Introduction
Cold stimuli are known to exacerbate chronic
obstructive pulmonary disease (COPD) and have been shown to promote
mucin (MUC) hypersecretion (1).
Excessive MUC production leads to airway obstruction and enhances
inflammation. Previous studies have mainly focused on secondary
inflammatory processes (2) and
bacterial (3) or viral infections
(4,5) that occur in the airways following
exposure to cold temperatures; however, the molecular mechanisms
behind cold-induced mucus secretion have not yet been fully
elucidated. Human organs express ion channels of the transient
receptor potential (TRP) family, including some ion channels that
respond at distinct temperature thresholds (6–9).
TRP melastatin 8 (TRPM8) is a member of this family that is
activated by temperatures ranging from 8 to 25°C. TRPM8 is a
ligand-gated cation channel with moderate to high selectivity for
calcium ions (10,11).
TRPM8 can be activated by cold temperatures and by
cooling agents, such as menthol, eucalyptol and icilin. In a
previous study, we found that TRPM8 plays a critical role in
cold-induced MUC secretion in the human respiratory system
(12). Human airway epithelial
cells exposed to cold temperatures exhibited activated TRPM8
channels, and MUC5AC expression was upregulated through the
TRPM8-Ca2+-phospholipase C (PLC)-phosphatidylinositol
bisphosphate (PIP2) pathway. Thus, we hypothesized that TRPM8 plays
a role in cold-induced MUC secretion. In this study, we aimed to
discover methods of inhibiting cold-induced MUC hypersecretion.
Salidroside (rhodioside) is a glycoside compound
primarily found in the plant, Rhodiola rosea, which grows in
cold, high-latitude areas. It is well known that salidroside has
pharmacological effects on many pathological conditions, including
radiation poisoning (13),
inflammation (14), fatigue,
aging and hypoxia (15,16). Other studies have found that
salidroside exerts its protective effects mainly by repressing the
inflow of intracellular free calcium (Ca2+)
([Ca2+]i) into cells. Considering what is already known
about salidroside and TRPM8, we hypothesized that salidroside has
the ability to protect cells from cold nociceptive stimuli and that
it may play a positive role in the airway system. In the present
study, we explored the regulation of TRPM8 gene transcription in
cold-assaulted cells and examined the effects of salidroside on
TRPM8 channels and MUC production. We used the HBE16 human airway
epithelial cell line for in vitro studies. The cells were
pre-treated with salidroside and exposed to cold temperatures; the
protein and mRNA levels of MUC5AC were then determined. The
expression levels of TRPM8 and the transcription factor, cAMP
response element-binding protein (CREB), were assayed to
investigate whether there were any differences in the levels of
these factors following treatment with salidroside. Furthermore, we
transfected the cells with CREB small interfering RNA (siRNA) to
determine whether the transcription factor, CREB, is involved in
TRPM8 gene transcription.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM)/Ham's F12
medium, HEPES, fetal bovine serum (FBS), anti-β-actin monoclonal
antibody, salidroside (>98% purity; National Institute for Food
and Drug Control), CREB siRNA and negative control siRNA were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Lipofectamine® 2000 and OPTI-MEM Reduced Serum Medium
were purchased from Invitrogen (San Diego, CA, USA). The rabbit
anti-TRPM8 polyclonal antibody was purchased from Abcam (Cambridge,
MA, USA) and the horseradish peroxidase (HRP)-goat anti-rabbit IgG
antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). CREB-Luc reporter constructs were purchased from
Biocat (Catalonia, Spain). The Dual Luciferase Reporter Gene Assay
kit was purchased from BioTime Technology (Beijing, China). The
pRL-TK Renilla Luciferase Reporter Vector was purchased from
Promega (Madison, WI, USA). The high-purity total RNA extraction
kit was purchased from Bioteke Biotechnology (Beijing, China). The
PrimeScript RT Reagent kit was purchased from Takara (Dalian,
China).
Cell culture
HBE16 human airway epithelial cells were plated in
6-well plates at a concentration of 5–6×105 cells/well
in 2 ml DMEM/Ham's F12 medium that contained 10% FBS. The cells
were then incubated at 37°C and 5% CO2. The culture
medium was changed to growth factor-free medium prior to the start
of each experiment. Cell supernatants and lysates were collected
and assays were performed, as described below.
Cell viability measurements
Confluent HBE16 cells were incubated with 10, 50,
100, or 200 μM salidroside for 24 h. We used the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reduction method to detect cell viability and to select the
appropriate dose of salidroside for the subsequent experiments.
siRNA transfection
Cells were plated at a density of 1–2×106
cells/ml and incubated at 37°C until the cells were 60–80%
confluent. CREB siRNA (0.5 μg) were diluted in 100 μl of siRNA
transfection medium (solution A); 6 μl siRNA transfection reagent
was diluted in 100 μl of siRNA transfection medium (solution B).
The solutions (A + B) were subsequently mixed gently and incubated
at room temperature for 45 min. The cells were washed once with 2
ml of siRNA transfection medium followed by the addition of 0.8 ml
of the siRNA transfection reagent mixture (solutions A + B). The
cells were then incubated for 7 h at 37°C in a CO2
incubator and were recovered in 2 ml of DMEM/Ham's F12 medium
containing 10% FBS for an additional 24 h. The medium was then
aspirated and replaced with fresh growth medium for the following
assays (see below for details).
RNA isolation and real-time PCR for
MUC5AC and TRPM8 mRNA
After the HBE16 cells were harvested, total RNA was
extracted from the cells using the Bioteke high-purity total RNA
extraction kit, and RNA integrity was verified using 1.5% agarose
gel electrophoresis. The absorbance at 260/280 nm
(A260/280) was in the range of 1.8–2.0. Total RNA was
primed with an oligo(dT) primer and reverse transcribed using the
PrimeScript RT Reagent kit. Real-time PCR was performed using the
SYBR Premix EX Taq™ II real-time PCR kit. The sequences of the
primers used for real-time PCR were as follows: MUC5AC (U06711)
forward, 5′-CAGCCACGTCCCC TTCAATA-3′ and reverse,
5′-ACCGCATTTGGGCATCC-3′; TRPM8 (NM024080) forward,
5′-ACTCAGAAGGCTGAGG TACA-3′ and reverse,
5′-TTCAGTCGGAGTCTCACTCT-3′; and GAPDH (BC026907) forward,
5′-GAAGGTGAAGGT CGGAGT-3′ and reverse, 5′-GAAGATGGTGATGGGA TTTC-3′.
GAPDH was used as the loading control. Relative mRNA expression was
determined by comparing it to a standard curve.
Enzyme-linked immunosorbent assay (ELISA)
for MUC5AC protein
MUC5AC protein levels in the cell lysates and
supernatants were measured using ELISA. Cell lysates at multiple
dilutions were prepared with phosphate-buffered saline (PBS). Cell
culture supernatants were collected for this assay. In addition, 50
μl of each sample were incubated with bicarbonate-carbonate buffer
(50 μl) at 40°C in a 96-well plate until dry. The wells were washed
3 times with PBS and blocked with 2% bovine serum albumin (BSA) for
1 h at room temperature. The wells were then incubated with mouse
anti-human MUC5AC monoclonal antibody (45M1) (10 μg/ml; Neomarkers
Inc., Fremont, CA, USA) diluted in PBS containing 0.05% Tween-20
for 1 h at room temperature. After washing 3 times, the wells were
incubated with HRP-conjugated goat anti-mouse IgG (1 μg/ml) for 1
h. HRP was developed with 3,3′,5,5′-tetramethylbenzidine (TMB)
peroxidase solution, quenched with 1 M H2SO4
and the absorbance at 450 nm was measured.
Western blot analysis for TRPM8 and
phosphorylated CREB (p-CREB) protein levels
Total protein was determined by BCA analysis. The
cells were lysed in lysate buffer, disrupted on ice for 20 min, and
then centrifuged at 12,000 rpm for 15 min at 4°C to remove the
nuclei and unbroken cells. The total protein concentration in the
cell lysates was determined by BCA assay. Equal amounts of protein
were diluted in SDS sample buffer and boiled for 5 min. Proteins
were resolved on 6% SDS gels. After running, the gels were
equilibrated in transfer buffer containing 25 mM Tris-HCl, 192 mM
glycine and 20% methanol (pH 8.3). The proteins were then
transferred by electrophoresis onto polyvinylidene difluoride
(PVDF) membranes. The membranes were incubated in 5% milk dissolved
in PBS and 0.05% Tween-20 for 1 h at room temperature and
subsequently incubated with rabbit anti-TRPM8 (diluted 1:200) or
rabbit anti-p-CREB (Ser-129) polyclonal antibodies (diluted 1:500)
overnight at 4°C. After washing 3 times, the membranes were
incubated with an HRP-labeled goat anti-rabbit IgG antibody
(diluted 1:1,000) for 2 h at room temperature. Enhanced
chemiluminescence (ECL) and autoradiography were performed to
visualize TRPM8 and p-CREB protein. The membranes were also blotted
for β-actin and α-tubulin to ensure equal protein loading.
Immunofluorescence for TRPM8
Cells were fixed with 4% paraformaldehyde and
subsequently permeabilized with 0.1% Triton X-100 in PBS for 30
min. The cells were rinsed, blocked in 1% BSA plus 1% normal goat
serum and incubated with rabbit anti-TRPM8 (diluted 1:500)
overnight at 4°C. After 3 washes of 10 min each in 0.1% Triton
X-100/PBS, the cells were incubated with FITC-conjugated
fluorescent goat anti-rabbit IgG (1:500) for 2 h at room
temperature followed by cellular nuclear staining with
4′6-diamidino-2-phenylindole (DAPI). The samples were examined
using a Leica inverted TCS-SP2 confocal microscope that was
equipped with appropriate fluorescence filters.
Determination of TRPM8 channel
conductivity
Whole-cell patch-clamp experiments were performed
using an EPC-10 amplifier and IGOR Pro 4.0 software for data
acquisition and analysis. Frequencies of 10 KHz were selected for
sampling and 2 KHz for filtration. The extracellular solution used
for electrophysiological recordings consisted of 140 mM NaCl, 5 mM
KCl, 2 mM CaCl2, 1 mM MgCl2, 0.3 mM
Na2HPO4, 0.4 mM KH2PO4,
4 mM NaHCO3, 5 mM glucose and 10 mM HEPES (the pH was
adjusted to 7.4 using NaOH). The intracellular solution filled into
patch-clamp pipettes contained 140 mM KCl, 1 mM MgCl2,
2.5 mM CaCl2, 4 mM EGTA and 10 mM HEPES. The calculated
free Ca2+ concentration in this solution was 150 nM, and
the pH was adjusted to 7.2 using KOH as previously described
(17). The borosilicate glass
patch pipettes used for the whole-cell recordings had resistances
of 2–4 MΩ when filled with intracellular solutions.
Ca2+ influx detection
The cells were exposed to cold temperatures (18°C)
or pre-treated with salidroside and collected at different time
intervals. We resuspended the cells in D-Hanks buffer containing
0.2% FBS (106 cells/ml), removed 1 ml of the cell
suspension to an Eppendorf tube and then added 2 μl Fura-2/AM stock
solution to the cells followed by incubation with shaking at 37°C
for 60 min. The cells were centrifuged at 1,300 rpm for 6 min,
washed twice with pre-heated D-Hanks buffer containing 0.2% BSA and
resuspended in D-Hanks buffer. The fluorescence intensity was then
observed using a dual wavelength spectrophotometer (Hitachi
F-3000). The 340/380 nm fluorescence intensity ratio of each sample
is abbreviated as R. The 380 nm fluorescence intensity of Fura-2
under CA2+-free and Ca2+ saturation
conditions are abbreviated as Fmin and Fmax, respectively. As the
controls, 40 μl 2.5% Triton X-100 were added to the cells to detect
the maximum fluorescence intensity ratio (Rmax) and 40 μl 250 mM
Ca2+ chelator EGTA were added to the cells to detect the
minimum fluorescence intensity ratio (Rmin). The [Ca2+]i
concentrations were calculated using the formula [Ca2+]i
= Kd[(R - Rmin)/(Rmax - R)](Fmin/Fmax).
CREB activity assay
CREB activity was determined using the
CREB-Luciferase Reporter Assay System (Biocat). The CREB-Luc and
pRL-TK Renilla Luciferase Reporter plasmids were transfected into
the HBE16 cells using Lipofectamine® 2000. Eighteen
hours after transfection, the cells were pre-incubated with 100 μM
salidroside for 30 min and exposed to cold temperatures (18°C).
CREB activity was assayed in accordance with the manufacturer's
instructions provided with the Luciferase Reporter Gene Assay kit.
The results were expressed as detected luciferase relative light
units (RLU)/Renilla luciferase RLU.
Statistical analysis
Data analysis was performed using the SPSS 17.0
statistical software package (SPSS, Inc., Chicago, IL, USA). All
in vitro experiments using cell lines included samples from
at least 6 separate wells and 2–3 independent experiments.
Comparisons of >2 groups were made using the Student's t-test or
a one-way analysis of variance (ANOVA) followed by Bonferroni's
analysis. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Viability of HBE16 cells following
exposure to salidroside
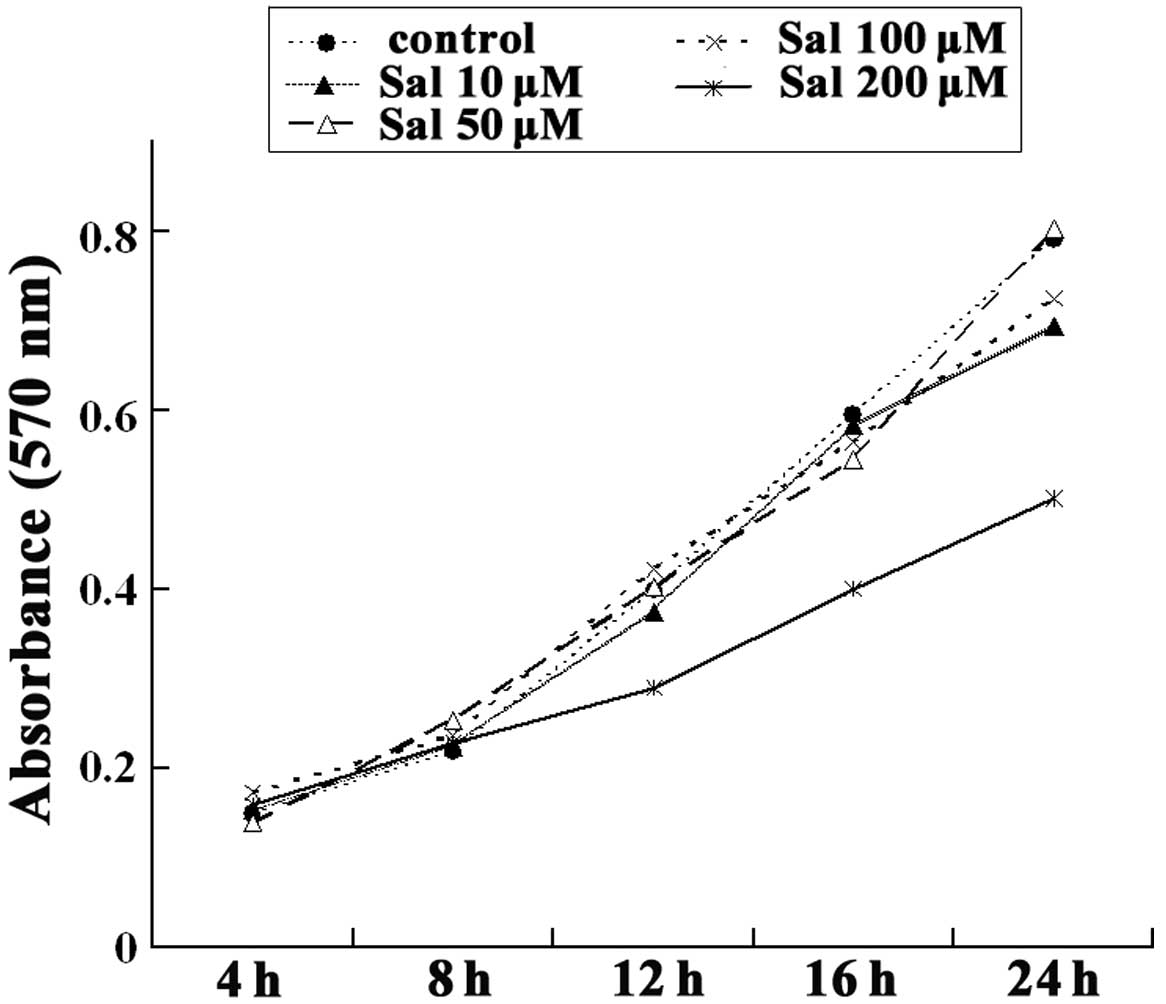
The cells were incubated with 10, 50, 100, or 200 μM
salidroside for various periods of time to determine cellular
proliferation rates following exposure to salidroside. Salidroside
at a concentration of 200 μM inhibited cell proliferation after 16
h. Following incubation with 10, 50, or 100 μM salidroside for 24
h, the viability of these cells did not differ significantly from
that of the non-treated cells (Fig.
1). These results indicate that lower doses of salidroside do
not affect cell viability. Therefore, we selected to use the
concentrations of 50 and 100 μM for the following experiments.
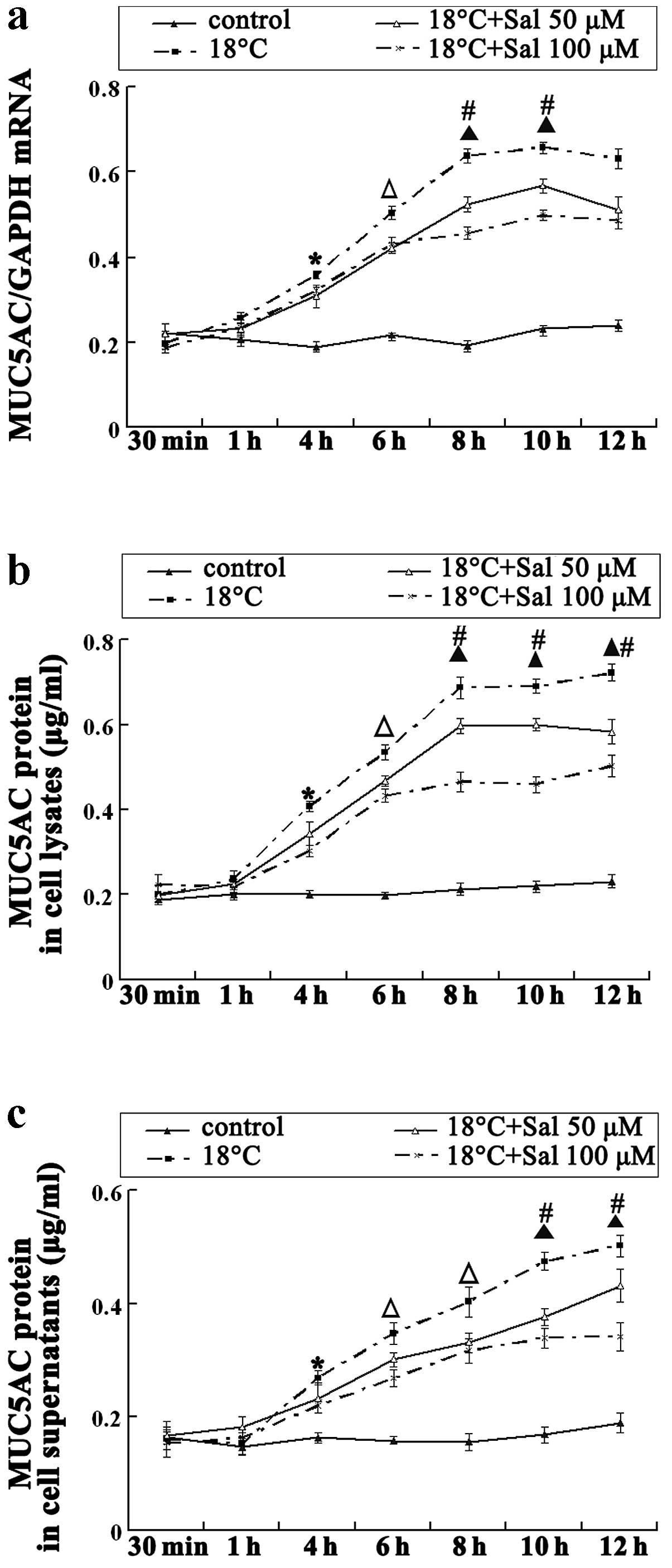
Salidroside decreases cold-induced MUC5AC
expression
To investigate the effects of cold temperatures on
MUC5AC expression in airway epithelial cells, we exposed the HBE16
cells to cold temperatures (18°C) for various periods of time.
MUC5AC mRNA levels were upregulated after 1 h of exposure to cold
temperatures, with the concentration of MUC5AC protein in the cell
lysates and culture supernatants increasing as well. The mRNA and
protein levels of MUC5AC continued to increase, reaching a plateau
at 8 h. These results indicate that exposure to cold temperatures
actively induces MUC5AC expression. The cells were then incubated
with 50 μM salidroside prior to exposure cold temperatures. The
MUC5AC protein and mRNA levels in the salidroside-treated cells
were lower than those observed in the cold-stimulated group at 6 h.
Treatment of the cells with 100 μM salidroside for 8 h
significantly attenuated the cold-induced intracellular synthesis
and secretion of the MUC5AC protein (Fig. 2), indicating that salidroside
time- and dose-dependently reduced MUC5AC expression induced by
cold stimulation.
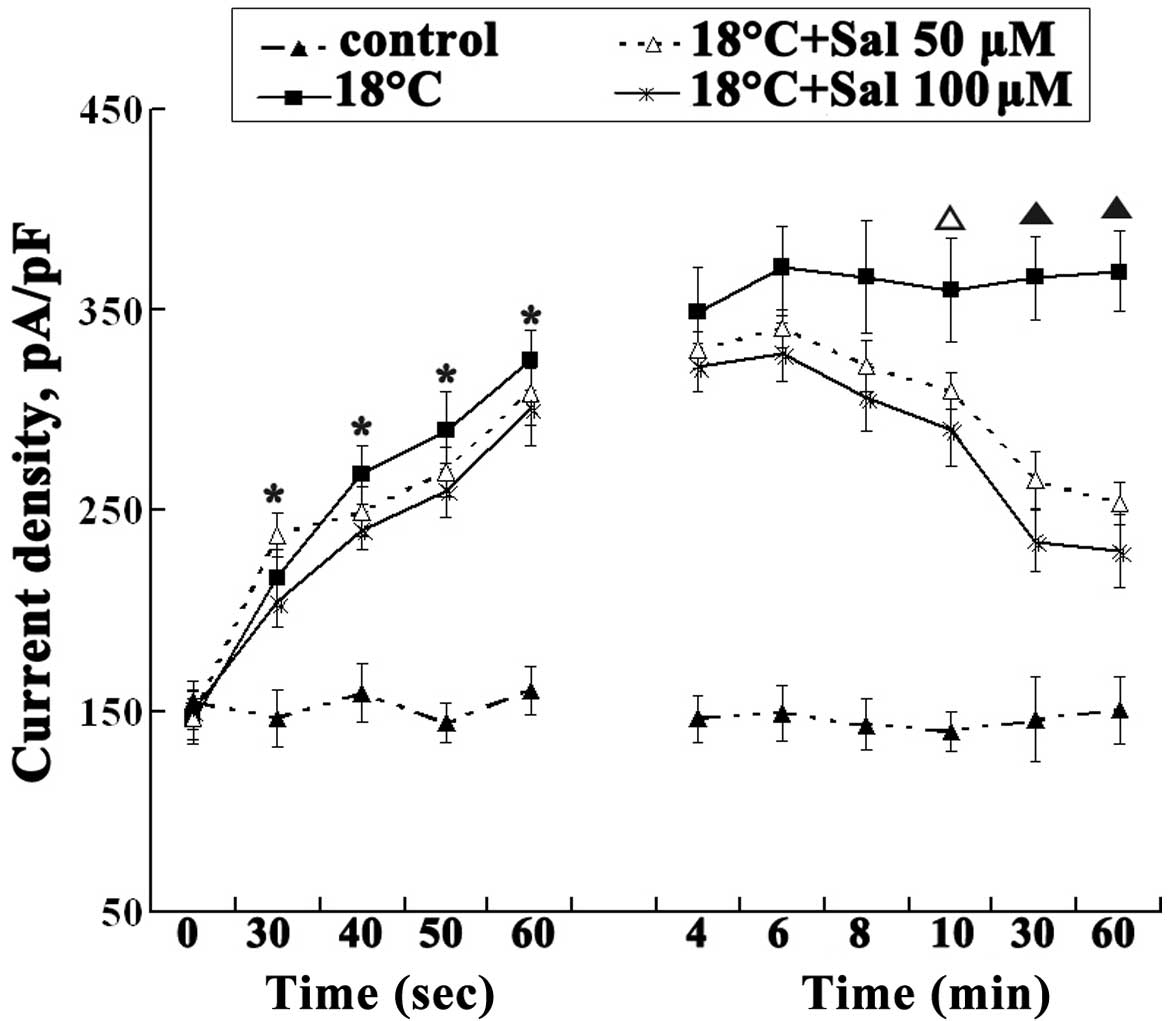
Salidroside decreases ITRPM8
membrane currents
In a previous study, we found that cold temperatures
induce TRPM8 activation (12). We
further assayed the activation of TRPM8 in cold-stimulated HBE16
cells by patch-clamping the cells at various time points following
exposrue to cold temperatures. The current density of
cold-stimulated TRPM8 channels increased at 15 sec and reached a
plateau at 6 min. In the salidroside-treated cells, TRPM8
activation began to decrease after 40 sec. Salidroside
significantly decreased the current through cold-stimulated TRPM8
channels at 10 min of recording in comparison with the untreated
cold-stimulated channels (P<0.05). The 100 μM concentration of
salidroside was more effective at reducing currents than the 50 μM
concentration, showing maximal effects at 20 min (Fig. 3). These results indicate that
TRPM8 activation is involved in cold-stimulated MUC expression and
that salidroside inhibits currents through TRPM8, suggesting a
previously unknown property of salidroside.
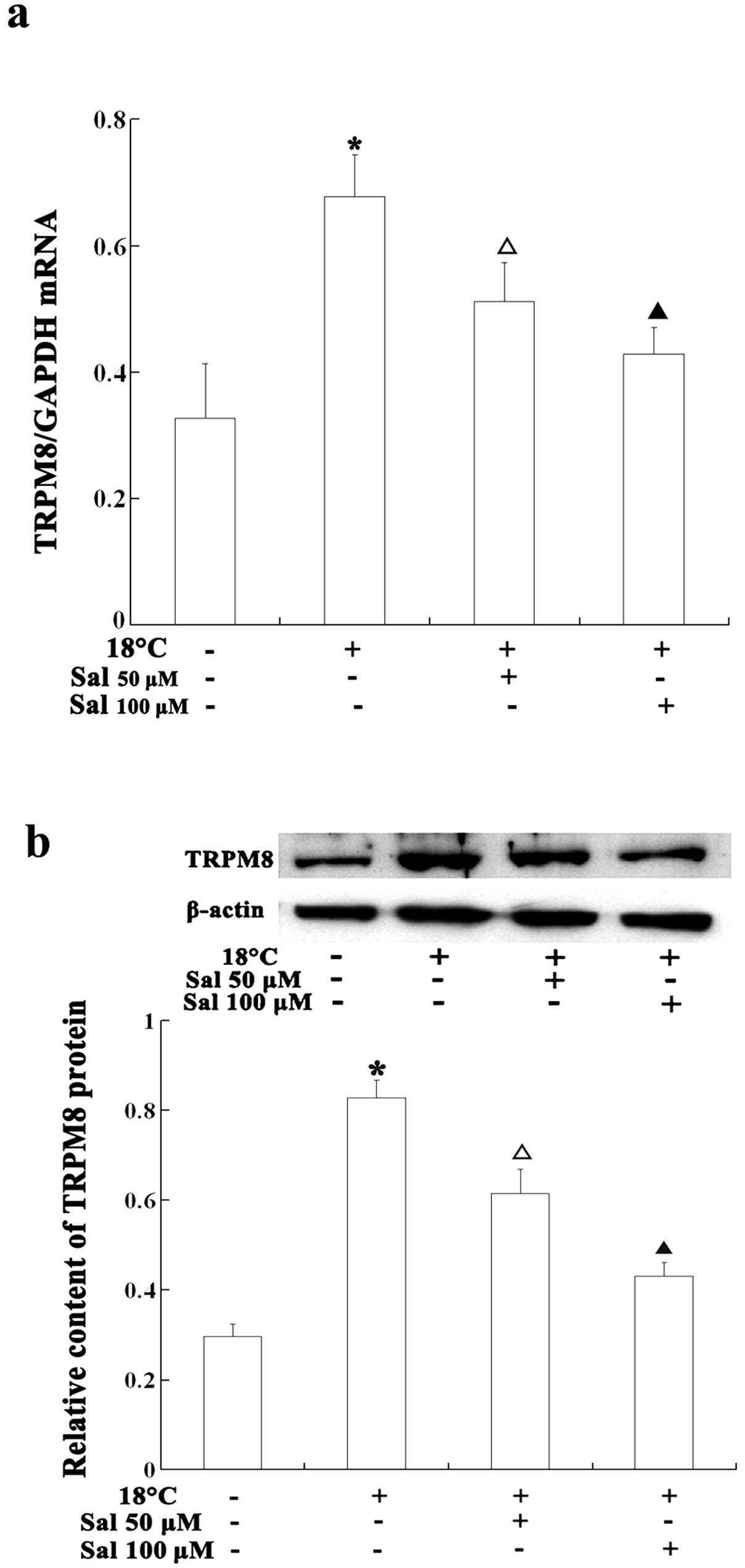
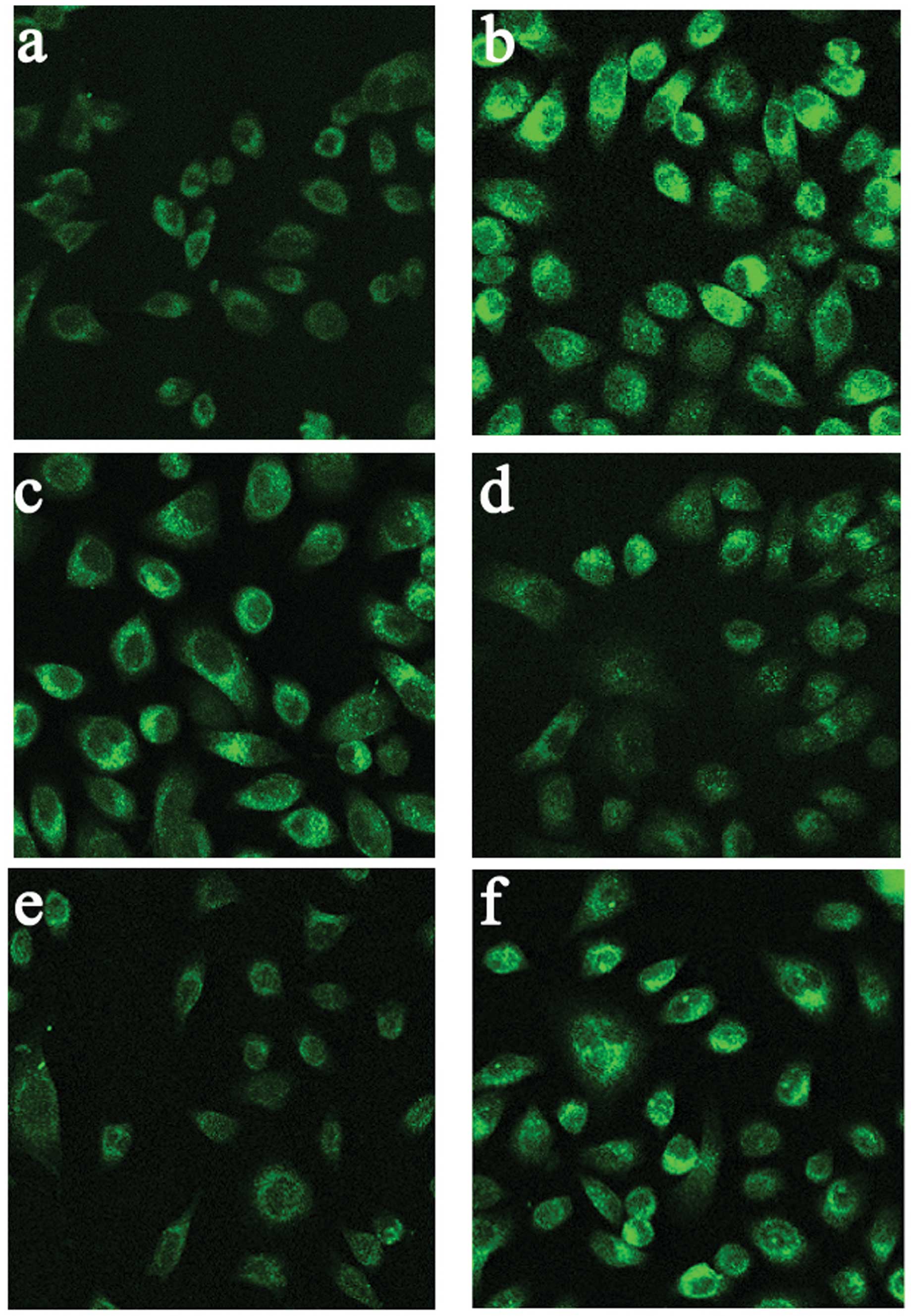
Salidroside reduces cold-induced TRPM8
expression in HBE16 cells
To further confirm the inhibitory effects of
salidroside on TRPM8, we investigated the mRNA and protein
expression of TRPM8 following incubation with salidroside. The
cells were pre-treated with salidroside and then exposed to cold
temperatures (18°C). The TRPM8 mRNA and protein levels were then
detected by real-time PCR, western blot analysis and
immunofluorescence assay, respectively. The cells were exposed to
cold temperatures (18°C) for 6 h; exposure to cold temperatures
significantly increased TRPM8 protein and mRNA levels compared with
the normal control group (P<0.05), and the cellular TRPM8 levels
continued to increase in a time-dependent manner. The cells
pre-treated with salidroside prior to exposure to cold temperatures
had lower TRPM8 expression levels than the non-treated cells
(P<0.05). The high dose of salidroside (100 μM) showed more
potent inhibitory effects on increased TRPM8 expression than the
low dose (50 μM) (Figs. 4 and
5). These results indicate that
salidroside represses TRPM8 not only by reducing TRPM8 activation
but also by inhibiting TRPM8 mRNA and protein expression.
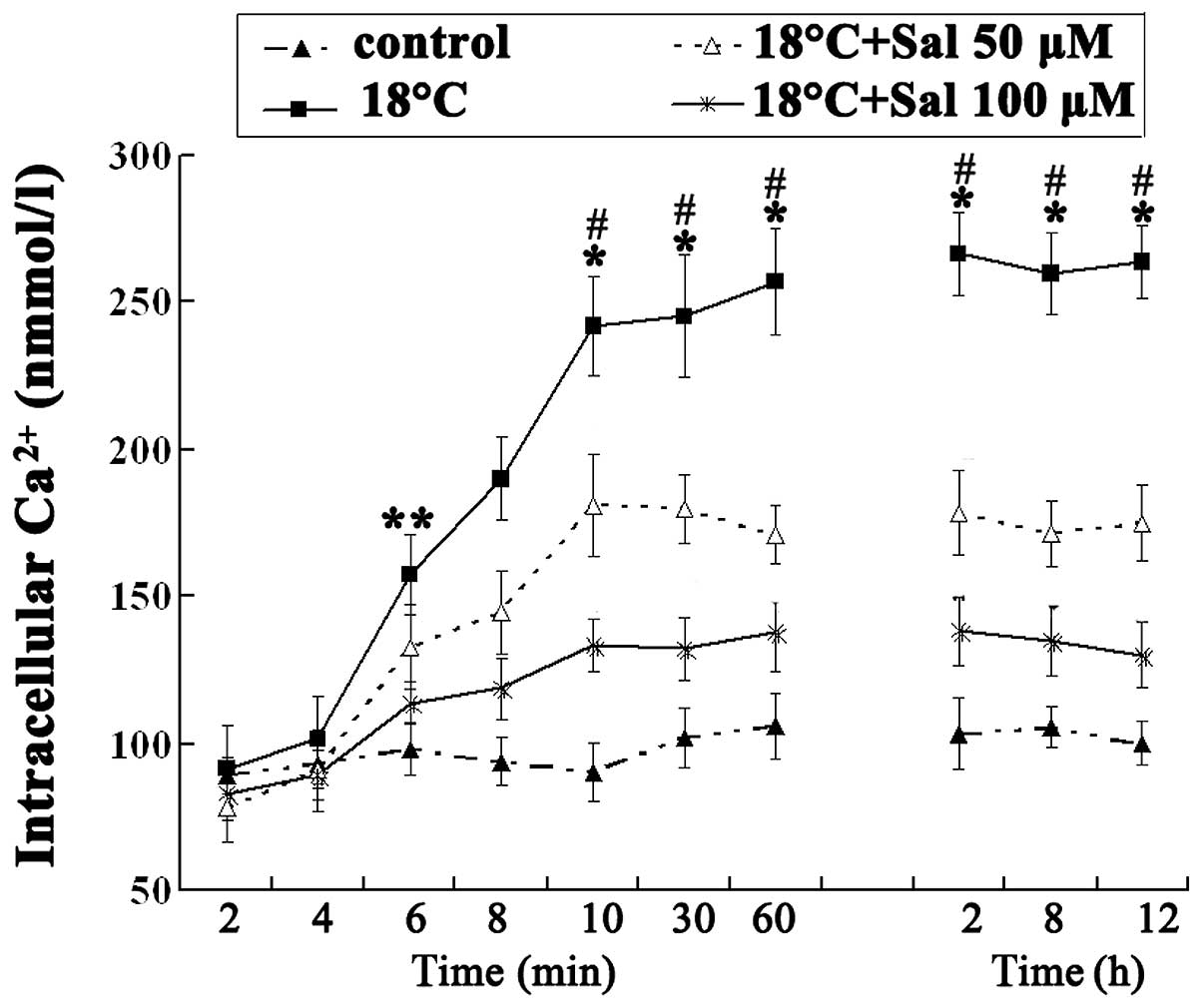
Salidroside inhibits [Ca2+]i
in HBE16 cells
To investigate the mechanisms underlying the
salidroside-mediated TRPM8 inhibition, we assayed the free calcium
influx in the cells, which is crucial to TRPM8 activation and is
known to play a role in many other salidroside-mediated protective
events. The HBE16 cells were loaded with Fura-2/AM to examine the
intracellular calcium concentration. When the HBE16 cells were
exposed to cold temperatures (18°C), we observed that the
[Ca2+]i concentration began to increase after 4 min and
continued to increase, reaching a plateau at 10 min, compared with
the normal group (P<0.01). In the HBE16 cells incubated with
salidroside prior to exposure to cold temperatures, the increase in
the [Ca2+]i concentration was significantly attenuated
at 8 min. Furthermore, pre-treatment of the cells with both
concentrations of salidroside (50 and 100 μM) decreased the
[Ca2+]i concentration in a dose-dependent manner
(Fig. 6).
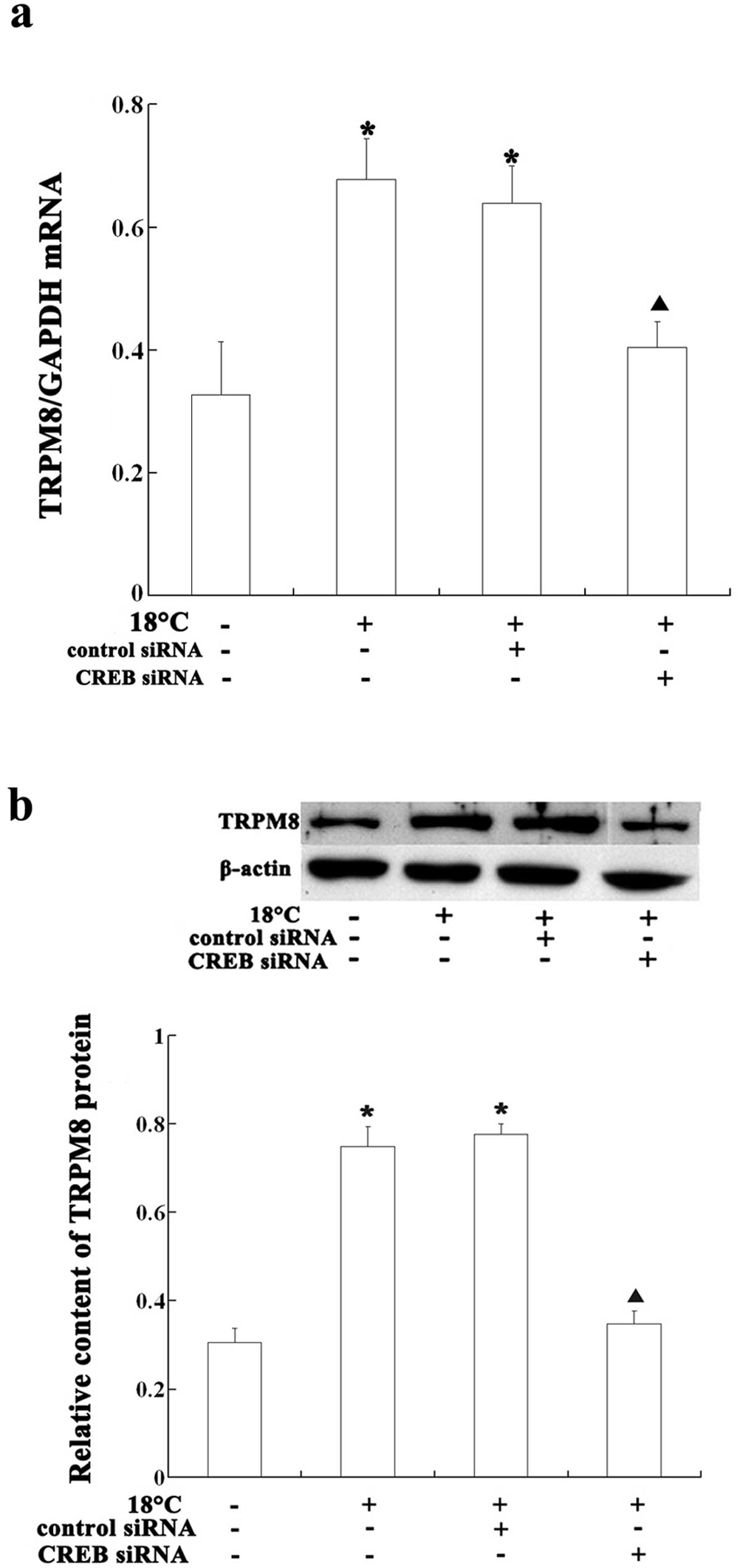
Salidroside decreases TRPM8 expression by
inhibiting CREB activity in HBE16 cells
Previous studies have reported that the TRPM8
promoter may have a CREB binding site (18). To determine whether CREB is
involved in TRPM8 activation, we first assayed the expression and
activity of CREB in the cells under cold assault, and then
transfected the cells with CREB siRNA to investigate the effects of
CREB on TRPM8 expression. Following the incubation of the HBE16
cells under cold conditions for 6 h, the mRNA and protein levels of
TRPM8 increased when compared with the control cells kept at warm
temperatures. When the cells were transfected with CREB siRNA prior
to exposure to cold temperatures (18°C), the TRPM8 mRNA and protein
levels were considerably lower than the levels observed in the
non-transfected cells (Figs. 5
and 7). Exposure to cold
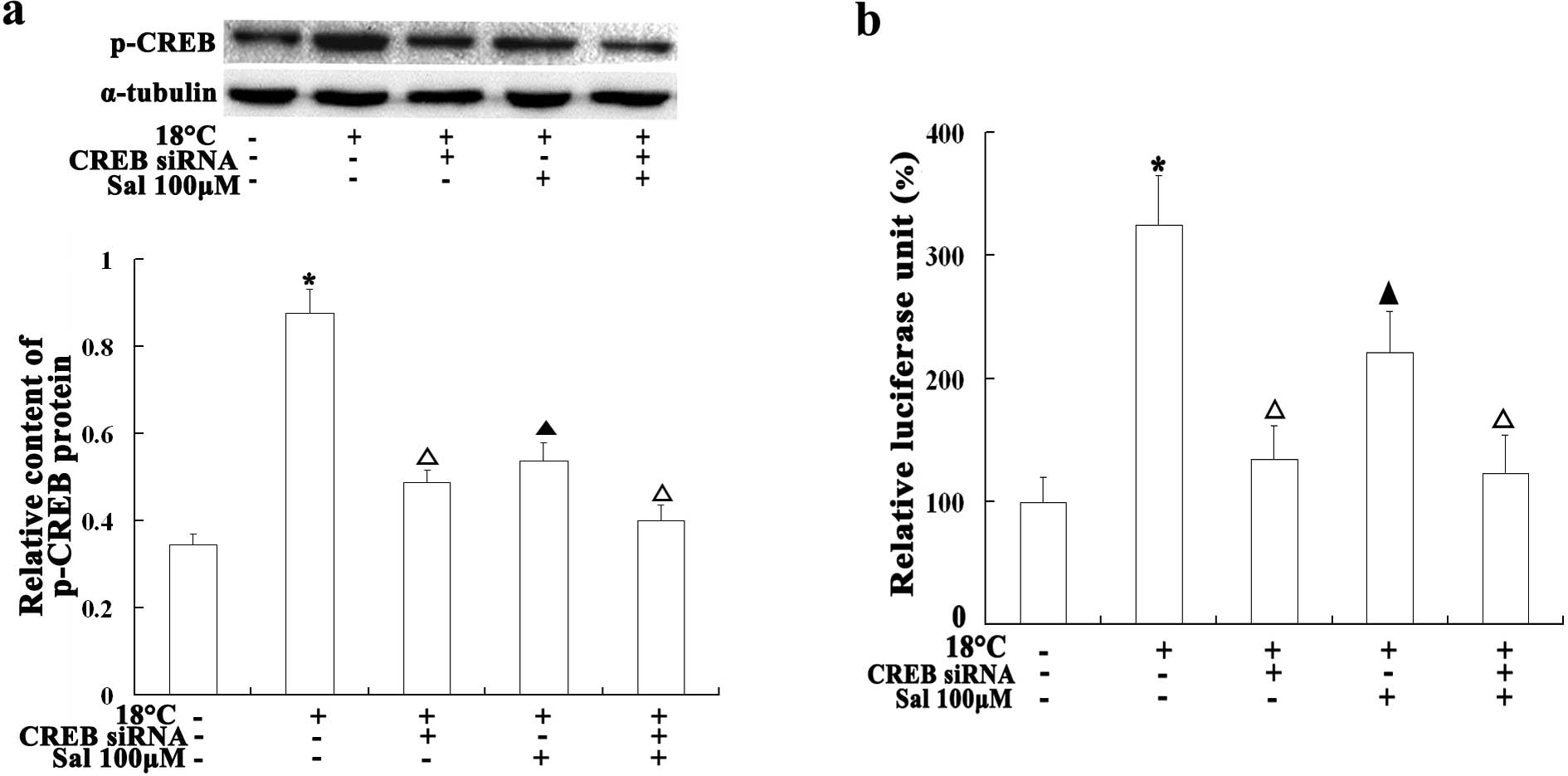
temperatures also increased the levels of p-CREB. The cells
transfected with CREB siRNA showed lower levels of p-CREB, an
effect similar to the one observed in the salidroside-treated cells
(P<0.05 compared with the cold-stimulated (18°C) cells (Fig. 8A). We additionally transfected the
cells with a CREB-Luc Reporter plasmid to measure the activity of
CREB in the cold-stimulated cells. The cells exposed to cold
temperatures (18°C) showed higher RLU levels than the normal
control cells, while the cells incubated with salidroside prior to
exposure to cold temperatures exhibited lower RLU levels than the
cold-stimulated cells not treated with salidroside (P<0.01)
(Fig. 8B). These results suggest
that CREB plays an important role in TRPM8 gene transcription and
that salidroside represses TRPM8 expression by inhibiting CREB
activity.
Discussion
Mucus overproduction is a clinical hallmark of acute
exacerbation of COPD. One of the important aggravating factors is
exposure to cold temperatures, which can cause mucociliary
dysfunction and the accumulation of inflammatory factors, which may
ultimately result in a bacterial infection in the respiratory
system. The effective management of COPD exacerbation requires a
thorough understanding of the underlying pathophysiological
mechanisms that shape its clinical manifestation. In a previous
study, we reported that cold temperatures induce MUC hypersecretion
in human airway epithelial cells through a TRPM8-mediated mechanism
(12). TRPM8 is a major cold
receptor in the body with variants that are expressed by the human
respiratory system (19). TRPM8
is activated by temperatures between 8 and 25°C (20) and is a member of the TRP family of
Ca2+-permeable, non-selective cation channels and the
TRPM subfamily of TRP proteins (21–23). We found that TRPM8 is highly
expressed in human airway epithelial cells following exposure to
cold stimuli. Functional assays revealed that TRPM8 activation
significantly reduced MUC secretion induced by cold temperatures;
however, the intervention mechanims for TRPM8-related events have
not yet been fully elucidated.
Rhodiola rosea has long been used in
traditional Chinese medicine and it primarily grows at high
altitude in thin air and cold weather conditions. Salidroside is
the major active ingredient isolated from the plant, Rhodiola
rosea, and possesses the ability to enhance the resistance of
the body to fatigue and cold (24). Salidroside has also been reported
to have potent antioxidant and anti-aging properties (25). Salidroside has been shown to
reduce H2O2-induced cell apoptosis in SH-SY5Y
human neuroblastoma cells (26,27). However, the potential effects of
salidroside on cold-induced airway inflammation have not yet been
fully elucidated. The respiratory epithelium communicates directly
with the external environment. When cold air is inhaled, the
respiratory tract loses a considerable amount of heat. This heat
loss may be followed by a series of stress reactions. Cold
air-induced respiratory symptoms include MUC oversecretion and
inflammation, and these phenomena are common in countries or areas
with a cold climate (28). It has
also been reported that people living at high latitudes exhibit
perturbed mucociliary function, placing them at a higher risk of
developing nasal obstruction and cough (29). Considering the known properties of
salidroside, we hypothesized that it may play a role in regulating
homeostasis in the airway system.
In this study, we found that HBE16 cells exposed to
cold temperatures exhibited a marked increase in MUC5AC levels and
TRPM8 activation. By contrast, cells pre-treated with various
concentrations of salidroside (10, 50 and 100 μM) prior to the cold
assault exhibited a dose- and time-dependent decrease in MUC5AC
expression, indicating that salidroside is capable of protecting
HBE16 cells from cold-induced MUC oversecretion. Furthermore, we
found that the [Ca2+]i concentration rapidly and
robustly increased in the cold-stimulated cells, reaching a plateau
at 6 to 10 min following exposure to cold temperatures. The cells
pre-treated with salidroside showed decreased levels of
[Ca2+]i and TRPM8 activation, with the
[Ca2+]i concentration decreasing as early as 1 min
following incubation with salidroside and reaching a minimal level
at 2 h post-treatment. High doses of salidroside inhibited the
inflow of Ca2+ almost completely.
[Ca2+]i is an important second messenger
in various types of cells, regulating functional and physiological
cellular activities, such as contraction, secretion, signal
transmission and transcriptional regulation. Under normal
circumstances, the extracellular concentration of calcium is
usually 0.1–10 mM and the intracellular calcium concentration is
10−7–10−8 M, much lower than the calcium
concentrations in the extracellular environment.
Ca2+-dependent cellular signaling pathways are activated
by alterations in the levels of [Ca2+]i. When cells are
exposed to stimuli such as inflammation (30), hypoxia (31) and cold temperatures (32), there is an influx of extracellular
calcium or a release of calcium from the cellular endoplasmic
reticulum, either of which overloads the [Ca2+]i level.
The [Ca2+]i concentration plays an important role in the
activation of TRPM8. Studies have also shown that TRPM8 mediates
transmembrane Ca2+ flux (33). However, the excessive inflow of
Ca2+ leads to desensitization of TRPM8, a process that
is mediated through the Ca2+-dependent activation of
protein kinase C (PKC) (34,35). TRPM8 is also regulated by a number
of kinases and second messengers, such as PLC, PIP2 (36,37), phospholipase A2 (PLA2),
lysophospholipids (LPLs) (38,39), arachidonic acid (AA) (40) and cAMP (41).
CREB is a 43-kDa leucine zipper transcription factor
that belongs to the activating transcription factor (ATF) subfamily
of the basic-region leucine zipper (bZIP) family. CREB primarily
responds to the cAMP signaling pathway, binding to CREs to increase
or decrease the transcription of certain genes (42). CREB-mediated transcription
regulates various cellular responses, including intermediary
metabolism, neuronal signaling, cell proliferation, apoptosis and
other molecular events. The CREB protein contains an N-terminal
transaction domain (kinase-induced domain), a basic region and a
leucine zipper motif at the C-terminus. The transaction domain
includes a phosphorylation site at Ser133 that is recognized by
certain protein kinases (43).
CREB can be activated by a variety of stimuli, including peptide
hormones, growth factors, forskolin, phorbol esters and a number of
protein kinases, including protein kinase A (PKA), pp90 ribosomal
S6 kinase (pp90RSK), and Ca2+/calmodulin-dependent
protein kinases (CaMKs) (44,45).
It has been reported that the TRPM8 core promoter
region contains a CRE, which is possibly located between base pairs
−1,417 and −1,406. It may play an important role in promoting the
transcription of TRPM8 (18,46). Therefore, we further examine
whether the transcription factor, CREB, is involved in TRPM8
expression. We found that the expression of p-CREB protein and CREB
activity were significantly elevated in the cells exposed to cold
temperatures compared with the control cells. CREB phosphorylation
and activity levels were lower in the cells pre-treated with
salidroside than the untreated cells. While TRPM8 expression
decreased in the cells transfected with CREB siRNA, the expression
of TRPM8 in the cells transfected with CREB siRNA and incubated
with salidroside prior to exposure to cold temperatures decreased
even more significantly. These results indicate not only that CREB
activation directly contributes to TRPM8 expression, but also that
salidroside inhibits TRPM8 expression by decreasing the activation
and activity of CREB.
The known chemical antagonists of TRPM8 are
capsazepine,
N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carboxamite
(BCTC) and thiol BCTC. While these are all synthesized chemicals,
salidroside is a component of the Chinese herb, Rhodiola
rosea, which may be a natural candidate for the treatment of
diseases involving TRPM8 overexpression. The regulation of TRPM8
expression by salidroside is important for the identification of
alternative treatment modalities for airway mucus hypersecretory
diseases induced by cold stimuli.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81100003 and
31171346), and the China-Russia Cooperation Research Foundation
(grant no. 31211120168), and the New Teacher Fund for Doctor
Station, the Ministry of Education, China (no. 20115503120006), and
Chongqing Nature Science Foundation (grant no.
cstc2011jjA10046).
References
|
1
|
Giesbrecht GG: The respiratory system in a
cold environment. Aviat Space Environ Med. 66:890–902.
1995.PubMed/NCBI
|
|
2
|
Voynow JA and Rubin BK: Mucins, mucus, and
sputum. Chest. 135:505–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhen G, Park SW, Nguyenvu LT, Rodriguez
MW, Barbeau R, Paquet AC and Erle DJ: IL-13 and epidermal growth
factor receptor have critical but distinct roles in epithelial cell
mucin production. Am J Respir Cell Mol Biol. 36:244–253. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu L, Lee PK, Lee WM, Zhao Y, Yu D and
Chen Y: Rhinovirus-induced major airway mucin production involves a
novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol.
40:610–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turner J and Jones CE: Regulation of mucin
expression in respiratory diseases. Biochem Soc Trans. 37:877–881.
2009. View Article : Google Scholar
|
|
6
|
Caterina MJ, Leffler A, Malmberg AB,
Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI
and Julius D: Impaired nociception and pain sensation in mice
lacking the capsaicin receptor. Science. 288:306–313. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caterina MJ, Rosen TA, Tominaga M, Brake
AJ and Julius D: A capsaicin-receptor homologue with a high
threshold for noxious heat. Nature. 398:436–441. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes
PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L,
Egerton J, Charles KJ, Smart D, Randall AD, Anand P and Davis JB:
TRPV3 is a temperature-sensitive vanilloid receptor-like protein.
Nature. 418:186–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Güler AD, Lee H, Iida T, Shimizu I,
Tominaga M and Caterina M: Heat-evoked activation of the ion
channel, TRPV4. J Neurosci. 2:6408–6414. 2002.PubMed/NCBI
|
|
10
|
McKemy DD, Neuhausser WM and Julius D:
Identification of a cold receptor reveals a general role for TRP
channels in thermosensation. Nature. 416:52–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peier AM, Moqrich A, Hergarden AC, Reeve
AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan
S and Patapoutian A: A TRP channel that senses cold stimuli and
menthol. Cell. 108:705–715. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li MC, Li Q, Yang G, Kolosov VP, Perelman
JM and Zhou XD: Cold temperature induces mucin hypersecretion from
normal human bronchial epithelial cells in vitro through a
transient receptor potential melastatin 8 (TRPM8)-mediated
mechanism. J Allergy Clin Immunol. 128:626–634. 2011. View Article : Google Scholar
|
|
13
|
Li YR, Cao W, Guo J, Miao S, Ding GR, Li
KC, Wang J and Guo GZ: Comparative investigations on the protective
effects of rhodioside, ciwujianoside-B and astragaloside IV on
radiation injuries of the hematopoietic system in mice. Phytother
Res. 25:644–653. 2011.PubMed/NCBI
|
|
14
|
Díaz Lanza AM, Abad Martínez MJ, Fernández
Matellano L, Recuero Carretero C, Villaescusa Castillo L, Silván
Sen AM and Bermejo Benito P: Lignan and phenylpropanoid glycosides
from Phillyrea latifolia and their in vitro anti
inflammatory activity. Planta Med. 67:219–223. 2001.
|
|
15
|
Kelly GS: Rhodiola rosea: a
possible plant adaptogen. Altern Med Rev. 6:293–302. 2001.
|
|
16
|
Kucinskaite A, Briedis V and Savickas A:
Experimental analysis of therapeutic properties of Rhodiola
rosea L. and its possible application in medicine. Medicina
(Kaunas). 40:614–619. 2004.(In Lithuanian).
|
|
17
|
Bavencoffe A, Kondratskyi A, Gkika D,
Mauroy B, Shuba Y, Prevarskaya N and Skryma R: Complex regulation
of the TRPM8 cold receptor channel: role of arachidonic acid
release following M3 muscarinic receptor stimulation. J Biol Chem.
286:9849–9855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bidaux G, Roudbaraki M, Merle C, Crépin A,
Delcourt P, Slomianny C, Thebault S, Bonnal JL, Benahmed M, Cabon
F, Mauroy B and Prevarskaya N: Evidence for specific TRPM8
expression in human prostate secretory epithelial cells: functional
androgen receptor requirement. Endocr Relat Cancer. 12:367–382.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sabnis AS, Shadid M, Yost GS and Reilly
CA: Human lung epithelial cells express a functional cold-sensing
TRPM8 variant. Am J Respir Cell Mol Biol. 39:466–474. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCoy DD, Knowlton WM and McKemy DD:
Scraping through the ice: uncovering the role of TRPM8 in cold
transduction. Am J Physiol Regul Integr Comp Physiol.
300:R1278–R1287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L and Barritt GJ: TRPM8 in prostate
cancer cells: a potential diagnostic and prognostic marker with a
secretory function? Endocr Relat Cancer. 13:27–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Behrendt HJ, Germann T, Gillen C, Hatt H
and Jostock R: Characterization of the mouse cold-menthol receptor
TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric
imaging plate reader (FLIPR) assay. Br J Pharmacol. 141:737–745.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andersson DA, Chase HW and Bevan S: TRPM8
activation by menthol, icilin, and cold is differentially modulated
by intracellular pH. J Neurosci. 24:5364–5369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tolonen A, Pakonen M, Hohtola A and
Jalonen J: Phenylpropanoid glycosides from Rhodiola rosea.
Chem Pharm Bull (Tokyo). 51:467–470. 2003. View Article : Google Scholar
|
|
25
|
Mao GX, Wang Y, Qiu Q, Deng HB, Yuan LG,
Li RG, Song DQ, Li YY, Li DD and Wang Z: Salidroside protects human
fibroblast cells from premature senescence induced by H(2)O(2)
partly through modulating oxidative status. Mech Ageing Dev.
131:723–731. 2010. View Article : Google Scholar
|
|
26
|
Li X, Ye X, Li X, Sun X, Liang Q, Tao L,
Kang X and Chen J: Salidroside protects against MPP(+)-induced
apoptosis in PC12 cells by inhibiting the NO pathway. Brain Res.
1382:9–18. 2011.
|
|
27
|
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan
C, Cao G and Wang Z: Protective effects of salidroside on hydrogen
peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells.
Eur J Pharmacol. 564:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koskela HO: Cold air-provoked respiratory
symptoms: the mechanisms and management. Int J Circumpolar Health.
66:91–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodway GW and Windsor JS: Airway
mucociliary function at high altitude. Wilderness Environ Med.
17:271–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dada LA and Sznajder JI: Mitochondrial
Ca2+ and ROS take center stage to orchestrate
TNF-α-mediated inflammatory responses. J Clin Invest.
121:1683–1685. 2011.PubMed/NCBI
|
|
31
|
Gusarova GA, Trejo HE, Dada LA, Briva A,
Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M and
Sznajder JI: Hypoxia leads to Na,K-ATPase downregulation via Ca(2+)
release-activated Ca(2+) channels and AMPK activation. Mol Cell
Biol. 31:3546–3556. 2011.PubMed/NCBI
|
|
32
|
Galli GL, Lipnick MS, Shiels HA and Block
BA: Temperature effects on Ca2+ cycling in scombrid
cardiomyocytes: a phylogenetic comparison. J Exp Biol.
214:1068–1076. 2011.PubMed/NCBI
|
|
33
|
Cho Y, Jang Y, Yang YD, Lee CH, Lee Y and
Oh U: TRPM8 mediates cold and menthol allergies associated with
mast cell activation. Cell Calcium. 48:202–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abe J, Hosokawa H, Sawada Y, Matsumura K
and Kobayashi S: Ca2+-dependent PKC activation mediates
menthol-induced desensitization of transient receptor potential M8.
Neurosci Lett. 397:140–144. 2006.
|
|
35
|
Premkumar LS, Raisinghani M, Pingle SC,
Long C and Pimentel F: Downregulation of transient receptor
potential melastatin 8 by protein kinase C-mediated
dephosphorylation. J Neurosci. 25:11322–11329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Daniels RL, Takashima Y and McKemy DD:
Activity of the neuronal cold sensor TRPM8 is regulated by
phospholipase C via the phospholipid phosphoinositol
4,5-bisphosphate. J Biol Chem. 84:1570–1582. 2009.PubMed/NCBI
|
|
37
|
Rohács T, Lopes CM, Michailidis I and
Logothetis DE: PI(4,5)P2 regulates the activation and
desensitization of TRPM8 channels through the TRP domain. Nat
Neurosci. 8:626–634. 2005.
|
|
38
|
Andersson DA, Nash M and Bevan S:
Modulation of the cold-activated channel TRPM8 by lysophospholipids
and polyunsaturated fatty acids. J Neurosci. 27:3347–3355. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vanden Abeele F, Zholos A, Bidaux G, Shuba
Y, Thebault S, Beck B, Flourakis M, Panchin Y, Skryma R and
Prevarskaya N: Ca2+-independent phospholipase
A2-dependent gating of TRPM8 by lysophospholipids. J Biol Chem.
281:40174–40182. 2006.
|
|
40
|
Bavencoffe A, Gkika D, Kondratskyi A, Beck
B, Borowiec AS, Bidaux G, Busserolles J, Eschalier A, Shuba Y,
Skryma R and Prevarskaya N: The transient receptor potential
channel TRPM8 is inhibited via the alpha 2A adrenoreceptor
signaling pathway. J Biol Chem. 285:9410–9419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De Petrocellis L, Starowicz K, Moriello
AS, Vivese M, Orlando P and Di Marzo V: Regulation of transient
receptor potential channels of melastatin type 8 (TRPM8): effect of
cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res.
313:1911–1920. 2007.PubMed/NCBI
|
|
42
|
Andrisani OM: CREB-mediated
transcriptional control. Crit Rev Eukaryot Gene Expr. 9:19–32.
1999.
|
|
43
|
Johannessen M and Moens U: Multisite
phosphorylation of the cAMP response element-binding protein (CREB)
by a diversity of protein kinases. Front Biosci. 12:1814–1832.
2007.PubMed/NCBI
|
|
44
|
Johannessen M, Delghandi MP, Seternes OM,
Johansen B and Moens U: Synergistic activation of CREB-mediated
transcription by forskolin and phorbol ester requires PKC and
depends on the glutamine-rich Q2 transactivation domain. Cell
Signal. 16:1187–1199. 2004. View Article : Google Scholar
|
|
45
|
Shaywitz AJ and Greenberg ME: CREB: a
stimulus-induced transcription factor activated by a diverse array
of extracellular signals. Annu Rev Biochem. 68:821–861. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang L and Barritt GJ: Evidence that
TRPM8 is an androgen-dependent Ca2+ channel required for
the survival of prostate cancer cells. Cancer Res. 64:8365–8373.
2004. View Article : Google Scholar : PubMed/NCBI
|