1. Introduction
High mobility group box 1 (HMGB1) was originally
described 30 years ago as a nonhistone DNA-binding protein with
high-electrophoretic mobility (1). It is present in the nucleus of
almost all metazoans and plants, where it exerts structural and
transcriptional activities (2–4).
In addition to its role in the nucleus, HMGB1 has recently emerged
as an extracellular signaling factor with key roles in cell
differentiation, proliferation and disease pathogenesis (5).
In this review, we focus on the involvement of HMGB1
in the pathogenesis of kidney diseases, as well as renal allograft
rejection after renal transplantation. An understanding of these
discoveries may shed new insight into the possibilities for
developing novel therapeutic strategies to mitigate or prevent
kidney diseases.
2. Structure of HMGB1
The HMGB1 protein, a member of the high mobility
group nuclear protein family, is one of the most evolutionarily
conserved proteins and shares 99% identity in the amino acid
sequence between rodents and humans (1). The human HMGB1 gene is located on
chromosome 13q12 (6), and six
polymorphic loci throughout the gene locus have recently been
identified (7).
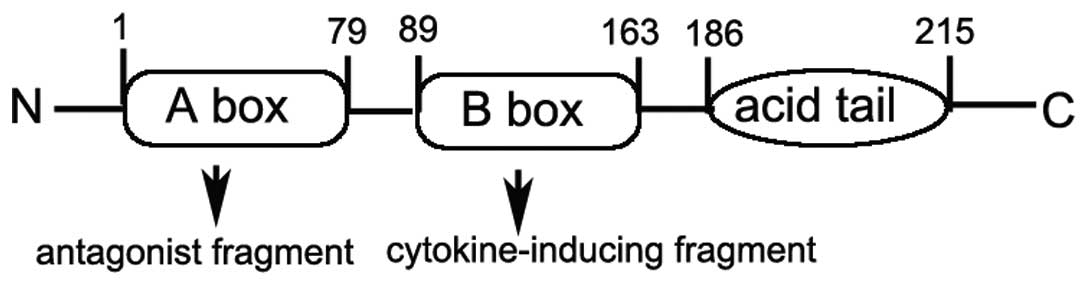
HMGB1 has a highly dipolar structure consisting of
215 residues organized into two basic DNA-binding domains, referred
to as the A and B box as well as a negatively charged C-acidic tail
(Fig. 1) (8). Each HMGB1 A or B box is
approximately 75–80 amino acids in length (9) and is formed by two short and one
long α-helix that upon folding produce an L- or V-shaped
three-dimensional domain structure (10,11). Research suggests that the B box
possesses the pro-inflammatory properties of HMGB1 including
cytokine release, and the A box instead competes with HMGB1 for
binding sites leading to attenuation of the inflammatory cascade
(12,13).
3. Function of intra-nuclear HMGB1
As the HMGB1 protein is essential for life,
HMGB1-knockout mice die shortly after birth (14). HMGB1 is fairly ubiquitous in
mammals and is almost always present in the nucleus (15)where HMGB1 plays an important role
in binding without sequence specificity to the minor groove of DNA
and induces bends in the helical structure, which regulate physical
interactions between DNA and transcription factors, including p53,
homeobox proteins, glucocorticoid receptor,
recombination-activating gene 1/2 (RAG1/2) proteins and steroid
hormone receptors (16,17). Even though HMGB1 is not essential
for the overall organization of chromatin in the nucleus, it is
critical for proper transcriptional control by specific
transcription factors (14,18).
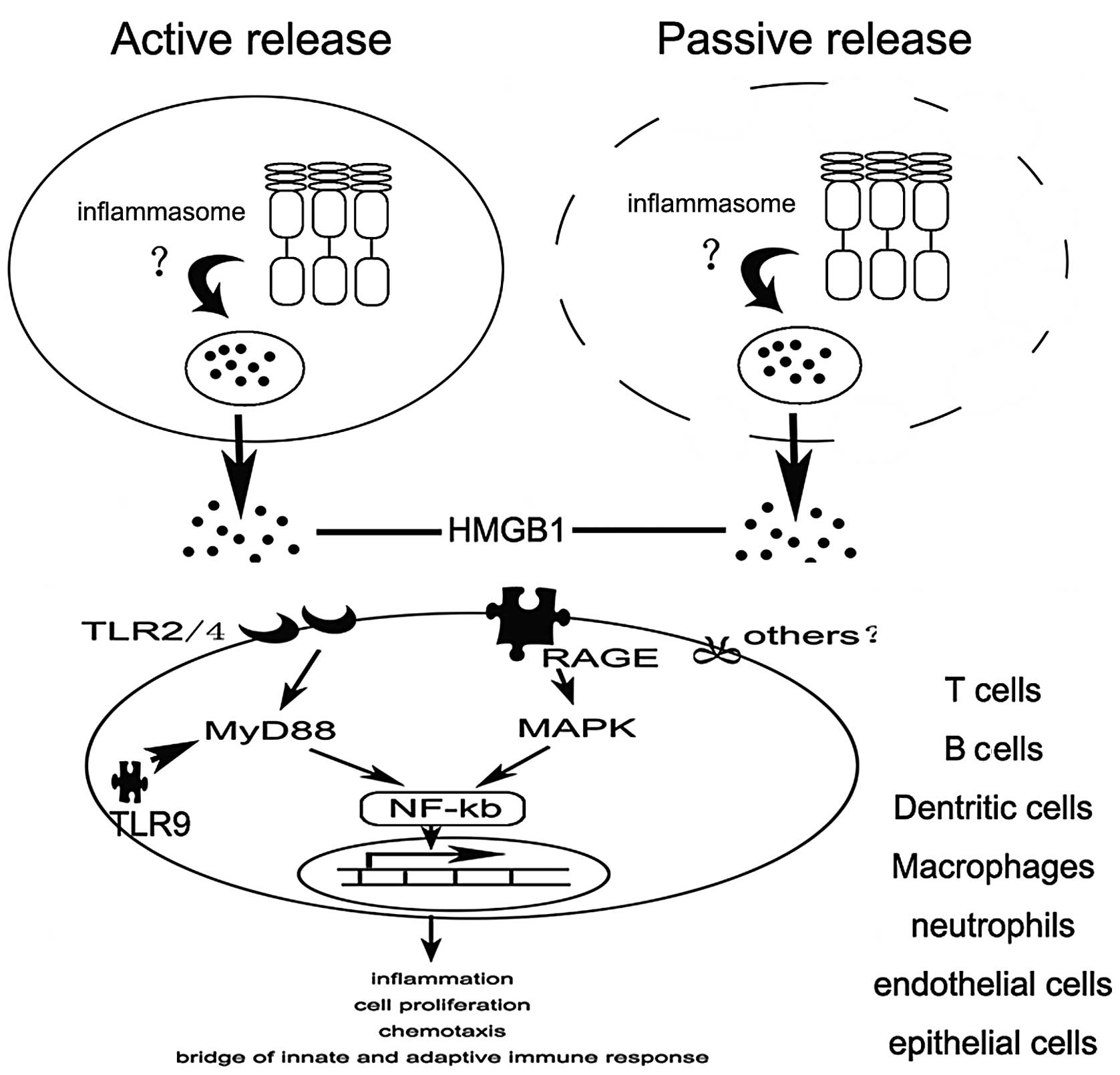
4. Release of HMGB1
In addition to its roles in regulating nucleosome
function and transcription, HMGB1 has recently emerged as an
extracellularly release mediator of inflammation, although it lacks
a classical secretion signal. There are two modes of HMGB1 release:
one active and one passive. HMGB1 not only is passively released
from damaged cells under conditions of injury, but can also be
actively secreted from activated immune cells in response to
inflammatory stimuli (18,19).
How is HMGB1 released? A growing body of evidence
indicates that inflammasomes play an important role in the release
of HMGB1. Recently, NLRP3 inflammasome activation was shown to be
essential for HMGB1 release from LPS-primed macrophages treated
with ATP or exposed to nigericin (20). Similarly, the extracellular
release of HMGB1 from S. typhimurium-infected macrophages
was found to rely on activation of caspase-1 by the NLRC4
inflammasome (20). However, the
exact mechanism remains unclear (Fig.
2).
5. Ligands and signaling of HMGB1
To date, several important surface receptors have
been implicated in HMGB1 signaling, including the receptor for
advanced glycation end products (RAGE), Toll-like receptor (TLR)2,
TLR4 and syndecan (21,22). Binding to these receptors results
in the activation of nuclear factor (NF)-κB, which induces the
upregulation of pro-inflammatory cytokines, thereby promoting
inflammation.
RAGE which is expressed on monocytes, macrophages,
neurons and endothelial cells, as well as on a variety of tumor
cells is thought to be one of the primary receptors for HMGB1
(23,24). Interaction of HMGB1 with RAGE can
activate two major signaling pathways, one encompassing CDC42/Rac
and the other involving diverse mitogen-activated protein kinases
(MAPKs) that finally leads to cytoskeletal changes and NF-κB
activation, respectively (25,26).
In addition to RAGE, TLR2 and 4 which are expressed
on antigen-presenting cells (APCs) were found to directly interact
with HMGB1 as determined by fluorescence resonance energy transfer
(FRET) and immunoprecipitation (27). Numerous studies have demonstrated
that HMGB1 signaling through TLR2 and TLR4 is mediated by the
Rac1/phosphoinositide-3-kinase (PI3K)/CDC42 pathway and
MyD88-dependent NF-κB activation pathway, respectively (21,28,29).
Recently, numerous studies have demonstrated that
HMGB1 acts as a CpG-ODN-binding protein, by which it interacts and
preassociates with TLR9 in the endoplasmic reticulum-Golgi
intermediate compartment (ERGIC), and as a result, forms a complex
within specialized vesicles (30)
which is considered as an accelerator of TLR9 response with CpG-DNA
(31). HMGB1 accelerates the
delivery of CpG-ODNs to its receptor, leading to a TLR9-dependent
augmentation of interleukin (IL)-6, IL-12 and tumor necrosis factor
(TNF)-α secretion (30).
More recently, apart from RAGE and TLRs, HMGB1 can
interact with a wide range of proteins with a phage display
approach (10). Yet, further
studies are required to determine the exact function of HMGB1
interaction with these proteins.
6. HMGB1 and kidney diseases
HMGB1 and glomerulonephritis
Granulomatous nephritis is triggered by a diverse
group of factors and results in renal failure. Granulomatous
inflammation is one of the most significant pathogenetic mechanisms
in nephritis. Recently, research revealed that the HMGB1 level in
urine and serum was elevated in crystal-induced granulomatous
nephritis caused by an adenine-rich diet, and HMGB1 induced
monocyte chemoattractant protein-1 (MCP-1) secretion through the
MAPK and PI3K pathways. The authors concluded that HMGB1 is a new
mediator involved in crystal-induced nephritis that amplifies
granulomatous inflammation through a cycle in which MCP-1 attracts
activated macrophages, resulting in excessive and sustained HMGB1
release. Thus, HMGB1 may be a novel target for the prevention or
treatment of granulomatous nephritis (32).
HMGB1 and secondary kidney diseases
HMGB1 and lupus nephritis
Lupus nephritis is common in systemic lupus
erythematosus (SLE) patients and manifests mainly by proteinuria,
hematuria, and, less commonly, severe renal failure. The mechanism
of lupus nephritis is not fully clear. Yet, accumulating data
suggest that HMGB1 may play an important role in lupus
nephritis.
Iwata et al (33) revealed that HMGB1 secreted by
dendritic cells via p38 MAPK activation participates in
autoimmunity in MRL-Fas(lpr) mice (a lupus-prone mouse model).
These results suggest that HMGB1 is involved in the progression of
autoimmune kidney diseases in MRL-Fas(lpr) mice (33).
Research reveals that high expression of HMGB1 (in
blood or renal biopsies) is positively correlated with MCP-1
expression and may contribute to the pathogenesis of lupus
nephritis (34,35). Recently, Feng et al
(36) demonstrated that HMGB1
mRNA and protein levels were increased in the glomeruli of lupus
nephritis patients and BXSB mice. Their findings indicate that
HMGB1 mediates interferon (IFN)-γ-induced cell proliferation in
mouse mesangial cells through regulation of the cyclin D1/CDK4/p16
pathway and promotion of cell cycle transition from G1 to S stage
in lupus nephritis (36).
Therefore, the above findings clearly indicate a key role of HMGB1
in lupus nephritis, and inhibition of HMGB1 or MCP-1 may be a novel
treatment strategy for lupus nephritis (34,37).
HMGB1 and antineutrophilic
cytoplasmatic antibody (ACNA)-associated vasculitis (AAV)
Cytokines, such as TNF and IL-6, are important in
ACNA-AAV. HMGB1 can induce the release of TNF and IL-6 by APCs.
Recently, Bruchfeld et al (38) concluded that the level of HMGB1 is
increased in AVV with renal manifestations. This suggests that
HMGB1 plays an important role in AVV.
HMGB1 and diabetic nephropathy
Research has shown that the expression of HSP70 and
HMGB1, endogenous ligands of TLRs, is significantly upregulated in
the kidneys of diabetic rats. These findings suggest that release
of hyperglycemia-induced HMGB1 may induce renal injury in diabetic
rats, and that the pathogenic role of HMGB1 may be dependent on
RAGE or TLR4 and through activation of NF-κB and may promote
tubulointerstitial inflammation in diabetic nephropathy (39,40). In contrast, other results found
that the level of HMGB1 in serum was decreased in patients with
diabetic nephropathy (41).
Further extensive study is required to explore the role of HMGB1 in
diabetic nephropathy.
HMGB1 and autosomal dominant
polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD)
is the most common monogenic kidney disease and the fourth leading
cause of end-stage kidney disease in adults worldwide (42). Recently, two research groups
reported that the serum level of HMGB1 was increased in ADPKD
patients (43,44) which suggests that HMGB1 may play a
role in ADPKD.
HMGB1 and acute kidney injury
In 1999, HMGB1 was implicated as a late mediator of
lethal systemic inflammation in sepsis (18). As acute kidney injury is a severe
complication of sepsis, there is evidence to indicate that HMGB1
plays a significant role in sepsis-mediated acute kidney injury
(45).
Wang et al (46) found that HMGB1 expression was
markedly increased in renal tissue and in acute kidney injury in
rats with delayed resuscitation after thermal injury. Chen et
al (47) found that HMGB1
released by injured renal cells in renal ischemia and reperfusion
injury (RIRI) can induce TLR4 (+/+) leukocytes producing IL-6 by
binding to its receptor TLR4. This underscores the importance of
HMGB1/TLR4 signaling in the pathogenesis of ischemic acute kidney
injury.
In addition, Chung et al (48) found that HMGB1 expression was
increased in the kidney 6 h after reperfusion and was decreased
gradually 1, 3 and 5 days following reperfusion. Moreover, Wu et
al (49) demonstrated that
mice treated with anti-HMGB1 antibody had significantly less
tubulointerstitial infiltration by neutrophils (day 1) and
macrophages (day 5) and markedly reduced apoptosis of tubular
epithelial cells. Furthermore, anti-HMGB1 antibody-treated IRI
kidneys had significantly lower levels of IL-6, TNF and MCP-1 mRNA,
which are downstream of HMGB1. Conversely, administration of rHMGB1
after reperfusion exacerbated kidney IRI in wild-type mice. They
conclude that HMGB1 contributes to kidney ischemia reperfusion
injury (50). These findings
demonstrate that HMGB1 plays an important role in acute kidney
injury.
HMGB1 and chronic kidney disease
Chronic kidney disease is a multifactorial disorder
occurring in the context of chronic conditions of co-morbidity.
Research has indicated that chronic kidney disease is an immune
inflammatory condition (51).
There is accumulating evidence linking HMGB1 and chronic kidney
disease.
Bruchfeld et al (52) revealed that HMGB1 is significantly
elevated in patients with chronic kidney disease and correlates
with GFR as well as markers of inflammation and malnutrition.
Another study found that HMGB1 was expressed in the serum of
patients with renal diseases who underwent renal biopsies,
particularly patients who suffered from vasculitis including
ANCA-GN, Henoch-Schonlein purpura nephritis, and IgAN with
glomerular crescents (53).
Moreover, Leelahavanichkul et al (54) suggested that HMGB1 is an important
common mediator for both chronic kidney disease and sepsis.
Nakamura et al (55) demonstrated that HMGB1 enhances the
accumulation of asymmetric dimethylarginine (ADMA) levels,
suggesting the active involvement of the AGE/HMGB1-RAGE-ADMA axis
in nondiabetic chronic kidney disease patients and that inhibition
of HMGB1/RAGE may be a strategy for the treatment of chronic kidney
disease (56).
In addition, research suggests that HMGB1 is a key
mediator of immune-mediated epithelial-mesenchymal transition (EMT)
of proximal tubular epithelial cells and a potentially important
signaling molecule in the development of renal fibrosis (57).
HMGB1 and clear cell renal cell
carcinoma
Renal cell carcinoma is the most common cancer of
the kidney. The main histological subtypes are clear cell (75%),
papillary (15%) and chromophobe renal cell carcinoma (5%) (58). Recently, Lin et al
(59) demonstrated that HMGB1
promotes the development and progression of clear cell renal cell
carcinoma via ERK1/2 activation, which is partially mediated by
RAGE. This suggests that HMGB1 is involved in clear cell renal cell
carcinoma.
HMGB1 and chronic allograft
dysfunction
Chronic allograft dysfunction, a leading cause of
chronic allograft failure among kidney transplant recipients, is a
multifactorial process associated with progressive interstitial
fibrosis and tubular atrophy (60). Recently, Wang et al
(61) found that the level of
HMGB1/TLR4 was increased in chronic renal transplantation patients.
Their findings indicate the MyD88 and TRIF signaling plays an
important role in graft-infiltrating mononuclear cells in the
pathophysiology of chronic allograft dysfunction (61). These findings suggest that HMGB1
may be an effective target for the prevention and treatment of
chronic allograft dysfunction.
7. Conclusion
In conclusion, the robust associations between HMGB1
and kidney diseases have been reviewed in this article (Table I). Although the mechanisms
promoting the release of HMGB1 and the signaling pathways it
activates require further elucidation, evidence suggests its
potential as a therapeutic target/agent in various kidney diseases.
Considering its notable role in kidney diseases, a therapeutic
approach involving the HMGB1-mediated signaling pathway may
constitute a new strategy for the treatment of kidney diseases.
Future research may aid in determining the feasibility of such an
approach.
 | Table IThe main role of HMGB1 in kidney
diseases. |
Table I
The main role of HMGB1 in kidney
diseases.
| Kidney
diseases | Role of HMGB1 |
|---|
| HMGB1 and
glomerulonephritis | Promotes
inflammation and induces MCP-1 secretion |
| HMGB1 and secondary
kidney diseases |
| HMGB1 and lupus
nephritis | Promotes
inflammation and induces MCP-1 secretion; mediates IFN-γ-induced
cell proliferation |
| HMGB1 and
ACNA-associated vasculitis | Mediates the immune
response |
| HMGB1 and diabetic
nephropathy | Activation of
NF-κB; chemotactic; promotes tubulointerstitial inflammation |
| HMGB1 and dominant
polycystic kidney disease | Promotes
inflammatory injury |
| HMGB1 and acute
kidney injury |
| Acute kidney
injury of sepsis | Mediates
inflammation; increases influx of neutrophils |
| Acute kidney
injury of ischemia and reperfusion injury | Activation of
macrophages; induces cytokine release; reduces apoptosis of tubular
epithelial cells |
| HMGB1 and chronic
kidney diseases | Promotes immune
response; enhances ADMA levels; immune-mediated EMT |
| HMGB1 and renal
cell carcinoma | Activation of
ERK1/2 |
| HMGB1 and chronic
allograft dysfunction | Promotes
inflammation through MyD88 and TRIF signaling |
References
|
1
|
Goodwin GH, Sanders C and Johns EW: A new
group of chromatin-associated proteins with a high content of
acidic and basic amino acids. Eur J Biochem. 38:14–19. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bustin M, Lehn D and Landsman D:
Structural features of the HMG chromosomal proteins and their
genes. Biochim Biophys Acta. 1049:231–243. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonaldi T, Längst G, Strohner R, Becker PB
and Bianchi ME: The DNA chaperone HMGB1 facilitates
ACF/CHRAC-dependent nucleosome sliding. EMBO J. 21:6865–6873. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travers AA: Priming the nucleosome: a role
for HMGB proteins? EMBO Rep. 4:131–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hock R, Furusawa T, Ueda T and Bustin M:
HMG chromosomal proteins in development and disease. Trends Cell
Biol. 17:72–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrari S, Finelli P, Rocchi M and Bianchi
M: The active gene that encodes human high mobility group 1 protein
(HMG1) contains introns and maps to chromosome 13. Genomics.
35:367–371. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kornblit B, Munthe-Fog L, Petersen S,
Madsen H, Vindeløv L and Garred P: The genetic variation of the
human HMGB1 gene. Tissue Antigens. 70:151–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Landsman D and Bustin M: A signature for
the HMG-1 box DNA-binding proteins. Bioessays. 15:539–546. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han J, Zhong J, Wei W, et al:
Extracellular high-mobility group box 1 acts as an innate immune
mediator to enhance autoimmune progression and diabetes onset in
NOD mice. Diabetes. 57:2118–2127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dintilhac A and Bernués J: HMGB1 interacts
with many apparently unrelated proteins by recognizing short amino
acid sequences. J Biol Chem. 277:7021–7028. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weir H, Kraulis P, Hill C, Raine A, Laue E
and Thomas J: Structure of the HMG box motif in the B-domain of
HMG1. EMBO J. 12:1311–1319. 1993.PubMed/NCBI
|
|
12
|
Messmer D, Yang H, Telusma G, et al: High
mobility group box protein 1: an endogenous signal for dendritic
cell maturation and Th1 polarization. J Immunol. 173:307–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang H, Ochani M, Li J, et al: Reversing
established sepsis with antagonists of endogenous high-mobility
group box 1. Proc Natl Acad Sci USA. 101:296–301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calogero S, Grassi F, Aguzzi A, et al: The
lack of chromosomal protein Hmg1 does not disrupt cell growth but
causes lethal hypoglycaemia in newborn mice. Nat Genet. 22:276–280.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Müller S, Scaffidi P, Degryse B, et al:
New EMBO member’s review: the double life of HMGB1 chromatin
protein: architectural factor and extracellular signal. EMBO J.
20:4337–4340. 2001.
|
|
16
|
Ulloa L, Batliwalla FM, Andersson U,
Gregersen PK and Tracey KJ: High mobility group box chromosomal
protein 1 as a nuclear protein, cytokine, and potential therapeutic
target in arthritis. Arthritis Rheum. 48:876–881. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brickman JM, Adam M and Ptashne M:
Interactions between an HMG-1 protein and members of the Rel
family. Proc Natl Acad Sci USA. 96:10679–10683. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Bloom O, Zhang M, et al: HMG-1 as
a late mediator of endotoxin lethality in mice. Science.
285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bianchi ME and Manfredi AA: High-mobility
group box 1 (HMGB1) protein at the crossroads between innate and
adaptive immunity. Immunol Rev. 220:35–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lamkanfi M, Sarkar A, Vande Walle L, et
al: Inflammasome-dependent release of the alarmin HMGB1 in
endotoxemia. J Immunol. 185:4385–4392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JS, Svetkauskaite D, He Q, et al:
Involvement of toll-like receptors 2 and 4 in cellular activation
by high mobility group box 1 protein. J Biol Chem. 279:7370–7377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kokkola R, Andersson A, Mullins G, et al:
RAGE is the major receptor for the proinflammatory activity of
HMGB1 in rodent macrophages. Scand J Immunol. 61:1–9. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hori O, Brett J, Slattery T, et al: The
receptor for advanced glycation end products (RAGE) is a cellular
binding site for amphoterin. J Biol Chem. 270:25752–25761. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang H, Wang H, Czura CJ and Tracey KJ:
The cytokine activity of HMGB1. J Leukoc Biol. 78:1–8. 2005.
View Article : Google Scholar
|
|
25
|
Merenmies J, Pihlaskari R, Laitinen J,
Wartiovaara J and Rauvala H: 30-kDa heparin-binding protein of
brain (amphoterin) involved in neurite outgrowth. Amino acid
sequence and localization in the filopodia of the advancing plasma
membrane. J Biol Chem. 266:16722–16729. 1991.
|
|
26
|
Taguchi A, Blood DC, del Toro G, et al:
Blockade of RAGE-amphoterin signalling suppresses tumour growth and
metastases. Nature. 405:354–360. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JS, Gamboni-Robertson F, He Q, et al:
High mobility group box 1 protein interacts with multiple Toll-like
receptors. Am J Physiol Cell Physiol. 290:C917–C924. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JS, Arcaroli J, Yum HK, et al:
Activation of gene expression in human neutrophils by high mobility
group box 1 protein. Am J Physiol Cell Physiol. 284:C870–C879.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu M, Wang H, Ding A, et al: HMGB1 signals
through toll-like receptor (TLR) 4 and TLR2. Shock. 26:174–179.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ivanov S, Dragoi AM, Wang X, et al: A
novel role for HMGB1 in TLR9-mediated inflammatory responses to
CpG-DNA. Blood. 110:1970–1981. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian J, Avalos AM, Mao SY, et al:
Toll-like receptor 9-dependent activation by DNA-containing immune
complexes is mediated by HMGB1 and RAGE. Nat Immunol. 8:487–496.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oyama Y, Hashiguchi T, Taniguchi N, et al:
High-mobility group box-1 protein promotes granulomatous nephritis
in adenine-induced nephropathy. Lab Invest. 90:853–866. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwata Y, Furuichi K, Sakai N, et al:
Dendritic cells contribute to autoimmune kidney injury in
MRL-Faslpr mice. J Rheumatol. 36:306–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou JG, Dong JY, Zhang LH and Wang J:
Expression of high mobility group box chromosomal protein 1 in mice
with lupus nephritis. Zhejiang Da Xue Xue Bao Yi Xue Ban.
40:200–206. 2011.(In Chinese).
|
|
35
|
Pisetsky DS: HMGB1: a smoking gun in lupus
nephritis? Arthritis Res Ther. 14:1122012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng X, Hao J, Liu Q, et al: HMGB1
mediates IFN-γ-induced cell proliferation in MMC cells through
regulation of cyclin D1/CDK4/p16 pathway. J Cell Biochem.
113:2009–2019. 2012.
|
|
37
|
Zickert A, Palmblad K, Sundelin B, et al:
Renal expression and serum levels of high mobility group box 1
protein in lupus nephritis. Arthritis Res Ther. 14:R362012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bruchfeld A, Wendt M, Bratt J, et al:
High-mobility group box-1 protein (HMGB1) is increased in
antineutrophilic cytoplasmatic antibody (ANCA)-associated
vasculitis with renal manifestations. Mol Med. 17:29–35. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim J, Sohn E, Kim CS, Jo K and Kim JS:
The role of high-mobility group box-1 protein in the development of
diabetic nephropathy. Am J Nephrol. 33:524–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin M, Yiu WH, Wu HJ, et al: Toll-like
receptor 4 promotes tubular inflammation in diabetic nephropathy. J
Am Soc Nephrol. 23:86–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Penfold SA, Coughlan MT, Patel SK, et al:
Circulating high-molecular-weight RAGE ligands activate pathways
implicated in the development of diabetic nephropathy. Kidney Int.
78:287–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Torres VE, Harris PC and Pirson Y:
Autosomal dominant polycystic kidney disease. Lancet.
369:1287–1301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakamura T, Kawagoe Y, Ueda Y, Yamada S
and Koide H: Hemoperfusion treatment in a septic shock patient with
autosomal dominant polycystic kidney disease and increased HMGB1
protein levels. Blood Purif. 32:139–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakamura T, Sato E, Fujiwara N, et al:
Changes in urinary albumin excretion, inflammatory and oxidative
stress markers in ADPKD patients with hypertension. Am J Med Sci.
343:46–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu YM, Pai MH, Yeh CL, Hou YC and Yeh SL:
Glutamine administration ameliorates sepsis-induced kidney injury
by downregulating the high-mobility group box protein-1-mediated
pathway in mice. Am J Physiol Renal Physiol. 302:F150–F158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Q, Yao YM, Wang YB, et al: Effect of
ethyl pyruvate on renal high mobility group box-1 protein
expression and acute kidney injury in rats with delayed
resuscitation after thermal injury. Zhonghua Wai Ke Za Zhi.
45:1210–1213. 2007.(In Chinese).
|
|
47
|
Chen J, Hartono JR, John R, et al: Early
interleukin 6 production by leukocytes during ischemic acute kidney
injury is regulated by TLR4. Kidney Int. 80:504–515. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chung KY, Park JJ and Kim YS: The role of
high-mobility group box-1 in renal ischemia and reperfusion injury
and the effect of ethyl pyruvate. Transplant Proc. 40:2136–2138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu H, Ma J, Wang P, et al: HMGB1
contributes to kidney ischemia reperfusion injury. J Am Soc
Nephrol. 21:1878–1890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li J, Gong Q, Zhong S, et al:
Neutralization of the extracellular HMGB1 released by ischaemic
damaged renal cells protects against renal ischaemia-reperfusion
injury. Nephrol Dial Transplant. 26:469–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bao YS, Na SP, Zhang P, et al:
Characterization of interleukin-33 and soluble ST2 in serum and
their association with disease severity in patients with chronic
kidney disease. J Clin Immunol. 32:587–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bruchfeld A, Qureshi AR, Lindholm B, et
al: High mobility group box protein-1 correlates with renal
function in chronic kidney disease (CKD). Mol Med. 14:109–115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sato F, Maruyama S, Hayashi H, et al: High
mobility group box chromosomal protein 1 in patients with renal
diseases. Nephron Clin Pract. 108:c194–c201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Leelahavanichkul A, Huang Y, Hu X, et al:
Chronic kidney disease worsens sepsis and sepsis-induced acute
kidney injury by releasing high mobility group box protein-1.
Kidney Int. 80:1198–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nakamura T, Sato E, Fujiwara N, et al:
Positive association of serum levels of advanced glycation end
products and high mobility group box-1 with asymmetric
dimethylarginine in nondiabetic chronic kidney disease patients.
Metabolism. 58:1624–1628. 2009. View Article : Google Scholar
|
|
56
|
D’Agati V and Schmidt AM: RAGE and the
pathogenesis of chronic kidney disease. Nat Rev Nephrol. 6:352–360.
2010.
|
|
57
|
Lynch J, Nolan S, Slattery C, Feighery R,
Ryan MP and McMorrow T: High-mobility group box protein 1: a novel
mediator of inflammatory-induced renal epithelial-mesenchymal
transition. Am J Nephrol. 32:590–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sandim V, Pereira D, Ornellas A and Alves
G: Renal cell carcinoma and proteomics. Urol Int. 84:373–377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin L, Zhong K, Sun Z, Wu G and Ding G:
Receptor for advanced glycation end products (RAGE) partially
mediates HMGB1-ERKs activation in clear cell renal cell carcinoma.
J Cancer Res Clin Oncol. 13:11–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ganji MR and Harririan A: Chronic
allograft dysfunction: major contributing factors. Iran J Kidney
Dis. 6:88–93. 2012.PubMed/NCBI
|
|
61
|
Wang S, Schmaderer C, Kiss E, et al:
Recipient Toll-like receptors contribute to chronic graft
dysfunction by both MyD88- and TRIF-dependent signaling. Dis Model
Mech. 3:92–103. 2010. View Article : Google Scholar : PubMed/NCBI
|