Introduction
Human corneal dystrophy (HCD) is a group of genetic
disorders characterized by a non-inflammatory, bilateral opacity of
the cornea (1). Many types of HCD
are associated with transforming growth factor, β-induced, 68 kDa
(TGFBI, also known as BIGH3) gene mutations. The symptoms may
appear before the age of 20, developing during adolescence and
gradually progressing throughout life. HCD often affects only one
layer of the cornea at first and then the disease progresses to the
remainder of the cornea. Histopathological studies have also proven
that the BIGH3 protein can be found in these corneal deposits.
Penetrating keratoplasty has commonly been performed for extensive
CD. However, recurrence and deterioration of the disease has often
been observed even after surgery (2).
Although studies of BIGH3-related HCD are of
great interest (3,4), the role of BIGH3 mutations in
HCD remains to be fully understood due to the lack of animal models
(5). Since there are many
limiting factors in the study of HCD, relevant animal models would
greatly contribute to the study of this pathogenesis. Transgenic
mice produced by microinjection are the most widely used animal
models. Therefore, in this study, a transgenic mouse model
expressing the human BIGH3 gene was established. Our data
also indicated that the human BIGH3 gene was overexpressed
in the mouse corneas and induced corneal opacity in the eyes of the
mice.
Materials and methods
Animals
C57BL/6J mice were purchased from the Shanghai
Biomodel Research Center (Shangai, China). All animals were fed
with sterile mouse food and water at pH 2.8–3.2 under specific
pathogen-free (SPF) conditions and were kept in an isolated room
with an automatic light control (14 h day light and 10 h dark
cycle). The animal experiments were approved by the Institutional
Animal Care and Use Commitee (IACUC) of the Shangai Biomodel
Research Center for Experimental Animal Management (permit no.
IACUC 2010-0001). All animal experiments were conducted in
compliance with the relevant provisions of the Association for
Research in Vision and Ophthalmology (ARVO; American Association of
Ophthalmology) for animal research.
Construction of the pPGK-BIGH3-IRES-EGFP
transgene vector
A 0.5 kb phosphoglycerate kinase (PGK) promoter
fragment was amplified by PCR using DNA polymerase Ex Taq (Takara
Bio, Inc., Shiga, Japan) and pPL451 as a template (Shanghai
Biomodel Research Center). The forward and reverse primers were as
follows: 5′-CGACTCGAGACC GGGTAGGGGAGGCGCTTT-3′ and 5′-GGCGTCGACTCG
AAAGGCCCGGAGATGAGG-3′, respectively. The amplicon flanked with
XhoI and SalI was recovered from the agarose gel and
ligated into the similarly double digested plasmid, pCAG-IRES-EGFP,
which was obtained from the same supplier. The ligation was
transformed into E. coli in order to isolate the recombinant
plasmid, pPGK-CAG-IRES-EGFP. The plasmid DNA was digested with
PhBI and purified to remove the CAG promoter fragment by
agarose gel electrophoresis. The recovered DNA was self-ligated to
generate the recombinant plasmid, pPGK-IRES-EGFP.
The cDNA fragment of BIGH3 (Source
BioScience, Nottingham, UK) was digested with SalI and
SacII and ligated to the similarly digested pPGK-IRES-EGFP.
The ligation mixture was transformed into E. coli to obtain
the recombinant plasmid, pPGK-BIGH3-IRES-EGFP. The plasmid
was sequenced for confirmation before being linearized with
XbaI, purified with phenol-chloroform extraction and used in
microinjection buffer (pH 7.4) at a concentration of 4 ng/μl to
produce the transgenic mice.
Generation of the transgenic animals
The purified pPGK-BIGH3-IRES-EGFP plasmid DNA
was microinjected into the fertilized eggs of C57BL/6J mice. After
the injection, the fertilized eggs that were in a good condition
were transplanted into the fallopian tubes of pseudo-pregnant
female mice of the same strain, to produce the F0
generation animals. All animals were kept in an isolated room at
22ºC, with an automatic light control (14 h day and 10 h dark
cycle). The F0 animals were mated with the wild-type
mice to generate F1 animals.
Genomic DNA extraction and PCR
genotyping
The transgenic founders and offsprings were
identified by PCR of the genomic DNA extracted from tail biopsies.
The tissues were digested overnight in a water bath at 55ºC in 500
μl of a lysis buffer, containing 20–100 mg/ml proteinase K, 50
mmol/l Tris, pH 8.0, 100 mmol/l EDTA, 100 mmol/l NaCl and 1% SDS
(Bio-Rad, USA). The digested tissues were extracted twice with a
double volume of phenol-chloroform and the supernatants were
diluted in a double volume of absolute ethanol to precipitate the
DNA. After having been washed twice with 70% ethanol, the pellets
were air-dried and dissolved in 100 μ1 TE buffer. The DNA
concentration was determined using a UV spectrophotometer. PCR was
conducted to confirm the presence of the exogenous gene in
pPGK-BIGH3-IRES-EGFP using the forward primer,
5′-GACTAGCCCCTGTCTATCAAAAGTT-3′ and the reverse primer,
5′-AACCTCGACTAAACACATGTAAAGC-3′, which produced a product of 578
bp. PCR was carried out under the following conditions: 95ºC for 3
min, followed by 25 cycles of 95ºC for 15 sec, 65ºC for 30 sec,
72ºC for 1 min and with an extension at 72ºC for 10 min at the end
of the reaction. The results of the reaction were visualized by gel
electrophoresis. Wild-type DNA was run as a negative control on a
1% agarose gel electrophoresis.
RT-PCR of human BIGH3 expression in the
cornea
Several corneas were removed from two randomly
selected wild-type and PCR-positive F1 transgenic mice,
which were euthanized, irrespective of their gender. Euthanasia was
performed by cervical dislocation. Total RNA was extracted using
TRIzol reagent and digested with DNase. Phenol-chloroform was used
to extract the residual genomic DNA before being subjected to
reverse transcription. cDNA was obtained using an RT-PCR kit and
Gapdh as the control. The following PCR primers were used for
RT-PCR: Gapdh forward, 5′-TGGGAAGCTGGTCATCAAC-3′ and reverse,
5′-GCATCACCCCATTTGATGTT-3′; and BIGH3 forward,
5′-CAGGCGTCAGCGTATTCC-3′ and reverse, 5′-CCTTCCCTACCCGTCCAA-3′. PCR
was conducted under the following conditions: 90ºC for 30 sec, 61ºC
for 20 min followed by pre-denaturation at 94ºC for 30 sec and 35
cycles of amplification at 55–61ºC for 30 sec, 72ºC for 30 sec and
then extension at 72ºC for 5 min. The products were analyzed on a
1% agarose gel. All experiments were repeated three times
independently.
Western blot analysis of human BIGH3
expression in the cornea
Three wild-type mice served as the controls. Corneas
were collected under a stereo microscope from the eye balls of
euthanized mice following cervical dislocation, each randomly
selected from wild-type and PCR-positive F1 transgenic
mice. The corneal tissues were separated using micro-tweezers,
weighed and cut into small sections using micro scissors. The
tissues were added with equal volumes of lysis buffer, homogenized
on ice and incubated for 30 min at 4ºC in a refrigerator. The
digested tissues were transferred to 1.5 ml centrifugation tubes
and spun for 1 min at 12,000 rpm at 4ºC. The supernatants were
heated for 5 min in a water bath at 100ºC before being used for
SDS-PAGE. The proteins were blotted onto nitrocellulose membranes
following electrophoresis and reacted with 1:500 diluted antibodies
against BIGH3 (Abcam, Cambridge, UK) to detect the expression
product. The images were scanned using the LI-COR
Odyssey® Infrared Laser Imaging System (LI-COR
Biotechnology; Lincoln, NE, USA). The results were expressed as a
percentage of the control optical density. All experiments were
repeated three times.
Corneal photography
The corneas of these transgenic mice were
photographed using a digital camera following anesthesia to
determine the gross appearance of the corneas.
Statistical analysis
Data were statistically analyzed using SPSS 17.0
software (SPSS, Chicago, IL, USA). Quantitative data were tested
using the F-test for homogeneity of variance. Data with homogeneous
variance were tested using the independent sample t-test, while
non-homogeneous data were analyzed by the Mann-Whitney U test. A
p-value <0.05 was considered to indicate a statistically
significant difference.
Results
Construction of PGK-BIGH3-IRES-EGFP
vector
The PGK promoter fragment and the XhoI and
SalI digested plasmid, pCAG-IRES-EGFP, were ligated to
produce pPGK-CAG-IRES-EGFP. The plasmid was digested and gel
purified to remove the CAG fragment and then self-ligated to obtain
pPGK-IRES-EGFP. Human BIGH3 cDNA was cloned into the
SalI/Sac II site of pPGK-IRES-EGFP to generate a new
7416 bp recombinant vector, pPGK-BIGH3-IRES-EGFP.
Identification of founder transgenic
mice
Following pre-nuclei microinjection of the
linearized plasmid pPGK-BIGH3-IRES-EGFP, 36 founder mice
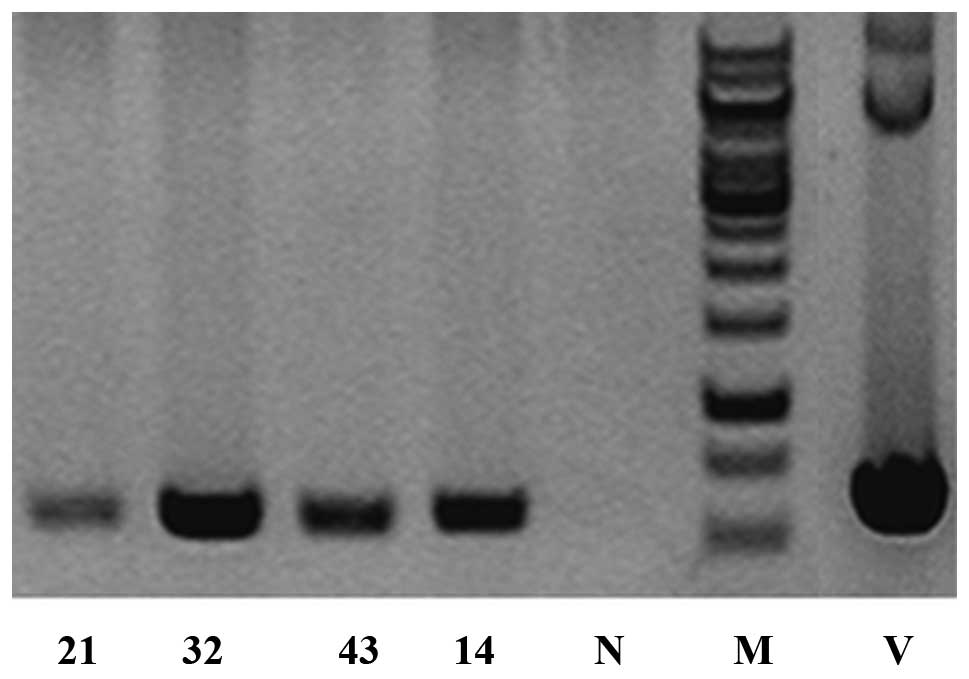
were generated. PCR indicated that four of them (M21, male; M32,
female; M43, female; M14, male) were transgenic founders. The
PCR-positive animals were confirmed twice along with three
wild-type animals using DNA prepared from the sampled tail tips
with an amplified product of 578 bp in the transgenic but not in
the wild-type mouse samples (Fig.
1).
Generation of F1 transgenic
mice
The four F0 transgenic mice were mated
with wild-type C57BL/6J mice to produce F1 animals and
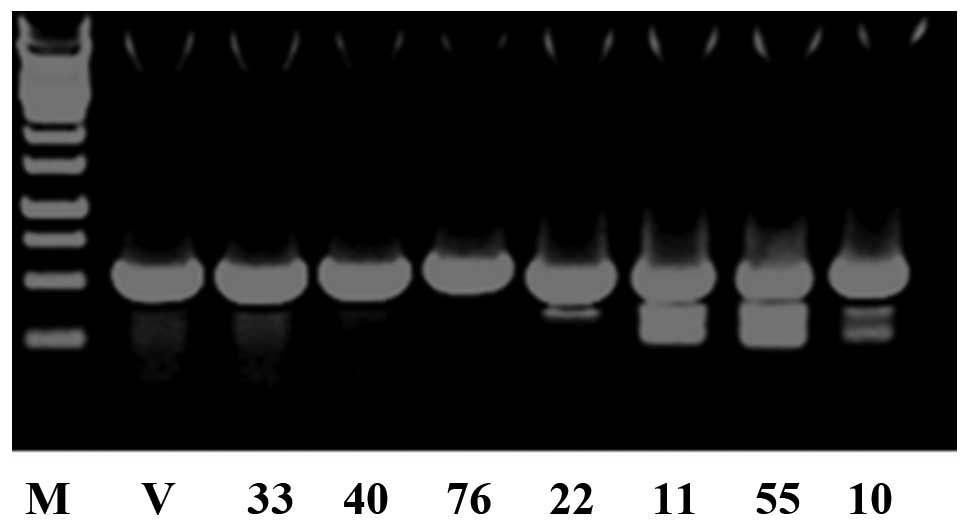
the offsprings were identified by PCR genotyping ten days after
birth. A total of 30 mice were born. PCR analysis indicated that
seven of them [M33, female; M40, female; M76, male; M22, male; M11,
female; M55, female; M10, male] were transgenic (Fig. 2).
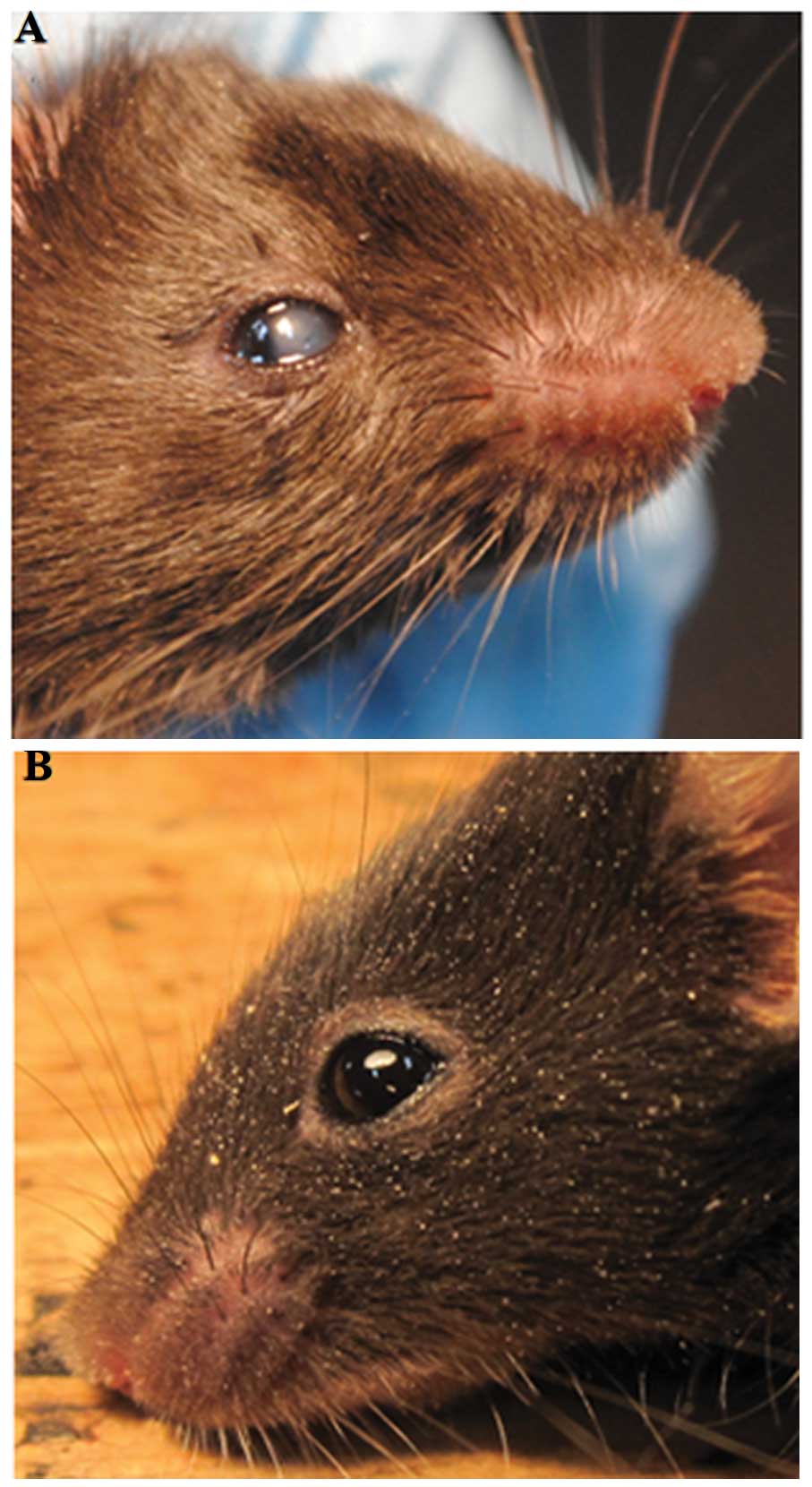
Gross phenotype of transgenic mice
The corneas of these transgenic mice were
photographed using a digital camera following anesthesia to
determine the gross appearance of the corneas. There were five
transgenic mice among the seven F1 transgenic mice, that
displayed a centrally reduced corneal transparency, which was
visible when the eyelids opened at approximately two weeks after
birth (Fig. 3).
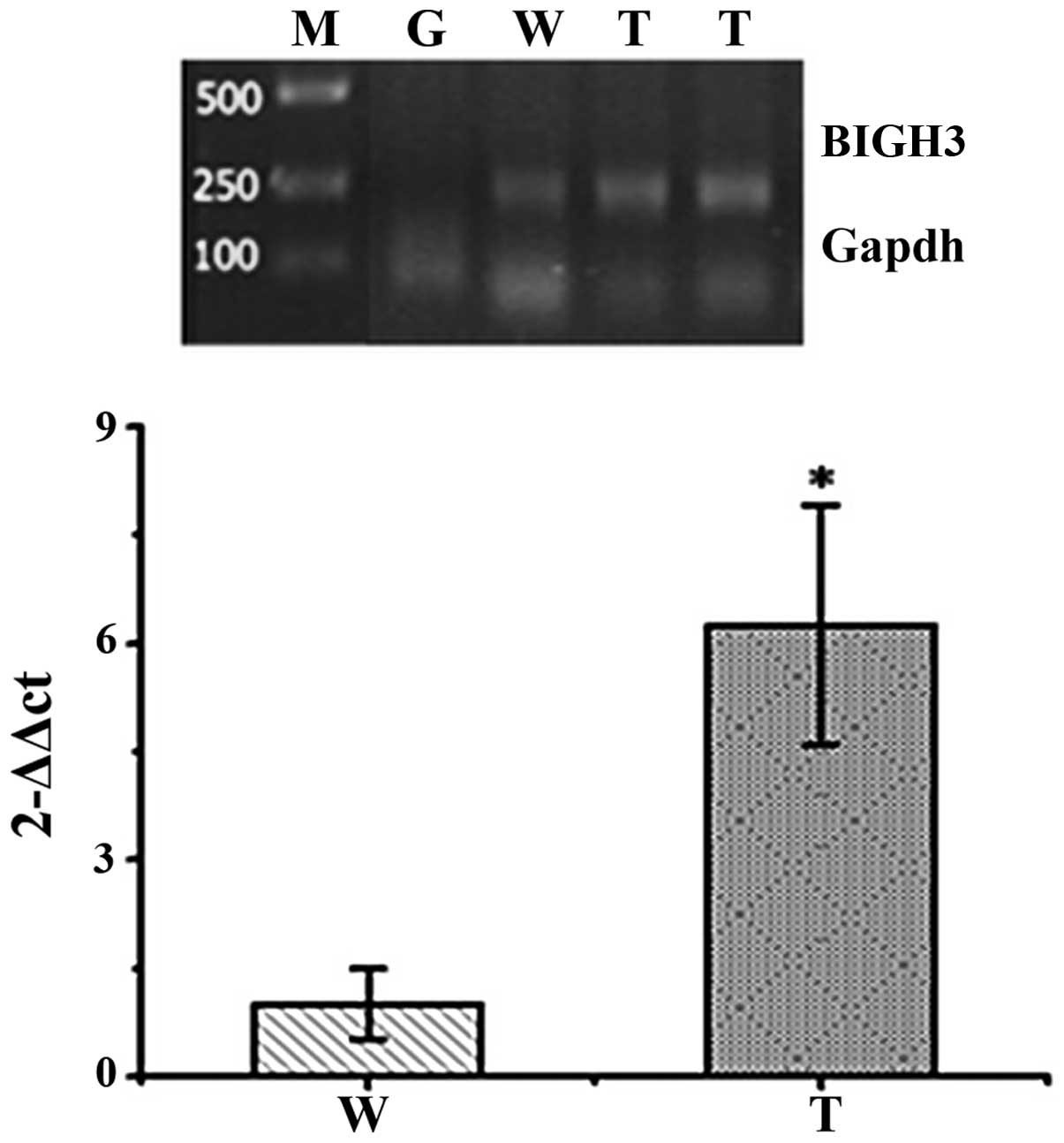
RT-PCR of corneal tissues from
F1 transgenic mice
The corneas were removed from the dissected
F1 transgenic mouse eyes. RT-PCR was performed to
examine the corneas of two F1 transgenic mice in order
to investigate the relative expression of human BIGH3 mRNA
in the corneas from wild-type and transgenic mice. As illustrated
in Fig. 4, compared with the
wild-type mice, the expression of BIGH3 in the transgenic mice was
significantly upregulated in their corneas (p<0.01).
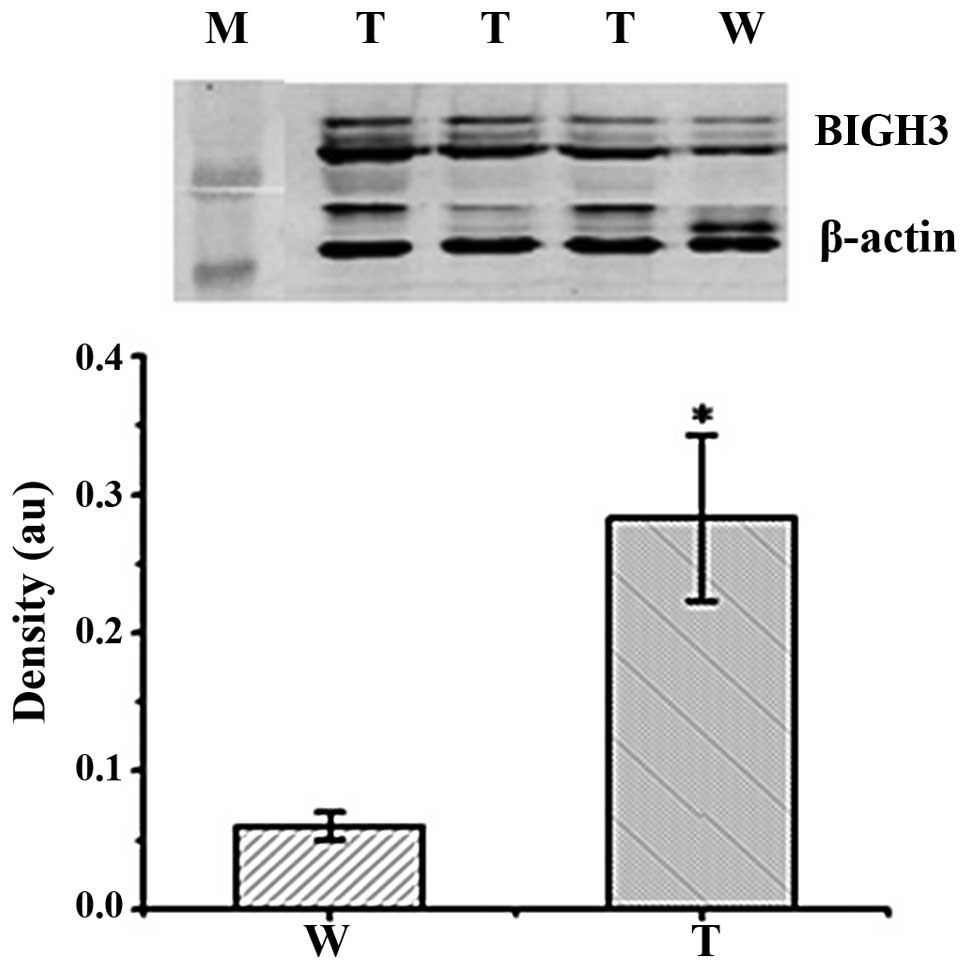
Western blot analysis of the corneas of
BIGH3 transgenic mice
Western blot analysis was performed to examine the
corneas of three F1 transgenic mice in order to
investigate the expression of the human BIGH3 gene in the
corneas from wild-type and transgenic mice. As the anti-human BIGH3
antibody partially cross-reacted with both the mouse and human
proteins, the amount of BIGH3 in the wild-type mice likely
represented the level of endogenous mouse BIGH3 in the corneas. As
illustrated in Fig. 5, in
comparison with the wild-type mice, the transgenic mice had higher
levels of BIGH3 in their corneas (p<0.01).
Discussion
BIGH3 (68 kDa), also known as TGFBI, is
located on chromosome 5q31, spanning a region of 30 kb (6). A mutation was initially discovered
when TGF was used to treat adenocarcinoma cell lines (7–9).
Research to date on BIGH3 has focused on two point mutations of
arginine residues 124 and 555 (10,11). They are the most common mutations
in the human population (12).
Few reports are available regarding the establishment of animal
models for CD. Kim et al (13) reported the use of the Alb promoter
to establish a transgenic mouse model of CD overexpressing
BIGH3. However, the mice were subsequently found to have
anterior segment disease, including corneal opacity, cataract and
iris abnormalities. Bustamante et al (14) also described the development of
the model with the mutated BIGH3 gene R555W using lentiviral
vectors. However, they found that the animals tended to age more
rapidly, with retinal degeneration, although their corneas were
normal.
To achieve a high level of expression of exogenous
genes and efficient secretion in mammalian cells, it is necessary
to use suitable vectors and recipient systems that enable the
effective induction of expression. The key element is a strong
promoter to drive the expression cassette. In this study, we used
the PGK promoter to drive the high level expression of the
BIGH3 gene. The PGK promoter has been widely used in yeast
expression systems (15,16) and is recognized as a strong
constitutive promoter with a wide range of hosts. It has three
transcriptional start sites with a high GC content. The GC content
upstream of the transcriptional start sites can reach 70%. It does
not contain conserved sequences, such as TATA or CAAT boxes as
often observed in other promoters. There is a repeat sequence
GGGGCGG upstream from the transcriptional start sites (17,18). All these features describe the
high efficiency of the PGK promoter and the reasons we selected
it.
The pCAG-IRES-EGFP vector, also used in this study,
has an enhanced GFP gene with an internal ribosomal entry site
(IRES). GFP is non-toxic, stable and easy to visualize (19). It has been widely used to monitor
transfection efficiency, expression levels and to sort the
transfected cells. IRES is a conserved cis-acting element
existing in the mRNA of eukaryotes or viral genomes. It binds to
ribosomes to mediate the 5′ cap-dependent translation of downstream
genes. The sequence has been widely used in various binary
expression vectors (20–22). In our study, we intended to use
EGFP as a reporter to measure the expression of the BIGH3
transgene. However, the GFP fluorescence observed in vivo
under a fluorescence microscope was too weak to fulfill this
purpose. We speculate that this may be due to the dark fur color of
the C57BL/6J mice, leading to the absorption of most of the GFP
fluorescence.
Male pronuclear microinjection was applied to
generate transgenic mice in our study, which is currently the most
commonly used method for transgenic mouse production. The
recombinant plasmid, PGK-BIGH3-IRES-EGFP, was injected into the
pronuclei of 85 fertilized eggs of C57BL/6J mice to produce
BIGH3 transgenic mice. Four transgenic founder mice (M21,
male; M32, female; M43, female; M14, male) of 36 born mice were
successfully generated, as confirmed by PCR analysis. After mating
with wild-type C57BL/6J mice, a total of 30 F1 offspring
mice were produced and seven of them were PCR-positive (M33,
female; M40, female; M76, male; M22; male, M11; female, M55;
female, M10; male), indicating that the human BIGH3 gene had
integrated into the mouse genome and was able to be transmitted to
the next generation. However, we also noted that only 6–25% of the
transgenic mice were able to transmit the BIGH3 gene to the
next generation, which is a low integration rate. We speculate that
this is due to the multiple integration and chimerical structure of
the foreign genes.
Subsequently, we observed that the BIGH3
transgenic mice did not present apparent macroscopic abnormalities
compared with the wild-type mice, suggesting that BIGH3
overexpression did not severely impair mouse development. A digital
camera was also applied to determine the gross appearance of the
corneas of these transgenic mice following anesthesia. There were
five among the seven F1 transgenic mice that displayed
centrally reduced corneal transparency, as illustrated in Fig. 3, which was similar to the symptoms
of HCD.
Furthermore, RT-PCR and western blot analysis were
also performed with the corneas of F1 transgenic mice to
investigate the expression of the human BIGH3 gene in the
corneas from wild-type and transgenic mice. As the anti-human BIGH3
antibody partially cross-reacted with both mouse and human
proteins, the amount of BIGH3 in the wild-type mice likely
represented the level of the endogenous mouse BIGH3 in the corneas.
We demonstrated that, in comparison with the wild-type mice, the
transgenic mice had a significantly increased expression of BIGH3
in their corneas (Fig. 5).
Taken together, the results from our study suggest
that we successfully generated a transgenic mouse model
overexpressing the human BIGH3 gene under the control of the
PGK promoter, which may serve as a practical model for studying
HCD. In addition, we believe that the BIGH3 gene is
essential for ocular development in vivo and pathologically
important to corneal disorganization. Therefore, this transgenic
mouse model is likely to contribute to further studies concerning
the detailed pathogenesis of the overexpression of BIGH3 protein in
corneal dystrophy.
Acknowledgements
The present study was supported by a grant from the
experimental animal project of the Shanghai Science and Technology
Commission (no. 10140903900).
References
|
1
|
Eiberg H, Moller HU, Berendt I and Mohr J:
Assignment of granular corneal dystrophy Groenouw type I (CDGG1) to
chromosome 5q. Eur J Hum Genet. 2:132–138. 1994.PubMed/NCBI
|
|
2
|
Yu P, Gu Y, Yang Y, et al: A clinical and
molecular-genetic analysis of Chinese patients with lattice corneal
dystrophy and novel Thr538Pro mutation in the TGFBI (BIGH3) gene. J
Genet. 85:73–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paliwal P, Sharma A, Tandon R, et al:
TGFBI mutation screening and genotype-phenotype correlation in
north Indian patients with corneal dystrophies. Mol Vis.
16:1429–1438. 2010.PubMed/NCBI
|
|
4
|
Vincent AL, de Karolyi B, Patel DV, et al:
TGFBI mutational analysis in a New Zealand population of inherited
corneal dystrophy patients. Br J Ophthalmol. 94:836–842. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Korvatska E, Munier FL, Djemai A, et al:
Mutation hot spots in 5q31-linked corneal dystrophies. Am J Hum
Genet. 62:320–324. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skonier J, Bennett K, Rothwell V, et al:
Beta-igh3: a transforming growth factor-beta responsive gene
encoding a secreted protein that inhibits cell attachment in vitro
and suppresses the growth of CHO cells in nude mice. DNA Cell Biol.
13:571–584. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grunauer-Kloevekorn C, Braeutigam S,
Weidle E, et al: Molecular genetic and histopathological
examinations for genotype-phenotype analysis in patients with
TGFBI-linked corneal dystrophy. Klin Monbl Augenheilkd.
223:829–836. 2006.(In German).
|
|
8
|
Kannabiran C and Klintworth GK: TGFBI gene
mutations in corneal dystrophies. Hum Mutat. 27:615–625. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel DA, Chang SH, Harocopos GJ, et al:
Granular and lattice deposits in corneal dystrophy caused by R124C
mutation of TGFBIp. Cornea. 11:1215–1222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gruenauer-Kloevekorn C, Clausen I, Weidle
E, et al: TGFBI (BIGH3) gene mutations in German families: two
novel mutations associated with unique clinical and
histopathological findings. Br J Ophthalmol. 93:932–937. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grunauer-Kloevekorn C, Brautigam S,
Wolter-Roessler M, et al: Molecular genetic analysis of the BIGH3
gene in lattice type I (Biber-Haab-Dimmer) and granular type II
(Avellino) corneal dystrophy: is indirect mutation analysis for hot
spots recommended? Klin Monbl Augenheilkd. 222:1017–1023. 2005.(In
German).
|
|
12
|
Munier FL, Korvatska E, Djemai A, et al:
Kerato-epithelin mutations in four 5q31- linked corneal
dystrophies. Nat Genet. 15:247–251. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JE, Han MS, Bae YC, et al: Anterior
segment dysgenesis after overexpression of transforming growth
factor-beta-induced gene, beta igh3, in the mouse eye. Mol Vis.
13:1942–1952. 2007.PubMed/NCBI
|
|
14
|
Bustamante M, Tasinato A, Maurer F, et al:
Overexpression of a mutant form of TGFBI/BIGH3 induces retinal
degeneration in transgenic mice. Mol Vis. 14:1129–1137.
2008.PubMed/NCBI
|
|
15
|
Pey AL, Mesa-Torres N, Chiarelli LR and
Valentini G: Structural and energetic basis of protein kinetic
destabilization in human phosphoglycerate kinase 1 deficiency.
Biochemistry. 52:1160–1170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schay G, Herenyi L, Fidy J and Osvath S:
Role of domain interactions in the collective motion of
phosphoglycerate kinase. Biophys J. 104:677–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei Y and Liu YG: Isolation, sequence
identification and tissue expression profile of a novel sheep
gene-PGK1. Res J Biotechnol. 8:38–41. 2013.
|
|
18
|
Singer-Sam J, Keith DH, Tani K, et al:
Sequence of the promoter region of the gene for human X-linked
3-phosphoglycerate kinase. Gene. 32:409–417. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takada T, Lida K, Awaji T, et al:
Selective production of transgenic mice using green fluorescent
protein as a marker. Nat Biotechnol. 15:458–461. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizuguchi H, Xu Z, Ishii-Watabe A, et al:
IRES-dependent second gene expression is significantly lower than
cap-dependent first gene expression in a bicistronic vector. Mol
Ther. 1:376–382. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borman AM, Bailly JL, Girard M and Kean
KM: Picornavirus internal ribosome entry segments: comparison of
translation efficiency and the requirements for optimal internal
initiation of translation in vitro. Nucleic Acids Res.
23:3656–3663. 1995. View Article : Google Scholar
|
|
22
|
Cao HQ and Ding JF: Construction strategy
of polygene co-expression vectors. Foreign Med Sci (Molecular
Biology Section). 24:1–4. 2000.
|