Introduction
Accumulating evidence suggests that endothelial
progenitor cells (EPCs) are recruited to the sites of injury,
participate in the repair of damaged tissue, form new vessels in
ischemic areas and attenuate the development and progression of
atherosclerosis (1–4). Several preclinical studies using
animal models have shown the therapeutic efficacy of EPCs in
ischemic disorders and vascular injury (1,4–6).
Additionally, small-scale clinical trials, including directly
injecting EPCs into infarcted tissue or injecting pro-angiogenic
cytokines into target tissue to increase EPC number and function,
have been performed successfully in patients with cardiovascular
diseases (7–9).
Previous studies have demonstrated that multiple
pathological conditions can increase the production of reactive
oxygen species (ROS) in the vascular wall, including
hypercholesterolemia, diabetes and hypertension (10). Increased oxidative stress impairs
endothelial function and mediates atherosclerosis, stroke,
cardiovascular diseases and numerous other vascular diseases
(11–13). Moreover, studies have demonstrated
that EPCs exhibit high resistance to oxidative stress, which is a
characteristic feature of stem cells (14–16). It has also been demonstrated that
the higher expression of intracellular antioxidative enzymes, such
as catalase, manganese superoxide dismutase (MnSOD) and glutathione
peroxidase-1 (GPx-1) in EPCs, as opposed to mature endothelial
cells, is a critical mechanism through which EPCs are protected
against oxidative stress (14–16). However, other studies have
suggested that the increased expression of apoptosis
signal-regulating kinase 1 (ASK1) diminishes the vessel-forming
ability of EPCs following exposure to oxidave stress (13). Nevertheless, despite the proteins
identified in EPCs upon exposure to oxidative stress, the global
protein profile in EPCs remains elusive. In this study, to
determine protein response to oxidative stress in EPCs, we
systematically surveyed the changes in protein expression patterns
in EPCs treated with H2O2 by two-dimensional
(2D) gel/matrix-assisted laser desorption-ionization time-of-flight
(MALDI-TOF/TOF) mass spectrometry (MS).
Materials and methods
Cell culture
EPCs (Lonza, Walkersville, MD, USA) were maintained
in pre-warmed ECFC growth medium (EBM-2 basal medium supplemented
with EGM-2 SingleQuot cryovials and ECFC serum supplement; Lonza)
and then seeded in T-75 culture flasks pre-coated with rat tail
collagen I (BD Biosciences, Bedford, MA, USA) in a 37°C humidified
atmosphere with 5% CO2. The culture medium was replaced
with fresh, warm medium every 2 days until the cells reached 70–80%
confluency. EPC monolayers were passaged.
MTT assay
The effect of H2O2 on EPC
proliferation was determined by MTT assay. Early- passage EPCs
(passages 4–8) were seeded in a 96-well tissue culture plate
pre-coated with type I rat tail collagen (6,000–7,000 cells/well).
The following day, the cells were either left untreated or treated
with increasing concentrations of H2O2 in
quintuplicate for 3 h. The medium was then replaced with fresh,
warm medium supplemented with 10 μl of MTT (5 mg/ml). Four hours
after incubation, the supernatant was discarded and the EPCs were
shaken with 200 μl of DMSO for 10 min. The OD value was measured at
490 nm.
Scratch-wound assay
To determine the migration activity of EPCs, the
cells were seeded in 24-well tissue culture plates. After reaching
80–90% confluency, the cells were pre-treated with
H2O2 for 3 h, then the monolayer EPCs were
scrape-wounded with a sterile micropipette tip to create a denuded
zone (gap) of constant width. After washing with phosphate-buffered
saline (PBS) to remove the cell debris, the cells were incubated in
fresh, warm medium for a further 18 h. The monolayer scratched
cells were observed under an inverted phase-contrast microscope
with a ×10 objective (Olympus, Tokyo, Japan), and images were
acquired using a color camera (Olympus) immediately after wounding
and 18 h after wounding. The percentage of wound closure was
estimated using the following equation: wound closure (%) = [1−
(wound area at Tt/wound area at T0)] ×100%,
where Tt is the time after wounding and T0 is
the time point of wounding. Five fields/well were examined and 5
independent experiments were performed.
Matrigel invasion assay
The EPCs that were either untreated or pre-treated
with an increasing concentrations of H2O2 for
3 h, were seeded at 15,000 cells/well in 96-well tissue culture
plates coated with 50 μl of Matrigel (BD Biosciences). Following
incubation at 37°C for 8 or 24 h, the cells in the central field of
each well were observed under an inverted phase-contrast microscope
with a ×10 objective (Olympus), and images were acquired
photographed using a color camera (Olympus). The total vessel
length of each image was calculated from the captured images using
Image-Pro Plus software. Closed network units of the central visual
field of each well were enumerated by visual inspection. Three
independent experiments were performed.
Proteomic sample preparation
The cells (passages 4–7) were harvested and washed 3
times with ice-cold PBS. The cells were then lysed with lysis
buffer (7 M urea, 2 M thiourea, 4% CHAPS, 30 mM Tris, pH 8.8)
containing 1% protease inhibitors and nuclease mix. Following
centrifugation at 25,000 × g for 30 min, the supernatants were
collected. Proteins in the supernatant were cleaned up and
quantified using a Clean-up kit and 2-D Quant kit (GE Healthcare,
Uppsala, Sweden) according to the manufacturer’s instructions.
Two-dimensional differential in-gel
electrophoresis (2D-DIGE)
Following the manufacturer’s instructions, 50 μg of
proteins from each sample were minimally labelled with fluorescent
dyes. Following incubation on ice for 30 min, the reaction was
terminated by the addition 1 μl of 10 mM lysine. Cy3-, Cy5- and
Cy2-labelled samples and internal standards were pooled and then
rehydration buffer [8 M urea, 2% CHAPS, 0.4% IPG buffer and 0.28%
dithiothreitol (DTT)] was added to gain equal volumes. The samples
were loaded on an IPG strip (24 cm, pH 4–7, linear) for isoelectric
focusing (IEF) on an IPGphor isoelectric focusing system (GE
Healthcare). The parameters were 30 V for 6 h (rehydration) at
20°C, 500 V for 1 h, gradient 1,000 V for 1 h, gradient 8,000 V for
2 h and 8,000 V for 7 h. Following IEF, the strips were first
equilibrated in equilibration solution of 50 mM Tris-HCl (pH 8.8),
6 M urea, 30% (v/v) glycerol, 2% (w/v) sodium dodecyl sulfate (SDS)
and 1% (w/v) DTT for 15 min and then again in the same solution
without DTT [DTT was replaced with 4% (w/v) iodoacetamide] for a
further 15 min. The IPG strips were then loaded onto 12.5%
polyacrylamide gels in an Ettan DALTsix system (GE Healthcare) for
electrophoresis at 1.5 W/gel overnight until the bromophenol blue
tracking dye reached the bottom of the gels.
Image acquisition and analysis
Fluorescent images were acquired using a Typhoon
9400 scanner (GE Healthcare) at a resolution of 100 μm. Matching,
quantification and statistical analysis were carried out using
DeCyder 2-D Differential Analysis software (GE Healthcare) and
images were scanned manually to eliminate artifacts. Protein spots
with at least a 1.3-fold increase or decrease in intensity between
the control and H2O2-treated groups
(P<0.05, Student’s t-test) were considered as significantly
differentially expressed proteins. Two other preparative gels with
the control and H2O2-treated groups were made
with 600 μg of protein under the same conditions. The gels were
fixed in 20% trichloroacetic acid (TCA) overnight and stained with
PhastGel Blue. For further identification, the spots of interest
were excised from the preparative gels using an Ettan Spot Picker
(GE Healthcare).
Tryptic in-gel digestion of
two-dimensional gel electrophoresis (2DE)-resolved proteins
Protein spots, which were excised from the
preparative gels, were destained in 100 mM
NH4HCO3/30% acetonitrile (ACN). After
removing the destaining buffer, the gel pieces were lyophilized and
rehydrated in 30 μl of 50 mM NH4HCO3
containing 50 ng of trypsin (sequencing grade; Promega, Madison,
WI, USA). Following overnight digestion at 37°C, the peptides were
extracted 3 times with 0.1% trifluoroacetic acid (TFA) in 60% ACN.
The extracts were pooled together and lyophilized. The resulting
lyophilized tryptic peptides were kept at −80°C until mass
spectrometric analysis was performed. A protein-free gel piece was
treated as described above and used as the control to identify
autoproteolysis products derived from trypsin.
MALDI-TOF/TOF MS and protein
identification
MS and MS/MS spectra were obtained using the ABI
4800 Proteomics Analyzer (MALDI-TOF/TOF; Applied Biosystems, Foster
City, CA, USA) operating in a result-dependent acquisition mode.
Peptide mass maps were acquired in the positive ion reflector mode
(20 kV accelerating voltage) with 1,000 laser shots/spectrum.
Monoisotopic peak masses were automatically determined within the
mass range of 800–4,000 Da with a signal-to-noise ratio minimum set
to 10 and a local noise window width of m/z 250. Up to 10 of the
most intense ions, with a minimum signal-to-noise ratio >50,
were selected as precursors for MS/MS acquisition, excluding common
trypsin autolysis peaks and matrix ion signals. In the
MS/MS-positive ion mode, spectra were averaged with 2 kV collision
energy, and default calibration was set. Monoisotopic peak masses
were automatically determined with a signal-to-noise ratio minimum
set to 5 and a local noise window width of m/z 250. The MS together
with MS/MS spectra were searched against the UniProtKB/Swiss-Prot
database (Swiss-Prot 56.9) using GPS Explorer software (version
3.6; Applied Biosystems) and Mascot software (version 2.1; Matrix
Science Inc., Boston, MA, USA) using the following parameter
settings: trypsin cleavage, 1 missed cleavage allowed,
carbamidomethylation set as fixed modification, oxidation of
methionines allowed as variable modification, peptide mass
tolerance set to 100 ppm, fragment tolerance set to ±0.3 Da and
minimum ion score confidence interval for MS/MS data set to
95%.
Western blot analysis
From the H2O2-treated group,
150 μg of protein were minimally labelled with Cy5 fluorescent dye.
Following incubation on ice for 30 min, the reaction was terminated
by the addition of 1 μl of 10 mM lysine. The proteins were then
pooled and rehydration buffer (8 M urea, 2% CHAPS, 0.4% IPG buffer
and 0.28% DTT) was added to gain 250 μl of final volume. The sample
was loaded onto an IPG strip (13 cm, pH 4–7, linear) for IEF on an
IPGphor isoelectric focusing system (GE Healthcare). The parameters
were 30 V for 6 h (rehydration) at 20°C, 500 V for 1 h, gradient
1,000 V for 1 h, grad 8,000 V for 1 h and 8,000 V for 5 h.
Following IEF, the strips were first equilibrated in equilibration
solution containing 50 mM Tris-HCl (pH 8.8), 6 M urea, 30% (v/v)
glycerol, 2% (w/v) SDS and 1% (w/v) DTT for 15 min and then again
in the same solution without DTT [DTT was replaced with 4% (w/v)
iodoacetamide] for a further 15 min. The IPG strips were loaded
onto 10% polyacrylamide gels for electrophoresis at 10 mA for 1 h
followed by 30 mA until the bromophenol blue tracking dye reached
the bottom of the gels. Fluorescent images were acquired using a
Typhoon 9400 scanner (GE Healthcare) at a resolution of 100 μm. A
6×6 cm gel containing protein was excised from the whole gel.
Proteins were electrophoretically transferred onto a PVDF membrane
(GE Healthcare). The membrane was blocked in TBS containing 0.1%
Tween-20 (TBS-T) and 5% fat-free milk for 1 h. After a brief wash
with TBS-T buffer, the membrane was incubated overnight at 4°C with
mouse anti-human peroxiredoxin-3/thioredoxin-dependent peroxide
reductase, mitochondrial (Prx-3) antibody (Abcam, Cambridge, UK)
diluted in TBS-T buffer (1:1,000 dilution). The primary antibody
was removed, and the membrane was washed extensively in TBS-T
buffer. Subsequent incubation with goat anti-mouse antibody (1:500)
pre-adsorbed with Cy3 (Abcam) was performed at room temperature for
2 h. The membrane was washed extensively in TBS-T buffer to remove
the secondary antibody. Fluorescent images were acquried using a
Typhoon 9400 scanner (GE Healthcare) at a resolution of 100 μm.
Statistical analysis
Data are expressed as the means ± SEM. Differences
between groups were analyzed using Student’s t-tests. A value of
P<0.05 was considered to indicate a statistical significant
difference.
Results
Tube-forming ability of EPCs is affected
to a greater extent following exposure to oxidative stress
To investigate the effects of oxidative stress on
EPCs, the cells were pre-treated with H2O2 at
different final concentrations (100–400 μM) for 3 h prior to the
following experiments. MTT, scratch-wound and Matrigel invasion
assay were performed to examine the proliferation, migration and
tube-forming ability of the EPCs, respectively. The results were
similar to those from our previous study (17). Treatment with
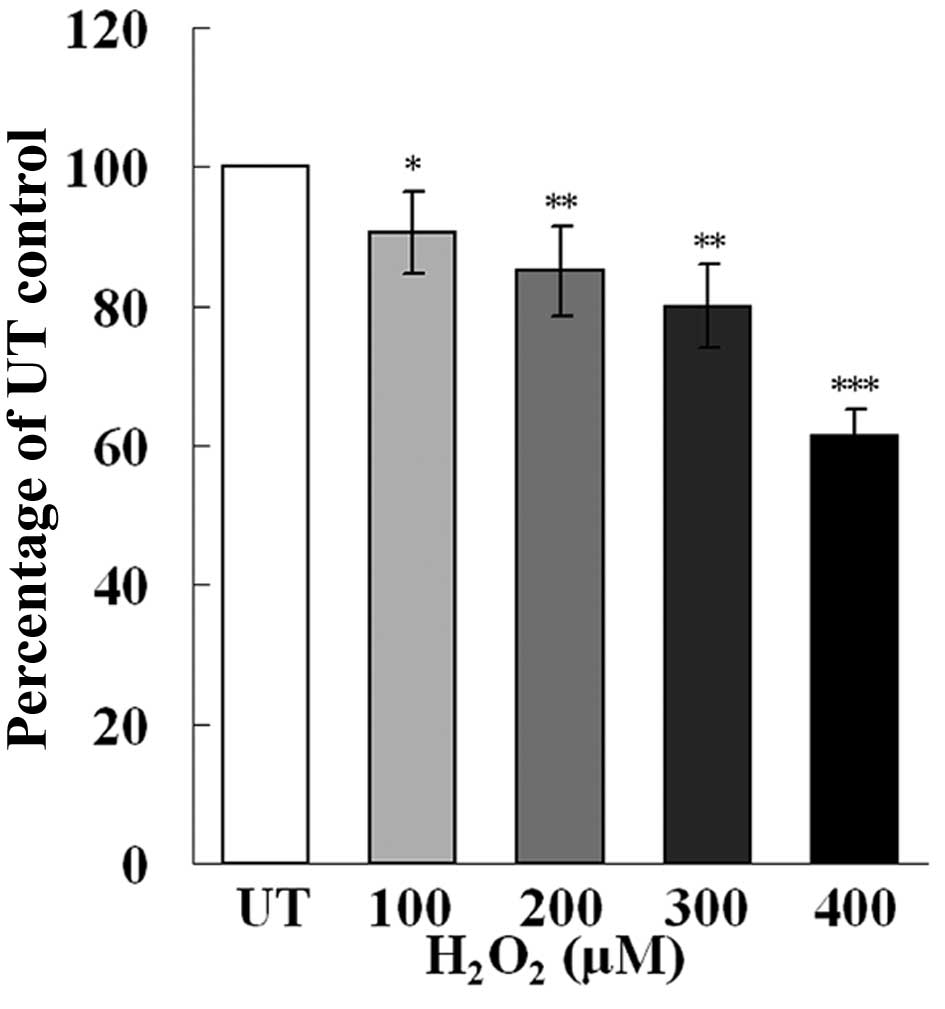
H2O2 resulted in a dose-dependent decrease in
cell proliferation (Fig. 1) and
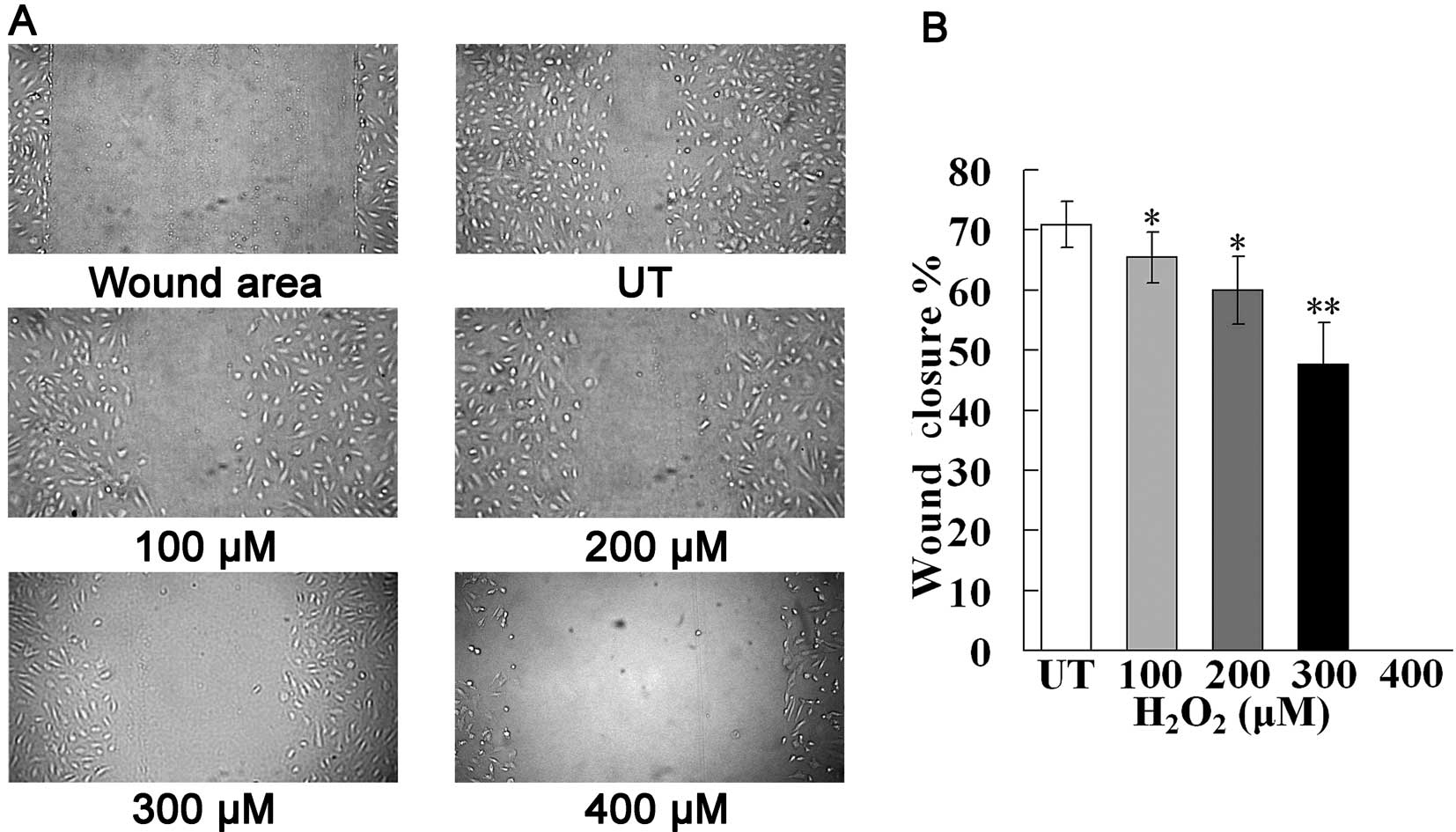
cell migration (Fig. 2).
Additionally, treatment with H2O2 at a final
concentration of 300 μM markedly impaired cell migration compared
with the control group (33% decrease). At a final
H2O2 concentration of 400 μM, cell migration
was completely suppressed. These data suggest that following
exposure to oxidative stress, the migration ability and survival of
EPCs is impaired.
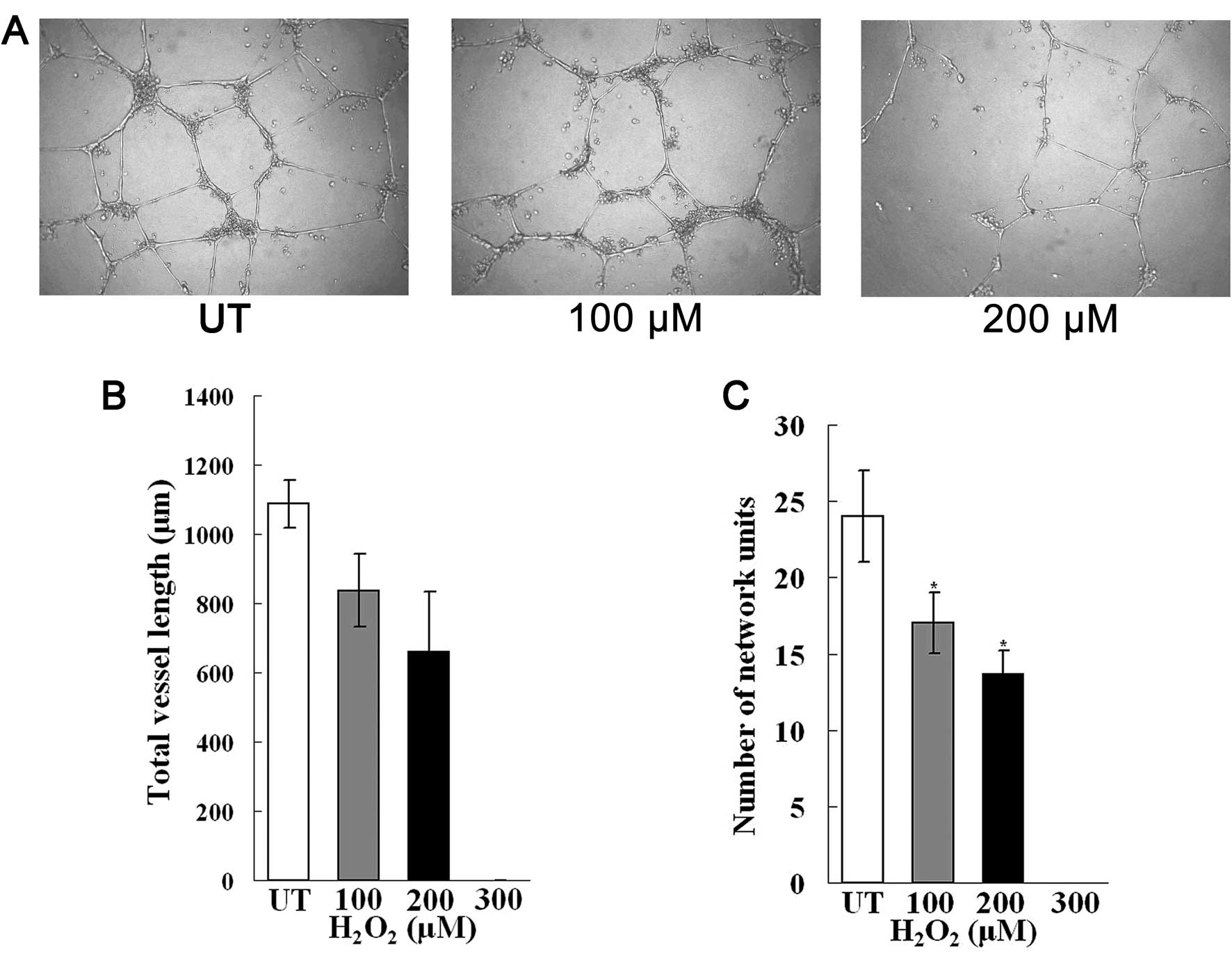
To examine the capillary tube-forming ability of
EPCs following exposure to oxidave stress, we performed Matrigel
invasion assay and the tube-forming ability of the EPCs was
observed over a period of 24 h. After 8 h, we observed the
initiation of capillary-like structures in the untreated and
H2O2-treated EPCs. However, after 24 h, the
EPCs treated with H2O2 exhibited reduced
vessel length and closed network units (Fig. 3), suggesting that the EPCs failed
to form effective tubules due to oxidative stress. In addition,
following treatment with H2O2 at a final
concentration of 300 μM, there was no part or closed network unit
formation, although cells still proliferated and migrated at this
concentration. This suggests that the tube-forming ability of the
EPCs is affected to greater extent in response to oxidative
stress.
2DE of H2O2-treated
EPCs and identification of differentially expressed proteins
To identify the proteins involved in the protection
of EPCs under oxidative stress conditions, comparative proteomic
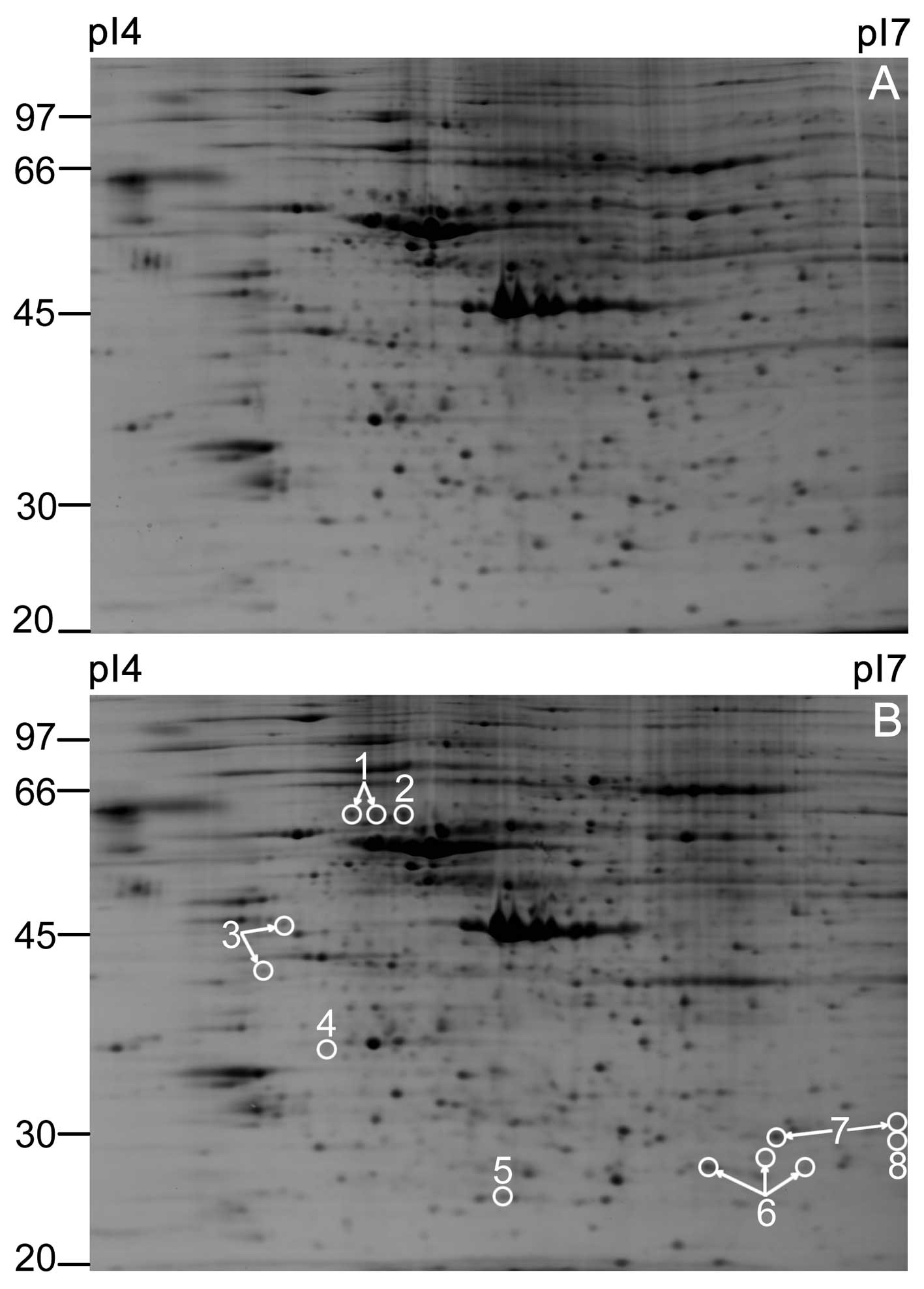
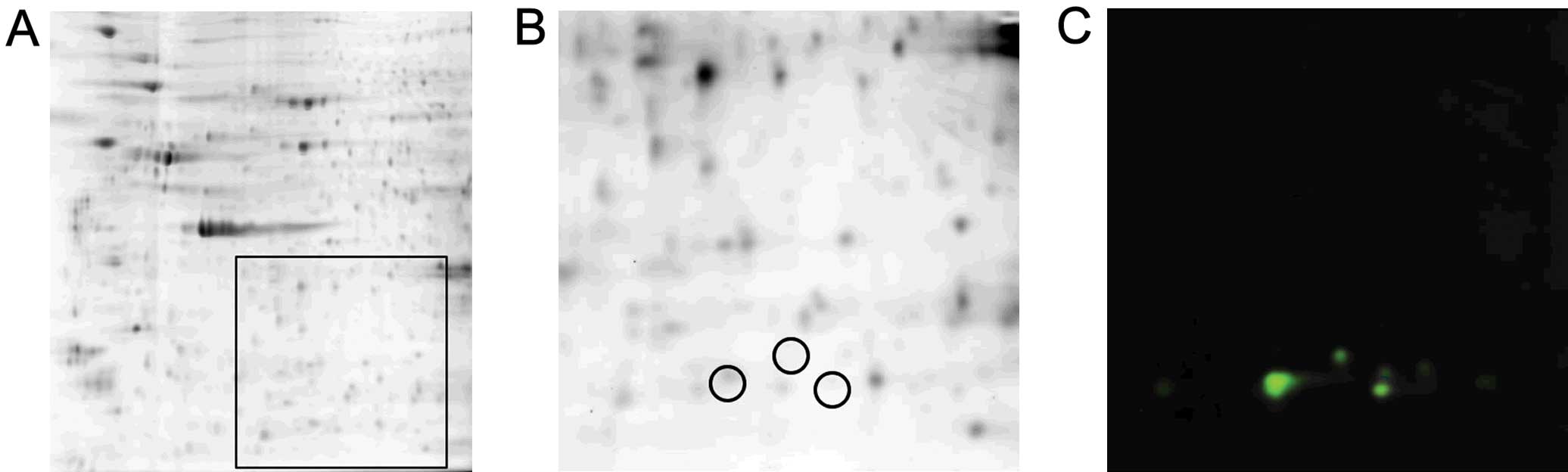
analysis was performed. Representative 2DE gel images from the
untreated control group and H2O2-treated
group are shown in Fig. 4. A
total of 11 upregulated protein spots and 2 downregulated protein
spots were observed in the H2O2-treated group
compared with control group, which are indicated by circles
(Fig. 4B). The pixel volume of
each spot was calculated, normalized and compared between the 2
groups using the Student’s t-test. For all the selected spots, the
P-values between the untreated control and
H2O2-treated groups were <0.05.
The differentially expressed protein spots were
excised from the preparative gels, subjected to trypsin digestion,
and identified using MALDI-TOF/TOF MS. The peptide mass peaks and
peptide sequences were compared with those in the Swiss-Prot
database. Eight proteins were identified successfully, 6 of which
were upregulated and the remainder were downregulated. Table I shows the Swiss-Prot accession
number, theoretical molecular weight and the isoelectric point (pI)
of each protein spot, altered ratio
(H2O2-treated/control group) and the protein
scores of each protein. In some cases, 1 protein was identified in
several discrete spots, presumably representing protein
modifications (such as spot 6), suggesting post-translational
modifications consisting of the addition or removal of a small
charged moiety. However, the specific nature of these modifications
cannot be ascertained from the present data.
 | Table ISummary of differentially expressed
proteins in EPCs in response to exposure to
H2O2-induced oxidative stress. |
Table I
Summary of differentially expressed
proteins in EPCs in response to exposure to
H2O2-induced oxidative stress.
| Spot no. | Protein
identity | Swiss-Prot no. | Relative spot
altered ratio % (H2O2/control, 100%) | pI/Mr | Mascot score |
|---|
| 1 | EGF-containing
fibulin-like extracellular matrix protein 1 | Q12805 | 146 | 4.95/56.9 | 440 |
| 2 | Rab GDP
dissociation inhibitor α | P31150 | 137 | 5/51.2 | 137 |
| 3 | Vimentin | P08670 | 151 | 5.06/53.7 | 351 |
| 4 | ADP-sugar
pyrophosphatase | Q9UKK9 | 69 | 4.87/24.6 | 200 |
| 5 |
Peroxiredoxin-2 | P32119 | 273 | 5.66/22.0 | 335 |
| 6 |
Thioredoxin-dependent peroxide reductase,
mitochondrial | P30048 | 286 | 7.67/28.0 | 277 |
| 7 |
Peroxiredoxin-6 | P30041 | 307 | 6/25.1 | 725 |
| 8 | Triosephosphate
isomerase | P60174 | 70 | 6.45/26.94 | 299 |
Proteins with altered expression patterns following
exposure to H2O2-induced oxidative stress,
were categorized into several groups according to their
putative/known functions. The major group contained anti-oxidative
proteins, including peroxiredoxin-2 (Prx-2), Prx-3 and
peroxiredoxin-6 (Prx-6), all of which were upregulated. Vimentin
and EGF-containing fibulin-like extracellular matrix protein 1
(EFEMP1), cytoskeleton proteins, were upregulated. In addition,
ADP-sugar pyrophosphatase (NUDT5) and triose-phosphate isomerase
(TIM) were downregulated.
Western blot analysis
In order to demonstrate that protein 6 (Fig. 4B) was Prx-3, western blot analysis
was performed. Firstly, whole cell proteins were separated by 2DE
and a fluorescent image was acquired (Fig. 5A). A 6×6 cm square area, indicated
in Fig. 5A, was excised. Proteins
were electrophoretically transferred onto a PVDF membrane and
scanned using Cy5 (Fig. 5B) and
Cy3 (Fig. 5C). Protein 6 was
detected both by Cy5 (Fig. 5B)
and Cy3 (Fig. 5C), suggesting
that protein 6 was actually Prx-3.
Discussion
EPCs, which circulate in peripheral blood vessels,
can be recruited to damaged vessels or tissues and participate in
vascular repair and neovascularization. Thus, the enhancement of
EPC number and function is a novel therapeutic approach to vascular
diseases. Studies have shown that vascular diseases, including
hypertension, diabetes and atherosclerosis, are characterized by
elevated levels of ROS (14). To
comprehensively determine alterations in protein expression in
response to oxidative stress in EPCs, we performed a proteomic
analysis using 2D-DIGE followed by MALDI-TOF/TOF MS. We identified
8 proteins which were differentially expressed in the untreated and
H2O2-treated cells, and 6 were upregulated
and 2 were downregulated. Finally, a spot from the 2D-DIGE gel
(protein 6) was selected to further confirm the results from MS by
western blot analysis.
It has been reported that several antioxidant
enzymes, including catalase, MnSOD and GPx-1 are highly expressed
in EPCs in response to oxidative stress (14–16). In the present study, 3 antioxidant
enzymes, Prx-2, Prx-3 and Prx-6, were found to be upregulated in
response to H2O2-induced oxidative stress.
All 3 enzymes belong to the peroxiredoxin (Prx) family, which is
involved in redox regulation of cell survival. They commonly
exhibit peroxidase activity, which is dependent on reduced forms of
thioredoxin and/or glutathione (18). Prx-2 reduces peroxides by reducing
equivalents through the thioredoxin system, plays an important role
in eliminating peroxides generated during metabolism, and
participates in the signaling cascades of growth factors and tumor
necrosis factor-α by regulating the intracellular concentrations of
H2O2 (19).
Prx-3 protects radical-sensitive enzymes from oxidative damage. Its
gene expression is induced by oxidative stress and appears to
function as an antioxidant in the cardiovascular system (18). Bovine aortic endothelial cells
with an elevated level of Prx-3 due to exposure to mild oxidative
stress have been shown to be more tolerant to subsequent exposure
to severe oxidative stress (19).
Prx-6 plays a role in the regulation of phospholipid turnover, as
well as in the protection against oxidative injury (18). Taken together, these data suggest
that the upregulation of antioxidant enzymes from the Prx family
protects EPCs from oxidative injury.
Apart from antioxidant enzymes, alterations in
protein expression induced by mild oxidative stress are also
observed in cytoskeleton proteins. EFEMP1 (fibulin-3), which
belongs to the fibulin family, is an extracellular glycoprotein and
is mainly localized in the walls of capillaries and basement
membrane of the large but not distal airways (20,21). Studies have demonstrated that
EFEMP1 promotes neovascularization by increasing the expression
level of vascular endothelial growth factor (VEGF) (22,23). Accordingly, the upregulation of
EFEMP1 may partially explain our discovery that there was no
difference in tube formation between the untreated EPCs and those
treated with 100 or 200 μM H2O2 at 8 h after
seeding on Matrigel.
Another upregulated cytoskeleton protein in our
study was vimentin. It belongs to the intermediate filaments (IFs)
family and is the only cytoplasmic IF found in macrophages,
lymphocytes, neutrophils, endothelial cells and fibroblasts
(24,25). It has been demonstrated that the
mitochondria are less sensitive to ROS in cells containing vimentin
than in cells devoid of vimentin, suggesting that vimentin protects
the mitochondria from oxidative stress (25). Therefore, the upregulation of
vimentin in the H2O2-treated EPCs may be a
compensatory mechanism in order to protect the cells from oxidative
damage. Consistent with our finding, an in vitro study using
HepG2 cells revealed that pre-treatment with low concentrations of
H2O2 (50 μM) induced increased levels of
vimentin, and reduced the cleavage of vimentin following exposure
to oxidative stress (26). It has
been reported that the cleavage of vimentin by caspases results in
the disruption of its filamentous structure, which may promote
apoptosis by facilitating nuclear condensation and subsequent
fragmentation and amplifying the cell death signal (26). Thus, fewer EPCs may undergo
apoptosis induced by oxidative stress through the upregulation of
vimentin. This may be a compensatory mechanism through which the
EPCs protect themselves from damage due to oxidative stress. In
addition, vimentin has been shown to be associated with the
organization of proteins that are involved in adhesion, migration
and cell signaling, and the loss of vimentin leads to a lack of
integrity in the vascular endothelium (27). In support of a role for vimentin
in the transmigration of myeloid cells, vimentin has been reported
to reside in the filopodia and podosomes of adherent macrophages
(27). Podosomes are early cell
adhesion structures found in myeloid cells that are crucial for
directional movement and transmigration (27). Therefore, the upregulation of
vimentin may partially enhance the migration ability and survival
ECPs following exposure to oxidative stress.
Though multiple defensive responses exist, our data
revealed that treatment with H2O2 induced a
concentration-dependent reduction in EPC number and function. In
addition, the tube-forming ability and function were found to be
affected to a greater extent following exposure to oxidative
stress, suggesting that EPCs have an impaired function in
neovascularization under oxidative stress conditions. These results
may explain the clinical findings that the number of EPCs inversely
correlates with the risk of cardiovascular and cerebrovascular
events (2,5,28–32). Thus, there may be proteins or
pathways through which H2O2 exerts its
negative effects on EPCs. In this study, we found 3 proteins that
may be involved in the loss of EPC number and function following
treatment with H2O2. Rab GDP dissociation
inhibitor α (Rab GDIα) was upregulated, and NUDT5 and TIM were
downregulated.
Rab proteins are small GTP-binding proteins that are
important in the vesicular trafficking of molecules between
cellular organelles (33). They
act as molecular switches, cycling between ‘inactive’ GDP-bound and
‘active’ GTP-bound states (34).
Rab GDIα is an important factor in the control of Rab and the
specificity of vesicular transport. By binding Rab, released after
membrane fusion, Rab GDIα prevents Rab from releasing its GDP
molecule until it has interacted with appropriate proteins in the
donor membrane (33,35,36). Therefore, an upregulation of Rab
GDIα may lead to the reduction of signal transduction among
cellular organelles and the survival of EPCs following oxidative
damage.
Human NUDT5, which has an intrinsic activity to
cleave ADP sugars to AMP and sugar phosphate, possesses the ability
to degrade 8-oxo-dGDP to monophosphate. Since 8-oxo-dGDP and
8-oxo-dGTP are inter-convertible by cellular enzymes, NUDT5 has the
potential to prevent errors during DNA replication. As a
‘sanitization enzyme’, NUDT5 has the potential to eliminate
endogenous toxic materials from the cell and the mutagenic oxidized
substrates from the nucleotide pool in order to prevent their toxic
effects (37, 38). In our study, the downregulation of
NUDT5 suggests a high error rate in DNA replication due to
oxidative stress, resulting in EPC apoptosis.
TIM is considered an important biocatalyst during
glycolysis and rapidly interconverts dihydroxyacetone phosphate and
D-glyceraldehyde-3-phosphate (39). It has been demonstrated that the
energy metabolism of endothelial cells is characterized by glucose
fermentation to lactate even under normoxic conditions (‘aerobic
glycolysis’). This is possibly an adaptation to the oxidative
environment since ‘aerobic glycolysis’ can help reduce the
production of oxygen radicals. ‘Aerobic glycolysis’ is also linked
to cell proliferation. A correlation has been established between
the amount of lactate produced and the cell doubling time (40). The downregulation of TIM induces a
reduction in energy production and reduces EPC proliferation, which
is consistent with the results from our study. We hypothesized that
even if vimentin and EFEMP1 expression is upregulated in the EPCs,
enhancing the migration and tube-forming ability and survival of
EPCs, the lack of energy may still lead to the impaired function of
EPCs under oxidative stress conditions.
In conclusion, our results demonstrated that
H2O2-induced oxidative stress modified the
levels of 8 proteins that are associated with defensive responses
against oxidative stress, energy production, signal transduction
and the elimination of oxidative nucleotides. Despite the defensive
responses, including the upregulation of antioxidant enzymes
(>2.5-fold) and cytoskeleton proteins, EPCs still dysfunction
due to the altered expression of proteins associated with energy
production, signal transduction and the elimination of oxidative
nucleotides. The data presented in this study indicate that in
order to increase the function and number of EPCs, we should focus
on defensive proteins, as well as protective proteins, such as Rab
GDIα, NUDT5 and TIM.
References
|
1
|
Shantsila E, Watson T and Lip GY:
Endothelial progenitor cells in cardiovascular disorders. J Am Coll
Cardiol. 49:741–752. 2007. View Article : Google Scholar
|
|
2
|
Rouhl RP, van Oostenbrugge RJ, Damoiseaux
J, Tervaert JW and Lodder J: Endothelial progenitor cell research
in stroke: a potential shift in pathophysiological and
therapeutical concepts. Stroke. 39:2158–2165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sen S, McDonald SP, Coates PT and Bonder
CS: Endothelial progenitor cells: novel biomarker and promising
cell therapy for cardiovascular disease. Clin Sci (Lond).
120:263–283. 2011.PubMed/NCBI
|
|
4
|
Zampetaki A, Kirton JP and Xu Q: Vascular
repair by endothelial progenitor cells. Cardiovasc Res. 78:413–421.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lapergue B, Mohammad A and Shuaib A:
Endothelial progenitor cells and cerebrovascular diseases. Prog
Neurobiol. 83:349–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ward MR, Stewart DJ and Kutryk MJ:
Endothelial progenitor cell therapy for the treatment of coronary
disease, acute MI, and pulmonary arterial hypertension: current
perspectives. Catheter Cardiovasc Interv. 70:983–998. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dobert N, Britten M, Assmus B, Berner U,
Menzel C, Lehmann R, Hamscho N, Schachinger V, Dimmeler S, Zeiher
AM and Grunwald F: Transplantation of progenitor cells after
reperfused acute myocardial infarction: evaluation of perfusion and
myocardial viability with FDG-PET and thallium SPECT. Eur J Nucl
Med Mol Imaging. 31:1146–1151. 2004. View Article : Google Scholar
|
|
8
|
Flores-Ramirez R, Uribe-Longoria A,
Rangel-Fuentes MM, Gutierrez-Fajardo P, Salazar-Riojas R,
Cervantes-Garcia D, Trevino-Ortiz JH, Benavides-Chereti GJ,
Espinosa-Oliveros LP, Limon-Rodriguez RH, et al: Intracoronary
infusion of CD133+ endothelial progenitor cells improves
heart function and quality of life in patients with chronic
post-infarct heart insufficiency. Cardiovasc Revasc Med. 11:72–78.
2010.PubMed/NCBI
|
|
9
|
Haendeler J, Hoffmann J, Diehl JF, Vasa M,
Spyridopoulos I, Zeiher AM and Dimmeler S: Antioxidants inhibit
nuclear export of telomerase reverse transcriptase and delay
replicative senescence of endothelial cells. Circ Res. 94:768–775.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogita H and Liao J: Endothelial function
and oxidative stress. Endothelium. 11:123–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madamanchi NR, Vendrov A and Runge MS:
Oxidative stress and vascular disease. Arterioscler Thromb Vasc
Biol. 25:29–38. 2005.PubMed/NCBI
|
|
12
|
Alexandrova ML and Bochev PG: Oxidative
stress during the chronic phase after stroke. Free Radic Biol Med.
39:297–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Case J, Ingram DA and Haneline LS:
Oxidative stress impairs endothelial progenitor cell function.
Antioxid Redox Signal. 10:1895–1907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dernbach E, Urbich C, Brandes RP, Hofmann
WK, Zeiher AM and Dimmeler S: Antioxidative stress-associated genes
in circulating progenitor cells: evidence for enhanced resistance
against oxidative stress. Blood. 104:3591–3597. 2004. View Article : Google Scholar
|
|
15
|
Imanishi T, Tsujioka H and Akasaka T:
Endothelial progenitor cells dysfunction and senescence:
contribution to oxidative stress. Curr Cardiol Rev. 4:275–286.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He T, Peterson TE, Holmuhamedov EL, Terzic
A, Caplice NM, Oberley LW and Katusic ZS: Human endothelial
progenitor cells tolerate oxidative stress due to intrinsically
high expression of manganese superoxide dismutase. Arterioscler
Thromb Vasc Biol. 24:2021–2027. 2004. View Article : Google Scholar
|
|
17
|
Wei J, Liu Y, Chang M, Sun CL, Li DW, Liu
ZQ and Hu LS: Proteomic analysis of oxidative modification in
endothelial colony-forming cells treated by hydrogen peroxide. Int
J Mol Med. 29:1099–1105. 2012.PubMed/NCBI
|
|
18
|
Fujii J and Ikeda Y: Advances in our
understanding of peroxiredoxin, a multifunctional, mammalian redox
protein. Redox Rep. 7:123–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rhee SG, Kang SW, Chang TS, Jeong W and
Kim K: Peroxiredoxin, a novel family of peroxidases. IUBMB Life.
52:35–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi N, Kostka G, Garbe JH, Keene DR,
Bachinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML and
Sasaki T: A comparative analysis of the fibulin protein family.
Biochemical characterization, binding interactions, and tissue
localization. J Biol Chem. 282:11805–11816. 2007. View Article : Google Scholar
|
|
21
|
Timpl R, Sasaki T, Kostka G and Chu ML:
Fibulins: a versatile family of extracellular matrix proteins. Nat
Rev Mol Cell Biol. 4:479–489. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song EL, Hou YP, Yu SP, Chen SG, Huang JT,
Luo T, Kong LP, Xu J and Wang HQ: EFEMP1 expression promotes
angiogenesis and accelerates the growth of cervical cancer in vivo.
Gynecol Oncol. 121:174–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roybal CN, Marmorstein LY, Vander Jagt DL
and Abcouwer SF: Aberrant accumulation of fibulin-3 in the
endoplasmic reticulum leads to activation of the unfolded protein
response and VEGF expression. Invest Ophthalmol Vis Sci.
46:3973–3979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muller K, Dulku S, Hardwick SJ, Skepper JN
and Mitchinson MJ: Changes in vimentin in human macrophages during
apoptosis induced by oxidised low density lipoprotein.
Atherosclerosis. 156:133–144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zamoner A, Barreto KP, Filho DW, Sell F,
Woehl VM, Guma FC, Silva FR and Pessoa-Pureur R: Hyperthyroidism in
the developing rat testis is associated with oxidative stress and
hyperphosphorylated vimentin accumulation. Mol Cell Endocrinol.
267:116–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Kang H and Zou F: Low
concentration of GA activates a preconditioning response in HepG2
cells during oxidative stress-roles of Hsp90 and vimentin. Cell
Stress Chaperones. 14:381–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ivaska J, Pallari HM, Nevo J and Eriksson
JE: Novel functions of vimentin in cell adhesion, migration, and
signaling. Exp Cell Res. 313:2050–2062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghani U, Shuaib A, Salam A, Nasir A,
Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B
and Todd K: Endothelial progenitor cells during cerebrovascular
disease. Stroke. 36:151–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Werner N, Wassmann S, Ahlers P, Schiegl T,
Kosiol S, Link A, Walenta K and Nickenig G: Endothelial progenitor
cells correlate with endothelial function in patients with coronary
artery disease. Basic Res Cardiol. 102:565–571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmidt-Lucke C, Rossig L, Fichtlscherer
S, Vasa M, Britten M, Kamper U, Dimmeler S and Zeiher AM: Reduced
number of circulating endothelial progenitor cells predicts future
cardiovascular events: proof of concept for the clinical importance
of endogenous vascular repair. Circulation. 111:2981–2987. 2005.
View Article : Google Scholar
|
|
32
|
Briguori C, Testa U, Riccioni R, Colombo
A, Petrucci E, Condorelli G, Mariani G, D’Andrea D, De Micco F,
Rivera NV, et al: Correlations between progression of coronary
artery disease and circulating endothelial progenitor cells. FASEB
J. 24:1981–1988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang P, Chintagari NR, Narayanaperumal J,
Ayalew S, Hartson S and Liu L: Proteomic analysis of lamellar
bodies isolated from rat lungs. BMC Cell Biol. 9:342008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seabra MC and Wasmeier C: Controlling the
location and activation of Rab GTPases. Curr Opin Cell Biol.
16:451–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamaguchi Y, Miyagi Y and Baba H:
Two-dimensional electrophoresis with cationic detergents: a
powerful tool for the proteomic analysis of myelin proteins. Part
2: analytical aspects. J Neurosci Res. 86:766–775. 2008. View Article : Google Scholar
|
|
36
|
Poirrier JE, Guillonneau F, Renaut J,
Sergeant K, Luxen A, Maquet P and Leprince P: Proteomic changes in
rat hippocampus and adrenals following short-term sleep
deprivation. Proteome Sci. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ito R, Sekiguchi M, Setoyama D, Nakatsu Y,
Yamagata Y and Hayakawa H: Cleavage of oxidized guanine nucleotide
and ADP sugar by human NUDT5 protein. J Biochem. 149:731–738. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kamiya H, Hori M, Arimori T, Sekiguchi M,
Yamagata Y and Harashima H: NUDT5 hydrolyzes oxidized
deoxyribonucleoside diphosphates with broad substrate specificity.
DNA Repair (Amst). 8:1250–1254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wierenga RK, Kapetaniou EG and Venkatesan
R: Triosephosphate isomerase: a highly evolved biocatalyst. Cell
Mol Life Sci. 67:3961–3982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peters K, Kamp G, Berz A, Unger RE, Barth
S, Salamon A, Rychly J and Kirkpatrick CJ: Changes in human
endothelial cell energy metabolic capacities during in vitro
cultivation. The role of ‘aerobic glycolysis’ and proliferation.
Cell Physiol Biochem. 24:483–492. 2009.PubMed/NCBI
|