Introduction
Influenza A viruses are a major cause of morbidity
and mortality globally. The most severe pandemic human influenza
outbreak occurred in 1918–1919, when the Spanish influenza caused
more than 20 million deaths worldwide (1). In June 2009, the pandemic H1N1 virus
spread rapidly throughout the world, and was characterized by a
unique triple re-assortment of gene segments that had never before
been identified in humans, pigs or birds (2). The rapid worldwide spread of the
virus in less than two months led the WHO to raise the alert level
of influenza pandemic from phase 3 to 6 (3). Although vaccination has been
extremely successful in reducing the human cost of influenza virus
infection, the search continues for effective therapeutic or
prophylactic agents that may further diminish the threat of
widespread outbreaks. Currently, adamantane derivatives (amantadine
and rimantadine) and neuraminidase inhibitors (zanamivir and
oseltamivir) are used for the prophylaxis and treatment of
influenza (4,5). However, viruses resistant to these
drugs have emerged due to mutations at amino acid residues encoding
the M2 ion channel or active sites of neuraminidase (6,7).
Another neuraminidase inhibitor, peramivir, has exhibited potent
antiviral activity against influenza A and B viruses, and viral
resistance has not been reported. Yet, due to the safety and
overall experience, peramivir has not been approved by the FDA
(8). For these reasons,
alternative methods and therapies to prevent and overcome these
obstacles have been suggested.
Isatis indigotica root (IIR, Radix
isatidis), also known as Ban-Lan-Gen in Chinese, belongs to the
family Cruciferae and is widely distributed in northern and central
China. This medicinal plant has been traditionally used for the
treatment of influenza, viral pneumonia, mumps, pharyngitis and
hepatitis (9). Over the last
decade, more than 50 chemical constituents have been identified in
IIR. The antiviral compounds isolated from IIR including:
2,4(1H,3H)-quinazolinedione (10), (3H)-quinazolinone (11), lignan glycoside A,
8′R-(−)-lariciresinol-4,4′-bis-O-β-D-glucopyranoside (clemastanin
B), 5-hydroxymethylfurfural, 5-hydroxymethyl-furoic acid,
epigoitrin (12,13), banlangen polysaccharide (14), uridine, hypoxanthine, anthranilic
acid, phenylformic acid and banlangen lectin (15). Among these, the antiviral monomers
have received much attention, such as epigoitrin,
2,4(1H,3H)-quinazolinedione, 4(3H)-quinazolinone, clemastanin B and
banlangen polysaccharide.
Clemastanin B was first isolated from the roots of
Clematis stans by Kizu et al (16). Our research has focused on the
antiviral effects of clemastanin B, which is one of the major
lignans (content 0.04%) extracted from IIR. Previously, He et
al (17) reported that
clemastanin B exhibited antioxidant bioactivities against free
radicals, protected cells and inhibited HSV-1, HSV-2 in
vitro; yet, its anti-influenza activity in vitro and the
underlying mechanism have not been clarified to date in any
published literature. The aim of the present study was to
investigate the anti-influenza activities of clemastanin B in more
detail against a variety of influenza strains (human and avian)
in vitro and to evaluate its potency against the emergence
of resistant strains in comparison with M2 inhibitors.
Materials and methods
Plant material, cells, reagents and
viruses
IIR, cultivated in Good Agricultural Practice (GAP)
farms in Fuyang, An-Hui Province, was obtained from Hutchison
Whampoa Guangzhou Baiyunshan Chinese Medicine Co., Ltd. It was
authenticated by Professor Ye Huagu at the Chinese Medicine
Research Institute.
Madin-Darby canine kidney (MDCK), HEp-2, LLC-MK2,
VERO-E6 and MRC-5 cells were purchased from the American Tissue
Culture Collection (ATCC, Manassas, VA, USA). Influenza virus
A/PR/8/34 (H1N1), A/FM/1/47 (H1N1), A/Aichi/2/68 (H3N2),
parainfluenza virus 3 (PIV3) and respiratory syncytial virus (RSV,
long strain) were purchased from ATCC and seasonal influenza virus
A/Guangzhou/GIRD/02/09 (H1N1), B/Guangzhou/GIRD/08/09, novel swine
influenza virus (A/Guangzhou/GIRD/07/09, H1N1, GenBank Accession
no. HM014332.1), adenovirus type 3 (ADV3), enterovirus 71 (EV71)
and human rhinovirus (HRV) were isolated from routine clinical
specimens. Avian influenza strains of H6N2 (A/Duck/Guangdong/2009),
H7N3 (A/Duck/Guangdong/1994) and H9N2 (A/Chicken/Guangdong/1996)
were a kind gift from Dr Jianxin Chen (South China Agricultural
University). The influenza viruses were propagated and passaged in
MDCK cells. HEp-2 cells were used as the host for RSV and ADV3.
LLC-MK2 cells were used for culturing PIV3. VERO-E6 and MRC-5 cells
were used to culture EV71 and HRV viruses. All cells were grown in
Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated
fetal calf serum (FCS). D101 macroporous resin, MCI gel and
Sephadex LH-20 were purchased from Beijing H&E Co., Ltd.
(Beijing, China).
Extraction and isolation of clemastanin
B
Initially, 10 kg IIR were dried and ground to a
power and extracted three times with 10 times water (v/w) for 2 h.
The extractions were combined and condensed to a proper volume
under reduced pressure. Second, the solution was transferred to the
D101 macroporous resin column and eluted with water, 10% EtOH and
30% EtOH, respectively. Third, the 30% EtOH elution was collected
and condensed to a proper volume under reduced pressure,
transferred to the MCI gel column and eluted with water, 10% MeOH
and 25% MeOH, respectively. Fourth, the 25% MeOH elution was
collected, condensed and dried under reduced pressure, dissolved in
MeOH and purified using the Sephadex LH-20 column. Finally, the
material was crystallized with MeOH to produce a yellow-white power
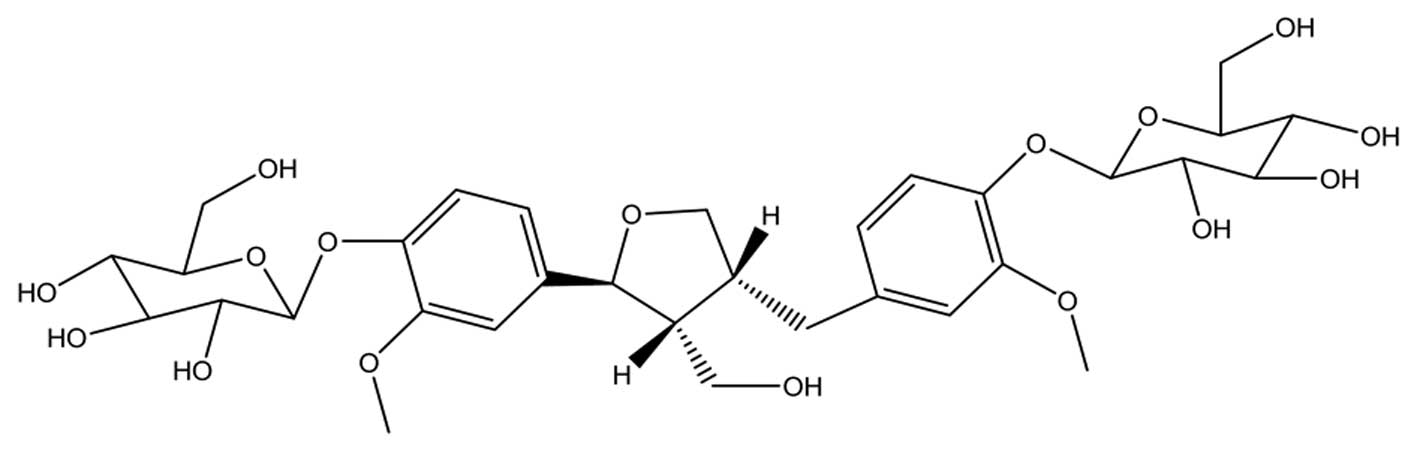
(2.5 g), which was identified as clemastanin B (Fig. 1).
Cytotoxicity assay
MDCK cells were left untreated or treated with the
indicated amounts of clemastanin B. Cell viability was measured
using the 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium
bromide (MTT; GBC Bio Co., Ltd.) assay. Briefly, cells were treated
with 5 mg/ml thiazole blue tetrazolium bromide in
phosphate-buffered saline (PBS) and incubated for 3 h at 37°C. The
reaction product was dissolved in DMSO and cells were further
incubated for 20 min at 37°C. The absorbance was measured using a
microplate reader at 570 nm (18).
Viral infections
For infection, cells were washed with PBS, incubated
with virus diluted in serum-free MEM containing 100 U/ml penicillin
and 0.1 mg/ml streptomycin for 1.5 h at 34°C at the indicated
multiplicities of infection (MOI). The inoculums were aspirated,
and the cells were incubated with MEM supplementing with 2 μg/ml
TPCK-trypsin.
Treatment of viruses and cells with
clemastanin B
MDCK cells (0.8–1.0×105) were seeded into
each well of 12-well plastic plates and cultured at 37°C for 24–48
h. For the anti-influenza activity assay and identification of the
affected viral life cycle, cells were treated with clemastanin B
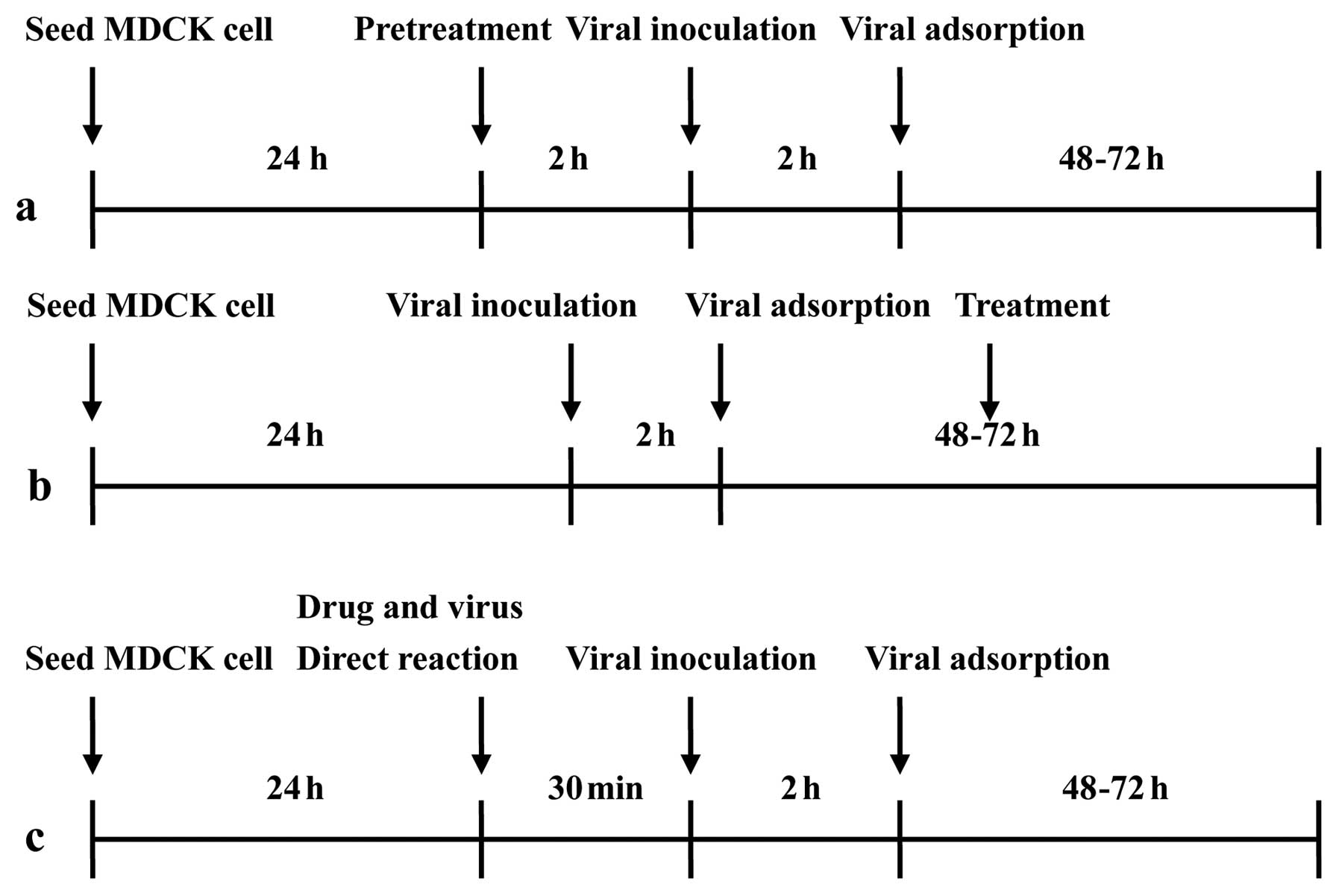
using three different protocols (19–21) (Fig.
2). First, before viral adsorption, the cells were
pre-incubated with clemastanin B for 2 h at 37°C. The treated cells
were then washed and inoculated with the virus (MOI, 0.01) for 2 h
in the absence of clemastanin B and further cultured for 48–72 h.
Second, cells were inoculated with the virus at 37°C for 2 h, and
then washed and cultured for 48–72 h in the presence of clemastanin
B. Third, diluted clemastanin B was mixed with the virus and
incubated at 37°C for 30 min, and the mixture was added to the
cells and further cultured for 48–72 h. The amount of progeny virus
was determined using plaque assay crystal violet staining.
Time course assay (time-of-addition)
MDCK cells in 24-well plates were prepared, then
infected with virus (A/PR/8/34, 0.01 MOI) for 1 h. After infection,
the medium was discarded and cells were washed with PBS three
times. Next, MEM was added to the cells, and incubation was carried
out in a CO2 incubator at 37°C. Clemastanin B was added
1 h before infection, or at the same time with the virus or at
indicated time points post-infection. At 12 h post-infection, the
supernatants were collected and infectious titers were determined
by plaque assay.
Activities against non-influenza
viruses
The activities of clemastanin B against RSV
(22), ADV (23), PIV3 (24), EV71 (25) and HRV (26) were evaluated. A total of 100
TCID50 (50% tissue culture infective dose) of the viral
infective titer was allowed to adsorb to the appropriate confluent
cell lines for 2 h, followed by washing of each virus with
serum-free medium. Then, the test medium containing the desired
concentration of clemastanin B was added. After appropriate periods
of incubation, the cytopathic effect (CPE) in the virally infected
cells was observed microscopically, and the TCID50 and
the 50% inhibitory concentration (IC50) were determined
using methods described by Reed and Muench (27).
Virus resistance assay
MDCK cells were infected with amantadine-sensitive
influenza A virus (A/FM/1/47, 0.01 MOI) and left untreated or
treated with clemastanin B (1 mg/ml), amantadine (0.03 mg/ml;
Sigma-Aldrich) or ribavirin (0.03 mg/ml) for 24 h. The
post-infection supernatants were removed and used for infection in
the second round of investigation. After infection, cells were left
untreated or treated again with the indicated amount of clemastanin
B or amantadine. This procedure was repeated six times.
Supernatants were assayed for progeny virus yields by plaque
assays. Virus yields of mock-treated cells were arbitrarily set as
100% (18).
Localization of influenza viral NP
protein
MDCK cells (0.8–1.0×105) grown on glass
coverslips were cultured at 37°C for 24–48 h, then infected with
virus (A/PR/8/34, 1 MOI) for 2 h. After infection, the medium was
discarded and cells were washed with PBS three times. Next,
clemastanin B was added to the cells and incubated in a
CO2 incubator at 37°C. At 8 h post-infection, cells were
washed with PBS three times and fixed with 4% paraformaldehyde for
30 min, and 1% Triton X-100 at room temperature. After blocking,
fixed cells were incubated with NP-specific antibody (Santa Cruz
Biotechnology, Inc.) overnight, washed with PBS three times, and
cells were incubated with FITC-labeled goat anti-mouse antibody for
30 min. Lastly, cells were incubated with DAPI
(4′,6-diamidino-2-phenylindole) DNA stain for 5 min and observed by
fluorescence microscopy (28).
Statistical analysis
All experiments were carried out in triplicate and
were representative of at least three separate experiments unless
otherwise mentioned. Statistical significance of the data was
determined by the one-way ANOVA method using SPSS 12.0 software. A
P-value of <0.05 was considered to indicate a statistically
significant result.
Results
Structural identification
The identification of the obtained materials was
carried out by IR, MS, 1H NMR and 13C NMR
spectra as follows. Yellow-white power, mp 201–202°C. IR
νKBr cm−1: 3401(OH), 1261, 1595, 1510. ESI-MS
m/z: 707[M+Na]+, 1391[2M+1]+,
ESI-MS2(m/z 707) m/z:
545[M+Na-Glc]+, 361[M-2Glc]+. 1H
NMR: (400 MHz, DMSO-d6+MeOD) δ: 7.195 (1H, d,
J=1.6 Hz, H-2), 7.31 (1H, d, J=8.4 Hz, H-5), 7.074
(1H, dd, J=8.4, 1.6 Hz, H-6), 5.024 (1H, d, J=6.4 Hz,
H-7), 2.537 (1H, m, H-8), 7.102 (1H, d, J=1.6 Hz, H-2′),
7.289 (1H, d, J=8.4, H-5′), 6.959 (1H, d, J=1.6 Hz,
H-7′α), 2.82 (1H, dd, J=11, 3.8, H-7′β), 2.791 (1H, m,
8′-H), 4.05 (9H, s, 3x-OCH3), 5.089 (1H, d, J=7.6
Hz, H-1″), 5.122 (1H, d, J=7.6 Hz, H-1‴). 13C
NMR: (125 MHz, DMSO-d6+MeOD) δ: 136.88 (C-1), 111.46
(C-2), 150.70 (C-3), 147.18 (C-4), 117.41 (C-5), 119.49 (C-6),
83.56 (C-7), 54.09 (C-8), 60.37 (C-9), 136.88 (C-1′), 114.39
(C-2′), 150.69 (C-3′), 117.72 (C-5′), 122.12 (C-6′), 33.63 (C-7′),
43.64 (C-8′), 73.53 (C-9′), 56.77 (OCH3), 102.59 (C-1″),
74.87 (C-2″), 56.77 (C-3″), 71.27 (C-4″), 78.26 (C-5″), 62.37
(C-6″), 102.45 (C-1‴), 74.87 (C-2‴), 56.77 (C-3‴), 71.27 (C-4‴),
77.99 (C-5‴), 62.37 (C-6‴). These data were consistent with the
literature (16,29). The purity was >98%, as
determined by HPLC analysis.
Cytotoxicity
Clemastanin B was tested for cytotoxicity against
MDCK, HEp-2, LLC-MK2, VERO-E6 and MRC-5 cells. The MTT data
indicated that clemastanin B did not negatively affect the
viability of the cells. The TC50 of MDCK, HEp-2,
LLC-MK2, VERO-E6 and MRC-5 cells was 7.5, 6.2, 7.5, 6.3, 7.5 and
7.5 mg/ml, respectively.
Anti-influenza activity of clemastanin
B
Clemastanin B was tested using a plaque assay for
inhibition against a series of human and avian influenza viruses at
different magnitudes of activity. The mode of action of clemastanin
B was studied. When MDCK cell lines were pre-treated with
clemastanin B before viral adsorption or with the influenza virus
pre-incubated with clemastanin B, no protective effect was observed
(Table I). However, when MDCK
cell lines were treated with clemastanin B after virus incubation,
a pronounced titer reduction of progeny virus was detected.
 | Table IAnti-influenza virus activities of
clemastanin B, amantadine and ribavirin. |
Table I
Anti-influenza virus activities of
clemastanin B, amantadine and ribavirin.
| IC50
(mg/ml)a |
|---|
|
|
|---|
| Virus type and
strain | Clemastanin
Bb | Clemastanin
Bc | Clemastanin
Bd | Amantadinec | Ribavirinc |
|---|
| A/PR/8/34
(H1N1) | >10 | 0.32 | >10 | >0.05 | 0.037 |
| A/FM/1/47
(H1N1) | >10 | 0.5 | >10 | 0.017 | 0.0068 |
|
A/Guangzhou/GIRD07/09 (H1N1) | >10 | 0.087 | >10 | >0.05 | 0.012 |
|
A/Guangzhou/GIRD02/2009 (H1N1) | >10 | 0.27 | >10 | >0.05 | 0.013 |
| A/Aichi/2/68
(H3N2) | >10 | 0.62 | >10 | 0.015 | 0.021 |
|
A/Duck/Guangdong/2009 (H6N2) | >10 | 0.37 | >10 | 0.015 | 0.009 |
|
A/Duck/Guangdong/1994 (H7N3) | >10 | 0.088 | >10 | 0.025 | 0.014 |
|
A/Chicken/Guangdong/1996 (H9N2) | >10 | 0.171 | >10 | >0.05 | 0.021 |
|
B/Guangzhou/GIRD08/09 | >10 | 0.72 | >10 | >0.05 | 0.021 |
Time course assay (time-of-addition) in
influenza virus-infected cells
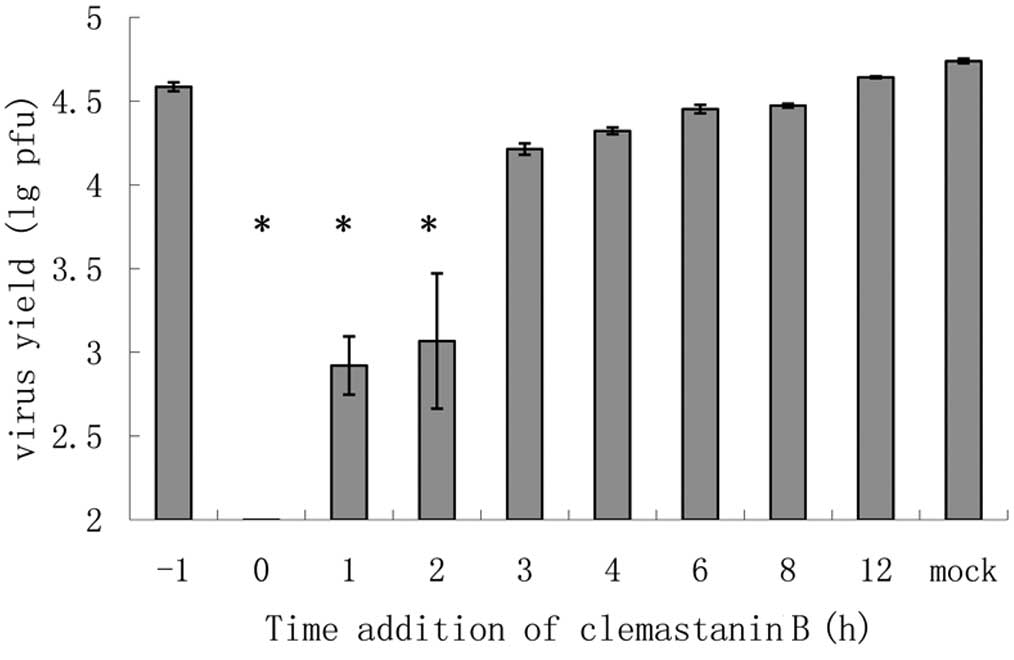
The antiviral mechanism of clemastanin B was
determined by a time course assay in a single infectious cycle
using an A/PR/8/34 (H1N1) infection model. A notable and
significant reduction was observed when clemastanin B was applied
between 0 and 2 h after viral adsorption, which indicated that
clemastanin B targets the early viral replication stage rather than
adsorption or late stage of viral replication (Fig. 3).
Spectrum of antiviral activity
The spectrum of antiviral activity of clemastanin B
was investigated. It did not inhibit the CPE caused by RSV, ADV,
PIV3, EV71, and the HRV IC50 values in all cases were
>10 mg/ml.
Tendency to induce viral resistance
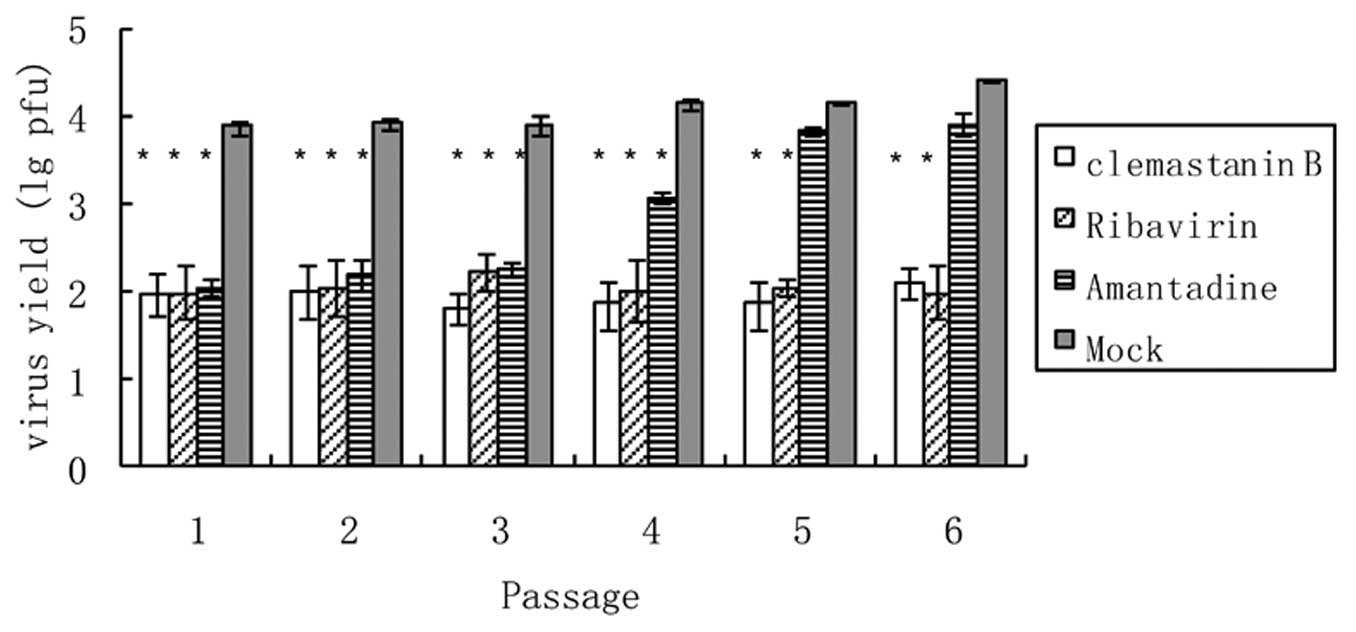
A multi-passage experiment to detect the emergence
of resistant viruses in cell culture was set up as a reference
(30). Virus titers from cells
treated with amantadine were at the same levels as untreated cells
after five passages, showing that this pool of viruses had become
fully resistant to the drug (Fig.
4). This was different from infected cells treated with
clemastanin B, where viral titers did not rise with increasing
passage numbers, indicating that the influenza virus was not
resistant to clemastanin B.
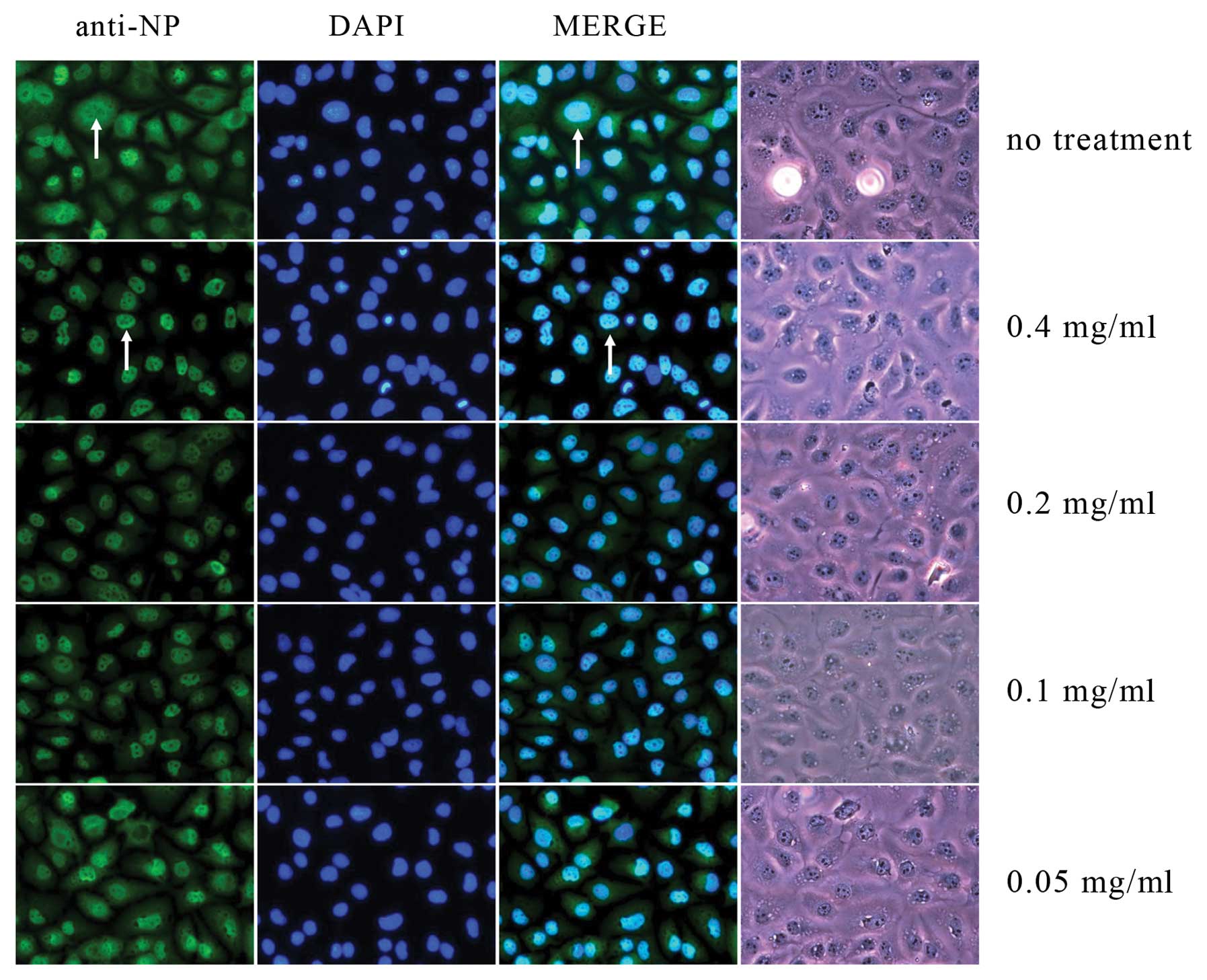
Clemastanin B treatment results in NP
distribution in the nuclei
The viral NP produced by influenza A/PR/8/34 was
evident and its expression persisted thereafter. NP was distributed
not only in the cytoplasm but also in the nuclei of the infected
cells (Fig. 5). However, in the
clemastanin B-treated cells, the results showed that NP production
was only distributed in the nuclei of the infected cells.
Discussion
The Chinese herbal medicine, Isatis
indigotica root, has been traditionally known to have an effect
on fever and sore throats. Many chemical compounds have been
isolated from IIR, including indigotin, indirubin, tryptanthrin,
isatin, isaindigotidione, epigoitrin, 2,4(1H,3H)-quinazolinedione,
clemastanin B, 4(3H)-quinazolinone and others. Among them,
tryptanthrin, indigo and indirubin are usually selected as markers
for quality control due to their unique pharmaceutical activities.
Indirubin has been shown to have potent anti-influenza viral
activities by inhibition of RANTES (also known as CCL5) expression
(31). Yamada (32) previously reported a glycoprotein
from Radix isatidis that showed useful antiviral activities
in the therapy and prevention of viral infection in vitro
and in vivo. Clemastanin B, a major lignan in IIR, has been
isolated using conventional methods. However, evidence of the
inhibition of influenza virus by clemastanin B from IIR has not
been previously reported and its mode of action remains
elusive.
Herein, we found that clemastanin B showed
inhibitory effects against different subtypes of human or avian
influenza viruses at different magnitudes of activity in
vitro. Among these, clemastanin B inhibited the swine-origin
H1N1 and avian influenza virus H7N3 with an IC50 of
0.087 and 0.088 mg/ml, respectively, indicating potent effects
against such pathogens. Further investigations are required to
determine this specificity.
In contrast, clemastanin B showed no antiviral
activity against RSV, PIV3, ADV3, EV71 and HRV. These results
suggest that clemastanin B exhibits a broad range and selective
antiviral activity against various subtypes of influenza viruses.
However, previous reports had shown that clemastanin B had
antiviral activity against HSV-1 and HSV-2 in vitro, but no
such activities resulted from our experimentation. These
contradictory results may be due to different assays or specificity
to strains.
Generally, the influenza virus replication cycle can
be divided into six steps: adsorption, endocytosis, uncoating,
packaging, budding and release (33). To determine at which step
clemastanin B acts in the viral life cycle, its mode of action was
observed. Pretreatment of cells following viral infection or at
late stages of viral infection did not affect viral replication.
This indicated that the viral adsorption, budding or release were
not affected by clemastanin B. Our results also revealed that
clemastanin B did not inhibit the influenza virus hemagglutinin
specificity as determined by the hemagglutinin inhibition assay and
the NA protein by NA inhibition (data not shown). Clemastanin B
displayed antiviral properties when the virus and drugs were added
to cells simultaneously, as well as post-infection, particularly in
the early replication stage. These results suggest that an early
stage of viral replication, for exampe endocytosis or uncoating,
were inhibited. In addition, the RNP remained in the nucleus of
clemastanin B-treated cells, which suggests that clemastanin B may
interfere with RNP export from the nucleus at an early stage and
persists throughout the rest of the infection. It was reported that
there were several cell signaling pathways related to RNP export:
Raf/MEK/ERK signaling (34), PKCα
(35), PI3/akt (36) and NF-κB pathways (37). These pathways influence the
expression of cytokines and chemokines. The Raf/MEK/ERK signaling
pathway induced by influenza virus is essential for viral RNP
export. Thus, we cannot exclude the possibility that clemastanin B
may effect these pathways.
It has been reported that amino adamantine-resistant
viruses readily emerge and were already prevalent worldwide during
the recent seasonal H1N1 and H3N2 influenza pandemics (38,39). The swine-origin H1N1 pandemic in
2009 was already amino adamantine-resistant (40). This feature was also replicated in
our in vitro study, as the viruses in the presence of
amantadine were resistant to amantadine at passage five, while the
viruses passaged in the presence of clemastanin B did not result in
the emergence of viral drug resistance until the sixth passage.
These results demonstrated that clemastanin B did not easily result
in the emergence of viral drug resistance in vitro and may
possess unique mechanisms against influenza viruses that are
different from the existing antiviral drugs such as M2 or
neuraminidase inhibitors. To our knowledge, this is the first
report showing that clemastanin B has antiviral effects against
various influenza A and B viruses.
Given that its probable mechanism of action differs
from current antiviral agents and it is derived from a common TCM
medicinal plant, clemastanin B could be developed as a moderate
antiviral drug candidate via inhibition of viral replication after
infection, particularly in the early replication stage, although it
was not a very potent antiviral compound when compared with
ribavirin or amantadine.
Additionally, clemastanin B did not easily result in
drug resistance compared with M2 inhibitors. The effects of
clemastanin B illustrated in this study promote the antiviral study
of IIR. However, additional studies are required to define the
anti-influenza mechanism(s).
Acknowledgements
This work was funded by the National Science and
Technology Major Project of the Ministry of Science and Technology
of China (Grant no. 2013ZX09201021, 2013ZX09304102), the National
Natural Science Foundation of China (Grant no. U1201227), Guangdong
Natural Science Foundation (Grant no. S2012010008276), the Joint
Research foundation of Department of Education, Guangdong Province
(Grants no.gxzd0901), Guangzhou Science and Technology Project
(Grants no.2011Y3-00039), the Science and Technology Development
Fund in Macao Special Administrative Region (Grant no.
043/2007/A3), Recruitment Project of Guangzhou Technology Bureau
for enterprises scientific and technological problems (Grant no.
2008Z1-I011). The funders had no role in study design, data
collection and analysis, decision to publish, or preparation of the
manuscript.
References
|
1
|
Nicholson KG, Wood JM and Zambon M:
Influenza. Lancet. 362:1733–1745. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trifonov V, Khiabanian H, Greenbaum B and
Rabadan R: The origin of the recent swine influenza seasonal
influenza A(H1N1) virus infecting humans. Euro Surveill.
14:191932009.PubMed/NCBI
|
|
3
|
Centers for Disease Control and Prevention
(CDC). Update: Infections with a Swine-Origin Influenza A (H1N1)
Virus, United States and other countries. 2009, http://www.cdc.gov/mmwr.
|
|
4
|
Hayden FG and Hay AJ: Emergence and
transmission of influenza A viruses resistant to amantadine and
rimantadine. Curr Top Microbiol Immunol. 176:119–130.
1992.PubMed/NCBI
|
|
5
|
de Jong MD, Tran TT, Truong HK, Vo MH,
Smith GJ, Nguyen VC, Bach VC, Phan TQ, Do QH, Guan Y, Peiris JS,
Tran TH and Farrar J: Oseltamivir resistance during treatment of
influenza A (H5N1) infection. N Engl J Med. 353:2667–2672.
2005.
|
|
6
|
Deyde VM, Xu X, Bright RA, Shaw M, Smith
CB, Zhang Y, Shu Y, Gubareva LV, Cox NJ and Klimov AI: Surveillance
of resistance to adamantanes among influenza A (H3N2) and A (H1N1)
viruses isolated worldwide. J Infect Dis. 196:249–257. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le QM, Kiso M, Someya K, Sakai YT, Nguyen
TH, Nguyen KH, Pham ND, Ngyen HH, Yamada S, Muramoto Y, Horimoto T,
Takada A, Goto H, Suzuki T, Suzuki Y and Kawaoka Y: Avian flu:
isolation of drug-resistant H5N1 virus. Nature. 437:11082005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shetty AK and Peek LA: Peramivir for the
treatment of influenza. Expert Rev Anti Infect Ther. 10:123–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang YS and Ho YL: Studies on the
homonymic Chinese crude drug species in Taiwan. Evaluation of the
quality of DA-Ching-Yeh and Ching-Dai. Anal Sci. 17:a423–a426.
2001.
|
|
10
|
Xu LH, Huang F, Cheng T and Wu J:
Antivirus constituents of radix of Isatis indigotica. Chin J
Nat Med. 3:359–361. 2005.
|
|
11
|
Li L, Dong TY, Li XL and Qiao CZ: Study of
quality control on herbal drugs and preparations of daqingye and
banlangen. Acta Pharm Sin. 28:229–233. 1993.(In Chinese).
|
|
12
|
Huang QS, Yoshihiro K and Natori S:
Isolation of 2-hydroxy -3-butenyl thiocyanate, epigoitrin and
adenosine from ‘banlangen’, Isatis indigotica root. Planta
Med. 42:308–310. 1981.PubMed/NCBI
|
|
13
|
Li X, He LW and Sun DD: The methods of
anti-virus extract from Radix and its use. P: CN1969923. pp. 05–30.
2007
|
|
14
|
Zuo Y, Dai M, Wang ZY and Liu J: Effects
of banlangen polysaccharide on mice resistance to Influenza virus
infection. WCJ PS. 23:666–667. 2008.
|
|
15
|
An YQ, Jia XB, Yuan HJ, Chen Y and Jin XY:
Thinking in studies on antiviral material base of Radix
isatidis. Chin Tradit Herbal Drugs. 39:616–619. 2008.
|
|
16
|
Kizu H, Shimana H and Tomimori T: Studies
on the constituents of clematis species. VI The Constituents of
Clematis stans Sieb et Zucc. Chem Pharm Bull. 43:2187–2194.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He LW, LI X, Chen JW, Xia Y and Wang YY:
Determination of lariciresinol-4,4′-bis-O-b-D-glucopyranoside from
Radix isatidis by HPLC. Chin Tradit Herbal Drugs.
39:1895–1897. 2008.
|
|
18
|
Ehrhardt C, Hrincius ER, Korte V, Mazur I,
Droebner K, Poetter A, Dreschers S, Schmolke M, Planz O and Ludwig
S: A polyphenol rich plant extract, CYSTUS052, exerts
anti-influenza virus activity in cell culture without toxic side
effects or the tendency to induce viral resistance. Antiviral Res.
76:38–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yingsakmongkon S, Miyamoto D,
Sriwilaijaroen N, Fujita K, Matsumoto K, Jampangern W, Hiramatsu H,
Guo CT, Sawada T, Takahashi T, Hidari K, Suzuki T, Ito M, Ito Y and
Suzuki Y: In vitro inhibition of human influenza A virus infection
by fruit-juice concentrate of Japanese plum (Prunus mume
SIEB. et ZUCC). Biol Pharm Bull. 31:511–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyamoto D, Hasegawa S, Sriwilaijaroen N,
Yingsakmongkon S, Hiramatsu H, Takahashi T, Hidari K, Guo CT,
Sakano Y, Suzuki T and Suzuki Y: Clarithromycin inhibits progeny
virus production from human influenza virus-infected host cells.
Biol Pharm Bull. 31:217–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knox YM, Suzutani T, Yosida I and Azuma M:
Anti-influenza virus activity of crude extract of Ribes
nigrum L. Phytother Res. 17:120–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Graham BS, Perkins MD, Wright PF and
Karzon DT: Primary respiratory syncytial virus infection in mice. J
Med Virol. 26:153–162. 1998. View Article : Google Scholar
|
|
23
|
Hui MB, Lien EJ and Trousdale MD:
Inhibition of human adenoviruses by
1-(2′-hydroxy-5′-methoxybenzylidene) amino-3-hydroxyguanidine
tosylate. Antiviral Res. 24:261–273. 1994.
|
|
24
|
Mao H, Thakur CS, Chattopadhyay S,
Silverman RH, Gudkov A and Banerjee AK: Inhibition of human
parainfluenza virus type 3 infection by novel small molecules.
Antiviral Res. 77:83–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho HY, Cheng ML, Weng SF, Leu YL and Chiu
DT: Antiviral effect of epigallocatechin gallate on enterovirus 71.
J Agric Food Chem. 57:6140–6147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaiser L, Crump CE and Hayden FG: In vitro
activity of pleconaril and AG7088 against selected serotypes and
clinical isolates of human rhinoviruses. Antiviral Res. 7:215–220.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reed LJ and Muench H: A simple method of
estimating fifty percent endpoints. Am J Hyg. 27:493–497. 1938.
|
|
28
|
Ludwig S, Wolff T, Ehrhardt C, Wurzer WJ,
Reinhardt J, Planz O and Pleschka S: MEK inhibition impairs
influenza B virus propagation without emergence of resistant
variants. FEBS Lett. 561:37–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng JP, Fan GR and Wu YT: Isolation and
purification of clemastanin B and indigoticoside A from Radix
isatidis by high-speed counter-current chromatography. J
Chromatogr A. 1091:89–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taubenberger JK: The origin and virulence
of the 1918 ‘Spanish’ influenza virus. Proc Am Philos Soc.
150:86–112. 2006.
|
|
31
|
Mak NK, Leung CY, Wei XY, Shen XL, Wong
RN, Leung KN and Fung MC: Inhibition of RANTES expression by
indirubin in influenza virus-infected human bronchial epithelial
cells. Biochem Pharmacol. 67:167–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamada H: Antiviral compositions
containing new glycoprotein from Isatis tinctoria. Acta
Pharm Sin. 1160:5991999.
|
|
33
|
Palese P: Influenza: old and new threats.
Nat Med. 10(Suppl 12): S82–S87. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pleschka S, Wolff T, Ehrhardt C, Hobom G,
Planz O, Rapp UR and Ludwig S: Influenza virus propagation is
impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat
Cell Biol. 3:301–305. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marjuki H, Alam MI, Ehrhardt C, Wagner R,
Planz O, Klenk HD, Ludwig S and Pleschka S: Membrane accumulation
of influenza A virus hemagglutinin triggers nuclear export of the
viral genome via protein kinase Calpha-mediated activation of ERK
signaling. J Biol Chem. 81:16707–16715. 2006. View Article : Google Scholar
|
|
36
|
Wu MS, Yen HR, Chang CW, Peng TY, Hsieh
CF, Chen CJ, Lin TY and Horng JT: Mechanism of action of the
suppression of influenza virus replication by Ko-Ken Tang through
inhibition of the phosphatidylinositol 3-kinase/Akt signaling
pathway and viral RNP nuclear export. J Ethnopharmacol.
134:614–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mazur I, Wurzer WJ, Ehrhardt C, Pleschka
S, Puthavathana P, Silberzahn T, Wolff T, Planz O and Ludwig S:
Acetylsalicylic acid (ASA) blocks influenza virus propagation via
its NF-kappaB-inhibiting activity. Cell Microbiol. 9:1683–1694.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bright RA, Medina MJ, Xu X, Perez-Oronoz
G, Wallis TR, Davis XM, Povinelli L, Cox NJ and Klimov AI:
Incidence of adamantane resistance among influenza A (H3N2) viruses
isolated worldwide from 1994 to 2005: a cause for concern. Lancet.
366:1175–1181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bright RA, Shay DK, Shu B, Cox NJ and
Klimov AI: Adamantane resistance among influenza A viruses isolated
early during the 2005–2006 influenza season in the United States.
JAMA. 295:891–894. 2006.
|
|
40
|
Dawood FS, Jain S, Finelli L, Shaw MW,
Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB and Uyeki TM:
Emergence of a novel swine-origin influenza A (H1N1) virus in
humans. N Engl J Med. 360:2605–2615. 2009. View Article : Google Scholar : PubMed/NCBI
|