Introduction
Angiopoietin-like 4 (Angptl4), one of the key
components of the renin-angiotensin system, regulates glucose
homeostasis, insulin sensitivity and lipid metabolism (1–4).
This multi-functional hormone also affects the proliferation and
apoptosis of vascular endothelial cells. A recent study
demonstrated that the overexpression of Angptl4 impairs tumor
growth associated with enhanced apoptosis, and plays a role in the
inflammatory response; however, the mechanisms involved remain
unknown (5). Angptl4 expression
is upregulated under a variety of conditions, including treatment
with glucocorticoids, peroxisome proliferator-activated receptor
(PPAR) agonists and transforming growth factor-β (TGF-β) (6–9).
Moreover, the expression of Angptl4 is markedly induced under
ischemic and hypoxic conditions (10).
Acute lung injury (ALI) is a very common clinical
presentation characterized by severe clinical symptoms.
Lipopolysaccharide (LPS), a major component of Gram-negative
bacterial outer membranes, is an endotoxin which is believed to be
the main initiator for the microcirculatory abnormalities in septic
ALI (11). It has been reported
that Angptl4 is a downstream target gene of the ligand-activated
transcription factor, PPAR-γ (12). Studies have shown that PPAR-γ
plays an anti-inflammatory role in gastric inflammation induced by
ischemia/reperfusion (I/R) (13–17), aspirin (18) and Helicobacter pylori
infection (19) in rats. The
PPAR-γ ligand, troglitazone, has been shown to markedly decrease
the severity of pancreatic and pulmonary injury in acute
pancreatitis (AP) by reversing the increase in the mRNA expression
of the pro-inflammatory cytokines, interleukin (IL)-6 and tumor
necrosis factor (TNF)-α, in cerulean-induced pancreatitis in mice
(20). The lung microvascular
endothelial cell response to sepsis or ALI is incompletely defined,
even though they appear to be the first cells of the lungs to be
altered during ALI. The responses of lung microvascular endothelial
cells lead to changes in the permeability of the vessels. This
raises some important issues: i) whether Angptl4 is expressed in
rat pulmonary microvascular endothelial cells (RPMVECs); and ii)
effects of Angptl4 overexpression that occur during the LPS-induced
injury of the pulmonary microvascular endothelium. This may be
important in the modulation of endothelial function during
LPS-induced ALI. Therefore, in this study, we used RPMVECs to
determine whether Angptl4 overexpression attenuates the
inflammatory response and whether the regulation of Angptl4 affects
the modulation of endothelial function during LPS-induced ALI.
Materials and methods
Reagents
LPS from Escherichia coli and
phalloidin-tetramethylrhodamine B isothiocyanate were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Polyclonal antisera
against Angptl4, p-MEK1/2, p-AKT, Bax, caspase-8 and -9 were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The Angptl4 gene was purchased from Source BioScience
(Nottingham, UK, BC078944). Extracellular matrix (ECM), fetal calf
serum, penicillin and streptomycin were purchased from ScienCell
Research Laboratories (Carlsbad, CA, USA). Plastic tissue culture
flasks were from Costar (Cambridge, MA, USA).
Isolation of RPMVCs and primary cell
culture
The isolation of microvascular endothelial cells was
performed according to a modified method originally developed by
Chen et al(21). Briefly,
the fresh lungs isolated from the sacrificed rats were washed with
50 ml serum-free DMEM. The pleura was discarded from the lung
tissue and the outer edges of the lung lobe, which did not contain
large blood vessels, cut off and minced in serum-free DMEM using
scissors. The pellet was rinsed followed by the addition of DMEM
containing 20% fetal calf serum, 100 U/ml of penicillin and 0.1
mg/ml streptomycin. The cells were incubated at 37°C in a 5%
CO2 incubator. After 60 h, the residue lung tissues were
removed and the cells were grown in plastic tissue culture flasks.
The culture medium was replaced every 3 days and after reaching
confluence, the cells were treated with a 0.25% solution of
trypsin. The RMVECs were identified according to morphological and
functional criteria. They were examined under an inverted
microscope by phase-contrast microscopy.
Experimental design
The cells were randomly divided into the following
groups: i) control (CON) group; ii) LPS group: cells were exposed
to 100 ng/ml LPS and incubated for 6, 12 and 24 h; iii) LPS +
rosiglitazone (ROZ) group: cells were exposed to 100 ng/ml LPS and
50 μg/ml ROZ and incubated for 6, 12 and 24 h; iv) LPS + GW9662
group: cells were exposed to 100 ng/ml LPS and 50 μg/ml GW9662 and
incubated for 6, 12 and 24 h; v) pcDNA3.1-eGFP group: cells were
transfected with pcDNA3.1-eGFP and incubated for 48 h; vi)
pcDNA3.1-Angptl4-eGFP group: cells were transfected with
pcDNA3.1-Angptl4-eGFP and incubated for 48 h; vii) LPS +
pcDNA3.1-eGFP group: cells were transfected with pcDNA3.1-eGFP and
incubated for 48 h; they were then exposed to 100 ng/ml LPS and
incubated for 6, 12 and 24 h; viii) LPS + pcDNA3.1-Angptl4-eGFP
group: cells were transfected with pcDNA3.1-Angptl4-eGFP and
incubated for 48 h; they were then exposed to 100 ng/ml LPS and
incubated for 6, 12 and 24 h.
Angptl4 gene transfection
Cells (3–5 generation) were used for transfection.
The day before transfection, cells were seeded at approximately
5×105 cells/well in a 60-mm dish with 2 ml appropriate
growth medium until the cells reached 40–80% confluence. The
following day, 5 μg pcDNA3.1-eGFP or pcDNA3.1-Angptl4-eGFP DNA were
separately dissolved in TE buffer, pH 7–8, with cell growth medium
containing no serum, proteins or antibiotics to a total volume of
150 μl. The solution was mixed and centrifuged briefly to remove
drops from the top of the tube. Subsequently, 30 μl
SuperFect transfection reagent (Qiagen, Hilden, Germany) were added
to the DNA solution. The solution was then mixed by pipetting up
and down 5 times and the samples were incubated for 5–10 min at
room temperature to allow transfection complex formation.
Subsequently, 1 ml cell growth medium (containing serum and
antibiotics) was added to the reaction tube containing the
transfection complexes, after being washed once with PBS. The
solution was then mixed by pipetting up and down twice and the
total volume was immediately transferred to the cells in the 60-mm
dish. The cells were incubated with the transfection complexes for
2–3 h at 37°C in a 5% CO2 incubator. The cells were
transfected with the pcDNA3.1-eGFP or pcDNA3.1-Angptl4-eGFP
constructs and incubated for 24–48 h post-transfection to detect
the levels of gene expression.
Determination of Angptl4 mRNA expression
by real-time PCR
Total cellular RNA was extracted from the cells 48 h
post-transfection using RNAiso Plus (Takara Bio, Inc., Shiga,
Japan) according to the manufacturer’s recommendations. The level
of Angptl4 mRNA expression was quantified by real-time PCR using
Thermo Scientific Maxima SYBR-Green/ROX q-PCR Master Mix (2X)
(Fisher Scientific, Vilnius, Lithuania). RNA was solubilized in
RNase-free water and quantified by measurement of the absorbance at
260 nm. The potency was 220–280 ng/μl (the purity of RNA was
assured by examining the OD 260/280 as 1.6/1.9). cDNA was
synthesized using an iScript™ cDNA synthesis kit and reverse
transcription was then performed on a 1 μl RNA sample by the
addition of iScript reagents. Amplification and detection were
performed using the Rotor Gene 3000™ sequence detection system
(Corbett Research, Berkeley, CA, USA) starting with 500 ng of cDNA.
The primers and probes used were: Angptl4 forward,
5′-GCCGCTACTATCCACTAC-3′ and reverse, 5′-CCTGTTGCTCTGACTGTT-3′; and
β-actin forward, 5′–GGAGATTACTGCCCTGGCTCCTA-3′ and reverse,
5′-GACTCATCGTACTCCTGCTTGCTG-3′. β-actin was used as an internal
control. For relative quantification, the copy ratios of
Angptl4/β-actin were calculated and used as an indication of the
relative expression levels.
Western blot analysis
After the cell monolayer reached confluence, the
RPMVECs were washed with ice-cold PBS and were then resuspended at
1×106 cells/100 μl in ice-cold lysis buffer (50 mmol/l
Tris-HCl, pH 8.0, 150 mmol/l NaCl, 1% Triton X-100, 1 mmol/l
phenylmethyl sulfonyl fluoride, 0.02% sodium azide and 1
μg/ml aprotinin) and the adherent cells were scraped off the
plate into 100 μl lysis buffer/50 cm2 culture plate
surface. The cell lysates were placed on ice for 20 min and then
centrifuged at 12,000 × g for 10 min at 4°C. The post-mitochondrial
supernatant fraction was removed and stored in aliquots at −20°C.
The protein concentration of the samples was estimated using an
ultraviolet spectrophotometer (Perkin-Elmer, Norwalk, CT, USA).
Cell lysates were separated by sodium dodecyl sulfate-10%
polyacrylamide gel electrophoresis (SDS-PAGE). Briefly, after the
SDS-PAGE gels were run, they were transferred onto polyvinylidene
difluoride (PVDF) membranes using a semi-dry blot system
(Trans-Blot SD Semi-dry Transfer Cell; Bio-Rad, Richmond, CA, USA).
The membrane was blocked with blocking buffer (0.4% gelatin) for 1
h at 37°C and then probed with primary antibodies. Dilutions for
primary antibodies were as follows: anti-rat p-MEK1/2 antibody (200
μg/ml; 1:400), anti-rat Bax antibody (200 μg/ml;
1:400), anti-rat p-AKT (T450) antibody (200 μg/ml; 1:400,
all from Santa Cruz Biotechnology, Inc.), anti-rat AKT antibody
(200 μg/ml; 1:400, Bioworld Technology, Minneapolis, MN,
USA), anti-rat casapase-8 and -9 antibody (200 μg/ml;
1:400), rabbit polyclonal anti-rat Angptl4 antibody (200
μg/ml; 1:300) and anti-mouse β-actin antibody (200
μg/ml; 1:400, all from Santa Cruz Biotechnology, Inc.),
overnight at 4°C. The membrane was washed with PBST (PBS containing
0.5% Tween-20) and then incubated with horseradish
peroxidase-conjugated goat anti-rabbit or rabbit anti-goat, rabbit
anti-mouse secondary antibodies (500 μg/ml; 1:10,000) at
37°C for 1 h. Following repeated washes with PBST, the
antibody-antigen complexes were detected with ECL reagent
(Immobilon™ Western Chemiluminescent HRP Substrate; Millipore,
Billerica, MA, USA) and exposed to X-OMAT BT film (Kodak). Films
were scanned and the optical density (OD) of each band was detected
using the ChemiDoc™ XRS+ imaging system (Bio-Rad). The molecular
weights of the proteins were estimated by comparison with the
positions of the standard.
Cell viability assay
The viability of the cultured cells was determined
by MTT assay. The RPMVECs (10×104 cells/well) were
plated in 24-well plates and incubated at 37°C in a 5%
CO2 incubator. The cells were then divided into the
normal, LPS, LPS + ROZ, LPS + GW9662, pcDNA3.1-eGFP,
pcDNA3.1-Angptl4-eGFP, LPS + pcDNA3.1-eGFP and LPS +
pcDNA3.1-Angptl4-eGFP group and incubated for different periods of
time (6, 12 and 24 h). Subsequently, 100 μl of MTT (5 mg/ml) were
added to each well for an additional 4 h at 37°C. The supernatant
was removed and DMSO was added to dissolve the formazan crystals.
The optical absorbance was measured at 540 nm.
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was performed to quantify the concentration of
TNF-α in the culture medium using commercially available kits. The
TNF-α kit was purchased from R&D Systems (Minneapolis, MN,
USA).
Immunofluorescence
The RPMVECs were washed once with PBS and fixed in
3.7% formaldehyde solution in PBS for 10 min. They were then washed
extensively with PBS. They were dehydrated with aceton and then
permeabilized by incubation in PBS containing 0.1% Triton X-100.
After being washed with PBS; the cells were stained with 50
μg/ml fluorescent phalloidin conjugate solution in PBS for
40 min at room temperature. They were then washed several times
with PBS to remove unbound phalloidin conjugate and incubated for
10 min with DAPI. They were then washed extensively with PBS. The
stained cells were then examined under a Leica confocal laser
scanning microscope (Leica Microsystems, Mannheim, Germany).
Statistical analysis
All data are expressed as the means ± SD and were
analyzed statistically using one-way ANOVA followed by the
Newman-Keuls test. A P-value <0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using Graph Pad Prism 5 software.
Results
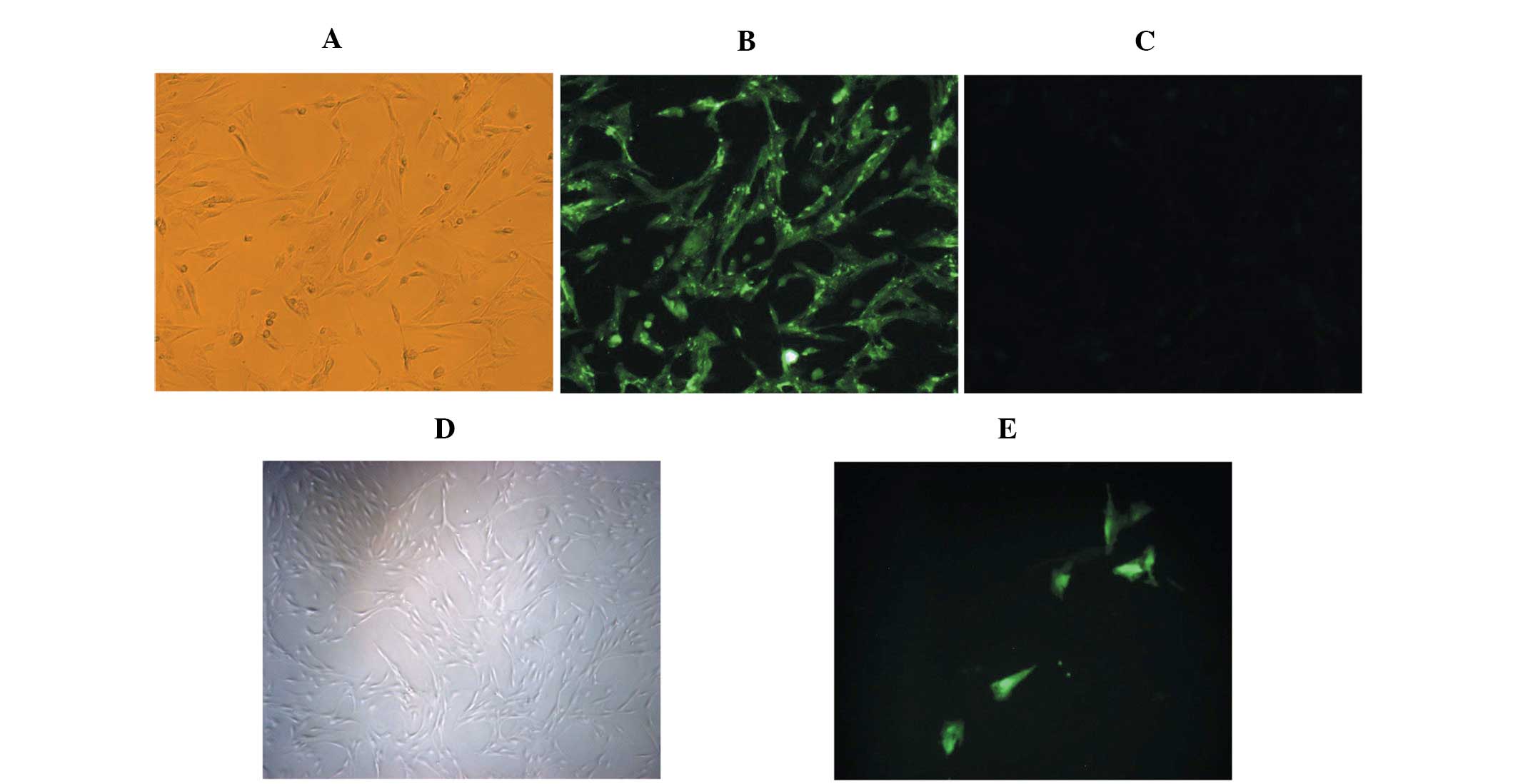
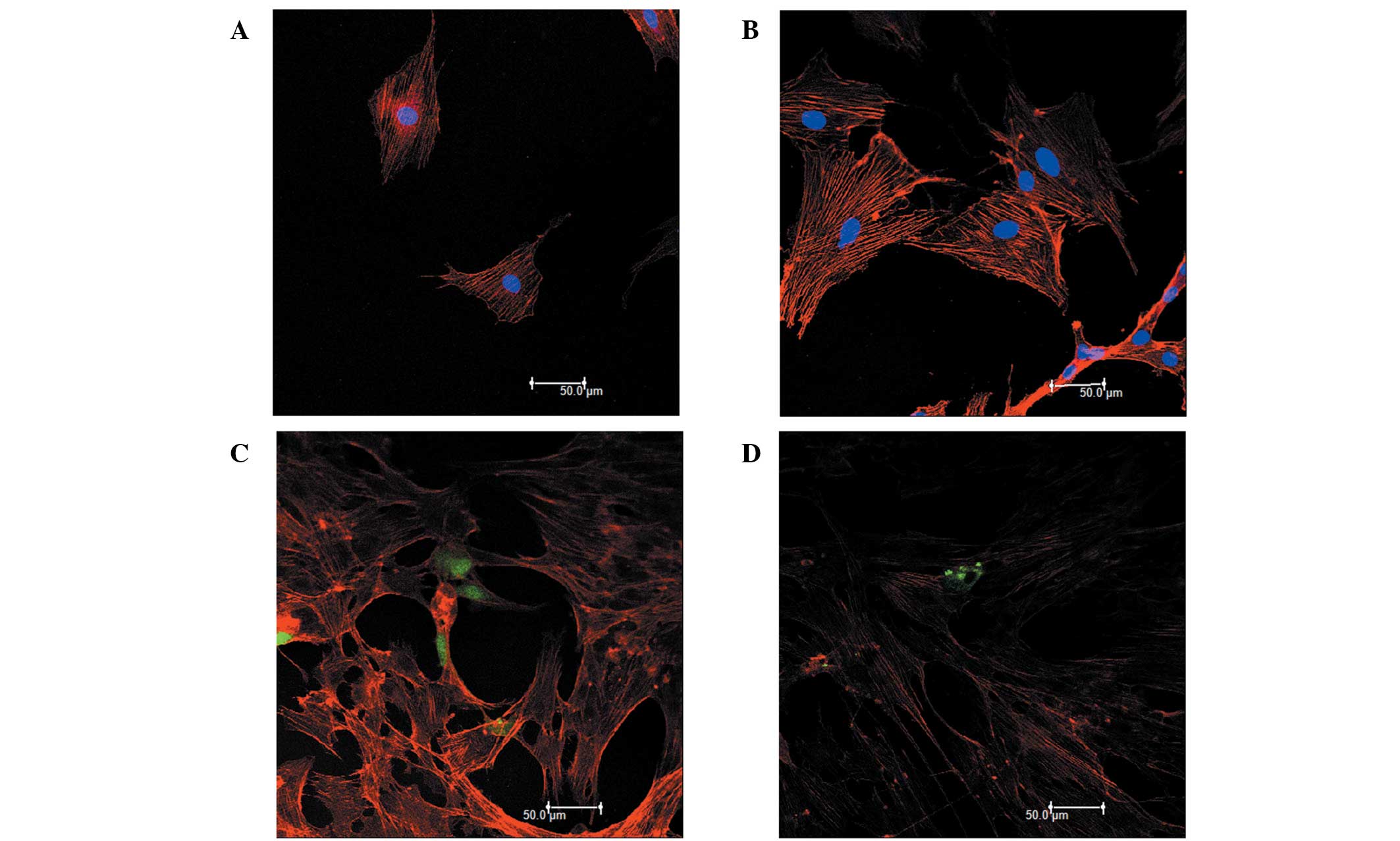
Characteristics of RPMVECs
The cells grew initially as capillary-like
structures and assumed the typical cobblestone morphology of
endothelial cells at confluence (Fig.
1A). These cells were characterized as endothelial cells by
CD31 antigen expression. Using immunofluorescence, the positive
expression of CD31 antigen in the RPMVECs was demonstrated by green
particles in the cytoplasm (Fig.
1B). The cells displayed negative staining by BSA (Fig. 1C). The cells were transfected with
the pcDNA3.1-eGFP or the pcDNA3.1-eGFP-Angptl4 vector and incubated
for 48 h. (Fig. 1E)
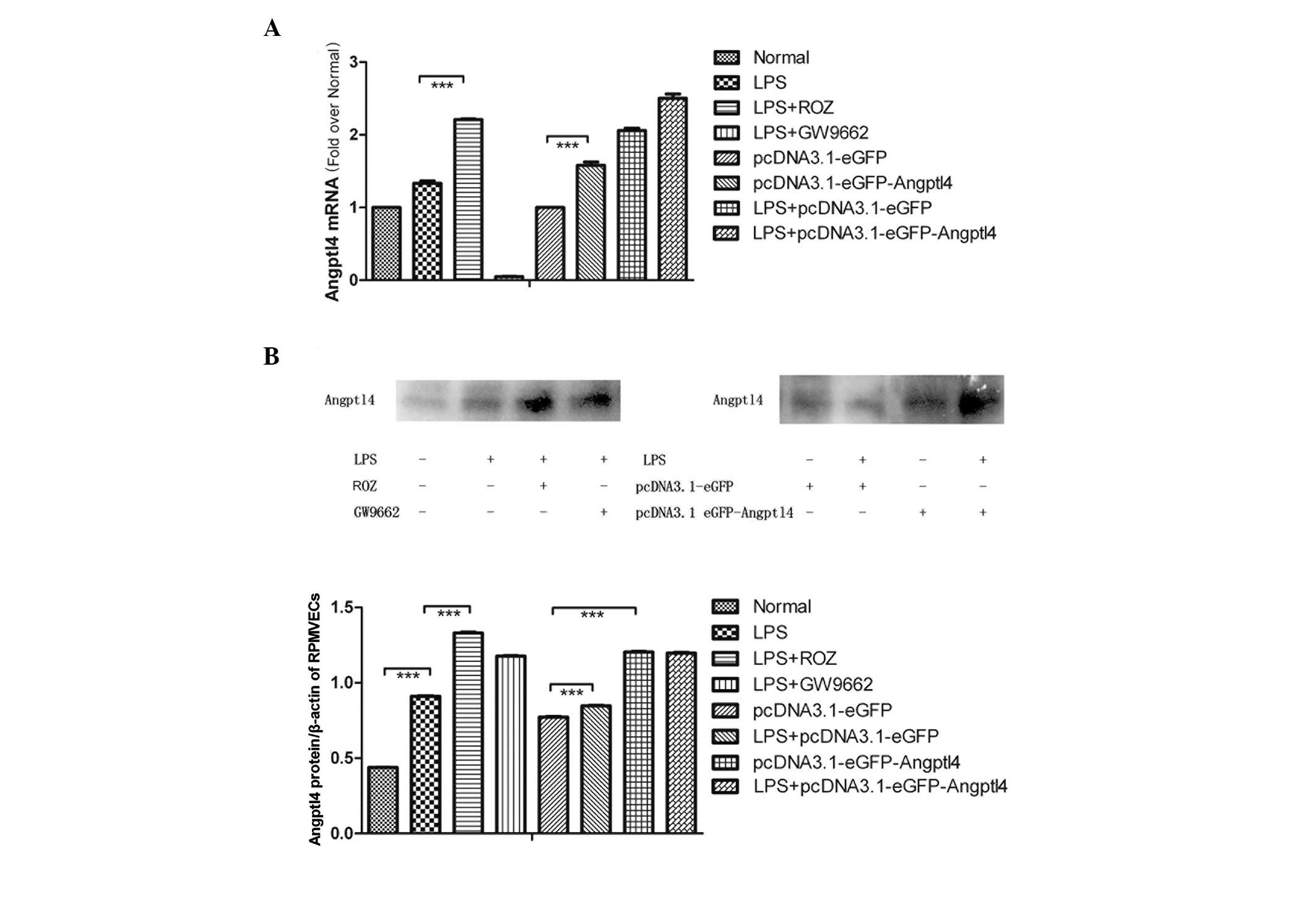
Effect of LPS on Angptl4 mRNA and protein
expression in RPMVECs
The RPMVECs were cultured and exposed to LPS for 24
h and 2 groups of cells were administered ROZ or GW9662. The
protein expression of Angptl4 was determined by western blot
analysis using Angptl4 antibodies as described in Materials and
methods. The mRNA expression of Angptl4 was determined by real-time
PCR (Fig. 2A). The mRNA and
protein expression of Angptl4 was slightly increased following
exposure to LPS. ROZ significantly increased the expression of
Angptl4 and GW9662 had the opposite effect. These results indicate
that LPS induces a slight increase in the expression of Angptl4 in
the PMVECs and ROZ markedly induces its expression.
Effect of LPS on upregulation of Angptl4
mRNA and protein expression in RPMVECs
The RPMVECs were cultured and transfected with the
pcDNA3.1-eGFP or the pcDNA3.1-eGFP-Angptl4 vector and then
stimulated with LPS (Fig. 2B). We
found that the mRNA levels increased by almost 2-fold compared with
the blank group, whereas the levels tripled following treatment
with ROZ and LPS. The protein levels were also altered.
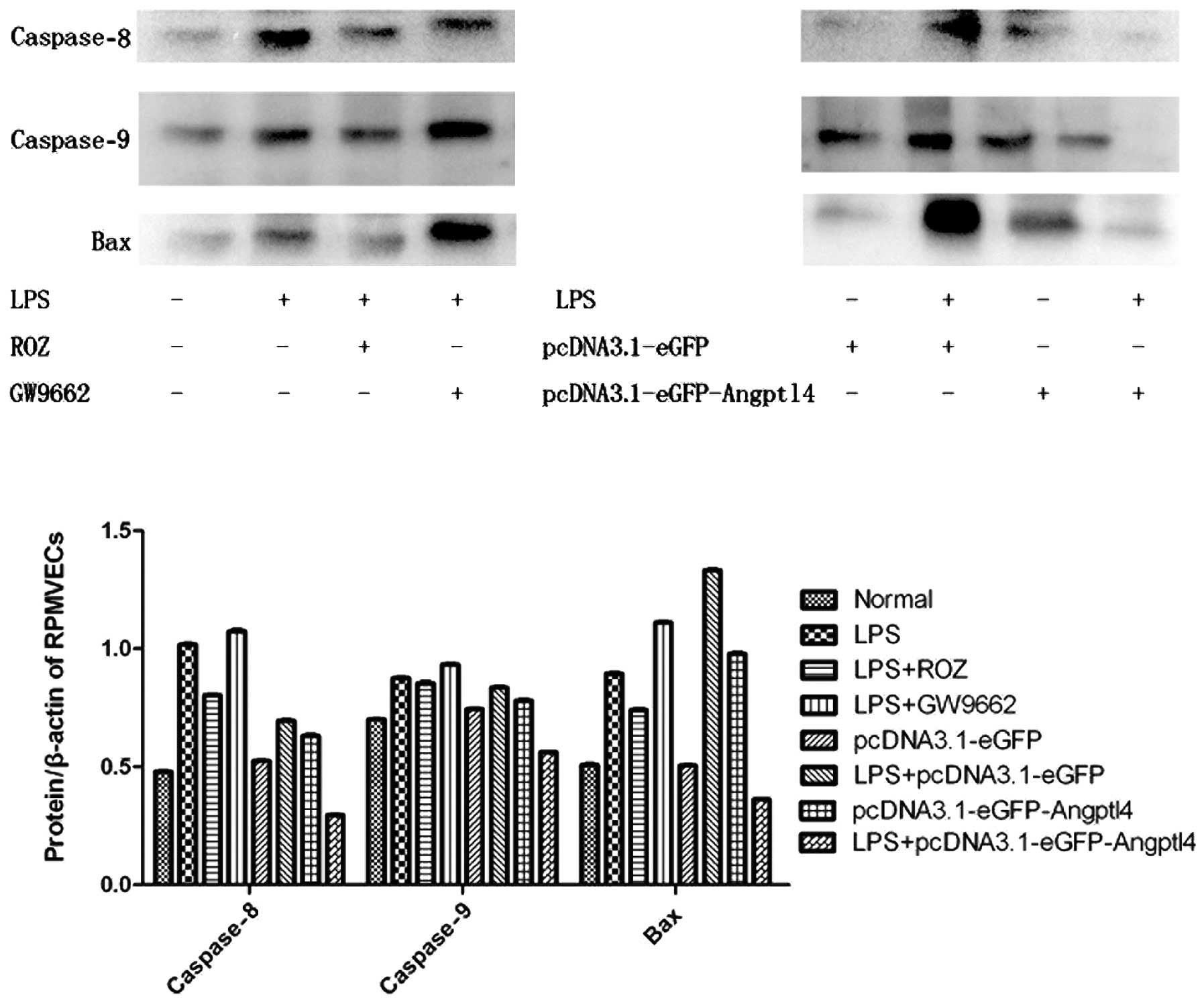
Anti-apoptotic effect of the
overexpression of Angptl4 on RPMVECs exposed to LPS
RPMVECs administered with ROZ and the transfected
cells were cultured and exposed to LPS for 24 h. We then detected
the expression levels of the pro-apoptotic factors, Bax and
casapase-8 and -9 by western blot analysis (Fig. 3). Our results revealed that the
expression of these factors was markedly decreased with the
upregulation of Angptl4. Of note, an opposite effect occurred after
transfection. This may be due to the secretion of Angptl4 protein
as a protective factor during acute injury.
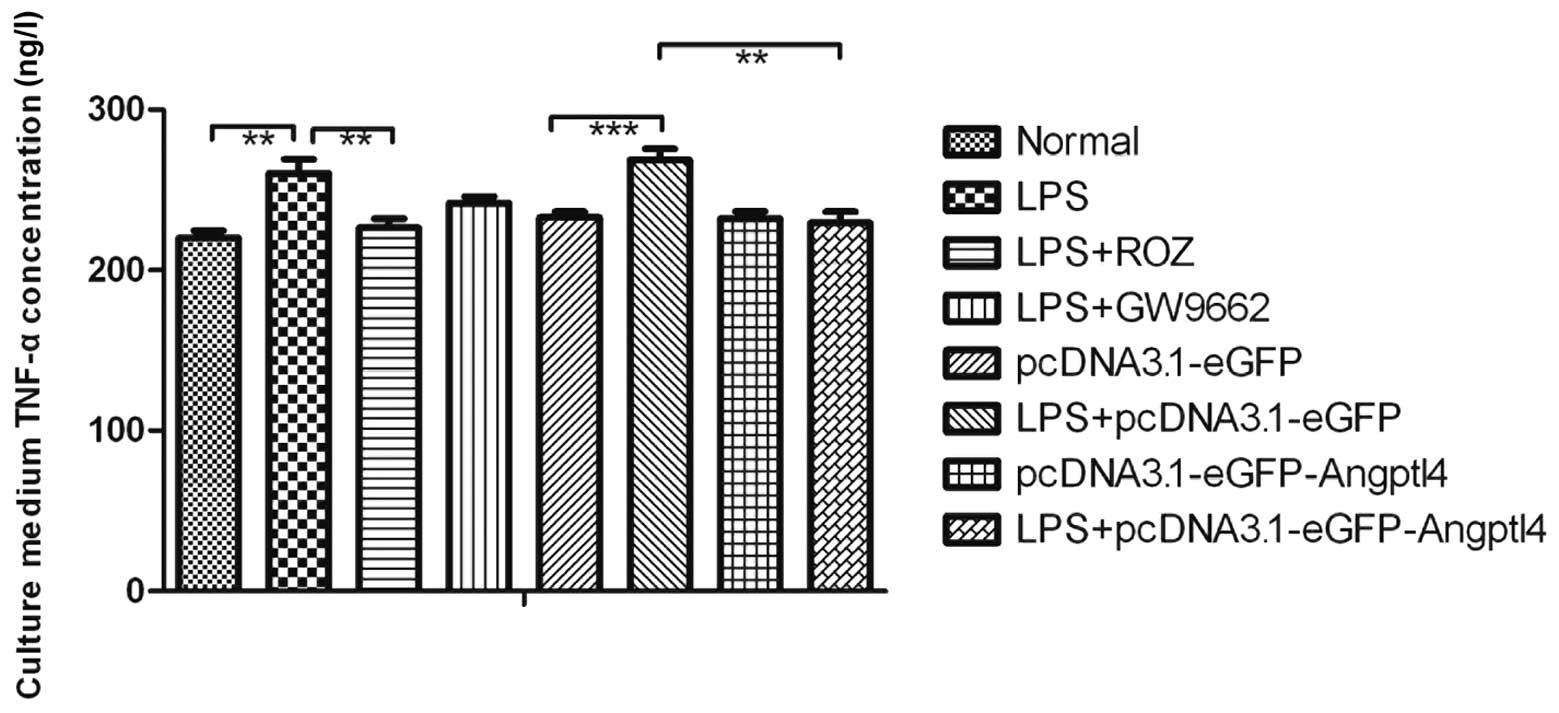
Measurement of TNF-α concentration in
culture medium by ELISA
To determine whether the overexpression of Angptl4
affects angiogenic and inflammatory molecules, such as TNF-α, ELISA
was carried out. The TNF-α concentration in the cell culture medium
in the LPS group was markedly increased compared with the CON group
(Fig. 4, P<0.01). However, the
decrease in the TNF-α concentration in the ROZ group and the group
transfected with Angptl4 and treated with LPS was significant
(P<0.01). These results suggest that the improved
anti-inflammatory functions may partly contribute to the beneficial
metabolic effects of Angptl4. These data demonstrate that the
secretion of Angptl4 in serum may influence the TNF-α
concentration; however, the exact mechanisms involved require
further investigation.
Angptl4 overexpression protects RPMVECs
against vascular permeability induced by LPS
Previously, it was shown that the stimulation of
RPMVECs with LPS results in cytoskeletal rearrangement, which can
destroy the F-actin cytoskeletan (22,23). Another study reported that the
inhibition of the phosphorylation of ERK1/2 attenuates the
polymerization of F-actin induced by LPS (24). In the current study, the
overexpression of Angptl4 was used to examine the hypothesis that
ERK1/2 activation is involved in LPS-induced actin cytoskeletal
rearrangement. As shown in Fig.
5, we found that the p-MEK1/2 protein expression was markedly
suppressed in the LPS-pcDNA3.1-Angptl4-eGFP group (P<0.01). As
shown in Fig. 6, the RPMVECs
exhibited a well organized actin cytoskeleton with F-actin fibers
crossing the body of the cells and forming a dense filamentous
network. The cells exposed to LPS showed a significant reduction in
the number of stress fibers and a different pattern of these stress
fibers, i.e., they were distributed in the periphery of the cells
(Fig. 6B). The RPMVECs
pre-treated with ROZ exhibited a morphology and actin stress fibers
similar to those of the control cells. Importantly, as shown in
Fig. 6D, transfection with
Angptl4 blocked the effects of LPS on the actin cytoskeleton. These
results indicate that the overexpression of Angptl4 inhibits ERK1/2
activation and plays a key role in actin cytoskeletal changes in
RPMVECs induced by LPS.
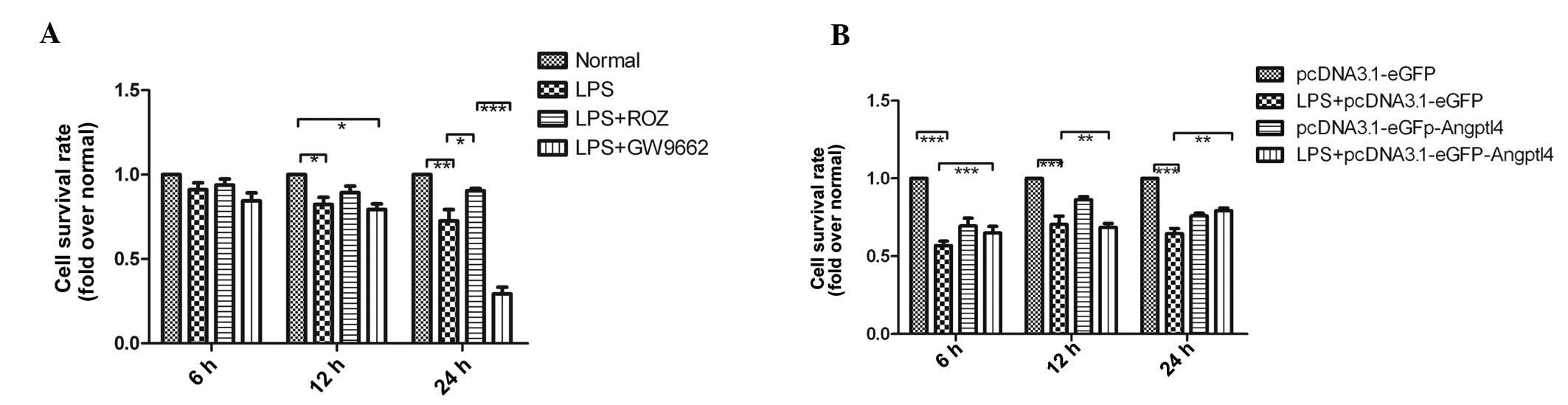
Effect of upregulation of Angptl4 on cell
viability
The effect of the upregulation of Angptl4 on cell
viability was dose- and time-dependent (Fig. 7). The absorption values
significantly increased following treatment with ROZ (50
μg/ml) and transfection with Angptl4 for 12 and 24 h
(P<0.05). The results revealed that the upregulation of Angptl4
improved the cell viability of the RPMVECs exposed to LPS following
treatment with ROZ and transfection with Angptl4 for 24 h.
Therefore, we selected the time point of 24 h to observe the
effects of ROZ on cell apoptosis.
Discussion
The results of the present study demonstrate the
effect of Angptl4 expression on RPMVECs during an acute
inflammatory stroke. This process needs to be explored further. The
pulmonary endothelium serves as a semi-selective barrier between
the plasma and interstitium of circulatory cells, macromolecules
and bioactive agents. The maintenance of this semi-selective
barrier represents an important physiological process for vessel
wall homeostasis and lung function. Injury to the endothelium
results in barrier dysfunction with exudation of proteins and fluid
within the interstitial tissue and alveolar space that contributes
to edema in lung injury.
LPS, a major component of Gram-negative bacterial
outer membranes, is an endotoxin that is believed to be the main
initiator of microcirculatory abnormalities in septic ALI. The
administration of LPS in various models has been shown to induce
profound vascular leakage in vivo(25) and to increase the permeability of
cultured endothelial cells. Studies have shown that in cultured
endothelial cells, an LPS-induced increase in endothelial
permeability occurs through several pathways, such as endothelial
contraction caused by the RhoA-dependent increase in myosin light
chain kinase (MLC) phosphorylation (26), reorganization of actin filaments,
protein tyrosine phosphorylation (27), etc.. We found that the F-actin
cytoskeleton in the RPMVECs in the LPS group was destroyed and that
the cell viability was markedly decreased.
In the present study, we aimed to determine the role
of Angptl4 in inflammatory responses. Angptl4 is a 50 kDa protein
that belongs to the angiopoietin-like family, all of which have a
secondary structural organization similar to angiopoietins,
including a NH2-terminal coiled domain and a
COOH-terminal fibrinogen-like domain (28,29). However, Angptl4 does not bind to
either the Tie1 or Tie2 angiopoietin receptors (30). Thus, it is currently considered an
orphan ligand whose functions differ from those of Angptl1 and
Angptl2. Over the last few years, a number of experimental studies
have further demonstrated that Angptl4 is involved in key events of
tumor growth (5,31). It has been suggested that Angptl4
inhibits VEGF-induced vascular leaks and neoangiogenesis in tumors,
while others have shown that Angptl4 hijacks integrin-mediated
signaling to maintain an elevated, oncogenic
O2:H2O2 ratio and therefore,
confers anoikis resistance to tumor cells, suggesting that Angptl4
is an important player in redox-mediated cancer progression
(5).
A previous study showed that Angptl4 is a positive
acute phase protein whose expression is increased in the liver,
heart, muscle and adipose tissue during the acute phase response
(32). In this study, we
demonstrate that Angptl4 expression increases in RPMVECs
administered with LPS during ALI. The mechanism accounting for the
increase in Angptl4 expression during the acute phase response is
unknown. Angptl4 expression is stimulated by PPARs (33), although previous studies have
shown it is unlikely that PPAR activation accounts for the observed
effects (34). The present study
confirmed the increased expression of Angptl4 at the mRNA and
protein level during the early stages of the LPS stimulation of
RPMVECs, which increased following the administration of ROZ and
transfection with pcDNA3.1-eGFP-Angptl4.
The molecular mechanisms that underlie the observed
functional response heterogeneity to Angptl4 in pulmonary
macrovascular cells are unknown but presumably involve the
differential activation of signals downstream of Angptl4. Since
Angptl4 is highly similar to angiopoietins structurally, it is not
surprising that human Angptl4, similar to Ang1, has anti-apoptotic
effects on endothelial cells, which can lead to the stabilization
of newly formed blood vessels. Consistent with this, human Angptl4
has been shown to promote the survival of endothelial cells and
blood vessel formation in in vivo experimental systems. Of
note, Yang et al(35)
showed that C-Angptl4 attenuates the bFGF-induced phosphorylation
of ERK1/2 MAP kinase, but not that of Akt and p38 MAP kinase. In
this study, immunofluorescence staining revealed that transfection
with Angptl4 and treatment with ROZ and LPS inhibited p-MEK1/2
expression. This suggests the Raf/MEK/ERK cascade inhibited
phosphorylated ERK polymerization with F-actin, inhibiting the
depolymerization and decreased density of central F-actin in the
PMVECs. It stabilized the formulation and protected the
cytoskeleton, while the permeability of RPMVECs was decreased
significantly. A previous study suggested that Angptl4 prevents
metastasis by inhibiting vascular leaks (36). We confer that the overexpression
Angptl4 protects the cytoskeleton of RPMVECs and decreases
pro-inflammatory cytokine leaks.
Previous studies have suggested the potential
pro-angiogenic activity of Angptl4, and data from several
independent laboratories have also demonstrated that Angptl4 is a
potent anti-angiogenic factor (34,37). Kim et al(30) showed that Angptl4 protects
endothelial cells from apoptosis through an endocrine action,
whereas Oike et al(29)
showed that Angptl4 inhibits VEGF-induced vascular leaks and
neoangiogenesis. In several studies, VEGF has been shown to induce
vascular permeability and lead to neutrophil infiltration, even
tissue edema. However, Angptl4 has been shown to inhibit the
VEGF-induced phosphorylation of ERK1/2, attributed to its specific
suppression of the ERK1/2 MAP kinase pathway (35,38).
In addition, we found that p-Akt (T450) decreased
following the induction of the overexpression of Angptl4 in the
cells stimulating with LPS. Nevertheless, there is a contradicting
result in vitro; the transfection of Angptl4 may induce
apoptopsis, but may inhibit apoptopsis following exposure to LPS,
which decreased Bax and caspase-8, and -9 protein expression in
this study. The reasons for these conflicting results and the
underlying mechanism of Angptl4 activity in the inflammatory
response, may be that p-AKT/AKT downregulation may have inactivated
the phosphorylation NF-κB and inhibited the downstream production
of inflammatory cytokines (39),
such as TNF-α. TNF-α is a pro-inflammatory mediator that promotes
the adhesion of leukocytes to the endothelium and plays a vital
role in the pathogenesis of severe acute pancreatitis (SAP)
(40). The increase in tissue and
serum TNF-α concentrations correlates directly with the severity of
pancreatic damage and inflammation in ALI (41). It can in turn damage the vascular
barrier, promoting leaks. As is already known, TNF-α is mediated by
a number of signaling pathways, such as the ERK1/2-MAPK and
TPK-Ras-MAPK pathways. The decrease in the TNF-α concentration
maybe be associated with the inhibition of the Raf/MEK/ERK cascade.
In this study, we found that the TNF-α concentration was markedly
decreased in the ROZ and pcDNA3.1-eGFP-Angptl4 groups, which
suggests that the overexpression of Angptl4 is associated with the
anti-inflammatory response.
In conclusion, the data from the present study
demonstrate that Angptl4 is a protective secreted protein that acts
downstream of other signaling events. Our results provide novel
evidence demonstrating that the possible anti-inflammatory
mechanisms of Angptl4 involve the protection of the cytoskeleton by
inhibiting cell contraction and decreasing the expression of
inflammatory cytokines. Further studies are required to fully
elucidate the signaling events underlying the anti-angiogenic
properties and anti-apoptotic activity of Angptl4.
Ackowledgements
This study was supported by The National Natural
Science Foundation of China (FNSFC_81173452). We completed this
study at the Laboratory of the First Affiliated Hospital of Dalian
Medical University and Dalian Center Hospital. The authors thank
HaiLong Li, Jun Ji and DongShang for their helpful discussions.
References
|
1
|
Ge H, Yang G, Yu X, Pourbahrami T and Li
C: Oligomerization state-dependent hyperlipidemic effect of
angiopoietin-like protein 4. J Lipid Res. 45:2071–2079. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu A, Lam MC, Chan KW, et al:
Angiopoietin-like protein 4 decreases blood glucose and improves
glucose tolerance but induces hyperlipidemia and hepatic steatosis
in mice. Proc Natl Acad Sci USA. 102:6086–6091. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sukonina V, Lookene A, Olivecrona T and
Olivecrona G: Angiopoietin-like protein 4 converts lipoprotein
lipase to inactive monomers and modulates lipase activity in
adipose tissue. Proc Natl Acad Sci USA. 103:17450–17455. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mandard S, Zandbergen F, van Straten E,
Wahli W, Kuipers F, Muller M and Kersten S: The fasting-induced
adipose factor/angiopoietin-like protein 4 is physically associated
with lipoproteins and governs plasma lipid levels and adiposity. J
Biol Chem. 281:934–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu P, Tan MJ, Huang RL, et al:
Angiopoietin-like 4 protein elevates the prosurvival intracellular
O2(−):H2O2ratio and confers
anoikis resistance to tumors. Cancer Cell. 19:401–415. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kersten S: Regulation of lipid metabolism
via angiopoietin-like proteins. Biochem Soc Trans. 33:1059–1062.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mandard S, Zandbergen F, Tan NS, et al:
The direct peroxisome proliferator-activated receptor target
fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in
blood plasma as a truncated protein that is increased by
fenofibrate treatment. J Biol Chem. 279:34411–34420. 2004.
View Article : Google Scholar
|
|
8
|
Koliwad SK, Kuo T, Shipp LE, et al:
Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a
direct glucocorticoid receptor target and participates in
glucocorticoid-regulated triglyceride metabolism. J Biol Chem.
284:25593–25601. 2009. View Article : Google Scholar
|
|
9
|
Padua D, Zhang XH, Wang Q, et al: TGF beta
primes breast tumors for lung metastasis seeding through
angiopoietin-like 4. Cell. 133:66–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belanger AJ, Lu H, Date T, et al: Hypoxia
up-regulates expression of peroxisome proliferator-activated
receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes:
role of hypoxia inducible factor 1alpha. J Mol Cell Cardiol.
34:765–774. 2002. View Article : Google Scholar
|
|
11
|
Liu F, Li W, Pauluhn J, Trubel H and Wang
C: Lipopolysaccharide-induced acute lung injury in rats:
comparative assessment of intratracheal instillation and aerosol
inhalation. Toxicology. 304:158–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feingold KR, Shigenaga JK, Cross AS, Moser
A and Grunfeld C: Angiopoietin like protein 4 expression is
decreased in activated macrophages. Biochem Biophys Res Commun.
421:612–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ichikawa H, Naito Y, Takagi T, et al: A
specific peroxisome proliferator-activated receptor-gamma
(PPAR-gamma) ligand, pioglitazone, ameliorates gastric mucosal
damage induced by ischemia and reperfusion in rats. Redox Rep.
7:343–346. 2002. View Article : Google Scholar
|
|
14
|
Konturek PC, Brzozowski T, Kania J, et al:
Pioglitazone, a specific ligand of the peroxisome
proliferator-activated receptor gamma reduces gastric mucosal
injury induced by ischaemia/reperfusion in rat. Scand J
Gastroenterol. 38:468–476. 2003. View Article : Google Scholar
|
|
15
|
Villegas I, Martin AR, Toma W and de la
Lastra CA: Rosiglitazone, an agonist of peroxisome
proliferator-activated receptor gamma, protects against gastric
ischemia-reperfusion damage in rats: role of oxygen free radicals
generation. Eur J Pharmacol. 505:195–203. 2004. View Article : Google Scholar
|
|
16
|
Wada K, Nakajima A, Takahashi H, et al:
Protective effect of endogenous PPARgamma against acute gastric
mucosal lesions associated with ischemia-reperfusion. Am J Physiol
Gastrointest Liver Physiol. 287:G452–G458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takagi T, Naito Y, Ichikawa H, et al: A
PPAR-gamma ligand, 15-deoxy-Delta12,14-prostaglandin J(2),
inhibited gastric mucosal injury induced by ischemia-reperfusion in
rats. Redox Rep. 9:376–381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Naito Y, Takagi T, Matsuyama K, Yoshida N
and Yoshikawa T: Pioglitazone, a specific PPAR-gamma ligand,
inhibits aspirin-induced gastric mucosal injury in rats. Aliment
Pharmacol Ther. 15:865–873. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Slomiany A, Nishikawa H and Slomiany BL:
Screening and modulation of extracellular signals by mucous
barrier. Serum glycosylphosphatidylinositol phospholipase D
(GPI-PLD) releases protective mucous barrier from oral mucosa. J
Physiol Pharmacol. 53:21–38. 2002.
|
|
20
|
Lappas M, Permezel M and Rice GE:
15-Deoxy-Delta(12,14)-prostaglandin J(2) and troglitazone
regulation of the release of phospholipid metabolites, inflammatory
cytokines and proteases from human gestational tissues. Placenta.
27:1060–1072. 2006. View Article : Google Scholar
|
|
21
|
Chen SF, Fei X and Li SH: A new simple
method for isolation of microvascular endothelial cells avoiding
both chemical and mechanical injuries. Microvasc Res. 50:119–128.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng C, Liu H, Ge H, et al:
Lipopolysaccharide induces expression of SSeCKS in rat lung
microvascular endothelial cell. Mol Cell Biochem. 305:1–8. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H and Sun GY: LPS induces
permeability injury in lung microvascular endothelium via AT(1)
receptor. Arch Biochem Biophys. 441:75–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adkison JB, Miller GT, Weber DS, et al:
Differential responses of pulmonary endothelial phenotypes to
cyclical stretch. Microvasc Res. 71:175–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao G, Kapushoc ST, Simpson AM, Thiemann
OH and Simpson L: Guide RNAs of the recently isolated LEM125 strain
of Leishmania tarentolae: an unexpected complexity. RNA.
7:1335–1347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Essler M, Staddon JM, Weber PC and
Aepfelbacher M: Cyclic AMP blocks bacterial
lipopolysaccharide-induced myosin light chain phosphorylation in
endothelial cells through inhibition of Rho/Rho kinase signaling. J
Immunol. 164:6543–6549. 2000. View Article : Google Scholar
|
|
27
|
Harhaj NS and Antonetti DA: Regulation of
tight junctions and loss of barrier function in pathophysiology.
Int J Biochem Cell Biol. 36:1206–1237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koishi R, Ando Y, Ono M, et al: Angptl3
regulates lipid metabolism in mice. Nat Genet. 30:151–157. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oike Y, Yasunaga K, Ito Y, et al:
Angiopoietin-related growth factor (AGF) promotes epidermal
proliferation, remodeling, and regeneration. Proc Natl Acad Sci
USA. 100:9494–9499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim I, Kim HG, Kim H, et al: Hepatic
expression, synthesis and secretion of a novel
fibrinogen/angiopoietin-related protein that prevents
endothelial-cell apoptosis. Biochem J. 346:603–610. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brunckhorst MK, Wang H, Lu R, et al:
Angiopoietin-4 promotes glioblastoma progression by enhancing tumor
cell viability and angiogenesis. Cancer Res. 70:7283–7293. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu B, Moser A, Shigenaga JK, Grunfeld C
and Feingold KR: The acute phase response stimulates the expression
of angiopoietin like protein 4. Biochem Biophys Res Commun.
391:1737–1741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hermann LM, Pinkerton M, Jennings K, et
al: Angiopoietin-like-4 is a potential angiogenic mediator in
arthritis. Clin Immunol. 115:93–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cazes A, Galaup A, Chomel C, et al:
Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial
cell adhesion, migration, and sprouting and alters actin
cytoskeleton. Circ Res. 99:1207–1215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YH, Wang Y, Lam KS, et al:
Suppression of the Raf/MEK/ERK signaling cascade and inhibition of
angiogenesis by the carboxyl terminus of angiopoietin-like protein
4. Arterioscler Thromb Vasc Biol. 28:835–840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galaup A, Cazes A, Le Jan S, et al:
Angiopoietin-like 4 prevents metastasis through inhibition of
vascular permeability and tumor cell motility and invasiveness.
Proc Natl Acad Sci USA. 103:18721–18726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li KQ, Li WL, Peng SY, et al: Anti-tumor
effect of recombinant retroviral vector-mediated human ANGPTL4 gene
transfection. Chin Med J (Engl). 117:1364–1369. 2004.PubMed/NCBI
|
|
38
|
Dhillon AS and Kolch W: Untying the
regulation of the Raf-1 kinase. Arch Biochem Biophys. 404:3–9.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu F, Wang H, Li J, Liang J and Ma S:
Homoplantaginin modulates insulin sensitivity in endothelial cells
by inhibiting inflammation. Biol Pharm Bull. 35:1171–1177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masamune A, Watanabe T, Kikuta K and
Shimosegawa T: Roles of pancreatic stellate cells in pancreatic
inflammation and fibrosis. Clin Gastroenterol Hepatol. 7:S48–S54.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Faulkner A, Rosen S and Norman C: The
right information may matter more than frequency-place alignment:
simulations of frequency-aligned and upward shifting cochlear
implant processors for a shallow electrode array insertion. Ear
Hear. 27:139–152. 2006. View Article : Google Scholar
|