiation successfully increased RANKL mRNA
expression levels (Fig. 4). The
protein level of RANKL was increased at the terminal stage of
osteoblast differentiation after 4 Gy of radiation exposure and was
decreased at the early and terminal stages of osteoblast
differentiation following 2 Gy of irradiation. Of note, 2 and 4 Gy
of irradiation did not change OPG mRNA and protein expression at
the early stage of osteoblast differentiation, but impaired OPG
levels at the terminal stage (Figs.
4 and 5).
Discussion
There is a high degree of structural homology in the
intra-cellular domain of the four Notch receptors. Although the
structural and functional properties of Notch1 have been
characterized to a greater extent than those of other Notch
receptors, their functional homology has not been explored in
detail. It has been previously shown that elevated levels of Notch1
impaired osteoblastogenesis by inhibiting Wnt/β-catenin signaling
(19,31). Wnt/β-catenin signaling is required
for osteoblastogenesis. Notch1 acts as a suppressor of osteoblast
differentiation, but the different timing of overexpression of NICD
is probably responsible for the differences in phenotype observed
(32). NICD overexpression
controlled by a 3.6-kb fragment of the collagen type 1α1 (Col1α1)
promoter, which is expressed during the early stages of osteoblast
differentiation, causes osteopenia due to decreased numbers of
osteoblasts (33). Overexpression
of NICD under the control of a 2.3-kb fragment of the Col1α1
promoter, which is active in mature osteoblasts, causes an increase
in osteoblasts synthesizing woven bone, possibly because of
impaired terminal osteoblast differentiation, an effect mediated by
the Notch canonical pathway (34,35).
In the present study, both early and terminal
osteoblast differentiation stages were investigated for the
response to radiation exposure. Our results did not show a strong
correlation between Notch1 regulation and suppression of osteoblast
differentiation. γ-irradiation at 4 Gy increased the expression of
Notch1 in osteoblast precursors, the early osteoblast
differentiation stage, but did not affect the expression level of
ALP, an osteoblast marker. In addition, 4 Gy of γ-radiation of
exposure decreased the ALP levels in osteoblasts at the terminal
differentiation stage, but did not significantly increase Notch1
expression. It is widely recognized that Notch1 may present the
main function of all four Notch receptors. However, we still
suspect that Notch2–4 proteins could play a role in the regulation
of skeletal development. The radiation effect on osteoblast
differentiation could be an integrated result of the four Notch
receptors or regulation by other molecular mechanisms. Although few
studies concerning the function of Notch2–4 in osteoblasts exist,
our laboratory investigated the radiation effects on Notch2–4 at
the early and late stage of osteoblast differentiation.
We also investigated the radiation effects on three
important Notch ligands, Jagged1, 2 and Delta1. Expression of the
Notch ligand Jagged1 has been detected in osteoblastic cells in
vivo and in vitro (31,36–40), and during bone regeneration
(41). There are still
controversies concerning the role of Jagged1 in osteoclastogenesis.
Sethi et al demonstrated that Jagged1-expressing cells
directly interact with osteoclast precursor cells to increase their
activity by accelerating osteoclast differentiation and maturation
(42). Bai et al
demonstrated that Jagged1-expressing cells inhibit the
differentiation of bone marrow macrophages to the osteoclast
phenotype in the presence of RANKL (43). Nobta et al suggested that
Jagged1 is capable of enhancing bone mineral deposition (41). Several studies have shown that
osteoblasts expressing the Notch ligand Jagged1 are part of the
hematopoietic stem cell (HSC) niche (36,44). However, Mancini et al
showed that HSC self-renewal and differentiation are independent of
Jagged1-dependent Notch signaling (45). Our study demonstrated that the
level of Jagged-1 expression increased in osteoblasts after 2 and 4
Gy of radiation and in osteoblast precursors after 4 Gy of
irradiation. Our results also showed their radiation induces
Jagged2 and Delta1 expression changes at the early and late stages
of osteoblast differentiation. Even though the function of the
Notch ligand in osteoblasts remains unclear, the ligands may play a
role in osteoblasts after radiation exposure.
The expression of Hes1, one of the targets of Notch
canonical signaling, was investigated after radiation. Hes1 plays
an important role in osteoblast differentiation and
osteoblast-osteoclast interaction. Hes1 overexpression impaired
osteoblastogenesis in vitro, and Hes1-overexpressing
transgenic osteoblasts enhanced the resorptive activity of
co-cultured osteoclast precursors (46). Additionally, Hes1 binds to the
osteocalcin promoter and suppresses trans activation of osteocalcin
(47). This indicates that Hes1
could suppress osteoblastogenesis and play a positive role in
osteoclastogenesis. We observed that 4 Gy of radiation increased
Hes1 expression by 2.2-fold. Of note, the expression level of ALP
decreased accompanied by increased Hes1 levels in osteoblasts after
4 Gy of irradiation. Therefore, we suspect that 4 Gy of radiation
can inhibit osteoblast differentiation at the terminal stage by
upregulating Hes1 expression. The upregulation of Hes1 in
osteoblasts indicated that Notch signaling was more active after 2
and 4 Gy of irradiation. The components in Notch signaling were not
directly transcribed upon radiation treatment, but this does not
mean that the pathway was not affected by irradiation.
Runx2 is an essential transactivator for osteoblast
differentiation and bone formation and is crucial for regulating
the expression of bone-specific genes (48,49). Runx2 has been shown to induce ALP
activity and mineralization in immature mesenchymal cells and
osteoblastic cells in vitro (50–52). Runx2 is an inhibitor of the Notch1
signaling pathway during normal osteoblast differentiation by
physically interacting with Notch1-IC and disrupting the
Notch1-IC-RBP-Jk transcription complex (53). We found that Runx2 expression is
repressed at the terminal stages of osteoblast differentiation
after exposure to 2 and 4 Gy of irradiation. Additionally, 4 Gy of
irradiation impaired both Runx2 and ALP expression at the late
stage of differentiation. We suspected that 4 Gy of irradiation
suppressed ALP expression levels and osteoblast differentiation at
the terminal stage by inhibition of Runx2 expression. Both 2 and 4
Gy of irradiation impaired Runx2 expression, but only 4 Gy of
irradiation decreased ALP expression. However, 2 Gy of irradiation
did not repress, but slightly increased ALP expression at the late
stage of osteoblast differentiation. This result indicated that the
increased ALP expression at the early stage of differentiation
after 2 Gy of irradiation was not induced by Runx2.
Irradiated osteoblasts may promote osteoclast
differentiation and proliferation mediated by RANKL/OPG and
macrophage colony stimulating factor (M-CSF). RANKL, OPG and M-CSF
are the key regulators of bone resorption and act directly on
osteoclast precursors. M-CSF produced by osteoblast cells is
required for survival of cells in the macrophage-osteoclast
lineage, and appears to be essential for the proliferation and
differentiation of osteoclast progenitors (54–58). In addition to M-CSF, RANKL has
been identified as a key cytokine that regulates osteoclastogenesis
and bone resorption (22,59–61).
Activated osteoblasts support osteoclast formation
and differentiation by expressing M-CSF and RANKL, but they also
inhibit this process through expression of OPG, which binds to and
inactivates RANKL. Changes in RANKL, OPG and M-CSF mRNA and protein
expression in early and terminally differentiated osteoblasts after
2 and 4 Gy of irradiation are listed in Table II. Our results showed that
osteoblasts receiving 2 and 4 Gy of irradiation at the terminal
differentiation stage undergo osteoclast differentiation and
proliferation by increased RANKL and M-CSF expression and decreased
OPG expression. Compared with the late stage of osteoblast
differentiation, the radiation effect on the early stage of
osteoblast differentiation was more pronounced. Irradiation at 2 Gy
decreased RANKL mRNA and protein expression levels and showed no
significant changes in OPG levels at the early stage of osteoblast
differentiation. Radiation exposure at 2 Gy induced M-CSF
downregulation at the mRNA level, but upregulation at the protein
level at the early stage of osteoblast differentiation. Even more,
4 Gy of irradiation increased M-CSF mRNA and protein expression and
showed no significant changes in OPG level in early stage
osteoblasts. Radiation exposure at 4 Gy induced RANKL upregulation
at the mRNA level, while RANKL was downregulated at the protein
level at the early stage of osteoblast differentiation. Irradiation
at 4 Gy stimulated osteoclast differentiation and functioned
through increasing M-CSF and RANKL levels. Radiated osteoblasts may
play a role in radiation-induced bone loss. Considering the
radiation effect on Notch signaling and its important role in
osteoblast regulation, we suspect that Notch signaling plays an
important role in osteoblast-osteoclast communication.
 | Table IIRANKL, OPG and M-CSF mRNA and protein
expression alterations at the early and terminal differentiation
stages following 2 and 4 Gy of irradiation. |
Table II
RANKL, OPG and M-CSF mRNA and protein
expression alterations at the early and terminal differentiation
stages following 2 and 4 Gy of irradiation.
| 2-Gy irradiation
| 4-Gy irradiation
|
|---|
| Early osteoblast
differentiation stage | Terminal osteoblast
differentiation stage | Early osteoblast
differentiation stage | Terminal osteoblast
differentiation stage |
|---|
| RANKL | | | | |
| mRNA level | ↓ | ↑ | ↑ | ↑ |
| Protein
level | ↓ | - | ↓ | ↑ |
| OPG | | | | |
| mRNA level | - | ↓ | - | ↓ |
| Protein
level | - | ↓ | - | ↓ |
| M-CSF | | | | |
| mRNA level | ↓ | ↑ | ↑ | ↑ |
| Protein
level | ↑ | - | ↑ | ↑ |
Many studies have reported that Notch signaling is
vital to osteoblast development and skeletal remodeling by loss and
gain methods. However, radiation may be more com plicated and could
cause a serial of changes in Notch signaling. Although our data
suggest that Notch1 may not be an important contribution to the
radiation effect of osteoblast differentiation, we still need to
understand how Notch receptors function together when subjected to
external stimuli, such as exposure to radiation. This study reports
the changes in Notch signaling following radiation. Our study did
not show a strong relation between the upregulation of Notch1
expression and suppression of osteoblast differentiation. We found
that Hes1 plays a role in the radiation effect on osteoblast
differentiation. The components in Notch signaling were not
directly transcribed upon radiation treatment, but this does not
mean that the pathway was not affected by irradiation. Moreover,
our result indicated that irradiated osteoblasts promote osteoclast
differentiation and proliferation. Our findings aid in the
understanding of the mechanism of the Notch signaling pathway in
radiation-impaired osteoblastogenesis.
Acknowledgements
The authors thank Dr Liqing Du and Dr
XiaoChun Wang for the helpful discussion. This study was supported
by grants from the National Nature Science Foundation of China (no.
30970867) (http://www.nsfc.gov.cn).
References
|
1
|
Parker RG and Berry HC: Late effects of
therapeutic irradiation on the skeleton and bone marrow. Cancer.
37(Suppl 2): 1162–1171. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baxter NN, Habermann EB, Tepper JE, Durham
SB and Virnig BA: Risk of pelvic fractures in older women following
pelvic irradiation. JAMA. 294:2587–2593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown SA and Guise TA: Cancer
treatment-related bone disease. Crit Rev Eukaryot Gene Expr.
19:47–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guise TA: Bone loss and fracture risk
associated with cancer therapy. Oncologist. 11:1121–1131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Florin TA, Fryer GE, Miyoshi T, Weitzman
M, Mertens AC, Hudson MM, Sklar CA, Emmons K, Hinkle A, Whitton J,
Stovall M, Robison LL and Oeffinger KC: Physical inactivity in
adult survivors of childhood acute lymphoblastic leukemia: a report
from the childhood cancer survivor study. Cancer Epidemiol
Biomarkers Prev. 16:1356–1363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oeffinger KC, Mertens AC, Sklar CA,
Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie
W, Kadan-Lottick NS, Schwartz CL, Leisenring W and Robison LL:
Childhood Cancer Survivor Study: chronic health conditions in adult
survivors of childhood cancer. N Engl J Med. 355:1572–1582. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ergün H and Howland WJ: Postradiation
atrophy of mature bone. CRC Crit Rev Diagn Imaging. 12:225–243.
1980.
|
|
8
|
Hopewell JW: Radiation-therapy effects on
bone density. Med Pediatr Oncol. 41:208–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Howland WJ, Loeffler RK, Starchman DE and
Johnson RG: Postirradiation atrophic changes of bone and related
complications. Radiology. 117:677–685. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitchell MJ and Logan PM:
Radiation-induced changes in bone. Radiographics. 18:1125–1136.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sams A: The effect of 2000 r of X-rays on
the internal structure of the mouse tibia. Int J Radiat Biol Relat
Stud Phys Chem Med. 11:51–68. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dudziak ME, Saadeh PB, Mehrara BJ,
Steinbrech DS, Greenwald JA, Gittes GK and Longaker MT: The effects
of ionizing radiation on osteoblast-like cells in vitro. Plast
Reconstr Surg. 106:1049–1061. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gal TJ, Munoz-Antonia T, Muro-Cacho CA and
Klotch DW: Radiation effects on osteoblasts in vitro: a potential
role in osteoradionecrosis. Arch Otolaryngol Head Neck Surg.
126:1124–1128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szymczyk KH, Shapiro IM and Adams CS:
Ionizing radiation sensitizes bone cells to apoptosis. Bone.
34:148–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakurai T, Sawada Y, Yoshimoto M, Kawai M
and Miyakoshi J: Radiation-induced reduction of osteoblast
differentiation in C2C12 cells. J Radiat Res. 48:515–521. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geblinger D, Zink C, Spencer ND, Addadi L
and Geiger B: Effects of surface microtopography on the assembly of
the osteoclast resorption apparatus. J R Soc Interface.
9:1599–1608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willey JS, Lloyd SA, Nelson GA and Bateman
TA: Space radiation and bone loss. Gravit Space Biol Bull.
25:14–21. 2011.
|
|
18
|
Canalis E, Giustina A and Bilezikian JP:
Mechanisms of anabolic therapies for osteoporosis. N Engl J Med.
357:905–916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deregowski V, Gazzerro E, Priest L,
Rydziel S and Canalis E: Notch 1 overexpression inhibits
osteoblastogenesis by suppressing Wnt/beta-catenin but not bone
morphogenetic protein signaling. J Biol Chem. 281:6203–6210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gazzerro E and Canalis E: Bone
morphogenetic proteins and their antagonists. Rev Endocr Metab
Disord. 7:51–65. 2006. View Article : Google Scholar
|
|
21
|
Krishnan V, Bryant HU and Macdougald OA:
Regulation of bone mass by Wnt signaling. J Clin Invest.
116:1202–1209. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong BR, Rho J, Arron J, Robinson E,
Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS III,
Frankel WN, Lee SY and Choi Y: TRANCE is a novel ligand of the
tumor necrosis factor receptor family that activates c-Jun
N-terminal kinase in T cells. J Biol Chem. 272:25190–25194. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caetano-Lopes J, Canhão H and Fonseca JE:
Osteoblasts and bone formation. Acta Reumatol Port. 32:103–110.
2007.
|
|
24
|
Zanotti S and Canalis E: Notch regulation
of bone development and remodeling and related skeletal disorders.
Calcif Tissue Int. 90:69–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hori K, Sen A, Kirchhausen T and
Artavanis-Tsakonas S: Regulation of ligand-independent Notch signal
through intra-cellular trafficking. Commun Integr Biol. 5:374–376.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schroeter EH, Kisslinger JA and Kopan R:
Notch-1 signalling requires ligand-induced proteolytic release of
intracellular domain. Nature. 393:382–386. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iso T, Kedes L and Hamamori Y: HES and
HERP families: multiple effectors of the Notch signaling pathway. J
Cell Physiol. 194:237–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zanotti S and Canalis E: Notch and the
skeleton. Mol Cell Biol. 30:886–896. 2010. View Article : Google Scholar
|
|
29
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: an in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murshed M, Harmey D, Millán JL, McKee MD
and Karsenty G: Unique coexpression in osteoblasts of broadly
expressed genes accounts for the spatial restriction of ECM
mineralization to bone. Genes Dev. 19:1093–1104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sciaudone M, Gazzerro E, Priest L, Delany
AM and Canalis E: Notch 1 impairs osteoblastic cell
differentiation. Endocrinology. 144:5631–5639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalajzic I, Kalajzic Z, Kaliterna M,
Gronowicz G, Clark SH, Lichtler AC and Rowe D: Use of type I
collagen green fluorescent protein transgenes to identify
subpopulations of cells at different stages of the osteoblast
lineage. J Bone Miner Res. 17:15–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zanotti S, Smerdel-Ramoya A, Stadmeyer L,
Durant D, Radtke F and Canalis E: Notch inhibits osteoblast
differentiation and causes osteopenia. Endocrinology.
149:3890–3899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tao J, Chen S, Yang T, Dawson B, Munivez
E, Bertin T and Lee B: Osteosclerosis owing to Notch gain of
function is solely Rbpj-dependent. J Bone Miner Res. 25:2175–2183.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Engin F, Yao Z, Yang T, Zhou G, Bertin T,
Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF and Lee B:
Dimorphic effects of Notch signaling in bone homeostasis. Nat Med.
14:299–305. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Calvi LM, Adams GB, Weibrecht KW, Weber
JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P,
Bringhurst FR, Milner LA, Kronenberg HM and Scadden DT:
Osteoblastic cells regulate the haematopoietic stem cell niche.
Nature. 425:841–846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pereira RM, Delany AM, Durant D and
Canalis E: Cortisol regulates the expression of Notch in
osteoblasts. J Cell Biochem. 85:252–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schnabel M, Fichtel I, Gotzen L and
Schlegel J: Differential expression of Notch genes in human
osteoblastic cells. Int J Mol Med. 9:229–232. 2002.PubMed/NCBI
|
|
39
|
Luo B, Aster JC, Hasserjian RP, Kuo F and
Sklar J: Isolation and functional analysis of a cDNA for human
Jagged2, a gene encoding a ligand for the Notch1 receptor. Mol Cell
Biol. 17:6057–6067. 1997.PubMed/NCBI
|
|
40
|
Dallas DJ, Genever PG, Patton AJ,
Millichip MI, McKie N and Skerry TM: Localization of ADAM10 and
Notch receptors in bone. Bone. 25:9–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nobta M, Tsukazaki T, Shibata Y, Xin C,
Moriishi T, Sakano S, Shindo H and Yamaguchi A: Critical regulation
of bone morphogenetic protein-induced osteoblastic differentiation
by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem.
280:15842–15848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sethi N, Dai X, Winter CG and Kang Y:
Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast
cancer by engaging notch signaling in bone cells. Cancer Cell.
19:192–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bai S, Kopan R, Zou W, Hilton MJ, Ong CT,
Long F, Ross FP and Teitelbaum SL: NOTCH1 regulates
osteoclastogenesis directly in osteoclast precursors and indirectly
via osteoblast lineage cells. J Biol Chem. 283:6509–6518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang J, Niu C, Ye L, Huang H, He X, Tong
WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM,
Mishina Y and Li L: Identification of the haematopoietic stem cell
niche and control of the niche size. Nature. 425:836–841. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mancini SJ, Mantei N, Dumortier A, Suter
U, MacDonald HR and Radtke F: Jagged1-dependent Notch signaling is
dispensable for hematopoietic stem cell self-renewal and
differentiation. Blood. 105:2340–2342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zanotti S, Smerdel-Ramoya A and Canalis E:
HES1 (hairy and enhancer of split 1) is a determinant of bone mass.
J Biol Chem. 286:2648–2657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Lian JB, Stein JL, van Wijnen AJ
and Stein GS: The Notch-responsive transcription factor Hes-1
attenuates osteocalcin promoter activity in osteoblastic cells. J
Cell Biochem. 108:651–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ito Y: Oncogenic potential of the RUNX
gene family: ‘overview’. Oncogene. 23:4198–4208. 2004.
|
|
49
|
Levanon D, Negreanu V, Bernstein Y, Bar-Am
I, Avivi L and Groner Y: AML1, AML2, and AML3, the human members of
the runt domain gene-family: cDNA structure, expression, and
chromosomal localization. Genomics. 23:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Banerjee C, McCabe LR, Choi JY, Hiebert
SW, Stein JL, Stein GS and Lian JB: Runt homology domain proteins
in osteoblast differentiation: AML3/CBFA1 is a major component of a
bone-specific complex. J Cell Biochem. 66:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: a transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Harada H, Tagashira S, Fujiwara M, Ogawa
S, Katsumata T, Yamaguchi A, Komori T and Nakatsuka M: Cbfa1
isoforms exert functional differences in osteoblast
differentiation. J Biol Chem. 274:6972–6978. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ann EJ, Kim HY, Choi YH, Kim MY, Mo JS,
Jung J, Yoon JH, Kim SM, Moon JS, Seo MS, Hong JA, Jang WG, Shore
P, Komori T, Koh JT and Park HS: Inhibition of Notch1 signaling by
Runx2 during osteoblast differentiation. J Bone Miner Res.
26:317–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yoshida H, Hayashi S, Kunisada T, Ogawa M
and Nishikawa S, Okamura H, Sudo T, Shultz LD and Nishikawa S: The
murine mutation osteopetrosis is in the coding region of the
macrophage colony stimulating factor gene. Nature. 345:442–444.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lagasse E and Weissman IL: Enforced
expression of Bcl-2 in monocytes rescues macrophages and partially
reverses osteopetrosis in op/op mice. Cell. 89:1021–1031. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Takahashi N, Udagawa N, Akatsu T, Tanaka
H, Isogai Y and Suda T: Deficiency of osteoclasts in osteopetrotic
mice is due to a defect in the local microenvironment provided by
osteoblastic cells. Endocrinology. 128:1792–1796. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hattersley G, Owens J, Flanagan AM and
Chambers TJ: Macrophage colony stimulating factor (M-CSF) is
essential for osteoclast formation in vitro. Biochem Biophys Res
Commun. 177:526–531. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tanaka S, Takahashi N, Udagawa N, Tamura
T, Akatsu T, Stanley ER, Kurokawa T and Suda T: Macrophage
colony-stimulating factor is indispensable for both proliferation
and differentiation of osteoclast progenitors. J Clin Invest.
91:257–263. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A,
Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N and Suda T:
Osteoclast differentiation factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar
|
|
60
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX,
Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J and
Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Anderson DM, Maraskovsky E, Billingsley
WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D
and Galibert L: A homologue of the TNF receptor and its ligand
enhance T-cell growth and dendritic-cell function. Nature.
390:175–179. 1997. View
Article : Google Scholar : PubMed/NCBI
|