Introduction
Trop2 is a cell-surface glycoprotein first
identified in trophoblast cells almost 30 years ago (1). It has been shown that Trop2 plays an
important role in the survival, metastasis and aggressiveness of
cancer cells (2–9). Previous reports indicated that Trop2
is also highly expressed in hepatic oval cells which are considered
to be facultative hepatic stem cells and prostate basal cells with
stem cell characteristics (10,11). It appears that Trop2 may provide
signals to cancer cells with requirement for proliferation as well
as to stem-like cells.
Previous studies found that Trop2 was similar to
integrins since both transduce signals through an increase in
cytoplasmic Ca2+ (12), as the function observed in other
progenitor cells, leading us to investigate the relationship
between cardiac progenitor cells (CPCs) and Trop2.
Herein we report that Trop2+ cells
represent a minor subpopulation of c-kit+ cells in
healthy adult hearts, but the subset increases following acute
myocardial infarction (AMI). Trop2 activation enhances
c-kit+ cells survival ability in vitro, and the
mitogen-activated protein kinase (MAPK) pathway may respond to the
potential molecular mechanism underlying this effect.
Materials and methods
Mice
The mice (C57BL/6J) were maintained in certified SPF
facilities and the experiments were approved by the Ethics
Committee of Animal Use for Teaching and Research, Tongji Medical
College of Huazhong University of Science.
Myocardial infarction (MI) was induced in male mice
at 12 months (26–28 g) by permanent ligation of the left anterior
descending (LAD) coronary artery as previously described (13). Briefly, mice were anaesthetized by
intraperitoneal injection with chloral hydrate (Sigma-Aldrich, St.
Louis, MO, USA) 300 mg/kg body weight and scopolamine hydrobromide
(Sigma-Aldrich) 3 mg/kg body weight, respectively. Following
thoracotomy, an 8/0 polypropylene monofilament suture (Jinhuan,
Shanghai, China) was tightened around the proximal LAD artery.
Sham-operated mice underwent the same surgical procedure without
tying the suture but moving it behind the LAD artery.
Electrocardiogram (ECG) was performed to verify the presence of
MI.
Isolation of cardiac c-kit+
cell subpopulations
Cardiac c-kit+/Trop2+ and
c-kit+/Trop2− cells were isolated from the
hearts of the mice at 12 months (26–28 g) by two-step
immunomagnetic microbead-based cell sorting, according to the
manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach,
Germany). First, a modified procedure was performed to isolate
cardiac c-kit+ cells as we previously described
(14). Cells were stained with
goat anti-Trop2 antibody (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and rat anti-goat immunomagnetic microbeads
(Miltenyi Biotec) for separation of
c-kit+/Trop2+ and
c-kit+/Trop2− cells. The harvested cells were
maintained in the same condition as described above, and the purity
of fractioned populations was assayed using flow cytometry.
Trop2 shRNA vector construction and
transfection
Mouse Trop2 short hairpin RNA (shRNA) plasmids were
constructed by Genechem (Shanghai, China). The validated target
sequences were designed according to the cDNA sequences of mouse
Trop2 (GenBank accession no. NM_020047). Each anti-Trop2 target
sequence corresponds to nt 300–318, 549–567 and 688–706 of Trop2
cDNA sequences. A scrambled shRNA sequence which was not present in
the murine or human genome databases was used as a negative
control. The schematic diagram of shRNA-expressing plasmid vector
together with target sequences is shown in Fig. 3B. The DNA sequences of 4 shRNA
plasmids were confirmed by sequencing.
c-kit+/Trop2+ cells grew in
6-well plates to 70% confluence. Five micrograms of each shRNA
plasmid diluted in 0.5 ml of Opti-MEM® (Gibco-BRL, Grand
Island, NY, USA) were mixed thoroughly with 7.5 μl of PLUS™
reagent (Life Technologies, Rockville, MD, USA) and incubated at
room temperature for 5 min. Then, 22.5 μl of Lipofectamine™
LTX (Life Technologies) was added, and the mixture was incubated at
room temperature for 30 min. The cells were incubated at 37°C in a
CO2 incubator and culture medium was changed after 6 h.
The silencing efficiency of Trop2 was monitored with western blot
analysis every day for a week.
Western blot analysis and kinase
assay
Western blot analysis was performed to assay the
silencing efficiency of transfected cardiac
c-kit+/Trop2+ cells and the expression level
of downstream effectors of signaling pathways. For the latter,
isolated Trop2+ and Trop2− cardiac
c-kit+ cells were washed once with serum-free DMEM/F12
medium and then returned to the same medium for 24 h prior to
stimulation with 10% FCS for 30 min. Cells were lysed and the
membranes were incubated with the primary antibodies in optimized
dilution, including goat-anti-mTrop2 antibody, and rabbit-anti-Rsk
antibody (Santa Cruz Biotechnology, Inc.), anti-Akt antibody,
anti-phospho-Akt antibody phosphorylated at Thr308 and at Ser473
(all from Cell Signaling Technology, Beverly, MA, USA).
Anti-β-actin (Sigma-Aldrich) was used as a loading control.
Immunoprecipitation kinase assay was performed to
detect the activity of Rsks as described by Shimamura et al
(15). The cleared lysates from
Trop2− and Trop2+ cells were incubated with
the Rsk antibody for 3 h, then incubated for an additional hour
with 50% slurry of Protein-A-Sepharose beads (Sigma-Aldrich) in
PBS. The beads were washed and the kinase assay was performed as
described (16). Reactions were
subjected to SDS-PAGE on 12% gels and quantitation was performed by
phosphorimaging.
Flow cytometric analysis
Hearts were extracted from mice at different time
points following MI or sham operation. Using small cells (<40
μm) isolated from 2 hearts, each independently after
digestion and sequential filtration, we stained cells with a
dual-color antibody panel composed of phycoerythrin (PE) conjugated
anti-mouse Abs c-kit (eBioscience, San Diego, CA, USA) and
allophycocyanin (APC) conjugated anti-mTrop2 (R&D Systems,
Minneapolis, MN, USA) or with single PE conjugated anti-mouse Abs
c-kit only for different purposes. Data were collected on a BD LSR
II and a number of 1×107 live events based on the
viability dye 7-amino-actinomycin D (7-AAD) (BD Biosciences)
negative staining were processed for each test. An experiment using
endothelial isolated from hearts of neonatal mice negative for
c-kit and Trop2 demonstrated minimal non-specific labeling with the
antibodies used. Data analysis was performed with standard
CellQuest software (version 3.4; BD Biosciences).
For the cell proliferation assay, the transfected
cardiac c-kit+/Trop2+ cells were harvested
following incubation with 10 μM bromodeoxyuridine (BrdU) and
the incorporated BrdU was revealed using APC anti-BrdU antibody
according to the BrdU Flow kit (BD Biosciences). To assess
apoptosis in vitro, the isolated cardiac
c-kit+/Trop2+ and
c-kit+/Trop2− cells were incubated in the
conditioned media (CM) derived from supernatant of LPS-stimulated
monocytes and DMEM/F12 containing 10% FCS in different ratios for 6
h prior to apoptosis assay using Annexin V-APC/propidium iodide
(PI) staining according to the manufacturer’s instructions (BD
Biosciences). Data were acquired and analyzed as described
above.
Statistical analysis
All data are expressed as the mean ± SD.
Significance between two comparisons was determined by Student’s
t-test and among multiple comparisons by Bonferroni test. P<0.05
was considered to indicate statistically significant
differences.
Results
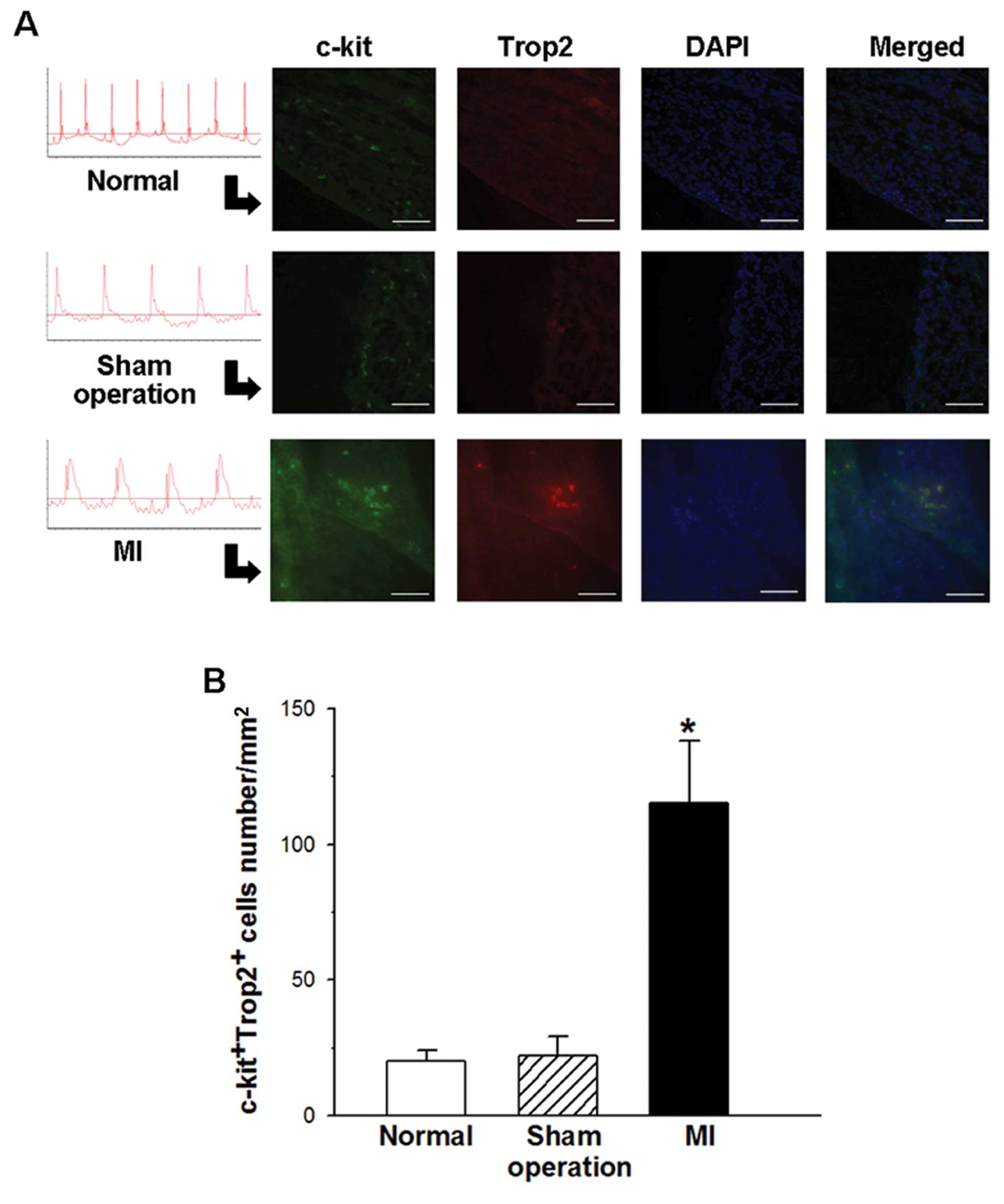
Trop2 is expressed in c-kit+
cells within the myocardium and is increased in post-infarct mouse
hearts
The experimental MI C57BL/6J mouse model was
inducted to investigate the cellular regulation of the cardiac
Trop2 receptor in response to acute ischemia injury. Under ECG
monitoring, the successful MI model characterized with the
ST-segment rose immediately after LAD ligation and kept elevating
during the procedure. Seven days after MI, immunofluorescence
staining was performed on heart sections of surviving mice using
Trop2 specific Abs combination with c-kit. The density of the
c-kit+ cells in the MI heart was greater than in the
heart without MI. Moreover, Trop2 was only detected in
c-kit+ cells (Fig.
1A). Compared with sham operation and normal hearts, the
frequency of c-kit+/Trop2+ cells was much
higher in MI hearts (Fig.
1B).
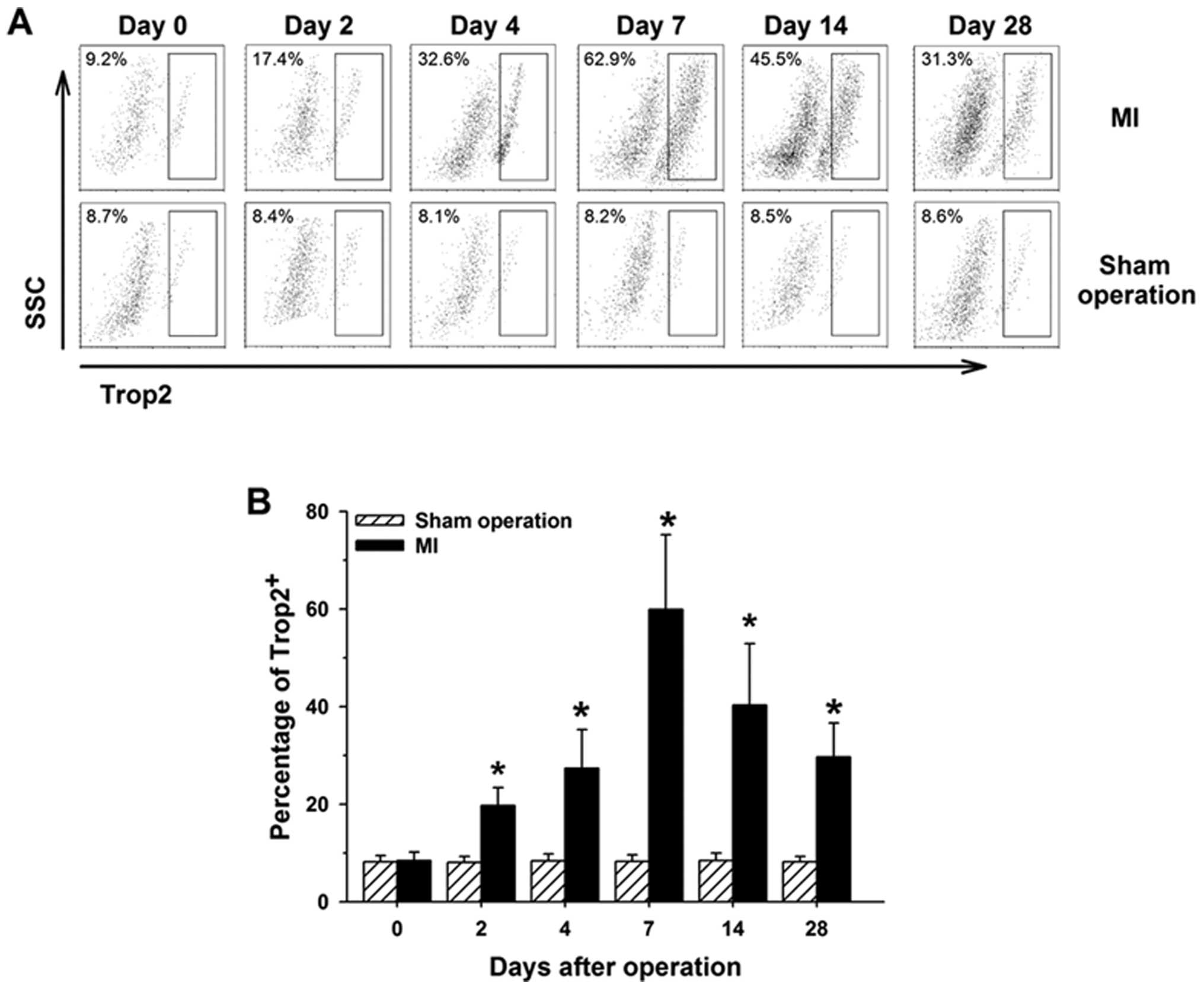
To quantify the rate change of
c-kit+/Trop2+ cells during the natural
history of MI, all the cells were isolated from the hearts of mice
0, 2, 4, 7, 14 and 28 days after the surgical procedure. We
collected the isolated heart cells for dual-color fluorescence flow
cytometry analysis. The percentage of
c-kit+/Trop2+ cells dramatically increased
and was maximal at 7 days after MI, then slightly decreased with
time. The c-kit-gated events increased with time following MI.
However, the percentage of c-kit+/Trop2+
cells or the total number of c-kit+ cells presented no
obvious change at different time points in sham-operated control
animals (Fig. 2). Notably, the
change trend of c-kit+/Trop2+ cells was
consistent with the infiltration pattern of inflammatory cells in
border zone of infarcts during the natural history of MI (17), suggesting that the Trop2 receptor
may play a critical role in response to acute inflammatory reaction
following MI.
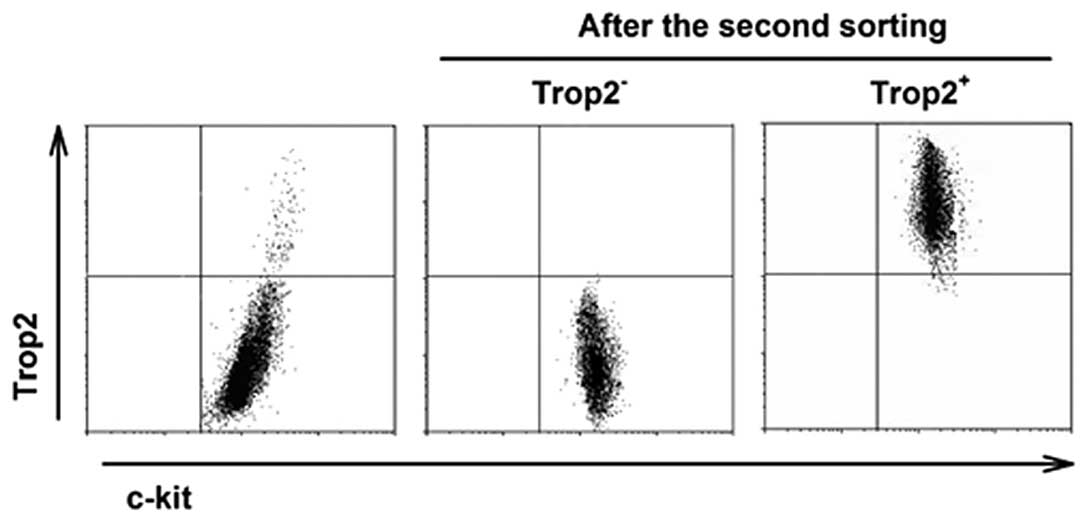
Isolation of high purity cardiac
c-kit+ cell subpopulations and silencing Trop2 of
c-kit+/Trop2+ cells
To ensure lineage-negative state, the two
subpopulations were isolated from healthy hearts. With two-step
immunomagnetic microbead-based cell sorting, the purity of sorted
Trop2 positive cells was >95% and of negative cells almost 100%
after the second round of immunomagnetic selection (Fig. 3).
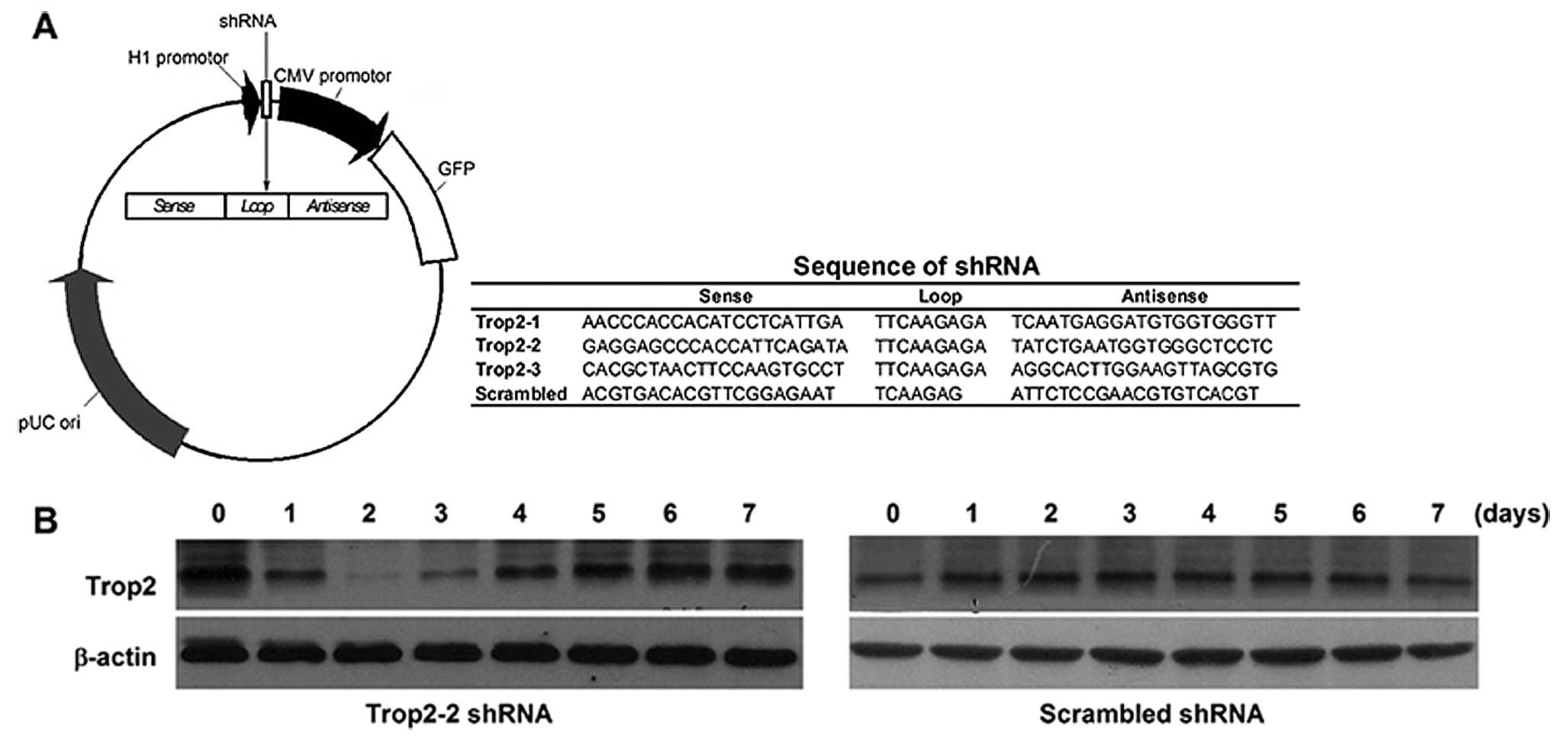
Constructed plasmid vectors transcribing shRNA
against Trop2 under H1 promoter (Fig.
4A) were generated and transduced to the
c-kit+Trop2+ cells between 0–2 passages. By
monitoring the silencing efficiency every day with the detection of
GFP (green fluorescent protein) expression, we found that Trop2 was
downregulated only using Trop2-2 shRNA plasmid vector. The Trop2
expression decreased to the minimum on the second day after
transfection of Trop2-2 shRNA plasmid vector, but returned to the
basal line within one week. However, there was no significant
silencing efficiency when using scrambled shRNA (Fig. 4B).
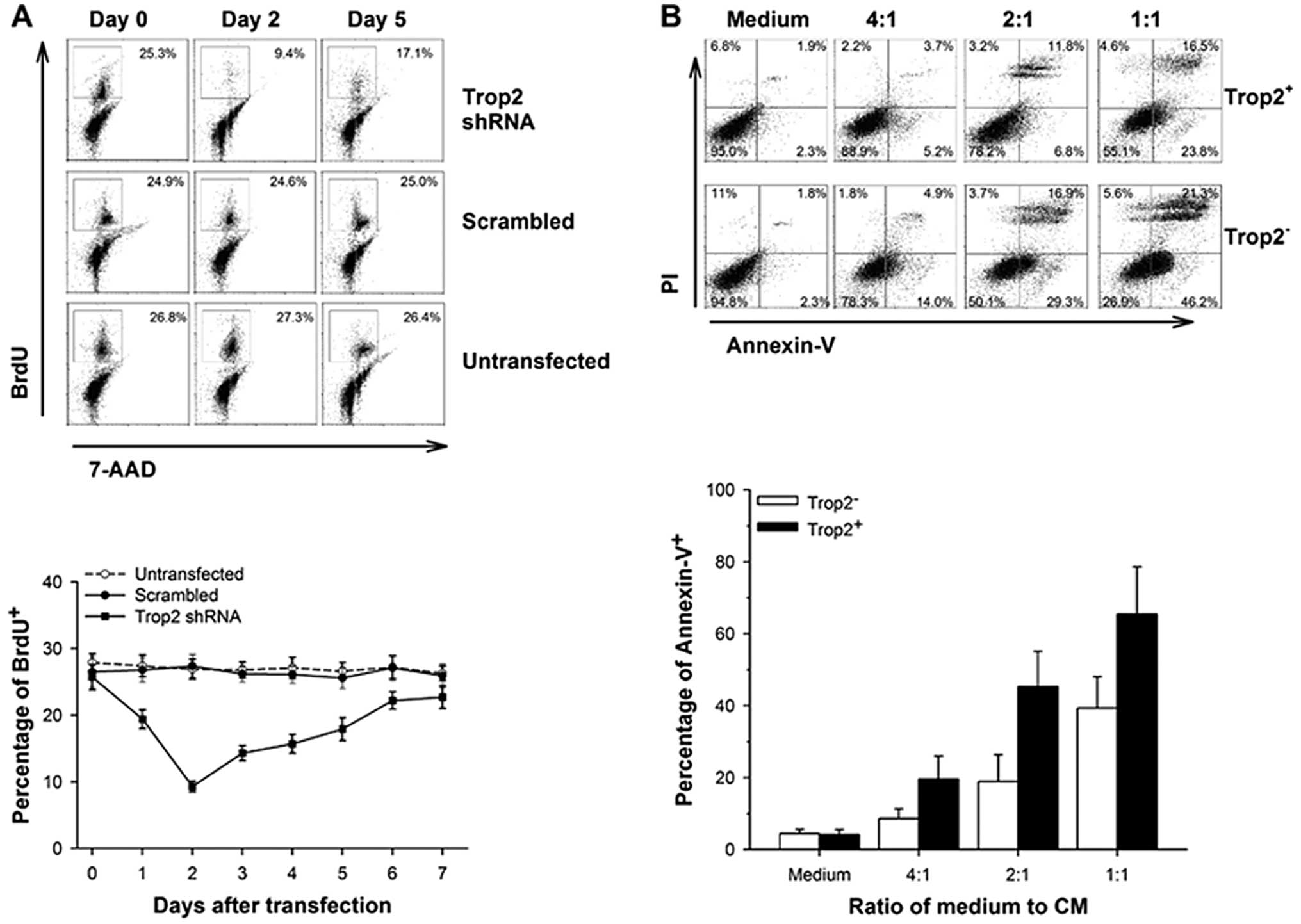
Inhibition of Trop2 significantly
suppresses proliferation of cardiac
c-kit+Trop2+ cells in vitro
To clarify whether Trop2 affects the proliferation
of cardiac c-kit+ cells, we measured the BrdU
incorporation following shRNA plasmid vectors transfected into
c-kit+/Trop2+ cells. BrdU incorporation
correlated with the expression of Trop2 in 2% FCS serum conditions
(Fig. 5A). The percentage of BrdU
positive cells decreased to the lowest at 9.3±0.8% on the second
day, while the values were 25.7±1.9 and 22.7±1.7% at 0 and 7 days,
respectively, after transfection of vector expressing Trop2 shRNA.
However, cells that were transfected with scrambled shRNA plasmid
vectors or that were untreated showed invariable growth rate. These
results show that downregulation of Trop2 significantly impairs the
proliferation of cardiac c-kit+ cells.
c-kit+/Trop2+
cardiac cells are more resistant to inflammatory cytokines in
vitro
We performed Annexin V assay to identify whether
Trop2 inhibits apoptosis in cardiac c-kit+ cells in MI.
Purified Trop2+- or Trop2−-c-kit+
cells were both treated with CM at 0, 1:4, 1:2 and 1:1 ratio
(vol vs. vol) respectively, related to DMEM/F12
medium in vitro and apoptosis examined at 6 h
post-treatment. The percentage of Trop2+ and
Trop2− cell apoptosis were similar in the absence of CM.
Although a positive correlation between the ratio of medium to CM
and the rate of apoptosis exists in both Trop2+ and
Trop2− subpopulations, the latter displays a stronger
upward trend (Fig. 5B). These
data highlight the crucial role of Trop2 in inhibiting cardiac
c-kit+ cell apoptosis mediated by inflammatory
cytokines.
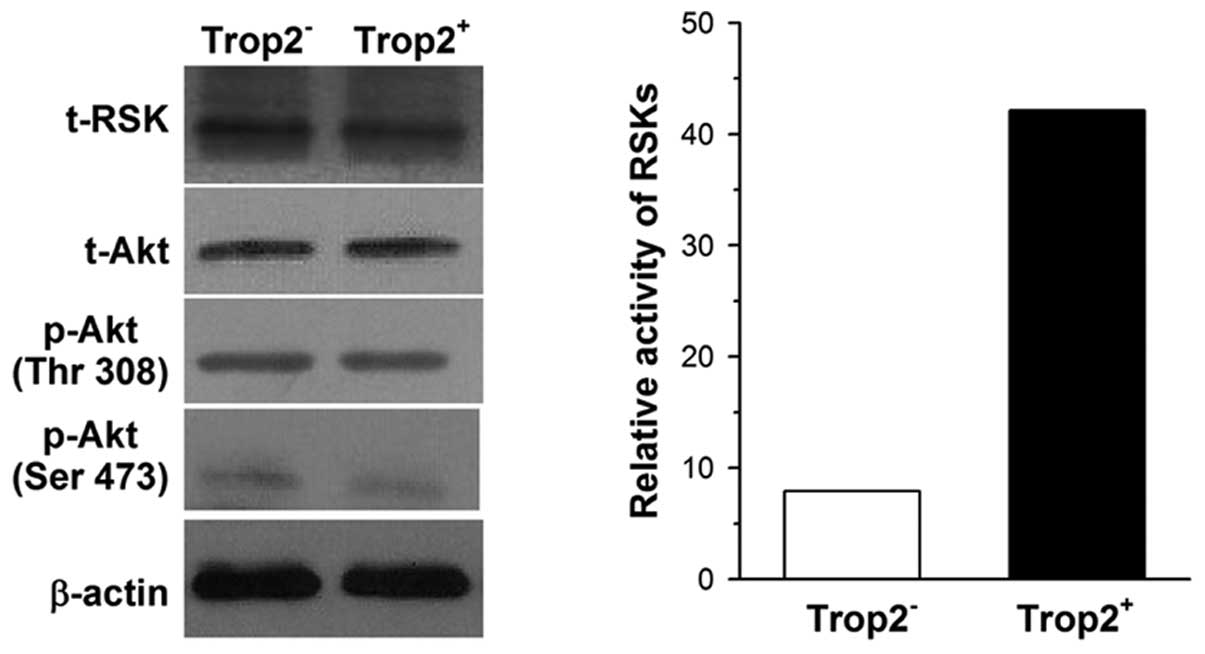
Activation of MAPK cascades is
responsible for protection of c-kit+ cells by Trop2
In the present study, we focused on the MAPK and
phosphatidylinositol 3-OH kinase (PI3K) pathways, since
both of them are involved in promoting proliferation and inhibiting
apoptosis, and Ca2+ serves as a model in these cascades
(18). We measured the activity
of ribosomal S6 kinases (RSKs) and the level of phosphorylated Akt
in cardiac c-kit+ cells as they are downstream effectors
of the MAPK and PI3K pathways, respectively. Cell
lysates from Trop2+ and Trop2− cells were
immunoprecipitated with RSK antibody and bacterially expressed
glutathione-S-transferase (GST)-conjugated substrate GST-S6 as
described by Shimamura et al (15). Immune-complex kinase assays
confirmed RSK kinase activity in Trop2+ cells is
approximately 3.7-fold compared with Trop2− cells.
However, there was no significant difference in total-Akt or
phosphorylated Akt expression between Trop2+ and
Trop2− cells when their lysates were subjected to
western blot analysis, whether the phosphorylation site was located
on Ser473 or Thr308 (Fig. 6).
These results suggest that the MAPK rather than the PI3K
signaling pathway corresponds to Trop2 activation in cardiac
c-kit+ cells.
Discussion
In the present study we demonstrated that
c-kit+ cells exclusively express Trop2 in
cardiomyocytes. c-kit+/Trop2+ cells are
rarely detected in normal myocardium, but its frequency increases
significantly following MI. Decreased expression of Trop2 cardiac
c-kit+ cells weaken its ability of proliferation and
survival response to the inflammation in vitro. The pathway
responsible for Trop2 signal transduction may be the MAPK
cascade.
In general, Trop2 expression is only associated with
aggressive malignant tumor behavior. Evidence of this hypothesis
was found in two organs (prostate and liver) with high regenerative
capability. In the prostate, only the basal cells expressing high
levels of Trop2 were able to efficiently form spheres in
vitro and regenerate prostatic tubules in vivo (11), and in the liver, Trop2 was
exclusively expressed on activating oval cells, but was not
detected in normal state (10).
Our data after experimental MI in mice also support this
possibility. Furthermore, the current study shows Trop2 is
exclusively present in c-kit+ cells in the heart, which
is similar to the liver since oval cells were reported to express
c-kit (19), indicating that
Trop2 is involved in the regulation of the biological behavior of
c-kit+ cells.
The recognition that a pool of undifferentiated
cells expressing stem cell surface antigens c-kit, Sca-1, MDR1 and
Isl-1 reside within the adult myocardium and that these cells form
myocytes, smooth muscle cells and endothelial vascular cells has
challenged the traditional concept of the heart as a postmitotic
organ (20). It has been
demonstrated these cells are involved in repairing damaged
myocardium (21,22) and cardiac c-kit+ cells
may be more relevant in emergencies than other CPCs (20). However, in most cases, the
resident CPCs are insufficient for rejuvenating cardiac performance
of injured heart. The reasons for this limited effect of
self-repair of the heart may be the rare number and intrinsic
properties of CPCs. Moreover, high oxidative stress in damaged
myocardium, such as infarcted lesion, further decreases the pool
size of CPCs available for cardiac repair (23). Although numerous compounds
including proteins and steroids and gene therapy coding for
antioxidants and apoptosis have been reported to have significant
cardioprotection in animal studies through augmenting role of CPCs
(23–27), further efforts are required for
their clinical application. In the present study, we observed Trop2
expression related to the proliferation potential of cardiac
c-kit+ cells. Compared with
c-kit+/Trop2− cells, double-positive cells
showed stronger survivability in the conditions that mimic
inflammatory microenvironment of infarct myocardium. These results
obtained from in vitro and in vivo experiments
suggest that activation of Trop2 could provide a protective role
for cardiac c-kit+ cells. These data also suggest that
the physiological ligand of Trop2 may be one or more cytokines
secreted by activated monocytes.
An important link between the MAPK pathway and the
function of Trop2 contributing to promoting proliferation and
inhibiting apoptosis of cardiac c-kit+ cells was made
following the discovery that activity of RSKs in Trop2+
cells was significantly higher than in Trop2− cells.
Activation of RSKs target genes implicated in the regulation of
diverse cellular processes according to phosphorylating targets,
including proliferation promoters such as cyclin D1 and cyclin E as
well as apoptosis inhibitors such as Bad, death-associated protein
kinase (28). Consistent with a
previous report (29), we were
able to identify that the MAPK cascade corresponds to the Trop2
signal transduction pathway. However, since only the cascades that
Ca2+ are involved in have been investigated, and
considering the versatility of the regulatory actions on
Ca2+ signaling (18),
this molecular mechanism may be only one repertoire between
extracellular stimuli of Trop2 and physiological phenotypes. Thus,
a precise and complete signaling network remains to be further
clarified.
In conclusion, this study reveals that activation of
Trop2 plays an important cardioprotective role after MI through
promoting proliferation and inhibiting apoptosis of cardiac
c-kit+ cells. These observations suggest that the import
of cardiac c-kit+ cells overexpressing Trop2 or
manipulation of autogenous cardiac c-kit+ cells using a
selective Trop2 agonist may be potential approaches for the
management of acute ischemic cardiomyopathy.
Abbreviations:
|
CPCs
|
cardiac progenitor cells;
|
|
MI
|
myocardial infarction;
|
|
KO
|
knockout;
|
|
PCR
|
polymerase chain reaction;
|
|
LAD
|
left anterior descending;
|
|
ECG
|
electrocardiogram;
|
|
shRNA
|
short hairpin RNA;
|
|
WT
|
wild-type;
|
|
BSA
|
bovine serum albumin;
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium;
|
|
bFGF
|
fibroblast growth factor;
|
|
LIF
|
leukemia inhibitory factor;
|
|
EGF
|
epidermal growth factor;
|
|
PBMCs
|
peripheral blood mononuclear
cells;
|
|
LPS
|
lipopolysaccharides;
|
|
CM
|
conditioned media;
|
|
PE
|
phycoerythrin;
|
|
APC
|
allophycocyanin;
|
|
7-AAD
|
7-aminoactinomycin D;
|
|
BrdU
|
bromodeoxyuridine;
|
|
PI
|
propidium iodide;
|
|
FSC
|
forward scatter;
|
|
SSC
|
side scatter;
|
|
GFP
|
green fluorescent protein;
|
|
PIP2
|
phosphatidylinositol
4,5-bisphosphate;
|
|
PKC
|
protein kinase C;
|
|
PLC
|
phospholipase C;
|
|
IP3
|
inositol 1,4,5-trisphosphate;
|
|
DAG
|
diacylglycerol;
|
|
ER
|
endoplasmic reticulum;
|
|
RSKs
|
ribosomal S6 kinases;
|
|
MAPK
|
mitogen-activated protein kinase;
|
|
PI3K
|
phosphatidylinositol 3-OH kinase;
|
|
GST
|
glutathione-S-transferase
|
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (no.
30571840) and the Specialized Research Fund for the Doctoral
Program of Higher Education (no. 20110142110009).
References
|
1
|
Lipinski M, Parks DR, Rouse RV and
Herzenberg LA: Human trophoblast cell-surface antigens defined by
monoclonal antibodies. Proc Natl Acad Sci USA. 78:5147–5150. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi H, Minami Y, Anami Y, et al:
Expression of the GA733 gene family and its relationship to
prognosis in pulmonary adenocarcinoma. Virchows Arch. 457:69–76.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muhlmann G, Spizzo G, Gostner J, et al:
TROP2 expression as prognostic marker for gastric carcinoma. J Clin
Pathol. 62:152–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Day R, Dong Y, Weintraub SJ and
Michel L: Identification of Trop-2 as an oncogene and an attractive
therapeutic target in colon cancers. Mol Cancer Ther. 7:280–285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohmachi T, Tanaka F, Mimori K, Inoue H,
Yanaga K and Mori M: Clinical significance of TROP2 expression in
colorectal cancer. Clin Cancer Res. 12:3057–3063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fong D, Moser P, Krammel C, et al: High
expression of TROP2 correlates with poor prognosis in pancreatic
cancer. Br J Cancer. 99:1290–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bignotti E, Todeschini P, Calza S, et al:
Trop-2 overexpression as an independent marker for poor overall
survival in ovarian carcinoma patients. Eur J Cancer. 46:944–953.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trerotola M, Rathore S, Goel HL, et al:
CD133, Trop-2 and alpha-2beta1 integrin surface receptors as
markers of putative human prostate cancer stem cells. Am J Transl
Res. 2:135–144. 2010.PubMed/NCBI
|
|
9
|
Fong D, Spizzo G, Gostner JM, et al:
TROP2: a novel prognostic marker in squamous cell carcinoma of the
oral cavity. Mod Pathol. 21:186–191. 2008.PubMed/NCBI
|
|
10
|
Okabe M, Tsukahara Y, Tanaka M, et al:
Potential hepatic stem cells reside in EpCAM+ cells of
normal and injured mouse liver. Development. 136:1951–1960. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldstein AS, Lawson DA, Cheng D, Sun W,
Garraway IP and Witte ON: Trop2 identifies a subpopulation of
murine and human prostate basal cells with stem cell
characteristics. Proc Natl Acad Sci USA. 105:20882–20887. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ripani E, Sacchetti A, Corda D and Alberti
S: Human Trop-2 is a tumor-associated calcium signal transducer.
Int J Cancer. 76:671–676. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tarnavski O, McMullen JR, Schinke M, Nie
Q, Kong S and Izumo S: Mouse cardiac surgery: comprehensive
techniques for the generation of mouse models of human diseases and
their application for genomic studies. Physiol Genomics.
16:349–360. 2004. View Article : Google Scholar
|
|
14
|
Han Y, Chen JD, Liu ZM, et al: Functional
ion channels in mouse cardiac c-kit(+) cells. Am J Physiol Cell
Physiol. 298:C1109–C1117. 2010.
|
|
15
|
Shimamura A, Ballif BA, Richards SA and
Blenis J: Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr
Biol. 10:127–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roux PP, Richards SA and Blenis J:
Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates
extracellular signal-regulated kinase docking and RSK activity. Mol
Cell Biol. 23:4796–4804. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alpert JS, Thygesen K, Antman E and
Bassand JP: Myocardial infarction redefined - a consensus document
of The Joint European Society of Cardiology/American College of
Cardiology Committee for the redefinition of myocardial infarction.
J Am Coll Cardiol. 36:959–969. 2000. View Article : Google Scholar
|
|
18
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petersen BE, Goff JP, Greenberger JS and
Michalopoulos GK: Hepatic oval cells express the hematopoietic stem
cell marker Thy-1 in the rat. Hepatology. 27:433–445. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anversa P, Kajstura J, Leri A and Bolli R:
Life and death of cardiac stem cells: a paradigm shift in cardiac
biology. Circulation. 113:1451–1463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Urbanek K, Torella D, Sheikh F, et al:
Myocardial regeneration by activation of multipotent cardiac stem
cells in ischemic heart failure. Proc Natl Acad Sci USA.
102:8692–8697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Urbanek K, Quaini F, Tasca G, et al:
Intense myocyte formation from cardiac stem cells in human cardiac
hypertrophy. Proc Natl Acad Sci USA. 100:10440–10445. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levonen AL, Vahakangas E, Koponen JK and
Yla-Herttuala S: Antioxidant gene therapy for cardiovascular
disease: current status and future perspectives. Circulation.
117:2142–2150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brinckmann M, Kaschina E, Altarche-Xifro
W, et al: Estrogen receptor alpha supports cardiomyocytes
indirectly through post-infarct cardiac c-kit+ cells. J
Mol Cell Cardiol. 47:66–75. 2009. View Article : Google Scholar
|
|
25
|
Altarche-Xifro W, Curato C, Kaschina E, et
al: Cardiac c-kit+AT2+ cell population is
increased in response to ischemic injury and supports cardiomyocyte
performance. Stem Cells. 27:2488–2497. 2009.
|
|
26
|
Padin-Iruegas ME, Misao Y, Davis ME, et
al: Cardiac progenitor cells and biotinylated insulin-like growth
factor-1 nanofibers improve endogenous and exogenous myocardial
regeneration after infarction. Circulation. 120:876–887. 2009.
View Article : Google Scholar
|
|
27
|
Lavu M, Gundewar S and Lefer DJ: Gene
therapy for ischemic heart disease. J Mol Cell Cardiol. 50:742–750.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anjum R and Blenis J: The RSK family of
kinases: emerging roles in cellular signalling. Nat Rev Mol Cell
Biol. 9:747–758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cubas R, Li M, Chen C and Yao Q: Trop2: a
possible therapeutic target for late stage epithelial carcinomas.
Biochim Biophys Acta. 1796:309–314. 2009.PubMed/NCBI
|