1. Introduction
Bone sarcomas
Sarcomas are a group of relatively rare
mesenchymal-derived tumors which account for <1% of all human
malignancies (1,2). They can be divided into three
categories, including intermediate tumors, malignant round-cell
tumors and malignant non-round-cell tumors based on differences in
their biological behavior and treatment (1). Osteosarcoma (OS), chondrosarcoma and
Ewing's sarcoma are the three most common tumor types in bone
sarcomas.
OS is the most frequent primary malignant bone
tumor, mainly occurring in children and adolescents (3). It is often localized to the distal
femur and proximal tibia regions with a high propensity for lung
metastasis, which is the leading cause of mortality and is detected
in 13–27% of patients with OS at diagnosis and in 40% of patients
at the developmental stage (4–6).
Despite advances in adjuvant chemotherapy and surgical-wide
resection, the five-year survival rate for patients with OS without
and with metastases is 60–65% and 20–29%, respectively (6).
Chondrosarcoma is the second most common primary
malignant bone tumor which predominantly occurs in adults over 40
years of age (7,8). Due to its poor response to both
chemotherapy and radiotherapy, surgical resection remains an
effective treatment for chondrosarcoma at present (9,10).
This mesenchymal malignancy has a poor prognosis with local
recurrence and the five-year survival rate being 24–33% and 64–77%,
respectively (11,12). The predilection sites of
chondrosarcomas are the pelvis and femur; nevertheless, the
majority of chondrosarcomas grow slowly. Although metastasis is
infrequent, the lungs are still the most common metastatic site in
chondrosarcomas (13–15).
Ewing's sarcoma, an aggressive round-cell sarcoma,
mostly occurs in children and young adults, and is characterized by
a high metastatic potential and unfavorable prognosis (16–18). The lungs and bone are the most
common target organs. Approximately 25% of patients with Ewing's
sarcoma suffer from metastatic disease at diagnosis, which is
usually associated with a fatal outcome (16,17).
Chemokines and their receptors
Chemokines are a superfamily of 8–12-kDa
chemoattractive cytokines constitutively secreted by stromal cells,
including fibroblasts and endothelial cells (19,20). At present, >50 chemokines have
been identified and they can be divided into four groups (C, CC,
CXC and CX3C) based on the number and position of conserved
cysteines, where C represents the number of cysteine residues and X
denotes the number of intervening amino acids between the conserved
cysteines (21–23). Chemokines were initially
discovered as essential mediators in the process of the directional
migration of leukocytes to the infection and inflammation sites
(24) and have been increasingly
demonstrated to regulate tumor development and metastasis (25).
Chemokine receptors are G protein-coupled
seven-transmemberane cell surface receptors to which their ligands
bind with high affinity. To date, at least 20 chemokine receptors
have been confirmed (22) and
these receptors can also be classified into four subtypes [CXC
chemokine receptors (CXCRs), CC chemokine receptors (CCRs), XCR and
CX3CR] on the basis of their specific preference for some
chemokines (26). Chemokine
receptors were originally identified on leukocytes, where they have
been proven to play a crucial role in inflammation (27). Not only can the same chemokines
bind to different receptors, but more than one chemokine is able to
bind to the same receptor to a certain extent (19). However, certain chemokines only
interact with a single receptor (22). The binding of chemokines to their
receptors stimulates the activation of several downstream signaling
pathways that regulate tumor progression and metastasis (21).
CXCL12
Chemokine 12 (CXCL12), also designated as stromal
cell-derived factor-1 (SDF-1), secreted by stromal cells including
fibroblasts and endothelial cells as mentioned above is a member of
the CXC subfamily of chemokines. It is widely expressed in a number
of organs, such as the lungs, liver, skeletal muscle, brain,
kidneys, heart, skin and bone marrow (19). Its primary role is in the homing
of hematopoietic stem cells to bone marrow (28). The involvement of CXCL12 in the
metastasis of various types of cancer has also been previously
demonstrated (20). Increasing
evidence indicates that CXCL12 can promote proliferation and
survival in ovarian cancer (29),
prostate cancer (30), breast
cancer (20,31,32), glioma (33) and glioblastoma (34). However, it has been reported that
there are minimal or negligible effects on the survival and growth
of myeloma in the presence of CXCL12 in vitro (35). On the one hand, the expression of
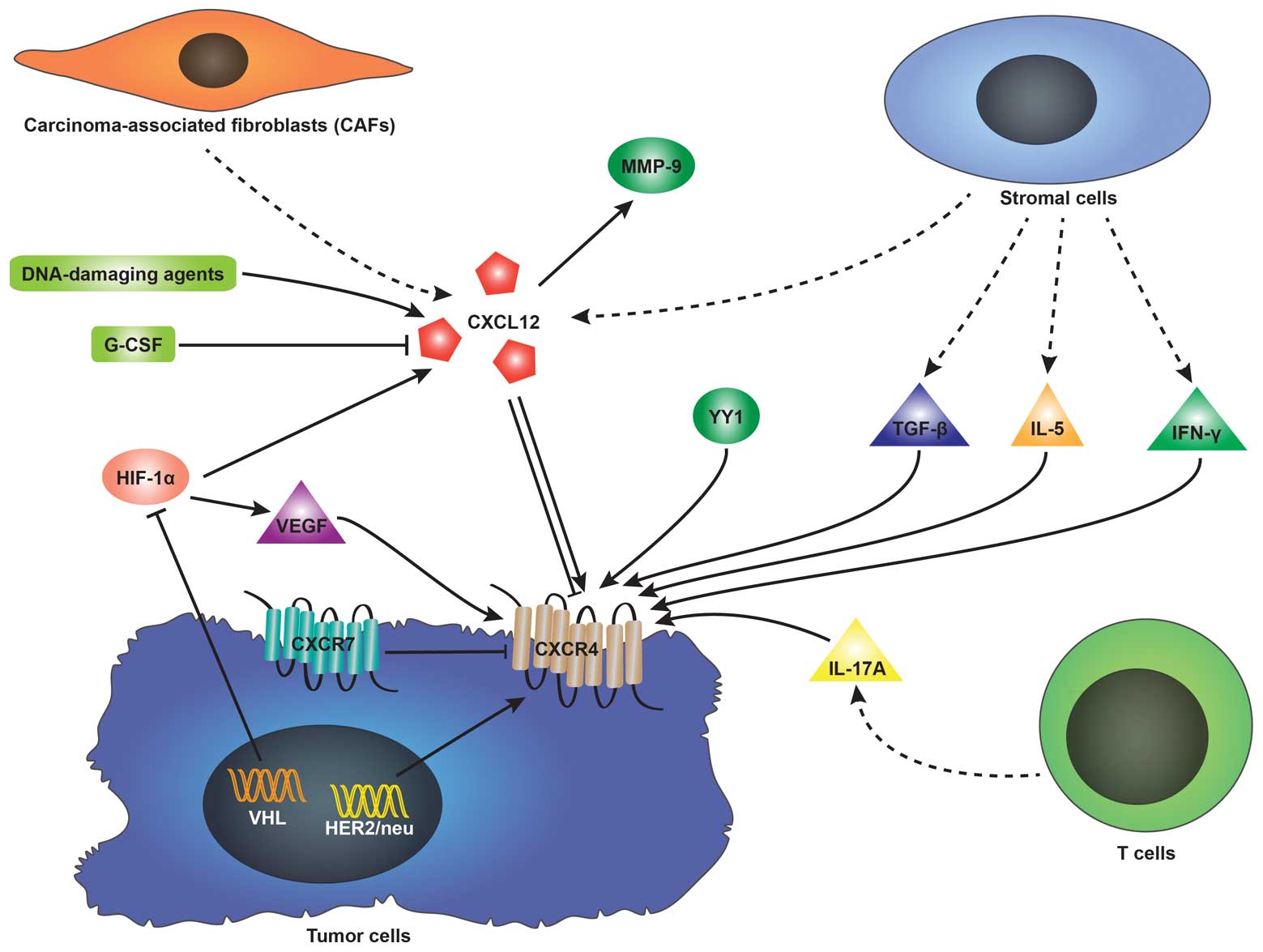
CXCL12 can be affected by a number of factors. It has been reported
that DNA-damaging agents, such as irradiation, cyclophosphamide, or
5-fluorouracil upregulate CXCL12 expression in mouse marrow and
cultured cells (36).
Hypoxia-inducible factor-1α (HIF-1α) induces CXCL12 expression in
hypoxic or damaged tissues (37).
Besides, it is carcinoma-associated fibroblasts (CAFs) rather than
normal fibroblasts that elevate CXCL12 expression (32,38). Nevertheless, CXCL12 expression is
reduced by granulocyte colony-stimulating factor (G-CSF) in the
process of inducing hematopoietic stem cell mobilization (39). On the other hand, CXCL12 can
stimulate the secretion of other factors. It has previously been
demonstrated that matrix metalloproteinase-9 (MMP-9) expression is
upregulated in the presence of CXCL12 when investigating the
involvement of the CXCL12-CXCR4 axis in the metastasis of prostate
cancer and OS (40,41).
CXCR4
Chemokine receptor 4 (CXCR4), initially discovered
as co-receptor facilitating the entry of T-tropic (X4) HIV viruses
into CD4+ T cells, is the cognate receptor of CXCL12
(42,43). It has been found that CXCR4 is
expressed in a wide range of tissues, including brain, lymph node
and small intestine tissues (21), as well as in monocytes, B cells,
naïve T cells and early hematopoietic progenitor cells in the
immune system (22). It should be
noted that the overexpression of CXCR4 can be detected in no less
than 23 different types of human cancer (44). Tumor cells expressing CXCR4 are
more likely to migrate to organs with an abundant source of CXCL12
(19). Similar to CXCL12, the
expression of CXCR4 is regulated by a number of factors, among
which HIF-1α is the most frequently mentioned. Under hypoxic
conditions, the von Hippel-Lindau (VHL) tumor suppressor gene,
which induces the degradation of HIF-1 is inactivated (26). Therefore, elevated levels of HIF-1
stimulate CXCR4 expression via the VHL-HIF-1 pathway in renal cell
carcinoma (RCC) (45,46) and non-small cell lung cancer
(NSCLC) (47). The vascular
endothelial growth factor (VEGF) regulated by HIF-1 can also induce
CXCR4 expression in breast cancer cells (48) and glioblastoma (49). It has been confirmed that human
epidermal growth factor receptor 2 (HER2)/neu detected in
approximately 30% of breast cancers elevates the expression of
CXCR4 by inhibiting its degradation (50). Additionally, transforming growth
factor-β (TGF-β) (51),
interleukin-5 (IL-5) and interferon-γ (IFN-γ) (52) released by stromal cells and
interleukin-17A (IL-17A) (53)
secreted by T cells induce the expression of CXCR4. Of note,
certain studies have reported that CXCL12 itself can alter CXCR4
expression in tumor cells. The increasing or reducing effect of
CXCL12 on CXCR4 expression largely depends on the type of tumor.
The surface expression of CXCR4 in oral squamous cell carcinoma
(54) and OS cells (55) has been shown to be induced by
CXCL12. However, Perissinotto et al (41) pointed out that CXCL12
downregulated CXCR4 expression in an OS cell line (SJSA) due to
CXCR4 internalization induced by the increase in intracellular
CXCR4 expression (Fig. 1).
CXCR7
CXCR4 has long been considered as the only receptor
which binds to CXCL12 and regulates the biological effects induced
by the CXCL12-CXCR4 pathway. However, this theory was challenged by
the fact that chemokine receptor 7 (CXCR7; (RDC-1) was identified
in 2005 as a novel decoy receptor of CXCL12 participating in
CXCL12-CXCR4 signaling and can bind to CXCL11 (I-TAC) with low
affinity (56,57). Similar to CXCR4, the elevated
CXCR7 expression can be detected in a number of tumors and plays an
important role in promoting growth and metastasis in tumor models
in vivo by regulating neoangiogenesis and organ-specific
metastasis (22,58–60). As opposed to the conclusions drawn
by the majority of reports that CXCR7 is a positive regulator in
the proliferation/metastasis-enhancing effects on tumors induced by
the CXCL12-CXCR4 interaction, it was revealed by Liberman et
al (24) that CXCR7 eliciting
anti-tumorigenic functions significantly reduced the
CXCL12-CXCR4-mediated growth of CXCR7-expressing neuroblastoma
cells in vitro and in vivo. Of note, in contrast to
CXCR4, CXCR7 expression did not correlate with neuroblastoma grades
but with tumor differentiation in their study. These results are
consistent with another report that CXCR7 acts as a negative
regulator of CXCR4 and abolishes the function of CXCL12 (61).
2. Downstream pathways involved in the
CXCL12-CXCR4/CXCR7 interaction
It is already accepted that the binding of CXCL12 to
CXCR4 or CXCR7 leads to the activation of several downstream
pathways that regulate cell chemotaxis, survival, proliferation and
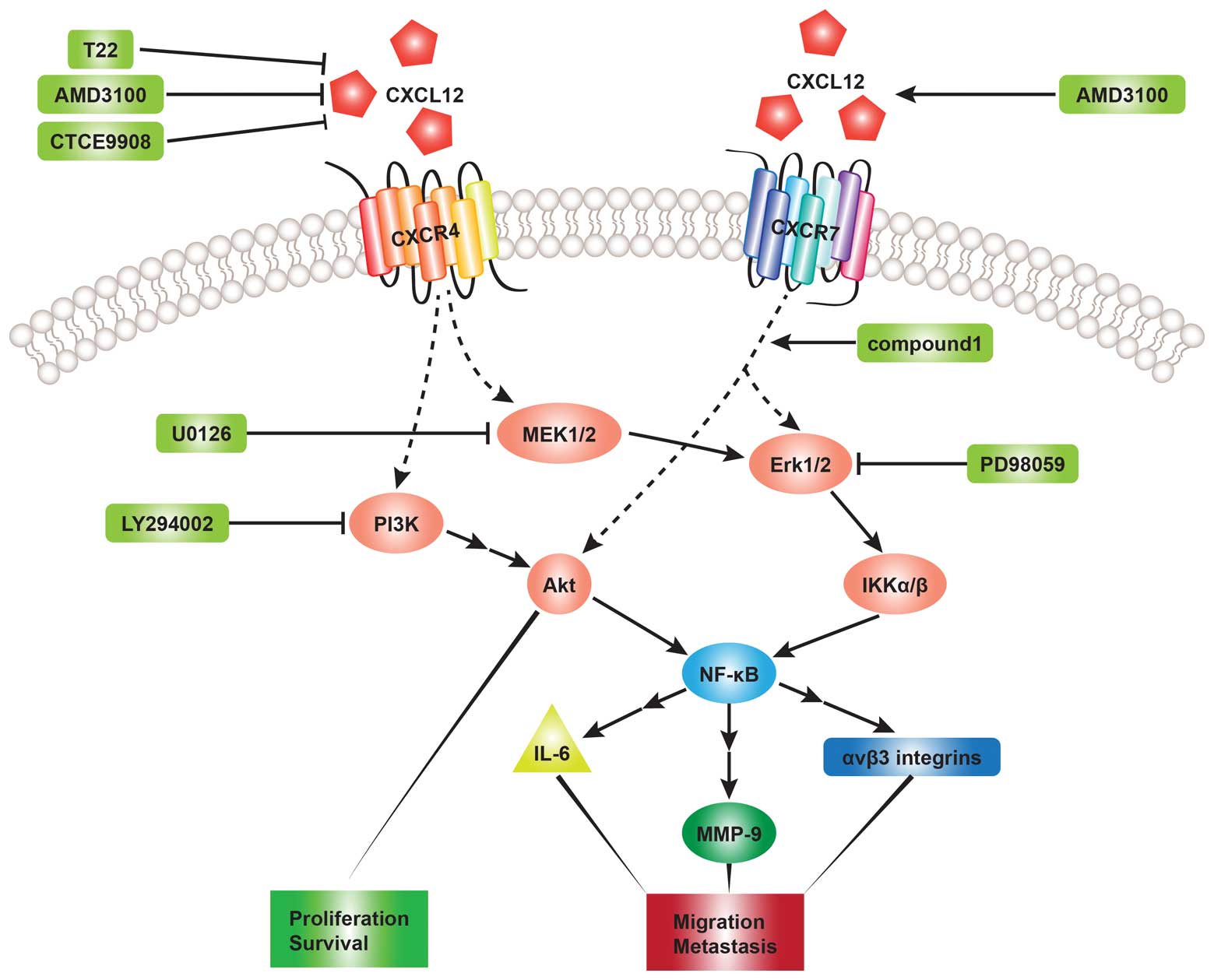
migration (19,62,63). The phosphoinositide 3-kinase
(PI3K) and mitogen-activated protein kinase (MAPK) pathways are
most frequently investigated in related studies. The PI3K and MAPK
pathways have been found to play a key role in tumor cell survival
and migration (34,40). PI3K activation can result in the
phosphorylation of Akt, which induces the activation of nuclear
factor-κB (NF-κB) transcription factors (19,64,65). MAPK pathways, including
extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal
kinase (JNK) and p38 can also stimulate the expression of NF-κB
transcription factors (66).
Chinni et al (40)
demonstrated that CXCL12 induced MMP-9 expression regulated by
NF-κB activity in prostate cancer cells by activating the
PI3K-Akt-NF-κB and MEK pathways and that pre-treatment with
LY294002 (PI3K inhibitor) and U0126 (MEK inhibitor) abolished the
effects induced by MMP-9 through the CXCL12-CXCR4 interaction in
PC-3 cells. They also suggested that PI3K may be upstream of the
MAPK-MEK pathway based on the result that the expression level of
MMP-9 regulated by PI3K activity was significantly higher than MAPK
activity. Consistent with their conclusions, Leelawat et al
(67) indicated that the binding
of CXCL12 to CXCR4 induced cholangiocarcinoma cell invasion by
triggering the ERK1/2 and PI3K signaling pathways. The stimulation
of ERK1/2/IκB kinase αβ (IKKαβ) and NF-κB mediated by the
CXCL12-CXCR4 interaction has been shown to lead to the upregulation
of interleukin-6 (IL-6), promoting osteoclastogenesis in human oral
cancer cells (54). Huang et
al (55) pointed out that the
activation of the MEK-ERK-IKKαβ-NF-κB pathway is involved in the
CXCL12-induced migration of human OS cells and the increased
expression of ανβ3 integrins, which has been found to play an
important role in human cancer migration and metastasis (68,69). Apart from the Akt and ERK
transduction pathways, p38 is also involved in the process of
CXCL12-mediated migration of human umbilical cord blood-mesenchymal
stem cells (hUCB-MSCs) (70).
This migration induced by CXCL12 was abrogated by LY294002 (PI3K
inhibitor), PD98059 (MAPK/ERK inhibitor) and SB203580 (p38
inhibitor) (70). However, much
less is known about the signaling pathways regulated by the
CXCL12-CXCR7 interaction. Heinrich et al (71) observed increased ERK1/2
phosphorylation in pancreatic cancer cells expressing CXCR4 and
CXCR7 following exposure to CXCL12. To further elucidate the role
of CXCR7 in CXCL12-induced pathways, the CXCR7 knockdown in PC
cells resulted in a markedly decreased ERK phosphorylation.
Therefore, they suggested that CXCR7 mediates ERK phosphorylation
in the presence of CXCL12. Another study (61) revealed that CXCR7 agonist compound
1 activated both Akt and ERK phosphorylation (Fig. 2).
3. Expression of CXCR4 in bone sarcomas
As described previously, it has been confirmed that
higher levels of CXCR4 expression can be detected in a various
types of human cancer compared with normal counterparts. To our
knowledge, CXCR4 expression can be evaluated by real-time PCR,
western blot analysis and flow cytometry in tumor cells, and
immunohistochemical staining and tissue microarray in tumor
tissues. Over the past decade, a number of studies have focused on
elucidating whether CXCR4 is expressed in bone sarcomas and whether
its expression level correlates with metastasis and the survival of
patients with bone sarcomas. It was first reported by Laverdiere
et al (72) that the mRNA
expression of CXCR4 was detected in 63% of OS samples, but was
detected at low levels in the cell lines by fluorescent
quantitative real-time PCR. Consistent with their findings, Lin
et al (73) and Baumhoer
et al (74) discovered
that 69.6% (39/56) and 73% (159/219) of OS samples expressed CXCR4
by tissue microarray and immunohistochemistry, respectively.
However, Perissinotto et al (41) detected CXCR4 expression in four
human OS cell lines (SJSA, MG-63, HOS and U2OS) among which SJSA
cells were found to express the highest levels by flow cytometry
and western blot analysis. They indicated that CXCR4 expression
levels in the cells was affected by culture conditions.
Specifically, CXCR4 expression in confluent cells is lower than
that in growing cells (41). Fan
et al (75) first reported
that five canine OS cells (POS, HMPOS, COS31, HOS and D17)
expressed CXCR4 mRNA and protein. The comparison of different CXCR4
expression levels between primary and metastatic tumors was
originally made by Oda et al (76), showing that approximately 66.6%
(20/30) of metastatic tumors were positive for CXCR4 compared with
only 33.3% (10/30) of primary ones. Based on these results, they
suggested that CXCR4 expression may be associated with the
metastatic progression of OS. This conclusion was supported by the
results obtained in the study by Lin et al (73), namely that the percentage of
CXCR4-positive samples in metastatic tumors was approximately 83.9%
(26/31), whereas it was 52% (13/25) in primary tumors. The positive
correlation between CXCR4 expression and metastasis was further
verified by Namløs et al (6). When making the comparison between
primary tumors and metastatic ones, they observed a significantly
increased CXCR4 gene expression in the metastatic samples. Of note,
they demonstrated that primary samples that developed metastases
later showed a higher CXCR4 expression than those that did not
metastasize. Their study was the first to evaluate the different
capabilities of primary OS samples to metastasize by detecting
their CXCR4 expression. However, Fan et al (75) observed that 8/11 canine primary OS
tumors expressed CXCR4 compared with only 2/8 in pulmonary
metastases. The reason why the expression level of CXCR4 in
metastatic tumors was lower than primary tumors may be explained by
one possibility that CXCR4 was involved in the initial step of
mediating CXCR4-positive tumor cells to metastasize to remote
organs, rather than the whole process and that tumor cells may
reduce its expression once reaching their target organs (75). Ma et al (77) also demonstrated that there was no
evidence to show the positive correlation between CXCR4 and
metastasis by observing that CXCR4 was expressed in 48/51
non-metastatic and 9/12 metastatic OS samples (Table I). In addition to OS, CXCR4
expression has also been detected in chondrosarcoma (22/22)
(78) and Ewing's sarcoma (28/44
in therapy-naïve and 7/15 in metastatic tumors) (16).
 | Table IExpression of chemokine receptor 4
(CXCR4) in osteosarcoma non-metastatic and metastatic samples. |
Table I
Expression of chemokine receptor 4
(CXCR4) in osteosarcoma non-metastatic and metastatic samples.
| CXCR4-negative | CXCR4-positive | CXCR4-positive
(%) |
|---|
|
|
|
|
|---|
|
Authors/(Refs.) | No metastasis | Metastasis | No metastasis | Metastasis | No metastasis | Metastasis | Total |
|---|
| Oda et al
(76) | 20 | 10 | 10 | 20 | 33.30 | 66.70 | 50.00 |
| Fan et al
(75) | 3 | 6 | 8 | 2 | 72.70 | 25.00 | 52.60 |
| Lin et al
(73) | 12 | 5 | 13 | 26 | 52.00 | 83.90 | 69.60 |
| Baumhoer et
al (74) | 41 | 33 | 42 | 29 | 50.60 | 46.80 | 49.00 |
| Namløs et al
(6) | 3 | 2 | 1 | 16 | 25.00 | 88.90 | 77.30 |
| Ma et al
(77) | 3 | 3 | 48 | 9 | 94.12 | 75.00 | 90.48 |
4. Correlation between CXCR4/CXCR7
expression and the survival of patients with bone sarcomas
A number of previous studies have demonstrated that
CXCR4 and CXCR7 expression is associated with the poor survival of
patients with bone sarcomas (1,16,72,73,78,79). Lin et al (73) made a comparison between the
two-year survival rate of patients with OS expressing CXCR4 and
those not expressing CXCR4. They found that the two-year survival
rate of CXCR4-positive patients (32.4%) was significantly lower
than that of CXCR4-negative ones (78.9%). In Ewing's sarcoma,
similar results were observed by Bennani-Baiti et al
(79), namely that the five-year
survival rate for patients with Ewing's sarcoma expressing low
levels of CXCR4 and CXCR7 was 90%, whereas for those with high
CXCR4 and CXCR7 expression, the survival rate was 54.5 and 45.4%,
respectively. Bai et al (78) also demonstrated that CXCR4
expression levels correlated with the chondrosarcoma grade. Given
the negative effects of CXCR4 expression on survival, it has been
suggested that CXCR4 may be used as a potential prognostic factor
in patients with bone sarcomas (1,72,73). Clark et al (80) indicated that compared with
traditional prognostic factors, such as metastases and response to
chemotherapy usually used during the late stages of disease, CXCR4
as a novel molecular prognostic indicator may facilitate earlier
diagnosis and treatment. However, certain studies have demonstrated
that there was no significant correlation between CXCR4 and CXCR7
expression and survival (74,76). Baumhoer et al (74) suggested that the ten-year survival
rate for CXCR4-positive and -negative patients with OS was 68 and
57%, respectively; for CXCR7-postive and -negative patients it was
57 and 61%, respectively.
5. Involvement of the CXCL12-CXCR4/CXCR7
axis in the growth and metastasis of bone sarcomas
The majority of studies have elucidated the role of
the CXCL12-CXCR4 axis in the metastasis of a number of types of
carcinoma, including bone sarcoma, as previously mentioned. The
disruption of the CXCL12-CXCR4 interaction by CTCE-9908, a small
peptide CXCR4 antagonist, has been shown to lead to a decrease in
the metastatic potential of OS K7M2 cells in vitro and in
vivo (81). The
downregulation of the expression of Yin Yang 1 (YY1) protein, which
strongly correlates with the malignant degree of bone tumors
induced by human SaOs-2 OS cells by small interfering RNA (siRNA),
has been shown to reduce CXCR4-mediated migration in vitro
(82). On the basis of the
results that mice implanted with YY1-silenced SaOs-2 cells produced
fewer vessels in vivo than those implanted with SaOs-2 cells
and injected with T22 peptide, a CXCR4 inhibitor, reduced the newly
formed vessels in SaOs-2-bearing mice, whereas it was ineffective
in decreasing vessel formation in YY1-silenced SaOs-2-bearing mice,
de Nigris et al (82)
suggested that YY1 was a positive regulator of both angiogenesis
and CXCR4 signal transduction. In addition, they found that 9/10
SaOs-2-bearing mice developed metastases compared with only 4/10
YY1-silenced SaOs-2-bearing mice. The treatment of OS cells with
CXCL12 induced migration by stimulating the MEK-ERK-IKKα/β-NF-κB
pathway, which was inhibited by CXCR4-neutralizing antibody,
CXCR4-specific inhibitor (AMD3100) and siRNA against CXCR4
(55). As regards tumor growth
and progression mediated by the CXCL12-CXCR4/CXCR7 axis, only a few
studies mention it. Miura et al (83) revealed that the ability to form
tumors in vivo positively correlated with the levels of
CXCR4 expression in human HOS OS cells. They determined the effect
of CXCR4 expression on HOS tumor growth by injecting intradermally
different HOS transfectant cells expressing CXCR4 at low,
intermediate and high levels into one flank of mice and control HOS
cells into the other flank of each mouse. The growth of tumors
injected with cells expressing low levels of CXCR4was greater than
the control cells eight and nine days after transplantation. The
tumor volume derived from the cells expressing intermediate levels
of CXCR4 was larger than the one derived from cells expressing low
levels of CXCR4 ten and 11 days post-transplantation. The growth of
cells expressing high levels of CXCR4 was significantly greater
than any other group 12 and 13 days after injection. Apart from OS,
Berghuis et al (16)
indicated that the activation of the CXCL12-CXCR4 interaction
induced the growth, rather than the metastasis of Ewing's sarcoma
cells.
6. Therapies targeting the
CXCL12-CXCR4/CXCR7 axis in preclinical studies
The CXCL12-CXCR4/CXCR7 axis is a potential target in
interference, resulting in the inhibition of downstream signaling,
which regulates tumor growth, survival and metastases. To our
knowledge, chemokine receptor-specific antagonists, neutralizing
antibodies and siRNA are the three most common methods widely
utilized in related studies.
The CXCR4 antagonist, AMD3100, a small bicyclam
molecule, was initially used to prevent X4-Tropic HIV-1 viruses
entering CD4+ T cells via CXCR4 (84). De Clercq (85) suggested that the effective
concentration range of AMD3100 used to inhibit HIV was 1–10 nM and
it was not toxic to the host cells even when AMD3100 was used at
concentration of up to 500 μM. Its safety and efficiency in
stimulating hematopoeitic stem cell mobilization in patients with
multiple myeloma and lymphoma has been demonstrated in clinical
trials (86,87). Due to the role of CXCR4 in tumor
growth and/or metastasis, a number of studies have reported that
the treatment of tumor cells with AMD3100 reduces the proliferation
and migration induced by CXCL12 in vitro (16,55). Berghuis et al (16) found that the
proliferation-increasing effect of CXCL12 on CXCR4-positive Ewing's
sarcoma cells in the absence of serum was abrogated by AMD3100.
Treatment of human OS cells with AMD3100 also inhibited the
CXCL12-induced migration, as indicated by Huang et al
(55). However, when exploring
the effects of AMD3100 on survival and proliferation of two myeloma
cell lines, Kim et al (88) for the first time observed that
AMD3100 at a high concentration initially promoted the
proliferation of myeloma cells under serum-deprived conditions for
up to five days and subsequently inhibited its proliferation by
blocking the binding of CXCL12 to CXCR4 in vitro. Of note,
Kalatskaya et al (89)
indicated that AMD3100 bound to CXCR7, as well as CXCR4, although
with opposite effects, indicating that AMD3100 is an allosteric
agonist of CXCR7.
siRNA is capable of inducing the constitutive
inhibition of CXCR4 expression which facilitates a more precise
assessment of the involvement of CXCR4 in tumor growth and
metastasis (90). To determine
the effects of CXCR4 knockdown by siRNA on tumor growth and
metastasis in vivo, Lapteva et al (90) injected CXCR4-negative MDA-MB-231
breast cancer cells (in which CXCR4 expression was downregulated
using siRNA) and CXCR4-positive MDA-MB-231 breast cancer cells into
mammary fat pads of mice. They found that none of the mice
implanted with CXCR4-negative cells developed tumors for up to 45
days, whereas all the mice inoculated with CXCR4-positive cells
developed tumors within three weeks. These results are consistent
with those of a previous study by Smith et al (31), indicating that the reduction of
CXCR4 expression in murine 4T1 breast cancer cells by siRNA delayed
tumor growth in mice. They also demonstrated that AMD3100 was less
effective than siRNA in delaying tumor growth in vivo by
blocking CXCR4 signaling; this was due to the variable antagonism
of CXCR4 produced by the rapid decrease in plasma levels of the
compound after dosing compared with the persistent inhibition of
CXCR4 expression by siRNA (31).
7. Conclusion
Bone sarcomas primarily including chondrosarcomas
and Ewing's sarcomas are a group of relatively rare
mesenchymal-derived tumors. Despite their low percentage in all
human malignancies and advances in adjuvant chemotherapy and
surgical-wide resection, prognosis remains poor, mainly due to the
high propensity for lung metastasis, which is the leading cause of
mortality in patients with bone sarcomas. It has been demonstrated
that the CXCL12-CXCR4/CXCR7 pathway plays a pivotal role in several
biological processes. CXCL12 and CXCR4 expression can be affected
by a number of factors and the binding of CXCL12 to CXCR4/CXCR7
stimulates the activation of several downstream signaling pathways
that regulate tumor progression and metastasis. The expression of
CXCR4 is detected in bone sarcomas and is associated with the
metastasis and survival of patients suffering from this type of
malignancy. Therefore, CXCR4 may be used as a potential prognostic
factor to facilitate earlier diagnosis and treatment in patients
with bone sarcomas. CXCR7, the second CXCL12 receptor, may serve as
a negative regulator of CXCR4 and plays an opposite role in
CXCL12-CXCR4 interaction. The disruption of the CXCL12-CXCR4
pathway by AMD3100 and siRNA abrogates the CXCL12-induced
proliferation and/or metastasis of bone sarcoma cells. It is
anticipated that the targeting of the CXCL12-CXCR4/CXCR7 pathway
may be utilized as a promising therapeutic strategy in the near
future.
Abbreviations:
|
CXCL12
|
chemokine 12
|
|
SDF-1
|
stromal cell-derived factor-1
|
|
CXCR4
|
chemokine receptor 4
|
|
CXCR7
|
chemokine receptor 7
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
OS
|
osteosarcoma
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
CAFs
|
carcinoma-associated fibroblasts
|
|
G-CSF
|
granulocyte colony-stimulating
factor
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
VHL
|
von Hippel-Lindau
|
|
RCC
|
renal cell carcinoma
|
|
NSCLC
|
non- small cell lung cancer
|
|
VEGF
|
vascular endothelial growth factor
|
|
TGF-β
|
transforming growth factor-β
|
|
IL-5
|
interleukin-5
|
|
IFN-γ
|
interferon-γ
|
|
IL-17A
|
interleukin-17A
|
|
NF-κB
|
nuclear factor-κB
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
IKKαβ
|
IκB kinase αβ
|
|
hUCB-MSCs
|
human umbilical cord blood-mesenchymal
stem cells
|
|
YY1
|
Yin Yang 1
|
References
|
1
|
Oda Y, Tateishi N, Matono H, et al:
Chemokine receptor CXCR4 expression is correlated with VEGF
expression and poor survival in soft-tissue sarcoma. Int J Cancer.
124:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim RH, Li BD and Chu QD: The role of
chemokine receptor CXCR4 in the biologic behavior of human soft
tissue sarcoma. Sarcoma. 2011:5937082011.PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mankin HJ, Hornicek FJ, Rosenberg AE,
Harmon DC and Gebhardt MC: Survival data for 648 patients with
osteosarcoma treated at one institution. Clin Orthop Relat Res.
429:286–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bentzen SM: Prognostic factor studies in
oncology: osteosarcoma as a clinical example. Int J Radiat Oncol
Biol Phys. 49:513–518. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Namløs HM, Kresse SH, Müller CR, et al:
Global gene expression profiling of human osteosarcomas reveals
metastasis-associated chemokine pattern. Sarcoma.
2012:6390382012.PubMed/NCBI
|
|
7
|
Clark JC, Akiyama T, Dass CR and Choong
PF: New clinically relevant, orthotopic mouse models of human
chondrosarcoma with spontaneous metastasis. Cancer Cell Int.
10:202010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hemmati M, Abbaspour A, Alizadeh AM, et
al: Rat xenograft chondrosarcoma development by human tissue
fragment. Exp Oncol. 33:52–54. 2011.PubMed/NCBI
|
|
9
|
Li TM, Lin TY, Hsu SF, et al: The novel
benzimidazole derivative, MPTB, induces cell apoptosis in human
chondrosarcoma cells. Mol Carcinog. 50:791–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergh P, Gunterberg B, Meis-Kindblom JM
and Kindblom LG: Prognostic factors and outcome of pelvic, sacral,
and spinal chondrosarcomas: a center-based study of 69 cases.
Cancer. 91:1201–1212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fiorenza F, Abudu A, Grimer RJ, et al:
Risk factors for survival and local control in chondrosarcoma of
bone. J Bone Joint Surg Br. 84:93–99. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruns J, Elbracht M and Niggemeyer O:
Chondrosarcoma of bone: an oncological and functional follow-up
study. Ann Oncol. 12:859–864. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qureshi A, Ahmad Z, Azam M and Idrees R:
Epidemiological data for common bone sarcomas. Asian Pac J Cancer
Prev. 11:393–395. 2010.PubMed/NCBI
|
|
14
|
Gelderblom H, Hogendoorn PC, Dijkstra SD,
et al: The clinical approach towards chondrosarcoma. Oncologist.
13:320–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozaki T, Hillmann A, Linder N, Blasius S
and Winkelmann W: Metastasis of chondrosarcoma. J Cancer Res Clin
Oncol. 122:625–628. 1996. View Article : Google Scholar
|
|
16
|
Berghuis D, Schilham MW, Santos SJ, et al:
The CXCR4-CXCL12 axis in Ewing sarcoma: promotion of tumor growth
rather than metastatic disease. Clin Sarcoma Res. 2:242012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hauer K, Calzada-Wack J, Steiger K, et al:
DKK2 mediates osteolysis, invasiveness, and metastatic spread in
Ewing sarcoma. Cancer Res. 73:967–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin Z, Zhao C, Han X and Han Y: Wnt5a
promotes ewing sarcoma cell migration through upregulating CXCR4
expression. BMC Cancer. 12:4802012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi
M and Yamamoto N: Stromal cell-derived factor-1 and CXCR4 receptor
interaction in tumor growth and metastasis of breast cancer. Biomed
Pharmacother. 60:273–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Loberg R and Taichman RS: The
pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis.
Cancer Metastasis Rev. 25:573–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun X, Cheng G, Hao M, et al:
CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer
Metastasis Rev. 29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Le Y, Zhou Y, Iribarren P and Wang J:
Chemokines and chemokine receptors: their manifold roles in
homeostasis and disease. Cell Mol Immunol. 1:95–104.
2004.PubMed/NCBI
|
|
24
|
Liberman J, Sartelet H, Flahaut M, et al:
Involvement of the CXCR7/CXCR4/CXCL12 axis in the malignant
progression of human neuroblastoma. PLoS One. 7:e436652012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar
|
|
26
|
Burger JA and Kipps TJ: CXCR4: a key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loetscher P, Moser B and Baggiolini M:
Chemokines and their receptors in lymphocyte traffic and HIV
infection. Adv Immunol. 74:127–180. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aiuti A, Webb IJ, Bleul C, Springer T and
Gutierrez-Ramos JC: The chemokine SDF-1 is a chemoattractant for
human CD34+ hematopoietic progenitor cells and provides
a new mechanism to explain the mobilization of CD34+
progenitors to peripheral blood. J Exp Med. 185:111–120.
1997.PubMed/NCBI
|
|
29
|
Scotton CJ, Wilson JL, Scott K, et al:
Multiple actions of the chemokine CXCL12 on epithelial tumor cells
in human ovarian cancer. Cancer Res. 62:5930–5938. 2002.PubMed/NCBI
|
|
30
|
Sun YX, Wang J, Shelburne CE, et al:
Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers
(PCa) in vivo. J Cell Biochem. 89:462–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith MC, Luker KE, Garbow JR, et al:
CXCR4 regulates growth of both primary and metastatic breast
cancer. Cancer Res. 64:8604–8612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
33
|
Zhou Y, Larsen PH, Hao C and Yong VW:
CXCR4 is a major chemokine receptor on glioma cells and mediates
their survival. J Biol Chem. 277:49481–49487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barbero S, Bonavia R, Bajetto A, et al:
Stromal cell-derived factor 1alpha stimulates human glioblastoma
cell growth through the activation of both extracellular
signal-regulated kinases 1/2 and Akt. Cancer Res. 63:1969–1974.
2003.PubMed/NCBI
|
|
35
|
Hideshima T, Chauhan D, Hayashi T, et al:
The biological sequelae of stromal cell-derived factor-1alpha in
multiple myeloma. Mol Cancer Ther. 1:539–544. 2002.PubMed/NCBI
|
|
36
|
Ponomaryov T, Peled A, Petit I, et al:
Induction of the chemokine stromal-derived factor-1 following DNA
damage improves human stem cell function. J Clin Invest.
106:1331–1339. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ceradini DJ, Kulkarni AR, Callaghan MJ, et
al: Progenitor cell trafficking is regulated by hypoxic gradients
through HIF-1 induction of SDF-1. Nat Med. 10:858–864. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Begley L, Monteleon C, Shah RB, Macdonald
JW and Macoska JA: CXCL12 overexpression and secretion by aging
fibroblasts enhance human prostate epithelial proliferation in
vitro. Aging Cell. 4:291–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Petit I, Szyper-Kravitz M, Nagler A, et
al: G-CSF induces stem cell mobilization by decreasing bone marrow
SDF-1 and up-regulating CXCR4. Nat Immunol. 3:687–694. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chinni SR, Sivalogan S, Dong Z, et al:
CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in
prostate cancer cells: the role of bone microenvironment-associated
CXCL12. Prostate. 66:32–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perissinotto E, Cavalloni G, Leone F, et
al: Involvement of chemokine receptor 4/stromal cell-derived factor
1 system during osteosarcoma tumor progression. Clin Cancer Res.
11:490–497. 2005.PubMed/NCBI
|
|
42
|
Feng Y, Broder CC, Kennedy PE and Berger
EA: HIV-1 entry cofactor: functional cDNA cloning of a
seven-transmembrane, G protein-coupled receptor. Science.
272:872–877. 1996. View Article : Google Scholar
|
|
43
|
Wegner SA, Ehrenberg PK, Chang G, Dayhoff
DE, Sleeker AL and Michael NL: Genomic organization and functional
characterization of the chemokine receptor CXCR4, a major entry
co-receptor for human immunodeficiency virus type 1. J Biol Chem.
273:4754–4760. 1998. View Article : Google Scholar
|
|
44
|
Balkwill F: The significance of cancer
cell expression of the chemokine receptor CXCR4. Semin Cancer Biol.
14:171–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schioppa T, Uranchimeg B, Saccani A, et
al: Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp
Med. 198:1391–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zagzag D, Krishnamachary B, Yee H, et al:
Stromal cell-derived factor-1alpha and CXCR4 expression in
hemangioblastoma and clear cell-renal cell carcinoma: von
Hippel-Lindau loss-of-function induces expression of a ligand and
its receptor. Cancer Res. 65:6178–6188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Phillips RJ, Mestas J, Gharaee-Kermani M,
et al: Epidermal growth factor and hypoxia-induced expression of
CXC chemokine receptor 4 on non-small cell lung cancer cells is
regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian
target of rapamycin signaling pathway and activation of hypoxia
inducible factor-1alpha. J Biol Chem. 280:22473–22481. 2005.
|
|
48
|
Bachelder RE, Wendt MA and Mercurio AM:
Vascular endothelial growth factor promotes breast carcinoma
invasion in an autocrine manner by regulating the chemokine
receptor CXCR4. Cancer Res. 62:7203–7206. 2002.PubMed/NCBI
|
|
49
|
Zagzag D, Lukyanov Y, Lan L, et al:
Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in
glioblastoma: implications for angiogenesis and glioma cell
invasion. Lab Invest. 86:1221–1232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li YM, Pan Y, Wei Y, et al: Upregulation
of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer
Cell. 6:459–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ao M, Franco OE, Park D, Raman D, Williams
K and Hayward SW: Cross-talk between paracrine-acting cytokine and
chemokine pathways promotes malignancy in benign human prostatic
epithelium. Cancer Res. 67:4244–4253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang L, Yeger H, Das B, Irwin MS and
Baruchel S: Tissue microenvironment modulates CXCR4 expression and
tumor metastasis in neuroblastoma. Neoplasia. 9:36–46. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang M, Wang L, Ren T, Xu L and Wen Z:
IL-17A/IL-17RA interaction promoted metastasis of osteosarcoma
cells. Cancer Biol Ther. 14:155–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang CH, Chuang JY, Fong YC, Maa MC, Way
TD and Hung CH: Bone-derived SDF-1 stimulates IL-6 release via
CXCR4, ERK and NF-kappaB pathways and promotes osteoclastogenesis
in human oral cancer cells. Carcinogenesis. 29:1483–1492. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang CY, Lee CY, Chen MY, et al: Stromal
cell-derived factor-1/CXCR4 enhanced motility of human osteosarcoma
cells involves MEK1/2, ERK and NF-kappaB-dependent pathways. J Cell
Physiol. 221:204–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Balabanian K, Lagane B, Infantino S, et
al: The chemokine SDF-1/CXCL12 binds to and signals through the
orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Burns JM, Summers BC, Wang Y, et al: A
novel chemokine receptor for SDF-1 and I-TAC involved in cell
survival, cell adhesion, and tumor development. J Exp Med.
203:2201–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Miao Z, Luker KE, Summers BC, et al: CXCR7
(RDC1) promotes breast and lung tumor growth in vivo and is
expressed on tumor-associated vasculature. Proc Natl Acad Sci USA.
104:15735–15740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang J, Shiozawa Y, Wang Y, et al: The
role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in
prostate cancer. J Biol Chem. 283:4283–4294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kollmar O, Rupertus K, Scheuer C, et al:
CXCR4 and CXCR7 regulate angiogenesis and CT26. WT tumor growth
independent from SDF-1. Int J Cancer. 126:1302–1315.
2010.PubMed/NCBI
|
|
61
|
Uto-Konomi A, McKibben B, Wirtz J, et al:
CXCR7 agonists inhibit the function of CXCL12 by down-regulation of
CXCR4. Biochem Biophys Res Commun. 431:772–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liekens S, Schols D and Hatse S:
CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell
mobilization. Curr Pharm Des. 16:3903–3920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Duda DG, Kozin SV, Kirkpatrick ND, Xu L,
Fukumura D and Jain RK: CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway
inhibition: an emerging sensitizer for anticancer therapies? Clin
Cancer Res. 17:2074–2080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gustin JA, Ozes ON, Akca H, et al: Cell
type-specific expression of the IkappaB kinases determines the
significance of phosphatidylinositol 3-kinase/Akt signaling to
NF-kappa B activation. J Biol Chem. 279:1615–1620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Y, Chinni SR and Sarkar FH: Selective
growth regulatory and pro-apoptotic effects of DIM is mediated by
AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci.
10:236–243. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
66
|
Katiyar SK and Meeran SM: Obesity
increases the risk of UV radiation-induced oxidative stress and
activation of MAPK and NF-kappaB signaling. Free Radic Biol Med.
42:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Leelawat K, Leelawat S, Narong S and
Hongeng S: Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4
induced cholangiocarcinoma cell invasion. World J Gastroenterol.
13:1561–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Burger M, Glodek A, Hartmann T, et al:
Functional expression of CXCR4 (CD184) on small-cell lung cancer
cells mediates migration, integrin activation, and adhesion to
stromal cells. Oncogene. 22:8093–8101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lai TH, Fong YC, Fu WM, Yang RS and Tang
CH: Stromal cell-derived factor-1 increase alphavbeta3 integrin
expression and invasion in human chondrosarcoma cells. J Cell
Physiol. 218:334–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ryu CH, Park SA, Kim SM, et al: Migration
of human umbilical cord blood mesenchymal stem cells mediated by
stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38
signal transduction pathways. Biochem Biophys Res Commun.
398:105–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Heinrich EL, Lee W, Lu J, Lowy AM and Kim
J: Chemokine CXCL12 activates dual CXCR4 and CXCR7-mediated
signaling pathways in pancreatic cancer cells. J Transl Med.
10:682012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Laverdiere C, Hoang BH, Yang R, et al:
Messenger RNA expression levels of CXCR4 correlate with metastatic
behavior and outcome in patients with osteosarcoma. Clin Cancer
Res. 11:2561–2567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lin F, Zheng SE, Shen Z, et al:
Relationships between levels of CXCR4 and VEGF and blood-borne
metastasis and survival in patients with osteosarcoma. Med Oncol.
28:649–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Baumhoer D, Smida J, Zillmer S, et al:
Strong expression of CXCL12 is associated with a favorable outcome
in osteosarcoma. Mod Pathol. 25:522–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fan TM, Barger AM, Fredrickson RL,
Fitzsimmons D and Garrett LD: Investigating CXCR4 expression in
canine appendicular osteosarcoma. J Vet Intern Med. 22:602–608.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Oda Y, Yamamoto H, Tamiya S, et al: CXCR4
and VEGF expression in the primary site and the metastatic site of
human osteosarcoma: analysis within a group of patients, all of
whom developed lung metastasis. Mod Pathol. 19:738–745. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ma Q, Zhou Y, Ma B, et al: The clinical
value of CXCR4, HER2 and CD44 in human osteosarcoma: A pilot study.
Oncol Lett. 3:797–801. 2012.PubMed/NCBI
|
|
78
|
Bai S, Wang D, Klein MJ and Siegal GP:
Characterization of CXCR4 expression in chondrosarcoma of bone.
Arch Pathol Lab Med. 135:753–758. 2011.PubMed/NCBI
|
|
79
|
Bennani-Baiti IM, Cooper A, Lawlor ER, et
al: Intercohort gene expression co-analysis reveals chemokine
receptors as prognostic indicators in Ewing's sarcoma. Clin Cancer
Res. 16:3769–3778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Clark JC, Dass CR and Choong PF: A review
of clinical and molecular prognostic factors in osteosarcoma. J
Cancer Res Clin Oncol. 134:281–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim SY, Lee CH, Midura BV, et al:
Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the
development of murine pulmonary metastases. Clin Exp Metastasis.
25:201–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
de Nigris F, Rossiello R, Schiano C, et
al: Deletion of Yin Yang 1 protein in osteosarcoma cells on cell
invasion and CXCR4/angiogenesis and metastasis. Cancer Res.
68:1797–1808. 2008.PubMed/NCBI
|
|
83
|
Miura K, Uniyal S, Leabu M, et al:
Chemokine receptor CXCR4-β1 integrin axis mediates tumorigenesis of
osteosarcoma HOS cells. Biochem Cell Biol. 83:36–48. 2005.
|
|
84
|
Hendrix CW, Collier AC, Lederman MM, et
al: Safety, pharmacokinetics, and antiviral activity of AMD3100, a
selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir
Immune Defic Syndr. 37:1253–1262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
De Clercq E: The AMD3100 story: the path
to the discovery of a stem cell mobilizer (Mozobil). Biochem
Pharmacol. 77:1655–1664. 2009.PubMed/NCBI
|
|
86
|
Devine SM, Flomenberg N, Vesole DH, et al:
Rapid mobilization of CD34+ cells following
administration of the CXCR4 antagonist AMD3100 to patients with
multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol.
22:1095–1102. 2004.PubMed/NCBI
|
|
87
|
Cashen A, Lopez S, Gao F, et al: A phase
II study of plerixafor (AMD3100) plus G-CSF for autologous
hematopoietic progenitor cell mobilization in patients with Hodgkin
lymphoma. Biol Blood Marrow Transplant. 14:1253–1261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim HY, Hwang JY, Kim SW, et al: The CXCR4
antagonist AMD3100 has dual effects on survival and proliferation
of myeloma cells in vitro. Cancer Res Treat. 42:225–234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kalatskaya I, Berchiche YA, Gravel S,
Limberg BJ, Rosenbaum JS and Heveker N: AMD3100 is a CXCR7 ligand
with allosteric agonist properties. Mol Pharmacol. 75:1240–1247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lapteva N, Yang AG, Sanders DE, Strube RW
and Chen SY: CXCR4 knockdown by small interfering RNA abrogates
breast tumor growth in vivo. Cancer Gene Ther. 12:84–89. 2005.
View Article : Google Scholar : PubMed/NCBI
|