Introduction
Asthma is a chronic inflammatory disorder of the
airways in which many cells and cellular elements play an important
role (1). Patients with severe
asthma with an incidence rate of 5–10% account for the high
morbidity and health costs; thus, novel treatment strategies for
this disorder are urgently required (2). The airway obstruction in asthma is
usually reversible; however, if left untreated, the obstruction may
become irreversible due to airway remodeling (3–5).
The process of airway remodeling in asthma includes subepithelial
basement membrane fibrosis, epithelial goblet cell hyperplasia, an
increase in the number of blood vessels and a proliferative state
of the airway smooth muscle with increased mass due to hyperplasia
and hypertrophy (5). Recent
studies have indicated that the increased mass of airway smooth
muscle cells (ASMCs) plays a critical role in the histopathological
characteristics of airway remodeling (2–4).
Thus, this may be a potential therapeutic target for asthma.
Several classes of drugs that target the airway smooth muscle,
including β-agonists, anti-cholinergics, antihistamines and
anti-leukotrienes were developed decades ago (6). However, these life-long therapies
only treat the symptoms, but have little or no effect on the
structural alterations in asthma.
Curcumin [diferuloylmethane
(C21H20O6)], a polyphenol, is
derived from the roots (rhizomes) of the plant, Curcuma
longa (7). Curcumin has been
used as a traditional medicine for the treatment of asthma in China
and India since 1900 BC (8,9).
Several studies have demonstrated that curcumin has wide range of
properties, including anti-inflammatory, anti-oxidant, anticancer,
chemopreventive and chemotherapeutic activities (7,9–12),
affecting multiple signaling pathways, such as the extracellular
signal-regulated kinase (ERK)1/2 pathway (12–16). The anti-proliferative effects of
curcumin have been investigated in various cell lines (17–20). Studies have shown that curcumin
inhibits vascular smooth muscle cell (VSMC) proliferation by
restoring the expression of caveolin-1, blocking the activation of
ERK1/2 (13,15,21–23). However, to our knowledge, whether
curcumin inhibits ASMC proliferation in asthma has not been
reported to date.
In this study, we investigated whether curcumin
inhibits the proliferation of ASMCs in vitro and in
vivo. Our results demonstrate that curcumin inhibits the
proliferation of ASMCs by upregulating the expression of caveolin-1
and thus blocking the ERK signaling pathway; these results may
promote the clinical application of curcumin in asthma.
Materials and methods
Animals
Eighty female BALB/c mice, weighing approximately
18–22 g, were purchased from the Guangdong Medical Laboratory
Animal Center, Foshan, China. The mice were divided into 5 groups
(n=16 per group): the control group (normal saline-challenged mice
treated with normal saline), model group [ovalbumin
(OVA)-challenged mice treated with normal saline] and the
curcumin-treated groups (OVA-challenged mice treated with 50, 100
and 150 mg/kg curcumin). All mice were kept in well-controlled
animal housing facilities, and had free access to tap water and
food pellets throughout the experimental period. All experimental
procedures involving animals were carried out in accordance with
the Guide for the Care and Use of Laboratory Animals and the
Institutional Ethical Guidelines for experiments with animals.
Sensitization and challenge with OVA
Mice underwent OVA sensitization and challenge as
previously described (24,25)
with slight modifications. Mice in the model and curcumin-treated
groups were immunized by an intraperitoneal (i.p.) injection of 0.2
ml of 50 μg/ml OVA (Sigma-Aldrich Co., St. Louis, MO, USA) on days
0 and 12. The control group received the same volume of
physiological saline at the same time. Mice in the model and
curcumin-treated groups were then challenged once a day with 5% OVA
(aerosolized for 30 min) via the airways between days 18 and 23,
while normal saline was administered to the control group in a
similar manner. Prolonged inflammation was induced by the
subsequent exposure of mice to aerosolized 5% OVA 3 times a week
for 30 min from day 26 onwards (chronic phase). Mice in the
curcumin-treated groups were administered with an i.p. injection of
curcumin 30 min prior to stimulation with OVA, while the others
were administered normal saline. The mice were sacrificed on day
55.
Airway hyper-responsiveness (AHR)
The activity of all the mice was observed closely
following challenge with OVA. Airway responsiveness was measured
indirectly by whole body plethysmography to calculate enhanced
pause (Penh: Buxco Technologies, Petersfield, UK). Response to
inhaled methacholine (Sigma-Aldrich Co.) at concentrations of 0.78,
1.56, 3.12, 6.25, 12.5, 25 and 50 mg/ml were measured for 1 min, as
described previously (26).
Airway responsiveness activities (Penh% values) were calculated by
the formula (P = m/s ×100%, in which P, m and s represent the Penh%
value, methacholine-stimulated Penh value and the saline-stimulated
Penh value, respectively).
Histology and immunohistochemistry
Paraffin-embedded lung sections from mice of the 5
groups were stained with hematoxylin and eosin (H&E) to observe
the pathological changes in the airways and lung tissue under an
optical microscope. We performed immunohistochemistry using the
monoclonal mouse antibody to α-smooth muscle actin (α-SMA) (1:100,
Sigma-Aldrich Co.) and the polyclonal rabbit antibodies to ERK
(1:100, Cell Signaling Technology, Inc., Danver, MA, USA). The
sections from mice in the 5 groups were deparaffinized, and a 3%
hydrogen peroxide solution was applied for 15 min to inhibit
endogenous peroxidase activity. Antigen retrieval was performed
with citrate solution for 45 min. After the sections were blocked
with 10% goat serum in phosphate-buffered saline (PBS) for 20 min,
they were incubated with the primary antibody overnight at 4°C. The
horseradish peroxidase (HRP)-conjugated rabbit-anti-mouse
immunoglobulin (Sigma-Aldrich Co.) and HRP-conjugated
goat-anti-rabbit immunoglobulin (Sigma-Aldrich Co.) were used as
the secondary antibodies and 3,3-diaminobenzidine (DAB)
(Sigma-Aldrich Co.) was used as the chromogen. Hematoxylin was used
for the counterstaining of the sections. For the negative control,
the primary antibody was replaced with PBS. The sections were
observed under a microscope. The thickness of the airway wall, the
thickness of the airway smooth muscle layer, the numbers of smooth
muscle cells and the intensity of ERK staining were measured as an
average optical density using Image-Pro Plus (IPP) software
(Image-Pro Plus 6.0; Media Cybernetics, Inc., Rockville, MD, USA).
A non-stained region was selected and set as the background.
Primary cell culture
The ASMCs were cultivated as an explant culture as
previously described with slight modifications (27). Briefly, primary rat airway smooth
muscle was isolated from the bronchial smooth muscle from
10–12-week-old specific-pathogen-free (SPF) Sprague-Dawley (SD)
male rats and the smooth muscle was isolated, cut into sections of
1–2 mm in cubic size, and placed on culture flasks with Dulbecco's
modified Eagle's medium/Ham's F-12 (DMEM/F-12) medium (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS)
in an incubator at 37°C, 5% CO2. The ASMCs migrated from
the tissue explants and approached confluence around the explants.
The culture medium was changed every 3 days. When the cells reached
90–100% confluence, they were passaged with 0.25% trypsin-EDTA
(Gibco). The cells at passage 3 to 6 were used in the following
experiments.
Immunofluorescence in vitro
The primary ASMCs were characterized by microscopy
and their purity was verified by the immunofluorescence staining of
α-SMA. The location of caveolin-1 in the ASMCs was also detected by
immunofluorescence. After the cells grew to approximately 50%
confluence, they were fixed in the culture plate using 4%
paraformaldehyde for 10 min, permeabilized with 0.3% Triton X-100,
blocked with 5% bovine serum albumin (BSA) at 37°C for 1 h,
incubated with monoclonal mouse antibodies against α-SMA (1:100,
Sigma-Aldrich Co.) and rabbit anti-rat caveolin-1 (1:500, Abcam,
Cambridge. UK) respectively overnight at 4°C, and then labeled with
fluorescein isothiocynate (FITC) secondary anti-mouse or
anti-rabbit antibody (1:100, Sigma-Aldrich Co.) at 37°C for 1 h in
the dark. The nuclei were counterstained with 20 μg/ml propidium
iodide (PI) and 1 μg/ml DAPI in methanol for 5 min. The culture
plate was washed 3 times between each step for 5 min in 0.1 M PBS
(pH 7.4). The cells were examined and images were acquired using a
fluorescence microscope.
Cell proliferation assay
The ASMCs were dispensed in a 96-well culture plate
at a cell density of 2×104/ml and incubated at 37°C, 5%
CO2. The cells were divided into 5 groups: i) negative
control group (DMEM/F-12 containing 2.5% FBS), ii) positive control
group (DMEM/F-12 containing 10% FBS), iii) proliferation group [25
ng/ml platelet-derived growth factor (PDGF) in DMEM/F-12
supplemented with 2.5% FBS], iv) curcumin-treated group with
caveolin-1 (25 μmol/l curcumin with 25 ng/ml PDGF in DMEM/F-12 2.5%
FBS), and v) curcumin-treated group without caveolin-1 [the cells
were pre-treated with 5 μmol/l methyl-β-cyclodextrin (MβCD) for 60
min, then 25 μmol/l curcumin were added with 25 ng/ml PDGF in
DMEM/F-12 containing 2.5% FBS]. Cell Counting Kit-8 (CCK-8) assays
(Dojindo Laboratories, Kumamoto Japan) were used to measure cell
proliferation according to the manufacturer's instructions. After
the cells were treated with the culture medium for 6, 12, 24 and 48
h, they were incubated in 10% CCK-8 diluted in normal culture
medium at 37°C for 2 h and the absorbance at 450 nm (OD at 450 nm)
was measured. Each group was analyzed in triplicate. The results
were expressed as OD values at 450 nm.
Real time PCR
Total RNA was extracted from the ASMCs in each group
at 5 time points (0, 4, 8, 12 and 24 h) using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
instructions. The concentration, purity and amount of total RNA
were determined by ultraviolet spectrometry (ND-1000
spectrophotometer; NanoDrop Technologies, Wilmington, DE, USA).
Reverse transcriptase PCR was carried out using the Prime Script™
RT reagent kit (Takara Bio Inc., Otsu, Japan). The sense and
antisense primers for caveolin-1 (GenBank ID: NM_133651) and
β-actin (GenBank ID: NM_031144.2) were synthesized by Takara Bio
Inc. The primers used are listed in Table I.
 | Table IOligonucleotide sequences of primers
used for real-time PCR. |
Table I
Oligonucleotide sequences of primers
used for real-time PCR.
| Gene name | Sense/antisense
primers |
|---|
| Caveolin-1 |
5′-GGGCAACATCTAGAAGCCCAACAA-3′
5′-CTGATGCACTGAATTCCAATCAGGAA-3′ |
| β-actin |
5′-CTTCCTTCCTGGGTATGGAATC-3′
5′-GAGATGATCTGGGGGTCTGA-3′ |
Real-time PCR analyses were performed using SYBR
Premix Ex Taq (Takara Bio Inc.). Amplification was performed in an
ABI PRISM 7500 Real-Time PCR System with the following temperature
profile: a pre-denaturation step of 30 sec at 95°C, extended at
60°C for 34 sec, and denaturion for 5 sec at 95°C for 40 cycles.
The data were calculated using the 2−ΔΔCt method
normalized to β-actin. Each experiment was performed 3 times.
Western blot analysis
The cells were lysed in cell lysis buffer (Cell
Signaling Technology, Inc.). Total protein was extracted by
sonication and centrifugation of the cell lysates at 12,000 × g for
10 min at 4°C. Whole-cell extracts were fractionated by 15% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Proteins were transferred onto a nitrocellulose membrane
(Millipore, Bedford, MA, USA) using a wet transfer apparatus. For
immune detection, the membranes were washed and incubated with
rabbit anti-rat ERK1/2 polyclonal antibody (1:4,000),
rabbit-anti-rat phospho-ERK1/2 polyclonal antibody (1:4,000),
anti-GADPH polyclonal antibody (1:2,000; all from Cell Signaling
Technology, Inc., USA), rabbit-anti-rat caveolin-1 polyclonal
antibody (1:500, Abcam) and anti-β-actin monoclonal antibody
(1:2,000, Sigma-Aldrich Co.) overnight at 4°C. The signals were
amplified using the appropriate HRP-conjugated secondary antibody,
and visualized by enhanced chemiluminescence (Pierce Biotechnology,
Inc., Rockford, IL, USA).
Statistical analysis
All statistical analyses were performed using SPSS
for Windows v.13.0 (SPSS, Chicago, IL, USA). All data are expressed
as the means ± SD. Statistical analyses were carried out using
one-way ANOVA, the rank-sum test or least significant difference
tests where appropriate. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
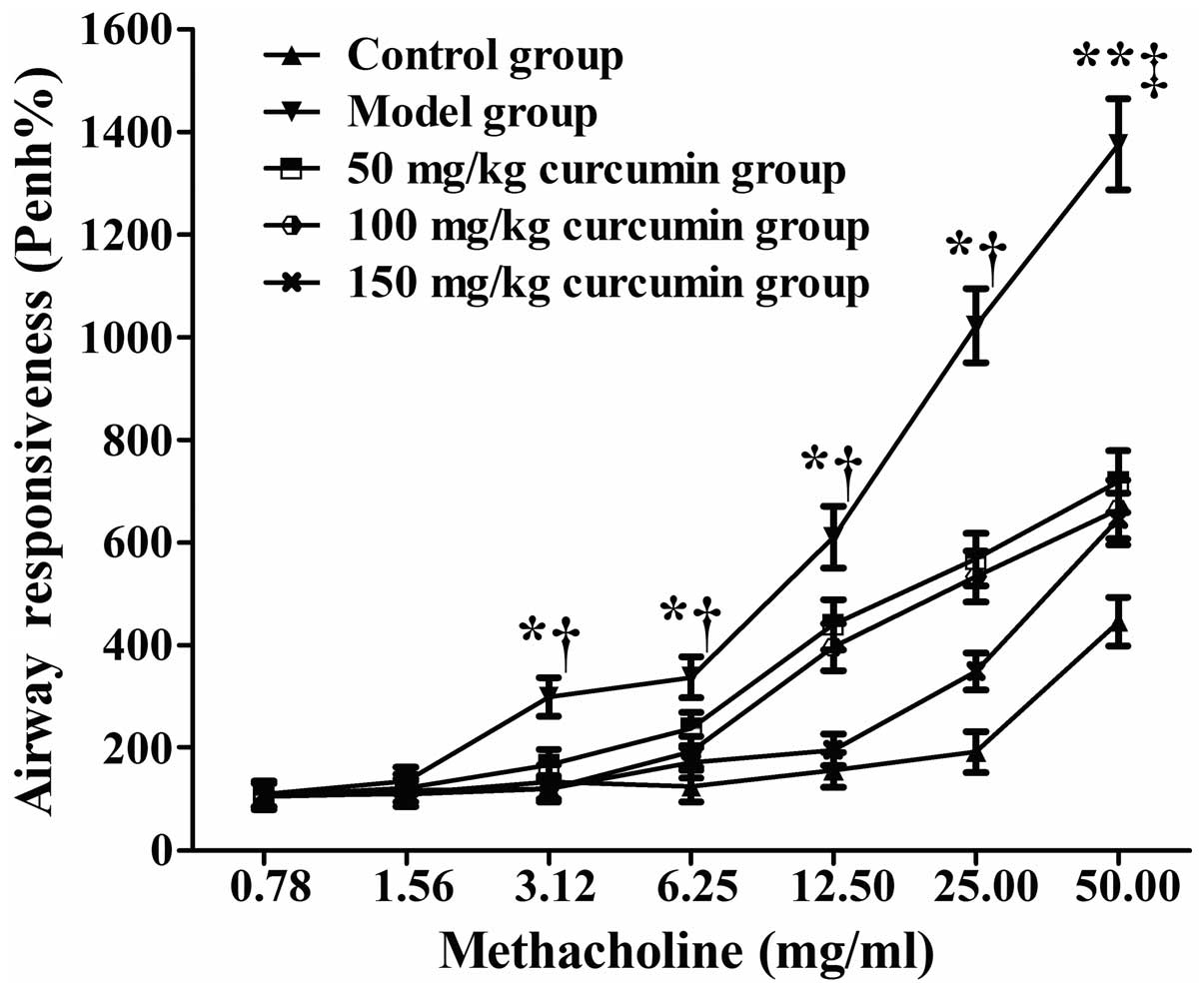
Curcumin decreases the airway
responsiveness in the mouse model of airway remodeling
Positive symptoms, such as dysphoria, tachypnea and
abdominal spasms were observed in the mice in the model group 5 to
10 min following challenge with OVA, whereas less severe symptoms,
including mild dysphoria and tachypnea were observed in the mice
treated with curcumin; all the symptoms soon dissipated.
The mice with asthma (model group), the mice with
asthma treated with curcumin (curcumin-treated groups) and the
untreated normal mice (control group) began to suffer from
breathing difficulties after inhaling methacholine at
concentrations of 1.56, 6.25 and 25 mg/ml. The airway
responsiveness (Penh%) of the model group and the curcumin-treated
groups (treated with various concentrations of curcumin)
significantly increased compared with the normal group when
methacholine was inhaled at the concentrations of 3.12, 6.25, 12.5
and 25 mg/ml (P<0.05) and at the concentration of 50 mg/ml
(P<0.01) (Fig. 1). The airway
responsiveness (Penh%) of the curcumin-treated groups significantly
decreased compared with the model group when methacholine was
inhaled at the concentrations of 3.12, 6.25, 12.5, 25 mg/ml
(P<0.05) and at the concentration of 50 mg/ml (P<0.01)
(Fig. 1).
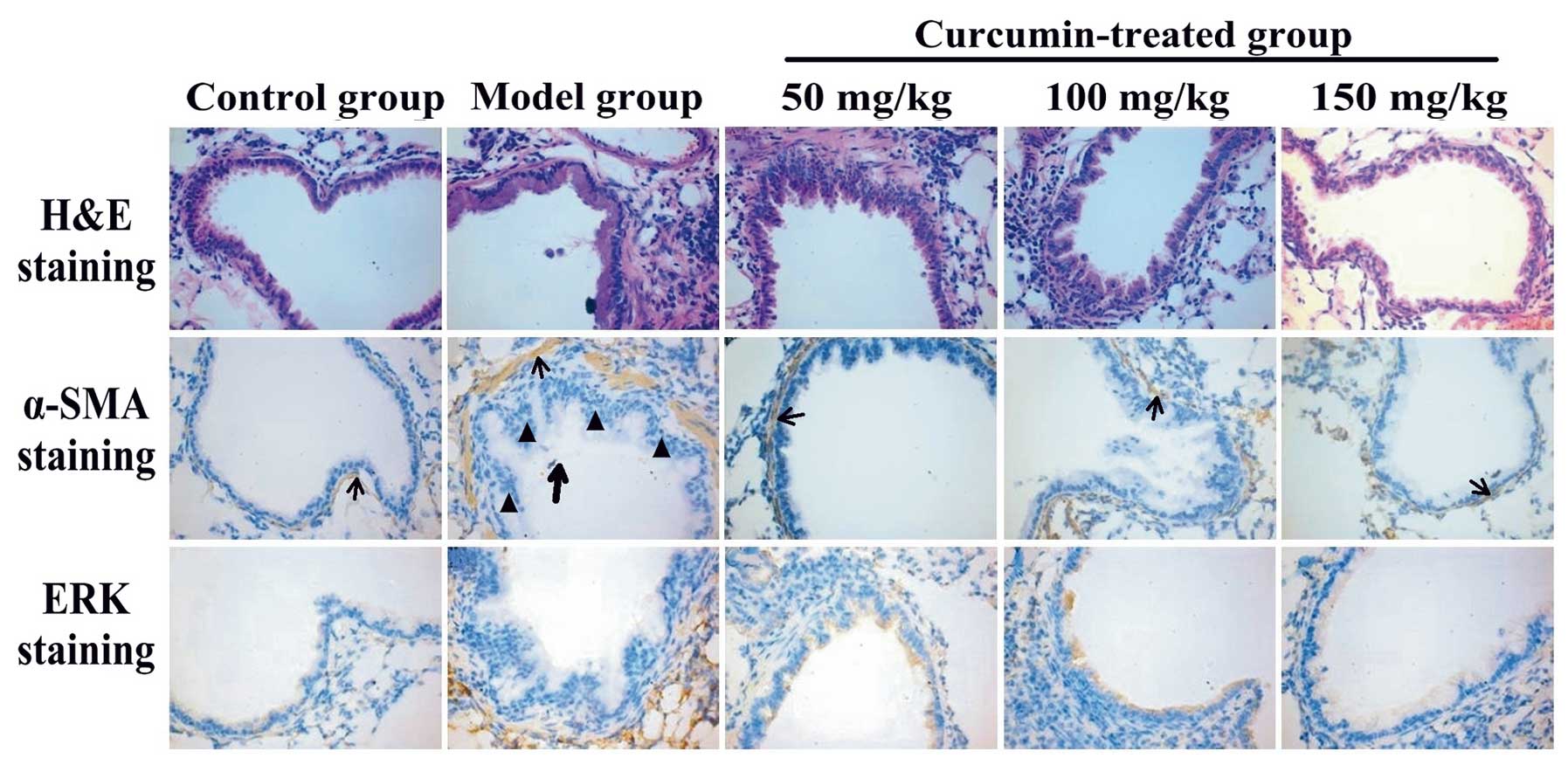
Effects of curcumin on OVA-induced
histopathological changes in lungs
To assess the anti-remodeling effects of curcumin,
histopathological experiments were performed. Using H&E
staining, inflammatory cell infiltration in the peribronchial and
perivascular areas was observed in OVA-challenged mice. Treatment
with curcumin (50, 100 and 150 mg/kg) markedly reduced the degree
of inflammatory cell infiltration in the peribronchial and
perivascular areas (Fig. 2, upper
panel). OVA induced the proliferation of ASMCs. In the
curcumin-treated groups, curcumin inhibited the OVA-induced ASMC
proliferation compared with the model group (Fig. 2, middle panel). The expression of
α-SMA and ERK was detected by immunohistochemistry and the
expression of ERK was increased in mice with asthma (Fig. 2, lower panel; ERK protein is
stained brown). Curcumin decreased the expression of ERK in the
curcumin-treated groups compared with the model group. The
bronchial wall thickness (WAi/Pi), the thickness of the smooth
muscle layer (WAm/Pi), the number of smooth muscle cells (N/Pi) and
the ERK gray values in the ASMCs in each group were measured using
IPP software (Table II). In the
model group, the thickness of the airway wall, the thickness of the
airway smooth muscle layer and the numbers of smooth muscle cells
were significantly increased compared with the control group
(P<0.01). In the curcumin-treated groups, the thickness of the
airway wall, the thickness of the airway smooth muscle layer and
the numbers of smooth muscle cells were significantly decreased
compared with the model group (P<0.05).
 | Table IILung pathology of the mice in the 5
groups. |
Table II
Lung pathology of the mice in the 5
groups.
| Group | WAi/Pi (μm) | WAm/Pi (μm) | N/Pi (/mm) | ERK gray-scale
value |
|---|
| Control | 5.6±0.5 | 1.2±0.5 | 12.4±1.2 | 68.5±6.4 |
| Model | 14.6±3.6b | 6.4±1.4b | 35.3±4.4b | 97.6±15.2b |
| 50 mg/kg
curcumin | 7.7±1.7a | 4.2±1.5a | 25.8±2.5a | 90.5±15.8a |
| 100 mg/kg
curcumin | 5.6±1.5a | 3.2±1.3a | 16.5±1.6a | 80.6±10.3a |
| 150 mg/kg
curcumin | 5.6±0.7a | 1.5±0.7a | 12.5±1.7a | 74.5±7.1a |
Correlation between ERK expression and
ASMC proliferation
Semi-quantitative image analysis demonstrated that
the expression level of ERK in the airways positively correlated
with the thickness of the airway smooth muscle layer (r=0.745,
P<0.05), and positively correlated with the number of ASMCs
(r=0.821, P<0.05).
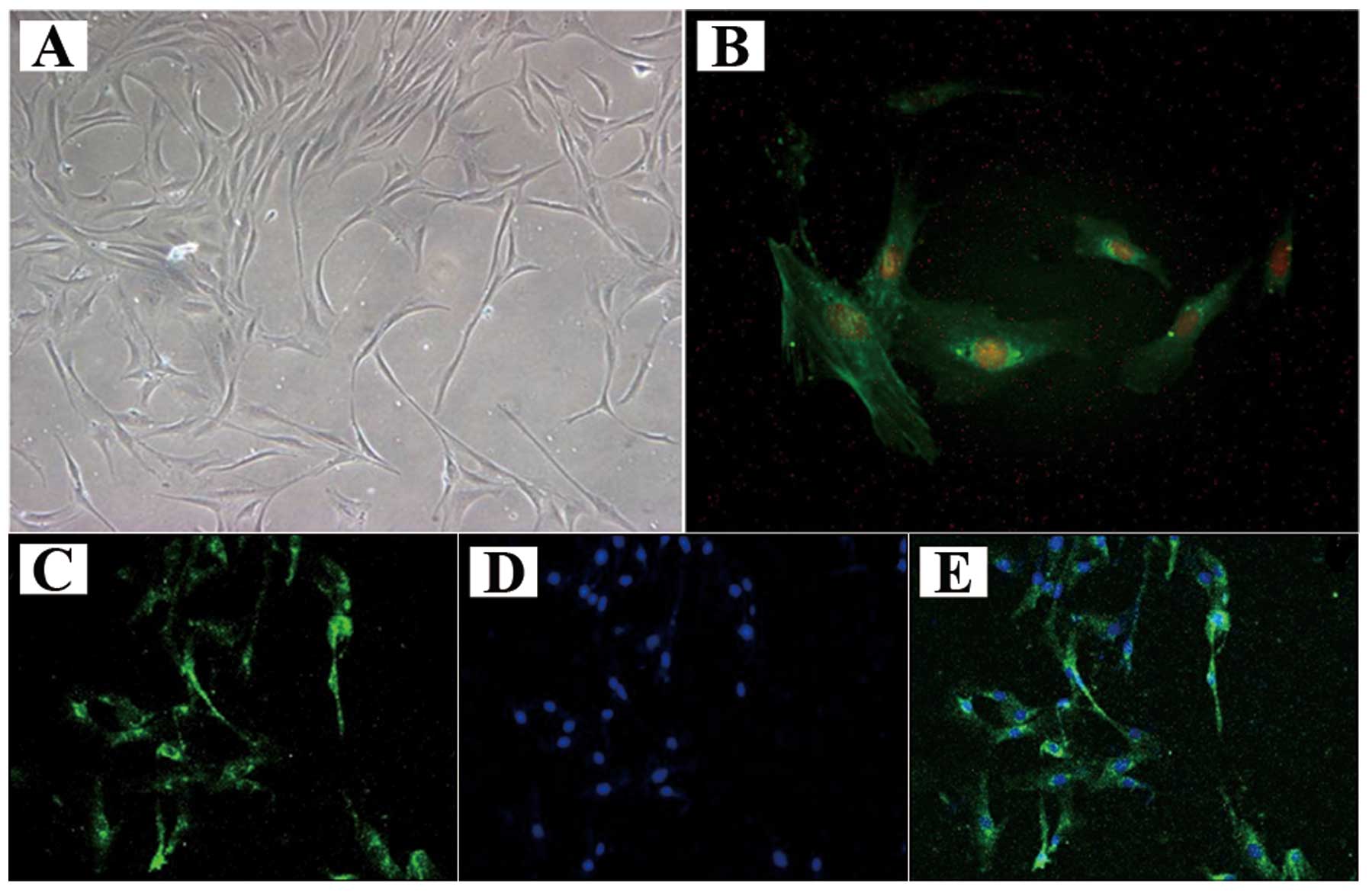
Rat primary ASMC identification and
location of caveolin-1 in ASMCs
The ASMCs generally became adherent within 2 days,
and assumed a stretched, spindle-shaped valley-like growth patterns
after 4–5 days (Fig. 3A). The
ASMCs grew to approximately 90% confluence after 8–10 days; they
were then digested by trypsin and passaged for immunofluorescence
staining (Fig. 3B). The
cytoplasmic filaments of positive cells were stained green. The
percentage of pure ASMCs was approximately 94.32% using Image Pro
Plus 6.0 software. Caveolin-1 was detected in the ASMC plasma
membrane under a fluorescence microscope (Fig. 3C–E).
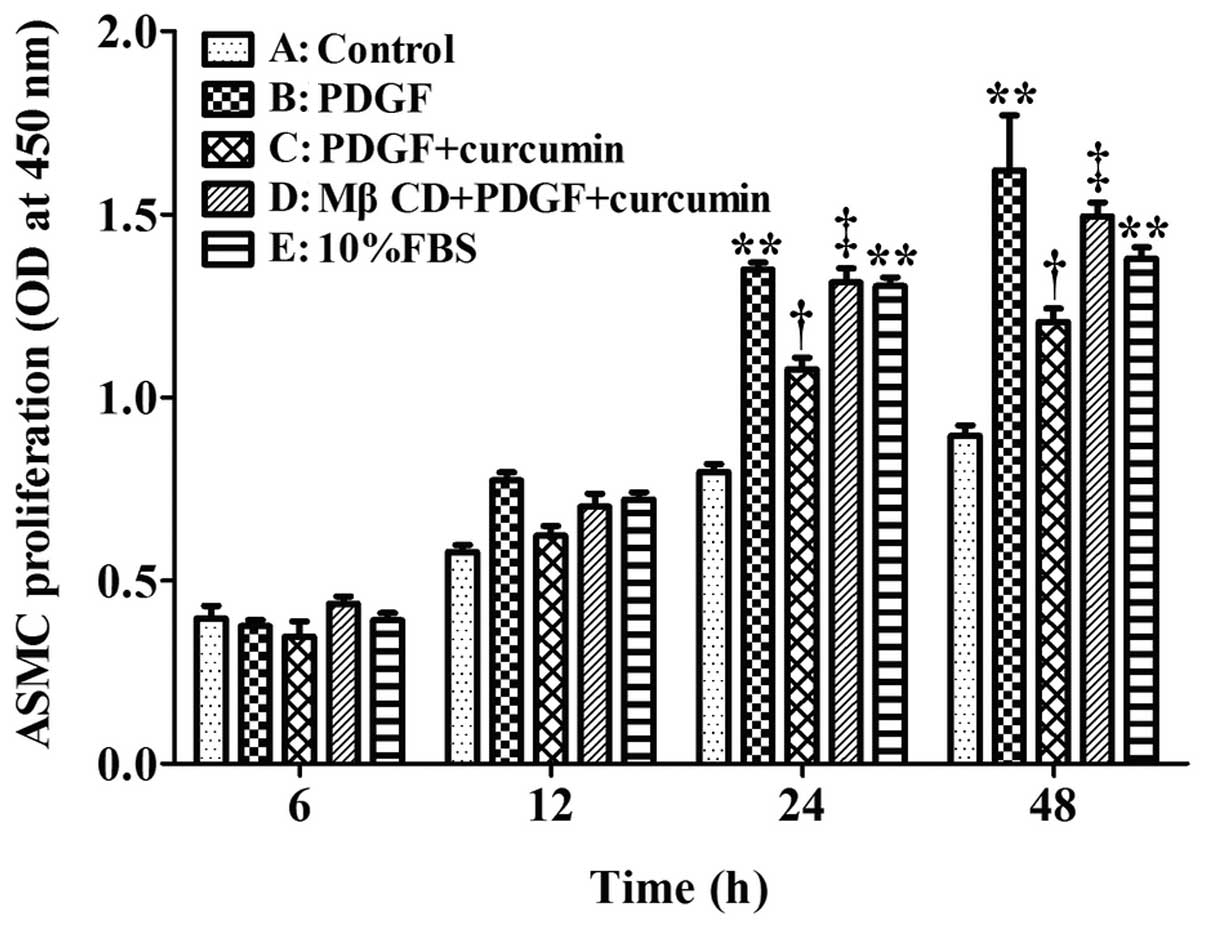
Effect of curcumin on PDGF-induced ASMC
proliferation
Cell proliferation was determined using the CCK-8
assay as shown in Fig. 4. At 12,
24 and 48 h, the absorbance at 450 nm was significantly higher in
the PDGF + curcumin group compared with the negative control group
(P<0.05). Of note, the ASMC proliferation induced by PDGF in
group B peaked at 48 h, compared with the negative control group
(P<0.01). Curcumin (25 μmol/l) significantly decreased the
PDGF-induced the proliferation of ASMCs at 24 h (P<0.05) (group
C compared with group B). After caveolae was disrupted by MβCD, the
PDGF-induced proliferation of ASMCs in group D was markedly
increased compared with group C. This suggests that the
anti-proliferative effects of curcumin are weakened without
caveolin-1; thus, caveolin-1 may play an important role in the
anti-proliferative effects of curcumin on ASMCs.
Effect of curcumin on the expression of
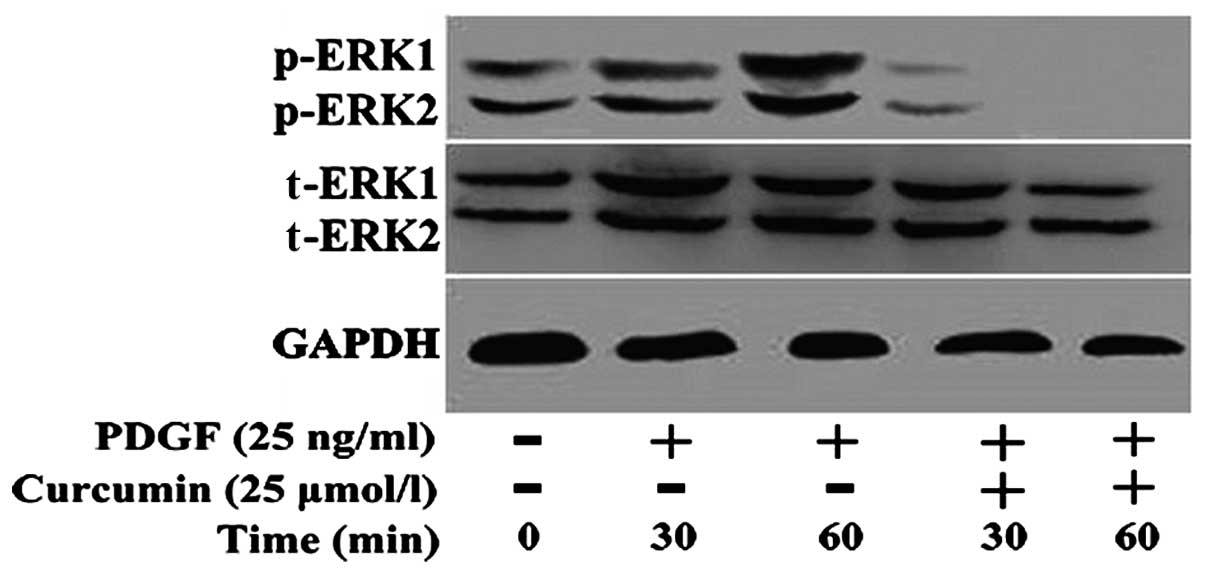
ERK1/2 protein in ASMCs
After the 4th passage ASMCs were treated with or
without curcumin (25 μmol/l) for 30 and 60 min, total protein was
extracted for western blot analysis to detect the expression of
total ERK1/2 protein and the phosphorylation of ERK1/2 protein. A
control group was set up. Treatment with PDGF increased ERK1/2
phosphorylation compared with the control group. The expression of
PDGF-induced phosphorylated-ERK was significantly decreased
following treatment with curcumin (Fig. 5), and phosphorylated ERK could not
be detected at 60 min. Curcumin had no effect on the expression of
total ERK1/2.
mRNA and protein expression of caveolin-1
following treatment with curcumin
After the rat ASMCs were treated with curcumin (25
μmol/l) for 0, 4, 8, 12 and 24 h, the mRNA expression of caveolin-1
in the ASMCs was measured by real-time PCR. The result revealed
that following treatment with PDGF with or without crucumin for 4
h, the caveolin-1 mRNA expression was increased (compared with the
control group) (P<0.01). The mRNA expression of caveolin-1 was
significantly increased in the PDGF + curcumin-treated group
compared with the PDGF group (P<0.05) (Fig. 6A). After the 4th passage ASMCs
were treated with PDGF or curcumin and PDGF for 24 h, total protein
was extracted for western blot analysis to detect the expression of
caveolin-1 protein. The results are shown in Fig. 6B. Compared with the PDGF group,
the protein expression of caveolin-1 in the cucumin + PDGF group
was significantly increased (P<0.01).
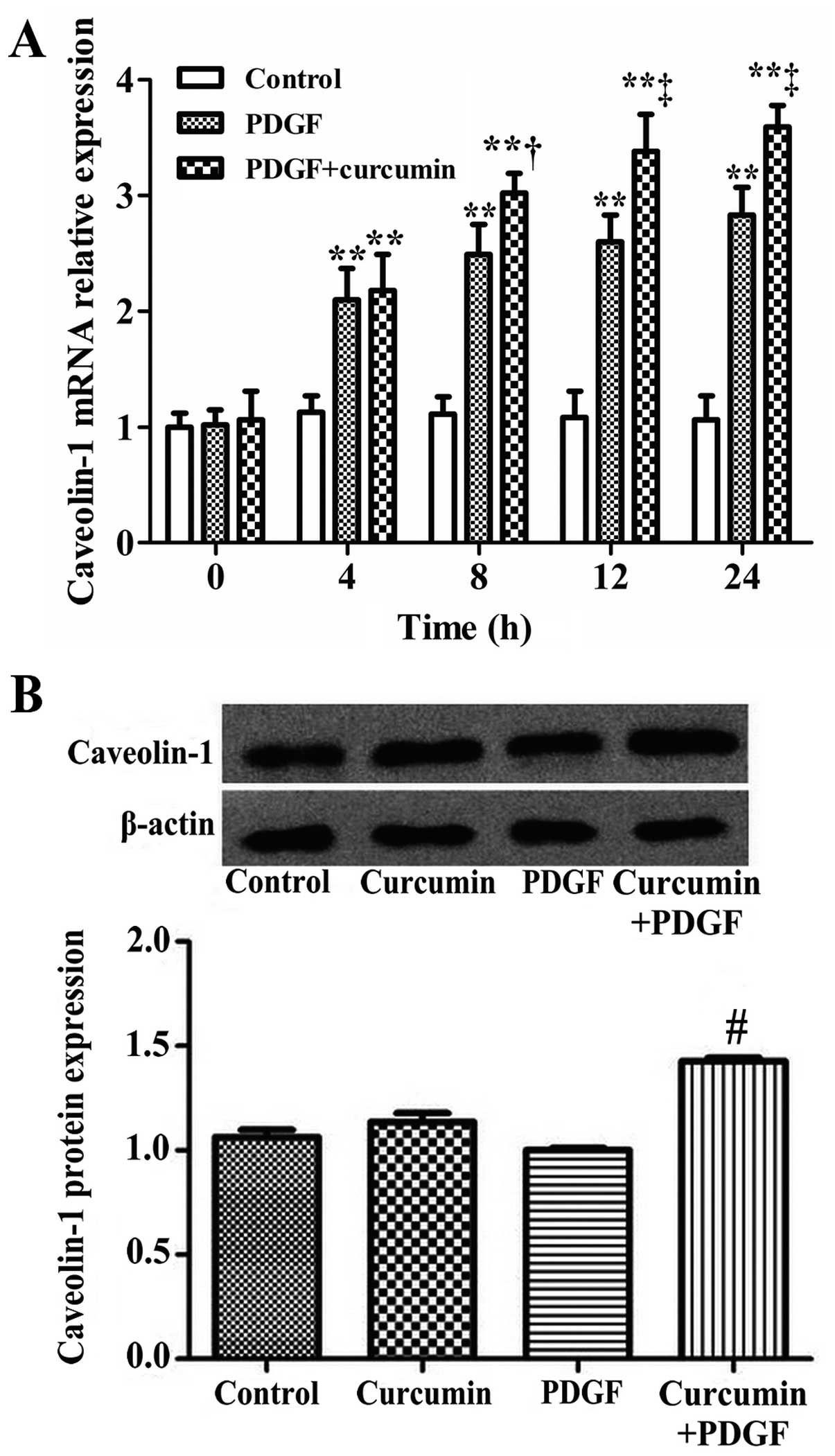 | Figure 6Curcumin increases the mRNA and
protein expression of caveolin-1 in airway smooth muscle cells
(ASMCs). (A) After the ASMCs were treated with curcumin for 0, 4,
8, 12 and 24 h, the mRNA expression of caveolin-1 in the ASMCs was
measured by real-time PCR. The data are expressed as the mean ± SD,
n=3. **P<0.01, compared with the control group;
†P<0.05, compared with the platelet-derived growth
factor (PDGF) group and ‡P<0.01, compared with the
PDGF group. (B) Total proteins from the 4th passage ASMCs were used
for western blot analysis to detect the protein expression of
caveolin-1. The data are expressed as the means ± SD, n=3,
#P<0.01, compared with the PDGF group. |
Discussion
Asthma is traditionally defined as a chronic disease
characterized by airway inflammation, AHR and airway remodeling. In
a previous study, Chung examined patients with asthma using airway
histopathology and lung function dynamic observation and indicated
that a series of structural changes could be found in the airway
wall in the patients with asthma (5). Among the histopathological
characteristics of airway remodeling, previous studies have
indicated that the increased mass of ASMCs plays a critical role
(2,4,5,28,29). Due to their important role in
airway remodeling, ASMCs may be a potential therapeutic target for
the treatment of patients with asthma.
Curcumin has been used in indigenous medicine for
the treatment of a variety of inflammatory conditions and chronic
diseases (9,13,14,23) and studies have mainly focused on
the effects of curcumin on inhibiting bronchial inflammation
(30–34). However, limited studies have
investigated the effects of curcumin on structural changes in
asthma, such as ASMC proliferation in airway remodeling. Therefore,
in this study, we performed in vitro and in vivo
experiments to examine the effects of curcumin on the proliferation
of ASMCs and to elucidate the underlying mechanisms. Our findings
demonstrate that curcumin inhibits the proliferation of ASMCs both
in vitro and in vivo.
In in vivo experiments, we used
OVA-challenged mice to establish a model of chronic asthma airway
remodeling; the curcumin-treated groups were administered various
doses of curcumin by an i.p. injection prior to challenge with OVA.
The mouse model of airway remodeling induced by OVA has previously
been used in a number of studiesl; however, the majority of these
studies focused on the inflammatory infiltration in this model. Our
results were identical with those from previous studies (30–33). Our findings indicated that the
symptoms of an asthma attack dissipated following treatment with
curcumin and that curcumin significantly reduced the airway
responsiveness. In addition, we paid particular attention to the
changes in airway smooth muscle. We found that following treatment
with curcumin, the thickness of the airway wall and the bronchial
smooth muscle layer became thinner and the number of smooth muscle
cells decreased; the most significant changes were observed in the
high-dose curcumin-treated group. These results demonstrate that
the administration of curcumin inhibits the proliferation of ASMCs
in vivo.
In in vitro experiments, we stimulated the
proliferation of primary rat ASMCs by PDGF and provided a
proliferative experimental model for the study of asthma at the
cellular and molecular level. Our results revealed that curcumin
inhibited the PDGF-induced proliferation of ASMCs in
vitro.
The mitogen-activated protein kinase (MAPK)
signaling cascade has been shown to play an important role in the
activation of various cells (35–38). It is activated by the 3-tiered
sequential phosphorylation of MAPK kinase, MAPK/ERK kinase (MEK)
and MAPK. There are 3 major groups of MAPK in mammalian cells,
including ERK, p38 MAPK and c-Jun N-terminal kinase. Accumulating
evidence has shown that ERK plays a crucial role in human ASMC
growth. A previous study demonstrated that ERK activity in the
lungs of asthmatic mice was significantly higher compared with
normal mice (39). ERK activation
is necessary for the proliferation of ASMCs (4). In addition, PDGF and other growth
factors are promoters of ERK1/2 (p42/44 MAPK) expression and the
activation of phosphorylation, which are closely associated with
the proliferation of smooth muscle cells (27,40–44). Thus, the ERK signaling pathway
plays a crucial role in the pathological process of airway
remodeling in asthma. The results of our study are in accordance
with these findings; we found that the phosphorylated-ERK1/2
protein levels were significantly decreased following the treatment
of ASMCs with curcumin. This suggests that the inhibition of the
activation of ERK1/2 by curcumin is involved in the inhibition of
ASMC proliferation. We provide evidence that curcumin inhibits ASMC
proliferation and that caveolin-1 plays a crucial role in this
process; the involvement of caveolin-1 is partly due to the fact
that it has the ability to regulate the ERK 1/2 pathway.
We found that curcumin significantly inhibited the
PDGF-induced proliferation of ASMCs and that this effect was
significantly attenuated by MβCD. The expression of caveolin-1 was
significantly increased in the curcumin-treated group as compared
with the PDGF group in our study. Caveolae are small vesicular
invaginations of the cell membrane. It is within this organelle
that cells perform transcytosis and signal transduction. Caveolae
are composed of a mixture of lipids and proteins (45–50). The chief structural proteins of
caveolae are caveolins (caveolin-1, caveolin-2 and caveolin-3), and
caveolin-1 appears to be an essential component of caveolae
(51–57). Evidence suggests that caveolin-1
regulates the ERK1/2 pathway during cell proliferation (55,58–60). Buitrago and Boland found that when
proliferating mouse skeletal myoblastic cells were pre-treated with
MβCD, a caveolae-disrupting agent, the
1α,25(OH)2D3-dependent activation of ERK1/2,
p38 MAPK and c-Src was suppressed (58). Furthermore, studies on human ASMCs
have shown that caveolae and caveolin-1 coordinate PDGF receptor
signaling, leading to myocyte proliferation, and inhibit the
constitutive activity of p42/p44 MAPK, sustaining cell quiescence
(55). These data are in
accordance with those in the study by Peterson et al, who
showed that the treatment of VSMCs with PDGF for 24 h resulted in a
loss of caveolin-1 protein expression and plasma
membrane-associated caveolae (21). The data mentioned above indicate
that caveolin-1 is an important negative regulator of cell
proliferation and PDGF signaling events. Our results therefore
suggest that caveolin-1 plays a crucial role in the
anti-proliferative effects of curcumin through the ERK1/2
pathway.
In conclusion, we demonstrate that curcumin inhibits
the proliferation of ASMCs in vitro and in vivo; the
possible mechanisms behind the inhibitory effects of curcumin may
be the upregulation of the expression of caveolin-1 and the
blocking of the ERK pathway, thereby inhibiting the proliferation
of ASMCs. Our findings provide new insight into the application of
curcumin in the prevention and treatment of asthma, particularly
airway remodeling in severe asthma.
Acknowledgements
This study was supported by grants from the
Guangzhou Science and Technology Committee (no. 2008J1-C261) and
the Guangzhou University Science and Technology Project (no.
10A149), China.
References
|
1
|
Bateman ED: Global strategy for asthma
management and prevention 2009 (update). Report of Global
Initiative for Asthma. 2009:22009.
|
|
2
|
Girodet PO, Ozier A, Bara I, Tunon de Lara
JM, Marthan R and Berger P: Airway remodeling in asthma: new
mechanisms and potential for pharmacological intervention.
Pharmacol Ther. 130:325–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delacourt C: Bronchial changes in
untreated asthma. Arch Pediatr. 11(Suppl 2): 71s–73s. 2004.(In
French).
|
|
4
|
Black JL, Roth M, Lee J, Carlin S and
Johnson PR: Mechanisms of airway remodeling. Airway smooth muscle.
Am J Respir Crit Care Med. 164:S63–S66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung KF: The role of airway smooth muscle
in the pathogenesis of airway wall remodeling in chronic
obstructive pulmonary disease. Proc Am Thorac Soc. 2:347–354. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siddiqui S, Redhu NS, Ojo OO, et al:
Emerging airway smooth muscle targets to treat asthma. Pulm
Pharmacol Ther. 26:132–144. 2012. View Article : Google Scholar
|
|
7
|
Bar-Sela G, Epelbaum R and Schaffer M:
Curcumin as an anti-cancer agent: review of the gap between basic
and clinical applications. Curr Med Chem. 17:190–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: an ‘old-age’
disease with an ‘age-old’ solution. Cancer Lett. 267:133–164.
2008.
|
|
9
|
Schaffer M, Schaffer PM, Zidan J and Bar
Sela G: Curcuma as a functional food in the control of cancer and
inflammation. Curr Opin Clin Nutr Metab Care. 14:588–597. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ammon HP and Wahl MA: Pharmacology of
Curcuma longa. Planta Med. 57:1–7. 1991.
|
|
11
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: from ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aggarwal BB and Harikumar KB: Potential
therapeutic effects of curcumin, the anti-inflammatory agent,
against neurodegenerative, cardiovascular, pulmonary, metabolic,
autoimmune and neoplastic diseases. Int J Biochem Cell Biol.
41:40–59. 2009. View Article : Google Scholar
|
|
13
|
Yang X, Thomas DP, Zhang X, et al:
Curcumin inhibits platelet-derived growth factor-stimulated
vascular smooth muscle cell function and injury-induced neointima
formation. Arterioscler Thromb Vasc Biol. 26:85–90. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong TF, Takeda T, Li B, et al: Curcumin
disrupts uterine leiomyosarcoma cells through AKT-mTOR pathway
inhibition. Gynecol Oncol. 122:141–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laporte JC, Moore PE, Baraldo S, et al:
Direct effects of interleukin-13 on signaling pathways for
physiological responses in cultured human airway smooth muscle
cells. Am J Respir Crit Care Med. 164:141–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weng SH, Tsai MS, Chiu YF, Kuo YH, Chen HJ
and Lin YW: Enhancement of mitomycin C-induced cytotoxicity by
curcumin results from down-regulation of MKK1/2-ERK1/2-mediated
thymidine phosphorylase expression. Basic Clin Pharmacol Toxicol.
110:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saab MB, Bec N, Martin M, et al:
Differential effect of curcumin on the nanomechanics of normal and
cancerous mammalian epithelial cells. Cell Biochem Biophys.
65:399–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhandapani KM, Mahesh VB and Brann DW:
Curcumin suppresses growth and chemoresistance of human
glioblastoma cells via AP-1 and NFkappaB transcription factors. J
Neurochem. 102:522–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang CL, Liu YY, Ma YG, et al: Curcumin
blocks small cell lung cancer cells migration, invasion,
angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3
signalling pathway. PLoS One. 7:e379602012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JW, Tang YL, Liu H, et al:
Anti-proliferative and anti-metastatic effects of curcumin on oral
cancer cells. Hua Xi Kou Qiang Yi Xue Za Zhi. 29:83–86. 2011.(In
Chinese).
|
|
21
|
Peterson TE, Guicciardi ME, Gulati R, et
al: Caveolin-1 can regulate vascular smooth muscle cell fate by
switching platelet-derived growth factor signaling from a
proliferative to an apoptotic pathway. Arterioscler Thromb Vasc
Biol. 23:1521–1527. 2003. View Article : Google Scholar
|
|
22
|
Luo DX, Cheng J, Xiong Y, et al: Static
pressure drives proliferation of vascular smooth muscle cells via
caveolin-1/ERK1/2 pathway. Biochem Biophys Res Commun.
391:1693–1697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin L, Yang YB, Tuo QH, et al: Effects and
underlying mechanisms of curcumin on the proliferation of vascular
smooth muscle cells induced by Chol:MbetaCD. Biochem Biophys Res
Commun. 379:277–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McMillan SJ and Lloyd CM: Prolonged
allergen challenge in mice leads to persistent airway remodelling.
Clin Exp Allergy. 34:497–507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lloyd CM, Gonzalo JA, Nguyen T, et al:
Resolution of bronchial hyperresponsiveness and pulmonary
inflammation is associated with IL-3 and tissue leukocyte
apoptosis. J Immunol. 166:2033–2040. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamelmann E, Schwarze J, Takeda K, et al:
Noninvasive measurement of airway responsiveness in allergic mice
using barometric plethysmography. Am J Respir Crit Care Med.
156:766–775. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen G and Khalil N: TGF-beta1 increases
proliferation of airway smooth muscle cells by phosphorylation of
map kinases. Respir Res. 7:22006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang ML, Wilson JW, Stewart AG and Royce
SG: Airway remodelling in asthma: current understanding and
implications for future therapies. Pharmacol Ther. 112:474–488.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nath P, Leung SY, Williams A, et al:
Importance of p38 mitogen-activated protein kinase pathway in
allergic airway remodelling and bronchial hyperresponsiveness. Eur
J Pharmacol. 544:160–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh SW, Cha JY, Jung JE, et al: Curcumin
attenuates allergic airway inflammation and hyper-responsiveness in
mice through NF-κB inhibition. J Ethnopharmacol. 136:414–421.
2011.PubMed/NCBI
|
|
31
|
Moon DO, Kim MO, Lee HJ, et al: Curcumin
attenuates ovalbumin-induced airway inflammation by regulating
nitric oxide. Biochem Biophys Res Commun. 375:275–279. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karaman M, Firinci F, Cilaker S, et al:
Anti-inflammatory effects of curcumin in a murine model of chronic
asthma. Allergol Immunopathol (Madr). 40:210–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Venkatesan N, Punithavathi D and Babu M:
Protection from acute and chronic lung diseases by curcumin. Adv
Exp Med Biol. 595:379–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharafkhaneh A, Velamuri S, Badmaev V, Lan
C and Hanania N: The potential role of natural agents in treatment
of airway inflammation. Ther Adv Respir Dis. 1:105–120. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nel AE: T-cell activation through the
antigen receptor. Part 1: signaling components, signaling pathways,
and signal integration at the T-cell antigen receptor synapse. J
Allergy Clin Immunol. 109:758–770. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jacob A, Cooney D, Pradhan M and
Coggeshall KM: Convergence of signaling pathways on the activation
of ERK in B cells. J Biol Chem. 277:23420–23426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gauld SB, Dal Porto JM and Cambier JC: B
cell antigen receptor signaling: roles in cell development and
disease. Science. 296:1641–1642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nadler MJ, Matthews SA, Turner H and Kinet
JP: Signal transduction by the high-affinity immunoglobulin E
receptor Fc epsilon RI: coupling form to function. Adv Immunol.
76:325–355. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duan W, Chan JH, Wong CH, Leung BP and
Wong WS: Anti-inflammatory effects of mitogen-activated protein
kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol.
172:7053–7059. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De S, Zelazny ET, Souhrada JF and Souhrada
M: Role of phospholipase C and tyrosine kinase systems in growth
response of human airway smooth muscle cells. Am J Physiol.
270:L795–L802. 1996.PubMed/NCBI
|
|
41
|
Walker TR, Moore SM, Lawson MF, Panettieri
RA Jr and Chilvers ER: Platelet-derived growth factor-BB and
thrombin activate phosphoinositide 3-kinase and protein kinase B:
role in mediating airway smooth muscle proliferation. Mol
Pharmacol. 54:1007–1015. 1998.
|
|
42
|
Kumar A, Lnu S, Malya R, et al: Mechanical
stretch activates nuclear factor-kappaB, activator protein-1, and
mitogen-activated protein kinases in lung parenchyma: implications
in asthma. FASEB J. 17:1800–1811. 2003. View Article : Google Scholar
|
|
43
|
Chiou YL, Shieh JJ and Lin CY: Blocking of
Akt/NF-kappaB signaling by pentoxifylline inhibits platelet-derived
growth factor-stimulated proliferation in Brown Norway rat airway
smooth muscle cells. Pediatr Res. 60:657–662. 2006. View Article : Google Scholar
|
|
44
|
Bai J, Liu XS, Xu YJ, Zhang ZX, Xie M and
Ni W: The effect of ERK signaling pathway on cell apoptosis in
airway smooth muscle cells of chronic asthmatic rats. Xi Bao Yu Fen
Zi Mian Yi Xue Za Zhi. 26:738–741. 2010.(In Chinese).
|
|
45
|
Schlegel A, Volonte D, Engelman JA, et al:
Crowded little caves: structure and function of caveolae. Cell
Signal. 10:457–463. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shaul PW and Anderson RG: Role of
plasmalemmal caveolae in signal transduction. Am J Physiol.
275:L843–L851. 1998.PubMed/NCBI
|
|
47
|
Anderson RG: The caveolae membrane system.
Annu Rev Biochem. 67:199–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Okamoto T, Schlegel A, Scherer PE and
Lisanti MP: Caveolins, a family of scaffolding proteins for
organizing ‘preassembled signaling complexes’ at the plasma
membrane. J Biol Chem. 273:5419–5422. 1998.
|
|
49
|
Couet J, Belanger MM, Roussel E and Drolet
MC: Cell biology of caveolae and caveolin. Adv Drug Deliv Rev.
49:223–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Razani B, Woodman SE and Lisanti MP:
Caveolae: from cell biology to animal physiology. Pharmacol Rev.
54:431–467. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schubert W, Frank PG, Woodman SE, et al:
Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice.
Treatment with a specific nitric-oxide synthase inhibitor, L-NAME,
restores normal microvascular permeability in Cav-1 null mice. J
Biol Chem. 277:40091–40098. 2002.
|
|
52
|
Ramirez MI, Pollack L, Millien G, Cao YX,
Hinds A and Williams MC: The alpha-isoform of caveolin-1 is a
marker of vasculogenesis in early lung development. J Histochem
Cytochem. 50:33–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thyberg J: Caveolin-1 and caveolae act as
regulators of mitogenic signaling in vascular smooth muscle cells.
Arterioscler Thromb Vasc Biol. 23:1481–1483. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Miyawaki-Shimizu K, Predescu D, Shimizu J,
Broman M, Predescu S and Malik AB: siRNA-induced caveolin-1
knockdown in mice increases lung vascular permeability via the
junctional pathway. Am J Physiol Lung Cell Mol Physiol.
290:L405–L413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gosens R, Stelmack GL, Dueck G, et al:
Role of caveolin-1 in p42/p44 MAP kinase activation and
proliferation of human airway smooth muscle. Am J Physiol Lung Cell
Mol Physiol. 291:L523–L534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sun Y, Hu G, Zhang X and Minshall RD:
Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary
vascular permeability via paracellular and transcellular pathways.
Circ Res. 105:676–685, 615 p following 685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Feng H, Guo L, Song Z, et al: Caveolin-1
protects against sepsis by modulating inflammatory response,
alleviating bacterial burden, and suppressing thymocyte apoptosis.
J Biol Chem. 285:25154–25160. 2010. View Article : Google Scholar
|
|
58
|
Buitrago C and Boland R: Caveolae and
caveolin-1 are implicated in 1α,25(OH)2-vitamin
D3-dependent modulation of Src, MAPK cascades and VDR localization
in skeletal muscle cells. J Steroid Biochem Mol Biol. 121:169–175.
2010.
|
|
59
|
Park JH, Ryu JM and Han HJ: Involvement of
caveolin-1 in fibronectin-induced mouse embryonic stem cell
proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. J
Cell Physiol. 226:267–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Watson CS, Jeng YJ, Hu G, Wozniak A,
Bulayeva N and Guptarak J: Estrogen- and xenoestrogen-induced ERK
signaling in pituitary tumor cells involves estrogen receptor-α
interactions with G protein-αi and caveolin I. Steroids.
77:424–432. 2012.PubMed/NCBI
|