Introduction
Nasopharyngeal carcinoma (NPC) is a significant
health burden in Southeast Asia and Southern China. Radiotherapy is
the primary treatment modality, and the use of radiation therapy in
combination with chemotherapy is recommended for the treatment of
locoregionally advanced tumors. However, in patients who develop
distant recurrence following radiotherapy, the median survival time
is ~12–15 months (1,2); thus, new treatments are needed.
Targeted therapy using specific inhibitors is currently in
development and has demonstrated promising antitumor efficacy. The
increasing knowledge of growth factor signal transduction pathways
has led to speculation that proteins in these pathways could offer
crucial targets for cancer therapy.
Cell death is the result of an unsuccessful
cytoprotective mechanism against intracellular and extracellular
stressors, which includes apoptosis, autophagy, necrosis and
mitotic catastrophe. Autophagic cell death is morphologically
characterized by a cell with an intact nucleus and an accumulation
of cytoplasmic double-membrane autophagic vacuoles called
autophagosomes. Apoptosis is characterized by chromatin
condensation and DNA fragmentation. Anticancer drugs have been
shown to induce not only apoptosis but also autophagy in cancer
cells (3–5). However, the relationship between
autophagy and apoptosis is complex as the molecular regulators of
both pathways are interconnected. The crosstalk between autophagy
and apoptosis can be in unison or in an opposing fashion, and it
varies depending on cell type and the type and duration of the
stimulus. Some published data suggest that tumor cell autophagy
induced by anticancer therapy inhibits tumor cell killing. However,
it has also been proposed that autophagy is a cell death mechanism
that could function as a backup mode of cell death when apoptosis
is disabled.
The mammalian target of rapamycin (mTOR), which is
well-known as a major negative regulator of autophagy, is a
serine/threonine protein kinase and is a key element of the
phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway
that integrates signals that govern protein biosynthesis, cell
division, motility, survival and angiogenesis (6). Therefore, mTOR acts as a central
regulator of cell growth and cell cycle progression. The inhibition
of mTOR prevents protein synthesis and cell proliferation.
Recently, it has been suggested that dysregulation of mTOR
contributes to oncogenesis in a broad range of cancers (7). Therefore, this protein is a novel
and validated therapeutic target for the treatment of cancer
(8–12).
RAD001 (everolimus) is the first oral mTOR inhibitor
to reach oncology clinics (13).
RAD001 inhibits mTOR complex 1 (mTORC1) and the phosphorylation of
its down-stream signaling mediators, ribosomal p70S6 kinase 1 (S6K)
and 4E-binding protein 1 (4E-BP1). Treatment with RAD001 has been
shown to induce autophagy in papillary thyroid cancer and enhance
the therapeutic response to cytotoxic chemotherapy and external
beam radiation (14). RAD001
inhibits growth in HPV-associated oral and cervical squamous
carcinomas, ovarian cancer, post-transplant Epstein-Barr
virus-related lymphoproliferative disorders, breast cancer and
pancreatic neuroendocrine tumors (15–19), as well as diminishes
lymphangiogenesis in primary tumors and prevents the dissemination
of head and neck squamous cell cancer cells to the cervical lymph
nodes (20). At present, RAD001
is currently undergoing evaluation studies in phases I–III as an
antitumor agent.
Previous studies have shown that Akt is frequently
activated in NPC tissues (21);
therefore, the inhibition of mTOR may be an appropriate treatment
strategy for this tumor type. In this study, we evaluated the
activity of the mTOR inhibitor RAD001 on NPC cell lines and
revealed that the inhibitory effect of RAD001 was mainly due to
apoptosis rather than autophagy. Our data demonstrated that the
combination of RAD001 and autophagy inhibitors may be a useful
therapeutic strategy for nasopharyngeal carcinoma.
Materials and methods
Cell lines and small-molecule
inhibitors
The human NPC cell lines HONE-1 and CNE-1 were grown
in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine
serum (FBS) (Hyclone, Logan, UT, USA). All cells were cultured in a
5% CO2 incubator at 37°C. The working stock of RAD001
(Selleck Chemicals, USA) was diluted to 20 mM using dimethyl
sulfoxide (DMSO) and stored at −20°C. The concentration of DMSO in
the final solution did not exceed 1% (v/v). 3-Methyladenine (3-MA,
#M9281) from Sigma-Aldrich was dissolved in hot water (70°C) to 30
mg/ml before use. Caspase inhibitor z-VAD-fmk (#sc-3067, 0.5 mg/0.1
ml) was purchased from Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA).
4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene
disulfonate (WST-1) assay
The cells were dispensed in 96-well, flat-bottom
microtiter plates at a density of 2×103 cells/well and
incubated with RAD001 at various dilutions for 20, 44 and 68 h
followed by an additional 4 h treatment with WST-1 (Roche,
Germany). The cleavage of WST-1 to formazan by metabolically active
cells was quantified by scanning the plates in a microtiter plate
reader at 440 and 620 nm (reference wavelength). The test medium
was used as the background control. Three independent sets of
experiments that were performed in triplicate were evaluated. The
viability of the treated cells was normalized to the untreated
control cells.
Cell shape assay
Twenty-four hours prior to drug treatment, the cells
were seeded directly into 6-well culture plates at a density of
1×105 cells/well. The cells were then treated with
various concentrations of RAD001 (0, 20 or 40 μM) for 24 h, stained
with DAPI, mounted, and analyzed using an inverted microscope
(IX71; Olympus).
Cytotoxicity assay
The cytotoxicity assay was performed using the
Cytotoxicity Detection KitPLUS LDH (#04744926001; Roche)
according to the manufacturer’s instructions. This assay is based
on the measurement of lactate dehydrogenase (LDH) activity released
from the cytosol of damaged cell. Three controls are included:
background control (assay medium), low control (untreated cells)
and high control (maximum LDH release). To determine the
experimental absorbance values, the average absorbance values of
the triplicate samples and controls were calculated and subtracted
from the absorbance values of the background control. The percent
cytotoxicity was determined using the following equation:
Cytotoxicity (%) = (exp. value − low control)/(high control − low
control) × 100.
Analysis of cell apoptosis
Cells (1×105 cells/ml) seeded in a 6-well
plate were treated with RAD001 for 24 h. For analysis of apoptosis,
staining was performed using the Annexin V-FITC/PI Apoptosis
Detection kit (#PF032; Merck) according to the manufacturer’s
instructions. Both cell cycle distribution and apoptosis were
analyzed using flow cytometry (FC500; Beckman Coulter, Brea, CA,
USA), and the results were displayed as histograms.
Western blot analysis
Lysates were prepared from 4×105 cells by
dissolving the cell pellets in 100 μl of cell lysis buffer (#9803;
Cell Signaling Technology) for 30 min on ice. The lysates were
centrifuged at 12,000 × g for 20 min and the supernatant was
collected. The protein content was determined using the Pierce BCA
Protein Assay (#23225; Thermo Scientific). A total of 30 μg of
protein was loaded into each well of an 8–15% SDS-PAGE gel. The
resolved proteins were electrophoretically transferred to PVDF
membranes and incubated sequentially with primary and secondary
antibodies [anti-rabbit IgG, HRP-linked antibody (#7074P2) or
anti-mouse IgG, HRP-linked antibody (#7076P2; both from Cell
Signaling Technology)]. After washing, the bound antibody complex
was detected using LumiGLO reagent (#7003; Cell Signaling
Technology) and XAR film (XBT-1; Kodak) as described by the
manufacturers. The following primary antibodies were used:
caspase-3 antibody (#9662; Cell Signaling Technology);
poly(ADP-ribose) polymerase (PARP) antibody (sc-7150; Santa Cruz
Biotechnology, Inc.); LC3 antibody (NB100-2220; Novus Biologicals);
and glyceraldehyde 3-phosphate dehydrogenase antibody (#32233;
Santa Cruz Biotechnology, Inc.).
Statistical analyses
All experiments were repeated three times. The
results of multiple experiments are presented as the mean ± SD.
Statistical analyses were performed using the SPSS 17.0 statistical
software. The P-values were calculated using a one-way analysis of
variance (ANOVA). A P-value of <0.05 was considered to indicate
a statistically significant result.
Results
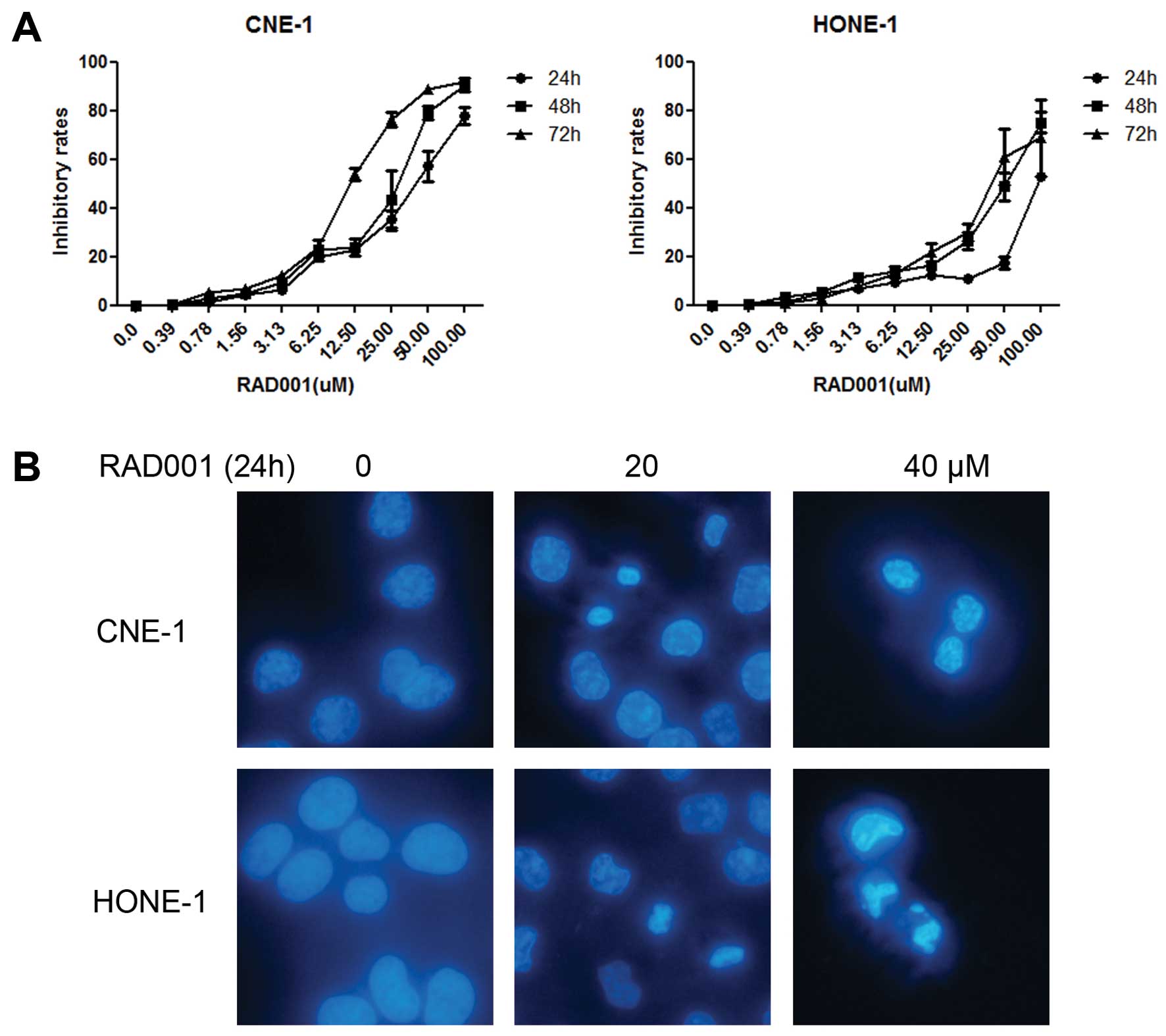
RAD001 inhibits the proliferation of NPC
cells
We first examined the viability of the two NPC cell
lines in the presence of different concentrations of RAD001 (0–100
μM) using the WST-1 assay. CNE-1 cells were more sensitive, with a
50% growth inhibition (GI50) of 30.0±1.0 μM at 72 h
compared to the GI50 of HONE-1, which was 56.9±13.1 μM
(Fig. 1A and Table I).
 | Table IAntiproliferative activities of
RAD001 in human nasopharyngeal carcinoma cell lines. |
Table I
Antiproliferative activities of
RAD001 in human nasopharyngeal carcinoma cell lines.
| GI50
(μM) |
|---|
|
|
|---|
| Time (h) | CNE-1 | HONE-1 |
|---|
| 24 | 51.7±3.5 | 96.6±2.0 |
| 48 | 40.3±2.9 | 59.1±3.8 |
| 72 | 30.0±1.0 | 56.9±13.1 |
Cell morphological change
To examine the lethal effect of RAD001 in CNE-1 and
HONE-1 cells, both cell lines were treated with different
concentrations of RAD001 (0, 20 and 40 μM) for 24 h. Cellular
apoptosis was determined using DAPI staining. Chromatin
condensation and cell shrinkage were clearly observed after RAD001
treatment (Fig. 1B).
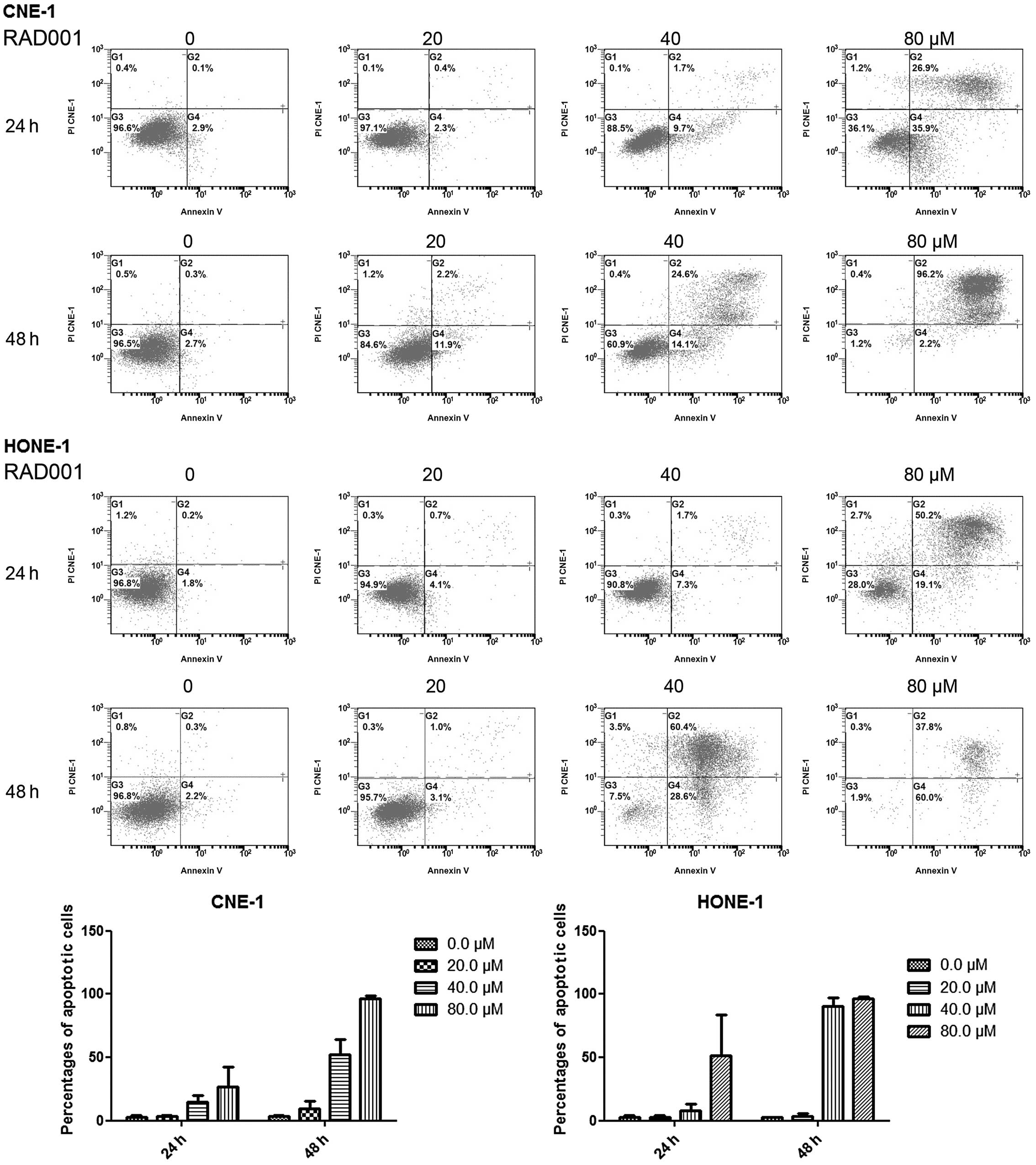
RAD001 induces cellular apoptosis
To identify whether mTOR inhibition induces
apoptosis, the treated cells were stained with Annexin V-FITC/PI
and the apoptotic cell population was analyzed using flow
cytometry. RAD001 treatment significantly increased the proportion
of apoptotic cells (Fig. 2). In
the control group, 3.1±1.1% cells were positive for Annexin V-FITC
staining, and the 20, 40 and 80 μM RAD001 treatments resulted in
Annexin V-FITC-positive rates of 3.5±0.9, 15.0±4.7 and 27.0±15.3%,
respectively, in the CNE-1 cells treated for 24 h. At 48 h
post-treatment, the apoptotic rates increased to 3.8±0.8% for the
control and to 9.5±6.3, 52.3±11.8 and 96.5±2.2%, respectively, for
the above-mentioned treatment groups. Similar results were observed
in HONE-1 cells (Fig. 2 and
Table II).
 | Table IIEffect of RAD001 on the apoptosis of
CNE-1 and HONE-1 cells. |
Table II
Effect of RAD001 on the apoptosis of
CNE-1 and HONE-1 cells.
| CNE-1 | HONE-1 |
|---|
|
|
|
|---|
| RAD001 (μM) | 24 h | 48 h | 24 h | 48 h |
|---|
| Annexin
V-FITC-positive staining cells (%) |
|---|
|
|
|---|
| |
|---|
| 0 | 3.1±1.1 | 3.8±0.8 | 3.0±1.4 | 2.4±0.7 |
| 20 | 3.5±0.9 | 9.5±6.3 | 3.1±1.2 | 3.5±1.9 |
| 40 | 15.0±4.7 | 52.3±11.8 | 8.3±5.0 | 90.6±6.5 |
| 80 | 27.0±15.3 | 96.5±2.2 | 51.2±32.4 | 96.3±1.4 |
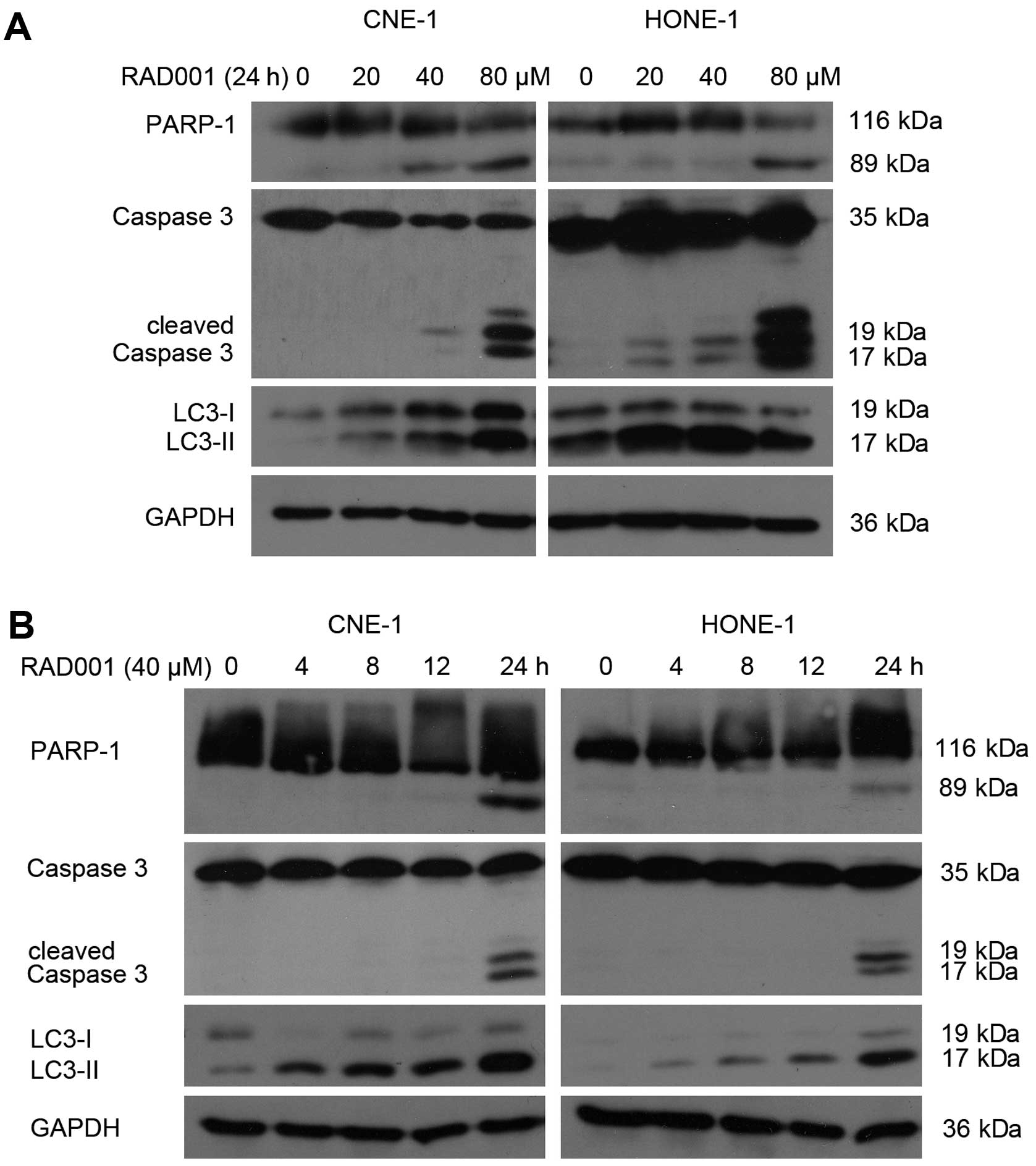
To demonstrate whether RAD001 treatment results in
increased activation of caspases, we analyzed both the cleavage of
PARP-1, a substrate cleaved by caspases during apoptosis, and the
activated cleavage of caspase-3. Our western blotting results
determined that procaspase-3 was cleaved to yield a 17 kDa fragment
and PARP-1 was cleaved to an 89 kDa fragment following treatment in
both cell lines (Fig. 3). These
results further confirmed that RAD001 induced caspase-3-dependent
cell death in these two nasopharyngeal carcinoma cell lines.
RAD001-treated NPC cells displayed typical signs of apoptotic cell
death including chromatin condensation, cell shrinkage, cleavage of
PARP and activation of caspase-3.
RAD001 induces cellular autophagy
mTOR is well known as a major negative regulator of
autophagy and acts as a central regulator of the PI3K/Akt pathway,
which can induce autophagy when inhibited. The membrane-associated
light chain 3 protein (LC3, Atg8) is a key marker of autophagy.
After the induction of autophagy, LC3-I is converted to LC3-II,
which is most likely conjugated to phosphatidylethanolamine (PE)
and tightly bound to the autophagosomal membranes, forming
ring-shaped structures in the cytoplasm. The amount of
PE-conjugated LC3 (LC3-II) correlates well with the number of
autophagosomes. Therefore, we examined LC3-I/II expression using
western blotting. RAD001 treatment caused the levels of LC3-II to
increase in a dose- and time-dependent manner in both cell lines
(Fig. 3).
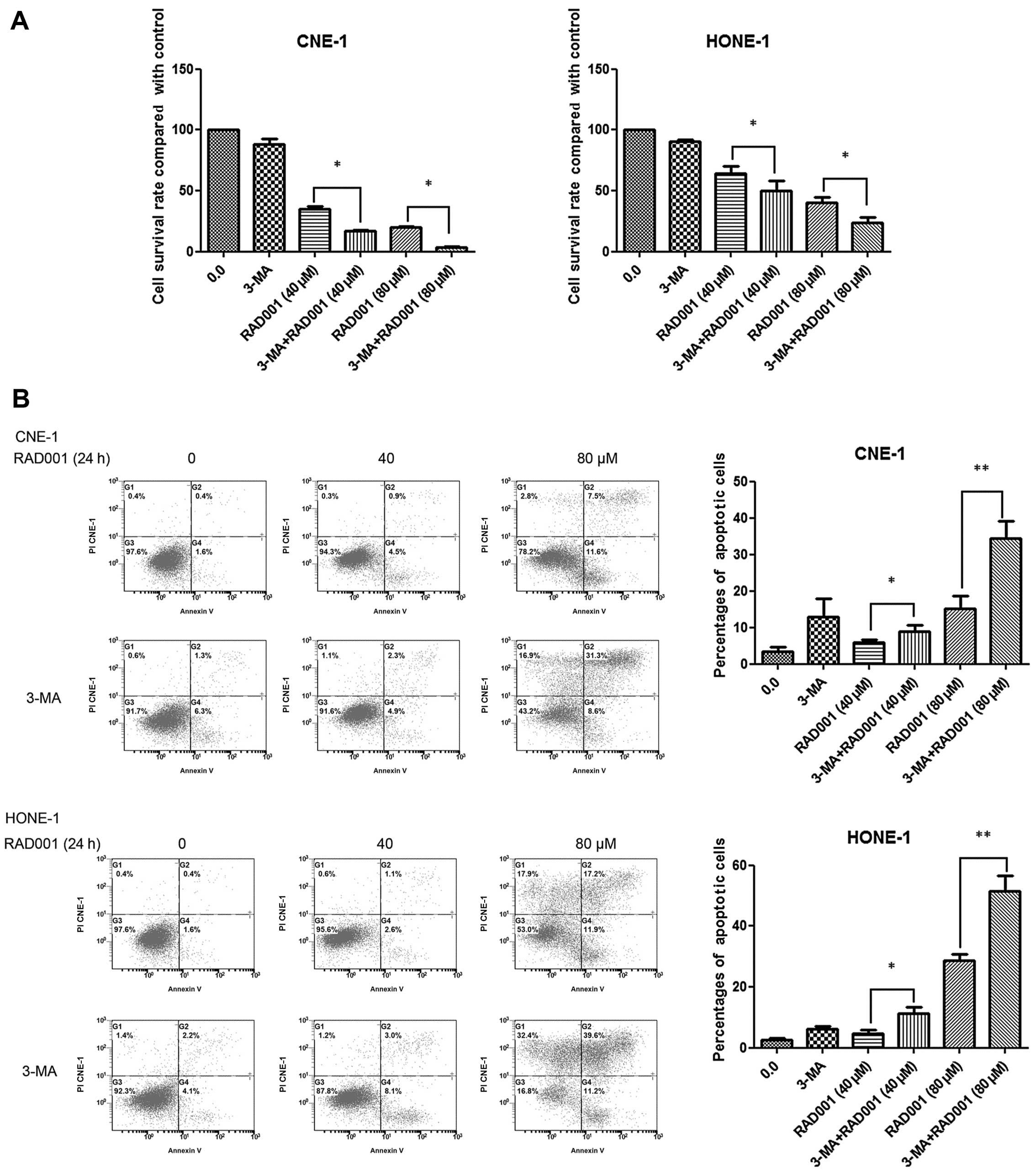
Inhibition of autophagy enhances
RAD001-induced cell growth inhibition and apoptosis
Since RAD001 induced both apoptosis and autophagy in
CNE-1 and HONE-1 cells, we aimed to identify which mechanism of
cell death was dominant. We first inhibited autophagy by combining
RAD001 with the autophagy inhibitor, 3-MA. 3-MA is used to inhibit
and study the mechanism of autophagy (lysosomal self-degradation).
It inhibits autophagy by blocking autophagosome formation via the
inhibition of type III PI3K. RAD001 of 40 and 80 μM resulted in
decreased survival rates of 34.9±2.4 and 19.9±0.9% compared with
the control group (100.0%) in the CNE-1 cells (Fig. 4A). Furthermore, there was
extensive growth inhibition in the combined treatment group. A
survival rate of 17.3±0.5% (P<0.05) and 3.9±0.2% (P<0.05) was
noted when 40 and 80 μM RAD001 was combined with 10 mM 3-MA in
CNE-1 cells. Similar results were observed in the HONE-1 cells
(Fig. 4A).
We next examined whether the combination was
effective at inducing cellular apoptosis. As displayed in Fig. 4B, 3.4±1.3% of the cells were
positive for Annexin V-FITC staining in the CNE-1 cell control
group. Treatment with 40 or 80 μM RAD001 resulted in Annexin
V-FITC-positive staining rates of 6.0±0.7 and 15.2±3.6% at 24 h.
When 40 or 80 μM RAD001 was combined with 3-MA, the Annexin
V-FITC-positive staining rates were 9.0±1.8% (P<0.05) and
34.5±4.7% (P<0.01), respectively. The combination treatment
significantly increased the proportion of apoptotic cells compared
to this proportion following RAD001 treatment (80 μM) alone
(P=0.0024). Regarding the HONE-1 cells, RAD001 of 40 or 80 μM
resulted in the Annexin V-FITC-positive staining rates of 4.6±2.0
and 28.6±3.8% compared with the control group (2.7±0.8%) at 24 h.
When 40 or 80 μM RAD001 was combined with 10 mM 3-MA, the Annexin
V-FITC-positive staining rates were 11.4±3.7% (P<0.05) and
51.5±9.0% (P=0.0076), respectively (Fig. 4B).
Inhibition of apoptosis decreases
RAD001-induced cell death
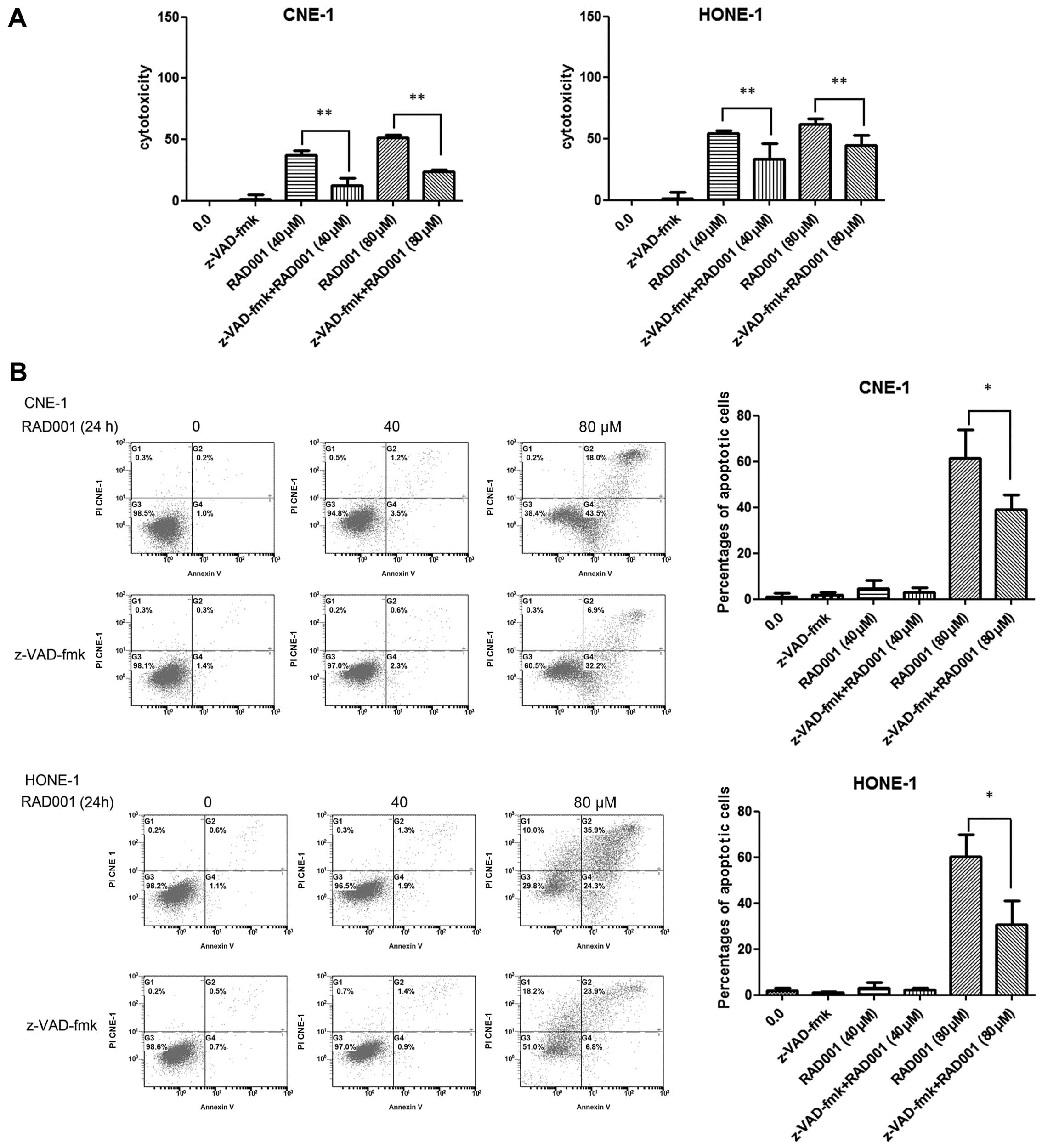
We next combined RAD001 with the caspase-3 inhibitor
z-VAD-fmk to determine whether inhibition of apoptosis decreases
RAD001-induced cell death. Using an LDH assay we determined that
the growth inhibition effect was markedly reduced in both CNE-1 and
HONE-1 cells. As shown in Fig.
5A, 40 and 80 μM RAD001 resulted in increased cytotoxicity
rates of 37.1±4.3 and 51.8±1.7% compared with the control group
(0.0%) in CNE-1 cells. When 40 or 80 μM RAD001 was combined with 20
μM z-VAD-fmk, the cytotoxicity rates were decreased to 12.5±5.9%
(P<0.01) and 23.8±1.7% (P<0.01), respectively. Similar
results were observed in HONE-1 cells (Fig. 5A).
Furthermore, using flow cytometry we examined
whether the combination of RAD001 and z-VAD-fmk was effective at
reducing cellular apoptosis. As shown in Fig. 5B, 1.2±1.3% of the cells were
positive for Annexin V-FITC staining in the CNE-1 cell control
group. In regards to the CNE-1 cells, treatment with 40 or 80 μM
RAD001 resulted in Annexin V-FITC-positive staining rates of
4.7±3.6 and 61.5±12.5% at 24 h. When 40 or 80 μM RAD001 was
combined with 20 μM z-VAD-fmk, the Annexin V-FITC-positive staining
rates were reduced to 2.9±2.0% (P>0.05) and 39.1±6.5%
(P<0.05), respectively. For HONE-1 cells, RAD001 of 40 or 80 μM
resulted in Annexin V-FITC-positive staining rates of 3.2±2.1 and
60.2±9.6% when compared to the rates in the control group
(1.7±1.4%) at 24 h. When 40 or 80 μM RAD001 was combined with
z-VAD-fmk, the Annexin V-FITC-positive staining rates were reduced
to 2.3±0.6% (P>0.05) and 30.7±10.3% (P<0.05), respectively
(Fig. 5B).
Discussion
Nasopharyngeal cancer was invariably lethal prior to
the advent of radiation. With improved knowledge and technology,
locoregional control of this disease exceeds 90%. However, further
reduction of distant failure and major late toxicities remain as
challenges, and the development of targeted therapy without
cytotoxicity is urgently needed. There is a paucity of data
concerning the preclinical activity of RAD001 in NPC. In the
present study, we investigated the antitumor effect of RAD001 in a
time- and dose-dependent manner in CNE-1 and HONE-1 cell lines.
RAD001 induced a significant increase in growth inhibition in the
two cell lines when used in combination with the autophagy
inhibitor 3-MA; however, the percentages of apoptotic cells
decreased when RAD001 was combined with the caspase inhibitor
z-VAD-fmk.
Consistent with a previous report (22), the main mechanism of RAD001 in our
study was the induction of apoptosis rather than autophagy.
Moreover, RAD001 had only a modest effect on the cell cycle (data
not shown), which is supported by both Beuvink et al
(23) and Mabuchi et al
(16). According to a recent
report (24), RAD001 was found to
increase both the cellular apoptotic rate and S phase cell cycle
arrest. However, Zhu et al (25) reported that RAD001 induced
G1 phase arrest and increased the number of cells
undergoing early apoptosis in breast cancer stem cells. Zhang et
al (26) treated stem cells
from primary breast cancer cells and breast cancer cell lines and
observed that the combination treatment of RAD001 with docetaxel
inhibited the growth of stem cells both in vitro and in
vivo by inhibiting cell proliferation and inducing apoptosis
and G2/M phase arrest. Others researchers described the
molecular mechanism of RAD001 as inducing G1 cell cycle
arrest in gastric cancer (27),
mantle cell lymphoma (28),
diffuse large B-cell lymphoma (29), esophageal cancer (30) and breast cancer (31). Still others have reported
alternate mechanisms of action, including the induction of
autophagy in prostate cancer (32), hepatocellular carcinoma (33) and acute lymphoblastic leukemia
(34).
Rosich et al (35) demonstrated that the selective
triple knockdown of the autophagy genes ATG7, ATG5
and ATG3 and pretreatment with the autophagy inhibitor
hydroxychloroquine effectively overcame RAD001 resistance in a
mantle cell lymphoma cell line, leading to the activation of the
mitochondrial apoptotic pathway. In this study, cells displayed
high levels of autophagy when RAD001 was used alone. We then
combined RAD001 with autophagy inhibitor 3-MA to determine whether
the response changed. We determined that the growth inhibition was
enhanced, which is consistent with the study by Rosich et
al. Because this growth inhibition was represented by
additional cellular apoptosis, we evaluated whether caspase-3
inhibition in combination with RAD001 impaired its effect. Using an
LDH assay and flow cytometry, we determined that the growth
inhibitory effect was markedly reduced when RAD001 was used in
combination with a caspase-3 inhibitor in both CNE-1 and HONE-1
cells.
Numerous reports suggest that autophagy is a
survival mechanism protecting cells from cell death due to DNA
damage. Other studies indicate that autophagy may be the mechanism
of cell death during tumor treatment or that autophagy may be
involved in the induction of apoptosis. Currently, both rapamycin
(an mTOR inhibitor) and chloroquine (an autophagosome-lysosome
fusion step blocker) are being used in combination with
chemotherapy in clinical trials for different cancer types. Whether
autophagy is an apoptosis-promoting mechanism per se or whether it
acts as a protective mechanism that reduces tumor cell death upon
treatment is still a controversial and complicated issue that
remains to be determined (36).
mTOR possesses both pro-apoptotic and anti-apoptotic
effects. On one hand, mTOR is capable of translocating into the
nucleus resulting in the phosphorylation and activation of p53.
This in turn results in the transcription of the pro-apoptotic
proteins BAX and others (37). On
the other hand, activated S6K is capable of binding to
mitochondrial membranes and phosphorylating BAD (38), which then becomes inactive and is
unable to promote apoptosis. In the present study, we combined
either an autophagy inhibitor or an apoptosis inhibitor with the
mTOR inhibitor RAD001 and observed more cell death when autophagy
was inhibited. This result suggests that mTOR possesses mainly
anti-apoptotic effects in CNE-1 and HONE-1 cell lines. RAD001
combined with an autophagy inhibitor aggravated mTOR inhibition and
thus enhanced the induction of apoptosis.
In conclusion, our results are the first to suggest
that the inhibition of mTOR by RAD001 may result in a potential
therapeutic benefit for NPC. The predominant mechanism involved was
the induction of cellular apoptosis rather than cell cycle arrest
or autophagy. The combination of RAD001 and the autophagy inhibitor
3-MA resulted in enhanced tumor inhibition by promoting tumor cell
cytotoxicity. Therefore, our present observations may provide
additional evidence to the growing amount of research that
indicates the effectiveness of mTOR inhibition for treating cancer
as well as a strong rationale for the clinical evaluation of RAD001
combined with autophagy inhibitors for the management of
nasopharyngeal malignancies. Further experiments will need to be
performed to verify our conclusion in vivo. Tumor samples
will be collected and autophagy- and apoptosis-related proteins
will be examined to confirm our conclusions.
Acknowledgements
This study was supported by the National Eleventh
Five Technology Major Project (grant no. 2008ZX09312-002) and the
National Natural Science Foundation of China (Young Scientist
Project #81201716)
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
mTOR
|
mammalian target of rapamycin
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
GI50
|
50% growth inhibition
|
|
PI
|
propidium iodide
|
|
WST-1
|
4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene
disulfonate
|
|
LDH
|
lactate dehydrogenase
|
References
|
1
|
Lee AW, Lin JC and Ng WT: Current
management of nasopharyngeal cancer. Semin Radiat Oncol.
22:233–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma BB, Hui EP and Chan AT: Systemic
approach to improving treatment outcome in nasopharyngeal
carcinoma: current and future directions. Cancer Sci. 99:1311–1318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qian W, Liu J, Jin J, Ni W and Xu W:
Arsenic trioxide induces not only apoptosis but also autophagic
cell death in leukemia cell lines via up-regulation of Beclin-1.
Leuk Res. 31:329–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basciani S, Vona R, Matarrese P, Ascione
B, Mariani S, Cauda R, Gnessi L, Malorni W, Straface E and Lucia
MB: Imatinib interferes with survival of multi drug resistant
Kaposi’s sarcoma cells. FEBS Lett. 581:5897–5903. 2007.PubMed/NCBI
|
|
5
|
Yang W, Monroe J, Zhang Y, George D,
Bremer E and Li H: Proteasome inhibition induces both pro- and
anti-cell death pathways in prostate cancer cells. Cancer Lett.
243:217–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar
|
|
7
|
Huang S and Houghton PJ: Targeting mTOR
signaling for cancer therapy. Curr Opin Pharmacol. 3:371–377. 2003.
View Article : Google Scholar
|
|
8
|
Gomez-Pinillos A and Ferrari AC: mTOR
signaling pathway and mTOR inhibitors in cancer therapy. Hematol
Oncol Clin North Am. 26:483–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grzybowska-Izydorczyk O and Smolewski P:
mTOR kinase inhibitors as a treatment strategy in hematological
malignancies. Future Med Chem. 4:487–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
from growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan X and Helman LJ: The biology behind
mTOR inhibition in sarcoma. Oncologist. 12:1007–1018. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan S: Targeting the mammalian target of
rapamycin (mTOR): a new approach to treating cancer. Br J Cancer.
91:1420–1424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vignot S, Faivre S, Aguirre D and Raymond
E: mTOR targeted therapy of cancer with rapamycin derivatives. Ann
Oncol. 16:525–537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin CI, Whang EE, Donner DB, Du J, Lorch
J, He F, Jiang X, Price BD, Moore FD Jr and Ruan DT: Autophagy
induction with RAD001 enhances chemosensitivity and
radiosensitivity through Met inhibition in papillary thyroid
cancer. Mol Cancer Res. 8:1217–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Molinolo AA, Marsh C, El Dinali M, Gangane
N, Jennison K, Hewitt S, Patel V, Seiwert TY and Gutkind JS: mTOR
as a molecular target in HPV-associated oral and cervical squamous
carcinomas. Clin Cancer Res. 18:2558–2568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mabuchi S, Altomare DA, Cheung M, Zhang L,
Poulikakos PI, Hensley HH, Schilder RJ, Ozols RF and Testa JR:
RAD001 inhibits human ovarian cancer cell proliferation, enhances
cisplatin-induced apoptosis, and prolongs survival in an ovarian
cancer model. Clin Cancer Res. 13:4261–4270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Götze KS, Hoffmann D, Schätzl HM, Peschel
C, Fend F and Decker T: Fatal Epstein-Barr virus-associated
lymphoproliferative disorder following treatment with a novel mTOR
inhibitor for relapsed chronic lymphocytic leukemia cells.
Haematologica. 92:1282–1283. 2007.
|
|
18
|
Ghayad SE, Bieche I, Vendrell JA, Keime C,
Lidereau R, Dumontet C and Cohen PA: mTOR inhibition reverses
acquired endocrine therapy resistance of breast cancer cells at the
cell proliferation and gene-expression levels. Cancer Sci.
99:1992–2003. 2008.PubMed/NCBI
|
|
19
|
Zitzmann K, De Toni EN, Brand S, Göke B,
Meinecke J, Spöttl G, Meyer HH and Auemhammer CJ: The novel mTOR
inhibitor RAD001 (everolimus) induces antiproliferative effects in
human pancreatic neuroendocrine tumor cells. Neuroendocrinology.
85:54–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patel V, Marsh CA, Dorsam RT, Mikelis CM,
Masedunskas A, Amornphimoltham P, Nathan CA, Singh B, Weigert R,
Molinolo AA and Gutkind JS: Decreased lymphangiogenesis and lymph
node metastasis by mTOR inhibition in head and neck cancer. Cancer
Res. 71:7103–7112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yip WK, Leong VC, Abdullah MA, Yusoff S
and Seow HF: Overexpression of phospho-Akt correlates with
phosphorylation of EGF receptors, FKHR and BAD in nasopharyngeal
carcinoma. Oncol Rep. 19:319–328. 2008.PubMed/NCBI
|
|
22
|
Ma BB, Lui VW, Hui EP, Lau CP, Ho K, Ng
MH, Cheng SH, Tsao SW and Chan AT: The activity of mTOR inhibitor
RAD001 (everolimus) in nasopharyngeal carcinoma and
cisplatin-resistant cell lines. Invest New Drugs. 28:413–420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beuvink I, Boulay A, Fumagalli S,
Zilbermann F, Ruetz S, O’Reilly T, Natt F, Hall J, Lane HA and
Thomas G: The mTOR inhibitor RAD001 sensitizes tumor cells to
DNA-damaged induced apoptosis through inhibition of p21
translation. Cell. 120:747–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinto-Leite R, Arantes-Rodrigues R,
Palmeira C, Gaivão I, Cardoso ML, Colaço A, Santos L and Oliveira
P: Everolimus enhances gemcitabine-induced cytotoxicity in
bladder-cancer cell lines. J Toxicol Environ Health A. 75:788–799.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu Y, Zhang X, Liu Y, Zhang S, Liu J, Ma
Y and Zhang J: Antitumor effect of the mTOR inhibitor everolimus in
combination with trastuzumab on human breast cancer stem cells in
vitro and in vivo. Tumour Biol. 33:1349–1362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Zhang S, Liu Y, Liu J, Ma Y, Zhu
Y and Zhang J: Effects of the combination of RAD001 and docetaxel
on breast cancer stem cells. Eur J Cancer. 48:1581–1592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee KH, Hur HS, Im SA, Lee J, Kim HP, Yoon
YK, Han SW, Song SH, Oh DY, Kim TY and Bang YJ: RAD001 shows
activity against gastric cancer cells and overcomes 5-FU resistance
by downregulating thymidylate synthase. Cancer Lett. 299:22–28.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haritunians T, Mori A, O’Kelly J, Luong
QT, Giles FJ and Koeffler HP: Antiproliferative activity of RAD001
(everolimus) as a single agent and combined with other agents in
mantle cell lymphoma. Leukemia. 21:333–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wanner K, Hipp S, Oelsner M, Ringshausen
I, Bogner C, Peschel C and Decker T: Mammalian target of rapamycin
inhibition induces cell cycle arrest in diffuse large B cell
lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br
J Haematol. 134:475–484. 2006. View Article : Google Scholar
|
|
30
|
Wang ZG, Fukazawa T, Nishikawa T, Watanabe
N, Sakurama K, Motoki T, Hatakeyama S, Omori O, Ohara T, Tanabe S,
Fujiwara Y, Takaoka M, Shirakawa Y, Yamatsuji T, Tanaka N and
Naomoto Y: RAD001 offers a therapeutic intervention through
inhibition of mTOR as a potential strategy for esophageal cancer.
Oncol Rep. 23:1167–1172. 2010.PubMed/NCBI
|
|
31
|
Liu H, Scholz C, Zang C, Schefe JH, Habbel
P, Regierer AC, Schulz CO, Possinger K and Eucker J: Metformin and
the mTOR inhibitor everolimus (RAD001) sensitize breast cancer
cells to the cytotoxic effect of chemotherapeutic drugs in vitro.
Anticancer Res. 32:1627–1637. 2012.PubMed/NCBI
|
|
32
|
Cao C, Subhawong T, Albert JM, Kim KW,
Geng L, Sekhar KR, Gi YJ and Lu B: Inhibition of mammalian target
of rapamycin or apoptotic pathway induces autophagy and
radiosensitizes PTEN null prostate cancer cells. Cancer Res.
66:10040–10047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Altmeyer A, Josset E, Denis JM, Gueulette
J, Slabbert J, Mutter D, Noël G and Bischoff P: The mTOR inhibitor
RAD001 augments radiation-induced growth inhibition in a
hepatocellular carcinoma cell line by increasing autophagy. Int J
Oncol. 41:1381–1386. 2012.PubMed/NCBI
|
|
34
|
Crazzolara R, Bradstock KF and Bendall LJ:
RAD001 (everolimus) induces autophagy in acute lymphoblastic
leukemia. Autophagy. 5:727–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosich L, Xargay-Torrent S, López-Guerra
M, Campo E, Colomer D and Roué G: Counteracting autophagy overcomes
resistance to everolimus in mantle cell lymphoma. Clin Cancer Res.
18:5278–5289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maycotte P and Thorburn A: Autophagy and
cancer therapy. Cancer Biol Ther. 11:127–137. 2011. View Article : Google Scholar
|
|
37
|
Castedo M, Roumier T, Blanco J, Ferri KF,
Barretina J, Tintignac LA, Andreau K, Perfettini JL, Amendola A,
Nardacci R, Leduc P, Ingber DE, Druillennec S, Roques B, Leibovitch
SA, Vilella-Bach M, Chen J, Este JA, Modjtahedi N, Piacentini M and
Kroemer G: Sequential involvement of Cdk1, mTOR and p53 in
apoptosis induced by the HIV-1 envelope. EMBO J. 21:4070–4080.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li B, Desai SA, MacCorkle-Chosnek RA, Fan
L and Spencer DM: A novel conditional Akt ‘survival switch’
reversibly protects cells from apoptosis. Gene Ther. 9:233–244.
2002.
|