Introduction
Diabetes mellitus (DM) is one of the most important
risk factors for heart failure and increased morbidity and
mortality (1). There is growing
evidence that the increased risk of heart failure may occur
independently of accelerated coronary artery disease and arterial
hypertension, suggesting that other mechanisms associated with
diabetes underlie the development of cardiomyopathy (2,3).
In addition, a number of studies have demonstrated
that hyperglycemia directly causes cardiac damage, contributing to
the development of diabetic cardiomyopathy (4,5).
However, numerous pathophysiological stimuli are involved in its
development and mediate cardiac injury, leading to systolic and
diastolic left ventricular (LV) dysfunction (6). The pathophysiology of diabetic
cardiomyopathy includes, for example, microangiopathy, endothelial
dysfunction, cardiac fibrosis and the disruption of intracellular
Ca2+ transport, all triggered by the diabetic milleu
(7–9). Moreover, structural changes in
extracellular matrix (ECM) regulation and the accumulation of
cardiac fibrosis, an intensified production of oxidative stress and
an overwhelming cardiac inflammatory immune response play a crucial
role in the pathogenesis of diabetic cardiomyopathy (6,7).
Previous studies have demonstrated the impaired LV
performance under basal conditions during the chronic stage of
streptozotocin (STZ)-induced diabetic cardiomyopathy. Furthermore,
in previous studies, we identified possible pathophysiological
mechanisms, resulting in the cardiac phenotype of diabetic
cardiomyopathy. Among others, we found that cardiac fibrosis,
endothelial dysfunction, cardiac inflammation, as well as
neurohumoral activation are greatly involved in the development of
cardiac dysfunction under those conditions (7,9–11).
The rat/mouse model of diabetic cardiomyopathy
induced by an injection of STZ is a well-established model for
investigating this condition. The cardiac phenotype under basal
conditions in the chronic stage has been sufficiently
characterized. Thus, in the current study, by time course analysis,
we investigated LV performance by in vivo pressure-volume
loops and adverse cardiac remodeling using a rat model of
STZ-induced diabetic cardiomyopathy. We measured LV function,
cardiac immune cell invasion, oxidative stress, as well as cardiac
remodeling under diabetic conditions.
Materials and methods
Animal characteristics and study
design
Six-week-old Sprague-Dawley rats (n=22) were
maintained on a 12:12 h light-dark cycle and fed with standard chow
ad libidum. In 12 rats, diabetes was induced by an
intraperitoneal (i.p.) single injection of STZ (70 mg/kg) diluted
in 0.1 M sodium citrate buffer, pH 4.5 (Sigma-Aldrich Chemie Gmbh,
Munich, Germany); the rats were then randomly divided into 2
experimental groups (STZ 2 weeks and STZ 6 weeks). Hyperglycemia
was measured 48 h later using a reflectance meter (Acutrend; Roche
Diagnostics GmbH, Mannheim, Germany). All diabetic animals
displayed a blood glucose level >550 mg/dl associated with
severe polyuria and polydipsia. For the controls, 6 vehicle-treated
animals (treated with citrate buffer only) were used. All animal
experiments were carried out in accordance with the Guide for the
Care and Use of Laboratory Animals published by the US NIH (NIH
Publication no. 85-23, revised 1996).
Surgical procedures and hemodynamic
measurements
Two (STZ 2 weeks) and 6 (STZ 6 weeks) weeks after
the STZ injection, the animals were anesthetized (pentobarbital 60
mg/kg, i.p. injection; and buprenorphine 0.1 mg/g, i.p. injection),
intubated and artificially ventilated. A 2 French micro-conductance
catheter (Aria SPR 858; Millar Instruments, Inc., Houston, TX, USA)
was positioned in the LV for the continuous registration of LV
pressure-volume (PV) loops in a closed-chest model. Calibration of
the volume signal was obtained using the hypertonic saline (10%)
wash-in technique. Indices of cardiac function were derived from PV
data obtained both at the basal steady state and during transient
preload reduction by temporary occlusion of the abdominal vena
cava. All hemodynamic measurements were performed during apnea.
After the euthanization of the mice, LV tissues were excised,
immediately snap-frozen in liquid nitrogen and stored at −80°C for
biological and immunohistological analyses.
Immunohistological measurements
The total collagen content of the Sirius red
(Polysciences, Inc., Warrington, PA, USA)-stained sections was
measured under circularly polarised light as previously described
(12). The data were then
quantified by digital image analysis as the percentage of area
fraction.
As previously described (13,14), LV tissue of the left ventricle was
embedded in Tissue-Tek (Dako) and immunohistochemistry was
performed with specific antibodies directed against CD3 (Bioss
Inc., Woburn, MA, USA), CD8a (Bioss Inc.), nitrotyrosin
(Sigma-Aldrich Chemie Gmbh), α-smooth muscle actin (SMA) (Abcam,
Cambridge, UK) and matrix metalloproteinase (MMP)-2 (Chemicon,
Temecula, CA, USA). Quantification was performed by digital image
analyses (13,14). In brief, the ratio between the
heart tissue area and the specific chromogen-positive area was
calculated (area fraction, %). The numbers of infiltrating cells
were calculated by measuring the number of cells/area of heart
tissue (cells/mm2).
Statistical analysis
Statistical analysis was performed using SPSS
version 12.0 software. Data are expressed as the means ± SEM.
Statistical differences were assessed using the Kruskal-Wallis test
in conjunction with the Mann-Whitney U post hoc test. Bonferroni
correction was applied to the post hoc Mann-Whitney U test to
adjust for multiple comparisons. Pearson’s correlation co-efficient
was used for linear regression analysis and Spearman’s correlation
co-efficient was used for non-linear correlations. Regression
analyses and curve fitting were performed to determine exact
correlations. Differences were considered statistically
significantly at a value of p<0.05.
Results
Animal characteristics and hemodynamic
measurements after the induction of diabetes
Two and 6 weeks after STZ-induced type I diabetes,
animal characteristics, systolic and diastolic LV function were
determined in all experimental groups using the conductance
catheter technique (Table I).
 | Table I.Animal characteristics and hemodynamic
results 2 and 6 weeks after the induction of diabetes. |
Table I.
Animal characteristics and hemodynamic
results 2 and 6 weeks after the induction of diabetes.
| Items | Control | STZ 2 weeks | STZ 6 weeks |
|---|
| Characteristics | | | |
| Body weight
(g) | 491±10 | 374±9a | 253±7a |
| Blood glucose
(mmol/l) | 4.1±0.3 | >30.5 | >30.5 |
| Mean blood
pressure | 113±7 | 94±8a | 73±9a,b |
| Heart weight
(mg) | 1223±16 | 1009±19a | 839±21a |
| LV weight
(mg) | 883±20 | 697±16a | 586±18a |
| Heart weight/body
weight ×10−3 | 2.49±0.06 | 2.67±0.09a | 3.31±0.07a |
| LV weight/heart
weight | 0.726±0.02 | 0.68±0.03a | 0.69±0.02a |
| Global LV
function | | | |
| Heart rate
(bpm) | 378±13 | 257±10a | 229±9a |
| LV end systolic
pressure (mmHg) | 116±4 | 97.4±6a | 76±7a,b |
| Cardiac output
(μl/min) | 95872±10723 | 50893±8983a | 53211±5213a |
| Ejection fraction
(%) | 52.7±4.8 | 45.2±5.5 | 49±3.9 |
| Systolic LV
function | | | |
| LV end systolic
volume (μl) | 256±42 | 272±52 | 268±32 |
| LV contractility
(mmHg/sec) | 7234±365 | 5671±331a | 4178±245a,b |
| LV end systolic
pressure (mmHg) | 115.3±3.9 | 94.8±4.2a | 76±6.1a,b |
| Diastolic LV
function | | | |
| LV end diastolic
volume (μl) | 489±46 | 446±61 | 477±51 |
| LV diastolic
relaxation (mmHg/sec) | −6598±195 | −3723±201a | −2874±202a,b |
| LV diastolic
relaxation time (msec) | 13.6±0.8 | 21.4±0.7a | 23.5±0.9a |
| LV end diastolic
pressure (mmHg) | 4.1±1 | 5.3±0.8 | 6.1±1 |
| LV pressure half
time (msec) | 8.2±0.3 | 12.8±0.7a | 14.2±1.1a |
| Stiffness
constant β | 0.0054±0.001 | 0.0086±0.003 |
0.0142±0.003a |
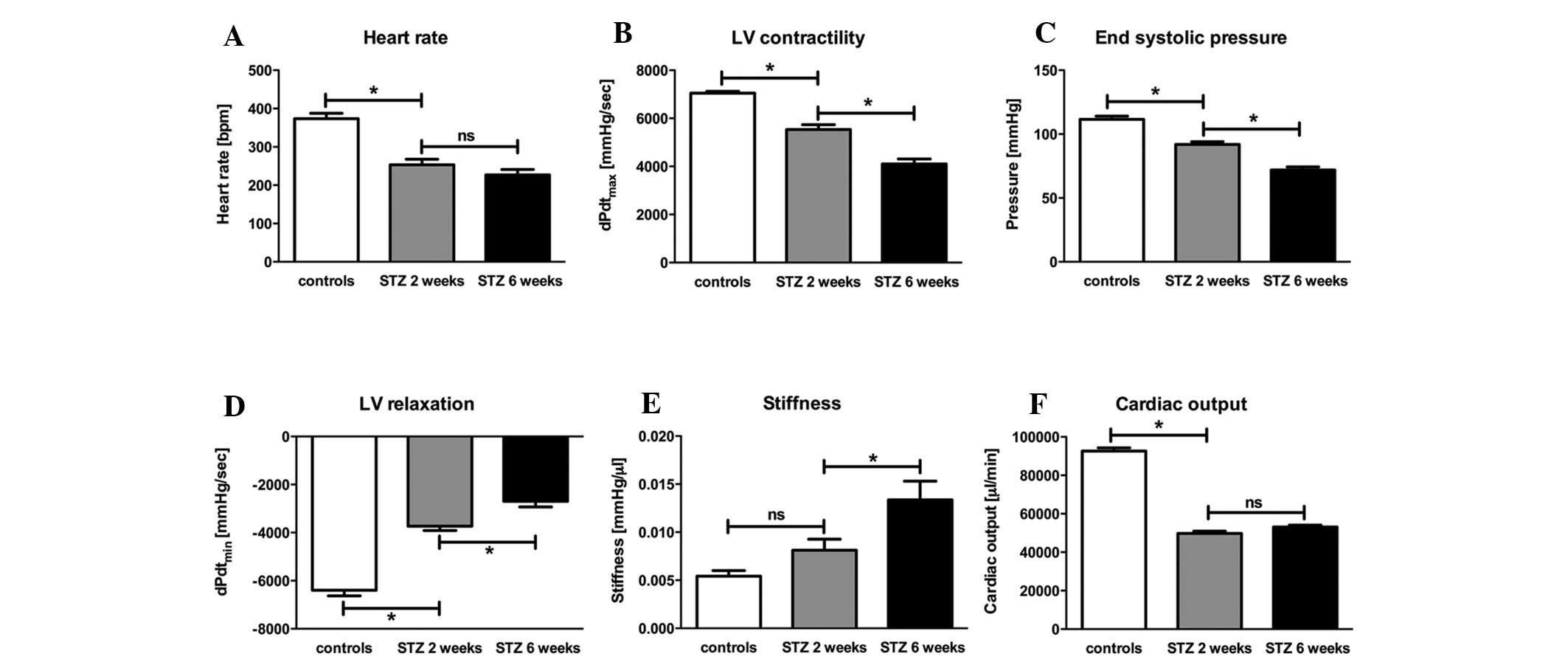
At the early time point of 2 weeks, a significant
decrease (−33%) in heart rate was observed in the diabetic animals.
At the later time point of 6 weeks, no change in heart rate was
observed (Fig. 1A). This was
associated with a reduction in diastolic LV function indicated by a
significant reduction of LV relaxation at the early time point
(−42%). Six weeks after STZ-induced diabetes, an additional
decrease in LV relaxation (−38%) was observed (Fig. 1D). In addition, STZ-induced
diabetes resulted in a significant increase (+38%) in cardiac
stiffness at the later time point (Fig. 1E). Systolic LV function, indexed
by LV contractility, displayed a significant reduction of −22% at
the early time point of the disease, leading to an additional
decrease of another −26% after 6 weeks (Fig. 1B); the end systolic pressure
exhibited the same pattern (Fig.
1C). The initial decrease (−18%) was followed by a second
significant (−26%) decrease, indicating global LV dysfunction. All
these results were associated with a significant reduction (−47%)
in cardiac output after 2 and 6 weeks (Fig. 1F).
ECM alterations and remodeling after the
induction of diabetes
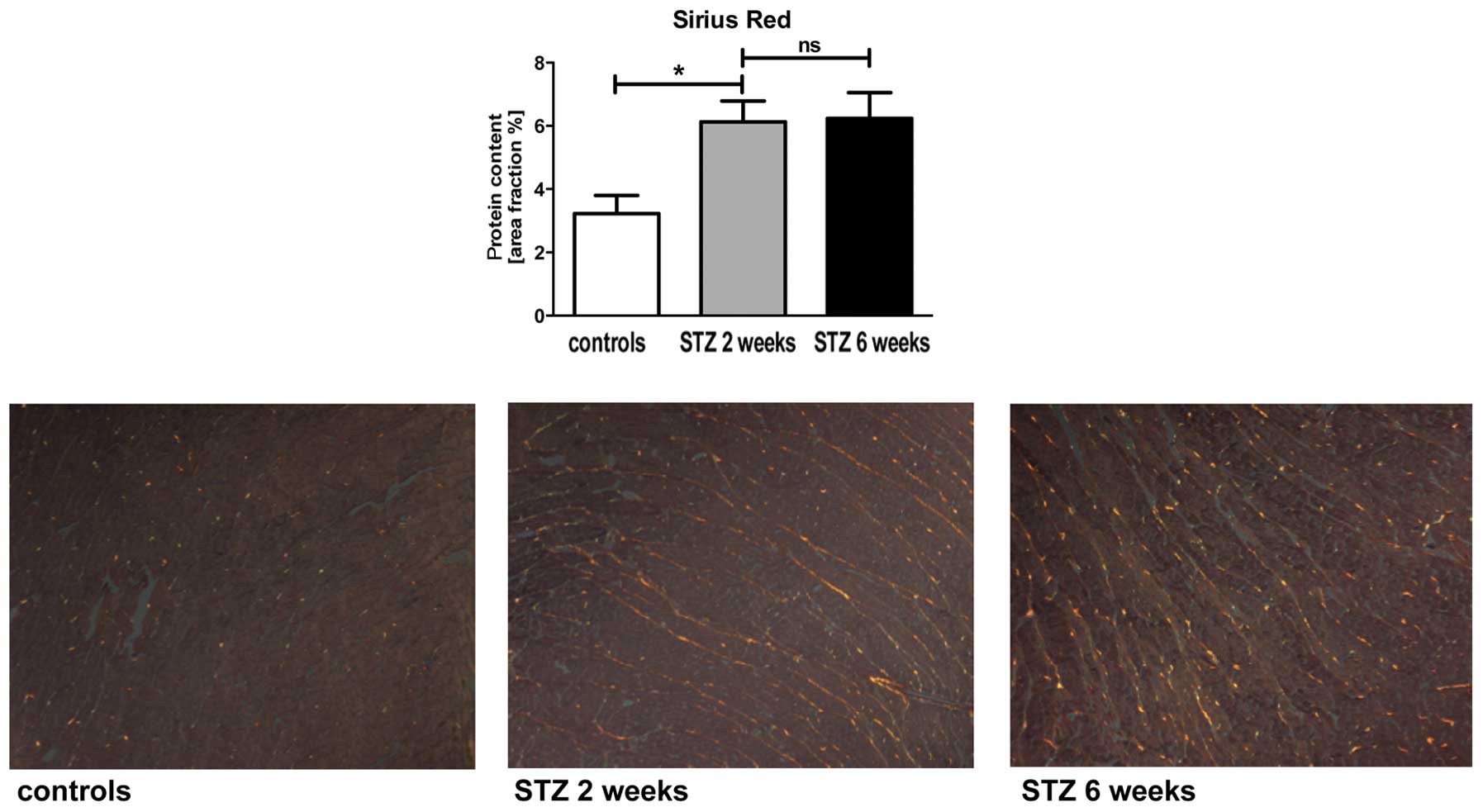
To examine the turn over of the ECM assembly,
cardiac tissue was stained by Sirius red measuring the total
content of collagen in the cardiac tissue. Moreover, α-SMA and
MMP-2 protein expression levels were measured by specific staining.
The diabetic animals showed an initial increase (1.9-fold) in total
collagen, but there was no additional increase at the later time
point (Fig. 2). By contrast, the
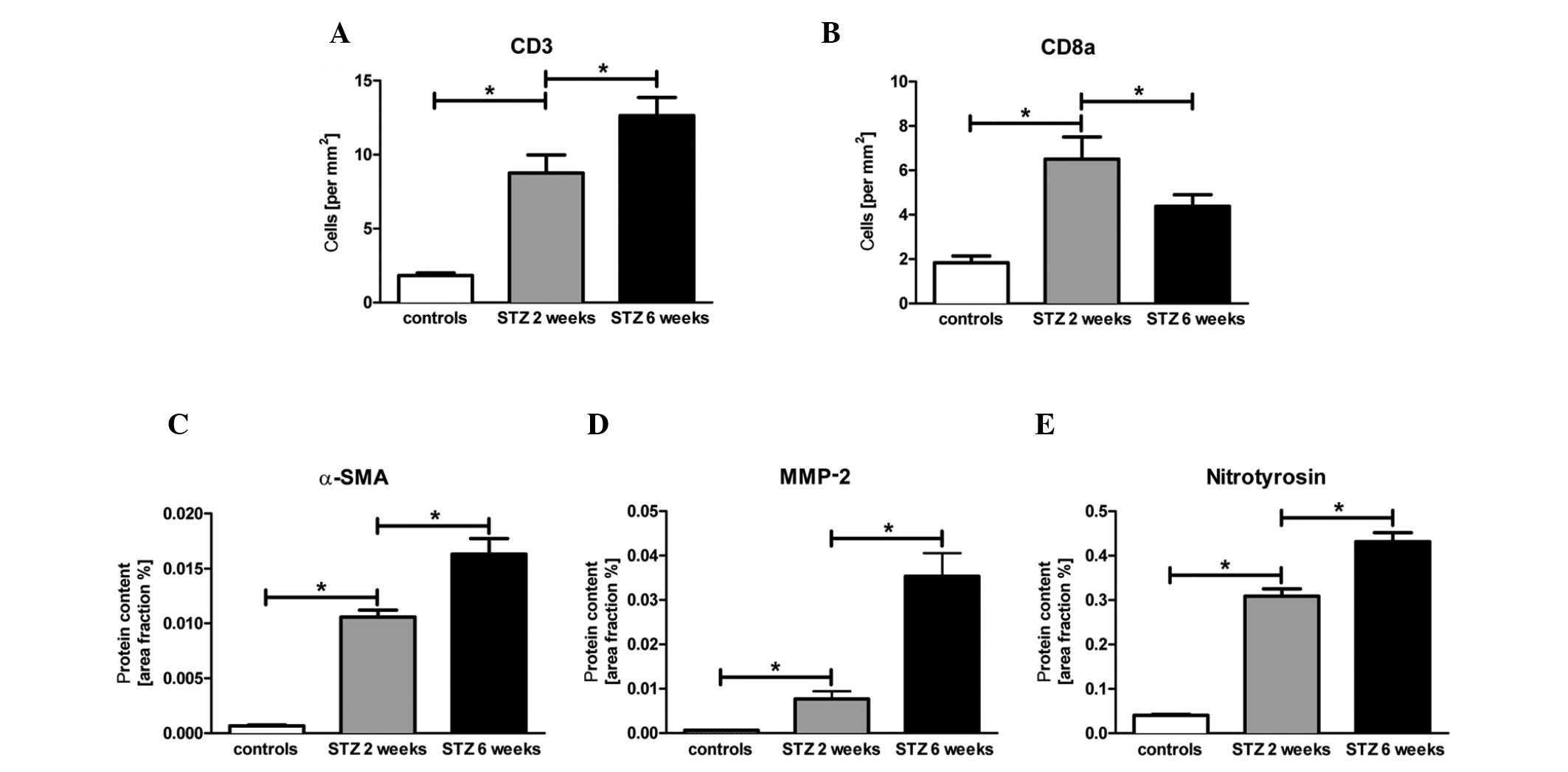
investigation of α-SMA and MMP-2 protein expression levels revealed
an initial 16.6-fold (p<0.05) and 11.6-fold (p<0.05) increase
after 2 weeks, respectively. Six weeks after the induction of
diabetes by STZ, we observed an additional 1.6-fold (p<0.05) and
5-fold (p>0.05) increase in α-SMA and MMP-2 protein expression
levels in the cardiac tissue, respectively (Fig. 3C and D).
Cardiac immune cell infiltration and
oxidative stress response after the induction of diabetes
To investigate the immune cell infiltration by T
cells, we measured the number of CD3+ and
CD8a+ immune cells in the cardiac tissue by specific
immunological staining, as well as the content of nitrotyrosin, to
evaluate oxidative stress response in the cardiac tissue. The
number of CD3+ immune cells significantly increased at 2
and 6 weeks after the induction of diabetes (Fig. 3A; p<0.05). Of note, the number
of CD8+ cells in the diabetic mice displayed an initial
3.6-fold (p<0.05) increase followed by a significant decrease
after 6 weeks (Fig. 3B). By
contrast, the level of nitrotyrosin showed a constant 7.8-fold
(p<0.05) increase followed by another 1.4-fold (p<0.05)
increase in the animals with STZ-induced diabetes compared with the
controls (Fig. 3E).
Correlations between hemodynamic and
morphological parameters after the induction of diabetes
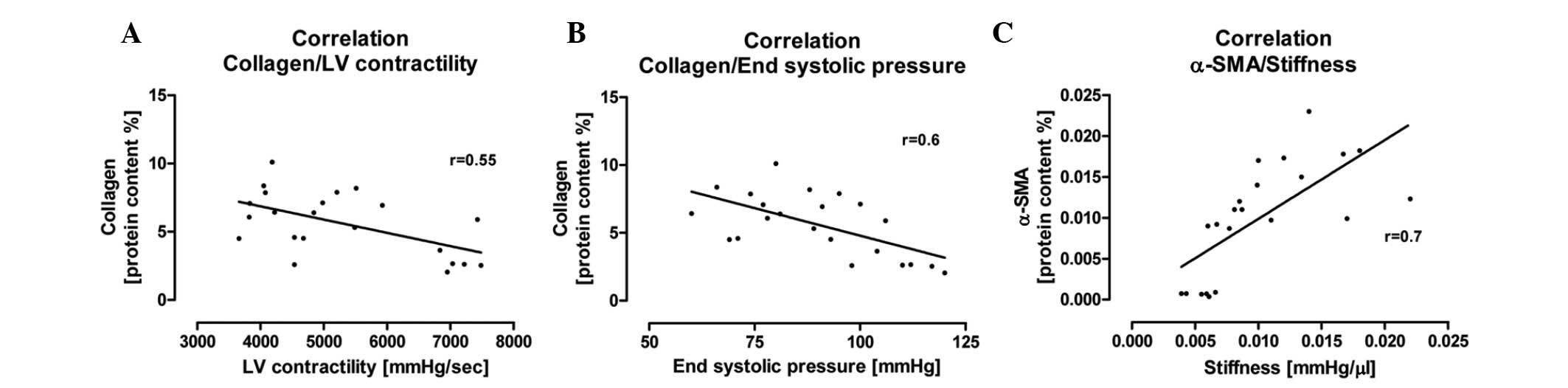
We examined the possible correlations between
hemodynamic and morphological parameters in this experimental
setting. We investigated the correlation between the total content
of collagen and systolic LV function, indexed by LV contractility
and end systolic pressure. We found that an increase in total
collagen content correlated with a significant reduction in
systolic LV function (r=0.55, r=0.6; Fig. 4A and B). Concerning the diastolic
LV function, we investigated the pathophysiological correlation
between the protein content of α-SMA and LV stiffness. We
determined a correlation between the increased number of
α-SMA-positive myofibroblasts and the increase in cardiac stiffness
(r=0.7; Fig. 4C).
Moreover, we performed correlation analyses to
determine the correlation between cardiac immune cell infiltration
and the parameters of systolic and diastolic LV function. We found
that an increased cardiac CD3+ immune cell infiltration
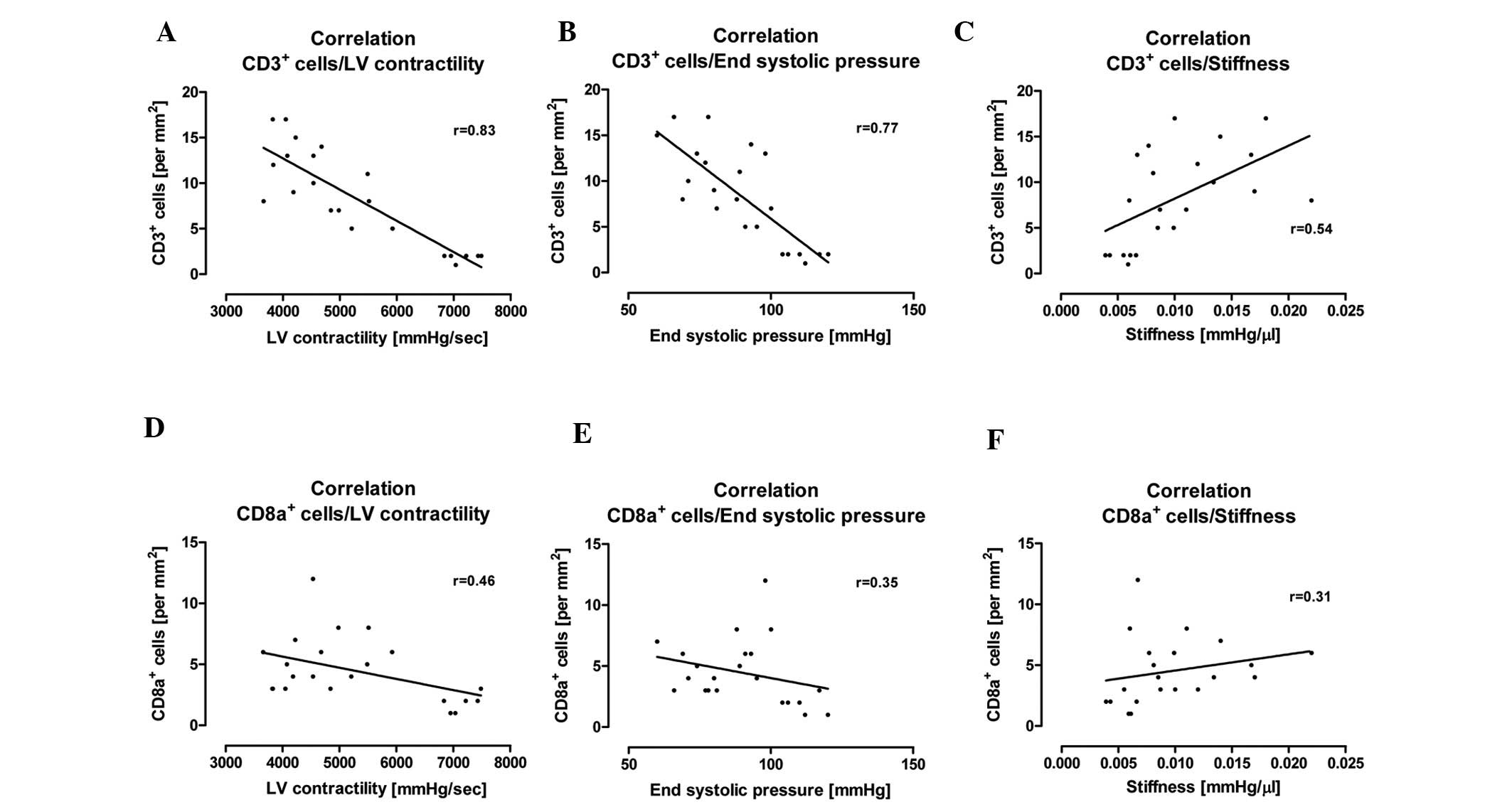
correlated with a reduction in LV contractility (r=0.83), end
systolic pressure (r=0.77) and an increase in cardiac stiffness
(r=0.54) (Fig. 5A–C). In
addition, we also investigated possible correlations between the
number of CD8a+ immune cells and these hemodynamic
parameters. It was also found that an increased number of
CD8a+ immune cells correlated with a reduction in LV
contractility (r=0.48), end systolic pressure (r=0.35) and an
increase in cardiac stiffness (r=0.31) (Fig. 5D–F). However, the correlation
between CD8a+ immune cell infiltration and hemodynamic
parameters was weaker when compared with the number of infiltrating
CD3+ immune cells and the hemodynamic parameters
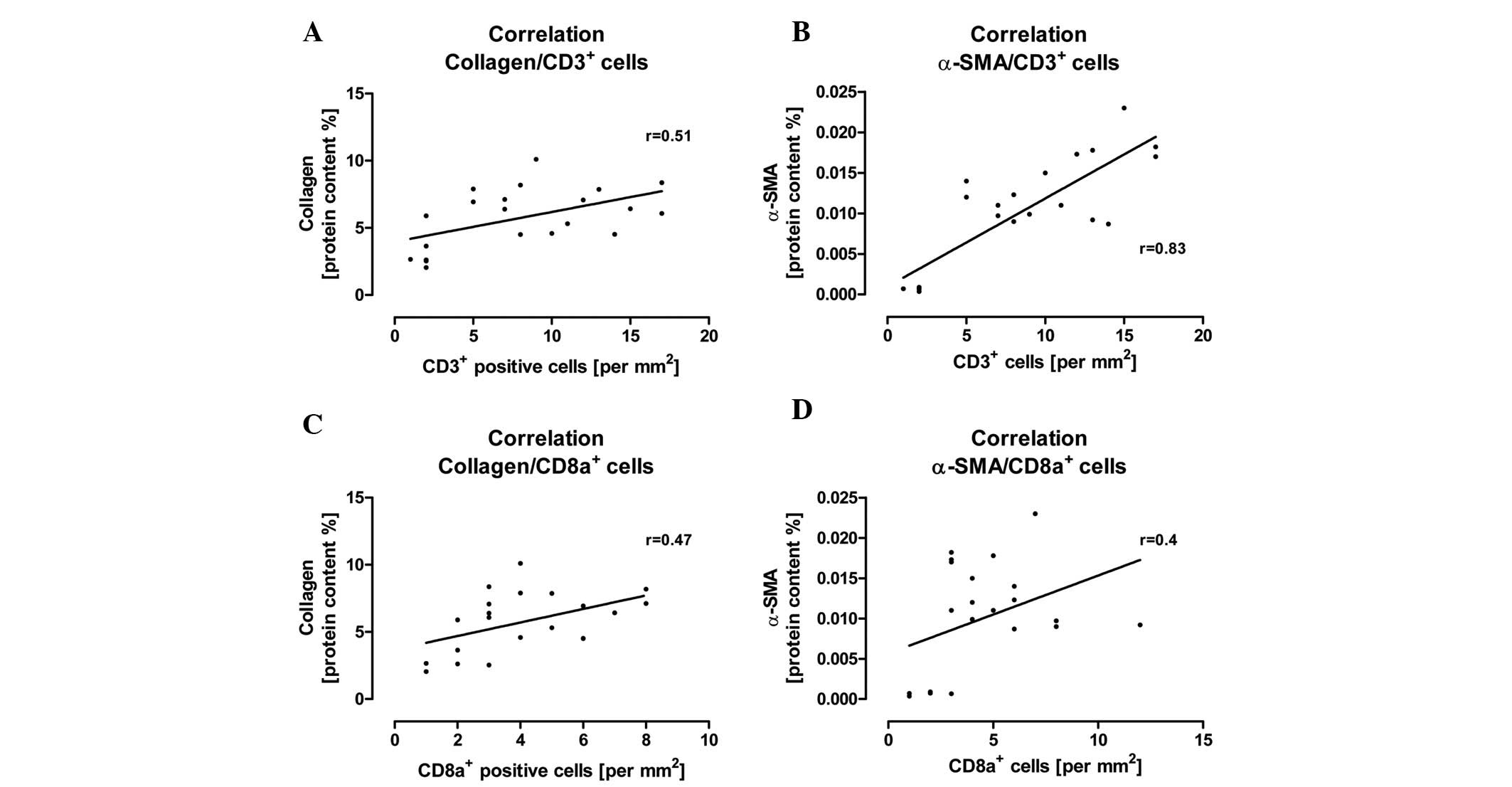
(Fig. 5). Furthermore, we
observed that a significant increase in CD3+ and
CD8a+ immune cells correlated with an increase in
collagen and α-SMA protein content in the cardiac tissue after the
induction of diabetes (Fig.
6).
Discussion
The salient finding of this study is that the model
of STZ-induced diabetic cardiomyopathy is a robust model for the
investigation of cardiac inflammation and remodeling processes. Our
data demonstrate that experimental diabetic cardiomyopathy is
characterized by an increase in cardiac inflammation and changes in
the regulation of the ECM over a time period of 6 weeks following
STZ-induced-diabetes.
Diabetic cardiomyopathy is associated with LV
dysfunction (12,15). The results of the present study
revealed an impairment in diastolic and systolic LV function at 2
and 6 weeks after the induction of diabetic cardiomyopathy. These
hemodynamic results are in line with those from previous studies
using the same experimental rat model (7,9,11,16). However, we also described the
concrete time course of the impairment in systolic and diastolic LV
performance over a period of 2–6 weeks, indicated by a decrease in
LV contractility, end systolic pressure, LV relaxation and an
increase cardiac stiffness, all resulting in a significant
reduction in cardiac output.
Our hemodynamic findings identified an early
diastolic LV dysfunction, which showed a clear progression over
time in this animal model. Active and passive diastolic LV
relaxation was affected by the STZ injection. LV relaxation as a
marker for active LV relaxation was significantly impaired 2 weeks
after the induction of diabetes, whereas cardiac stiffness as a
marker for passive LV relaxation displayed a tendency of
deterioration after 2 weeks, but was only impaired 6 weeks after
the induction of diabetes. The end diastolic pressure was
unaffected in this experimental setting; there was no significant
increase in end systolic pressure following STZ-induced diabetes
(Table I). This may be explained
by chronic dehydration in the animals with STZ-induced diabetes,
leading to a reduction in afterload. In previous studies, we
demonstrated that an induction of cardiac inflammation under
diabetic conditions was associated with an impairment in systolic
LV function (17,18). In the current study, we confirmed
the hemodynamic profile of systolic and diastolic LV dysfunction
under diabetic conditions.
Cardiac inflammation is one of the hallmarks of
heart failure (6,9). Intensified pro-inflammatory cytokine
expression levels, as well as increased immune cell infiltration,
such as cytotoxic T lymphocytes and macrophages, has been observed
in the inflamed heart in diabetic cardiomyopathy (19–21). As regards this finding, we
examined the invasion of CD3+ and CD8a+
immune cells into the heart in this disease model. The induction of
diabetes led to an uninterrupted increase in CD3+ immune
cell invasion over the 6-week observation period. In addition, the
induction of diabetes by STZ led to a significant increase in the
number of CD8a+ T lymphocytes 2 weeks after the STZ
injection. Of note, 6 weeks after the induction of diabetes, the
cardiac amount of this cell population was reduced when compared to
the time point of 2 weeks after the STZ injection, indicating an
important role of CD8a+ immune cells predominantly
during the early stages of diabetic cardiomyopathy.
A large body of evidence indicates that LV
remodeling accompanied with changes in ECM regulation is an
important factor for LV function in diabetic cardiomyopathy
(11,16). In this study, the induction of
diabetes led to a significant increase in cardiac fibrosis, indexed
by an increase in total collagen content at the time points of 2
and 6 weeks after the induction of diabetes. Moreover, we matched
the values of total collagen and LV contractility. We showed that
the total collagen content correlated with a reduction in LV
contractility and end systolic pressure, suggesting that the
corrrelation between systolic LV function and myocardial fibrosis
plays a pathophysiological role in this experimental setting.
In the clinical course of heart failure, MMPs are
upregulated by intense cardiac inflammation and may contribute to
cardiac remodeling under diabetic conditions (22,23). We therefore analysed the protein
expression levels of MMP-2 and found increased protein expression
levels of MMP-2 in this experimental setting.
However, the persistence of an abnormally high
number of myofibroblasts is a hallmark of fibrotic disease in other
organs, as well as the heart (24,25). In the current study, we identified
that STZ-induced diabetes led to an increase in the number of
α-SMA-positive myofibroblasts in the heart 2 weeks after the STZ
injection. However, an additional marked increase in the number of
α-SMA-positive myofibroblasts was documented at the time point of 6
weeks after the STZ injection. Concerning these findings, we also
observed a correlation between the number of α-SMA-positive
myofibroblasts and cardiac stiff-myofibroblasts stiffness, as a
strong marker for diastolic LV dysfunction. Of note, an increase in
the number of α-SMA-positive myofibroblasts correlated with an
increase in the cardiac stiffness index, suggesting a
pathophysiological impact of myofibroblasts in adverse myocardial
remodeling during diabetic conditions in rats. Moreover, we
verified that an increased cardiac immune cell invasion of
CD3+ and CD8+ cells correlated with an
increased protein content of collagen and α-SMA in the cardiac
tissue at 2 and 6 weeks after the induction of diabetes. In
addition, we also showed that an increased infiltration of these
immune cell populations was significantly associated with an
impairment in systolic and diastolic LV performance. These results
indicate an important role of CD3+ and CD8a+
immune cells in the development and progression of LV dysfunction
and adverse cardiac remodeling under diabetic conditions.
DM is associated with an exponential increase in
oxidative damage (26). Previous
studies have demonstrated that nitrotyrosin, as a maker of
oxidative stress, can participate in adverse remodeling,
contributing to the development of heart failure (27,28). In line with this finding, we
observed that the STZ injection led to an increase in nitrotyrosin
protein expression levels, suggesting an important role of
oxidative stress in the pathogenesis of diabetic
cardiomyopathy.
Previous studies have reported the effects of
exogenous insulin therapy. It has also been shown that insulin
treatment alone cannot normalize heart function under diabetic
conditions (29–31). However, future studies should
include a third experimental group with insulin treatment.
In conclusion, the current study displayed the
cardiac phenotype of rats with STZ-induced diabetes rats in a time
course analysis. The induction of diabetes by STZ led to an
impairment in systolic and diastolic LV function, associated with
an increase in immune cell invasion and adverse cardiac remodeling.
This study reveals an important role of the maintenance of cardiac
structure, by regulating the ECM assembly, in diabetic
cardiomyopathy. We hope that these new findings of cardiac
performance and remodeling will increase our understanding of the
pathophysiology and development of diabetic cardiomyopathy.
Acknowledgements
We thank Kerstin Puhl, Georg Zingler
and Nadine Orrin for their excellent technical assistance. This
study was funded by FP7-Health-2010, MEDIA (261409).
References
|
1.
|
Poornima IG, Parikh P and Shannon RP:
Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ
Res. 98:596–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Grundy SM, Benjamin IJ, Burke GL, et al:
Diabetes and cardiovascular disease: a statement for healthcare
professionals from the American Heart Association. Circulation.
100:1134–1146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Howard BV and Wylie-Rosett J: Sugar and
cardiovascular disease: a statement for healthcare professionals
from the Committee on Nutrition of the Council on Nutrition,
Physical Activity, and Metabolism of the American Heart
Association. Circulation. 106:523–527. 2002. View Article : Google Scholar
|
|
4.
|
Devereux RB, Roman MJ, Paranicas M, et al:
Impact of diabetes on cardiac structure and function: the strong
heart study. Circulation. 101:2271–2276. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cai L, Li W, Wang G, Guo L, Jiang Y and
Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium:
mitochondrial cytochrome C-mediated caspase-3 activation pathway.
Diabetes. 51:1938–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Heymans S, Hirsch E, Anker SD, et al:
Inflammation as a therapeutic target in heart failure? a scientific
statement from the Translational Research Committee of the Heart
Failure Association of the European Society of Cardiology. Eur J
Heart Fail. 11:119–129. 2009. View Article : Google Scholar
|
|
7.
|
Tschope C, Walther T, Koniger J, et al:
Prevention of cardiac fibrosis and left ventricular dysfunction in
diabetic cardiomyopathy in rats by transgenic expression of the
human tissue kallikrein gene. FASEB J. 18:828–835. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Tschope C, Spillmann F, Rehfeld U, et al:
Improvement of defective sarcoplasmic reticulum Ca2+
transport in diabetic heart of transgenic rats expressing the human
kallikrein-1 gene. FASEB J. 18:1967–1969. 2004.PubMed/NCBI
|
|
9.
|
Westermann D, Rutschow S, Jager S, et al:
Contributions of inflammation and cardiac matrix metalloproteinase
activity to cardiac failure in diabetic cardiomyopathy: the role of
angiotensin type 1 receptor antagonism. Diabetes. 56:641–646. 2007.
View Article : Google Scholar
|
|
10.
|
Riad A, Westermann D, Van Linthout S, et
al: Enhancement of endothelial nitric oxide synthase production
reverses vascular dysfunction and inflammation in the hindlimbs of
a rat model of diabetes. Diabetologia. 51:2325–2332. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Westermann D, Van Linthout S, Dhayat S, et
al: Cardioprotective and anti-inflammatory effects of interleukin
converting enzyme inhibition in experimental diabetic
cardiomyopathy. Diabetes. 56:1834–1841. 2007. View Article : Google Scholar
|
|
12.
|
Westermann D, Rutschow S, Van Linthout S,
et al: Inhibition of p38 mitogen-activated protein kinase
attenuates left ventricular dysfunction by mediating
pro-inflammatory cardiac cytokine levels in a mouse model of
diabetes mellitus. Diabetologia. 49:2507–2513. 2006. View Article : Google Scholar
|
|
13.
|
Westermann D, Becher PM, Lindner D, et al:
Selective PDE5A inhibition with sildenafil rescues left ventricular
dysfunction, inflammatory immune response and cardiac remodeling in
angiotensin II-induced heart failure in vivo. Basic Res Cardiol.
107:3082012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Becher PM, Lindner D, Miteva K, et al:
Role of heart rate reduction in the prevention of experimental
heart failure: comparison between If-channel blockade and
beta-receptor blockade. Hypertension. 59:949–957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wang J, Song Y, Elsherif L, et al: Cardiac
metallothionein induction plays the major role in the prevention of
diabetic cardiomyopathy by zinc supplementation. Circulation.
113:544–554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Westermann D, Van Linthout S, Dhayat S, et
al: Tumor necrosis factor-alpha antagonism protects from myocardial
inflammation and fibrosis in experimental diabetic cardiomyopathy.
Basic Res Cardiol. 102:500–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Van Linthout S, Riad A, Dhayat N, et al:
Anti-inflammatory effects of atorvastatin improve left ventricular
function in experimental diabetic cardiomyopathy. Diabetologia.
50:1977–1986. 2007.PubMed/NCBI
|
|
18.
|
Dorenkamp M, Riad A, Stiehl S, et al:
Protection against oxidative stress in diabetic rats: role of
angiotensin AT(1) receptor and beta 1-adrenoceptor antagonism. Eur
J Pharmacol. 520:179–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Savvatis K, Westermann D, Schultheiss HP
and Tschope C: Kinins in cardiac inflammation and regeneration:
insights from ischemic and diabetic cardiomyopathy. Neuropeptides.
44:119–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yeghiazaryan K, Bauriedel G, Schild HH and
Golubnitschaja O: Prediction of degeneration of native and
bioprosthetic aortic valves: issue-related particularities of
diabetes mellitus. Infect Disord Drug Targets. 8:88–99. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Marwick TH: Diabetic heart disease. Heart.
92:296–300. 2006.
|
|
22.
|
Tyagi SC and Hayden MR: Role of nitric
oxide in matrix remodeling in diabetes and heart failure. Heart
Fail Rev. 8:23–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Tsioufis C, Bafakis I, Kasiakogias A and
Stefanadis C: The role of matrix metalloproteinases in diabetes
mellitus. Curr Top Med Chem. 2:1159–1165. 2012. View Article : Google Scholar
|
|
24.
|
Leask A: Potential therapeutic targets for
cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF,
partners in fibroblast activation. Circ Res. 106:1675–1680. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hammoud L, Lu X, Lei M and Feng Q:
Deficiency in TIMP-3 increases cardiac rupture and mortality
post-myocardial infarction via EGFR signaling: beneficial effects
of cetuximab. Basic Res Cardiol. 106:459–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bugger H and Abel ED: Mitochondria in the
diabetic heart. Cardiovasc Res. 88:229–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Henderson BC and Tyagi SC: Oxidative
mechanism and homeostasis of proteinase/antiproteinase in
congestive heart failure. J Mol Cell Cardiol. 41:959–962. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
La Rocca G, Di Stefano A, Eleuteri E, et
al: Oxidative stress induces myeloperoxidase expression in
endocardial endothelial cells from patients with chronic heart
failure. Basic Res Cardiol. 104:307–320. 2009.PubMed/NCBI
|
|
29.
|
Regan TJ, Wu CF, Yeh CK, Oldewurtel HA and
Haider B: Myocardial composition and function in diabetes. The
effects of chronic insulin use. Circ Res. 49:1268–1277. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Iribarren C, Karter AJ, Go AS, et al:
Glycemic control and heart failure among adult patients with
diabetes. Circulation. 103:2668–2673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Dhalla NS, Pierce GN, Innes IR and Beamish
RE: Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J
Cardiol. 1:263–281. 1985.PubMed/NCBI
|