Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related mortality in the US (1,2).
Although many efforts have been made to improve the clinical
treatment of this disease, pancreatic cancer remains a challenging
malignancy (3–5). Due to the fact that the majority of
diagnoses are made during the late stages of disease and poor
response to current chemotherapeutic medicine (such as
gemcitabine), the 1-year survival rate is only 18% and the overall
5-year survival rate is 3–5% (6,7).
Sann-Joong-Kuey-Jian-Tang (SJKJT), a traditional Chinese medicinal
prescription, consists of 17 species of medicinal herbs:
Phellodendron amurense Rupr., Glycyrrhiza uralensis
Fisch, Sparganium stoloniferum Buch., Curcuma
aeruginosa Roxb., Laminaria japonica Aresch,
Bupleurum scorzoneri folium Willd (Bupleurum chinense
DC), Coptis chinensis Franch, Angelica sinensis
Diels, Cimicifuga heracleifolia Komar, Trichosanthes
cucumeroides Maxim, Anemarrhena asphodeloides Bunge,
Scutellaria baicalensis Georgi, Gentiana scabra
Bunge, Platycodon grandiflorus, Forsythia suspensa
Vahl, Paeonia lactiflora Pall and Pueraria lobata
Ohwi. It has been shown to inhibit the proliferation of human
breast cancer cells by blocking cell cycle progression and inducing
apoptosis (8). It has been
documented that SJKJT does not exert significant toxic effects on
certain types of normal cells (9). In our previous studies, we showed
that SJKJT inhibited the proliferation of colo 205 human colon
cancer cells by increasing the protein expression of
microtubule-associated protein II light chain 3 (LC3-II) in
vitro (10). SJKJT increased
the protein expression levels of tumor necrosis factor-α (TNF-α),
caspase-8 and caspase-3 in colo 205, inducing apoptosis in
vitro and in vivo (11). SJKJT has been prescribed as
complementary medicine for patients with solid tumors in Taiwan. In
a recent study of ours, we showed that SJKJT inhibited the
proliferation of Hep-G2 hepatocellular carcinoma cells by
increasing TNF-α, caspase-8, caspase-3 and Bax expression and
decreasing translationally controlled tumor protein (TCTP) and
myeloid cell leukemia 1 protein (Mcl-1) expression in vitro
(12). However, the anticancer
effects of SJKJT on human pancreatic cancer have not yet been
elucidated. The present study focused on the anticancer effects and
molecular mechanisms of action of SJKJT in human pancreatic cancer,
using BxPC-3 human pancreatic cancer cells.
Materials and methods
Crude extract of SJKJT was obtained from Chuang Song
Zong Pharmaceutical Co., Ltd. (Ligang Shiang, Taiwan). The BxPC-3
human pancreatic cancer cell line (BCRC no. 60283) was obtained
from the Food Industry Research and Development Institute (Hsinchu,
Taiwan). Potassium phosphate and TE buffer were purchased from
Merck Co. (Darmstadt, Germany). Fetal bovine serum (FBS) and
glutamine were from Gibco-BRL (Grand Island, NY, USA).
3-(4,5-Dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT),
sodium deoxycholate, leupeptin, Triton X-100, Tris-HCl,
ribonuclease-A and sodium pyruvate, HEPES, dimethyl sulfoxide
(DMSO) and RPMI-1640 were from Sigma-Aldrich (St. Louis, MO, USA).
Mouse anti-β-actin, and penicillin-streptomycin were obtained from
Sigma-Aldrich. BioMax film was from Kodak. The antibodies used were
antibodies against: Bax (no. 2774), Bcl-xL (no. 2764), Mcl-1 (no.
2764), TCTP (no. 2764), caspase-8 (no. 9502) and TNF-α (no. 3707)
(all from Cell Signaling Technology, Beverly, MA, USA); as well as
antibodies against caspase-3 (Lot: NB500-210) (Novus Biologicals,
LLC, Littleton, CO, USA); other materials and reagents not
specified were obtained from Sigma-Aldrich or Merck.
Cell culture
The BxPC-3 cells obtained from the Food Industry
Research and Development Institute, were maintained in RPMI-1640
medium containing 10% FBS, 1% penicillin/streptomycin (10,000 U/ml
penicillin, 10 mg/ml streptomycin) at 37°C in a humidified
atmosphere containing 5% CO2.
Cytotoxicity assay
The BxPC-3 cells were plated in 96-well plates at a
density of 1×104 cells/well for 16–20 h, and treated
with various concentrations (0, 0.1, 0.2, 0.4, 0.6, 0.8 and 1
mg/ml) of SJKJT for different periods of time (24, 48 and 72 h).
The cells were then incubated with 1 mg/ml of MTT in fresh
RPMI-1640 medium for 2 h. The surviving cells were measured at 590
nm using a microplate reader. The relative percentage of cell
viability was calculated by dividing the absorbance of the treated
cells by that of the control cells in each experiment, using the
following formula: proliferation rate (%) = (OD test − OD blank)
×100, where OD test and OD blank are the optical density of the
test substances and the blank control, respectively.
Cell cycle analysis
BxPC-3 cells were treated with various
concentrations (0, 0.3, 0.6 and 1.2 mg/ml) of SJKJT for 48 h or
with various concentrations (0, 0.1, 0.3 and 0.6 mg/ml) of SJKJT
for 72 h, and were then collected and fixed with ice-cold ethanol
(70%) overnight at −20°C; the cell pellets were then treated with
propidium iodide (PI) solution (containing 100 μg/ml RNase)
for 30 min at 37°C. Subsequently, the samples were analyzed using a
Cytomics TM FC 500 flow cytometer (Beckman Coulter Inc., Brea, CA,
USA). A minimum of 10,000 cells was analyzed for DNA content, and
the percentage of cells in each cell cycle phase was
quantified.
Immunocytochemistry (ICC)
BxPC-3 cells were treated with various
concentrations (0, 0.3, 0.6 and 1.2 mg/ml) of SJKJT for 48 h or
with various concentrations (0, 0.1, 0.3 and 0.6 mg/ml) of SJKJT
for 72 h and were then washed with PBS. Following fixation with 50%
acetone and 50% methanol solution overnight at 4°C, the cells were
washed 3 times with PBS, and non-specific binding sites were
blocked in PBS containing 0.1% BSA for 1 h at room temperature.
Thereafter, the cells were separately incubated with rabbit
anti-caspase-3 (1:20) antibody in PBS containing 0.1% BSA overnight
at 4°C, and washed 3 times with PBS. They were then incubated with
anti-rabbit FITC antibody (1:200) in PBS containing 0.1% BSA for 1
h at room temperature, and washed 3 times with PBS. The nuclei were
stained with 5 μg/ml PI. After staining, the samples were
immediately examined under an Olympus IX81 microscope (Olympus,
Tokyo, Japan).
Western blot analysis
The effects of SJKJT on the protein expression
levels of TNF-α, caspase-8, Bax, caspase-3, Mcl-1, TCTP and Bcl-xL
in the BxPC-3 cells were examined by western blot analysis. The
BxPC-3 cells were treated with various concentrations (0, 0.3, 0.6
and 1.2 mg/ml) of SJKJT for 48 h or with various concentrations (0,
0.1, 0.3 and 0.6 mg/ml) of SJKJT for 72 h, and the protein
expression levels of TNF-α, caspase-8, Bax, caspase-3, Mcl-1, TCTP
and Bcl-xL were then evaluated by western blot analysis. The
procedure was follows: following treatment with the drug, the cells
were lysed on ice-cold whole cell extract buffer containing
protease inhibitors. The lysate was then vibrated for 30 min at 4°C
and centrifuged at 10,000 rpm for 10 min. The protein concentration
was measured using the BCA protein assay kit (Pierce, Rockford, IL,
USA). Equal amounts of protein were subjected to electrophoresis
using 12% sodium dodecyl sulfate-polyacrylamide gels. To verify
equal protein loading and transfer, the proteins were then
transferred onto polyvinylidene difluoride membranes and the
membranes were blocked overnight at 4°C using blocking buffer [5%
non-fat dried milk in solution containing 50 mM Tris/HCl (pH 8.0),
2 mM CaCl2, 80 mM sodium chloride, 0.05% Tween-20 and
0.02% sodium azide]. The membranes were then incubated for 2 h at
25°C with specific primary antibody followed by anti-rabbit or
anti-mouse immunoglobulin G-horseradish peroxidase conjugated
secondary antibodies. The membranes were washed 3 times for 10 min
with washing solution. Finally, the protein bands were visualized
on X-ray film using the enhanced chemiluminescence detection system
(PerkinElmer Life and Analytical Sciences, Boston, MA, USA).
Statistical analysis
Values are presented as the means ± SD. The
Student’s t-test was used to analyze statistical significance. A
P-value <0.05 was considered to indicate a statistically
significant difference for all the tests.
Results and Discussion
Effects of SJKJT on the viability of
BxPC-3 cells
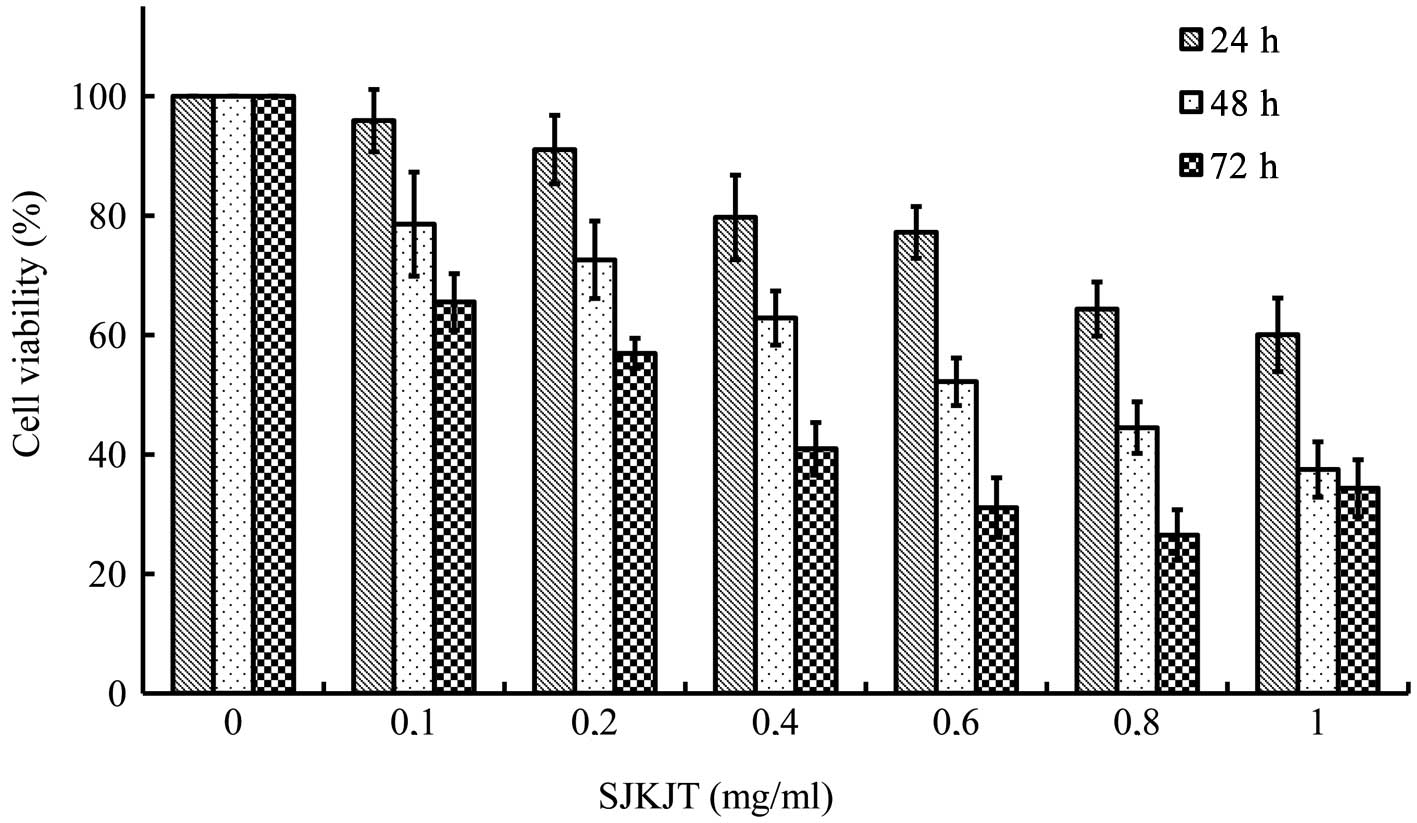
The results revealed that SJKJT inhibited the
proliferation of BxPC-3 cells in a time- and dose-dependent manner.
The half-maximal inhibitory concentration (IC50) was
1.38, 0.59 and 0.26 mg/ml at 24, 48 and 72 h, respectively
(Fig. 1).
SJKJT induces the apoptosis of BxPC-3
cells
The BxPC-3 cells were plated in 6-cm dish at a
density of 1×106 cells/dish and were then treated with
various concentrations of SJKJT for different periods of time (48
and 72 h). The cell cycle was analyzed by FACS. When the BxPC-3
cells were cultured with various concentrations (0, 0.3, 0.6 and
1.2 mg/ml) of SJKJT for 48 h, the percentage of cells in the subG1
phase was 3.97, 16.87, 32.2 and 57.13%, respectively. When the
BxPC-3 cells were cultured with various concentrations (0, 0.1, 0.3
and 0.6 mg/ml) of SJKJT for 72 h, the percentage of cells in the
sub-G1 phase was 2.33, 10.33, 14.37 and 32.06%, respectively
(Fig. 2). These results
demonstrated that treatment of the BxPC-3 cells with SJKJT
increased the percentage of cells in the subG1 phase. This
indicates that SJKJT induces the apoptosis of BxPC-3 cells.
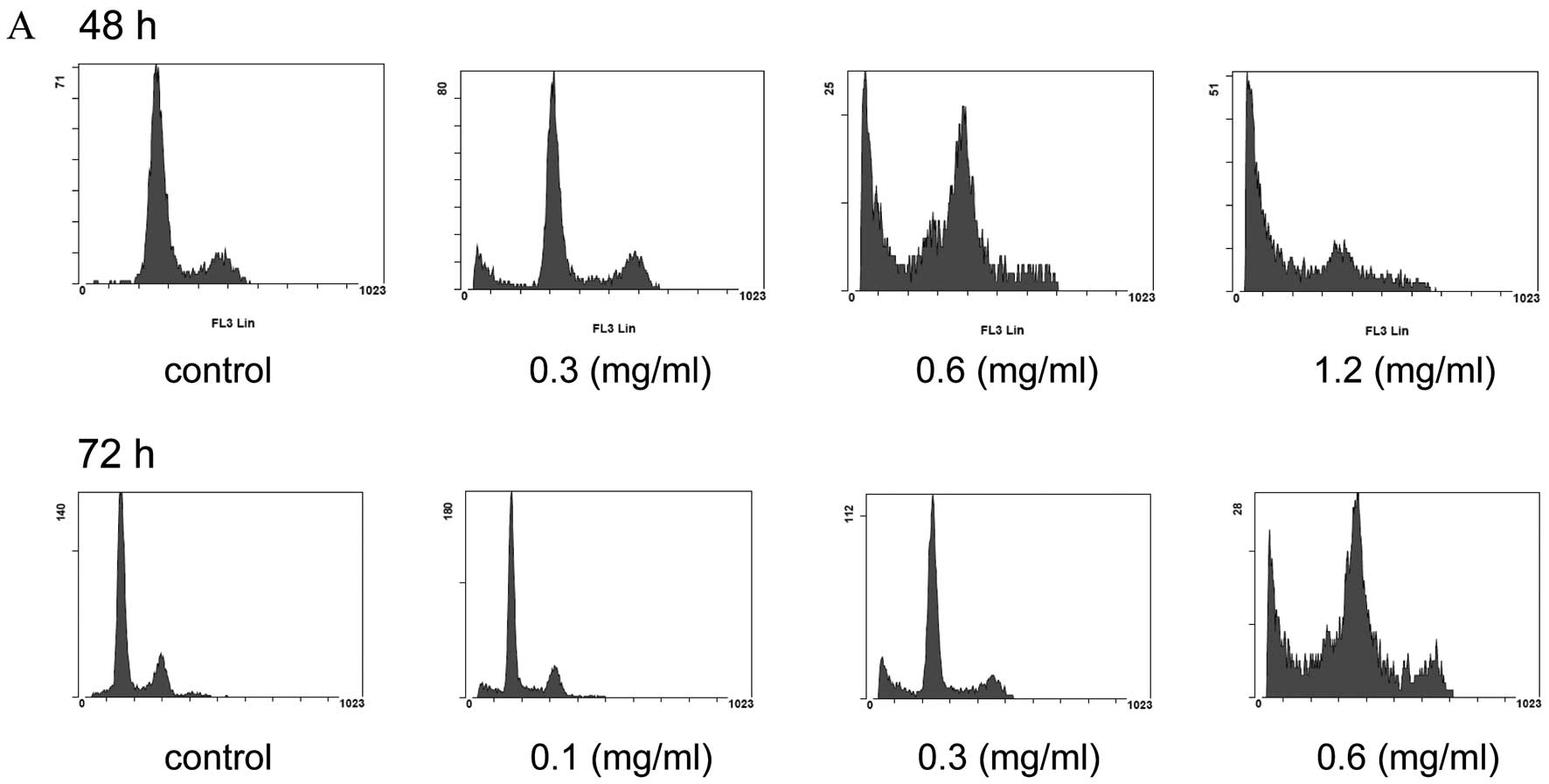 | Figure 2.Effect of Sann-Joong-Kuey-Jian-Tang
(SJKJT) on the cell cycle in BxPC-3 cells. BxPC-3 cells were plated
in 6-cm dish and were then treated with various concentrations of
SJKJT for different periods of time (48 and 72 h). (A) The cell
cycle was analyzed by FACS as described in Materials and methods.
When the BxPC-3 cells were cultured with various concentrations (0,
0.3, 0.6 and 1.2 mg/ml) of SJKJT for 48 h, the percentage of cells
in the subG1 phase was 3.97, 16.87, 32.2 and 57.13%, respectively.
(B) When the BxPC-3 cells were cultured with various concentrations
(0, 0.1, 0.3 and 0.6 mg/ml) of SJKJT for 72 h, the percentage of
cells in the sub-G1 phase was 2.33, 10.33, 14.37 and 32.06%,
respectively. |
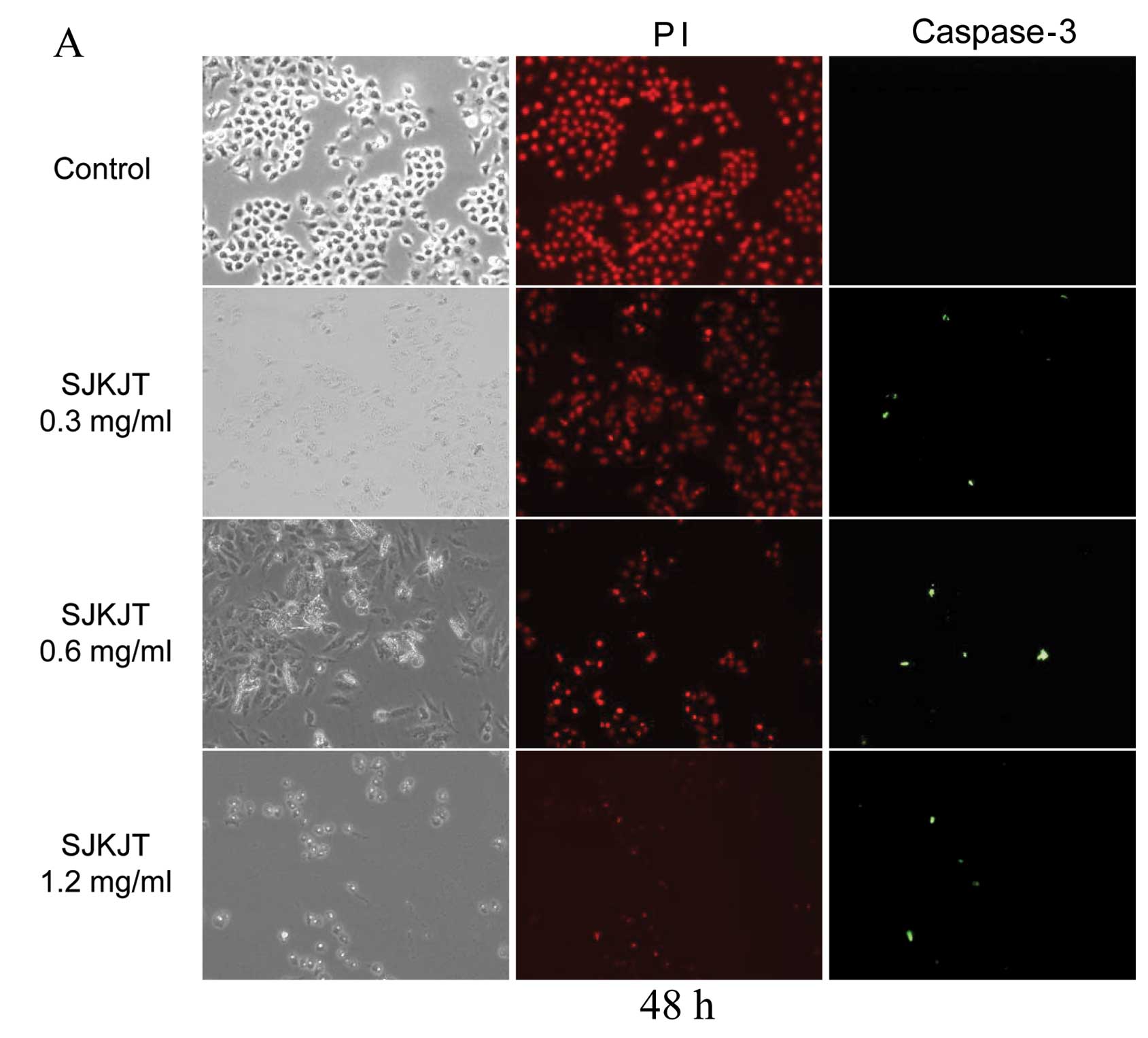
ICC analysis
The BxPC-3 cells were treated with various
concentrations of SJKJT (0, 0.3, 0.6 and 1.2 mg/ml) for 48 h or
with various concentrations of SJKJT (0, 0.1, 0.3 and 0.6 mg/ml)
for 72 h; the cells were then fixed with 4% paraformaldehyde for
the detection of the protein expression of caspase-3. The results
revealed that SJKJT increased caspase-3 expression in a
dose-dependent manner in the BxPC-3 cells (Fig. 3). These findings also suggest that
SJKJT induces the apoptosis of BxPC-3 cells in vitro.
Effects of SJKJT on the protein
expression of TNF-α, caspase-8, Bax, caspase-3, Mcl-1, TCTP and
Bcl-xL in BxPC-3 cells
The BxPC-3 cells were treated with SJKJT (0, 0.3,
0.6 and 1.2 mg/ml) for 48 h and the protein expression levels of
TNF-α, caspase-8, Bax, caspase-3, Mcl-1, TCTP and Bcl-xL were
evaluated by western blot analysis. The results revealed that SJKJT
increased the protein expression levels of Bax (Fig. 4A), caspase-9 (Fig. 4B) and caspase-3 (Fig. 4C), but decreased Mcl-1 (Fig. 4D) levels. The protein expression
levels of Bcl-2, TCTP, Bcl-xL (Fig.
4E), TNF-α (Fig. 4F) and
caspase-8 (Fig. 4G) were not
altered significantly. The BxPC-3 cells were then treated with
SJKJT (0, 0.1, 0.3 and 0.6 mg/ml) for 72 h. The results revealed
that SJKJT increased the protein expression levels of Bax (Fig. 5A), caspase-9 (Fig. 5B), TNF-α (Fig. 5C), caspase-8 (Fig. 5D) and caspase-3 (Fig. 5E), but decreased Mcl-1 (Fig. 5F), Bcl-2, Bcl-xL and TCTP levels
(Fig. 5G).
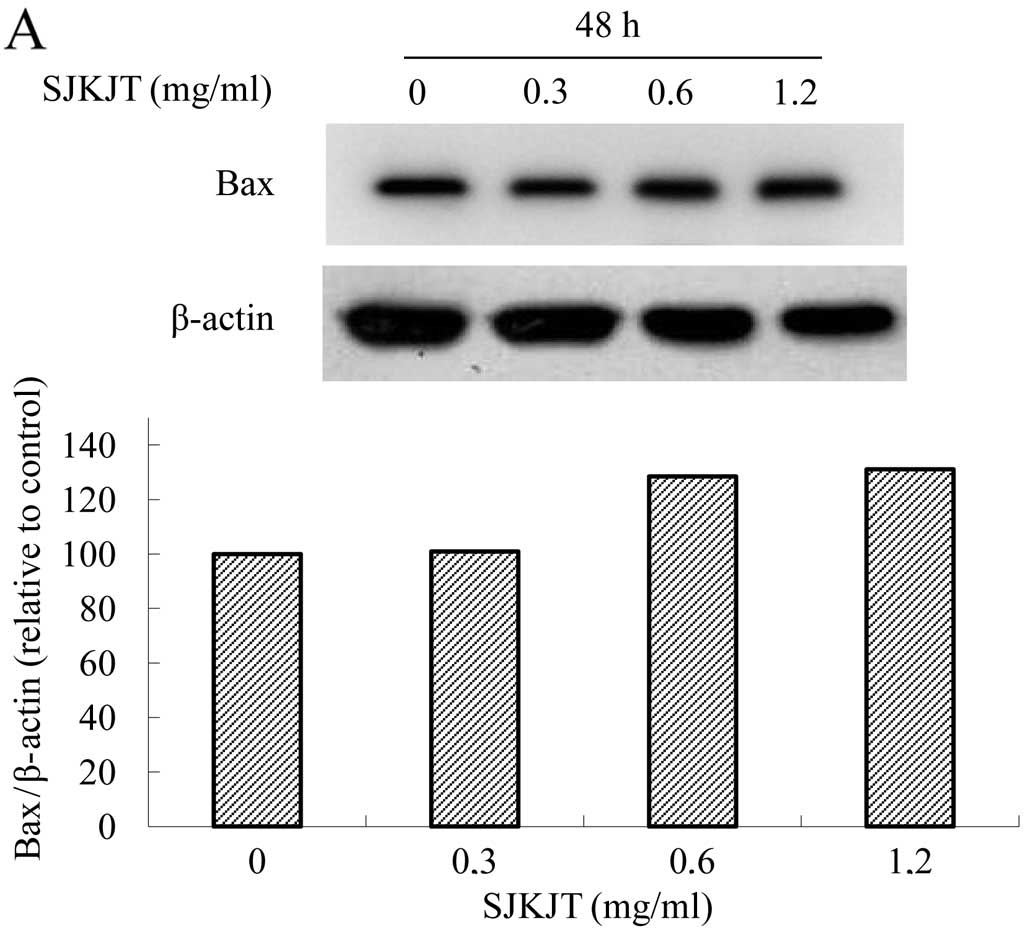 | Figure 4.Protein expression of TNF-α,
caspase-8, Bax, caspase-3, Mcl-1, TCTP and Bcl-xL in BxPC-3 cells.
The BxPC-3 cells were treated with SJKJT (0, 0.3, 0.6 and 1.2
mg/ml) for 48 h, and the protein expression levels were then
evaluated by western blot analysis as described in Materials and
methods. The results revealed that SJKJT increased the protein
expression levels of (A) Bax, (B) caspase-9 and (C) caspase-3, but
decreased (D) Mcl-1 levels. The protein expression levels of (E)
Bcl-2, TCTP, Bcl-xL, (F) TNF-α and (G) caspase-8 were not altered
significantly. |
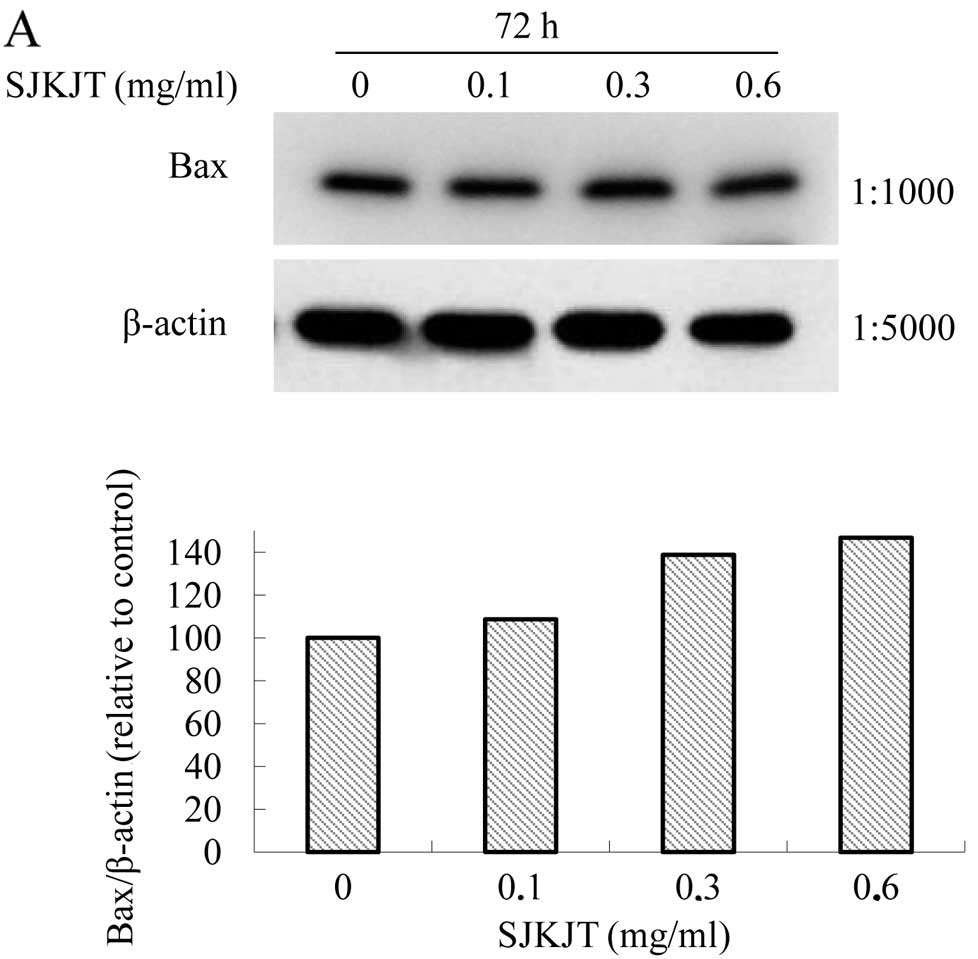 | Figure 5.Protein expression of TNF-α,
caspase-8, Bax, caspase-3, Mcl-1, TCTP and Bcl-xL in BxPC-3 cells.
The BxPC-3 cells were treated with SJKJT (0, 0.1, 0.3 and 0.6
mg/ml) for 72 h, and the protein expression levels were then
evaluated by western blot analysis as described in Materials and
methods. The results revealed that SJKJT increased the protein
expression levels of (A) Bax, (B) caspase-9, (C) TNF-α, (D)
caspase-8 and (E) caspase-3, but decreased (F) Mcl-1, (G) Bcl-2,
Bcl-xl and TCTP levels. |
It has been well documented that TNF-α binds to TNF
receptor type 1, resulting in the activation of caspase-8 and
caspase-3, thus inducing apoptosis (13,14). Our results demonstrated that the
treatment of BxPC-3 cells with SJKJT increased the protein
expression levels of TNF-α, caspase-8 and caspase-3. These findings
indicate that one of the molecular mechanisms of action of SJKJT
involved in the inhibition of BxPC-3 human pancreatic cancer cell
proliferation may be through the extrinsic pathway.
TCTP is a hydrophilic protein, widely expressed in
all eukaryotic organisms. It was discovered in Ehrlich ascites
tumor cells, and has been implicated in the protection of cells
against various stress conditions and apoptosis (15–18). It has been well documented that
TCTP binds to Bcl-xL and Mcl-1, antagonizing Bax, and thus
inhibiting the induction of apoptosis (19–22). Our results demonstrated that the
treatment of BxPC-3 cells with SJKJT decreased the protein
expression levels of Mcl-1, Bcl-xL and TCTP, but increased Bax,
caspase-9 and caspase-3 levels. Our results also indicated that
SJKJT induced the apoptosis of BxPC-3 cells. Therefore, one of the
molecular mechanisms of action of SJKJT involved in the inhibition
of BxPC-3 human pancreatic cancer cell proliferation may be through
the downregulation of Mcl-1, Bcl-xL and TCTP, and the upregulation
of Bax and caspase-9 protein expression, thus inducing
apoptosis.
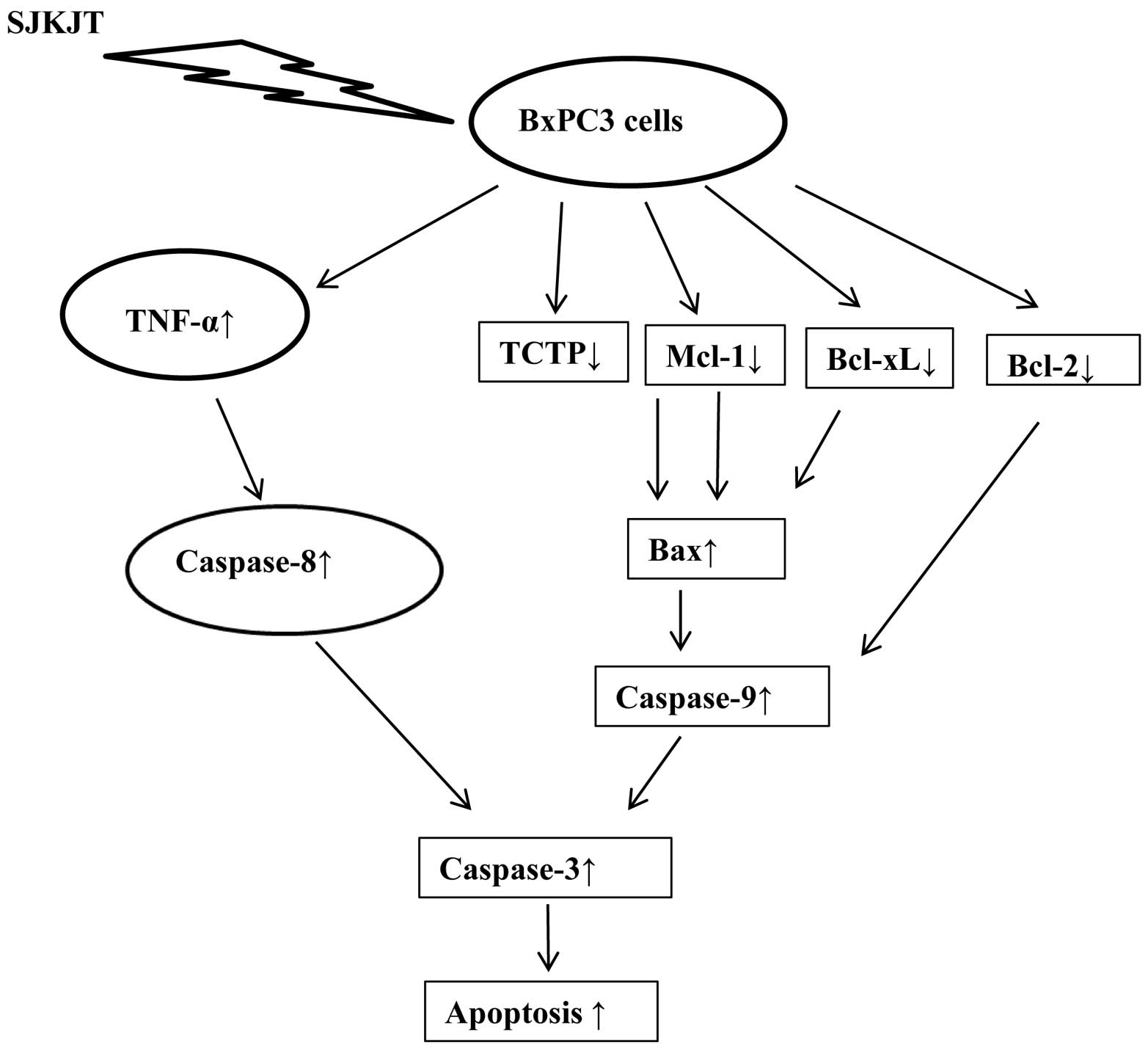
The results of present study, using BxPC-3 human
pancreatic cancer cells, demonstrate that SJKJT has potential as a
therapeutic agent for the treatment of pancreatic cancer in
vitro. One of the molecular mechanisms behind the
anti-proliferative effects of SJKJT may through the intrinsic
pathway; another may be through the extrinsic pathway. The proposed
signaling pathway through which SJKJT exerts its anti-proliferative
effects on BxPC-3 human pancreatic cancer cells is shown in
Fig. 6. To our knowledge, this is
the first report to demonstrate that SJKJT inhibits the
proliferation of BxPC-3 human pancreatic cancer cells. Further
sutdies are warranted to fully elucidate its mechanisms of
action.
Acknowledgements
The present study was supported by a
grant (100-CCH-ICO-06-1) from the Changhua Christian Hospital,
Changhua, Taiwan, R.O.C.
References
|
1.
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
3.
|
Louvet C, Labianca R, Hammel P, et al:
Gemcitabine in combination with oxaliplatin compared with
gemcitabine alone in locally advanced or metastatic pancreatic
cancer: results of a GERCOR and GISCAD phase III trial. J Clin
Oncol. 23:3509–3516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Heinemann V, Quietzsch D, Gieseler F, et
al: Randomized phase III trial of gemcitabine plus cisplatin
compared with gemcitabine alone in advanced pancreatic cancer. J
Clin Oncol. 24:3946–3952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Herrmann R, Bodoky G, Ruhstaller T, et al:
Gemcitabine plus capecitabine compared with gemcitabine alone in
advanced pancreatic cancer: a randomized, multicenter, phase III
trial of the Swiss Group for Clinical Cancer Research and the
Central European Cooperative Oncology Group. J Clin Oncol.
25:2212–2217. 2007. View Article : Google Scholar
|
|
6.
|
Burris HA III, Moore MJ, Andersen J, et
al: Improvements in survival and clinical benefit with gemcitabine
as first-line therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
7.
|
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi
AS, Kong D and Sarkar FH: Pancreatic cancer: understanding and
overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 8:27–33.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hsu YL, Yen MH, Kuo PL, et al:
San-Zhong-Kui-Jian-Tang, a traditional Chinese medicine
prescription, inhibits the proliferation of human breast cancer
cells by blocking cell cycle progression and inducing apoptosis.
Biol Pharm Bull. 29:2388–2394. 2006. View Article : Google Scholar
|
|
9.
|
Yang CH and Craise LM: Development of
human epithelial cell systems for radiation risk assessment. Adv
Space Res. 14:115–120. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cheng CY, Lin YH and Su CC:
Sann-Joong-Kuey-Jian-Tang increases the protein expression of
microtubule-associated protein II light chain 3 in human colon
cancer colo 205 cells. Mol Med Rep. 2:707–711. 2009.PubMed/NCBI
|
|
11.
|
Cheng CY, Lin YH and Su CC:
Sann-Joong-Kuey-Jian-Tang up-regulates the protein expression of
Fas and TNF-α in colo 205 cells in vivo and in vitro.
Mol Med Rep. 3:63–67. 2010.PubMed/NCBI
|
|
12.
|
Chen YL, Yan MY, Chien SY, Kuo SJ, Chen
DR, Cheng CY and Su CC: Sann-Joong-Kuey-Jian-Tang inhibits
hepatocellular carcinoma Hep-G2 cell proliferation by increasing
TNF-α, Caspase-8, Caspase- 3 and Bax but by decreasing TCTP and
Mcl-1 expression in vitro. Mol Med Rep. 7:1487–1493.
2013.PubMed/NCBI
|
|
13.
|
Carswell EA, Old LJ, Kassel RL, et al: An
endotoxin-induced serum factor that causes necrosis of tumors. Proc
Natl Acad Sci USA. 72:3666–3670. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gaur U and Aggarwal BB: Regulation of
proliferation, survival and apoptosis by members of the TNF
superfamily. Biochem Pharmacol. 66:1403–1408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yenofsky R, Cereghini S, Krowczynska A and
Brawerman G: Regulation of mRNA utilization in mouse
erythroleukemia cells induced to differentiate by exposure to
dimethyl sulfoxide. Mol Cell Biol. 3:1197–1203. 1983.PubMed/NCBI
|
|
16.
|
Chitpatima ST, Makrides S, Bandyopadhyay R
and Brawerman G: Nucleotide sequence of a major messenger RNA for a
21 kilo-dalton polypeptide that is under translational control in
mouse tumor cells. Nucleic Acids Res. 16:23501988. View Article : Google Scholar
|
|
17.
|
Bommer UA, Lazaris-Karatzas A, De
Benedetti A, et al: Translational regulation of the mammalian
growth-related protein P23: involvement of eIF-4E. Cell Mol Biol
Res. 40:633–641. 1994.PubMed/NCBI
|
|
18.
|
Bommer UA and Thiele BJ: The
translationally controlled tumour protein (TCTP). Int J Biochem
Cell Biol. 36:379–385. 2004. View Article : Google Scholar
|
|
19.
|
Zhang D, Li F, Weidner D, et al: Physical
and functional interaction between myeloid cell leukemia 1 protein
(MCL1) and Fortilin. The potential role of MCL-1 as a fortilin
chaperone. J Biol Chem. 277:37430–37438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Graidist P, Phongdara A and Fujise K:
Antiapoptotic protein partners fortilin and MCL1 independently
protect cells from 5-fluorouracil-induced cytotoxicity. J Biol
Chem. 279:40868–40875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Liu H, Peng HW, Cheng YS, et al:
Stabilization and enhancement of the antiapoptotic activity of
mcl-1 by TCTP. Mol Cell Biol. 25:3117–3126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Susini L, Besse S, Duflaut D, et al: TCTP
protects from apoptotic cell death by antagonizing bax function.
Cell Death Differ. 15:1211–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|