Introduction
Peritoneal dialysis (PD) has been used as the main
therapy for the treatment of end-stage renal disease (ESRD). A
single layer of mesothelial cells (MCs) covers an entire peritoneal
cavity. This layer of MCs can serve not only as a biological
barrier but also as a secretory organ. In addition, MCs have the
ability to synthesize and secrete various substances, such as
vascular endothelial growth factor (VEGF) and transforming growth
factor (TGF)-β and these substances participate in the functional
alteration of the peritoneal membrane (1–3).
Sterile dialysis solutions universally applied are
non-biocompatible, and can cause inflammation in the
sub-mesothelium. The inflammation process may sequentially lead to
fibrosis and angiogenesis, eventually leading to ultrafiltration
failure (4). In patients on
long-term PD using conventional peritoneal dialysis solutions
(PDS), alterations in the structure and function of the peritoneal
membrane, including increased peritoneal membrane permeability and
ultrafiltration failure, eventually lead to peritoneal fibrosis
(6,7). The substances secreted by MCs play
an important role in this process. Previous studies have suggested
that VEGF plays an important role in the alteration of peritoneal
membrane permeability (8,9). Pleiotrophin (PTN), initially
identified as a neurite growth/guidance-regulating protein, is an
18- or 15-kDa heparin-binding protein, which belongs to the midkine
family (10,11). PTN plays a variety of roles,
including roles in proliferation and apoptosis and has mitogenic,
angiogenic and oncogenic activities (12). In the studies by Kohashi et
al (13) and Henger et
al (14), PNT was reported to
participate in fibrosis in different organs. A previous study
demonstrated that the increase in peritoneal membrane permeability
and fibrosis induced by chlorhexidine gluconate (CG) was attenuated
in PTN knockout mice (15).
In this study, we investigated whether a
high-glucose-based peritoneal dialysis solution (HGPDS) directly
affects VEGF and PTN expression and whether this effect occurs
through the modulation of the serum/glucocorticoid-regulated kinase
1 (SGK1)-ERK1/2 signaling pathway in human peritoneal mesothelial
cells (HPMCs). We further explored the role of PTN in the
modulation of VEGF expression in HPMCs.
Materials and methods
Materials
Fluvastatin (Flu) was obtained as a gift from
Novartis Pharma Ltd., Shangai, China. Dulbecco’s modified Eagle’s
medium (DMEM) and fetal bovine serum (FBS) were purchased from
Gibco, Karlsruhe, Germany. Peritoneal dialysis fluids (4.25%)
(Baxter Corp., Deerfield, IL, USA) were used as co-culture medium.
Rabbit polyclonal anti-PTN (Proteintech, Manchester, UK), rabbit
polyclonal anti-VEGF (Abcam, Cambridge, MA, USA) and mouse
anti-GAPDH monoclonal antibody (Wuhan Boster Biological Technology
Ltd., Wuhan, China) were used for western blot analysis. GSK650394
(a competitive inhibitor of SGK1; Santa Cruz Biotechnology, Inc.,
Santa Cruz, USA) and PD98059 (an ERK specific inhibitor; Gibco)
were used to observe the effects of SGK1 and ERK1/2 in HPMCs. Mouse
anti-phosphorylated ERK1/2 (p-ERK) monoclonal antibody and mouse
anti-ERK monoclonal antibody were purchased from Cell Signaling
Technology, Inc., Danvers, MA, USA. A reverse transcription (RT)
kit, TRIzol reagent and PremixTaq version 2.0 were purchased from
Takara Bio, Inc., Shiga, Japan.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma, St. Louis, MO, USA) was used to assess cell viability.
Cell culture
HPMCs were obtained from the ATCC cell bank (no.
CRL-9444) and were routinely grown in DMEM supplemented with fetal
bovine serum (10% FBS), 100 UI/ml penicillin and 100 μg/ml
streptomycin. The cells were incubated at 37°C in a 5%
CO2 atmosphere and the culture medium was changed every
2–4 days. Cells were liberated with trypsin-EDTA to subculture in
new dishes with a subcultivation ratio of 1:3 to 1:4. All
experiments were conducted using cells at passage 5–10.
MTT
Cells were seeded into 96-well plates (4,000
cells/well) and cultured to 70–80% confluence. Following incubation
in DMEM with 0.01% FBS for 48 h, the cells were divided into
different groups : i) control (a 1:1 mixture of DMEM and total
culture fluid); ii) HGPDS (a 1:1 mixture of 4.25% PDS and total
culture fluid); iii) HGPDS plus Flu
(10−8–10−6 mol/l), HGPDS plus GSK650394
(10−5 mol/l) or HGPDS plus PD98059 (10−5
mol/l); iv) Flu (10−8–10−6 mol/l) (a 1:1
mixture of DMEM and total culture fluid), GSK650394
(10−5 mol/l) (a 1:1 mixture of DMEM and total culture
fluid) or PD98059 (10−5 mol/l). Each treatment group had
6 replicate wells. Following incubation for 6, 12, 24, 36, 48 or 72
h, 20 μl of MTT (5 mg/ml) were added to each well and the plates
were incubated for an additional 4 h. Subsequently, the medium was
discarded, 100 μl of DMSO were added to each well and mixed
thoroughly. The absorbance value of the wells was read at 490 nm
using a microplate reader (Bio-Rad, Hercules, CA, USA).
RNA extraction and RT-PCR
Cultured HPMCs were randomly divided into the
following groups: i) HPMCs were treated with HGPDS for 0, 6, 12 and
24 h; ii) HPMCs were grown in medium with HGPDS and/or the addition
of Flu (10−8–10−6 mol/l), HGPDS plus
GSK650394 (10−5 mol/l), HGPDS plus PD98059
(10−5 mol/l), PTN (10–30 nmol/l) with or without the
blocking peptide of PTN, Flu (10−6 mol/l), GSK650394
(10−5 mol/l) or PD98059 (10−5 mol/l) without
HGPDS. First-strand cDNA synthesis was performed using 1 μg of each
RNA sample. A 2 μl mixture was used in the PCR reaction with
PremixTaq version 2.0 (loading dye mix). All specific primers for
human PTN, VEGF, fibronectin (FN) and GAPDH were designed according
to the sequences in GenBank: PTN: forward, 5′-CTTGGCATTCATTTTCAT-3′
and reverse, 5′-GATCTT ACATCTCTGGGTCTT-3′; VEGF forward, 5′-ATGACGA
GGGCCTGGAGTGT-3′ and reverse, 5′-GGGATTTCTT GGGCTTTCGTTT-3′; FN
forward, 5′-AGCCGCCACGTG CCAGGATTAC-3′ and reverse,
5′-CTTATGGGGGTGGC CGTTGTGG-3′ and GAPDH forward, 5′-AGGTCGGAG
TCAACGGATTTG-3′ and reverse, 5′-GTGATGGCAT GGACTGTGGT-3′. The
annealing temperature was 51, 54, 59.4 and 60°C, respectively. PCR
was amplified using primers for human PTN, VEGF, FN and GAPDH and
the products were subjected to electrophoresis on 2.5% agarose
gels. The bands were visualized by ethidium bromide. Finally,
analysis was performed by densitometric gel scanning. GAPDH was
used as the housekeeping gene to normalize target gene expression.
The results were expressed as the ratio of PTN, VEGF and FN to
GAPDH in each sample analyzed.
Western blot analysis
Cells were divided into the same groups as for
RT-PCR. Protein from the cells was homogenized in lysis buffer and
was quantified. Each protein sample (60 μg, apart from PTN 200 μg)
was separated by 12% SDS-PAGE and transferred onto a nitrocellulose
membrane for 60 min (apart from PTN 40 min) at 100 V (apart from
PTN 90 V). Non-specific protein binding was blocked by incubating
the membranes in blocking solution [5% non-fat milk in TBS-0.1%
Tween-20 (TBS-T)] for 1 h at room temperature. The membrane was
exposed overnight to a 1:500 dilution of rabbit polyclonal
anti-human PTN, a 1:1,000 dilution of rabbit polyclonal anti-human
VEGF antibody, a 1:2,000 dilution of mouse monoclonal anti-human
ERK1/2 and p-ERK1/2 or a 1:500 dilution of mouse monoclonal
anti-GAPDH antibody at 4°C. After being rinsed with TBS-T solution
3 times, each membrane was incubated with horseradish
peroxidase-conjugated secondary antibody [sheep anti-rabbit IgG
(1:10,000) or sheep anti-mouse IgG (1:10,000)] for 60 min at room
temperature. After another wash with TBS-T solution, specific
signals were detected using an enhanced chemiluminescence western
blotting detection system. The bands were scanned using a laser
densitometer to assess the density.
ELISA
Cells were disseminated into 12-well plates
(105 cells/well) and divided into the control group (a
1:1 mixture of DMEM and total culture fluid), HGPDS group (a 1:1
mixture of 4.25% PDS and total culture fluid) and 20 nmol/l PTN
group (a 1:1 mixture of DMEM and total culture fluid with the
addition of 20 nmol/l PTN). After the cells were cultured under
different conditions, the supernatants were collected and the FN
protein from the supernatants was determined using a human
fibronectin ELISA kit (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s instructions. To control cell
number differences in the supernatants, all proteins in each group
were extracted and quantified by the BCA method. The FN levels were
then expressed as ng/mg protein.
Statistical analysis
We used SPSS 13.0 software to analyze the results
and all data are expressed as the means ± SD. Comparisons among
groups were performed by one-way ANOVA. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
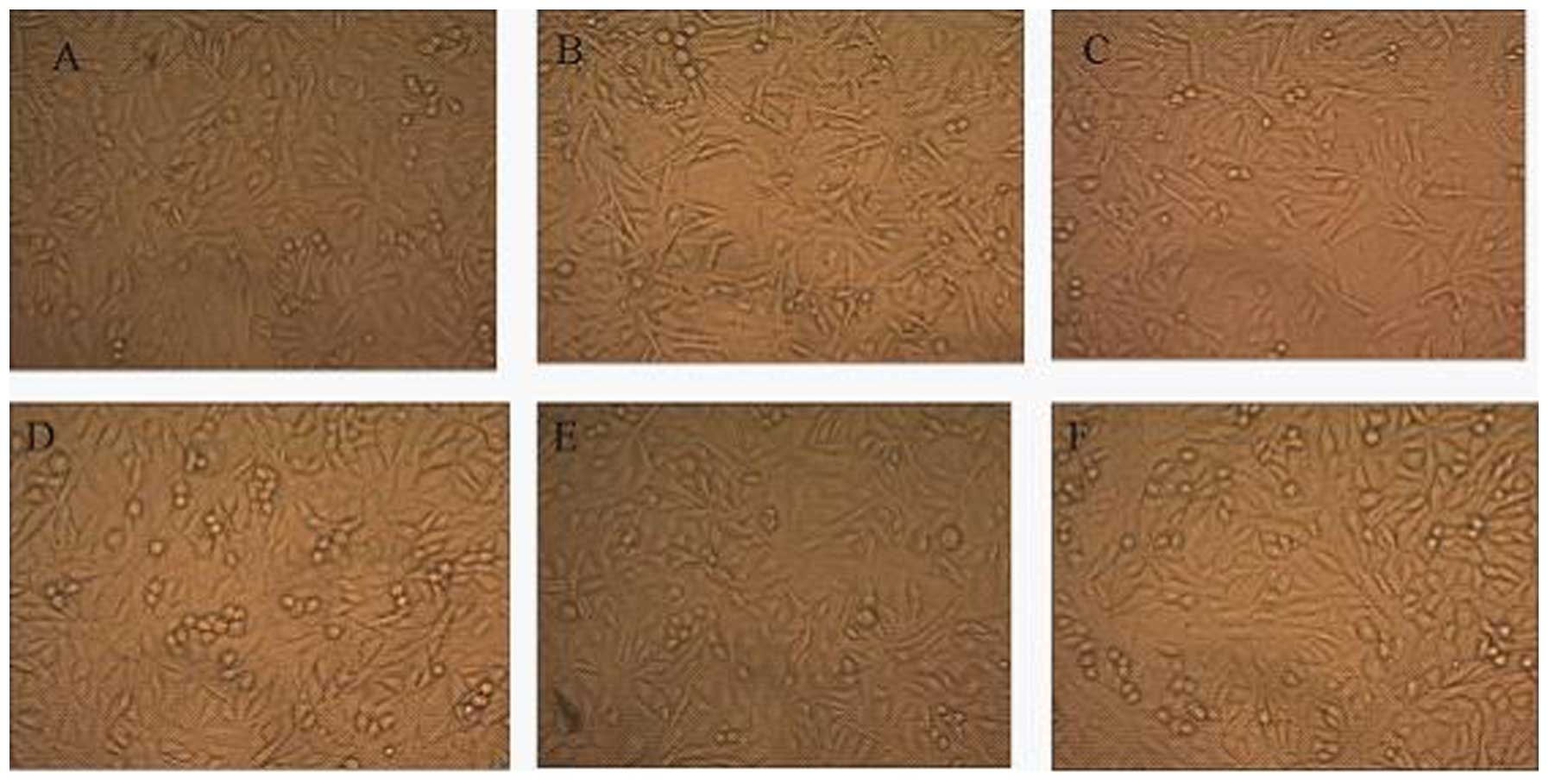
Changes in phenotypic characteristics of
HPMCs
The normal HPMC monolayer showed a characteristic
cobblestone-like appearance; however, the loss of cell contacts
with an acquisition of an elongated fibroblastic morphology was
observed after 48 h of incubation with HGPDS. A similar morphology
alteration was observed following incubation with PTN. Flu
(10−6 mol/l), GSK650394 (10−5 mol/l) and
PD98059 (10−5 mol/l) reversed the changes in cell
morphology induced by HGPDS. GSK650394 (10−5 mol/l),
PD98059 (10−5 mol/l) or Flu (10−6 mol/l)
alone had no effect on the phenotypic characteristics of the HPMCs
(Fig. 1).
Effect of HGPDS and Flu on cell
viability
Cell viability was affected by HGPDS, GSK650394
(10−5 mol/l), PD98059 (10−5 mol/l) and Flu
(10−6 mol/l). Compared with the control group, a
reduction in HPMC viability was observed in the HGPDS groups and
this reduction was partially reversed in the groups treated with
various concentrations of Flu, PD98059 and GSK650394. After 24 h of
incubation, the decrease in cell viability induced by HGPDS was
reversed with the concentration of Flu (10−6 mol/l),
GSK650394 (10−5 mol/l) and PD98059 (10−5
mol/l) (P<0.05) (Fig. 2).
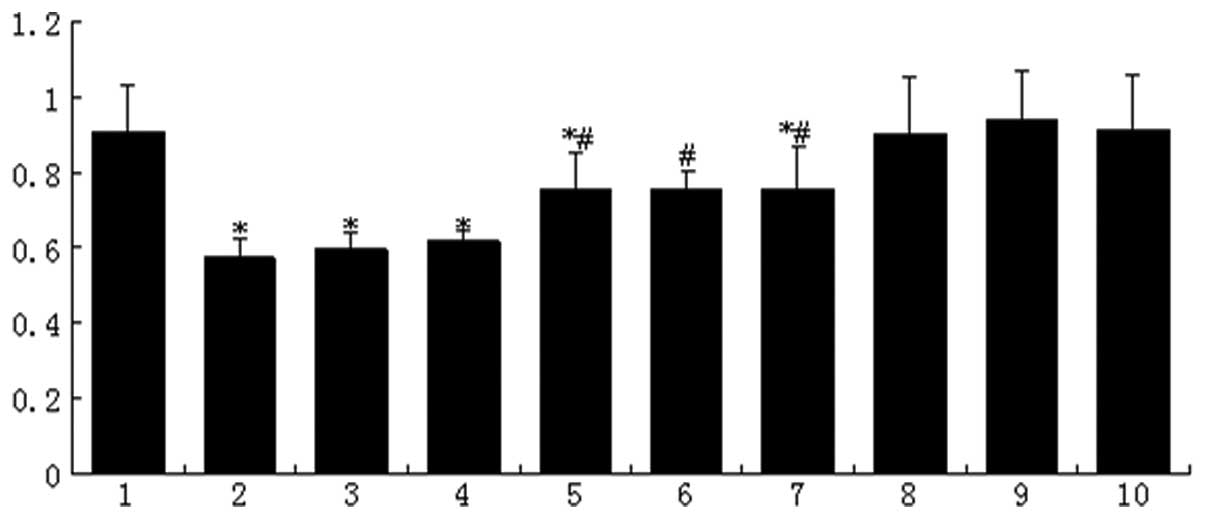 | Figure 2Cell viability decreased by
high-glucose-based peritoneal dialysis solution (HGPDS). Lane 1,
control; lane 2, HGPDS; lane 3, HGPDS + fluvastatin (Flu)
10−8 mol/l; lane 4, HGPDS + Flu 10−7 mol/l;
lane 5, HGPDS + Flu 10−6 mol/l; lane 6, HGPDS +
GSK650394 (a competitive inhibitor of SGK1) 10−5 mol/l;
lane 7, GPDS + PD98059 (a competitive inhibitor of ERK1/2)
10−5 mol/l; lane 8, Flu 10−6 mol/l; lane 9,
GSK650394 10−5 mol/l; lane 10, PD98059 10−5
mol/l. Cell viability was decreased by HGPDS, while it was
partially restored in the groups treated with 3 different
concentrations of Flu or GSK650394. The decrease in cell viability
induced by HGPDS was significantly reversed by the concentration of
Flu 10−6 mol/l, PD98059 and GSK650394 10−5
mol/l. *P<0.05 vs. control, #P<0.05 vs.
HGPDS. |
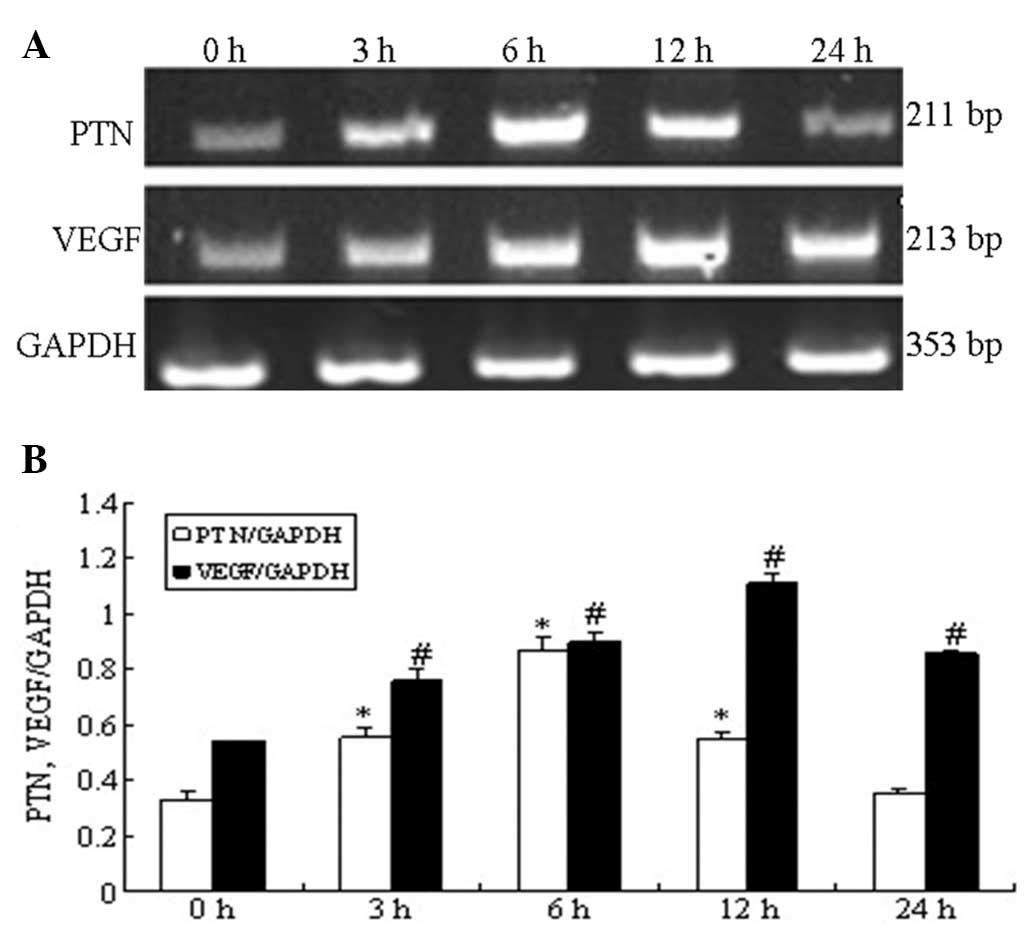
Effect of HGPDS and Flu on the mRNA
expression of PTN and VEGF
The mRNA expression of PTN measured by RT-PCR in the
HPMCs increased significantly following exposure to HGPDS, peaking
at 6 h. The VEGF mRNA levels were significantly elevated and peaked
at 12 h following exposure to HGPDS (P<0.05) (Fig. 3). The effects of HGPDS in
conjuction with 3 different concentrations of Flu, GSK650394 and
PD98059 on PTN mRNA expression were examined in the HPMCs.
GSK650394 (10−5 mol/l), PD98059 (10−5 mol/l)
and Flu (10−6 mol/l) significantly suppressed the
upregulation in the expression of PTN and VEGF induced by HGPDS
(P<0.05). However, Flu at the concentration of 10−7
mol/l and 10−8 mol/l had no significant effects on the
elevated PTN and VEGF mRNA expression (P>0.05). Compared with
the control group, Flu (10−6 mol/l), GSK650394
(10−5 mol/l) or PD98059 (10−5 mol/l) alone
had no significant effects on PTN, VEGF and FN mRNA expression in
the HPMCs (P>0.05) (Fig. 4).
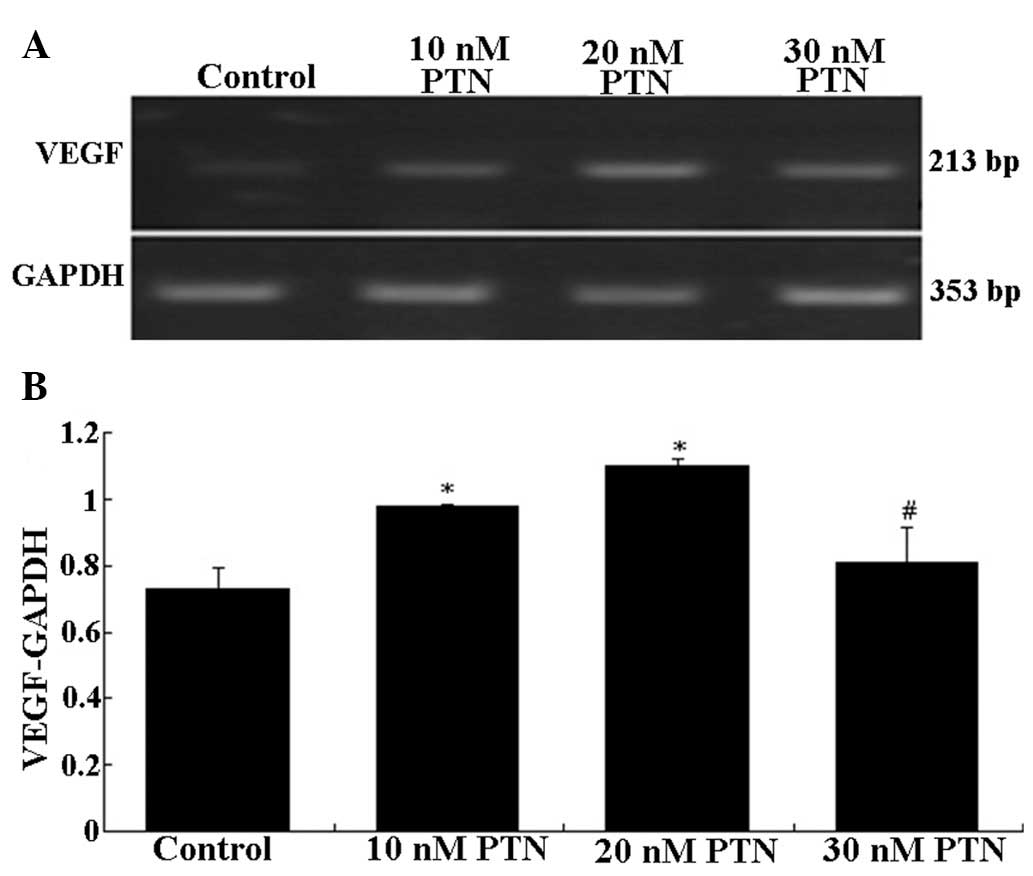
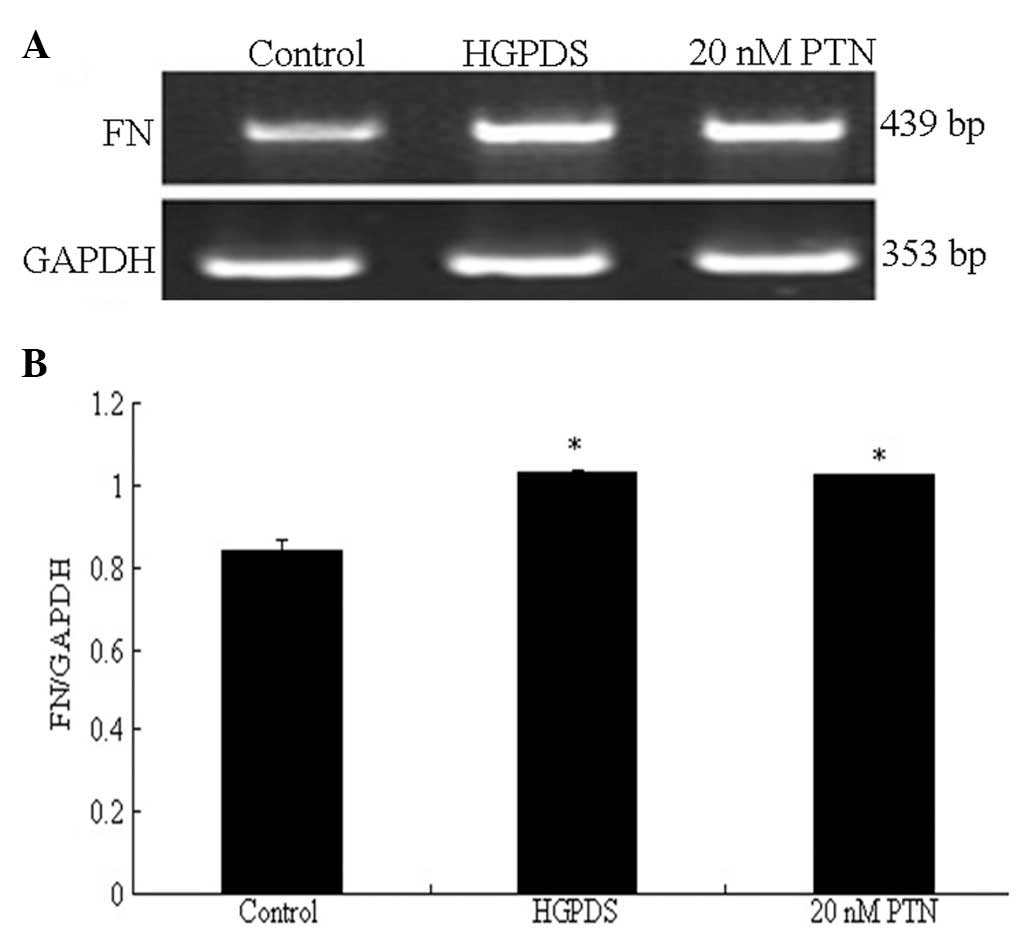
Compared with the control group, the concentration of PTN (10–30
nmol/l) which had the most significant effect on the mRNA
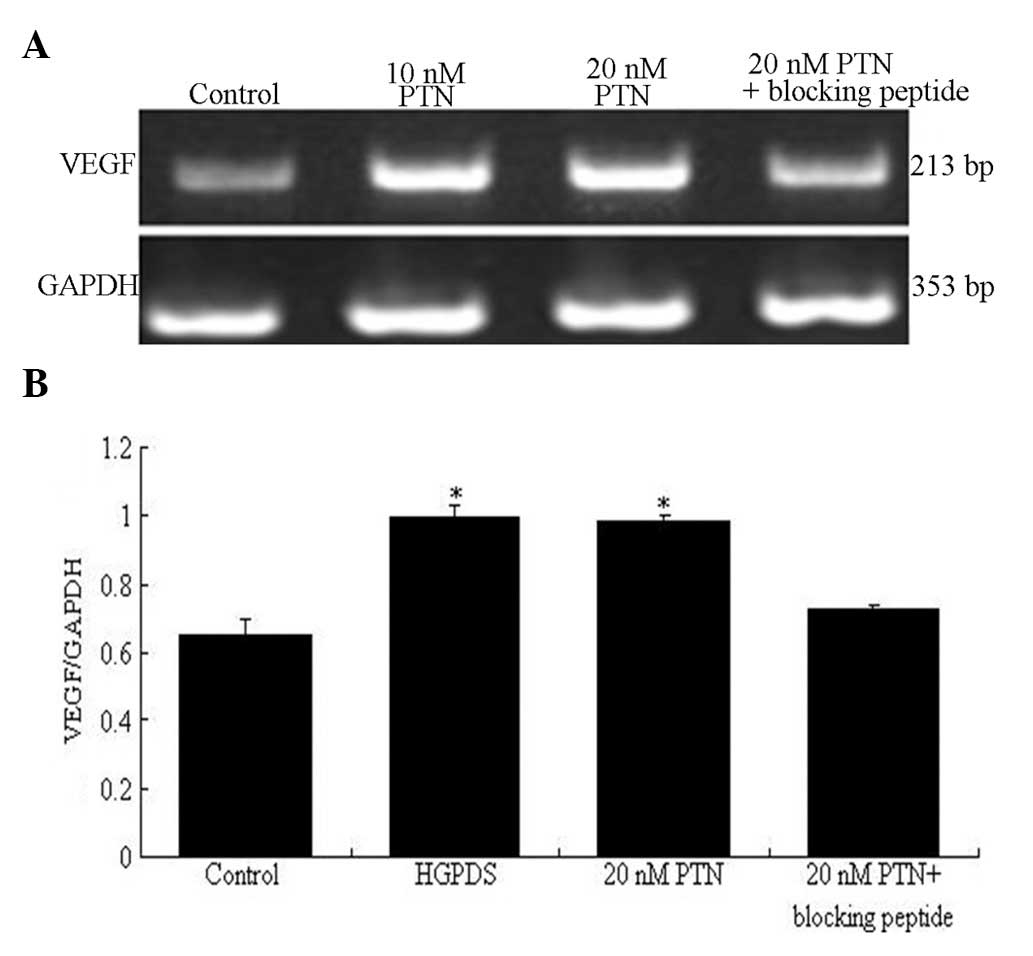
expression of VEGF was 20 nmol/l (P<0.05) (Fig. 5). The blocking peptide of PTN
decreased the mRNA expression of VEGF which had been increased by
PTN (20 nmol/l) (P<0.05) (Fig.
6). However, it had no effect on the mRNA expression of FN
which had been increased by PTN (Fig.
7).
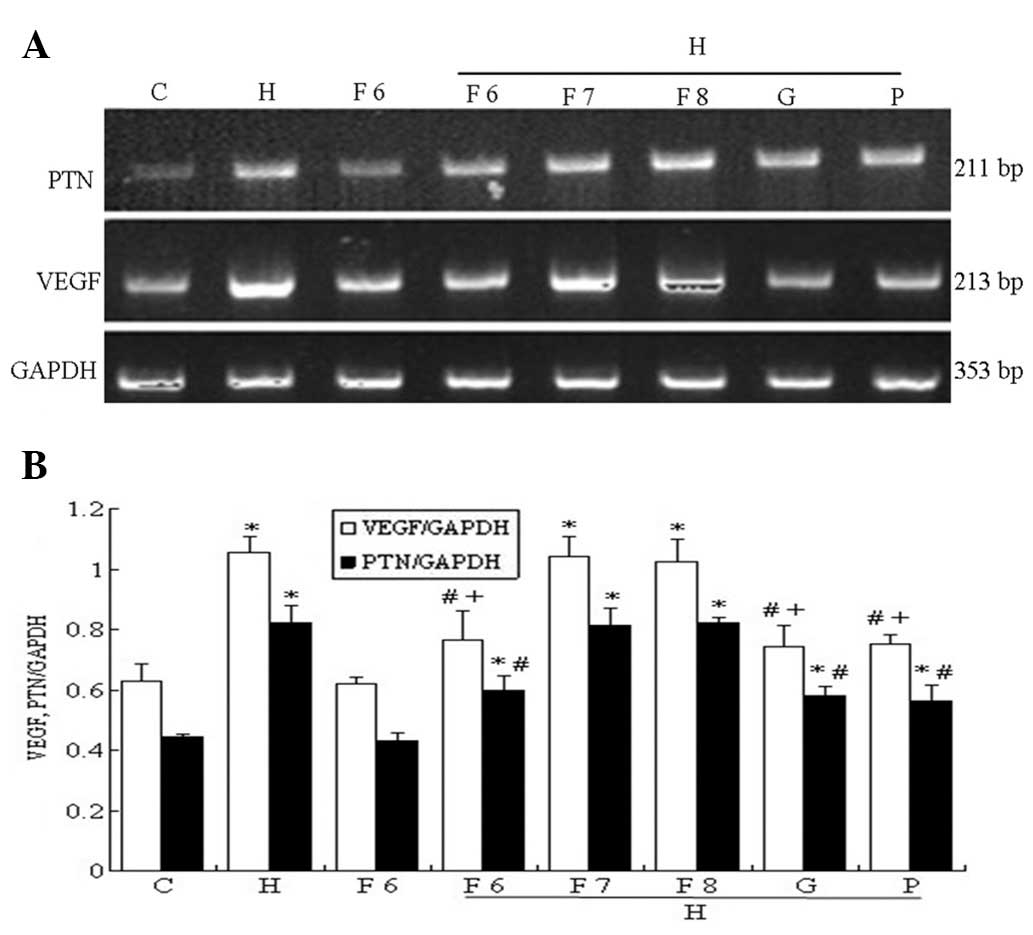 | Figure 4Effect of fluvastatin (Flu),
GSK650394 (a competitive inhibitor of SGK1) and PD98059 (a
competitive inhibitor of ERK1/2) on the high-glucose-based
peritoneal dialysis solution (HGPDS)-stimulated mRNA expression of
pleiotrophin (PTN) and vascular endothelial growth factor (VEGF).
C, control; H, HGPDS; F6, Flu 10−6 mol/l; H + F6, HGPDS
+ Flu 10−6 mol/l; H + F7, HGPDS + Flu 10−7
mol/l; H + F 8, HGPDS + Flu 10−8 mol/l; H + G, HGPDS +
GSK650394 10−5 mol/l; H + P, HGPDS + PD98059
10−5 mol/l. (A) Analysis of PTN and VEGF mRNA expression
by RT-PCR. (B) Densitometry analysis of PTN and VEGF mRNA. GAPDH
was used as the housekeeping gene to normalize target gene
expression. Flu inhibited the elevated PTN and VEGF mRNA expression
induced by HGPDS in a dose-dependent manner. *P<0.01
vs. control, #P<0.01, +P<0.05 vs.
HGPDS. |
Effect of HGPDS and Flu on the protein
expression of PTN and VEGF
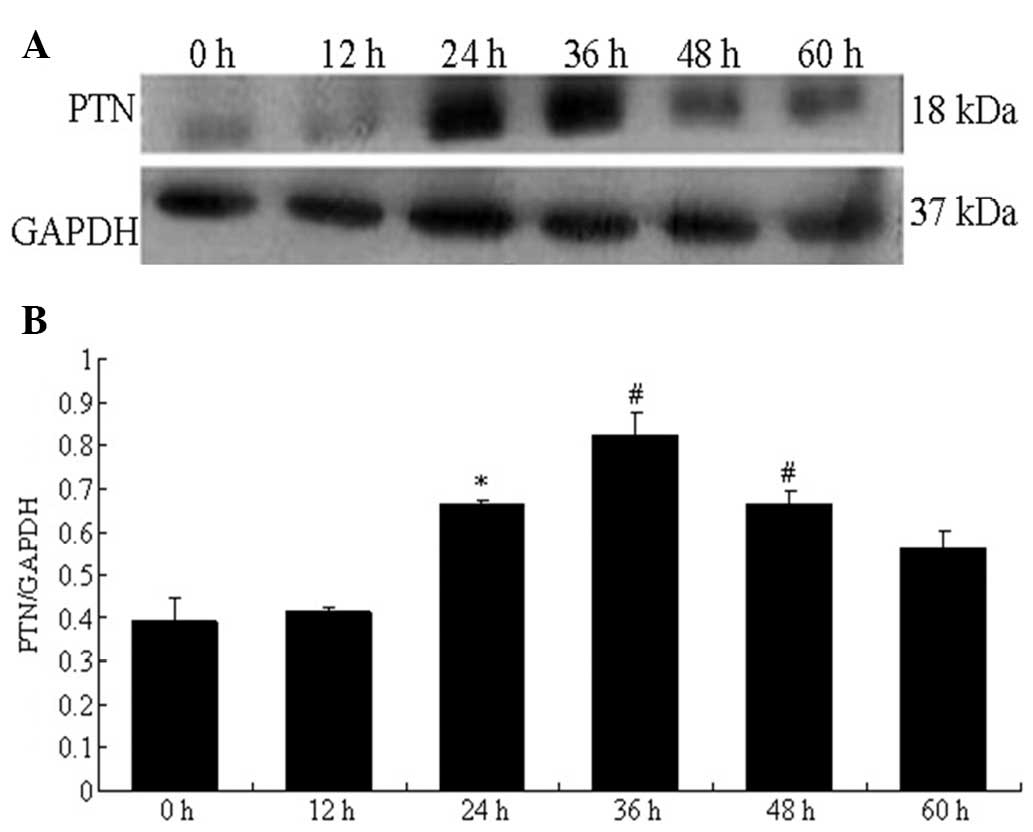
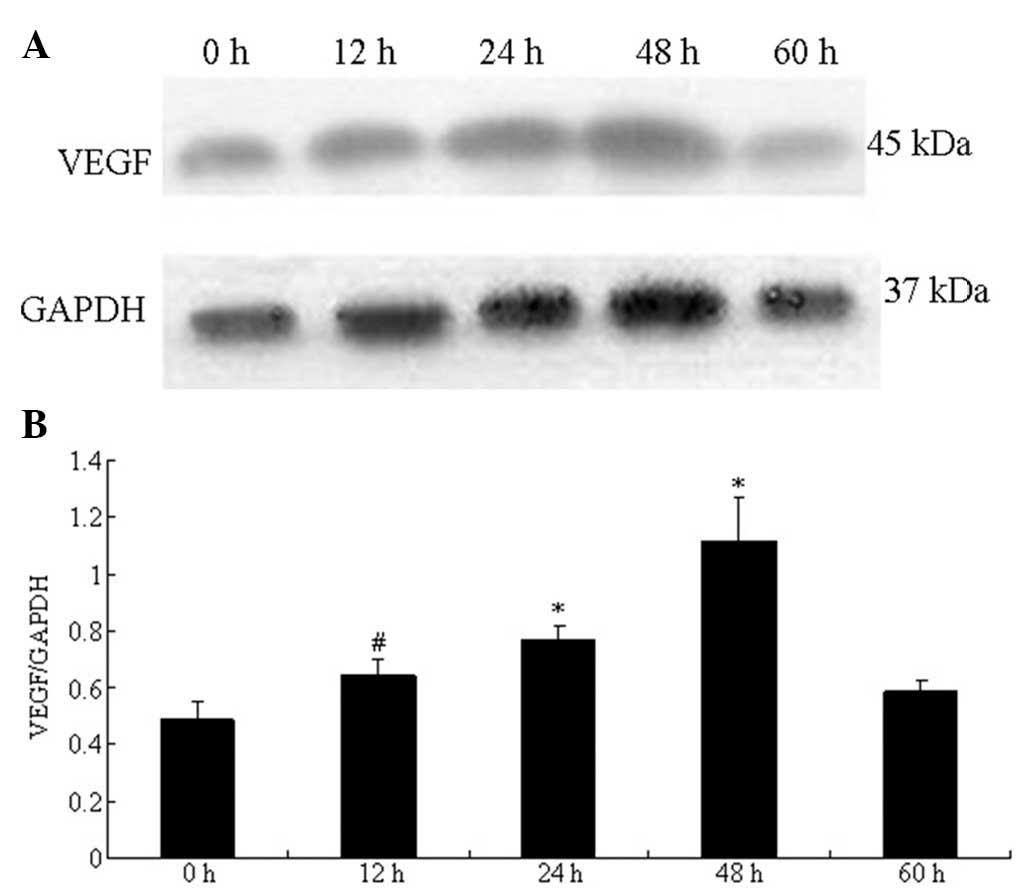
The protein expression of PTN measured by western
blot analysis in the HPMCs increased significantly and peaked at 36
h following exposure to HGPDS. The VEGF protein levels were
significantly elevated and peaked at 48 h following exposure to
HGPDS (P<0.05) (Figs. 8 and
9). Based on the results
(Figs. 8 and 9), we measured PTN and VEGF protein
expression following exposure to HGPDS in conjunction with 3
different concentrations of Flu, GSK650394 and PD98059 in the HPMCs
for 36 h and 48 h. GSK650394 (10−5 mol/l), PD98059
(10−5 mol/l) and Flu (10−6 mol/l)
significantly inhibited the upregulation in PTN and VEGF expression
induced by HGPDS (P<0.05). However, Flu at the concentration of
10−7 and 10−8 mol/l had no significant
effects on the elevated PTN and VEGF protein expression levels
(P>0.05). Compared with the control group, Flu (10−6
mol/l), PD98059 (10−5 mol/l) or GSK650394
(10−5 mol/l) alone had no significant effects on PTN and
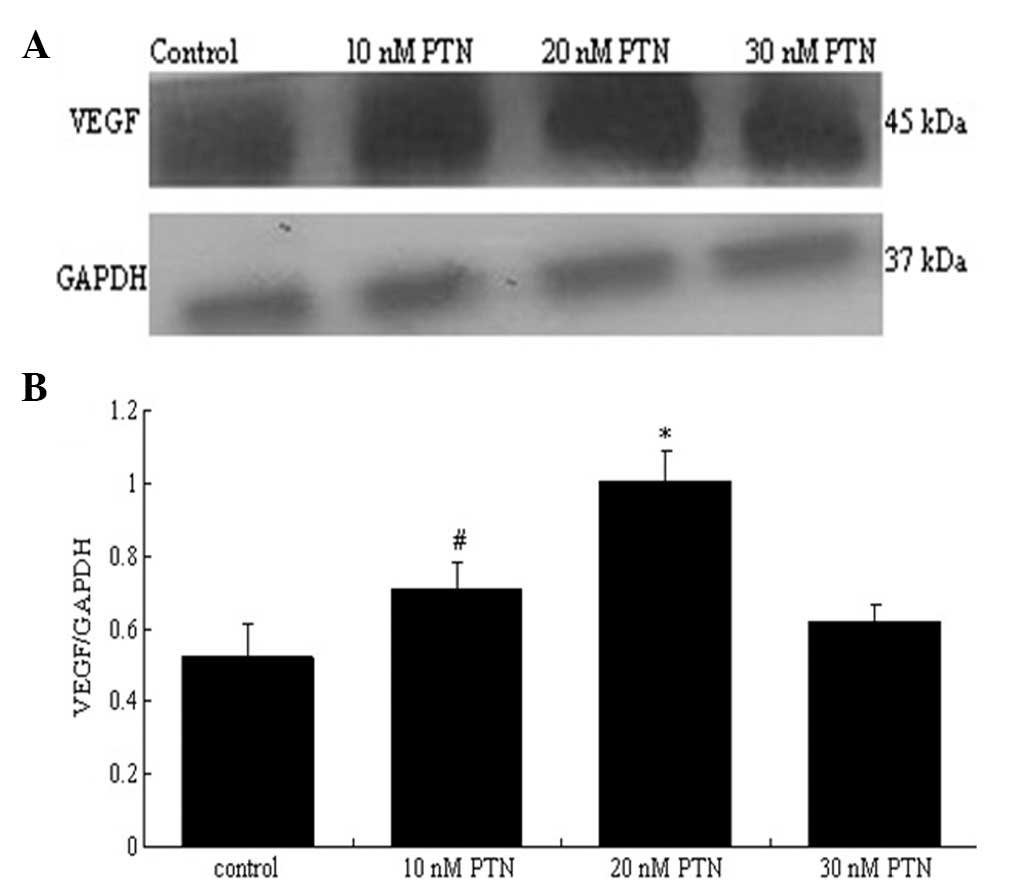
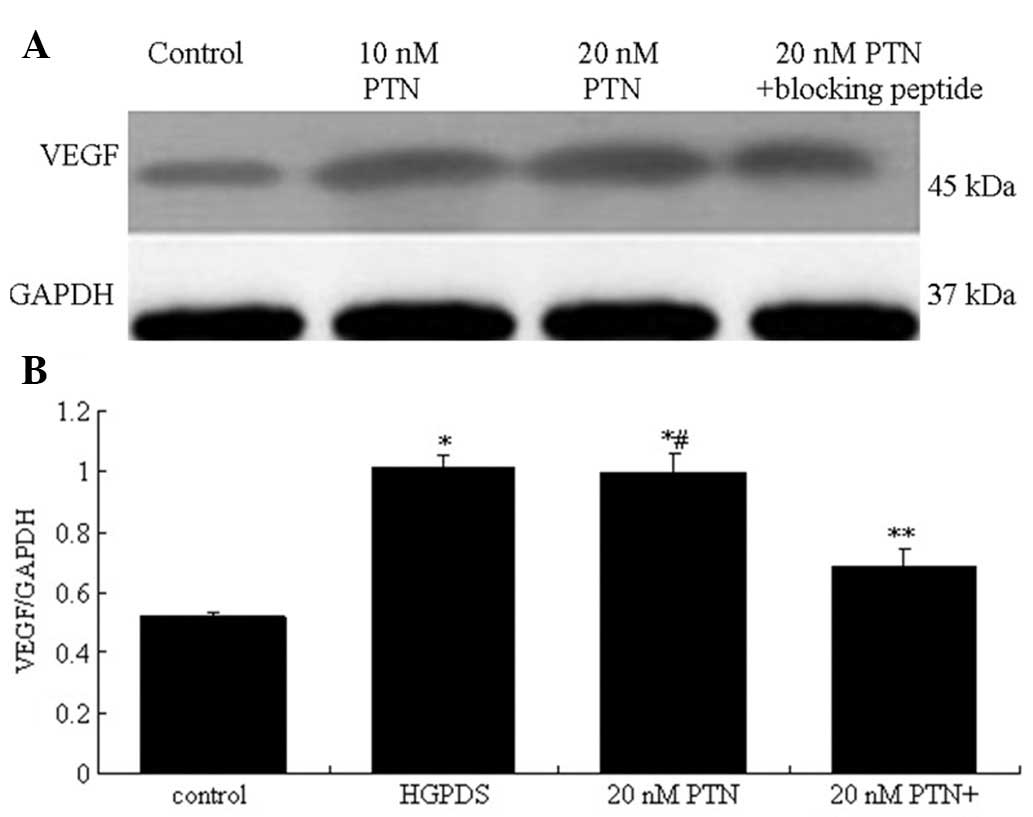
VEGF protein expression in HPMCs (P>0.05) (Fig. 10). The effect of PTN on the
expression of VEGF and FN was also examined. PTN (10–30 nmol/l)
increased the expression of VEGF in the HPMCs; the concentration of
20 nmol/l PTN induced the most significant increase (P<0.05)
(Fig. 11). The blocking peptide
of PTN decreased the expression of VEGF which had been increased by
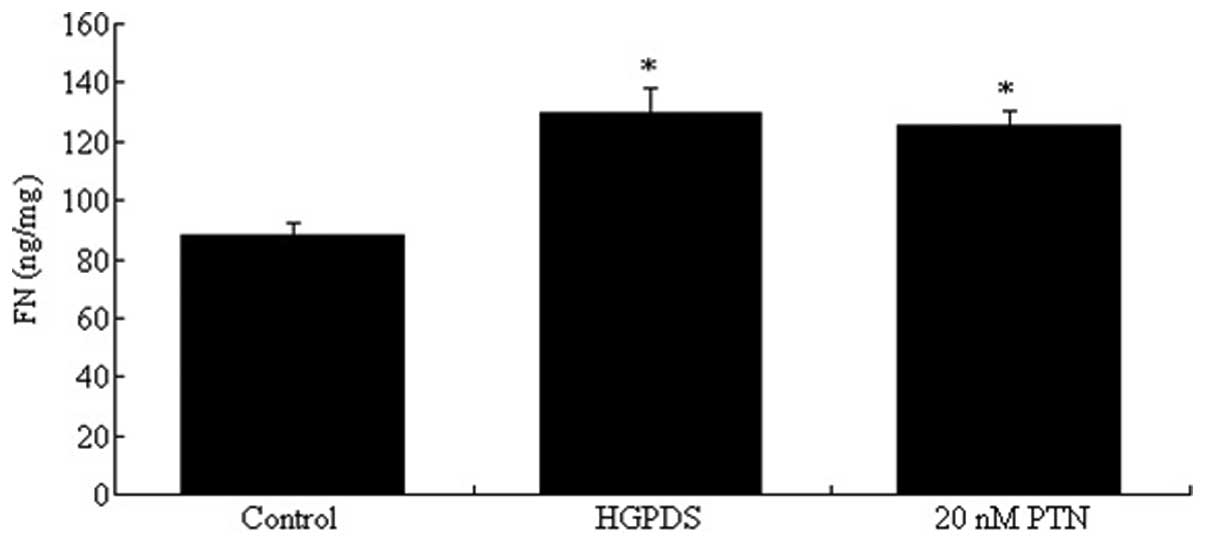
PTN (Fig. 12). Compared with the
control, 20 nmol/l of PTN increased the expression of FN
(P<0.05). Our results indicated that PTN and HGPDS had the same
effect on the expression of FN (Fig.
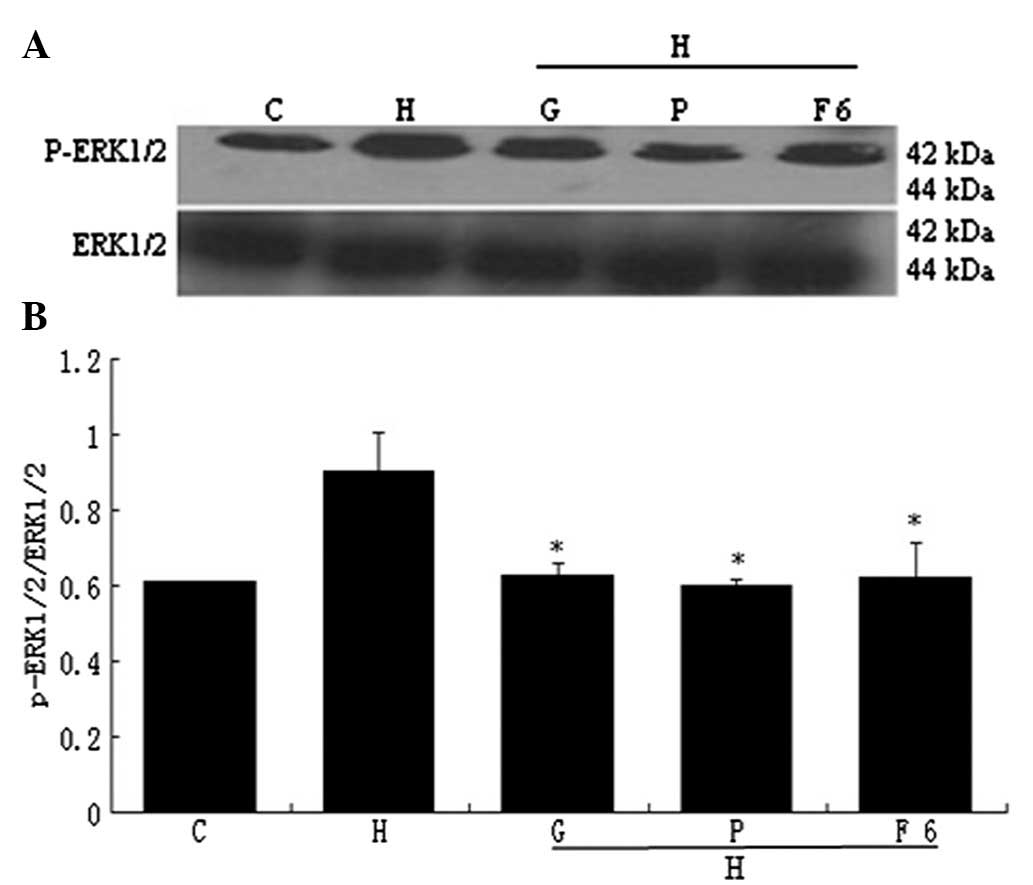
13). Flu (10−6 mol/l), PD98059 and GSK650394
decreased the expression of p-ERK1/2 which had been increased by
HGPDS (P<0.05) (Fig. 14).
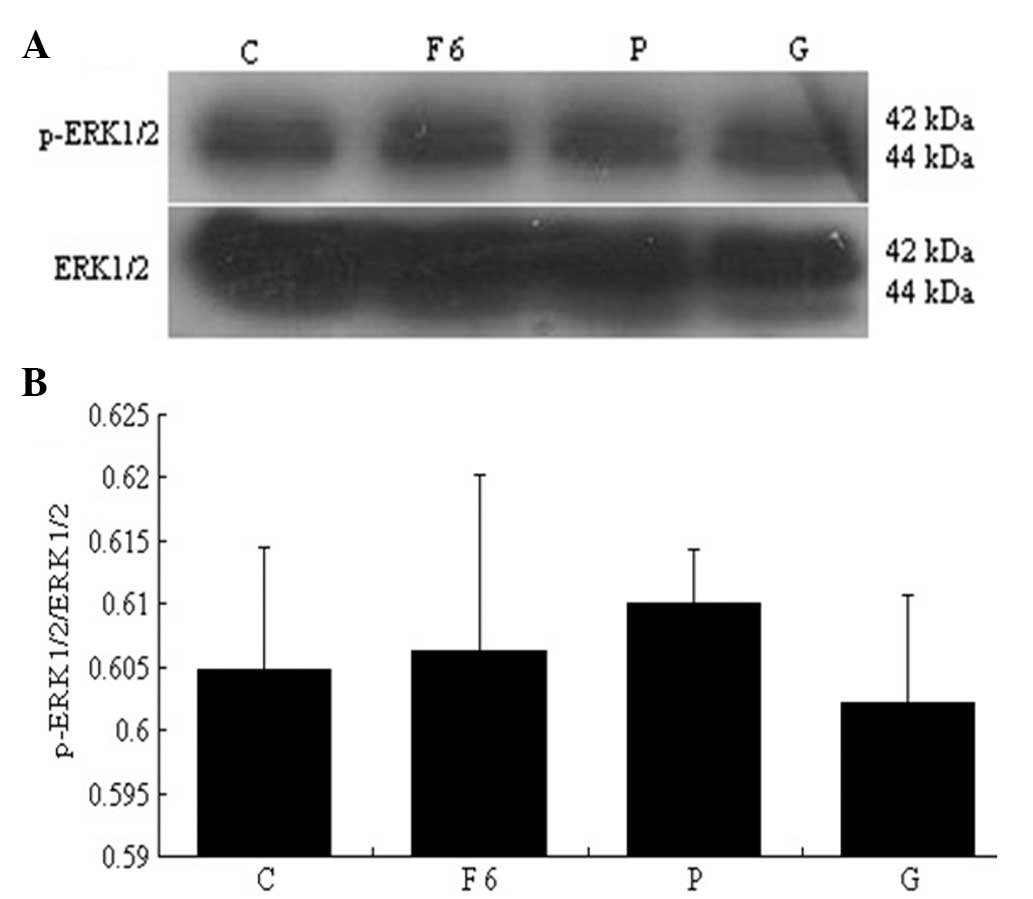
Flu, PD98059 and GSK650394 alone had no significant effect on the
expression of p-ERK1/2 compared with the control group (P>0.05)
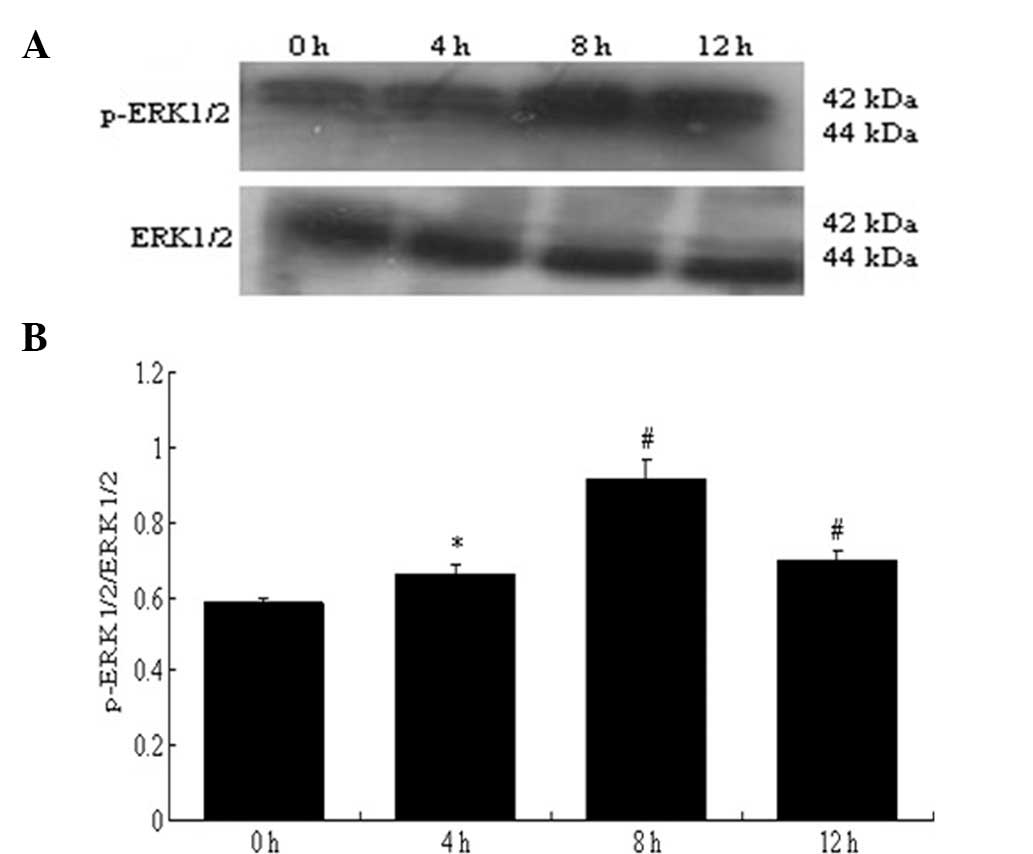
(Fig. 15). The expression of
p-ERK1/2 which had been increased by HGPDS peaked at 8 h (P<0.01
or P<0.05) (Fig. 16).
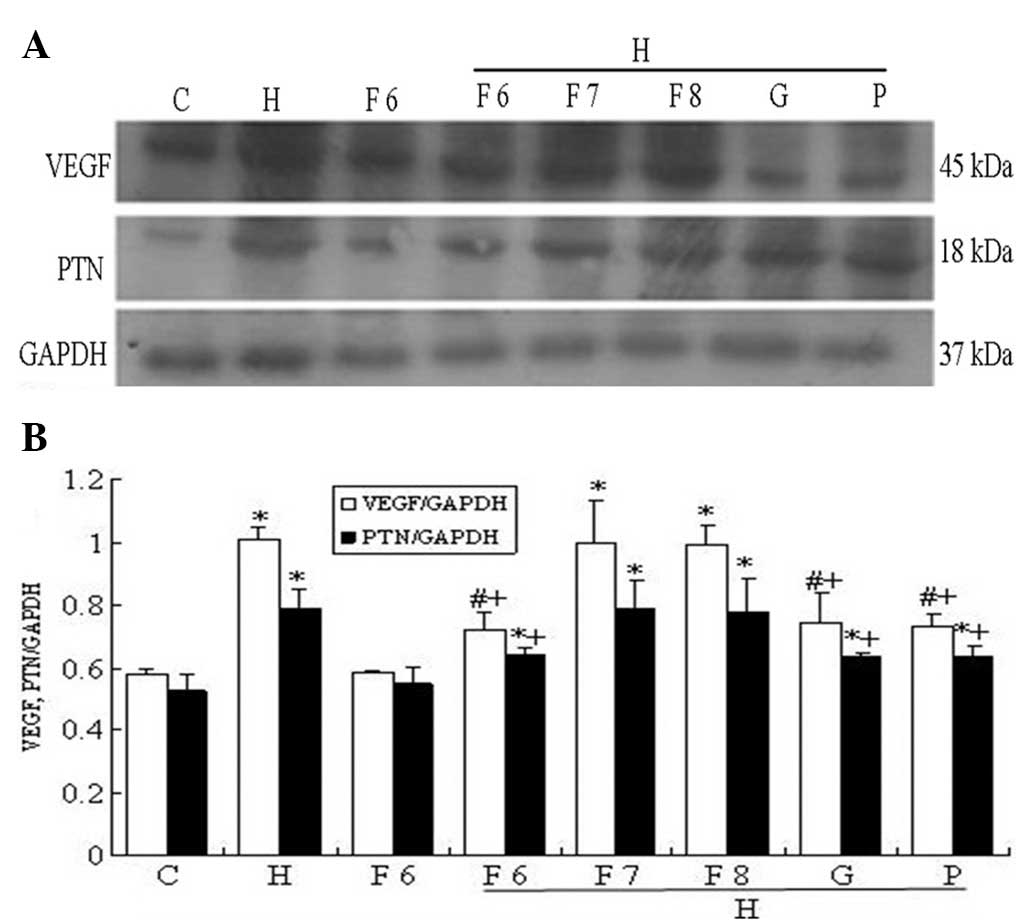 | Figure 10Effect of fluvastatin (Flu),
GSK650394 (a competitive inhibitor of SGK1) and PD98059 (a
competitive inhibitor of ERK1/2) on the high-glucose-based
peritoneal dialysis solution (HGPDS)-stimulated protein expression
of pleiotrophin (PTN) and vascular endothelial growth factor
(VEGF). C, control; H, HGPDS; F6, Flu 10−6 mol/l; H +
F6, HGPDS + Flu 10−6 mol/l; H + F7, HGPDS + Flu
10−7 mol/l; H + F8, HGPDS + Flu 10−8 mol/l; H
+ G, HGPDS + GSK650394 10−5 mol/l; H + P, HGPDS +
PD98059 10−5 mol/l. (A) Analysis of PTN and VEGF protein
expression by western blot analysis. (B) Densitometry analysis of
VEGF and PTN protein. GAPDH was used as the housekeeping gene to
normalize target gene expression. After incubation for 36 h or 48
h, Flu, GSK650394 and PD98059 inhibited the elevated PTN and VEGF
protein expression induced by HGPDS in a dose-dependent manner.
*P<0.05 vs. control, #P<0.05 vs. HGPDS,
+P<0.05 vs. HGPDS. |
Discussion
If the peritoneum is exposed to HGPDS continuously,
HGPDS will lead to the alteration of the structure and function of
the membrane. HGPDS may have the potential to increase peritoneal
permeability, resulting in the rapid dissipation of the osmotic
gradient and, eventually, causing ultrafiltration failure and
inadequate dialysis (16).
Decreased ultrafiltration has is regarded as evidence of the
augmentation of the peritoneal surface area available for the
diffusion exchange (17). An
increased vascular surface area within the peritoneal membrane may
thus account for the loss of ultrafiltration. Within this
framework, VEGF may play a critical role in ultrafiltration
failure, mainly as VEGF increases vascular permeability (18,19). In the present study, we focused on
the direct short-term effects of HGPDS on the expression of VEGF.
Modulation of the expression and organization of VEGF by PTN in
colorectal cancer has been reported (20). It has been reported that HPMCs
secrete PTN, then PTN increases peritoneal permeability which leads
to peritoneal fibrosis in mice (15). Pleiotrophin (PTN the protein,
ptn the gene), a 136 amino acid, secreted heparin-binding
cytokine, can signal diverse functions, such as neurite outgrowth
and angiogenesis. PTN may be a candidate ‘tumor promoter’ and may
initiate an ‘angiogenic switch’ through its C-terminal angiogenesis
domain (12).
In this study, we demonstrate that HGPDS upregulates
VEGF expression in HPMCs in a time-dependent manner. Our in
vitro data further confirm that HGPDS upregulates PTN
expression in HPMCs. HGPDS significantly inhibited the viability of
the HPMCs. In addition, the morphology of the HPMCs was altered
from a typical cobblestone-like appearance to a fibroblast-like
appearance following incubation with HGPDS for 48 h. The same
morphological changes were observed in the cells incubated in DMEM
supplemented with 20–30 nmol/l PTN. Coinciding with the
morphological changes, the mRNA and protein expression of PTN and
VEGF increased significantly in the HPMCs in response to HGPDS. The
increase in PTN expression occurred in a time-dependent manner and
peaked prior to VEGF, which indicated that VEGF expression may be
partially dependent on the secretion of PTN by HPMCs. When the
HPMCs were incubated with PTN, the expression of VEGF significantly
increased, particularly with the concentration of 20 nmol/l PTN.
The blocking peptide of PTN blocked the effects of the C-terminal
of PTN, which partially restored the morphological changes and
suppressed the expression of VEGF in the HPMCs incubated with PTN.
Our results also indicated that PTN significantly increased the
expression of FN in the HPMCs; however, this increase was not
reversed by the blocking peptide of PTN. Further investigation is
required to determine whether any other blocking peptide can
inhibit the expression of FN induced by PTN. Our results
demonstrate that PTN may mediate the process of peritoneal membrane
injury induced by HGPDS. However, the mechanisms behind the
increase in PTN expression induced by HGPDS in HPMCs remain
unclear.
SGK is a new member of the serine/threonine kinase
gene family and was originally identified as an immediate early
gene under acute transcriptional control by serum and
glucocorticoids (21). Human
Sgk-1 was cloned as a cell volume-sensitive gene which is
upregulated by hypertonic cell shrinkage (22). Evidence suggests that the
expression, enzymatic activity and cellular localization of SGK1
are regulated in response to various stimuli. These stimuli include
cell volume, epithelial transport, cardiac action potential and
cell proliferation, survival and apoptosis (23,24). SGK1 is expressed in a variety of
fibrosing tissues, such as tissues in Crohn’s disease, lung
fibrosis, liver cirrhosis and glomerulonephritis (25,26). ERK1 and ERK2 belong to the
protein-serine/threonine kinase family which participates in the
Ras-Raf-MEK-ERK signal transduction cascade. This cascade is
involved in the regulation of a large variety of processes, such as
cell cycle progression, cell migration, cell survival,
differentiation, proliferation and transcription (27). Advanced glycation end products
(AGEs) can induce ERK1/2 phosphorylation in skin cells (28). In the present study, GSK650394 or
PD98059 decreased the mRNA and protein expression of PTN in the
HPMCs which had been increased by HGPDS. The correlation between
SGK1 and ERK1/2 is poorly understood. Therefore, the correlation
between SGK1 and ERK1/2 was investigated further in this study. We
found that p-ERK1/2 expression was reduced by the addition of
GSK650394, an inhibitor of SGK1, suggesting that SGK1 may regulate
the phosphorylation of ERK1/2 and that the SGK1-ERK1/2 pathway may
play a role in the increased expression of PTN in HPMCs induced by
HGPDS. GSK650394 or PD98059 only partially inhibited the expression
of PTN and VEGF in the HPMCs which had been increased by HGPDS.
Therefore, there are many other signaling pathways that participate
in this injury process. Further studies are required to elucidate
this issue.
Statins inhibit the enzyme,
3-hydroxy-3-methylglutarylcoenzyme A (HMG-CoA) reductase, which is
required for cholesterol biosynthesis. However, there is increasing
evidence of the beneficial effects of statins, unrelated to their
lipid-lowering capacity, such as anti-fibrinolytic,
anti-proliferative and ant-inflammatory effects (29). By inhibiting HMG-CoA reductase,
statins can also inhibit the synthesis of isoprenoids, which are
important for intracellular signaling molecules, such as ERK, Rho,
Ras and Rac. Therefore, statins have various
cholesterol-independent effects, such as the reduction of
extracellular matrix synthesis, the regulation of cytokines and
inflammatory factors through the direct inhibition of these small
GTP-binding proteins (30).
Previous studies have demonstrated that SGK1 was the functional
cross of multiple signaling pathways (31,32). It can be activated by bone marrow
kinase/extracellular signal-regulated kinase 5 (BK/ERK5) or p38
MAPK. In addition, the small G protein Rac1 activates SGK1 through
a PI3-kinase-independent pathway (33,34). Additional activators of SGK1
include neuronal depolarization, cAMP, lithium and oxidation
(35). The present study did not
explore the underlying mechanisms of PTN regulation in response to
Flu. Further studies on statins are required to investigate the
mechanisms by which statins mediate PTN expression. Our study
confirms that statins reduce PTN expression in HPMCs induced by
HGPDS.
In conclusion, the present study demonstrates that
PTN expression induced by HGPDS consequently alters the expression
of VEGF in HPMCs. This upregulation in PTN expression induced by
HGPDS provides further support for the role of PTN in peritoneal
permeability and fibrosis in continuous ambulatory peritoneal
dialysis (CAPD). Our findings provide additional insight into the
mechanisms involved in the induction of the expression of PTN and
VEGF in HPMCs by HGPDS and the prevention of ultrafiltration
failure in CAPD, although the exact pathophysiological effects of
the long-term exposure of HPMCs to glucose degradation products
remain to be elucidated.
Acknowledgements
The present study was funded by the Priority
Academic Program Development (PAPD) of Jiangsu Higher Education
Institutions.
References
|
1
|
He Z, Potter R, Li X and Flessner M:
Stretch of human mesothelial cells increases cytokine expression.
Adv Perit Dial. 28:2–9. 2012.PubMed/NCBI
|
|
2
|
Loureiro J, Aguilera A, Selgas R, et al:
Blocking TGF-β1 protects the peritoneal membrane from
dialysate-induced damage. J Am Soc Nephrol. 22:1682–1695. 2011.
|
|
3
|
Mizutani M, Ito Y, Mizuno M, et al:
Connective tissue growth factor (CTGF/CCN2) is increased in
peritoneal dialysis patients with high peritoneal solute transport
rate. Am J Physiol Renal Physiol. 298:F721–F733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yokoi H, Kasahara M, Mori K, et al:
Peritoneal fibrosis and high transport are induced in mildly
pre-injured peritoneum by 3,4-didexoxyglucosone-3-ene in mice.
Perit Dial Int. 33:143–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heimbürger O, Waniewski J, Werynski A, et
al: Peritoneal transport in CAPD patients with permanent loss of
ultrafiltration capacity. Kidney Int. 38:495–506. 1990.
|
|
6
|
Davies SJ, Phillips L, Naish PF and
Russell GI: Peritoneal glucose exposure and changes in membrane
solute transport with time on peritoneal dialysis. J Am Soc
Nephrol. 12:1046–1051. 2001.PubMed/NCBI
|
|
7
|
Plum J, Hermann S, Fussholler A, et al:
Peritoneal sclerosis in peritoneal dialysis patients related to
dialysis settings and peritoneal transport properties. Kidney Int
Suppl. 78:S42–S47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zweers MM, de Waart DR, Smit W, et al:
Growth factors VEGF and TGF-beta1 in peritoneal dialysis. J Lab
Clin Med. 134:124–132. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zweers MM, Struijk DG, Smit W and Krediet
RT: Vascular endothelial growth factor in peritoneal dialysis: a
longitudinal follow-up. J Lab Clin Med. 137:125–132. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milner PG, Li YS, Hoffman RM, et al: A
novel 17 kD heparin-binding growth factor (HBGF-8) in bovine
uterus: purification and N-terminal amino acid sequence. Biochem
Biophys Res Commun. 165:1096–1103. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rauvala H: An 18-kd heparin-binding
protein of developing brain that is distinct from fibroblast growth
factors. EMBO J. 8:2933–2941. 1989.PubMed/NCBI
|
|
12
|
Deuel TF, Zhang N, Yeh HJ, et al:
Pleiotrophin: a cytokine with diverse functions and a novel
signaling pathway. Arch Biochem Biophys. 397:162–171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kohashi T, Tateaki Y, Tateno C, et al:
Expression of pleiotrophin in hepatic nonparenchymal cells and
preneoplastic nodules in carbon tetrachloride-induced fibrotic rat
liver. Growth Factors. 20:53–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henger A, Kretzler M, Doran P, et al: Gene
expression fingerprints in human tubulointerstitial inflammation
and fibrosis as prognostic markers of disease progression. Kidney
Int. 65:904–917. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yokoi H, Kasahara M, Mori K, et al:
Pleiotrophin triggers inflammation and increased peritoneal
permeability leading to peritoneal fibrosis. Kidney Int.
81:160–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jorres A and Witowski J: PD membrane:
biological responses to different PD fluids. Contrib Nephrol.
150:48–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vrtovsnik F, Coester AM, Lopes-Barreto D,
et al: Induction of chronic kidney failure in a long-term
peritoneal exposure model in the rat: effects on functional and
structural peritoneal alterations. Perit Dial Int. 30:558–569.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Vriese AS, Mortier S and Lameire NH:
Glucotoxicity of the peritoneal membrane: the case for VEGF.
Nephrol Dial Transplant. 16:2299–2302. 2001.PubMed/NCBI
|
|
19
|
Szeto CC, Wong TY, Lai KB, et al: The role
of vascular endothelial growth factor in peritoneal
hyperpermeability during CAPD-related peritonitis. Perit Dial Int.
22:265–267. 2002.PubMed/NCBI
|
|
20
|
Kong Y, Bai PS, Nan KJ, et al:
Pleiotrophin is a potential colorectal cancer prognostic factor
that promotes VEGF expression and induces angiogenesis in
colorectal cancer. Int J Colorectal Dis. 27:287–298. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saad S, Stevens VA, Wassef L, et al: High
glucose transactivates the EGF receptor and up-regulates serum
glucocorticoid kinase in the proximal tubule. Kidney Int.
68:985–997. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waldegger S, Barth P, Raber G and Lang F:
Cloning and characterization of a putative human serine/threonine
protein kinase transcriptionally modified during anisotonic and
isotonic alterations of cell volume. Proc Natl Acad Sci USA.
94:4440–4445. 1997.
|
|
23
|
Firestone GL, Giampaolo JR and O’Keeffe
BA: Stimulus-dependent regulation of serum and glucocorticoid
inducible protein kinase (SGK) transcription, subcellular
localization and enzymatic activity. Cell Physiol Biochem. 13:1–12.
2003. View Article : Google Scholar
|
|
24
|
Lang F and Cohen P: Regulation and
physiological roles of serum- and glucocorticoid-induced protein
kinase isoforms. Sci STKE. 2001:re172001.PubMed/NCBI
|
|
25
|
Waerntges S, Klingel K, Weigert C, et al:
Excessive transcription of the human serum- and glucocorticoid
dependent kinase hSGK1 in lung fibrosis. Cell Physiol Biochem.
12:135–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fillon S, Klingel K, Warntges S, et al:
Expression of the serine/threonine kinase hSGK1 in chronic viral
hepatitis. Cell Physiol Biochem. 12:47–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roskoski R Jr: ERK1/2 MAP kinases:
structure, function, and regulation. Pharmacol Res. 66:105–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu P, Ren M, Yang C, et al: Involvement
of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation
in HaCaT keratinocytes. Exp Dermatol. 21:123–129. 2012.
|
|
29
|
Vasan RS, Sullivan LM, Roubenoff R, et al:
Inflammatory markers and the risk of heart failure in elderly
subjects without prior myocardial infarction: the Framingham heart
study. Circulation. 107:1486–1491. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yokota T, Utsunomiya K, Murakawa Y, et al:
Mechanism of preventive effect of HMG-CoA reductase inhibitor on
diabetic nephropathy. Kidney Int Suppl. 71:S178–S181. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayashi M, Tapping RI, Chao TH, et al:
BMK1 mediates growth factor-induced cell proliferation through
direct cellular activation of serum and glucocorticoid-inducible
kinase. J Biol Chem. 276:8631–8634. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mizuno H and Nishida E: The ERK MAP kinase
pathway mediates induction of SGK (serum- and
glucocorticoid-inducible kinase) by growth factors. Genes Cells.
6:261–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng F, Yamagiwa Y, Taffetani S, et al:
IL-6 activates serum and glucocorticoid kinase via p38alpha
mitogen-activated protein kinase pathway. Am J Physiol Cell
Physiol. 289:C971–C981. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shelly C and Herrera R: Activation of SGK1
by HGF, Rac1 and integrin-mediated cell adhesion in MDCK cells:
PI-3K-dependent and -independent pathways. J Cell Sci.
115:1985–1993. 2002.PubMed/NCBI
|
|
35
|
Prasad N, Topping RS, Zhou D and Decker
SJ: Oxidative stress and vanadate induce tyrosine phosphorylation
of phosphoinositide-dependent kinase 1(PDK1). Biochemistry.
39:6929–6935. 2000. View Article : Google Scholar : PubMed/NCBI
|