Introduction
Acute kidney injury (AKI) is a clinical complication
with a high morbidity and mortality rate (1,2).
Patients with advanced age, diabetes or vascular diseases are at
high risk of AKI. The pathophysiology of AKI is very complex, and
includes tubular and vascular cell damage and an intense
inflammatory reaction (3).
Current therapies for AKI mainly include supportive care and renal
replacement therapy. Despite these therapies, the five-year
mortality rate for patients with AKI remains >50% (4). Hence, it is important to develop
novel therapeutic interventions for improving survival outcomes for
patients with AKI.
Stem cell-based therapy has sparked great interest
in AKI treatment over the years. A number of studies have
demonstrated that stem cells can prevent and repair damage to renal
tubular cells in AKI induced by ischemia-reperfusion (IR) (5–7),
or chemicals such as cisplatin (8–10)
and glycerol (11,12). Different types of stem cells, such
as embryonic stem cells (13),
amniotic fluid stem cells (14),
hematopoietic stem and progenitor cells (15), and mesenchymal stem cells (MSCs)
(5,9,10),
have been investigated and have shown to be promising in AKI
treatment. Among the different types of stem cells, bone
marrow-derived MSCs (BM-MSCs) have gained great popularity. BM-MSCs
are multipotent cells that can be easily isolated from bone marrow
and expanded ex vivo(16).
As BM-MSCs can be isolated from the bone marrow of patients, their
source and safety are well known. Several studies have used BM-MSCs
to treat AKI in animal models and have found that renal function
and structure can be improved by infusion with BM-MSCs (8,9,17,18). Despite evidence for the
therapeutic potential of BM-MSCs, the mechanisms underlying the
improvement in kidney function and structure remain unclear.
Previous studies have indicated that apoptotic
proximal tubular cell death is a prominent and characteristic
feature of AKI induced by cisplatin (19,20). Certain evidence indicates that
while cisplatin enhances the number of apoptotic cells in the
kidneys, the infusion of MSCs reduces the number of apoptotic cells
(9,10,21). Several pathways are involved in
cisplatin-induced AKI. Mitogen-activated protein kinase (MAPK)
signaling pathways play an important role in cisplatin-induced
renal injury. Both p38 and extracellular signal-regulated kinase
(ERK) are activated in cisplatin-induced AKI (22,23). Treatment with cisplatin leads to
the decrease or degradation of anti-apoptotic proteins, such as
Bcl-2, whereas the levels of pro-apoptotic proteins, such as Bax
are increased (24).
MSCs have the ability to differentiate into tubular
epithelial cells in vitro(25). However, integration and
differentiation into tubular epithelial cells are rarely reported
in experimental AKI models in vivo(7). It is generally considered that MSCs
promote tubular regeneration mainly through a paracrine mechanism
(26). MSCs can secrete several
types of growth factors and cytokines, such as vascular endothelial
growth factor (VEGF), basic fibroblast growth factor, insulin-like
growth factor-1 (IGF-1), interleukin (IL)-6 and IL-11 (27,28). The infusion of MSC-conditioned
medium (CM) has been reported to improve renal function and prolong
life span in cisplatin-induced AKI (29). For example, heme oxygenase-1
(HO-1)++ MSC-CM has been shown to protect against
cisplatin-induced renal injury, while HO-1−/− MSC-CM
failed to induce renoprotective effects (30). Another study indicated that the
knockdown of IGF-1 expression in MSCs by small-interfering RNA
(siRNA) led to a significant decrease in the protective effects of
MSCs on nephrotoxicity induced by cisplatin (31).
In order for MSCs to be clinically applied in the
treatment of AKI more effectively and safely in the future, further
understanding of the mechanisms responsible for the renoprotective
effects of MSCs is necessary. In the present study, we hypothesized
that the infusion of BM-MSCs alleviates damage to renal tubules in
cisplatin-induced AKI by inhibiting apoptotic pathways involved in
AKI. First, the renoprotective effects of BM-MSCs on
cisplatin-induced renal injury were evaluated in vivo.
Second, tubular cell apoptosis and proliferation after the infusion
of BM-MSCs were evaluated. Third, the expression and activation of
proteins related to cisplatin-induced renal cell apoptosis were
detected, including p38, ERK, caspase-3, Bax and Bcl-2. Finally,
the ability of BM-MSC-CM alone to reduce rat kidney cell apoptosis
induced by cisplatin was examined in vitro.
Materials and methods
Ethics
All experiments were performed using Sprague-Dawley
(SD) rats and were conducted according to the Wuhan University
Guide for the Care and Use of Laboratory Animals. All experimental
animal procedures were approved by the Animal Care and Use
Committee of Wuhan University, Wuhan, China.
Preparation of BM-MSCs
MSCs were collected from three- to four-week-old,
male SD rats (80–100 g). Briefly, the rats were euthanized by
cervical dislocation and their tibias and femurs were cleared of
muscle and connective tissue. Bone marrow cells were aspirated
using an 18-gauge needle with phosphate-buffered saline (PBS) and
passed through a 70-μm nylon gauze (BD Pharmingen, Bedford, MA,
USA). The cells were washed twice for 5 min each by centrifugation
at 150 × g and resuspended in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Sigma Chemical Co., St.
Louis, MO, USA), 2 mM glutamine (Gibco) and penicillin/streptomycin
(100 U/ml; Gibco). The bone marrow was plated on 25 cm2
culture flasks (Corning; Corning, NY, USA) at a density of
2×106 cells/ml. The flasks were incubated at 37°C with
5% CO2 in a humidified atmosphere. Four days after
plating, the medium was replaced to remove free-floating cells, and
then replenished every two to three days. After growing to 80–90%
confluence, the cells were washed with PBS and incubated with
trypsin (Gibco) for 5 min at 37°C. Trypsin was neutralized by
adding fresh complete medium. The cellular suspension was diluted
1:2 at each passage. DMSO (final concentration, 10%) was added to
the cells and they were then frozen in aliquots and stored in
liquid nitrogen.
Phenotypic analysis of BM-MSCs
Flow cytometric analysis was performed to
characterize the phenotype of MSCs. Cell suspensions were washed
twice with PBS containing 0.1% bovine serum albumin (BSA; Sigma
Chemical Co.). For direct assays, aliquots of cells at a
concentration of 1×106 cells/ml were incubated at 4°C
for 30 min with the following antibodies: fluorescein
isothiocyanate (FITC)-conjugated CD34 (Santa Cruz Biotechnology,
Santa Cruz, CA, USA), FITC-conjugated CD45 (eBioscience, San Diego,
CA, USA), PE-conjugated CD44 (Santa Cruz Biotechnology) and
PE-conjugated CD29 (eBioscience). Rat immunoglobulin G1-FITC and
rat immunoglobulin G1-PE (BD Biosciences Pharmingen) were used as
an isotype-matched control. Labeled cells were analyzed by a
FACSCalibur flow cytometer with the use of CellQuest software
(Beckman Coulter, Brea, CA, USA).
Cell lineage differentiation
The adipogenic and osteogenic differentiation
potential of the BM-MSCs was investigated at the third passage. To
induce adipogenic differentiation, the BM-MSCs were incubated at
100% confluency with rat BM-MSC adipogenic induction medium
(Cyagen, Guangzhou, China) for three days and maintenance medium
(Cyagen) for one day. After three cycles of induction and
maintenance, the cells were cultured in maintenance medium for an
additional seven days by replacing the medium every three days. Oil
Red O staining (Sigma Chemical Co.) was used to detect lipid
vacuoles. For osteogenic differentiation, the BM-MSCs were grown
with rat BM-MSC osteogenic differentiation medium (Cyagen) which
was replaced every three days. After two to three weeks of
differentiation, calcium deposits were stained with Alizarin red
(Sigma Chemical Co.).
Treatment of animals
Male SD rats (weighing, 190–220 g) were used in the
experiments. During the experiments, the animals were housed under
standard conditions with a 12-h light/12-h dark cycle. Water and
food were provided ad libitum. AKI was induced in the SD
rats by an intraperitoneal injection of 6 mg cisplatin/kg body
weight (Sigma Chemical Co.) (32). To investigate the effects of
BM-MSCs in the cisplatin-induced AKI animal model, the rats were
divided into the following three groups: i) the control group,
which received a single intraperitoneal injection of saline (n=8);
ii) the cisplatin group, which received a single intraperitoneal
injection of cisplatin and was administered 500 μl saline by tail
vein injection on day 1 after the cisplatin injection (n=8); and
iii) the cisplatin and BM-MSC group, which received a single
intraperitoneal injection of cisplatin and was administered BM-MSCs
(1×106 cells/500 μl) by tail vein injection 24 h after
the cisplatin injection (n=8). All animals were sacrificed on day 4
after the cisplatin injection. Blood, urine and tissue samples were
collected for the determination of renal function and tissue
damage.
Determination of renal function
Serum levels of creatinine, blood urea nitrogen
(BUN), urine levels of creatinine and urinary microalbumin levels
were measured using standard diagnostic kits in an autoanalyzer
(Olympus AU400; Olympus, Tokyo, Japan).
Renal histology
Kidney specimens from all animals were fixed in 10%
buffered formalin and embedded in paraffin. Tissues were cut into
5-μm-thick slices and then stained with hematoxylin and eosin
(H&E) for light microscopic analysis. Tubular injury was
defined as tubular epithelial necrosis, cast formation, tubular
dilatation and the loss of the brush border. Tubular injury was
scored by grading the percentage of affected tubules under ten
randomly selected, non-overlapping fields (magnification, ×200) as
follows: 0, 0%; 1, ≤10%; 2, 11–25%; 3, 26–45%; 4, 46–75%; and 5,
76–100%. To score injured tubules, whole tubule numbers per field
were considered as standard under a magnification of ×200. The
grading percentage was calculated in each field as follows: injury
score (%) = (number of injured tubules/number of whole tubules)
×100.
Apoptosis assay
Apoptosis was determined by a terminal
deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay
kit (Roche, Indianapolis, IN, USA). Accordingly, the kidney
sections were deparaffinized, rehydrated, digested with proteinase
K and labeled with a TUNEL reaction mixture for 60 min at 37°C.
TUNEL-positive, apoptotic tubular epithelial cells were counted in
ten high-power (x400) fields per section in the cortex.
Proliferation assay
To determine the number of proliferating tubular
cells, the expression of proliferating cell nuclear antigen (PCNA)
was detected by immunohistochemistry. Paraffin-embedded kidney
sections were first deparaffinized with xylene and rehydrated in a
series of alcohol and water. After blocking with goat serum for 30
min, the slides were incubated with an anti-PCNA antibody (1:200;
Sigma Chemical Co.) at 4°C overnight and anti-rabbit secondary
antibody (1:200; Santa Cruz Biotechnology) for 1 h. PCNA signal was
detected using a diaminobenzidine (DAB) kit (Beyotime, Haimen,
China). Nuclei were visualized by counterstaining with Harris’s
hematoxylin. Scoring for PCNA-positive cells was carried out by
counting the number of positive nuclei in the renal cortex in ten
high-power (x400) fields per section.
Labeling and preparation of BM-MSCs for
transplantation
To examine intrarenal localization and to quantify
the BM-MSCs, the BM-MSCs were labeled with a PKH-26 red
fluorescence cell linker kit (Invitrogen, Carlsbad, CA, USA) one
day after the cisplatin injection and were then infused into the
cisplatin-treated rats (n=5). Labeling efficacy was >98%, as
assessed by flow cytometry. Viability was >96%, as assessed by
trypan blue exclusion. After four days, the rats were sacrificed
and samples were immediately frozen in liquid nitrogen, embedded in
optimal cutting temperature (OCT) compound, sliced into 5-μm-thick
sections, fixed in acetone (10 min) and incubated with FITC-labeled
wheat germ agglutinin (WGA Lectin; Vector Laboratories, Burlingame,
CA, USA) for 30 min. Nuclei were stained with
4,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI; Sigma
Chemical Co.). PKH-26-positive cells were counted in six frozen
renal sections per rat (n=5). Data are expressed as the number of
PKH-26-positive cells per renal section.
Western blot analysis
Immunoblot analysis was performed as previously
described (33). Briefly, the
tissues or cultured cells were lysed in a buffer containing 20 mM
Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100,
25 mM sodium pyrophosphate, 1 mM NaF, 1 mM β-glycerophosphate, 0.1
mM sodium orthovanadate, 1 mM phenylmethyl sulfonyl fluoride, 2
μg/ml leupeptin and 10 μg/ml aprotinin. In all, 50 μg of total cell
lysate were separated on SDS-PAGE gels and transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore; Billerica,
MA, USA). The membranes were blocked with 5% non-fat dry milk in
Tris-buffered saline and Tween-20 (TBS-T) buffer and then incubated
with the following primary antibodies: phosphorylated ERK (p-ERK)
1:1,000; ERK, 1:1,000; phosphorylated p38 (p-p38), 1:1,000; p38,
1:1,000; Bcl-2, 1:1,000; Bax, 1:1,000; and caspase-3, 1:1,000 (Cell
Signaling Technology, Beverly, MA, USA); β-actin, 1:5,000 (Santa
Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The
samples were then incubated for 1 h at room temperature with
horseradish peroxidase (HRP)-conjugated anti-rabbit secondary
antibody (1:5,000; Santa Cruz Biotechnology) as a secondary
antibody. Signals were detected using an ECL Western Blotting Kit
(Amersham Pharmacia Biotech, Piscataway, NJ, USA). For
quantification, the density of the bands was measured using
Quantity One software (Bio-Rad, Hercules, CA, USA).
Collection of BM-MSC-CM
The BM-MSCs were cultured in a 75-cm2
culture flasks in 15 ml complete medium. The medium was changed for
15 ml fresh complete medium when the cells grew to 50% confluence
and was collected 72 h later when the cells were 90% confluent.
Floating cells were removed by centrifugation at 3,000 rpm for 10
min and the supernatant was frozen at −80°C for use in later
experiments.
Effects of BM-MSC-CM on NRK-52E cell
viability and apoptosis
NRK-52E cells (ATCC, Manassas, VA, USA), a rat renal
proximal tubular cell line, were cultured in DMEM (Gibco; Carlsbad,
CA, USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; Gibco), 2 mM glutamine (Gibco) and penicillin/streptomycin
(100 U/ml; Gibco). When the cultured cells were grown to 80%
confluence, the cells were incubated in CM or complete medium in
the presence or absence of cisplatin at a final concentration of 50
μM for 24 and 48 h (34).
Subsequently, a water-soluble tetrazolium salt-1 (WST-1) assay
(29), Annexin V and propidium
iodide staining and western blot analysis were performed.
The viability of the NRK-52E cells was assessed
using a WST-1 assay kit (Beyotime, Haimen, China). Briefly, the
cells were plated in 96-well tissue culture plates. To each well
was added 10 μl WST-1 solution at 24 or 48 h after treatment with
cisplatin. Following incubation with WST-1 solution for 2 h, the
absorbance values were measured at a wavelength of 450 nm.
The percentage of apoptotic cells was assessed by
Annexin V and propidium iodide staining (Annexin V-FITC apoptosis
detection kit; BestBio, Shanghai, China) by flow cytometry.
Briefly, both adherent and floating cells were collected and
resuspended in 400 μl of 1X binding buffer. Annexin V-FITC (5 μl)
was then added and the cells were incubated in the dark at 4°C for
15 min. Subsequently, 10 μl of propidium iodide were added and the
solution was incubated in the dark at 4°C for 5 min. The cells were
analyzed by a FACSCalibur flow cytometer (Beckman Coulter) using a
488 nm excitation wavelength for the Annexin V-FITC-positive cells
and propidium iodide-positive cells.
Statistical analysis
Data are expressed as the means ± SEM. Data were
analyzed using a t-test and one-way analysis of variance (ANOVA).
Statistical analysis was performed using SPSS 13.0 software (IBM;
Chicago, IL, USA). A value of P<0.05 was considered to indicate
a statistically significant difference.
Results
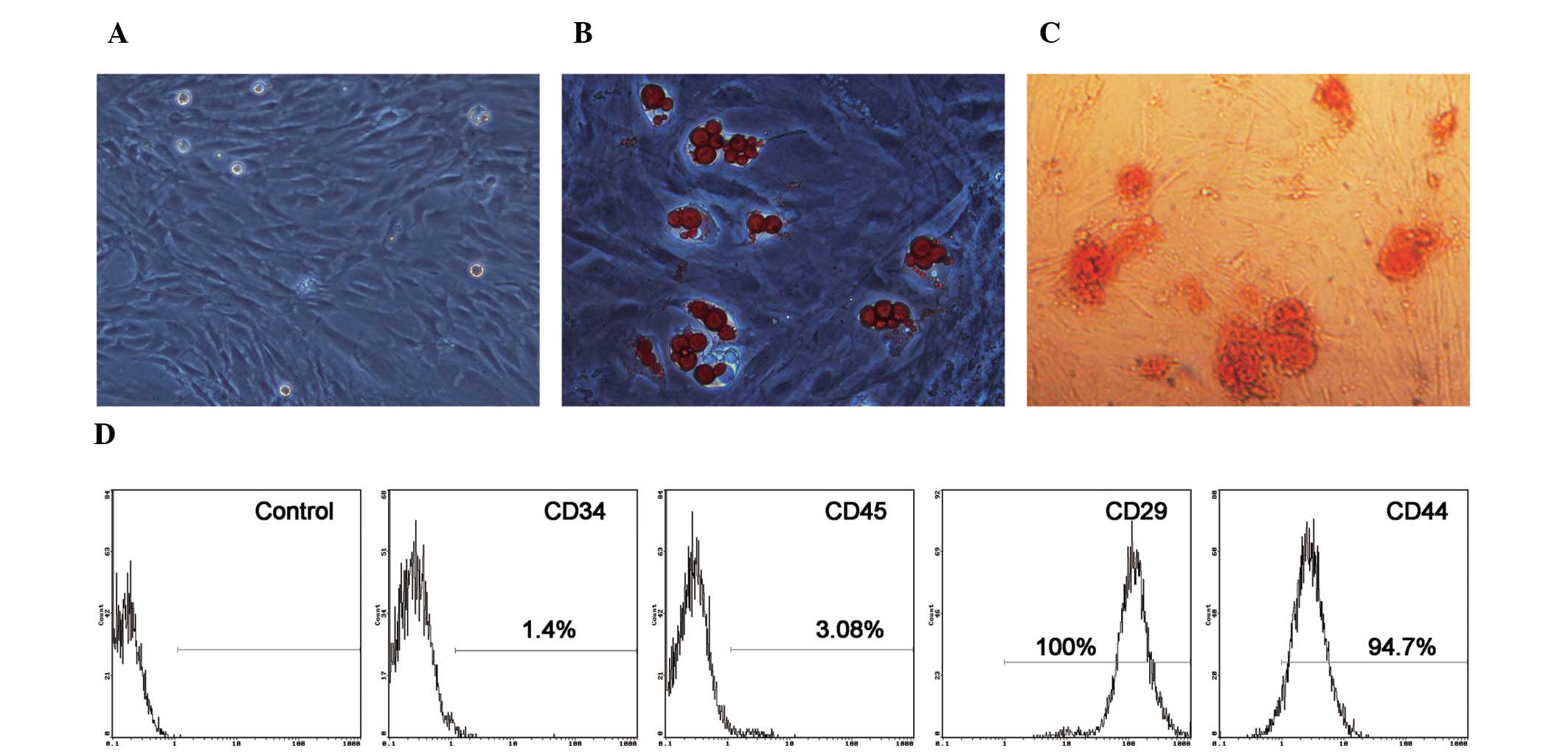
Morphology, immunophenotype and
differentiation status of BM-MSCs
The BM-MSCs were isolated and expanded from
four-week-old male rats. MSCs cultured as plastic-adherent cells
showed a flattened and spindle-shaped morphology in vitro.
The morphological features of the MSCs are shown in Fig. 1A. To verify the pluripotential
capacity of the cultured cells, the cells were exposed to the
appropriate induction medium. The BM-MSCs differentiated into
adipogenic and osteoblastic lineages two to three weeks after
induction (Fig. 1B and C). The
expression of cell surface markers on BM-MSCs was evaluated by flow
cytometry. As illustrated in Fig.
1D, the BM-MSCs weakly expressed CD45 and CD34, which are two
specific cell surface markers of hematopoietic cells. The cells
also strongly expressed CD29 and CD44, which are important cell
surface markers of MSCs. These results demonstrate the
spindle-shaped morphology, purity and differentiation potential of
the MSCs (35).
Infusion of BM-MSCs preserves renal
function in rats with cisplatin-induced AKI
The cisplatin-treated rats administered saline or
BM-MSCs were sacrificed four days after the cisplatin injection.
The control rats were also sacrificed four days after the saline
injection. Serum and urine samples were collected for the analysis
of serum BUN, serum creatinine, urinary creatinine and urinary
microalbumin levels. As shown in Fig.
2, the BM-MSCs exerted a renoprotective effect, as reflected by
significantly lower BUN, serum creatinine and urinary microalbumin
levels (P<0.01) and higher urinary creatinine levels (P<0.05)
compared with the cisplatin-treated rats adminstered saline. These
data indicate that the infusion of BM-MSCs preserves renal function
in rats with cisplatin-induced AKI.
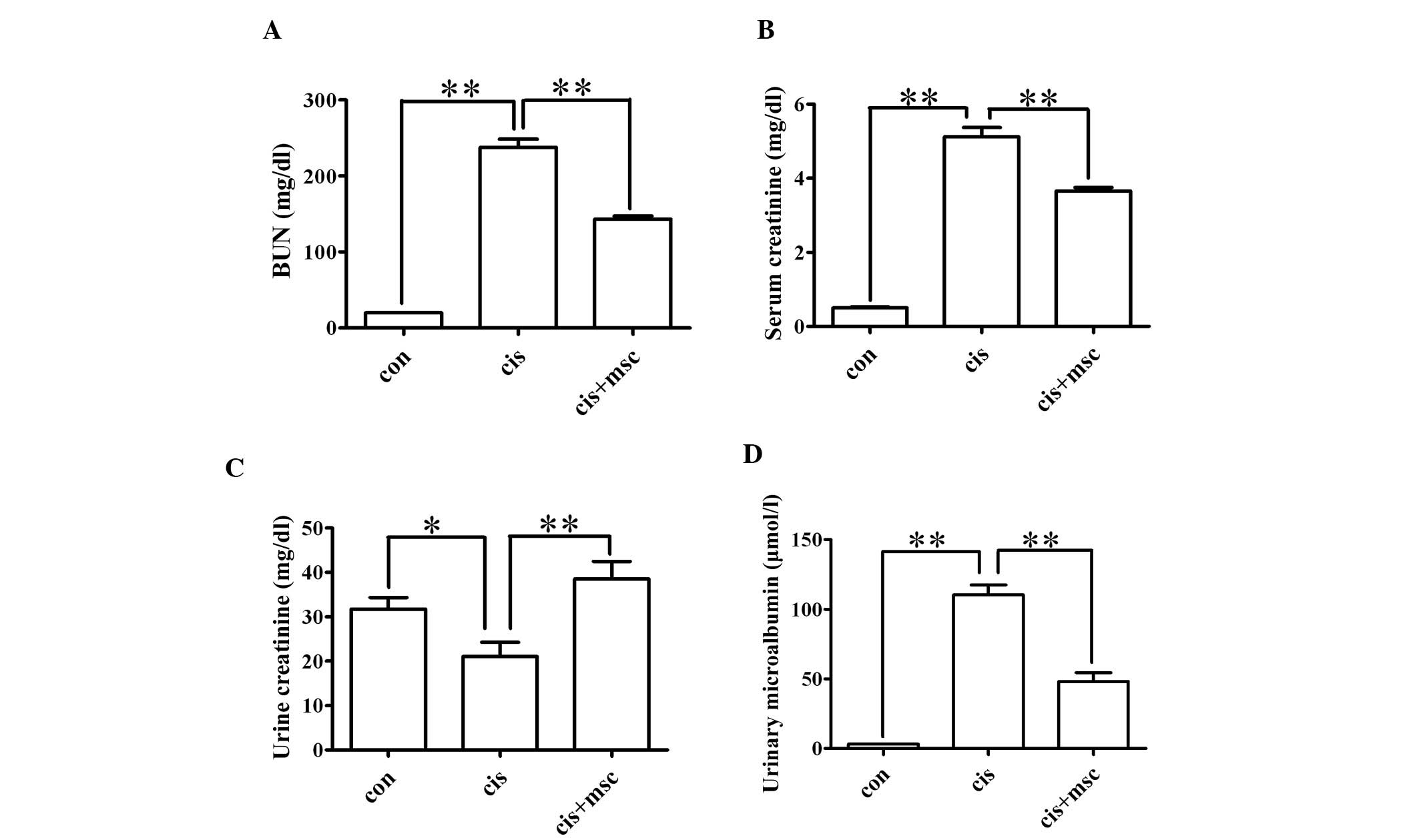 | Figure 2Protective effects of bone
marrow-derived mesenchymal stem cells (BM-MSCs) on renal function
in rats with acute kidney injury (AKI). Rats were divided into
three groups: the control group (con, n=8), cisplatin-treated rats
administered saline (cis, n=8) and cisplatin-treated rats
administered BM-MSCs (cis + msc, n=8). Renal function was assessed
by measuring blood urea nitrogen (BUN), serum creatinine, urinary
creatinine and urinary microalbumin levels. Data are presented as
the means ± SEM. (A) Serum levels of BUN; con vs. cis,
**P<0.01 between the indicated groups; cis vs. cis +
msc, **P<0.01 between the indicated groups. (B) Serum
levels of creatinine; con vs. cis, **P<0.01 between
the indicated groups; cis vs. cis + msc, **P<0.01
between the indicated groups. (C) Urine levels of creatinine; con
vs. cis, *P<0.05 between the indicated groups; cis
vs. cis + msc, **P<0.01 between the indicated groups.
(D) Urine levels of microalbumin; con vs. cis,
**P<0.01 between the indicated groups; cis vs. cis +
msc, **P<0.01 between the indicated groups. |
Infusion of BM-MSCs ameliorates
cisplatin-induced renal tubular lesions
To evaluate the effects of the infusion of BM-MSCs
on histological impairment in the kidneys induced by cisplatin, all
the rats were sacrificed four days after the cisplatin injection
and the kidneys were collected for H&E staining. The kidneys of
the cisplatin-treated rats administered saline showed typical
cisplatin-induced renal injury, including tubular necrosis, cast
formation, the loss of brush border in renal tubules and tubular
dilatation (Fig. 3A). The
infusion of BM-MSCs significantly attenuated renal tubular damage,
as reflected by the light microscopy images and much lower
histopathological scoring compared with the cisplatin-treated rats
administered saline (P<0.01; Fig.
3A and B). These results suggest that the infusion of BM-MSCs
significantly protects the kidneys from cisplatin-induced renal
histological damage.
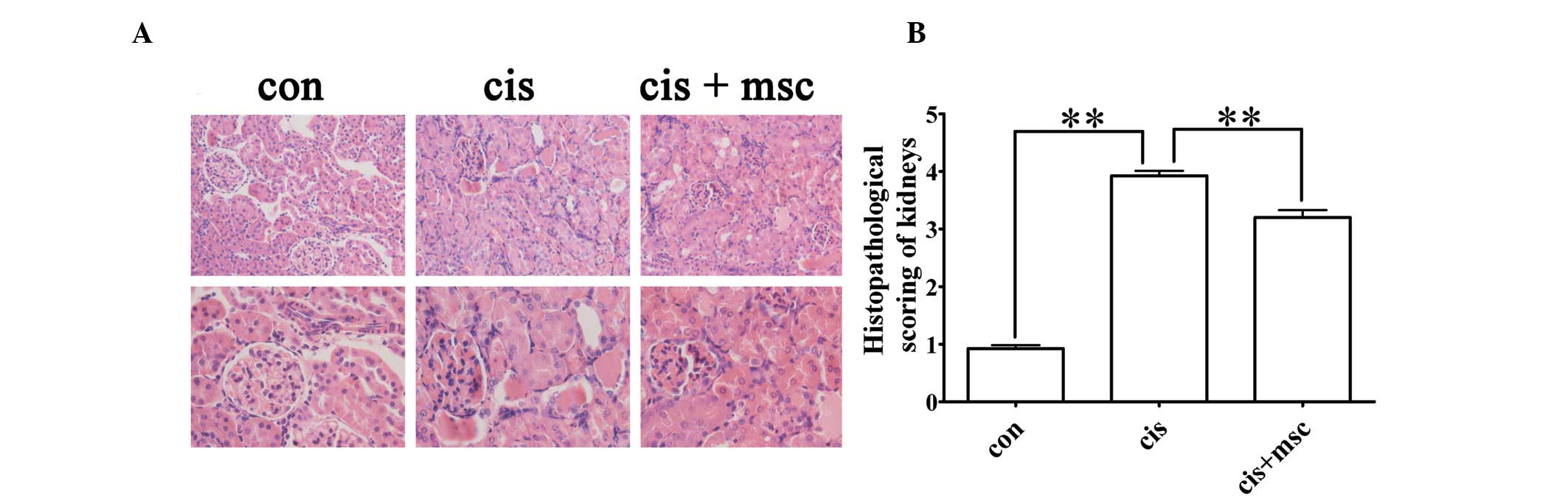 | Figure 3Histological changes and scoring of
kidney sections from the control and cisplatin-treated rats
administered saline or bone marrow-derived mesenchymal stem cells
(BM-MSCs), respectively, four days after the cisplatin injection.
(A) Representative hematoxylin and eosin (H&E) staining of
kidney sections from the control, cisplatin and cisplatin + BM-MSCs
group. Original magnification of images on upper panel, ×200;
original magnification of images from lower panel, ×400. (B)
Histopathological scoring of cisplatin-induced renal injury.
Tubular injury was defined as tubular necrosis, cast formation,
loss of brush border in renal tubules and tubular dilatation.
Histopathological scoring was based on the percentage of affected
tubules in the kidney sections, as described in ‘Materials and
methods’. Data are presented as the means ± SEM. con vs. cis,
**P<0.01 between the indicated groups; cis vs. cis +
msc, **P<0.01 between the indicated groups. |
BM-MSCs reduce apoptosis and accelerate
tubular cell regeneration in cisplatin-treated rats
The apoptosis of renal tubular cells is partly
responsible for cisplatin-induced AKI. Using TUNEL assay to detect
apoptotic renal tubular cells in the kidney sections, the number of
TUNEL-positive cells markedly increased in the cisplatin-treated
rats administered saline four days after the cisplatin injection
compared with the control rats (P<0.01; Fig. 4A and C). The infusion of BM-MSCs
significantly reduced the number of TUNEL-positive cells as
compared with the cisplatin-treated rats administered saline on day
4 (P<0.01; Fig. 4A and C).
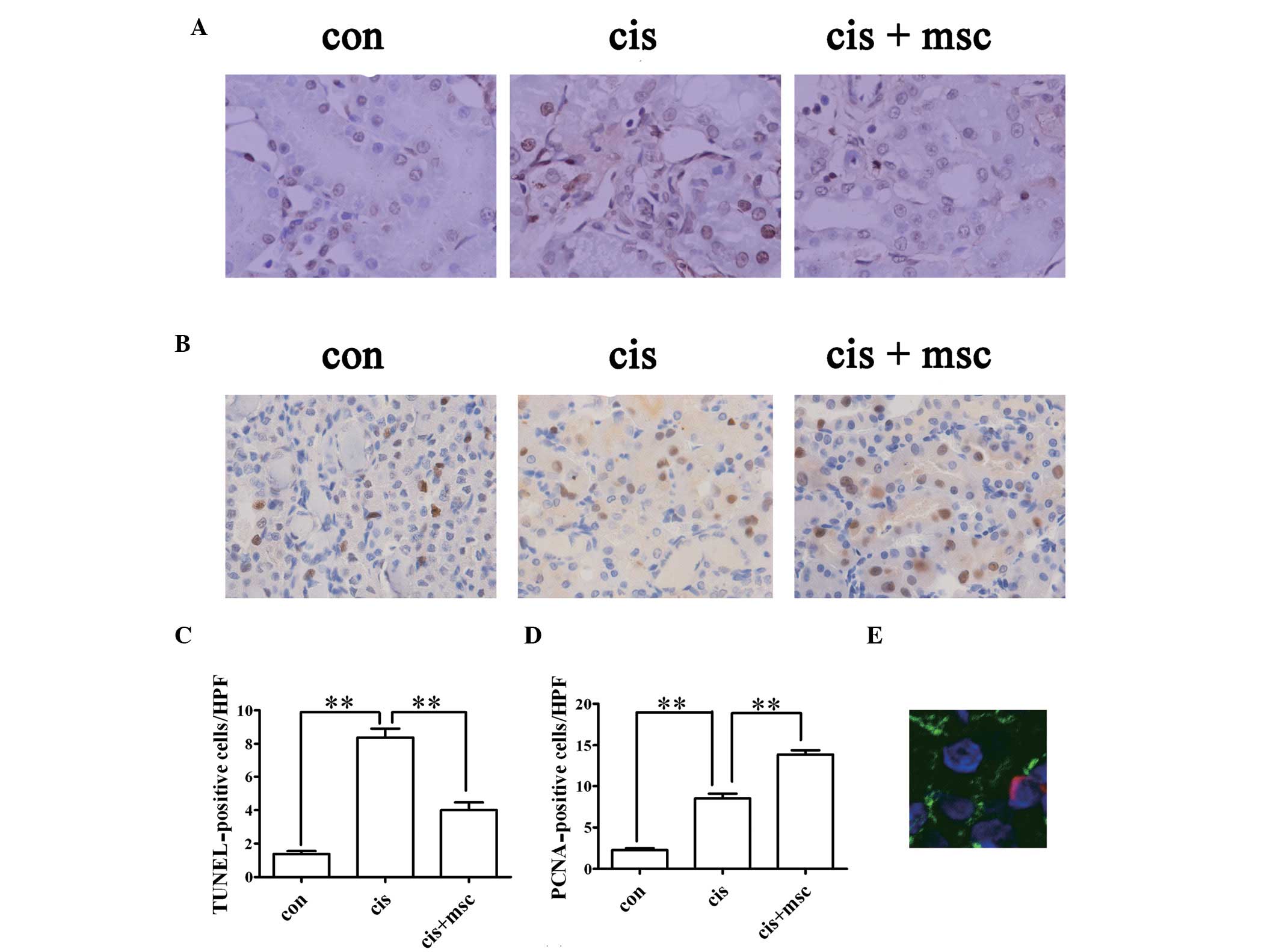 | Figure 4Effects of infusion of bone
marrow-derived mesenchymal stem cells (BM-MSCs) on
cisplatin-induced tubular apoptosis and tubular cell proliferation
four days after the cisplatin injection. (A) Representative images
of terminal dexynucleotidyltransferase-mediated dUTP nick
end-labeling (TUNEL) staining in the kidney sections from the
control and cisplatin-treated rats administered saline or BM-MSCs.
Original magnification, ×400. (B) Representative images of
proliferating cell nuclear antigen (PCNA) in the kidney sections
from the control and cisplatin-treated rats given administered or
BM-MSCs. Original magnification, ×400. (C) Quantification of
apoptotic cells in kidney sections, identified by TUNEL assay. Data
are presented as the means ± SEM. con vs. cis,
**P<0.01 between the indicated groups; cis vs. cis +
msc, **P<0.01 between the indicated groups. (D)
Quantification of PCNA-positive tubular cells in kidney sections.
Data are presented as the means ± SEM. Con vs. cis,
**P<0.01 between the indicated groups; cis vs. cis +
msc, **P<0.01 between the indicated groups. (E)
Representative image of PKH-26-labeled MSCs in the kidney sections
from rats injected with PKH-26-labeled BM-MSCs four days after the
cisplatin injection. PKH-26 labeled cells were stained red.
Sections were stained with fluorescein isothiocyanate
(FITC)-labeled lectin wheat germ agglutinin (WGA; green), and
4,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI) for
nuclei (blue). Original magnification, ×630. |
Tubular cell regeneration was evaluated by PCNA
staining. PCNA-positive cells increased in the renal sections from
the cisplatin-treated rats administered saline four days after the
cisplatin injection compared with the control group (P<0.01;
Fig. 4B and D). The number of
PCNA-positive cells in the cisplatin-treated rats administered
BM-MSCs was much higher than that in the cisplatin-treated rats
administered saline four days after the cisplatin injection
(P<0.01; Fig. 4B and D). These
results indicate that BM-MSCs promote regeneration and exert an
anti-apoptotic effect in cisplatin-induced nephrotoxicity.
Distribution of BM-MSCs infused in
cisplatin-treated rats
MSCs have the capacity of migrating into the injured
tissue (12). The existence of
BM-MSCs in the renal parenchyma was evaluated by the presence of
PKH-26-labeled cells in the kidney sections three days after the
administration of BM-MSCs. PKH-26-positive cells were counted and
were observed in 3±0.5 out of 105 renal cells in renal
tissues of cisplatin-treated rats on day 4. MSCs were predominantly
localized in the peritubular areas and rarely within the tubular
epithelium (Fig. 4E), which
suggested that it is unlikely that the MSCs differentiated into
renal cells.
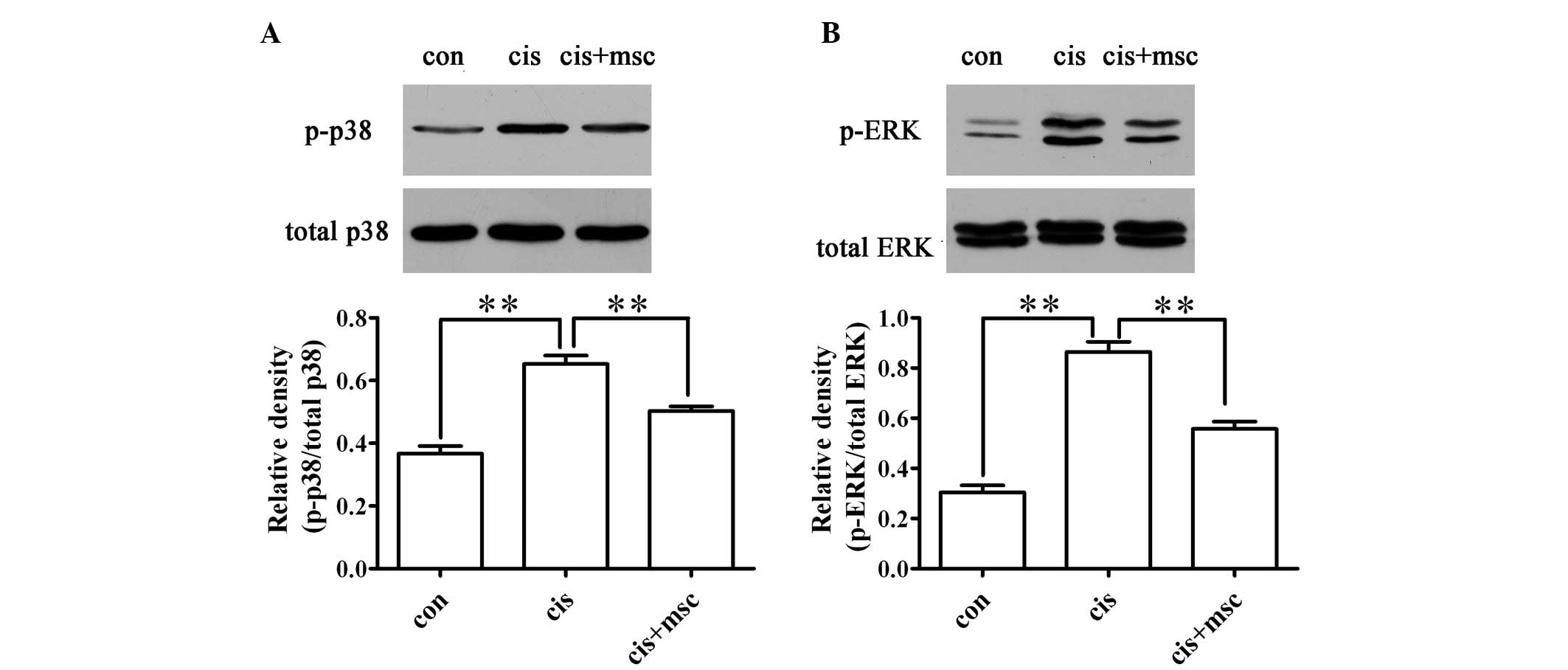
Infusion of BM-MSCs decreases the
phosphorylation of MAPKs, including p-ERK and p-p38 in the
cisplatin-treated rats
The phosphorylation of ERK and p38 has been
demonstrated to be involved in cisplatin-induced renal tubular cell
apoptosis (22). As shown in
Fig. 5, the infusion of BM-MSCs
inhibited the phosphorylation of ERK and p38 compared with the
cisplatin-treated rats administered saline. These results suggest
that BM-MSCs reduce renal tubular apoptosis by inhibiting the
activation of ERK and p38.
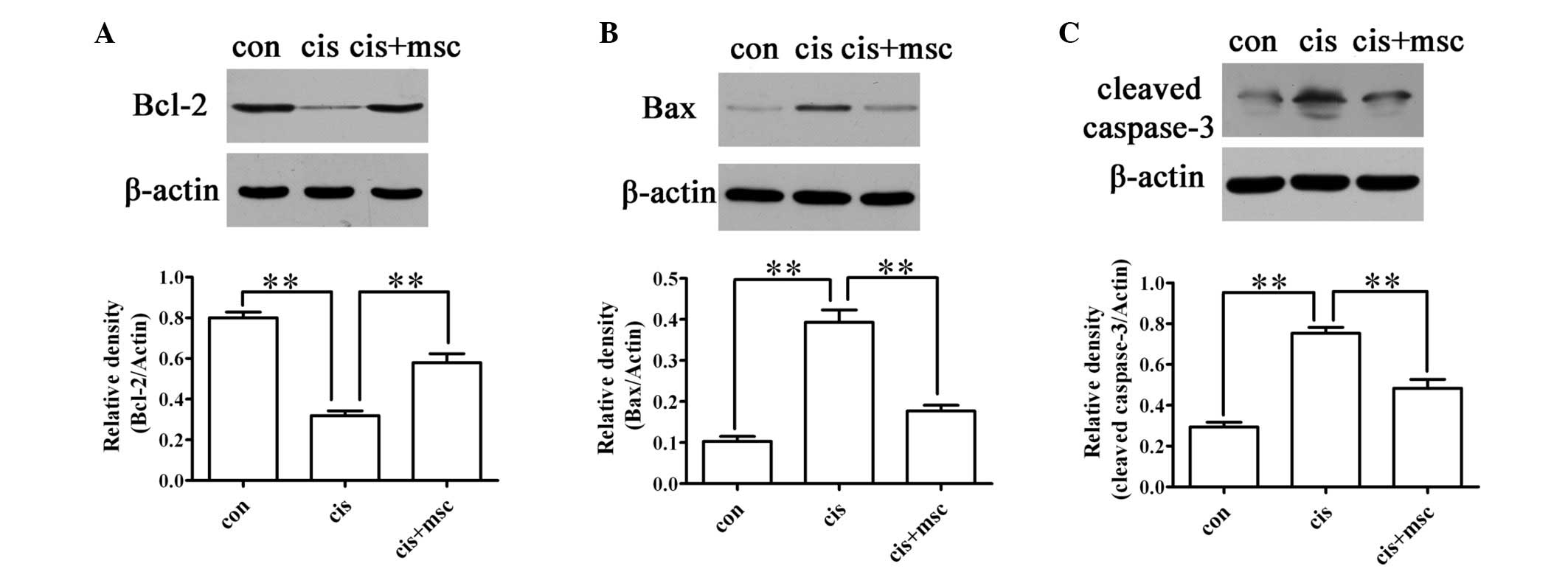
BM-MSCs influence the expression of
apoptosis-related proteins, such as Bax and Bcl-2
The pro-apoptotic gene, Bax, and the anti-apoptotic
gene, Bcl-2, play key roles in cisplatin-induced AKI (36). Caspase-3 acts as an executioner
protein in the apoptotic pathway. The downregulation of Bax and
caspase-3 and the upregulation of Bcl-2 were observed in the
cisplatin-treated rats administered BM-MSCs as compared with the
cisplatin-treated rats administered saline (Fig. 6). These results demonstrate that
another mechanism through which BM-MSCs reduce cell apoptosis in
cisplatin-treated rats is by regulating the expression of Bax and
Bcl-2.
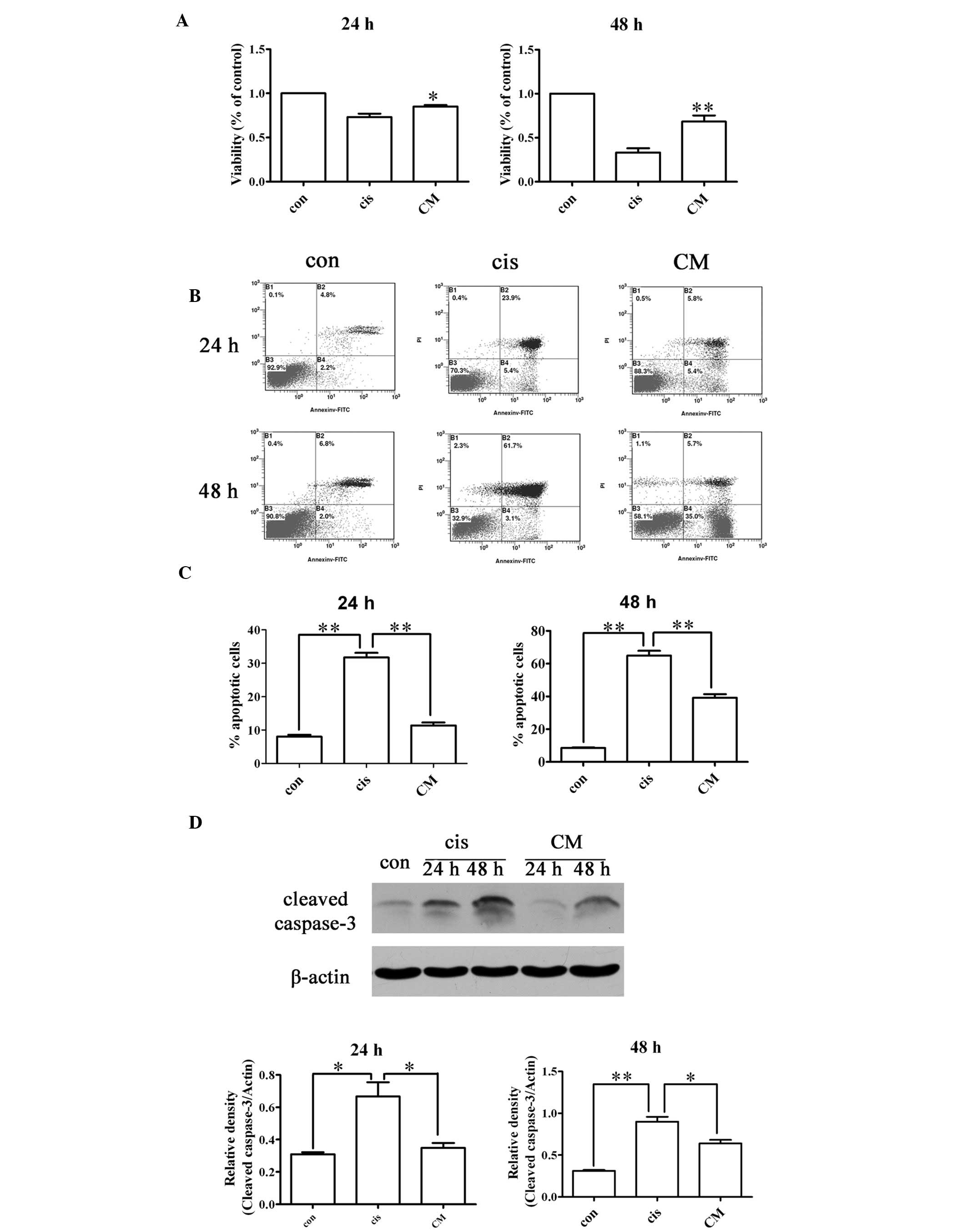
Effects of BM-MSC-CM on NRK-52E cell
viability and apoptosis in vitro
To investigate the effects of BM-MSC-CM on
cisplatin-treated NRK-52E cells, the NRK-52E cells were exposed to
50 μM cisplatin in the presence or absence of BM-MSC-CM for 24 or
48 h. The viability of the cisplatin-treated NRK-52E cells exposed
to CM was significantly higher than those not exposed to CM
(P<0.01; Fig. 7A).
As illustrated in Fig.
7B and C, BM-MSC-CM reduced the percentage of apoptotic cells
compared with the cisplatin-treated NRK-52E cells in the absence of
CM (P<0.01), as shown by flow cytometry analysis (FACS)
following Annexin V and propidium iodide staining. BM-MSC-CM also
reduced the expression of cleaved caspase-3 in the
cisplatin-treated NRK-52E cells (Fig.
7D). The addition of BM-MSC-CM alone was able to reduce renal
tubular cell apoptosis. These data indicate that BM-MSCs secrete
factors that provide renal protection, thereby attenuating
cisplatin-induced renal nephrotoxicity by inducing paracrine
effects in vivo.
Discussion
BM-MSCs are multipotent stem cells with
immunomodulatory ability, the capacity for expanding easily in
vitro and the potential for differentiation into cells of
mesenchymal or other lineage. Such unique properties have made
BM-MSCs an attractive candidate for stem cell-based therapy.
Previous studies have shown that the infusion of BM-MSCs is
effective in treating myocardial infarction (37,38), neurological diseases (39,40), diabetic nephropathy (41,42) and AKI. In agreement with previous
studies investigating the effects of BM-MSCs on AKI, this study
suggested that the infusion of BM-MSCs improves kidney function and
ameliorated renal injury induced by cisplatin. However, little is
known at present about the mechanisms underlying the therapeutic
effects of BM-MSCs on cisplatin nephropathy. The present study
focuses on the anti-apoptotic mechanisms responsible for the
therapeutic effects of BM-MSCs in cisplatin nephropathy.
Tubular cell apoptosis is a characteristic feature
of cisplatin nephrotoxicity, which results in the loss of renal
endothelial cells and renal dysfunction. The apoptosis of renal
cells induced by cisplatin has been detected both in vivo
and in vitro(43–45). Several therapeutic interventions
targeting apoptotic pathways involved in AKI have demonstrated
beneficial effects on renal injury induced by cisplatin in animal
models and cultured renal tubular cells (46–48). In the present study, the number of
TUNEL-positive cells increased significantly after the cisplatin
injection. The infusion of BM-MSCs resulted in a significant
reduction of cells positive for TUNEL staining. The decreased
cleaved caspase-3 expression also confirmed that the infusion of
BM-MSCs reduced apoptosis in the kidneys injured by cisplatin. PCNA
expression is an index of renal regeneration. The administration of
BM-MSCs significantly increased the number of PCNA-positive cells,
suggesting that BM-MSCs strongly promoted tubular cell
proliferation while inhibiting cell apoptosis. These findings
suggest that the renoprotective effects of BM-MSCs may rely on
their anti-apoptotic function.
To further elucidate the anti-apoptotic mechanisms
through which BM-MSCs exert renoprotective effects, we examined
certain pathways involved in renal cell apoptosis induced by
cisplatin. ERK and p38 are members of the MAPK family. The
activation of MAPKs regulates cellular homeostasis and processes,
such as proliferation, differentiation and apoptosis (49,50). The phosphorylation of ERK and p38
increases in kidney tissue or in cultured renal tubular cells
following the administration of cisplatin, while MEK inhibitors or
p38 inhibitors attenuate cisplatin-induced renal injury by
decreasing apoptosis (22,23,51,52).
Our study confirmed the results of previous studies, namely that
the phosphorylation of ERK and p38 increased following treatment
with cisplatin. The infusion of BM-MSCs resulted in a reduction of
ERK phosphorylation and p38 phosphorylation in parallel with a
reduction of apoptosis and an improvement of renal function.
Cisplatin also induces outer mitochondrial membrane
injury, suggesting that the activation of the mitochondrial pathway
of apoptosis plays an important role in cisplatin-induced
nephrotoxicity. In vivo, the ratio between Bcl-2 and Bax is
reduced following treatment with cisplatin. Bax−/− mice
are protected from cisplatin-induced renal injury (36,48,53). In vitro, treatment with
cisplatin leads to a decrease or the degradation of anti-apoptotic
proteins, whereas the levels of pro-apoptotic proteins are
increased (24). Our study
demonstrated that the administration of BM-MSCs upregulated the
anti-apoptotic protein, Bcl-2, and downregulated the pro-apoptotic
protein, Bax, which corresponded with reduced apoptosis and
improved renal function. These results suggest a correlation
between the changes in the mitochondrial pathway of apoptosis and
the infusion of BM-MSCs.
There are two potential, distinct mechanisms
responsible for the renoprotective effects of BM-MSCs against
cisplatin-induced nephrotoxicity: transdifferentiation or through a
paracrine process. Certain studies have reported that exogenous
BM-MSCs can engraft into injured tubules and it has been proposed
that the ability of the BM-MSCs to transdifferentiate explains
their protective effects (8,11,54). By contrast, other studies have
shown that BM-MSCs protect against acute tubular injury through a
differentiation-independent process (7,26,55,56). In our study, we observed the
renoprotective effects of BM-MSCs only three days after their
administration. This is too short a time period for the BM-MSCs to
transdifferentiate into renal tubular cells. The distribution of
BM-MSCs in kidney sections further confirmed that they had little
opportunity to differentiate into renal cells. The injected cells
were mainly located in the peritubular areas, not in the content of
the tubules. We then examined whether CM derived from BM-MSCs
protects renal tubular cells and attenuates cell apoptosis in
vitro. NRK-52E cells, an immortalized cell line derived from
rat proximal tubules, were incubated with cisplatin in the presence
or absence of CM. The results revealed that CM alone increased cell
viability and reduced cell apoptosis, as assessed by Annexin V and
propidium iodide staining and the reduced expression of cleaved
caspase-3. These results support the idea that BM-MSCs protect
tubular cells by inhibiting cell apoptosis through a paracrine
mechanism.
In conclusion, we demonstrate that the infusion of
BM-MSCs partially protects cisplatin-treated rats from AKI by
inhibiting tubular cell apoptosis. ERK, p38, Bcl-2 and Bax are
involved in the anti-apoptotic effects of BM-MSCs in cisplatin
nephrotoxicity. Furthermore, BM-MSCs may act by secreting factors
(i.e., a paracrine mechanism) that subsequently cause the changes
in the expression or activation of key proteins that inhibit
apoptosis and promote proliferation. Our study provides a
preliminary understanding of the role of BM-MSCs in the treatment
of AKI.
Acknowledgements
We thank Medjaden Bioscience Limited for assisting
in the preparation of this manuscript.
References
|
1
|
Bagshaw SM: Short- and long-term survival
after acute kidney injury. Nephrol Dial Transplant. 23:2126–2128.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chertow GM, Burdick E, Honour M, Bonventre
JV and Bates DW: Acute kidney injury, mortality, length of stay,
and costs in hospitalized patients. J Am Soc Nephrol. 16:3365–3370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tögel FE and Westenfelder C: Kidney
protection and regeneration following acute injury: progress
through stem cell therapy. Am J Kidney Dis. 60:1012–1022.
2012.PubMed/NCBI
|
|
4
|
Waikar SS, Liu KD and Chertow GM:
Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J
Am Soc Nephrol. 3:844–861. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YT, Sun CK, Lin YC, et al:
Adipose-derived mesenchymal stem cell protects kidneys against
ischemia-reperfusion injury through suppressing oxidative stress
and inflammatory reaction. J Transl Med. 9:512011. View Article : Google Scholar
|
|
6
|
Lange C, Togel F, Ittrich H, et al:
Administered mesenchymal stem cells enhance recovery from
ischemia/reperfusion-induced acute renal failure in rats. Kidney
Int. 68:1613–1617. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tögel F, Hu Z, Weiss K, Isaac J, Lange C
and Westenfelder C: Administered mesenchymal stem cells protect
against ischemic acute renal failure through
differentiation-independent mechanisms. Am J Physiol Renal Physiol.
289:F31–F42. 2005.
|
|
8
|
Morigi M, Imberti B, Zoja C, et al:
Mesenchymal stem cells are renotropic, helping to repair the kidney
and improve function in acute renal failure. J Am Soc Nephrol.
15:1794–1804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morigi M, Introna M, Imberti B, et al:
Human bone marrow mesenchymal stem cells accelerate recovery of
acute renal injury and prolong survival in mice. Stem Cells.
26:2075–2082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morigi M, Rota C, Montemurro T, et al:
Life-sparing effect of human cord blood-mesenchymal stem cells in
experimental acute kidney injury. Stem Cells. 28:513–522.
2010.PubMed/NCBI
|
|
11
|
Herrera MB, Bussolati B, Bruno S, Fonsato
V, Romanazzi GM and Camussi G: Mesenchymal stem cells contribute to
the renal repair of acute tubular epithelial injury. Int J Mol Med.
14:1035–1041. 2004.PubMed/NCBI
|
|
12
|
Herrera MB, Bussolati B, Bruno S, et al:
Exogenous mesenchymal stem cells localize to the kidney by means of
CD44 following acute tubular injury. Kidney Int. 72:430–441. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lazzeri E, Crescioli C, Ronconi E, et al:
Regenerative potential of embryonic renal multipotent progenitors
in acute renal failure. J Am Soc Nephrol. 18:3128–3138. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rota C, Imberti B, Pozzobon M, et al:
Human amniotic fluid stem cell preconditioning improves their
regenerative potential. Stem Cells Dev. 21:1911–1923. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Black R, Ma Z, Yang Q, Wang A and
Lin F: Use of mouse hematopoietic stem and progenitor cells to
treat acute kidney injury. Am J Physiol Renal Physiol. 302:F9–F19.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horwitz EM, Le Blanc K, Dominici M, et al:
Clarification of the nomenclature for MSC: the international
society for cellular therapy position statement. Cytotherapy.
7:393–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia X, Xie X, Feng G, et al: Bone
marrow-derived cells can acquire renal stem cells properties and
ameliorate ischemia-reperfusion induced acute renal injury. BMC
Nephrology. 13:1052012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yadav N, Rao S, Bhowmik DM and
Mukhopadhyay A: Bone marrow cells contribute to tubular epithelium
regeneration following acute kidney injury induced by mercuric
chloride. Indian J Med Res. 136:211–220. 2012.
|
|
19
|
Bonegio R and Lieberthal W: Role of
apoptosis in the pathogenesis of acute renal failure. Curr Opin
Nephrol Hypertens. 11:301–308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Havasi A and Borkan SC: Apoptosis and
acute kidney injury. Kidney Int. 80:29–40. 2011. View Article : Google Scholar
|
|
21
|
Yuan L, Wu MJ, Sun HY, et al:
VEGF-modified human embryonic mesenchymal stem cell implantation
enhances protection against cisplatin-induced acute kidney injury.
Am J Physiol Renal Physiol. 300:F207–F218. 2011. View Article : Google Scholar
|
|
22
|
Jo SK, Cho WY, Sung SA, Kim HK and Won NH:
MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by
decreasing inflammation and apoptosis. Kidney Int. 67:458–466.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramesh G and Reeves WB: p38 MAP kinase
inhibition ameliorates cisplatin nephrotoxicity in mice. Am J
Physiol Renal Physiol. 289:F166–F174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang M, Wei Q, Wang J, et al: Regulation
of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis.
Oncogene. 25:4056–4066. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan JX, Zou ZH, You DY, Cui J and Pan YB:
Bone marrow-derived mesenchymal stem cells differentiation into
tubular epithelial-like cells in vitro. Cell Biochem Funct.
30:129–138. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bi B, Schmitt R, Israilova M, Nishio H and
Cantley LG: Stromal cells protect against acute tubular injury via
an endocrine effect. J Am Soc Nephrol. 18:2486–2496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haynesworth SE, Baber MA and Caplan AI:
Cytokine expression by human marrow-derived mesenchymal progenitor
cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell
Physiol. 166:585–592. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weimar IS, Miranda N, Muller EJ, et al:
Hepatocyte growth factor/scatter factor (HGF/SF) is produced by
human bone marrow stromal cells and promotes proliferation,
adhesion and survival of human hematopoietic progenitor cells
(CD34+). Exp Hematol. 26:885–894. 1998.
|
|
29
|
Kim JH, Park DJ, Yun JC, et al: Human
adipose tissue-derived mesenchymal stem cells protect kidneys from
cisplatin nephrotoxicity in rats. Am J Physiol Renal Physiol.
302:F1141–F1150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zarjou A, Kim J, Traylor AM, et al:
Paracrine effects of mesenchymal stem cells in cisplatin-induced
renal injury require heme oxygenase-1. Am J Physiol Renal Physiol.
300:F254–F262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yasuda H, Kato A, Miyaji T, Zhou H, Togawa
A and Hishida A: Insulin-like growth factor-I increases p21
expression and attenuates cisplatin-induced acute renal injury in
rats. Clin Exp Nephrol. 8:27–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nemmar A, Al-Salam S, Zia S, Yasin J, Al
Husseni I and Ali BH: Diesel exhaust particles in the lung
aggravate experimental acute renal failure. Toxicol Sci.
113:267–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du H, Yao W, Fang M and Wu D: ARF triggers
cell G1 arrest by a P53 independent ERK pathway. Mol Cell Biochem.
357:415–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuruya K, Yotsueda H, Ikeda H, et al:
Involvement of p53-transactivated Puma in cisplatin-induced renal
tubular cell death. Life Sci. 83:550–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The international society for cellular therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
36
|
Sheikh-Hamad D, Cacini W, Buckley AR, et
al: Cellular and molecular studies on cisplatin-induced apoptotic
cell death in rat kidney. Arch Toxicol. 78:147–155. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dill T, Schachinger V, Rolf A, et al:
Intracoronary administration of bone marrow-derived progenitor
cells improves left ventricular function in patients at risk for
adverse remodeling after acute ST-segment elevation myocardial
infarction: results of the Reinfusion of Enriched Progenitor cells
And Infarct Remodeling in Acute Myocardial Infarction study
(REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart
J. 157:541–547. 2009.
|
|
38
|
Drexler H, Meyer GP and Wollert KC:
Bone-marrow-derived cell transfer after ST-elevation myocardial
infarction: lessons from the BOOST trial. Nat Clin Pract Cardiovasc
Med. 3(Suppl 1): S65–S68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng YB, Liu XG, Liu ZG, Liu XL, Liu Y and
Zhou GQ: Implantation of BM mesenchymal stem cells into injured
spinal cord elicits de novo neurogenesis and functional recovery:
evidence from a study in rhesus monkeys. Cytotherapy. 8:210–214.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Chen J, Zhang CL, et al: Gliosis and
brain remodeling after treatment of stroke in rats with marrow
stromal cells. Glia. 49:407–417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ezquer F, Ezquer M, Simon V, et al:
Endovenous administration of bone marrow-derived multipotent
mesenchymal stromal cells prevents renal failure in diabetic mice.
Biol Blood Marrow Transplant. 15:1354–1365. 2009. View Article : Google Scholar
|
|
42
|
Ezquer FE, Ezquer ME, Parrau DB, Carpio D,
Yañez AJ and Conget PA: Systemic administration of multipotent
mesenchymal stromal cells reverts hyperglycemia and prevents
nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant.
14:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lieberthal W, Triaca V and Levine J:
Mechanisms of death induced by cisplatin in proximal tubular
epithelial cells: apoptosis vs. necrosis. Am J Physiol.
270:F700–F708. 1996.PubMed/NCBI
|
|
44
|
Ramesh G and Reeves WB: TNFR2-mediated
apoptosis and necrosis in cisplatin-induced acute renal failure. Am
J Physiol Renal Physiol. 285:F610–F618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu H and Baliga R: Cytochrome P450 2E1
null mice provide novel protection against cisplatin-induced
nephrotoxicity and apoptosis. Kidney Int. 63:1687–1696. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hamar P, Song E, Kokeny G, Chen A, Ouyang
N and Lieberman J: Small interfering RNA targeting Fas protects
mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci
USA. 101:14883–14888. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Price PM, Safirstein RL and Megyesi J:
Protection of renal cells from cisplatin toxicity by cell cycle
inhibitors. Am J Physiol Renal Physiol. 286:F378–F384. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Santos NA, Bezerra CS, Martins NM, Curti
C, Bianchi ML and Santos AC: Hydroxyl radical scavenger ameliorates
cisplatin-induced nephrotoxicity by preventing oxidative stress,
redox state unbalance, impairment of energetic metabolism and
apoptosis in rat kidney mitochondria. Cancer Chemother Pharmacol.
61:145–155. 2008. View Article : Google Scholar
|
|
49
|
Owens DM and Keyse SM: Differential
regulation of MAP kinase signalling by dual-specificity protein
phosphatases. Oncogene. 26:3203–3213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rodriguez-Garcia ME, Quiroga AG, Castro J,
Ortiz A, Aller P and Mata F: Inhibition of p38-MAPK potentiates
cisplatin-induced apoptosis via GSH depletion and increases
intracellular drug accumulation in growth-arrested kidney tubular
epithelial cells. Toxicol Sci. 111:413–423. 2009. View Article : Google Scholar
|
|
52
|
Sohn SI, Rim HK, Kim YH, et al: The
ameliorative effect of 23-hydroxytormentic acid isolated from
Rubus coreanus on cisplatin-induced nephrotoxicity in rats.
Biol Pharm Bull. 34:1508–1513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wei Q, Dong G, Franklin J and Dong Z: The
pathological role of Bax in cisplatin nephrotoxicity. Kidney Int.
72:53–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yokoo T, Ohashi T, Shen JS, et al: Human
mesenchymal stem cells in rodent whole-embryo culture are
reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci
USA. 102:3296–3300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Duffield JS and Bonventre JV: Kidney
tubular epithelium is restored without replacement with bone
marrow-derived cells during repair after ischemic injury. Kidney
Int. 68:1956–1961. 2005. View Article : Google Scholar
|
|
56
|
Duffield JS, Park KM, Hsiao LL, et al:
Restoration of tubular epithelial cells during repair of the
postischemic kidney occurs independently of bone marrow-derived
stem cells. J Clin Invest. 115:1743–1755. 2005. View Article : Google Scholar : PubMed/NCBI
|