Introduction
Cardiovascular complications in diabetes mellitus
are one of the leading causes of patient mortality. Vascular
endothelial injury and dysfunction are important early pathological
manifestations of the cardiovascular complications of diabetes
(1). Increased apoptosis is an
early manifestation of endothelial injury, leading to endothelial
dysfunction (2).
Advanced glycation end products (AGEs) are the
product of non-enzymatic glycosylation on the amino groups of
macromolecules such as proteins and nucleic acids (3). In diabetic patients, sustained high
blood glucose significantly increases production of AGEs (4). Epidemiological studies show that the
presence of AGEs is highly correlated with diabetic cardiovascular
complications (4). Previous
studies found that AGEs increase endothelial cell (EC) apoptosis
and dysfunction (5). In addition,
our previous study found that AGEs increase apoptosis and
dysfunction of endothelial precursor cells (6). The generation of intracellular
reactive oxygen species (ROS) increases in AGE-induced cells, and
oxidative stress and ROS production, in turn, contribute to
AGE-induced apoptosis of ECs and endothelial precursor cells
(6).
Removal of AGEs from the body is challenging. One
strategy to reduce AGE-induced endothelial injury is to antagonize
AGE-activated oxidative stress (7). Hydrogen (H2) is the
smallest gas molecule in nature. Studies have shown that
H2 has antioxidant activities in living organisms; it
can specifically neutralize the most potent oxidative free radicals
(•OH and ONOO−) and it can also attenuate the
superoxidant anion level in certain pathophysiological conditions
(8). Moreover, it is easy for
H2 to pass through membrane structures such as cell
membranes and the mitochondrial membrane, where it can neutralize
intracellular ROS, thereby maintaining normal mitochondrial
function and preventing apoptosis (9). Numerous studies have shown that
H2 or hydrogen-containing solution can alleviate
ischemia-reperfusion injury (10–12) of the heart (13), brain (14,15), kidney, small intestine, and liver
(16); it can also antagonize
irradiation-induced cell injury (17). Inhalation of H2 also
slows down the growth of atherosclerotic plaque in
apoE−/− mice (18).
Our previous studies also found that hydrogen-rich saline prevented
neointima formation following carotid balloon injury (19). Oxidative stress plays an important
role in ischemia-reperfusion injury, irradiation-induced injury,
atherosclerosis and neointima formation (17). The ability of H2 to
antagonize these pathological reactions is closely related to the
anti-ROS effects of H2. However, whether H2
can ameliorate AGE-induced ROS generation and apoptosis of ECs has
yet to be elucidated.
The purpose of the present study was to determine
whether use of hydrogen-rich medium (HRM) can protect the ECs from
AGE-induced apoptosis, by detecting ROS production and
antioxidant-related gene expression.
Materials and methods
Animals
This study used 2–3-year-old Sprague-Dawley rats
purchased from the Experimental Animal Center of the Third Military
Medical University, Chongqing, China. All protocols conformed to
the Guide for the Care and Use of Laboratory Animals published by
the US National Institutes of Health (NIH Publication no. 85-23,
revised 1996).
Preparation of HRM
HRM was prepared as previously described (18). Briefly, H2 was
dissolved in low-glucose Dulbecco’s modified Eagle’s medium
(DMEM-L; Hyclone, Logan, UT, USA) supplemented with 20% fetal
bovine serum (FBS) for 6 h under high pressure (0.4 Mpa). The
saturated HRM was stored at 4°C under atmospheric pressure in an
aluminum bag with no dead volume. To ensure an H2
concentration of >0.6 mmol/l, the medium was freshly prepared
every week. H2 concentration in the prepared medium was
confirmed with gas chromatography as previously described (8).
Primary cell culture
Rat aortic ECs were isolated and cultured as
previously described (20). In
brief, the thoracic aorta was removed from the Sprague-Dawley rats
and placed into a 100-mm culture dish (Corning, Inc., Corning, NY,
USA) filled with serum-free DMEM-L (Hyclone) on ice. The adipose
tissue and adventitia of the aorta were removed. The aorta was
placed intimal side down on a sterile plate containing 0.2%
collagenase type I (Sigma, St. Louis, MO, USA) and incubated at
37°C for 30 min. Detached ECs were collected, cultured in DMEM-L
supplemented with 20% FBS (Gibco, USA), 100 U/ml penicillin, 100
μg/ml streptomycin and 75 μg/ml EC growth supplement
(Sigma) and placed in a 50 ml culture flask (Corning, Inc.). ECs at
passages 3–5 were used in the experiments. The purity of ECs was
evaluated by detection of von Willebrand factor (vWF) expression by
fluorescence immunocytochemistry using anti-vWF antibody (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). More than 95% of
ECs in the culture were vWF positive. In the experiments, cells
were incubated with various concentrations of AGEs (Jingmei Biotech
Co., Ltd, China) for 24 h.
Apoptosis assays
For Annexin V and 7-aminoactinomycin D (7-AAD)
assays, Annexin V binding and 7-AAD staining were carried out using
the Annexin V-APC/7-AAD Apoptosis Detection Kit (Keygen
Biotechnology, Nanjing, China). ECs following treatment were
trypsinized, washed twice and resuspended in binding buffer to a
concentration of 1×106 cells/ml. A volume of 500
μl of cells was mixed with 5 μl of Annexin V-APC and
7-AAD for 10 min at room temperature in the dark. After adding
binding buffer (400 μl), cells were analyzed by flow
cytometry (FACSCalibur; BD Biosciences). For terminal uridine
deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)
staining, cells were plated on cover-glasses for 16 h and fixed and
were then incubated with 0.1% Triton X-100 for 2 min and washed
twice with PBS. TUNEL staining was performed as previously
described (21).
Detection of ROS
DCF-DA (Sigma) was used as a fluorescent probe to
detect intracellular ROS, and flow cytometry was used to determine
the fluorescence intensity of cells. The spontaneous fluorescence
intensity of the negative control tube without DCF-DA was defined
as 1, and the fluorescence intensity values of other groups were
the values relative to that of the negative control (detected
fluorescence intensity/the fluorescence intensity of the negative
control). The treated cells in each group were collected and 5 ml
serum-free medium and 10 mM DCF-DA were added into a 50 ml culture
flask. The cells were incubated with 5% CO2 at 37°C for
45 min; the cells were then washed with PBS and digested with 0.25%
trypsin. After the digestion was terminated, the cells were washed
with PBS twice and were resuspended in 2 ml serum-free medium. In
the positive control group, ROS inducer Rosup (50 μg/ml) was
added together with DCF-DA, and the cells were incubated at room
temperature for 1 h prior to fluorescent detection. The fluorescent
intensity of the sample tubes represents the amount of
intracellular ROS.
Detection of antioxidative enzymes
Levels of superoxide dismutase (SOD) and glutathione
peroxidase (GSH-PX) expression were analyzed by real-time PCR
analysis. Total RNA was purified from cultured ECs with TRIzol
(Invitrogen, USA) according to the manufacturer’s protocol. Total
RNA was reverse-transcribed into cDNA, and the cDNA product was
amplified by SYBR-Green I fluorescence real-time PCR. The PCR
reaction [containing 12.5 μl RNase-free distilled water, 10
μl SYBR-Green I master mix (Toyobo Co., Ltd., Osaka, Japan),
0.75 μl of 5 μM forward and reverse primer, and 1
μl cDNA] was directly monitored by the Bioer FQD-66A
detection system. The primers used were: rat SOD, forward,
5′-CCACTGCAGGACCTCATTTT-3′ and reverse, 5′-CACCTTTGCCCAAGTCATCT-3′;
rat GSH-PX, forward, 5′-GTCCACCGTGTATGCCTTCT-3′ and reverse,
5′-CATTCACCTCGCACTTCTCA-3′; GAPDH, forward,
5′-ATTGTCAGCAATGCATCCTGCA-3′ and reverse,
5′-AGACAACCTGGTCCTCAGTGTA-3′. After an initial denaturation step at
95°C for 15 min, temperature cycling was initiated. Each cycle
consisted of denaturation at 94°C for 3 min, annealing at 56°C for
30 sec, and elongation at 72°C for 30 sec. A total of 40 cycles
were performed. We used GAPDH to normalize mRNA. Relative
quantification of mRNA expression levels was determined using the
relative standard curve method according to the manufacturer’s
instructions.
Activity of SOD and GSH-PX was assessed by
spectrophotometry analysis using commercial kits: superoxide
dismutase enzymatic activity assay kit, cat. no. A001-1 (Jiancheng
Biotech, Nanjing, China); GSH-PX enzymatic activity assay kit, cat.
no. A005 (Jiancheng Biotech). These two kits detected the remaining
substrate quantity following SOD or GSH-PX enzymatic activity. An
increase in optical density indicates a reduction of enzymatic
activity. Optical density values were measured at emission
wavelengths of 550 nm for SOD or 412 nm for GSH-PX.
Western blot analysis
The expressions of Bcl-2 and Bax were analyzed by
western blotting. Briefly, total cell lysate was separated by
SDS-PAGE (10% resolving gel) and transferred to a polyvinylidine
fluoride (PVDF) membrane (Roche, Basel, Switzerland) by
electroblotting for 2 h at 100 mA. The membrane was immunoblotted
with antibodies against Bcl-2 or Bax (both from Santa Cruz
Biotechnology, Inc.) at 4°C overnight. Immunoreactivity was
detected using the enhanced chemiluminescence reaction system
(Amersham Pharmacia Biotech, Piscataway, NJ, USA) according to the
manufacturer’s instructions. GAPDH was used as a loading control.
The expression of each protein was quantified by scanning
densitometry and normalized against GAPDH. Data were expressed as a
relative optical density value.
Statistical analysis
Data are presented as the means ± SEM. SPSS v13.0
software (SPSS, Inc., Chicago, IL, USA) was used for statistical
analysis. Differences among groups were evaluated by the unpaired
Student’s t-test or one-way ANOVA followed by a post hoc test.
Values of P<0.05 were considered to indicate statistically
significant differences.
Results
HRM protects AGE-induced endothelial
apoptosis
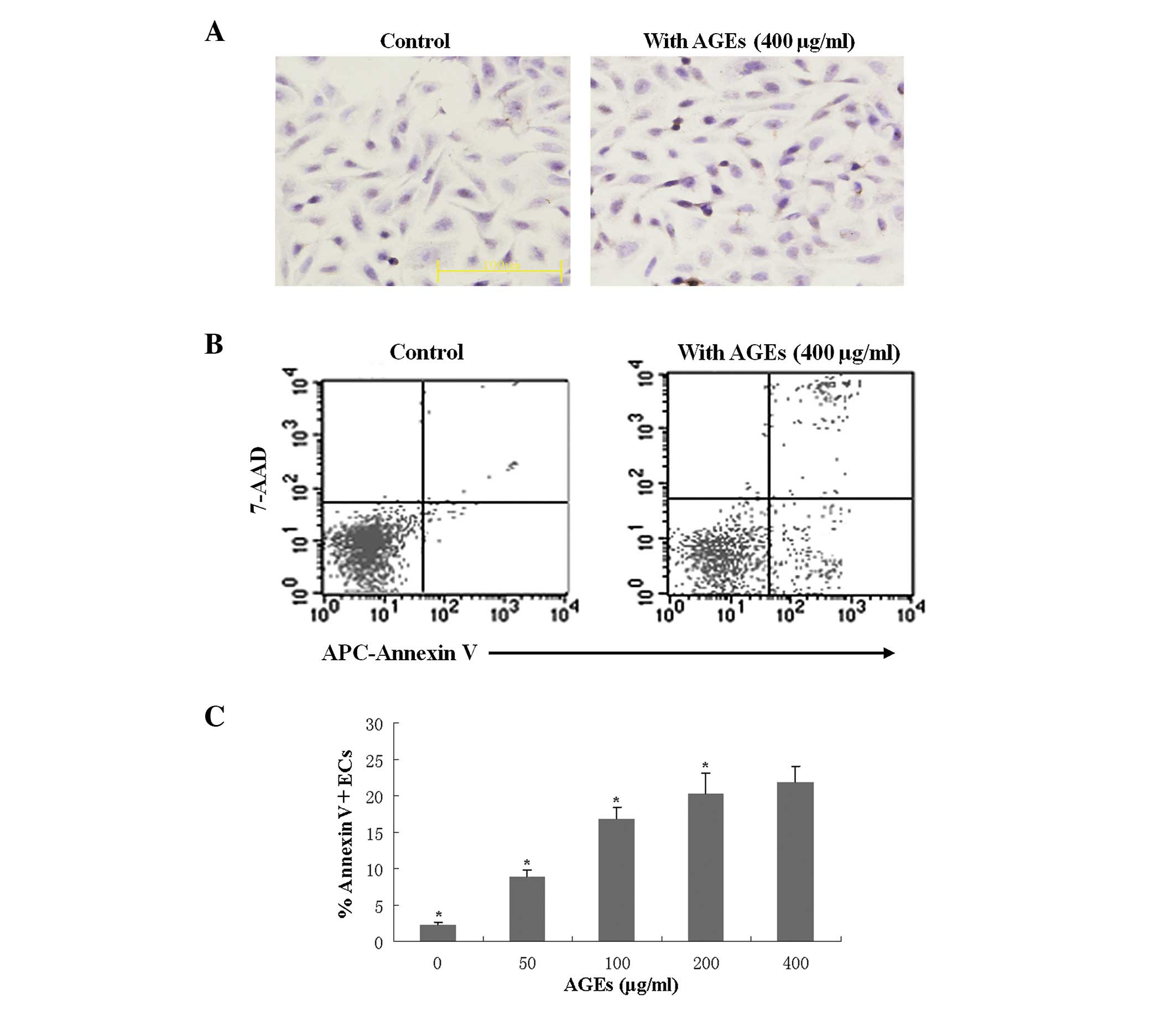
To detect the EC apoptosis induced by the presence
of AGEs, cultured ECs were treated with AGEs and cultured in the
normal or HRM-containing media prior to TUNEL staining. TUNEL
staining showed that AGEs (400 μg/ml) induced apoptosis of
ECs (Fig. 1A, right panel). In
addition, flow cytometry analysis revealed that AGEs induced EC
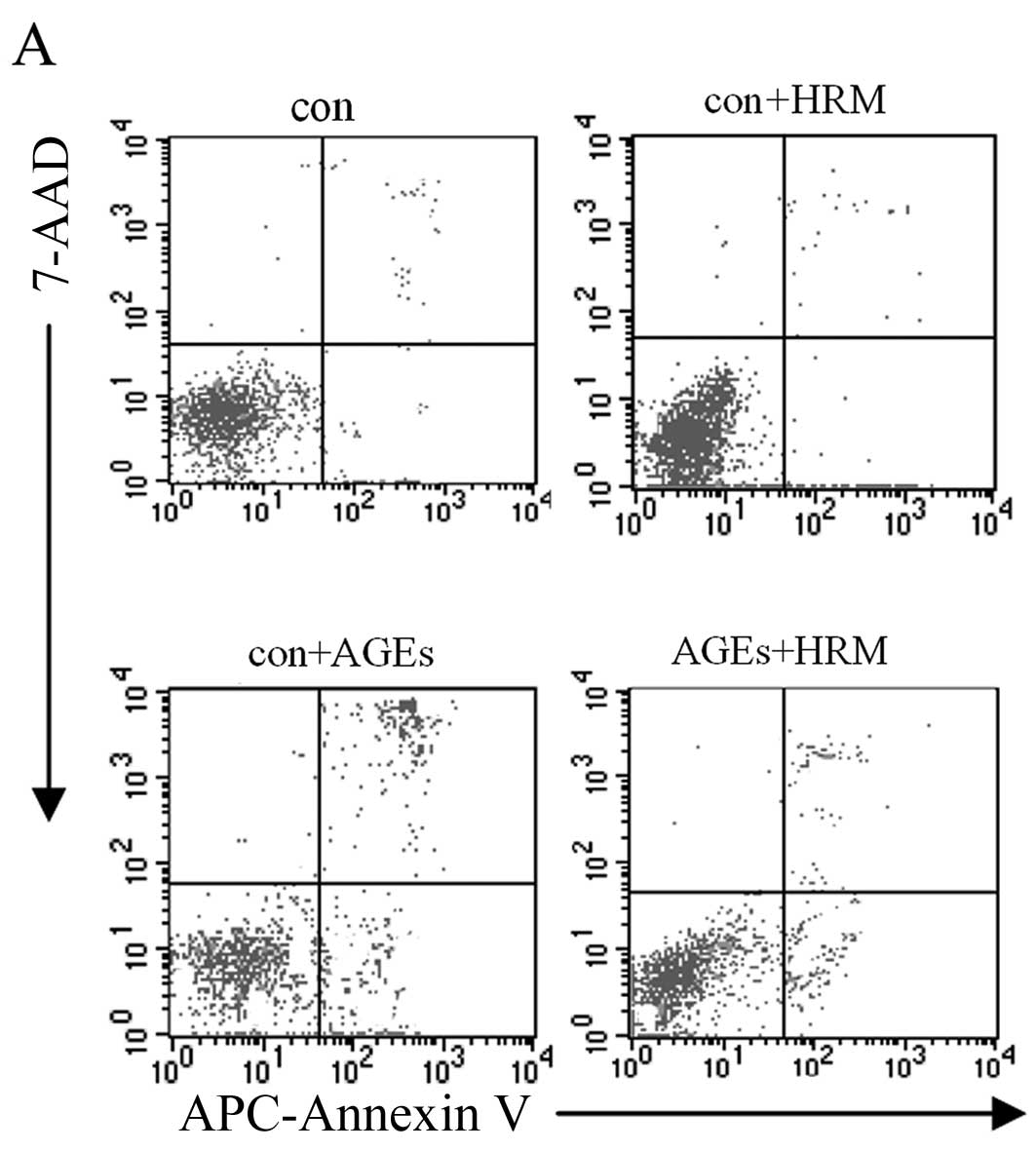
apoptosis in a concentration-dependent manner (Fig. 1B and C). After 24 h, evaluation of
the 4 EC groups, including the AGEs (400 μg/ml) group, HRM
group, HRM + AGEs group, and normal control group, revealed that
the cell apoptosis rate detected by flow cytometry was not
significantly different between the HRM group and the control group
when AGEs were absent. However, the presence of AGEs increased the
rate of EC apoptosis, and the addition of HRM reduced the apoptosis
rate of AGE-treated ECs from 21.61±2.52 to 11.32±1.75% (Fig. 2).
HRM reduces AGE-induced ROS generation in
ECs
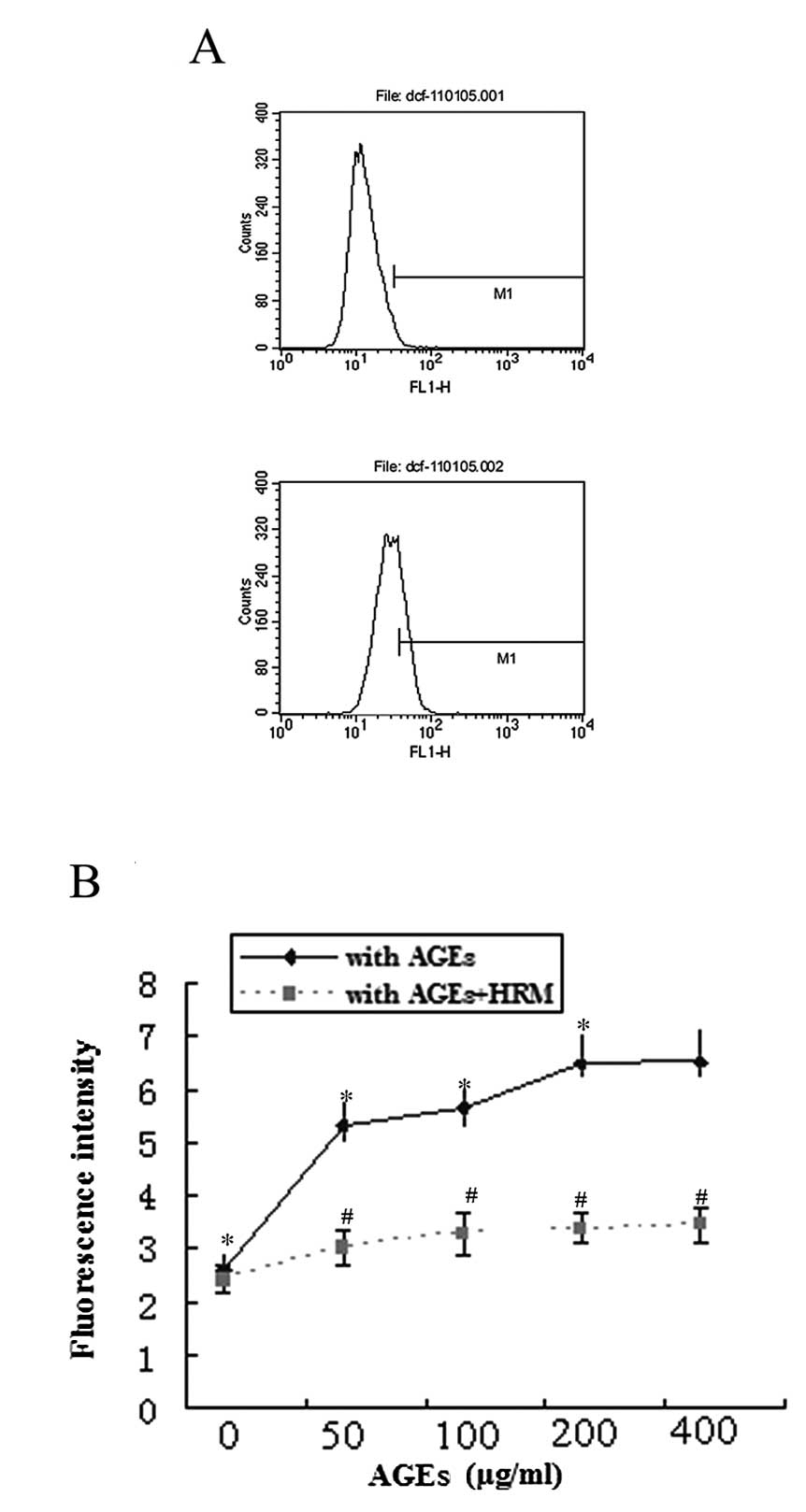
The production of intracellular ROS was measured by
detection of DCF-DA by flow cytometry analysis. Treatment of ECs
with AGEs significantly increased the generation of intracellular
ROS in a dose-dependent manner (Fig.
3A). However, HRM significantly decreased AGE-induced
intracellular ROS generation (Fig.
3B).
Effects of HRM on antioxidative enzymes
in ECs exposed to AGEs
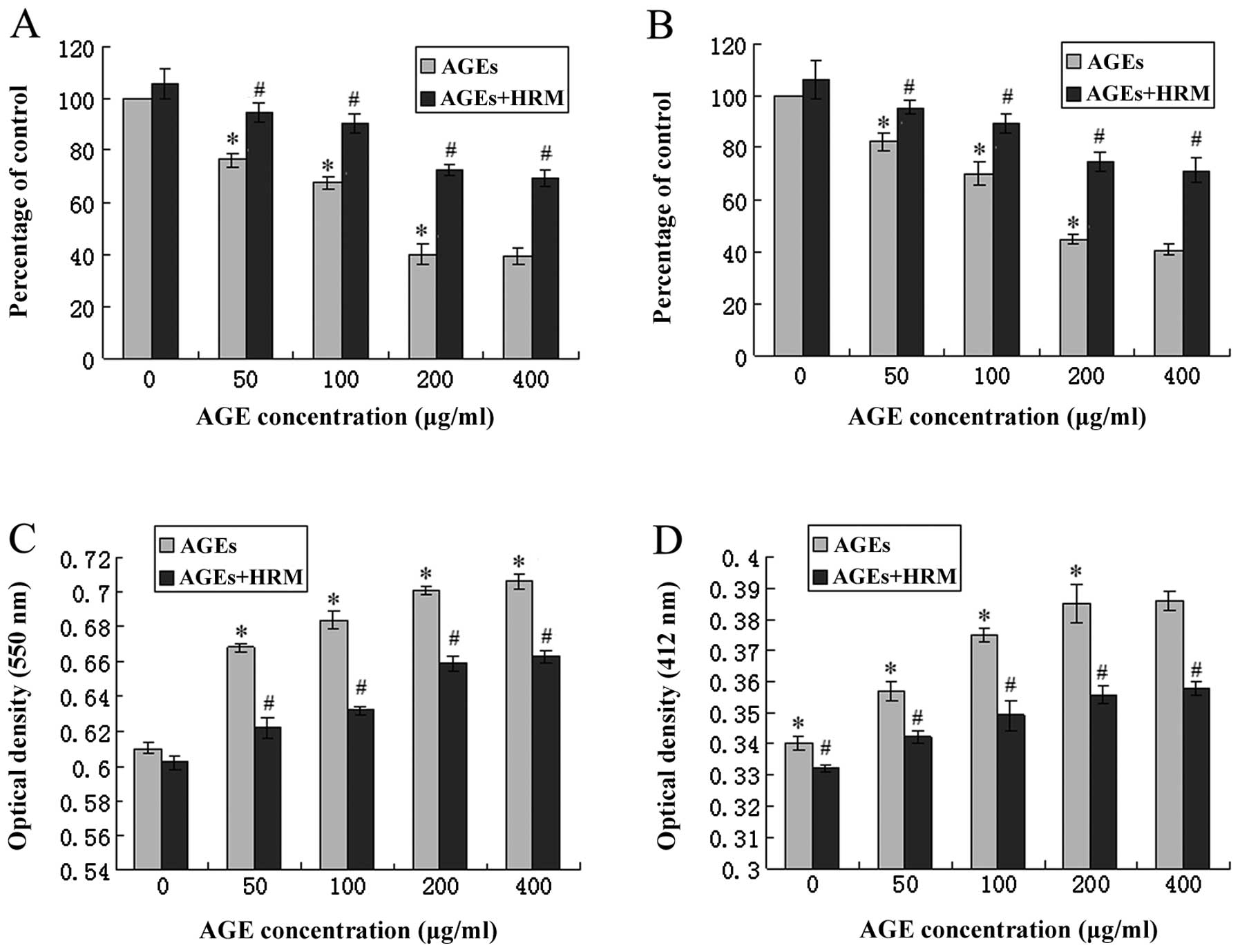
Co-culture with HRM and AGEs significantly reduced
the level of SOD mRNA, and 50, 100, 200 and 400 μg/ml AGEs
decreased the endothelial SOD mRNA levels to 76.34, 67.52, 40.03
and 39.15% of the baseline levels, respectively. However, following
HRM intervention, the SOD expression reduction caused by AGEs was
significantly attenuated (Fig.
4A). In addition, the enzyme activities of SOD and GSH-PX were
determined by enzymatic activity assay, in which the optical
density increases as the enzyme activity levels decrease. AGE
treatment significantly increased the optical density of
antioxidative enzymes. HRM treatment was also able to partly
obstruct this effect (Fig. 4C and
D).
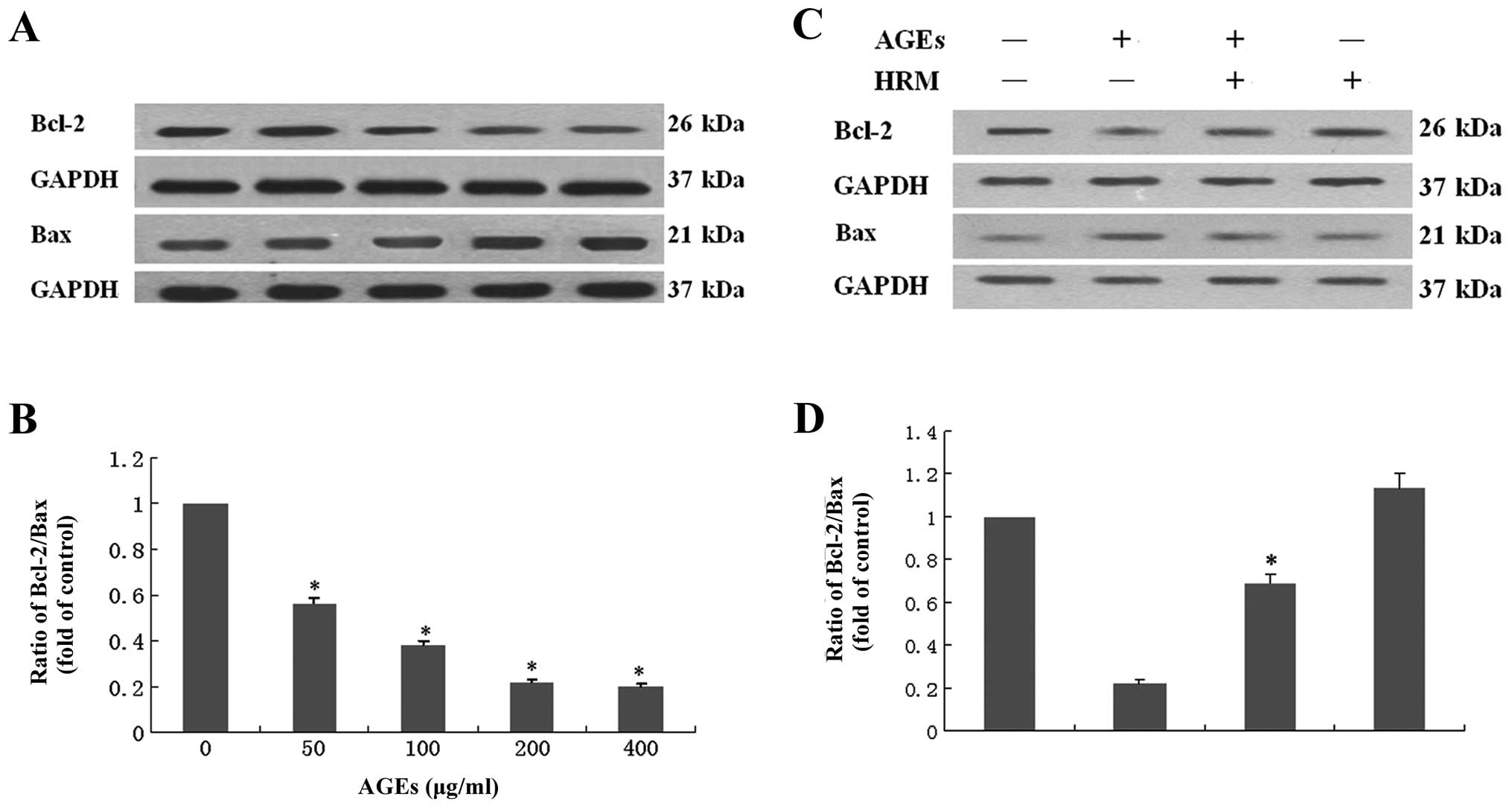
Effects of HRM on the ratio of Bcl-2/Bax
in ECs exposed to AGEs
Examination of the anti-apoptotic protein Bcl-2 and
the pro-apoptotic protein Bax in ECs revealed that ECs co-cultured
with AGEs showed decreased Bcl-2 protein levels and increased Bax
protein expression. The ratio of Bcl-2/Bax was decreased by ∼50%
following exposure to 400 μg/ml AGEs (Fig. 5A and B), indicating that EC
apoptosis induced by AGEs occurs partly through the mitochondrial
apoptotic pathway. HRM was shown to attenuate AGE-induced decrease
in the ratio of Bcl-2/Bax (Fig. 5C
and D).
Discussion
To the best of our knowledge, this study is the
first to report that HRM reduces AGE-induced apoptosis in ECs, and
this reduction in EC apoptosis was associated with the reduction of
ROS and increase of the Bcl-2/Bax ratio. Evidence from the present
study as well as from our previous studies (6,22)
support this conclusion. Although HRM has no significant protective
effects against endothelial apoptosis under normal culture
conditions, it can significantly reduce EC apoptosis induced by
large doses of AGEs. AGEs increase intracellular ROS in a
concentration-dependent manner, and HRM is able to reduce ROS
increases induced by different concentrations of AGEs (6,22).
AGEs also reduce expression of SOD and GSH-PX in ECs, which is
important as these enzymes have been shown to play a role in
antagonizing ROS (23,24). SOD and GSH-PX activity is reduced
by AGEs in a concentration-dependent manner, and HRM intervention
is shown to partially antagonize AGE-induced reduction of
antioxidant enzyme expression and activity. Additionally, AGEs can
reduce the expression of anti-apoptotic protein Bcl-2 and increase
the expression of pro-apoptotic protein Bax, thereby reducing the
Bcl-2/Bax ratio. HRM was also able to ameliorate this activity,
which partially reverses the AGE-induced reduction in the Bcl-2/Bax
ratio.
A high glucose environment can lead to excessive
production of AGEs in the body. It has been reported that AGEs can
significantly increase the ROS content in ECs and promote
apoptosis, and EC injury is closely associated with atherosclerosis
(4). Results of our previous
study showed that the receptor for AGEs (RAGE) is key to the
inflammatory process and endothelial activation, making it likely
to accelerate atherosclerosis, particularly in diabetes patients
(6). Results of that study also
showed that high concentrations of C-reactive protein (CRP) may
decrease the antioxidant defenses of endothelial progenitor cells
(EPCs) by upregulating RAGE, promoting EPC sensitivity toward
apoptosis mediated by oxidative stress (6). Oxidative stress and EC injury have
been extensively investigated (4,6,22,25), and ROS play an important role in
AGE-induced cell injury (25).
The RAGE activation mechanism was shown to decrease antioxidative
enzyme activities, not only through increasing ROS production, but
also by downregulating antioxidative enzyme mRNA expression
(6). In the present study, we
found that AGEs can lead to increased ROS production in rat ECs,
resulting in apoptosis. Therefore, inhibiting the increase of
AGE-caused ROS generation may be an effective method to mitigate EC
injury.
Several studies have demonstrated the protective
effects of H2 by using HRM to elevate cellular
antioxidative defense mechanisms (8,13,16,18,26–31). Yu et al (28) identified the protective effects of
HRM on human epidermal fibroblasts under oxidative stress caused by
diabetes. The investigators suggested that H2 was a
potential protective antioxidant for both preventive and
therapeutic applications through its reduction of the hydroxyl
radical, which is the most cytotoxic of ROS (8). Similar protective effects were
demonstrated in liver injury models as H2 selectively
reduced the strongest oxidants (•OH and ONOO−) without
interfering with metabolic oxidation-reduction reactions or ROS
cell signaling (16).
Hydrogen-rich PBS exhibited protective effects against
radiation-induced cellular damage, suggesting that H2
may be an effective radioprotective agent (27). H2 also has protective
effects against myocardial ischemia and reperfusion (30). Additionally, hydrogen-rich saline
protected the myocardium against ischemia/reperfusion in a rat
model, resulting in improved left ventrical systolic and diastolic
pressure (13). Chronic
hydrogen-rich saline treatment was also shown to reduce left
ventricular hypertrophy caused by hypertension by ablating
oxidative stress, suppressing inflammation and preserving
mitochondrial function (29).
H2 has also been shown to reduce the plasma glucose
levels of diabetic patients (26), alleviate atherosclerosis in
apolipoprotein E knockout mice (18), and provide protective effects
against high-fat, diet-induced atherosclerosis (31). Moreover, to date, no significant
side-effects or toxic effects of H2 have been found.
H2 reacts with •OH, yielding water; H2 has a
low molecular weight so that it easily enters intracellular
structures where it plays an active role (32).
The present study adopted HRM to observe the
protective effects of H2 on AGE-treated ECs. We showed
that increasing H2 levels through the use of HRM
alleviated AGE-induced oxidative injury of ECs. HRM attenuated ROS
increases in ECs following AGE treatment, increased SOD and GSH-PX
expression, reduced apoptosis, and simultaneously elevated the
Bcl-2/Bax ratio. Briefly, our results showed that H2 can
significantly reduce intracellular ROS levels and AGE-induced
apoptosis, indicating that H2 may reduce AGE-induced
cell apoptosis and protect ECs through mitigating cellular
oxidative stress. This is in accordance with the results of a
previous report, which showed that AGEs can reduce the generation
of antioxidant enzymes in the cells, and destroy the balance of
cellular oxidation within the antioxidant system, which affects
endothelial function (33). The
expression of antioxidant enzymes, SOD and GSH-PX, protects against
glucose-induced oxidative stress in vascular contractile cells and
AGE-induced endothelial injury (34). As an essential active reductase in
cell metabolism, the enzyme SOD plays an important role in
preventing organisms from oxidative damage, and GSH-PX clears lipid
peroxides induced by ROS and •OH, protecting the integrity of cell
membrane structure and function (28). Liu et al (12) also showed that hydrogen-rich
saline markedly increased the activities of antioxidant enzymes SOD
and GSH in a rat model of liver damage. In the present study, HRM
significantly reduced the degree to which SOD and GSH-PX were
inactivated in cells treated with AGEs. We found that the
application of H2 significantly precluded AGE-induced
decline in the levels of those two enzymes in ECs, indicating that
H2 protects the EC antioxidant system from AGE-induced
injury and stabilizes cell function.
AGE-induced apoptosis in ECs has been shown to be
related to decreased Bcl-2/Bax ratio (35). The Bcl-2 protein exerts
anti-apoptotic effects through antioxidant activity or inhibition
of ROS generation (36). By
contrast, the Bax gene plays an inductive role, promoting apoptosis
through antagonizing the Bcl-2 gene (23). A previous study by Oltvai et
al (24) suggested that the
ratio of Bcl-2 to Bax determines apoptosis or inhibition of
apoptosis. In the present study, we examined the anti-apoptotic
protein Bcl-2 and the pro-apoptotic protein Bax in ECs, finding
that ECs co-cultured with AGEs had decreased Bcl-2 protein levels
and increased Bax protein expression. The ECs cultured with AGEs in
HRM had significantly decreased apoptosis levels, while the
Bcl-2/Bax ratio was elevated. We suggest that it is possible that
the H2 protective mechanism is to upregulate Bcl-2/Bax,
thereby reducing EC apoptosis induced by AGEs and protecting EC
function.
In conclusion, H2 has significant
protective effects against AGE-caused EC injury. The mechanism of
its protective effects may be to reduce ROS generation, protect the
intracellular antioxidant enzyme system, and elevate the Bcl-2/Bax
ratio. Further studies are required to explore the specific
mechanism of the protective effects of H2 against
AGE-induced EC injury.
Acknowledgements
This study was supported in part by
the National Natural Science Foundation of China (Grants 30872710,
30770852).
References
|
1
|
Faria AM, Papadimitriou A, Silva KC, Lopes
de Faria JM and Lopes de Faria JB: Uncoupling endothelial nitric
oxide synthase is ameliorated by green tea in experimental diabetes
by re-establishing tetrahydrobiopterin levels. Diabetes.
61:1838–1847. 2012. View Article : Google Scholar
|
|
2
|
Caporali A, Pani E, Horrevoets AJ, et al:
Neurotrophin p75 receptor (p75NTR) promotes endothelial cell
apoptosis and inhibits angiogenesis: implications for
diabetes-induced impaired neovascularization in ischemic limb
muscles. Circ Res. 103:e15–e26. 2008. View Article : Google Scholar
|
|
3
|
Csiszar A and Ungvari Z: Endothelial
dysfunction and vascular inflammation in type 2 diabetes:
interaction of AGE/RAGE and TNF-alpha signaling. Am J Physiol Heart
Circ Physiol. 295:H475–H476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiang M, Yang M, Zhou C, Liu J, Li W and
Qian Z: Crocetin prevents AGEs-induced vascular endothelial cell
apoptosis. Pharmacol Res. 54:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Huang L, Song M, Yu S, Gao P and
Jing J: C-reactive protein upregulates receptor for advanced
glycation end products expression and alters antioxidant defenses
in rat endothelial progenitor cells. J Cardiovasc Pharmacol.
53:359–367. 2009. View Article : Google Scholar
|
|
7
|
Tan AL, Forbes JM and Cooper ME: AGE,
RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 27:130–143.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohsawa I, Ishikawa M, Takahashi K, et al:
Hydrogen acts as a therapeutic antioxidant by selectively reducing
cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007. View Article : Google Scholar
|
|
9
|
Xie K, Yu Y, Huang Y, et al: Molecular
hydrogen ameliorates lipopolysaccharide-induced acute lung injury
in mice through reducing inflammation and apoptosis. Shock.
37:548–555. 2012.PubMed/NCBI
|
|
10
|
Cai J, Kang Z, Liu K, et al:
Neuroprotective effects of hydrogen saline in neonatal
hypoxia-ischemia rat model. Brain Res. 1256:129–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Sun YP, Li Y, et al: Hydrogen-rich
saline ameliorates the severity of l-arginine-induced acute
pancreatitis in rats. Biochem Biophys Res Commun. 393:308–313.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Q, Shen WF, Sun HY, et al:
Hydrogen-rich saline protects against liver injury in rats with
obstructive jaundice. Liver Int. 30:958–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Q, Kang Z, Cai J, et al: Hydrogen-rich
saline protects myocardium against ischemia/reperfusion injury in
rats. Exp Biol Med. 234:1212–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Wang C, Zhang JH, Cai JM, Cao YP and
Sun XJ: Hydrogen-rich saline improves memory function in a rat
model of amyloid-beta-induced Alzheimer’s disease by reduction of
oxidative stress. Brain Res. 1328:152–161. 2010.PubMed/NCBI
|
|
15
|
Sun Q, Cai J, Zhou J, Tao H, Zhang JH,
Zhang W and Sun XJ: Hydrogen-rich saline reduces delayed neurologic
sequelae in experimental carbon monoxide toxicity. Crit Care Med.
39:765–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun H, Chen L, Zhou W, et al: The
protective role of hydrogen-rich saline in experimental liver
injury in mice. J Hepatol. 54:471–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terasaki Y, Ohsawa I, Terasaki M, et al:
Hydrogen therapy attenuates irradiation-induced lung damage by
reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol.
301:L415–L426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohsawa I, Nishimaki K, Yamagata K,
Ishikawa M and Ohta S: Consumption of hydrogen water prevents
atherosclerosis in apolipoprotein E knockout mice. Biochem Biophys
Res Commun. 377:1195–1198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin ZX, Yu P, Qian DH, et al:
Hydrogen-rich saline prevents neointima formation after carotid
balloon injury by suppressing ROS and the TNF-α/NF-κB pathway.
Atherosclerosis. 220:343–350. 2012.PubMed/NCBI
|
|
20
|
Wu X, Zhou Q, Huang L, Sun A, Wang K, Zou
Y and Ge J: Ageing-exaggerated proliferation of vascular smooth
muscle cells is related to attenuation of Jagged1 expression in
endothelial cells. Cardiovasc Res. 77:800–808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang X, Guo Y, Nakamura K, et al:
Nitroalkenes induce rat aortic smooth muscle cell apoptosis via
activation of caspase-dependent pathways. Biochem Biophys Res
Commun. 397:239–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Song M, Yu S, Gao P, Yu Y, Wang H
and Huang L: Advanced glycation endproducts alter functions and
promote apoptosis in endothelial progenitor cells through receptor
for advanced glycation endproducts mediate overpression of cell
oxidant stress. Mol Cell Biochem. 335:137–146. 2010. View Article : Google Scholar
|
|
23
|
Marsh SA, Laursen PB, Pat BK, Gobe GC and
Coombes JS: Bcl-2 in endothelial cells is increased by vitamin E
and alpha-lipoic acid supplementation but not exercise training. J
Mol Cell Cardiol. 38:445–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jandeleit-Dahm K, Watson A and
Soro-Paavonen A: The AGE/RAGE axis in diabetes-accelerated
atherosclerosis. Clin Exp Pharmacol Physiol. 35:329–334. 2008.
View Article : Google Scholar
|
|
26
|
Kajiyama S, Hasegawa G, Asano M, et al:
Supplementation of hydrogen-rich water improves lipid and glucose
metabolism in patients with type 2 diabetes or impaired glucose
tolerance. Nutr Res. 28:137–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian L, Li B, Cao F, Huang Y, Liu S, Cai J
and Gao F: Hydrogen-rich PBS protects cultured human cells from
ionizing radiation-induced cellular damage. Nucl Technol Radiat
Prot. 25:23–29. 2010. View Article : Google Scholar
|
|
28
|
Yu P, Wang Z, Sun X, Chen X, Zeng S, Chen
L and Li S: Hydrogen-rich medium protects human skin fibroblasts
from high glucose or mannitol induced oxidative damage. Biochem
Biophys Res Commun. 409:350–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Ys and Zheng H: Chronic hydrogen-rich
saline treatment reduces oxidative stress and attenuates left
ventricular hypertrophy in spontaneous hypertensive rats. Mol Cell
Biochem. 365:233–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Sun Q, He B, Xiao J, Wang Z and
Sun X: Anti-inflammatory effect of hydrogen-rich saline in a rat
model of regional myocardial ischemia and reperfusion. Int J
Cardiol. 148:91–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zong C, Song G, Yao S, et al:
Administration of hydrogen-saturated saline decreases plasma
low-density lipoprotein cholesterol levels and improves
high-density lipoprotein function in high-fat diet-fed hamsters.
Metabolism. 61:794–800. 2012. View Article : Google Scholar
|
|
32
|
Schoenfeld MP, Ansari RR, Zakrajsek JF, et
al: Hydrogen therapy may reduce the risks related to
radiation-induced oxidative stress in space flight. Med Hypotheses.
76:117–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hess DA and Hegele RA: Linking diabetes
with oxidative stress, adipokines, and impaired endothelial
precursor cell dunction. Can J Cardiol. 28:629–630. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharpe PC, Liu WH, Yue KK, et al:
Glucose-induced oxidative stress in vascular contractile cells:
comparison of aortic smooth muscle cells and retinal pericytes.
Diabetes. 47:801–809. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li BY, Li XL, Cai Q, et al: Induction of
lactadherin mediates the apoptosis of endothelial cells in response
to advanced glycation end products and protective effects of grape
seed procyanidin B2 and resveratrol. Apoptosis. 16:732–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deng X, Gao F and May WS Jr: Bcl2 retards
G1/S cell cycle transition by regulating intracellular ROS. Blood.
102:3179–3185. 2003. View Article : Google Scholar : PubMed/NCBI
|