Introduction
Human bone morphogenetic protein 2 (hBMP2) is a
protein of ossification differentiation, is secreted by human bone
marrow stromal cells (BMSCs), and is an early regulatory substance
(1,2). The ossification differentiation of
BMSCs is a process that involves various types of BMPs that adjust
to each other in a manner similar to that of a reticular system
(3,4). The BMP2 improves its own output as
well as that of other endogenous BMPs secreted from BMSCs (5). Other BMPs have shown an increase
with an increase in the endogenous BMP2, while no marked difference
exists in the ability of the body to respond to BMP2 at different
ages (6). Therefore, BMP2 was
used as the target gene for the treatment of local osteoporosis in
rat femurs. Recent studies have confirmed that BMP2 is capable of
promoting ossification (1,2,7,8).
However, a number of limiting factors, including the difficulty of
isolating natural BMP, tolerating the temperature at 37°C in in
vivo environments and maintaining effective density as well as
short half-life, have made the application of BMP difficult
(9).
If the target gene were imported, coding the cell
factor protein into appropriate target cells through transgenic
techniques, the target cells would continuously secrete this
protein in order to act on the target cells in an autocrine or
paracrine manner. Therefore, by utilizing this process, the problem
of local application of exogenous cell factor would likely be
resolved (10–12). In this study, the density of hBMP2
in marrow cavity was adjusted according to the average volume of
the grown Sprague-Dawley rat femoral marrow cavity as well as the
expression level of hBMP2 secreted by hBMP2 gene-modified BMSCs of
osteoporotic rats. The density of hBMP2 proteins in the rat femoral
marrow cavity was set to 0.15 mg/ml, making the BMSCs osteogenic
and enabling the treatment of local osteoporosis without any side
effects, such as cyst-like bone formation or soft tissue swelling
(13,14).
Lentivirus is a common transgenic carrier used in
laboratories due to its higher infection efficiency and more stable
expression of transfected genes (10,12–15). There are two methodologies for
lentivirus transfection, genetic transfection in vivo and
in vitro (16,17). The definition of genetic
transfection in vivo is the injection of the lentivirus
carrying the target gene into the relevant body part according to a
certain multiplicity of infection (MOI), which could have a
transgenic effect on stem cells. The definition of genetic
infection in vitro is the isolation of the stem cells first
and then mixing of the lentivirus with these cells according to a
certain MOI, followed by transplantation of the infected cells into
the relevant body part. The two transgenic effects caused by these
two procedures have been previously demonstrated (18–21). Additionally, a comparison of the
two transgenic effects may be crucial to determining the lentivirus
infection method to be used.
In most in vitro or in vivo genetic
infection experiments with lentivirus, normal BMSCs were used as
the experimental materials (20–23). However, if normal human BMSCs were
to be used in clinical practice, osteoporosis patients could face
many practical issues, including shortage of normal BMSCs,
excessive treatment costs and ethical/moral questions. Since the
twentieth century, medical technology has been radically
transformed as a modern science with a focus on numerous
technological breakthroughs. However, the accessibility of such
technology, its cost-effectiveness and the adverse effects to the
society remain to be clarified. Subsequently, the BMSCs of
osteoporotic rats were used as the experimental materials to treat
local osteoporosis in rat femurs. The long-term purpose of our
experiment was to lay the foundation for the treatment of local
osteoporosis in patients by transplanting their own BMSCs infected
with overexpressed genes that could ameliorate local osteoporosis.
Additionally, the aim was to identify an economically viable
treatment for local osteoporosis.
Materials and methods
Study design
Sprague-Dawley rats (SD rats) were divided into the:
osteoporosis, normal and sham groups. Postmenopausal osteoporosis
models were constructed by removing the ovaries in the osteoporotic
group. The control group was created by removing the adipose tissue
around the ovaries in the sham group. After three months, the rat
BMSCs (rBMSCs) were isolated from the femurs in the osteoporotic
and normal groups and were compared by colony-forming
units-fibroblastic (CFU-F) counting, osteoblast ALP staining
(improved Gomori calcium-cobalt method), and osteogenic capability
analysis (ALP activity, OCN level). The average number of rBMSCs
isolated from a femur in each group was calculated based on the
CFU-F counting. The osteogenic capability of rBMSCs was compared
among groups by osteoblast ALP staining and osteogenic capability
analysis. The lentiviral vector-mediated hBMP2 overexpressed in
rBMSCs was constructed to be used in the osteoporotic group in
vitro (rBMSCs in OE group). The osteogenic capability (ALP
activity, OCN level) of rBMSCs in the OE group was compared to that
of the MSCs CON lengthways. After the comparison, five study groups
were designed using SD rats, including the transplanted group of
lentiviral vector-mediated hBMP2 overexpressed (OE) cells,
transplanted group of lentiviral vector-mediated hBMP2 negative
control (NC) cells, injected group of lentivirus, transplanted
group of rat bone marrow stromal (MSCs OVX) cells and the saline
control group. Postmenopausal osteoporosis models were constructed
again with all five groups. The rBMSCs in the OE group (transplants
of genetic infection in vitro), lentivirus-containing
solution (injected material of genetic infection in vivo)
and some control materials were injected into the osteoporotic
femurs of the corresponding groups. After three months, the
treatment effects were compared in each group through whole femoral
BMD and bone histomorphometry in the distal part of the femur.
Animals and osteoporosis models
Forty-one female SD rats of ~6-month-old, weighing
400–430 g at the start of the experiment, were obtained from the
Amimal Experiment Center of the Second Affiliated Hospital, Harbin
Medical University. Rats were randomly divided into the
osteoporosis (MSCs OVX), normal (MSCs CON) and sham (SHAM CON)
groups. The MSCs OVX and MSCs CON groups comprised 17 rats each and
the SHAM CON comprised 7 rats. The rats in the MSCs OVX and SHAM
CON groups were anesthetized by intraperitoneal injection of 5%
chloralic hydras (0.66 ml/100 g). The ovaries of rats in MSCs OVX
were removed to construct the postmenopausal osteoporosis models
(PO models) and the adipose tissue around the ovaries of rats in
SHAM CON were removed in order to create a control group. After
three months, 7 rats were sacrificed from each group. Measurements
were obtained for: content of estrogens in serum, bone mineral
density (BMD) of the right femoral distal 1/3 section, uterus wet
weight and left femur dry weight. Furthermore, sections of the
right femur were stained to verify the PO models. Subsequent to
verification of the PO models, 10 rats were left in the MSCs OVX
and MSCs CON groups, respectively, while no rats were left in the
SHAM CON group. The use of the samples was approved by the local
healthcare authorities and Ethics Committee (The Guide for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health, NIH Publication No. 85-23, revised 1985).
Isolation of rBMSCs
The nucleated cells containing rBMSCs were isolated
from the left femurs of the remaining SD rats in the MSCs OVX and
MSCs CON groups under sterile conditions according to the protocol
reported by Maniatopoulos et al (24). Briefly, the two ends of the femora
were cut off at the epiphysis and marrow was flushed out using low
glucose Dulbecco's modified Eagle's medium (L-DMEM) (Gibco BRL,
Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine
serum (FBS) (HyClone, Logan, UT, USA) and 200 U/ml of heparin
(Sigma, St. Louis, MO, USA). The cells were gently kneaded in
L-DMEM containing 10% FBS in order to obtain a single-cell
suspension. Trypan blue (Guangzhou Genebase Bioscience Co., Ltd.,
Guangzhou, China) staining was used to analyze the cell activity
prior to cell culture. The percentage of cell activity was
calculated according to the formula: Cell activity (%) = [(total
cells - colored cells)/total cells] × 100%. The percentage of cell
activity was >96% in all the groups. Procedures were performed
at a facility accredited by the Institutional Animal Care and Use
Committee of Harbin Medical University. The culture medium was
changed on the 6th day for the first time and then non-adherent
cells were abandoned. When 90% confluency was reached, the BMSCs
were released from the culture substratum using trypsin/EDTA (0.25%
w/v trypsin, 0.02% EDTA) and were moved to culture dishes (10 cm in
diameter) at 1.0×105 cells/ml in 10 ml. The
characterization of rat BMSCs was validated by a flow cytometry
assay, as previously described (25).
Counting of CFU-Fs
Passage (P) 0 was randomly selected from each group
in rBMSCs (n=5). The method of CFU-Fs counting was performed
according to the protocol reported by Nishida et al
(26). The cells isolated from
the rat femur were gently kneaded and 10 ml of single-cell
suspension was prepared with all the cells (suspension A). We drew
10 μl of suspension A (suspension B) and dispersed it in 10 ml of
L-DMEM containing 10% FBS to prepare suspension C. A total of 2.5
ml of suspension C was cultured in each well of a 6-well plate. The
media were changed on the sixth day for the first time and the
non-adherent cells were abandoned. On the 10th day, the plate was
placed under an inverted microscope and the number of CFU-F was
counted in every well.
ALP staining of osteoblasts
The improved Gomori calcium-cobalt method (27) was utilized to perform ALP staining
in osteoblasts. P2 rBMSCs were chosen in each group (n=3) to
produce rBMSCs suspension in osteogenic induction medium (OI
medium) containing high-glucose Dulbecco's modified Eagle's medium
(H-DMEM) (Gibco BRL) including 0.1 μmol/l dexamethasone, 10 mmol/l
β-glycerophosphate, and 50 mg/l ascorbic acid. The rBMSCs were
cultured at a density of 1×105/ml. The OI medium was
changed every three days. After 28 days, the cells were removed and
cultured in 24-well plates containing sterile cell slides (Nunc,
Denmark) at a density of 2×104/ml with the same OI
medium for five days. The cell slides were prepared with 95%
ethanol, incubation solution (5 ml of 3% β-glycerophosphate; 5 ml
of 2% barbital sodium; 10 ml of distilled water; 10 ml of 2%
CaCl2; and 1 ml of 2% MgSO4), 2% cobalt
nitrate, and 1% ammonium sulphate. Images of the stained rBMSCs
after OI were captured in each group under a confocal laser
scanning microscope (FV1000, Olympus, Tokyo, Japan).
Osteogenic capability analysis
ALP activity and the osteocalcin (OCN) level were
detected, which reflected the osteogenic ability of rBMSCs. P3
rBMSCs in each group (n=5) were chosen to produce the rBMSC
suspensions at a density of 1×105/ml by OI media. The OI
media were changed every three days. The rBMSC ALP activity was
separately detected on the 7th and 14th day of OI. The rBMSC
supernatants were separately collected on the 14th, 21st, and 28th
day in order to detect the OCN level. The ALP activity assay kit
(Sigma) using p-nitrophenyl phosphate (p-NPP) as a substrate in
order was employed to determine the rBMSC ALP activity. Rat OCN
enzyme-linked immunoabsorbent assay (ELISA) kit (Westang Bio-tech,
Shanghai, China) was used to detect the OCN level.
Lentiviral vector construction, virus
production and infection
The hBMP2 cDNA was amplified from the human
osteosarcoma MG-63 cell line by polymerase chain reaction (PCR)
with the primers described in Table
I. The hBMP2 cDNA was subcloned in the lentiviral
vector-plasmid (GV208, Ubi-MCS-EGFP, Genechem, Shanghai, China),
and the hBMP2 gene overexpressing the lentiviral vector-plasmid
(Ubi-MCS-hBMP2-EGFP) was then utilized.
 | Table IhBMP2 primers.a |
Table I
hBMP2 primers.a
| Gene | Primers |
|---|
| hBMP2 |
5′-GAGGATCCCCGGGTACCGGTCGCCACCA
TGGTGGCCGGGACCCGCTGTC-3′ (forward) |
|
5′-TCACCATGGTGGCGACCGGGCGACACCC
ACAACCCTCCAC-3′ (reverse) |
Lentiviral vector-plasmid (Ubi-MCS-EGFP) and hBMP2
gene overexpressing lentiviral vector-plasmid (Ubi-MCS-hBMP2-EGFP)
were used to transfect the 293T cells using Lipofectamine™ 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). Therefore, the
lentivirus containing hBMP2 cDNA (Lv-BMP) as well as that
containing GFP cDNA (Lv-GFP) were obtained for subsequent analysis.
The lentivirus titer was detected by quantitative PCR (qPCR) (the
titer of Lv-GFP: 8 E+8 TU/ml, the titer of Lv-BMP: 2 E+8
TU/ml).
At MOI of 20 and 40, in the presence of 8 μg/ml
polybrene (Sigma), P3 rBMSCs of MSCs OVX were infected with Lv-GFP
and designated as the hBMP2-negative control group (NC group).
Similarly, the P3 rBMSCs of MSCs OVX were infected with Lv-BMP and
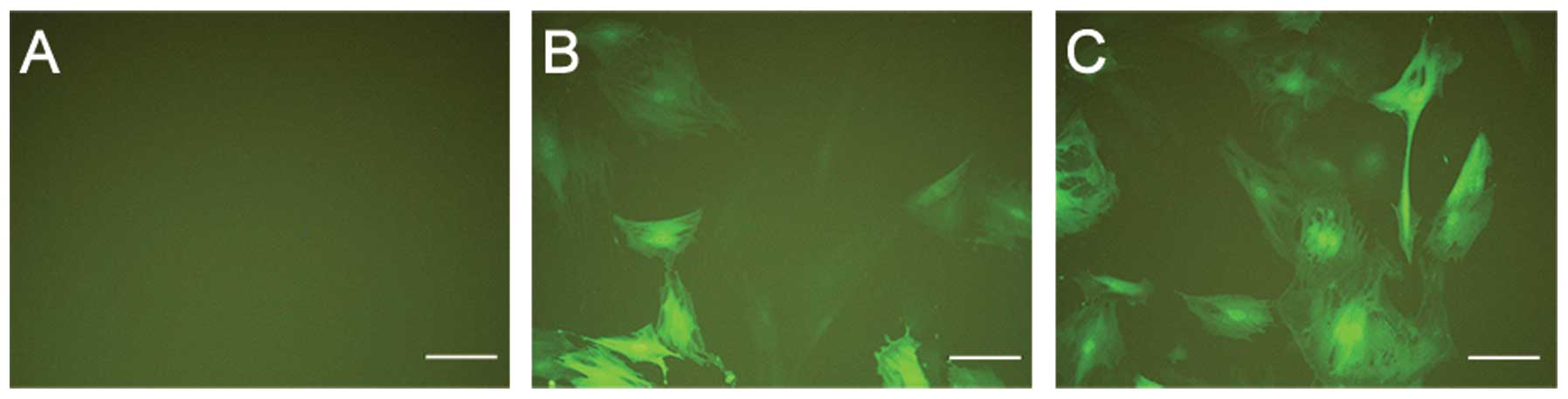
were designated as the hBMP2 OE group (Fig. 1). According to the MOI gradient,
five groups (n=3 per group) were identified: rBMSCs of MSCs OVX not
infected with lentivirus (control group), rBMSCs of MSCs OVX
infected with Lv-BMP at a MOI of 20 (low OE group), rBMSCs of MSCs
OVX infected with Lv-BMP at a MOI of 40 (high OE group), rBMSCs of
MSCs OVX infected with Lv-GFP at a MOI of 20 (low NC group) and
rBMSCs of MSCs OVX infected with Lv-GFP at a MOI of 40 (high NC
group). The aim was to identify a better MOI capable of producing
better infection by detecting and comparing the relative mRNA
expression in these groups.
qPCR analysis
Gene-specific primer sets were designed for hBMP2
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table II) to detect the relative mRNA
expression. The rBMSCs were collected from all five groups
described above on day seven after the infection. Total RNA was
extracted using the TRIzol (Invitrogen Life Technologies) from each
cell sample and the cDNA was synthesized from the total RNA. qPCR
was carried out using SYBR-Green I (Molecular Probes, Eugene, OR,
USA) in a Rotor-Gene 3000 (Corbett Research, Sydney, Australia). To
correct for differences in the RNA quality and quantity among
samples, the data were normalized to those of the GAPDH. The
melting curves were made after the qPCR was finished.
 | Table IIGene-specific primers for hBMP2 and
GAPDH.a |
Table II
Gene-specific primers for hBMP2 and
GAPDH.a
| Gene | Primers |
|---|
| hBMP2 |
5′-CATGCCATTGTTCAGACG-3′
(forward)
5′-TGTACTAGCGACACCCACA-3′ (reverse) |
| GAPDH |
5′-TTCAACGGCACAGTCAAGG-3′
(forward)
5′-CTCAGCACCAGCATCACC-3′ (reverse) |
ELISA analysis
According to the improved MOI detected by qPCR
analysis, the P3 rBMSCs in MSCs OVX were separately infected by
Lv-BMP and Lv-GFP. The infected rBMSCs were separately designated
as P3′ rBMSCs in the OE and NC groups. The rBMSCs lost contact
inhibition, i.e., the cell growth rate was retarded and the cells
began to overlap and gather on the 9th day as verified through
direct observation of the P3′ rBMSCs in the OE group with OI media.
While the BMSCs lost contact inhibition, the cells began to form
osteoid granules. The density of hBMP2 proteins secreted by the
rBMSCs with OI media was detected in each group (n=5 per group) on
the 7th and 9th day of OI, followed by the transplantation of the
cells on the 9th day. The groups included P3′ rBMSCs in the OE
group, P3′ rBMSCs in the NC group and P3 rBMSCs in MSCs OVX. The
rats hBMP2 ELISA kit (R&D Systems, Minneapolis, MN, USA) was
used to detect the density of hBMP2 proteins. The media were
changed every 24 h during the ELISA detection period.
rBMSCs transplantation
The ovaries of 35 SD rats were removed six months
later and were denoted second MSCs OVX. After three months, seven
rats were randomly selected from the second MSCs OVX to be compared
with the second MSCs CON (n=7) and second SHAM CON (n=7) via the
experimental indices described above. After construction of the
osteoporosis models, the remaining rats in the second MSCs OVX
group (n=28) were equally divided into four groups. These groups
(n=7 per group) were designated as: transplanted group of OE cells
(group of genetic infection in vitro), transplanted group of
NC cells, injected group of Lv-BMP (group of genetic infection
in vivo) and transplanted group of MSCs OVX cells.
The rats were anesthetized with 5% chloral hydrate
in 0.66 ml/100 g through introperitoneal injection. The rats were
placed in prone position and the posterolateral side of the hip was
chosen for the surgical approach. After the skin, fascia and
muscular tissues were removed, we found the trochanter and smoothed
its interior. The interior part of the trochanter connected with
the femoral neck was determined to be the point of penetration. A
mini electric drill (E-504, Shuangxi, Shanghai, China) containing a
1-mm diameter drill was used to rub the cortical bone in order to
expose the spongy bone at this point. A syringe needle was then
used to drill through this point.
To achieve an improved effect in treating local
osteoporosis, without introducing side effects such as cyst-like
bone formation and soft tissue swelling, the density of hBMP2
proteins in the marrow cavity had to be maintained at ~0.15 mg/ml
(13,14). Therefore, the total number of
hBMP2 proteins secreted by the transplanted cells had to be
maintained at ~0.12 mg in the adult rat femoral cavity. The number
of transplanted rBMSCs (5.5×108 cells) in the femur was
counted, according to the average hBMP2 protein expression level of
the OE group, which was counted via ELISA analysis on the 9th day
of the OI. On the 9th day of OI, suspensions of P3 (P3′) rBMSCs
from three groups (OE, NC and MSCs OVX) were separately created
with 0.5 ml saline at a density of 1.1×109/ml. The 10 μl
finnpipettes (MC, Anting finnpipettes factory, Shanghai, China)
were used to inject the rBMSCs suspensions in the left femurs of
three corresponding groups (group of genetic infection in
vitro, transplanted group of NC cells and transplanted group of
MSCs OVX cells). Furthermore, phosphate-buffered saline was
injected in the right femurs of all four groups. The range of the
injection (from the spongy bone on the top of femur to the spongy
bone in the bottom) was divided into five equal sections, where 0.1
ml of the suspension was injected in each section. Some scraps were
made by muscle and fascia tissues, which were used to fill the
point left by the drill. After the incisions were washed by saline,
they were sutured.
The amount of Lv-BMP liquid (8 μl) injected in the
femur in the group of genetic infection in vivo was
calculated according to the average amount of P0 rBMSCs in the
femur of MSCs OVX, which was calculated by CFU-Fs counting. The 10
μl finnpipettes were used to inject 1.6 μl of the Lv-BMP liquid in
each section according to the method described above. After the
rats were awake, they were fed with fresh water and kept in
separate cages. A total of 160,000 units of penicillin was injected
in each rat's inner thigh via intramuscular injection (two times a
day, for 7 days) and they were then left in their groups. After ~2
weeks, the walking abilities of all the rats were recovered.
Whole femoral BMD
After three months, the rats were anesthetized by 5%
chloral hydrate in 0.66 ml/100 g through introperitoneal injection.
The femoral BMD was measured in the rats through a dual energy
X-ray absorptiometry (QDR-2000 PLUS, Hologic Inc., Waltham, MA,
USA) with the rat whole body software package (version 5.73).
Bone histomorphometry analysis
Bone histomorphometry was used in the distal part of
the femur (28,29) to compare the change of trabeculars
in the groups. The femur was fixed in 70% ethanol and was cut in
half through the median sagittal plane. The experimental material,
which was located 3–7 mm from the lowest point of the growth plate
and 1 mm from the lateral cortex, was collected, with the exception
of the endocortical region. These materials were embedded and were
classified as undecalcified bone sections. A microtome (SM 2000R,
Leica, Mannheim, Germany) was used to prepare the bone sections. A
total of 12 sections (5 μm), were prepared from each material. Two
sections were randomly selected and placed on a slide and a total
of six slides were prepared. The first and the second sections were
used to prepare the toluidine blue stain. The third and fourth
sections were used to prepare the hematoxylin and eosin stain (HE
stain). The 5th and 6th sections were used to prepare the bone
histomorphometry. Five visual fields (the selected area was the
secondary spongiosa area, which was rich in trabecular bone) were
randomly selected under microscope for each slide and a digital
camera connected to the microscope was used to capture images. A
computerized automatic image analyzing system (VIDAS2.1, Opton,
Germany) was used to detect the area percentage of the bone
trabeculars (Tb.Ar/T.Ar), trabecular number (Tb.N), trabecular
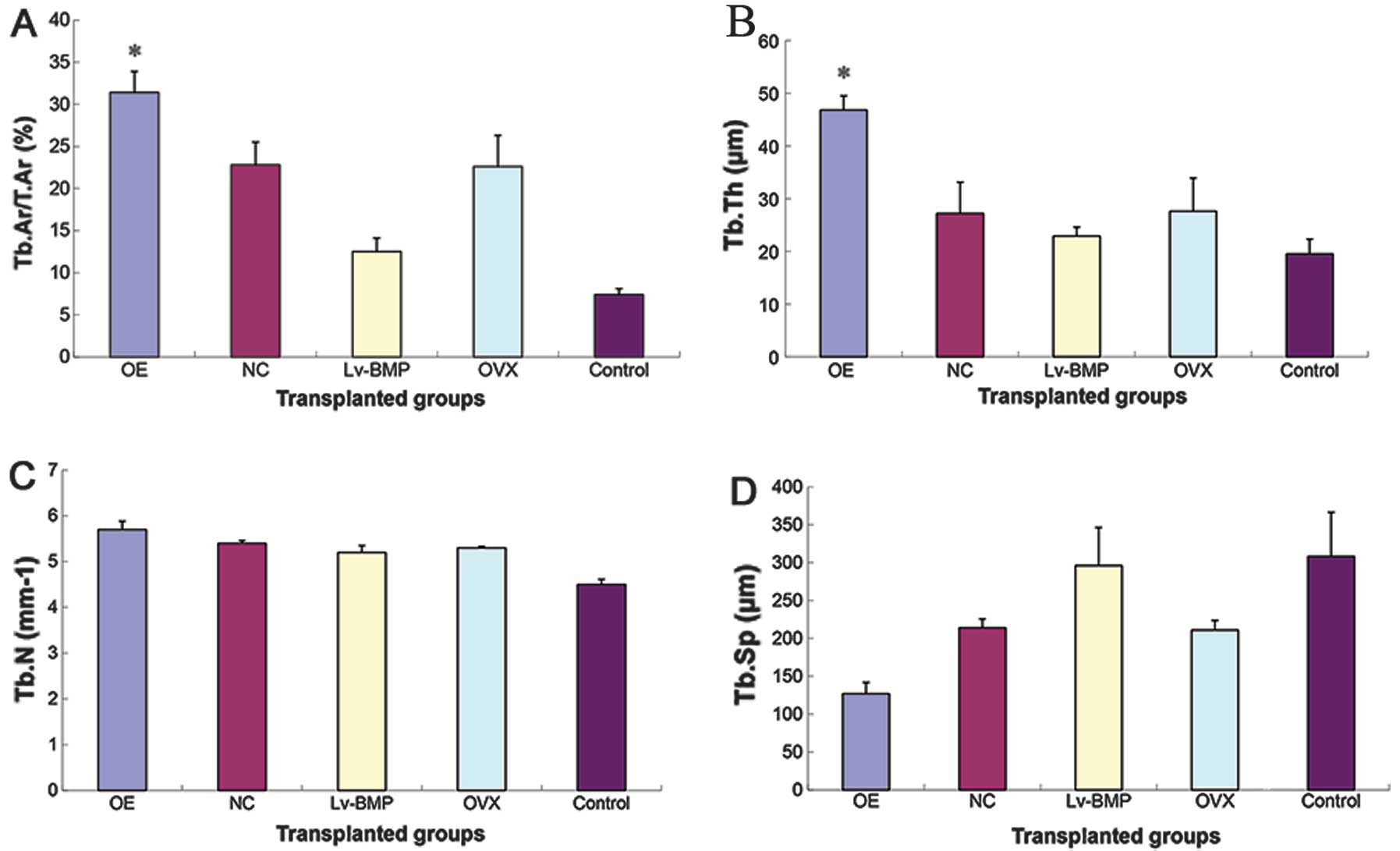
thickness (Tb.Th), and trabecular separation (Tb.Sp).
Statistical analysis
Data are expressed as means ± standard deviation
(SD). Statistical analysis was performed by analysis of variance
(ANOVA) using SPSS16.0 statistical software. Differences were
considered statistically significant when P<0.05.
Results
Osteoporosis models
The PO models of SD rats were constructed twice. The
first construction of PO models (Table III) was performed before the
isolation of rBMSCs, and the second construction of PO models
(Table IV) was performed before
the rBMSCs transplantation. There were no statistical differences
between MSCs CON (2nd MSCs CON) and SHAM CON (2nd SHAM CON)
(P>0.05), thus the influence of the surgery on the result of PO
models was eliminated. The results showed that the serum content of
estrogens and uterus wet weight were lower in MSCs OVX (2nd MSCs
OVX) as compared to that of the MSCs CON (2nd MSCs CON)
(P<0.05), suggesting that the content of estrogens clearly
decreased after the construction of PO models. Furthermore, the
observation of a lower BMD in the right femoral distal 1/3 section
and left femur dry weight in MSCs OVX (2nd MSCs OVX) as compared to
that of the MSCs CON (2nd MSCs CON) (P<0.05) suggested a
decrease of bone tissue quantity in unit volume after the
construction of PO models. The results of these experimental
indices (Tables III and
IV; Fig. 2) demonstrated that the
construction of PO models was successful.
 | Table IIIStatistical results of experiment
indices after the first construction of the postmenopausal
osteoporosis models. |
Table III
Statistical results of experiment
indices after the first construction of the postmenopausal
osteoporosis models.
| Experiment indices
(n=7) | MSCs CON | MSCs OVX | SHAM CON |
|---|
| Content of
estrogens (OD) | 0.670±0.01 | 0.563±0.047a | 0.653±0.023c |
| Wet weight of
uterus (g) | 0.342±0.025 | 0.133±0.007a | 0.336±0.057c |
| Dry weight of femur
(g/cm3) | 1.58±0.08 | 1.24±0.03b | 1.55±0.11c |
| BMD
(g/cm2) | 0.236±0.008 | 0.178±0.004a | 0.227±0.006c |
 | Table IVStatistical results of experiment
indices after the second construction of the postmenopausal
osteoporosis models. |
Table IV
Statistical results of experiment
indices after the second construction of the postmenopausal
osteoporosis models.
| Experiment indices
(n=7) | 2nd MSCs CON | 2nd MSCs OVX | 2nd SHAM CON |
|---|
| Content of
estrogens (OD) | 0.639±0.041 | 0.522±0.031a | 0.627±0.063c |
| Wet weight of
uterus (g) | 0.331±0.018 | 0.205±0.027a | 0.329±0.015c |
| Dry weight of femur
(g/cm3) | 1.53±0.12 | 1.21±0.007b | 1.49±0.16c |
| BMD
(g/cm2) | 0.243±0.015 | 0.171±0.005a | 0.24±0.011c |
Counting of CFU-Fs, osteoblast ALP
staining and osteogenic capability analysis
One CFU-F is formed by cells proliferating from a
single P0 of BMSCs in vitro, such that the number of CFU-Fs
stands for the number of BMSCs in marrow cavity (30,31). The number of CFU-Fs in MSCs CON
and MSCs OVX were 53.74±3.95/well and 39.89±1.72/well,
respectively. The average amount of rBMSCs in the MSCs OVX and MSCs
CON femurs (1.6×104±153.27 in MSCs OVX vs.
2.1×104±114.6 in MSCs CON, P<0.01) was counted
according to the number of CFU-Fs.
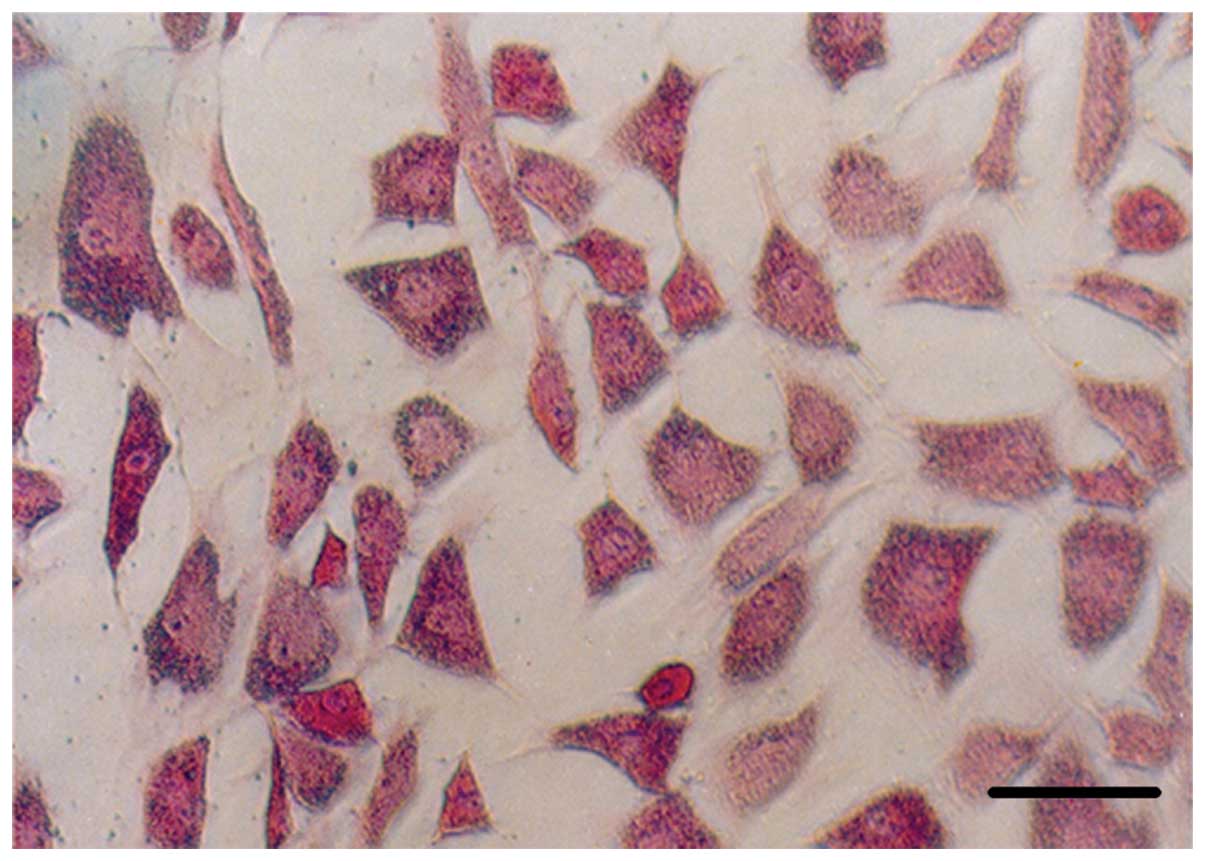
The OI osteoblasts in each group presented stacked
layouts and there were no differences in the shape of the cells or
in their CFU-F growth form between the two groups. Over 90% of the
cells in each group presented as ALP-positive stained and there
were dark gray or dark black granules in the cytoplasm of the
osteoblast in each group (Fig.
3). There was no difference between the two groups after OI and
ALP staining.
An important osteogenic indication of BMSCs may be
that the cells synthesized and secreted special osteogenic proteins
and extracellular matrices including ALP, type I collagen, BMP
(appeared early in the process of bone formation) and OCN (appeared
late). ALP has been reported to reflect the osteogenic level of
BMSCs (32,33) and OCN has been reported to promote
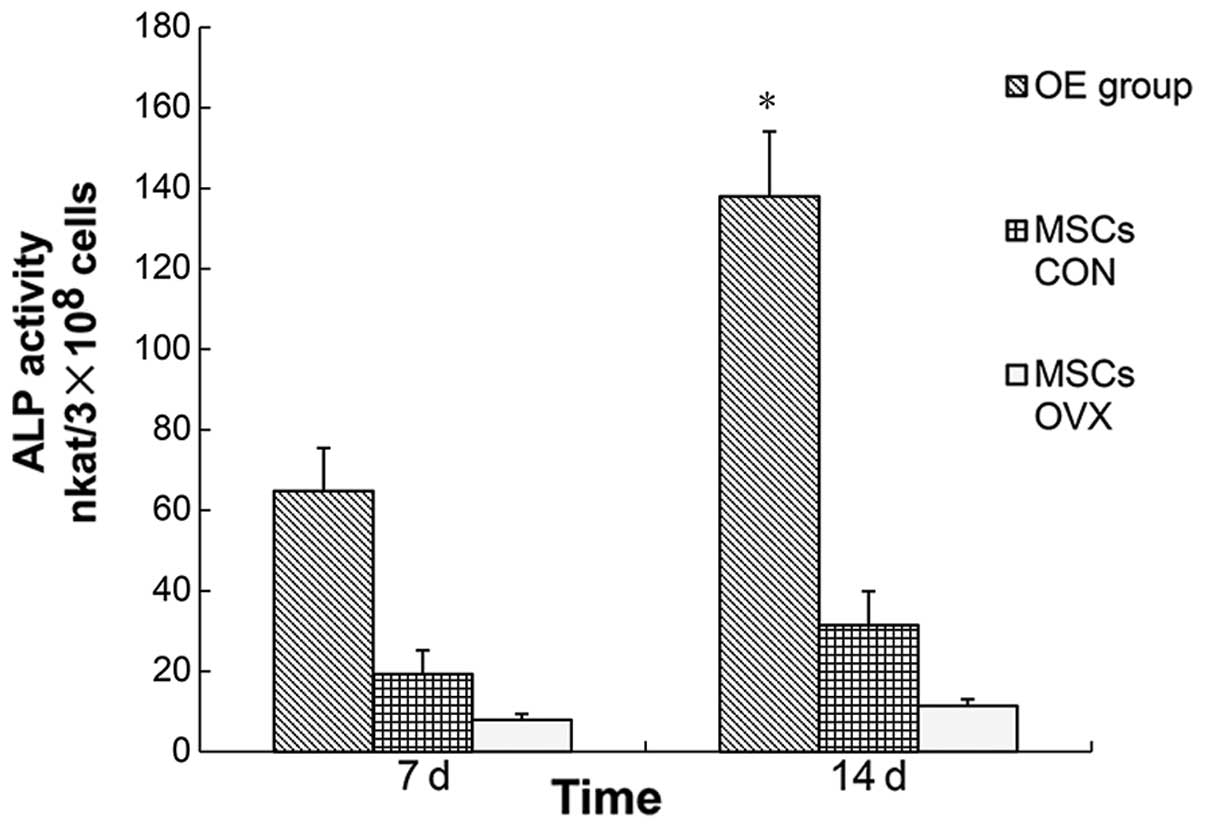
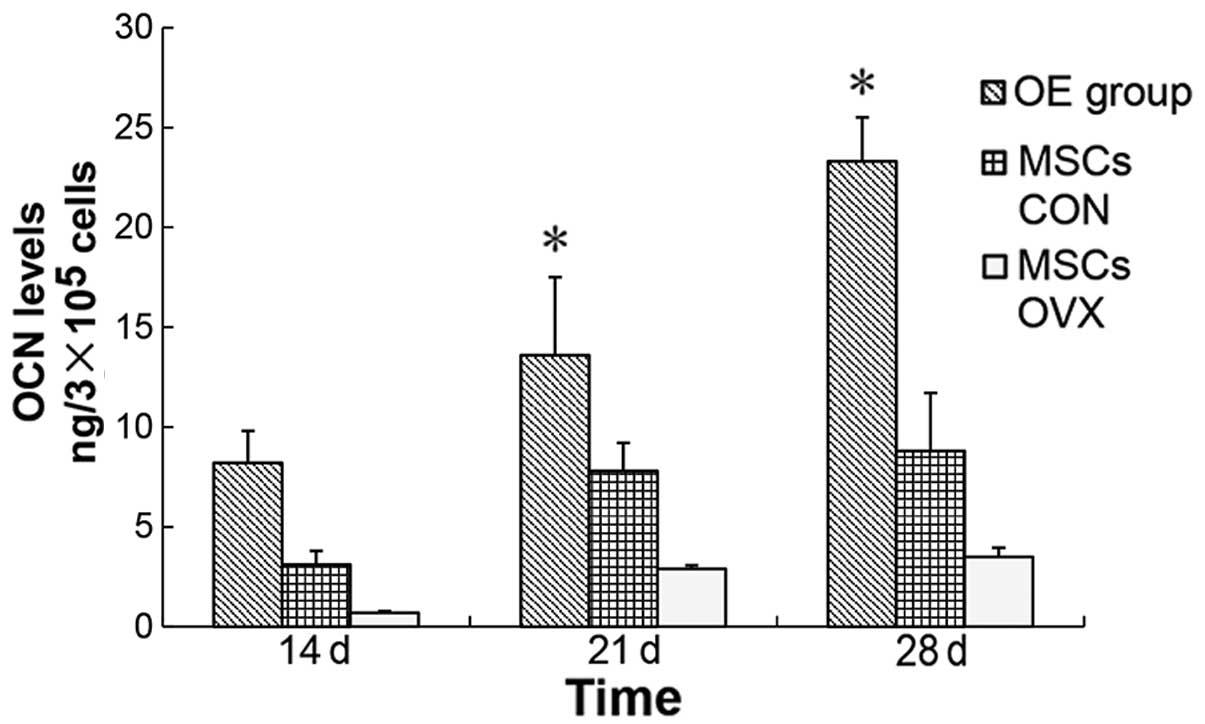
a normal process of mineralization in bone (34). We detected ALP activity (Fig. 4) and the OCN level (Fig. 5) to reflect the osteogenic ability
of rBMSCs. When the osteogenic capabilities were detected in MSCs
CON and MSCs OVX, statistical differences were evident (ALP
activity: 29.14 vs. 10.15 nkat/3×108 cells, P<0.05;
OCN level: 8.35 vs. 2.47 ng/3×105 cells, P<0.05).
Therefore, if rBMSCs in MSCs OVX were used to treat local
osteoporosis, the effect would have not been ideal. Since BMP gene
delivery increased ALP expression in BMSCs (35), we constructed Lv-BMP to infect the
rBMSCs in MSCs OVX and the osteogenic capabilities between the OE
group and MSCs CON were compared. The results of this comparison
indicated that the osteogenic capability of rBMSCs in the OE group
markedly increased and statistical differences (ALP activity:
137.57 vs. 29.14 nkat/3×108 cells, P<0.01; OCN level:
24.16 vs. 8.35 ng/3×105 cells, P<0.01) were reported
as compared to that of the MSCs CON. Therefore, if rBMSCs were used
as the transplanted materials in order to treat the local
osteoporosis, rBMSCs in the OE group would potentially be more
appropriate.
qPCR and ELISA
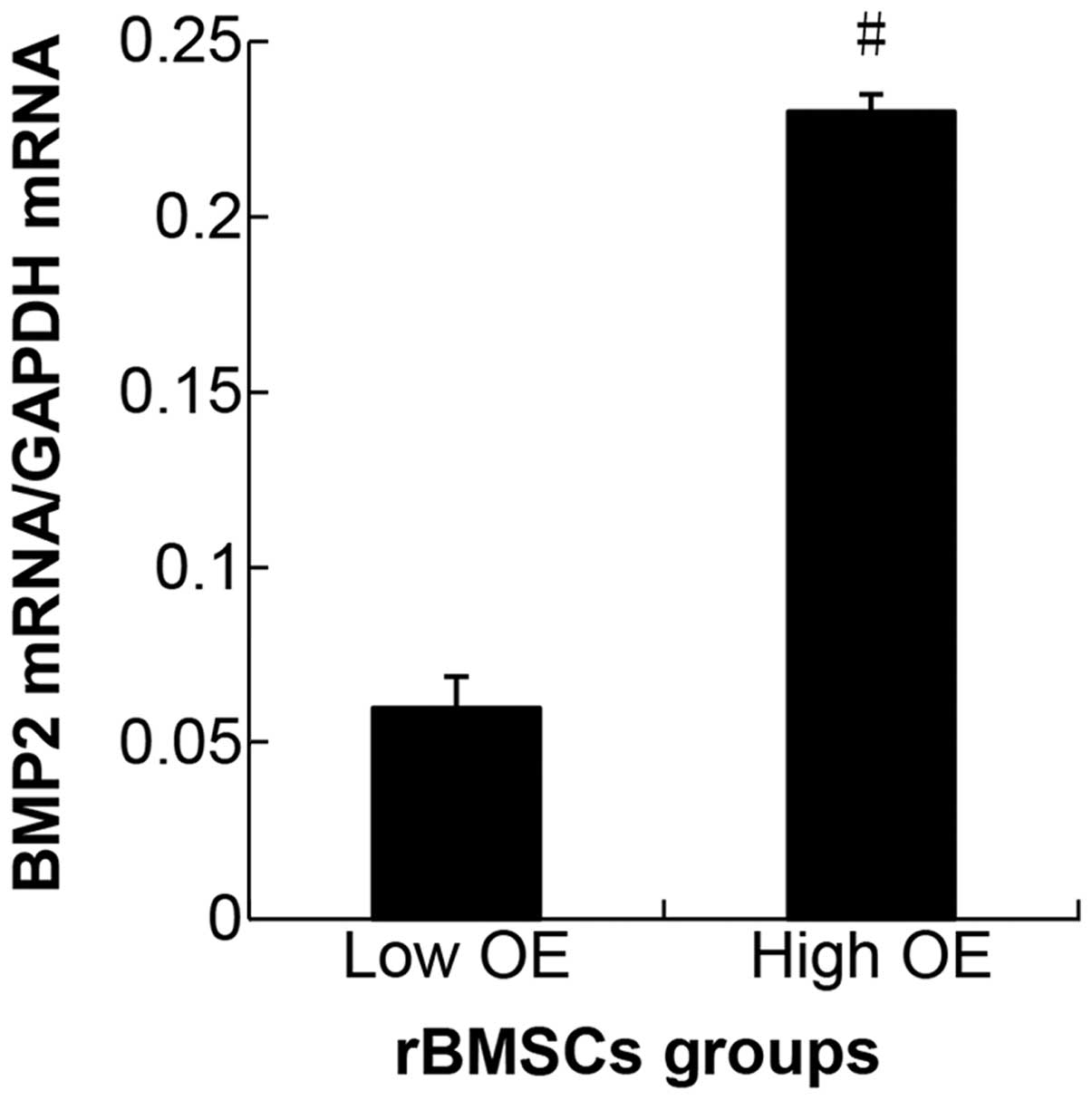
The qPCR results are shown in Fig. 6. The mRNA expression level of
hBMP2 gene in the control and NC groups was undetectable. A
significant difference of hBMP2 expression was detected between the
high and low OE groups (P<0.05). As a result, the mRNA
expression level of the hBMP2 gene reflected a higher lentiviral
gene transfer efficiency that was obtained at a MOI of 40. On the
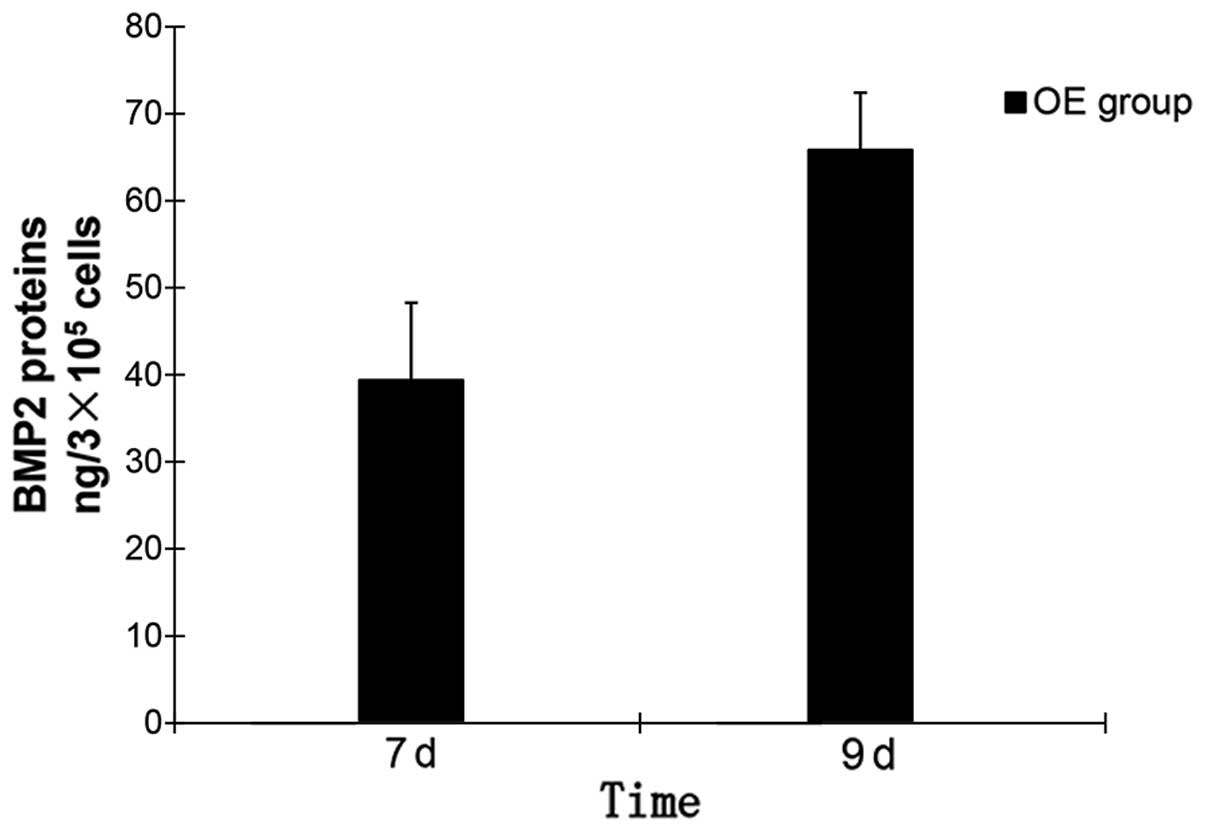
9th day of OI, the ELISA result (Fig.
7) showed that the density of hBMP2 proteins in the OE group
was 65.79 ng/3×105 cells on average. This indicated that
1×105 cells in OE group secreted an average of 21.93 ng
hBMP2 protein on the 9th day of OI. The density of these hBMP2
proteins in MSCs OVX and the NC group were negative. The qPCR and
ELISA results showed that the hBMP2 gene was successfully expressed
in rBMSCs through lentivirus-mediated infection.
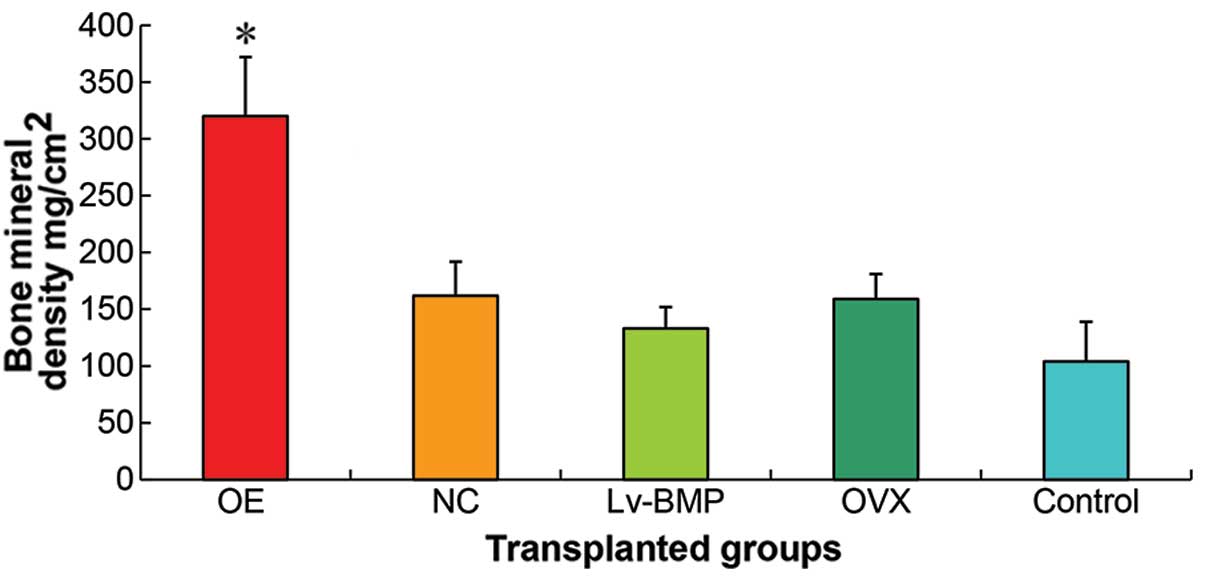
Whole femoral BMD
Results of the whole femoral BMD (Fig. 8) suggested that the group of
genetic infection in vitro had a higher expression as
compared to the group of genetic infection in vivo
(P<0.01). There were no statistical differences (P>0.05)
between the transplanted group of MSCs OVX cells and transplanted
group of NC cells, thus they were designated as the transplanted
group of OVX cells (NC cells). The whole femoral BMD in the
transplanted group of OVX cells (NC cells) was higher than that of
the group of genetic infection in vivo (P<0.05).
Statistical differences (P<0.05) were observed between the group
of genetic infection in vivo and the control group of
saline.
Bone histomorphometry
The results of bone histomorphometry (Fig. 9) suggested there were no
statistical differences in Tb.N (P>0.05) between the groups.
Results of the other three structural bone histomorphometric
parameters (Tb.Ar/T.Ar, Tb.Th, Tb.Sp) showed that the group of
genetic infection in vitro had the highest Tb.Ar/T.Ar and
Tb.Th among all the groups, but the lowest Tb.Sp. Obvious
statistical differences (P<0.01) were observed between the group
of genetic infection in vitro and the remaining groups. No
statistical differences (P>0.05) were observed between the
transplanted group of MSCs OVX cells and the transplanted group of
NC cells, thus these two groups were designated as the transplanted
group of OVX cells (NC cells). The transplanted group of OVX cells
(NC cells) had obvious statistical differences (P<0.01) with
respect to Tb.Ar/T.Ar, Tb.Sp as compared to the group of genetic
infection in vivo and the control group of saline.
Similarly, statistical difference (P<0.05) with respect to Tb.Th
was observed. The group of genetic infection in vivo had
statistical differences (P<0.05) with respect to the Tb.Ar/T.Ar
and Tb.Th as compared to the control group of saline. However, with
respect to the Tb.Sp, no statistical differences were observed
(P>0.05).
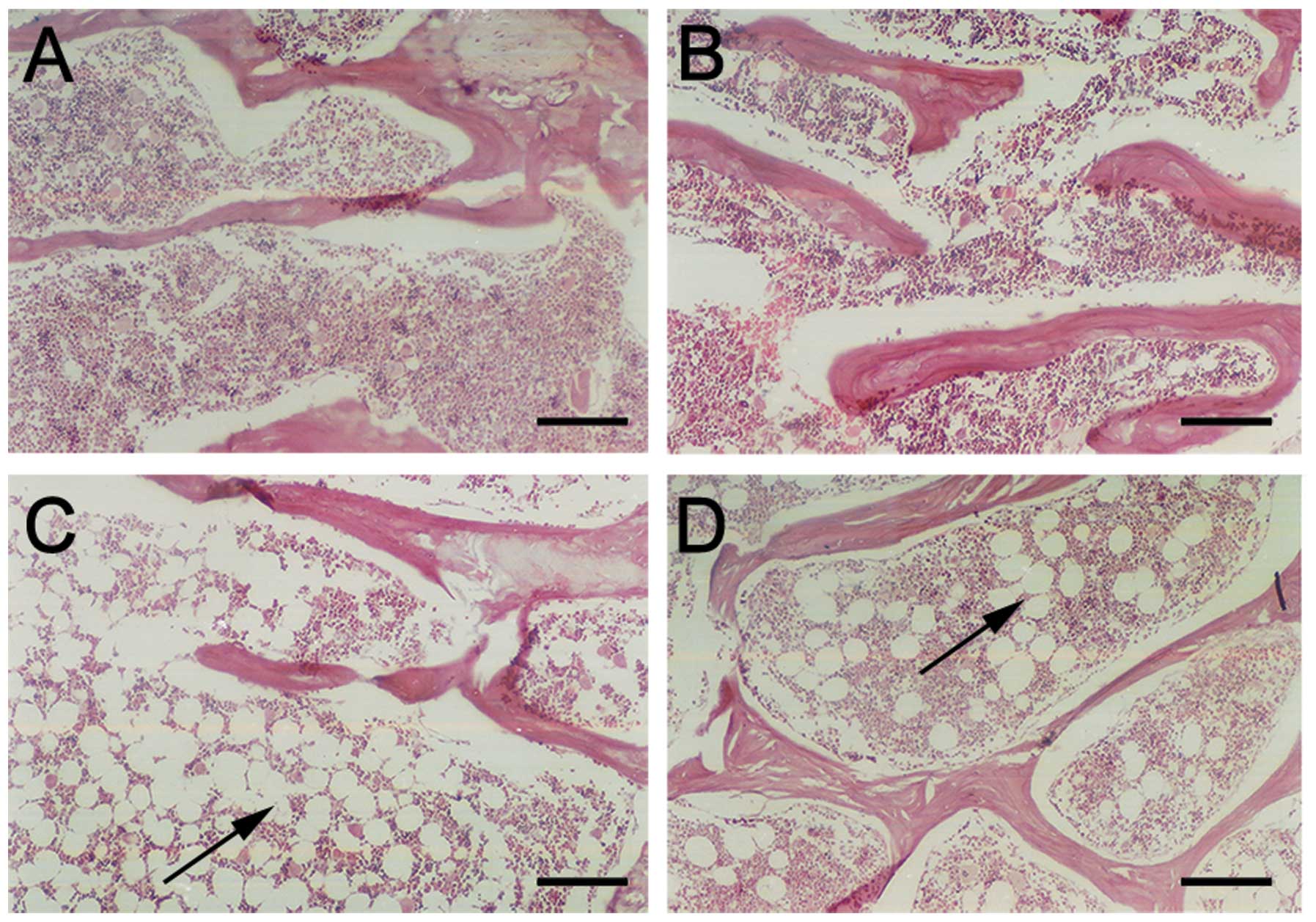
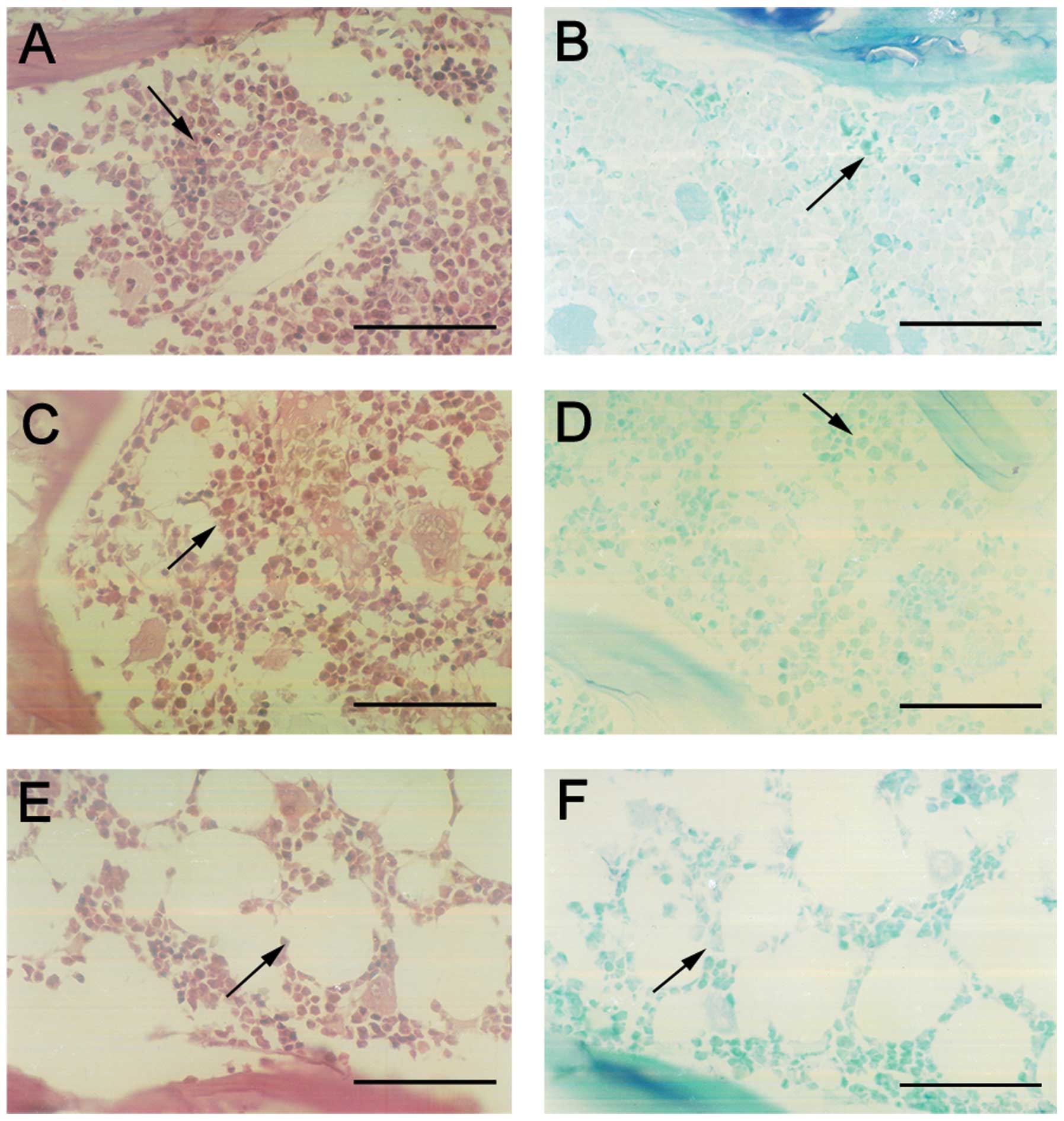
Femoral section stain
The femoral sections were stained by toluidine blue
and HE stains (Fig. 10). The
bone trabeculars in the group of genetic infection in vitro
were thicker, closer and in alignment as compared to the other
groups, while the symptoms of osteoporosis, such as rupture and
blind ends occured less than that of the other groups. The bone
trabeculars in the group of genetic infection in vivo were
the most similar to those of the control group of saline. The shape
of the bone trabeculars exhibited symptoms of osteoporosis such as
thinning, rupture, sparsity and blind ends. No cyst-like bone
formation and soft tissue swelling were observed.
Discussion
The present study demonstrated that the treatment
effect of Lv-BMP-mediated hBMP2 gene overexpression in the
osteoporotic rBMSCs, which was created by in vitro genetic
infection in local osteoporosis was improved compared with that
created by genetic infection in vivo. We analyzed three
possible causes for this observation. The amount of rBMSCs in MSCs
OVX and MSCs CON (1.6×104±153.27 vs.
2.1×104±114.6, P<0.01) suggested that rBMSCs in the
femoral marrow cavity of MSCs OVX were rare. Even if all the rBMSCs
were to be infected, the hBMP2 proteins secreted by these cells
could not have achieved the level [0.15 mg/ml (13,14)] required for the treatment of local
osteoporosis. However, if the rBMSCs were isolated from the MSCs
OVX, and infected by Lv-BMP in vitro, the infected rBMSCs
would have increased and the hBMP2 proteins secreted by them would
have achieved the level needed for the treatment of the local
osteoporosis. The OI of rBMSCs may therefore have been conducted
in vitro. A comparison of the rBMSCs in MSCs OVX to MSCs CON
in terms of osteogenic capability, would yield a lower ability in
the rBMSCs in MSCs OVX compared with those in MSCs CON (P<0.01),
whereas a comparison of the rBMSCs with OI in these two groups, may
yield a statistical difference (P<0.05), that may have been not
so obvious (36). This result may
have been a possible indication that the osteogenic ability of the
rBMSCs with OI in MSCs OVX approached that of the MSCs CON. The
Lv-BMP infection efficiency was also mentioned. The rBMSC quantity
was different in the MSCs OVX of individual rat femurs.
Additionally, the individualized quantity of Lv-BMP could not be
evaluated. Subsequently, the Lv-BMP infection efficiency was not
proven. Even if the individualized quantity of Lv-BMP had been
realized, Lv-BMP targeting would have to be resolved. The in
vivo genetic infection omitted the processes of subculture, OI
and genetic infection in vitro. Furthermore, it was
convenient and cost-effective; however, the result was not
satisfactory. By contrast, the results of the in vitro
genetic infection demonstrated an improved treatment effect on
local osteoporosis compared with that of the other methods
employed. This laid a foundation for treating the local
osteoporosis of patients by using their own BMSCs.
BMP2 participates in a number of molecular pathways
that might contribute to certain adverse clinical effects,
including cyst-like bone formation, significant soft tissue
swelling, as well as the resorption of excessive bone and vertebral
subsidence or collapse (37,38). Since BMP2 is a known
chemoattractant for monocytes, macrophages and lymphocytes, its
inflammatory response is not unexpected. Furthermore, by
potentiating the receptor activation of nuclear factor-κB ligand (a
cytokine essential for inducing osteoclast differentiation), BMP2
is capable of inducing osteoclastogenesis (39). BMP2 is also able to inhibit Wnt
signaling by upregulating PPARγ, thereby increasing osteoclasts
since PPARγ upregulates the expression of c-fos (an essential
mediator of osteoclastogenesis) (40). High-dose BMP2 (≥1.5 mg/ml)
decreases the osteoblast expression of osteoprotegerin (a member of
the tumor necrosis factor receptor family that inhibits the
receptor activator of nuclear factor-κB ligand) by inhibiting Wnt
signaling (41). Therefore,
high-dose BMP2 induces significant tissue inflammation, which
explains the adverse clinical effects including cyst-like bone
formation and significant soft tissue swelling, and increases
osteoclastogenesis, which may clinically manifest as vertebral
subsidence, collapse and excessive bone resorption.
In the present study, hBMP2 proteins at a density of
0.15 mg/ml were enough to produce osteogenesis by potentiating the
rBMSCs transplanted or contained through autocrine or paracrine
effects. At this density, hBMP2 had treatment effects on the local
femoral osteoporosis, with the exception of adverse clinical
effects including cyst-like bone formation and significant soft
tissue swelling. However, in order to produce the treatment effect
on local osteoporosis in human, the density of hBMP2 proteins was
required to be at least 1.5 mg/ml in the bone marrow cavity. Thus,
clinical adverse effects could be introduced at this high dose of
hBMP2 proteins. Furthermore, the quality of regenerated bone could
be reduced, with the cyst-like bone void formation and inflammation
resulting in life-threatening outcomes. Therefore, the method of
in vitro genetic infection with human BMSCs through the
lentivirus-carrying hBMP2 gene in order to treat human local
osteoporosis is not appropriate. However, if this in vitro
method of genetic infection were used to infect human BMSCs with
the hBMP2 gene and other growth factors in order to suppress the
non-osteogenic BMP2 signaling targets or to decrease the BMP2 dose
requirement, unwanted inflammatory and adipogenetic induction could
be reduced. Using this approach, improvements in the treatment of
human local osteogenesis could be achieved.
In conclusion, the results of this study have shown
that the hBMP2-modified osteoporotic rat BMSCs formed by in
vitro genetic infection had an improved treatment effect as
compared with that of the in vivo genetic infection (BMD:
0.315 vs. 0.19 g/cm2, P<0.01; bone histomorphometry:
Tb.Ar/T.Ar: 0.301 vs. 0.114, P<0.01. Tb.Th: 43.54 vs. 21.39 μm,
P<0.01. Tb.Sp: 115.7 vs. 304.87 μm, P<0.01). We concluded
that the treatment effect of the Lv-BMP-mediated hBMP2 gene that
was overexpressed in osteoporotic rBMSCs formed by in vitro
genetic infection on local osteoporosis was better than that
performed by in vivo genetic infection.
Acknowledgements
This study was supported by the National Science
Foundation of China (grant no. NFSC-30227738) and a grant from the
Heilongjiang Education Bureau, China (grant no. 12521180). The
authors would like to thank Professor Tianzun Tao and Wanli Tian
for assistance in collecting the experimental data.
References
|
1
|
Kim S, Kang Y, Krueger CA, et al:
Sequential delivery of BMP-2 and IGF-1 using a chitosan gel with
gelatin microspheres enhances early osteoblastic differentiation.
Acta Biomater. 8:1768–1777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song I, Kim BS, Kim CS and Im GI: Effects
of BMP-2 and vitamin D3 on the osteogenic differentiation of
adipose stem cells. Biochem Biophys Res Commun. 408:126–131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edgar CM, Chakravarthy V, Barnes G, Kakar
S, Gerstenfeld LC and Einhorn TA: Autogenous regulation of a
network of bone morphogenetic proteins (BMPs) mediates the
osteogenic differentiation in murine marrow stromal cells. Bone.
40:1389–1398. 2007. View Article : Google Scholar
|
|
4
|
Ben-David D, Srouji S, Shapira-Schweitzer
K, et al: Low dose BMP-2 treatment for bone repair using a
PEGylated fibrinogen hydrogel matrix. Biomaterials. 34:2902–2910.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuji K, Bandyopadhyay A, Harfe BD, et al:
BMP2 activity, although dispensable for bone formation, is required
for the initiation of fracture healing. Nat Genet. 38:1424–1429.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto A, Yamaji K, Kawanami M and Kato
H: Effect of aging on bone formation induced by recombinant human
bone morphogenetic protein-2 combined with fibrous collagen
membranes at subperiosteal sites. J Periodontal Res. 36:175–182.
2001. View Article : Google Scholar
|
|
7
|
Bai Y, Li P, Yin G, et al: BMP-2, VEGF and
bFGF synergistically promote the osteogenic differentiation of rat
bone marrow-derived mesenchymal stem cells. Biotechnol Lett.
35:301–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lieberman JR, Daluiski A, Stevenson S, et
al: The effect of regional gene therapy with bone morphogenetic
protein-2-producing bone-marrow cells on the repair of segmental
femoral defects in rats. J Bone Joint Surg Am. 81:905–917.
1999.PubMed/NCBI
|
|
9
|
Tsuda H, Wada T, Ito Y, et al: Efficient
BMP2 gene transfer and bone formation of mesenchymal stem cells by
a fiber-mutant adenoviral vector. Mol Ther. 7:354–365. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang J, Fan CY and Zeng BF: Osteogenic
differentiation effects on rat bone marrow-derived mesenchymal
stromal cells by lentivirus-mediated co-transfection of human BMP2
gene and VEGF165 gene. Biotechnol Lett. 30:197–203. 2008.
View Article : Google Scholar
|
|
11
|
Zhu C, Chang Q, Zou D, et al: LvBMP-2
gene-modified BMSCs combined with calcium phosphate cement
scaffolds for the repair of calvarial defects in rats. J Mater Sci
Mater Med. 22:1965–1973. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu WK, Sugiyama O, Park SH, et al:
Lentiviral-mediated BMP-2 gene transfer enhances healing of
segmental femoral defects in rats. Bone. 40:931–938. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oluigbo CO and Solanki GA: Use of
recombinant human bone morphogenetic protein-2 to enhance posterior
cervical spine fusion at 2 years of age: technical note. Pediatr
Neurosurg. 44:393–396. 2008.PubMed/NCBI
|
|
14
|
Zara JN, Siu RK, Zhang X, et al: High
doses of bone morphogenetic protein 2 induce structurally abnormal
bone and inflammation in vivo. Tissue Eng Part A. 17:1389–1399.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuda H, Wada T, Yamashita T and Hamada H:
Enhanced osteoinduction by mesenchymal stem cells transfected with
a fiber- mutant adenoviral BMP2 gene. J Gene Med. 7:1322–1334.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yukawa Y, Lou J, Fukui N and Lenke LG:
Optimal treatment timing to attenuate neuronal apoptosis via Bcl-2
gene transfer in vitro and in vivo. J Neurotrauma. 19:1091–1103.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sapet C, Pellegrino C, Laurent N, Sicard F
and Zelphati O: Magnetic nanoparticles enhance adenovirus
transduction in vitro and in vivo. Pharm Res. 29:1203–1218. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Liu HJ, Li TJ, et al: Lentiviral
vector-mediated siRNA knockdown of SR-PSOX inhibits foam cell
formation in vitro. Acta Pharmacol Sin. 29:847–852. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou S, Mizuno S and Glowacki J: Wnt
pathway regulation by demineralized bone is approximated by both
BMP-2 and TGF-β1 signaling. J Orthop Res. 31:554–560.
2013.PubMed/NCBI
|
|
20
|
Jiang XQ, Chen JG, Gittens S, Chen CJ,
Zhang XL and Zhang ZY: The ectopic study of tissue-engineered bone
with hBMP-4 gene modified bone marrow stromal cells in rabbits.
Chin Med J (Engl). 118:281–288. 2005.PubMed/NCBI
|
|
21
|
Krebsbach PH, Zhang K, Malik AK and
Kurachi K: Bone marrow stromal cells as a genetic platform for
systemic delivery of therapeutic proteins in vivo: human factor IX
model. J Gene Med. 5:11–17. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao L, Wang J, Zou Z, et al:
Transplantation of BMSCs expressing hPDGF-A/hBD2 promotes wound
healing in rats with combined radiation-wound injury. Gene Ther.
16:34–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Q, Liu XZ, Kang CS, Wang GX, Zhong Y
and Pu PY: The anti-glioma effect of suicide gene therapy using
BMSC expressing HSV/TK combined with overexpression of Cx43 in
glioma cells. Cancer Gene Ther. 17:192–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maniatopoulos C, Sodek J and Melcher AH:
Bone formation in vitro by stromal cells obtained from bone marrow
of young adult rats. Cell Tissue Res. 254:317–330. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zou D, Han W, You S, et al: In vitro study
of enhanced osteogenesis induced by HIF-1α-transduced bone marrow
stem cells. Cell Prolif. 44:234–243. 2011.
|
|
26
|
Nishida S, Endo N, Yamagiwa H, Tanizawa T
and Takahashi HE: Number of osteoprogenitor cells in human bone
marrow markedly decreases after skeletal maturation. J Bone Miner
Metab. 17:171–177. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu HC, ELL, Wang DS, et al:
Reconstruction of alveolar bone defects using bone morphogenetic
protein 2 mediated rabbit dental pulp stem cells seeded on
nano-hydroxyapatite/collagen/poly(L-lactide). Tissue Eng Part A.
17:2417–2433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamminen IS, Isaksson H, Aula AS, Honkanen
E, Jurvelin JS and Kröger H: Reproducibility and agreement of
micro-CT and histomorphometry in human trabecular bone with
different metabolic status. J Bone Miner Metab. 29:442–448. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hapidin H, Othman F, Soelaiman IN, Shuid
AN and Mohamed N: Effects of nicotine administration and nicotine
cessation on bone histomorphometry and bone biomarkers in
Sprague-Dawley male rats. Calcif Tissue Int. 88:41–47. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Owen M and Friedenstein AJ: Stromal stem
cells: marrow-derived osteogenic precursors. Ciba Found Symp.
136:42–60. 1988.PubMed/NCBI
|
|
31
|
Derubeis AR and Cancedda R: Bone marrow
stromal cells (BMSCs) in bone engineering: limitations and recent
advances. Ann Biomed Eng. 32:160–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zou L, Zou X, Chen L, et al: Multilineage
differentiation of porcine bone marrow stromal cells associated
with specific gene expression pattern. J Orthop Res. 26:56–64.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stucki U, Schmid J, Hämmerle CF and Lang
NP: Temporal and local appearance of alkaline phosphatase activity
in early stages of guided bone regeneration. A descriptive
histochemical study in humans. Clin Oral Implants Res. 12:121–127.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luu HH, Song WX, Luo X, et al: Distinct
roles of bone morphogenetic proteins in osteogenic differentiation
of mesenchymal stem cells. J Orthop Res. 25:665–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ying X, Cheng S, Wang W, et al: Effect of
boron on osteogenic differentiation of human bone marrow stromal
cells. Biol Trace Elem Res. 144:306–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li DJ, Ge DX, Wu WC, Wu J and Li L:
Osteogenic potential of bone marrow mesenchymal stem cells from
ovariectomied osteoporotic rat. Sichuan Da Xue Xue Bao Yi Xue Ban.
36:318–321. 2005.(In Chinese).
|
|
37
|
Vaidya R, Weir R, Sethi A, Meisterling S,
Hakeos W and Wybo CD: Interbody fusion with allograft and rhBMP-2
leads to consistent fusion but early subsidence. J Bone Joint Surg
Br. 89:342–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vaidya R, Carp J, Sethi A, Bartol S, Craig
J and Les CM: Complications of anterior cervical discectomy and
fusion using recombinant human bone morphogenetic protein-2. Eur
Spine J. 16:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Irie K, Alpaslan C, Takahashi K, et al:
Osteoclast differentiation in ectopic bone formation induced by
recombinant human bone morphogenetic protein 2 (rhBMP-2). J Bone
Miner Metab. 21:363–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wan Y, Chong LW and Evans RM: PPAR-gamma
regulates osteoclastogenesis in mice. Nat Med. 13:1496–1503. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Glass DA II and Karsenty G: Canonical Wnt
signaling in osteoblasts is required for osteoclast
differentiation. Ann N Y Acad Sci. 1068:117–130. 2006. View Article : Google Scholar : PubMed/NCBI
|