Introduction
Gastric cancer is one of the most common malignant
tumors worldwide. It was estimated that in 2008, 998,000 new cases
of gastric cancer were diagnosed (representing 7.8% of all new
tumor cases) and 736,000 patients died of this malignancy
(accounting for 9.7% of all tumor deaths) (1). Invasion and metastasis are major
factors for poor prognosis and mortality in gastric cancer. Thus,
understanding the molecular mechanisms involved in the invasion and
metastasis of gastric cancer is necessary for developing effective
treatment options to combat this disease (2).
Cancer invasion and metastasis are generally
associated with molecular abnormalities in adhesion molecules.
E-cadherin is one of the most important adhesion molecules and is
essential for the maintenance of intact cellular functionality. The
inhibition of tumor invasion and metastasis are among the most
important biological functions of E-cadherin, as its abnormal
expression and function have been observed in several
epithelium-derived cancers including gastric cancer (2–4).
A number of studies have shown that cyclooxygenase-2
(COX-2) affects many aspects of fundamental cellular processes,
such as the promotion of cell proliferation, inhibition of
apoptosis and the enhancement of neovascularization. Thus, COX-2
significantly contributes to carcinogenesis (5,6).
Previous studies have indicated that COX-2 participates in cancer
invasion and metastasis by decreasing the expression of E-cadherin
(7–9). However, the molecular mechanisms
through which COX-2 regulates E-cadherin expression and function
have not yet been fully elucidated.
In a previous study, we focused on the role of
inflammation-related gastric cancer, with particular interest in
the role of COX-2 and NF-κB in the development of gastric cancer
(10). In this study, we aimed to
determine the correlation between COX-2, nuclear factor-κB (NF-κB),
Snail and E-cadherin expression in gastric cancer tissues and cells
in order to elucidate the possible molecular mechanisms through
which COX-2 regulates E-cadherin expression. The data presented in
this study may aid in the development of novel therapeutic
approaches for combating the invasion and metastasis of gastric
cancer.
Materials and methods
Materials
The SGC7901, AGS, BGC823 and MGC803 gastric cancer
cell lines were purchased from the Shanghai Institute of Life
Sciences of Chinese Academy of Sciences (Shanghai, China).
Celecoxib, a selective COX-2 inhibitor, was a gift from the Faculty
of Medicine, University of Hong Kong, Hong Kong, China. RPMI-1640
and other cell culture supplements were purchased from
Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). Calf serum was
purchased from Hangzhou Sijiqing Biological Engineering Materials
Co., Ltd. (Hangzhou, China). The RNA extraction kit and protein
extraction kit were purchased from Shanghai Biological Engineering
Materials Co., Ltd. (Shanghai, China). The reverse transcription
kit and quantitative PCR (qPCR) kit were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). Polyclonal antibodies for
COX-2 and Snail were purchased from Abcam Ltd. (Cambridge, UK).
Polyclonal antibodies against NF-κB and E-cadherin, as well as
monoclonal antibody against β-actin were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Millicell cell culture
inserts were obtained from Millipore (Billerica, MA, USA). ECM Gel
was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Healthy gastric mucosa, gastric cancer
tissues and immunostaining
Paraffin-embedded blocks of healthy human gastric
mucosa (n=32) and gastric cancer tissue (n=189) were used to
construct tissue microarrays (TMAs). Gastric cancer tissues were
obtained from patients with gastric cancer who underwent surgery at
the Wuwei Tumor Hospital, Wuwei, China. The healthy gastric mucosal
tissues were obtained from individuals who underwent gastric cancer
screening endoscopy at the same hospital. The diagnosis of gastric
adenocarcinoma was based on the WHO diagnosis criteria, and was
confirmed by two independent pathologists. Patient demographics and
TMA construction were described in our previous study (10). For immunohistochemical staining,
the TMA slides were incubated for 1 h at 37°C with primary antibody
against the following proteins: COX-2 (1:200), NF-κB p65 (1:200),
Snail (1:200) and E-cadherin (1:250). The slides were then washed
three times with phosphate-buffered saline (PBS), incubated with
biotin-conjugated secondary antibody (1:150) for 60 min at 37°C,
washed with PBS and then incubated with strepavidin horseradish
peroxidase (SHRP) (Thermo Scientific, USA) for 40 min at room
temperature, developed with 2,3-diaminobenzidine tetrahydrochloride
(DAB) (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
China) and counterstained with hematoxylin. The tissue diagnosis
and immunostaining results were evaluated by two independent
pathologists, and representative images were aquired for data
presentation. Written informed consent was obtained from all
participants prior to enrollment in the study. The study was
approved by the Institutional Human Ethics Committee of the First
Hospital of Lanzhou University, Lanzhou, China.
Treatment of cells with celecoxib
The dose- and time-response of SGC7901 and BGC803
cells to celecoxib was examined using various concentrations of
celecoxib for various periods of time, as detailed in the relevant
figures and figure legends. The effect of celecoxib on the
expression of COX-2, NF-κB, Snail and E-cadherin was examined by
qPCR and western blot analysis as described below.
qPCR
Total cellular RNA was extracted using a commercial
RNA extraction kit. Approximately 30 ng of total RNA was
reverse-transcribed into cDNA. The PCR reaction system contained
the following: 12.5 μl SYBR® Premix Ex Taq™ II,
0.5 μl of each primer, 2 μl DNA template and 9.5
μl dH2O. The qPCR conditions were as follows:
95°C, 5 sec; 62°C, 30 sec, 40 cycles. The qPCR primers are
presented in Table I. Data were
analyzed according to the comparative Ct method and were normalized
to β-actin expression in each sample.
 | Table I.Primer sequences for quantitative
PCR. |
Table I.
Primer sequences for quantitative
PCR.
| Primer | Forward | Reverse | Product size
(bp) |
|---|
| COX-2 |
5′-TGGTGCCTGGTCTGATGATGTATGC-3′ |
5′-ATCTGCCTGCTCTGGTCAATGGAAG-3′ | 493 |
| E-cadherin |
5′-TTAAACTCCTGGCCTCAAGCAATC-3′ |
5′-TCCTATCTTGGGCAAAGCAACTG-3′ | 139 |
| NF-κB/p65 |
5′-TCAGTCAGCGCATCCAGACC-3′ |
5′-CAGAGCCGCACAGCATTCA-3′ | 91 |
| Snail |
5′-CGCGCTCTTTCCTCGTCAG-3′ |
5′-TCCCAGATGAGCATTGGCAG-3′ | 181 |
| β-actin |
5′-TGGCACCCAGCACAATGAA-3′ |
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ | 186 |
Western blot analysis
Total cellular protein was extracted using a
commercial kit, and the protein concentration was measured using
the BCA protein assay (Beyotime Institute of Biotechnology, Haimen,
China). Approximately 25 μg of denaturalized protein was
separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
and transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore). The blots were blocked with 5% skim milk in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 2 h at
room temperature before being incubated overnight with primary
antibodies against COX-2 (1:500), E-cadherin (1:500), NF-κB
(1:500), Snail (1:1000) and β-actin (1:500) at 4°C. After being
washed in TBST three times, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody (Santa
Cruz Biotechnology, Inc.) (1:5,000) for 1 h at room temperature.
The protein bands were then detected using the ECL detection
system.
Immunofluorescence and confocal laser
scanning microscopy
Cells were harvested at the logarithmic growth phase
and placed on glass coverslips in a 6-well plate (1×105
cells/well) and incubated in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS). After treatment with 75 μM
celecoxib for 12, 24 and 48 h, the coverslips were washed three
times with cold PBS and fixed with cold-acetone:methanol (1:1) for
10 min. The cells were then blocked in 10 g/l BSA solution for 30
min and then incubated with rabbit anti-E-cadherin polyclonal
antibody (1:100) at 4°C overnight. The cells were then washed three
times in PBS and incubated with FITC conjugated goat anti-rabbit
antibody (1:200) for 2 h at 37°C. After three washes in PBS, the
coverslips were sealed by glycerol carbonic acid. Fluorescence was
observed using a Leica TCS SP2 confocal microscope. PBS was used as
the negative control.
Invasion assay
Single cell suspension (5×105/ml) was
prepared with RPMI-1640 supplemented with 1% FBS. Two hundred
microliters of cell suspension containing celecoxib (75 μM)
were added to the upper chamber of each well. The lower chambers
were filled with RPMI-1640 containing 10% FBS. The plates were
incubated in a 5% CO2 container at 37°C. After 24 h, the
cells on the upper membrane surface were removed by wiping with a
cotton swab, and the filters were stained with Swiss dye solution
for 20 min. The invasive cells on the undersurface of filter were
observed under an inverted microscope (×200) and the average number
of invasive cells was calculated from five different fields. All
assays were performed in triplicate.
Statistical analysis
Data analysis was performed using SPSS11.0 software
(IBM SPSS Statistics, Armonk, NY, USA). All data are expressed as
the means ± standard deviation. A comparison of the differences
between each group was performed using the Student’s t-test.
Results
Analysis of the expression of COX-2,
NF-κB, Snail and E-cadherin by immunohistochemistry
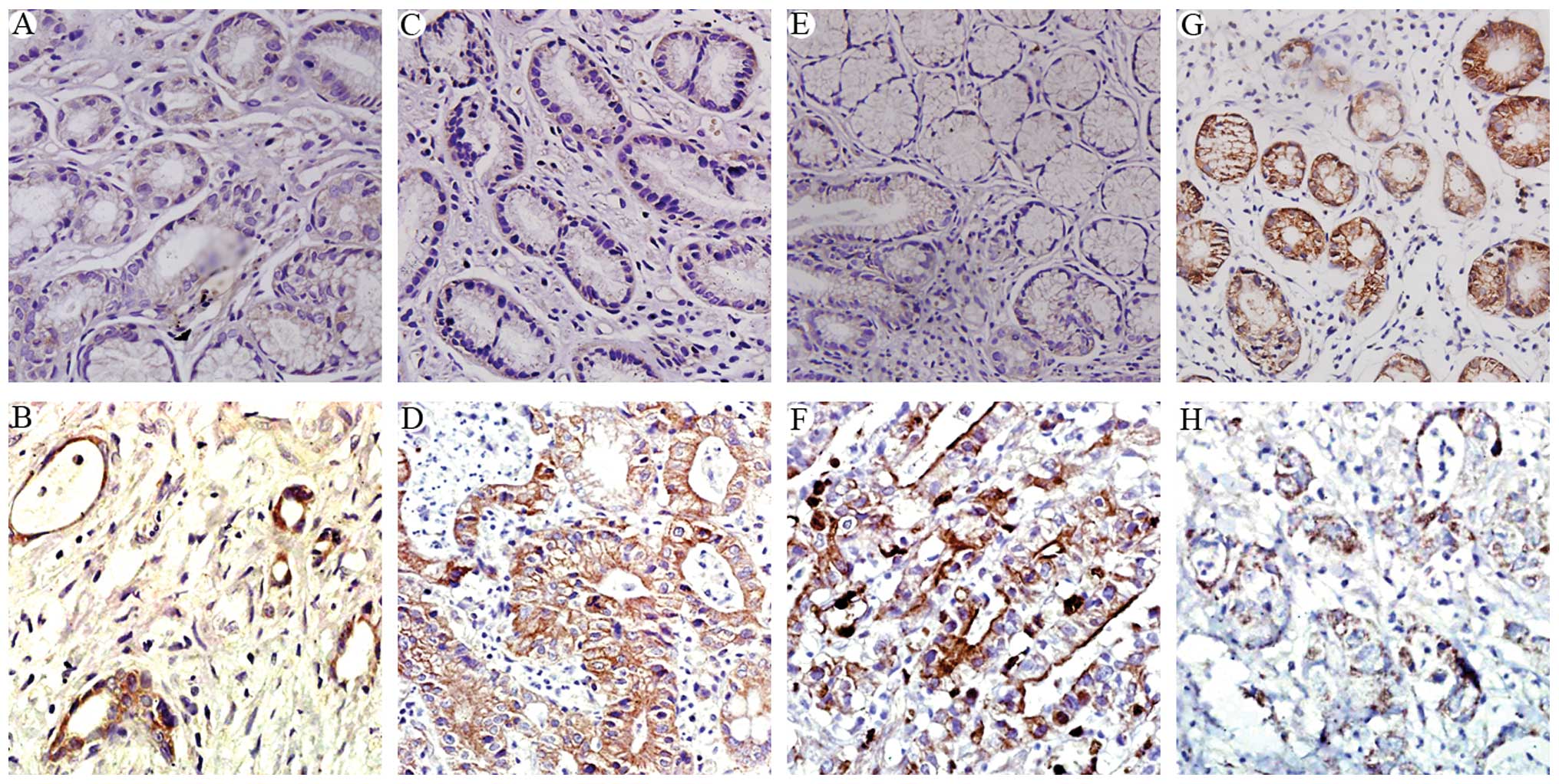
The gastric cancer tissues generally expressed
increased levels of COX-2 (Fig.
1B), NF-κB (Fig. 1D), and
Snail (Fig. 1F), compared to the
controls (Fig. 1A, C and E,
respectively). These proteins were expressed mostly in the
cytoplasmic compartments of the tumor cells. By contrast, a high
expression of E-cadherin was detected in the normal gastric
epithelial tissues, mostly as a membranous protein (Fig. 1G), whereas the expression of
E-cadherin was mostly lost in the gastric cancer tissues (Fig. 1H).
Expression of COX-2 in the four gastric
cancer cells with varying degrees of differentiation
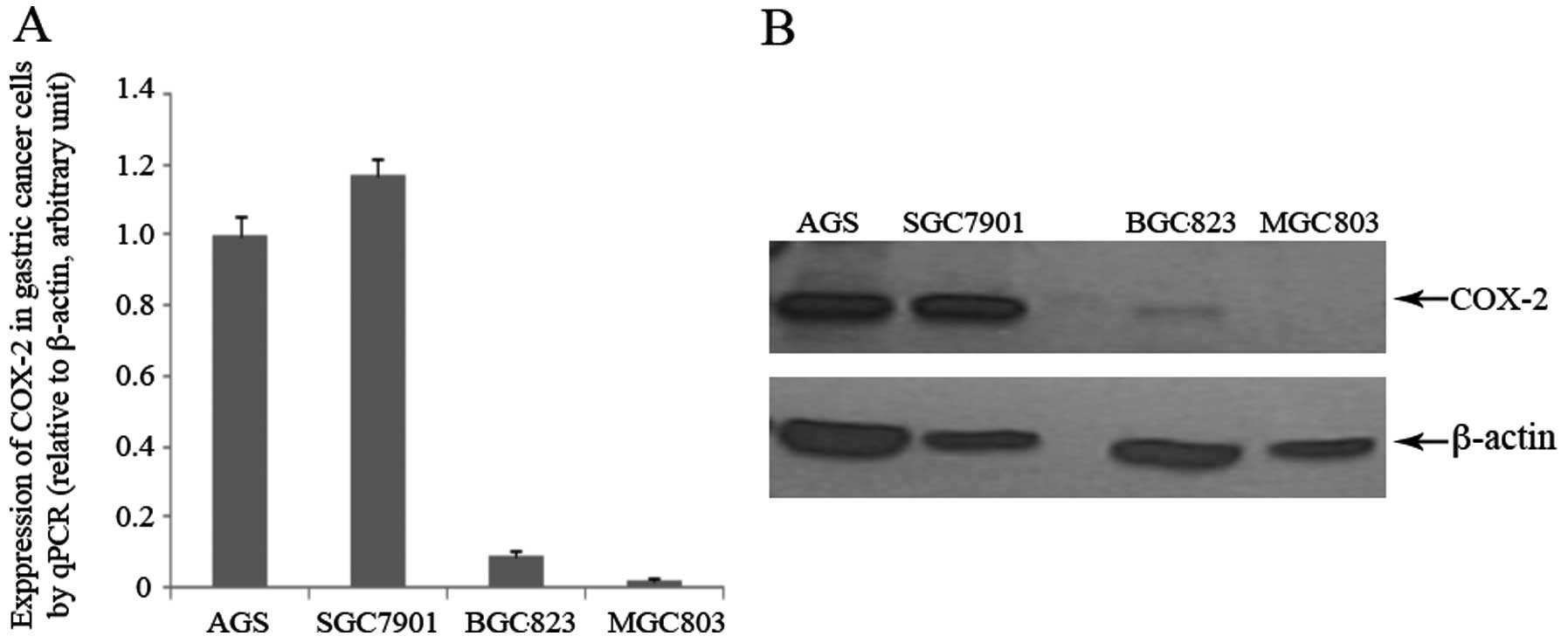
Using western blot analysis and qPCR, we
investigated the expression of COX-2 in the gastric cancer cells
with varying degrees of differentiation: AGS (well differentiated),
SGC7901 (moderately differentiated), BGC823 (poorly differentiated)
and MGC803 cells (undifferentiated). Using qPCR, we observed a
gradual decrease in the mRNA expression of COX-2 as the degree of
cell differentiation decreased, with the highest expression being
found in the SGC7901 cells, and the lowest in the MGC803 cells
(Fig. 2A). At the protein level,
high levels of COX-2 were observed in the SGC7901 and AGS cells;
the BGC823 cells had a very low expression, whereas no COX-2
expression was detected in the MGC803 cells (Fig. 2B). Based on these results, we
selected the SGC7901 cells as the COX-2-rich cells and the BGC803
cells as the COX-2-deficient cells for subsequent experiments.
Effect of COX-2 inhibition by celecoxib
on the expression of NF-κB, Snail and E-cadherin in the SGC7901 and
BGC803 cells
In our previous studies, we demonstrated that COX-2
and NF-κB regulate the expression of E-cadherin via the Snail
signaling pathway (10), and that
celecoxib restored the lost E-cadherin expression in gastric cancer
cells (7). In this study, we
treated the SGC7901 and BGC803 cells with celecoxib, and examined
its effects on the expression of COX-2, NF-κB, Snail and E-cadherin
in gastric cancer cells. Our results may provide clues on the
innate correlation between these genes.
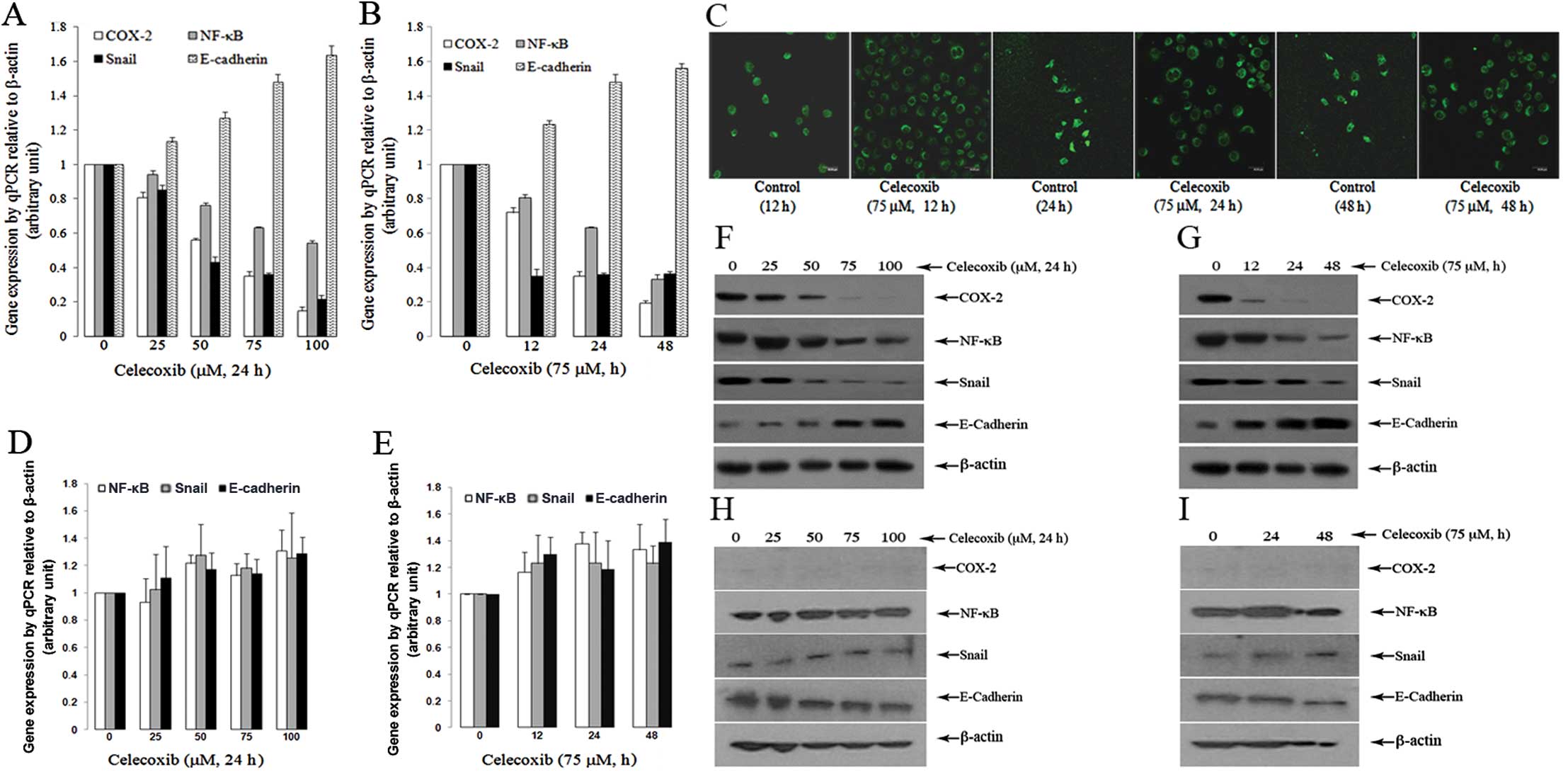
When the SGC7901 cells were treated with celecoxib,
there was a dose-dependent decrease in the mRNA expression of
COX-2, NF-κB and Snail (P<0.05, compared to the controls)
(Fig. 3A). Further experiments
revealed that the treatment of SGC7901 cells with 75 μM of
celecoxib for 12, 24 and 48 h led to a marked reduction in the mRNA
expression of COX-2, NF-κB and Snail; this reduction occurred in a
time-dependent manner, although not for Snail (Fig. 3B) (P<0.05, compared to the
controls). Notably, treatment of the SGC7901 cells with celecoxib
rendered a significant dose- and time-dependent increase in the
expression of E-cadherin (Fig. 3A and
B) (P<0.05, compared to the controls). The increased
expression of E-cadherin following treatment with celecoxib was
also observed under a confocal fluorescent microscope (Fig. 3C).
By contrast, the same treatment regimen did not
alter the mRNA expression of COX-2, NF-κB, Snail and E-cadherin in
the BGC803 cells (P>0.05, compared to the controls) (Fig. 3D and E).
The above changes were further confirmed at the
protein level by western blot analysis. Treatment of the SGC7901
cells with celecoxib caused a dose- and time-dependent decrease
(Fig. 3F and G) in the expression
of COX-2, NF-κB and Snail. On the other hand, the expression of
E-cadherin was increased by celecoxib in a dose- and time-dependent
manner (Fig. 3F and G). However,
the protein expression of COX-2, NF-κB, Snail and E-cadherin was
not significantly altered in the BGC803 cells after the same
treatment regimen (Fig. 3H and I)
(P>0.05, compared to the controls).
Effect of COX-2 inhibition by celecoxib
on cell invasion
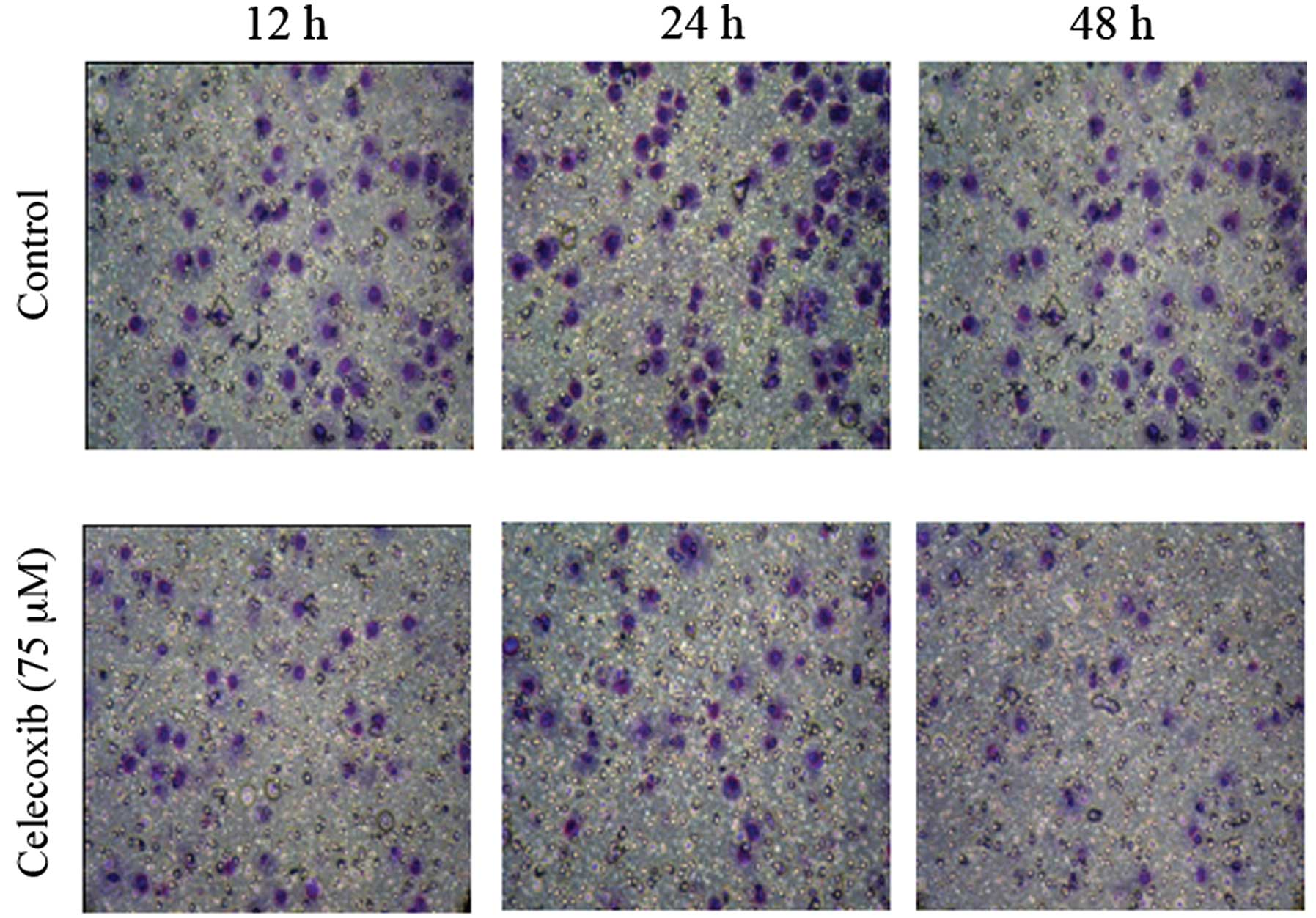
Treatment of the SGC7901 cells with celecoxib led to
an increase in the expression of E-cadherin in the SGC7901 cells
(P<0.05) (Fig. 3). We then
examined the effects of celecoxib on cell invasion. Celecoxib
decreased the invasive ability of the SGC7901 cells (P<0.01)
(Fig. 4).
Effect of COX-2 inhibition by celecoxib
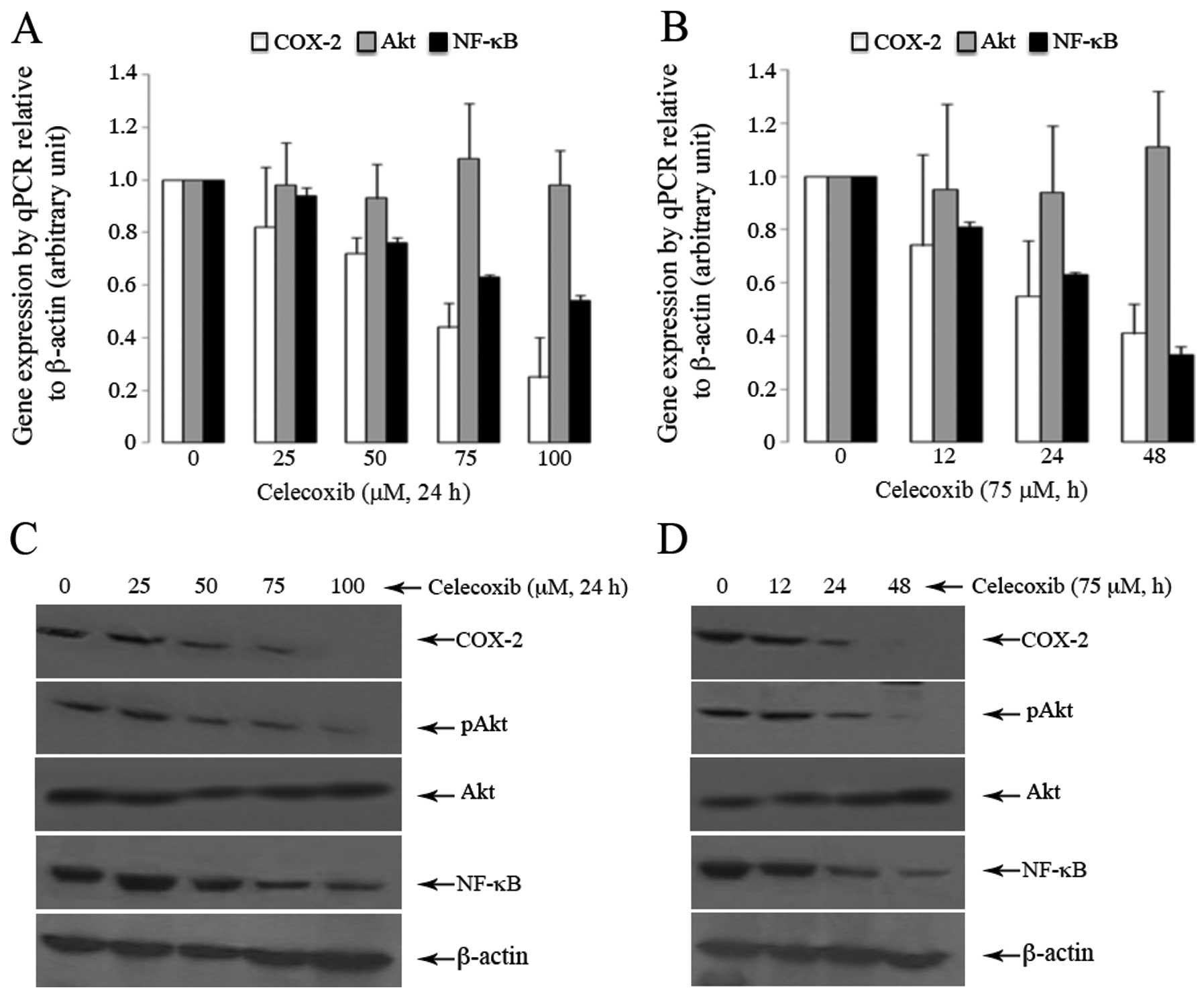
on the expression of Akt in SGC7901 cells
Treatment of the SGC7901 cells with celecoxib led to
a dose- and time-dependent decrease in the mRNA expression of COX-2
and NF-κB, but not in the expression of Akt (Fig. 5A and B) (P>0.05). However, at
the protein level, celecoxib caused a dose- and time-dependent
decrease in the expression of p-Akt (Fig. 5C and D) (P<0.05, compared to
the controls), and these changes were in parallel with the altered
expression patterns of COX-2 and NF-κB.
Discussion
COX-2 plays an important role in carcinogenesis and
metastasis in many types of cancer, including malignancies derived
from the gastrointestinal tract (11,12). COX-2 has been reported to induce
cell proliferation, inhibit apoptosis and facilitate angiogenesis
(5,6). The increased COX-2 expression has
been widely reported in gastric cancer; hence, the inhibition of
COX-2 has been suggested as a promising approach for the prevention
and treatment of gastric cancer (13–15). However, the mechanisms through
which COX-2 regulates gastric carcinogenesis have not yet been
fully elucidated.
The high expression level of COX-2 has been shown to
correlate with the downregulation of E-cadherin in prostate cancer
(8). E-cadherin is an important
molecule in the control of normal cell adhesion and tissue
integrity. The loss of E-cadherin has been well recognized in
gastric cancer and this has been linked to cancer progression,
invasion and metastasis (7,2,16).
The pre-operative administration of celecoxib in patients with
gastric cancer for seven days has been shown to significantly
decrease the expression of COX-2, VEGF and angiogenesis, but
increase E-cadherin expression and apoptosis in post-operative
gastric cancer tissues (7). In
the current study, we observed that celecoxib inhibited COX-2
expression in the SGC7901 cells and that this was associated with
the restoration of E-cadherin expression; this in turn, paralleled
with a decrease in cell invasion. These data further verify that
the role of COX-2 in gastric cancer development is likely
associated with the observed loss of E-cadherin expression.
In order to investigate the mechanisms through which
COX-2 regulates E-cadherin expression, we measured the expression
of Snail, a zinc finger transcription factor of the Snail super
family which includes Snail, E12/E47, zinc finger E-box binding
homeobox 1 (ZEB1), Smad interacting protein 1 (SIP1) and Slug.
Snail binds to the promoters of various effector genes and thereby
regulates the transcription and expression of that partner protein
(17–19). It has been shown that Snail binds
to the E-box of the E-cadherin promoter region and inhibits the
transcription and expression of the latter, and thus it is
considered a direct inhibitor of E-cadherin (20,21). The downregulation of E-cadherin
has indeed been causally linked to the abnormal activity of Snail
in several types of cancer (22,23).
In the current study, we observed that COX-2 not
only regulates E-cadherin, but also regulates the expression of
Snail in gastric cancer cells. Celecoxib, a selective COX-2
inhibitor, was used to treat the SGC7901 cells, in which COX-2 is
highly expressed. We found that celecoxib rendered a dose- and
time-dependent decrease in the mRNA and protein expression of
Snail. By contrast, the BGC803 cells (in which COX-2 is barely
detectable) did not show the same response to celecoxib, and this
agent did not cause any changes in Snail expression. These data
suggest that COX-2 closely correlates with Snail. This is
consistent with a previous report on non-small cell lung cancer,
showing that COX-2 regulates the expression of E-cadherin via Snail
(24). We therefore hypothesized
that Snail may be a critical mediator in the COX-2-induced loss of
E-cadherin expression in gastric cancer.
Our results revealed that celecoxib inhibited the
activity of COX-2 and that this effect was not only associated with
the reduced expression of Snail, but also with a marked reduction
in the expression of NF-κB subunit p65. Again, in the SGC7901
cells, the mRNA and the protein expression of p65 showed a dose-
and time-dependent decrease in response to celecoxib. By contrast,
the BGC803 cells did not exhibit a similar response to celecoxib,
suggesting that COX-2 regulates the expression of NF-κB.
As NF-κB/p65 directly binds to the promoter region
of Snail and thus induces its transcription in cancer (25,26), we hypothesized that in gastric
cancer, the interaction between NF-κB/p65 and Snail may play a role
in the COX-2-mediated loss of E-cadherin expression. The
interaction between COX-2 and NF-κB has not yet been fully
elucidated. Previous studies have provided some clues: COX-2
activates IκB kinase (IKK) through the activation of the Akt
pathway (27) and the treatment
of liver cancer cells with celecoxib has been shown to decrease the
phosphorylation level of Akt (28). In this study, we showed that
celecoxib did not change the total level of Akt as revealed by
qPCR, but induced a time- and dose-dependent downregulation of
phosphorylated Akt, and this change was associated with the
parallel inhibition of COX-2 and NF-κB. These data suggest that
under physiological conditions, COX-2 interacts with its target
proteins through the activation of the Akt pathway.
In view of these data, and considering our finding
that in gastric cancer tissues, COX-2, NF-κB and Snail showed a
very similar expression pattern, and as the same pattern was
maintained when the cells were exposed to the COX-2 inhibitor,
celecoxib, it can be hypothesized that a complex interaction
between COX-2, NF-κB, Snail and E-cadherin exists. These factors
may not operate alone during the development of gastric cancer.
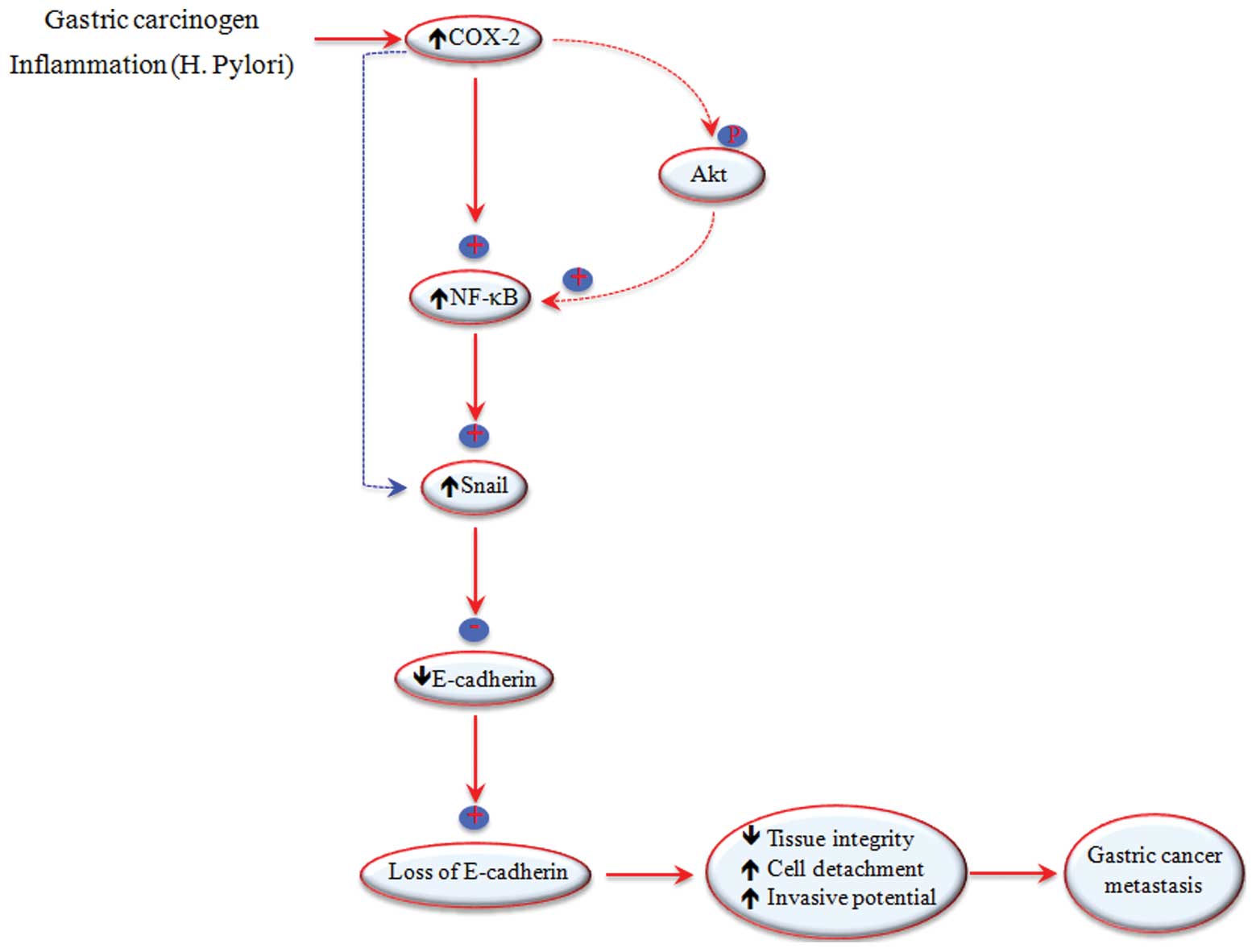
In conclusion, based on the published data and those
from our study, we suggest that COX-2 activates NF-κB, thus
regulating the transcription and expression of E-cadherin through
the Snail signaling pathway (Fig.
6), although the direct effect of COX-2 on Snail activation may
also play a role in the loss of E-cadherin expression during
gastric cancer. Further studies (e.g., experiments involving Snail
modulation) are warranted to clarify the mechanisms through which
COX-2 interacts with NF-κB, Snail and E-cadherin during the
development of gastric cancer.
Abbreviations:
|
COX-2
|
cyclooxygenase-2;
|
|
NF-κB
|
nuclear factor-κB
|
Acknowledgements
The study was sponsored by the
National Natural Science Foundation of China (grant ID, 30872478)
and the Gansu Special Program for High Technology Research and
Development (ID, 0912TCYA027). We thank Dr Lina Wang and Dr Meikai
Zhou from the same institution for their assistance in TMA
construction and immunohistochemistry.
References
|
1.
|
The GLOBOCAN project. http://globocan.iarc.fr/.
2008
|
|
2.
|
Gumbiner BM: Regulation of
cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol.
6:622–634. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Doukoumetzidis K and Hengartner MO: Cell
biology: dying to hold you. Nature. 451:530–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Winter JM, Ting AH, Vilardell F, et al:
Absence of E-cadherin expression distinguishes noncohesive from
cohesive pancreatic cancer. Clin Cancer Res. 14:412– 418. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Müller-Decker K and Fürstenberger G: The
cyclooxygenase-2-mediated prostaglandin signaling is causally
related to epithelial carcinogenesis. Mol Carcinog. 46:705–710.
2007.PubMed/NCBI
|
|
6.
|
Muraki C, Ohga N, Hida Y, et al:
Cyclooxygenase-2 inhibition causes antiangiogenic effects on tumor
endothelial and vascular progenitor cells. Int J Cancer. 130:59–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zhou Y, Ran J, Tang C, et al: Effect of
celecoxib on E-cadherin, VEGF, microvessel density and apoptosis in
gastric cancer. Cancer Biol Ther. 6:269–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rao DS, Gui D, Koski ME, et al: An inverse
relation between COX-2 and E-cadherin expression correlates with
aggressive histologic features in prostate cancer. Appl
Immunohistochem Mol Morphol. 14:375–383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Erdem H, Gündogdu C and Sipal S:
Correlation of E-cadherin, VEGF, COX-2 expression to prognostic
parameters in papillary thyroid carcinoma. Exp Mol Pathol.
90:312–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hu Z, Liu X, Tang Z, et al: Possible
regulatory role of Snail in NF-κB-mediated changes in E-cadherin in
gastric cancer. Oncol Rep. 29:993–1000. 2013.PubMed/NCBI
|
|
11.
|
Thiel A, Mrena J and Ristimäki A:
Cyclooxygenase-2 and gastric cancer. Cancer Metastasis Rev.
30:387–395. 2011. View Article : Google Scholar
|
|
12.
|
Li Y, Tan BB, Fan LQ, et al: Expression of
COX-2, survivin in regional lymph node metastases of gastric
carcinoma and the correlation with prognosis.
Hepatogastroenterology. 57:1435–1441. 2010.PubMed/NCBI
|
|
13.
|
Cho SJ, Kim N, Kim JS, et al: The
anti-cancer effect of COX-2 inhibitors on gastric cancer cells. Dig
Dis Sci. 52:1713–1121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yashiro M, Nakazawa K, Tendo M, et al:
Selective cyclooxygenase-2 inhibitor downregulates the paracrine
epithelial-mesenchymal interactions of growth in scirrhous gastric
carcinoma. Int J Cancer. 120:686–693. 2007. View Article : Google Scholar
|
|
15.
|
Jiménez P, García A, Santander S and
Piazuelo E: Prevention of cancer in the upper gastrointestinal
tract with COX-inhibition. Still an option? Curr Pharm Des.
13:2261–2273. 2007.PubMed/NCBI
|
|
16.
|
Winter JM, Ting AH, Vilardell F, et al:
Absence of E-cadherin expression distinguishes noncohesive from
cohesive pancreatic cancer. Clin Cancer Res. 14:412–418. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Spaderna S, Schmalhofer O, Wahlbuhl M, et
al: The transcriptional repressor ZEB1 promotes metastasis and loss
of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Neal CL, Henderson V, Smith BN, et al:
Snail transcription factor negatively regulates maspin tumor
suppressor in human prostate cancer cells. BMC Cancer. 12:3362012.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Dhasarathy A, Phadke D, Mav D, et al: The
transcription factors Snail and Slug activate the transforming
growth factor-beta signaling pathway in breast cancer. PLoS One.
6:e265142011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Becker KF, Rosivatz E, Blechschmidt K, et
al: Analysis of the E-cadherin repressor Snail in primary human
cancers. Cells Tissues Organs. 185:204–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Blechschmidt K, Kremmer E, Hollweck R, et
al: The E-cadherin repressor snail plays a role in tumor
progression of endometrioid adenocarcinomas. Diagn Mol Pathol.
16:222–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mazda M, Nishi K, Naito Y and Ui-Tei K:
E-cadherin E-cadherin is transcriptionally activated via
suppression of ZEB1 transcriptional repressor by small RNA-mediated
gene silencing. PLoS One. 6:e286882011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Francí C, Gallén M, Alameda F, et al:
Snail1 protein in the stroma as a new putative prognosis marker for
colon tumours. PLoS One. 4:e55952009.PubMed/NCBI
|
|
24.
|
Dohadwala M, Yang SC, Luo J, et al:
Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin
E(2) induces transcriptional repressors ZEB1 and snail in non-small
cell lung cancer. Cancer Res. 66:5338–5345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wu Y, Deng J, Rychahou PG, et al:
Stabilization of snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Stanisavljevic J, Porta-de-la-Riva M,
Batlle R, et al: The p65 subunit of NF-κB and PARP1 assist Snail1
in activating fibronectin transcription. J Cell Sci. 124:4161–4171.
2011.
|
|
27.
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
subunit of NF-kappaB through utilization of the IkappaB kinase and
activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Leng J, Han C, Demetris AJ, et al:
Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth
through Akt activation: evidence for Akt inhibition in
celecoxib-induced apoptosis. Hepatology. 38:756–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|