Introduction
Previous studies on animals and humans have
demonstrated that cold air elicits a series of respiratory
pathological and physiological changes. Prolonged exposure to cold
air can induce the activation of macrophages, an increase in
inflammatory factors and granulocytes, as well as the recruitment
of alveolar macrophages in the airway (1–3).
Long-term and continuous cold stimulation may cause a series of
morphological changes in the airways, such as an increase in the
number of goblet cells and mucus glands, hypertrophy of the airway
muscular fascicles, and a thickening of the muscle layers of the
terminal arteries and arterioles. Gradually, these changes may play
a role in the symptoms of chronic obstructive pulmonary disease and
bronchitis (4). Moreover, low
ambient temperatures are associated with a reduction in lung
function and an increased frequency of exacerbation in patients
with chronic obstructive pulmonary disease (COPD) (5). Therefore, exposure to cold air is a
major environmental factor that exacerbates chronic inflammatory
airway diseases, such as COPD and asthma (5).
Studies on the expression of the functional
cold-sensing transient receptor potential melastatin 8 (TRPM8)
variant in human lung epithelial cells have demonstrated that the
TRPM8 channel is involved in an underlying molecular mechanism of
respiratory cold temperature detection (6). The transient receptor potential
(TRP) channels are a family of cation channels that are involved in
diverse cellular functions, such as vision, taste, olfaction,
hearing, touch, pain and thermo- and osmosensation (7,8).
TRPM8 and TRPA1 have been reported to be the molecular transducers
of innocuous cooling perception and painfully cold temperatures,
respectively (9–13). TRPM8 is a non-selective
calcium-permeable cation channel that is expressed in a subset of
sensory neurons, including the dorsal root and trigeminal ganglia
(9–14), as well as in non-neuronal areas
(15–17). TRPM8 is activated by cold
temperatures below 25ºC and cooling agents, such as menthol,
eucalyptol and icilin agents (9,18,19).
In our previous study, we demonstrated that
upregulated TRPM8 expression in bronchial epithelial cells of
subjects with COPD can provoke mucus hypersecretion through the
cold-mediated activation of the TRPM8 channel (20). These findings provide a molecular
mechanism by which cold air increases the susceptibility of the
airways to exacerbation in subjects with COPD. However, the direct
effects of cold air on the ultrastructure of cilia, which is
involved in the function of mucociliary clearance, and the
mechanisms that underlie the upregulated expression of TRPM8 in
COPD are currently unknown.
In this study, we identified the direct effects of
cold air on the ultrastructure of cilia. We present the hypothesis
that cigarette smoke, which is a well-established risk factor for
COPD (21,22), is responsible for the enhanced
basal expression of the TRPM8 receptor. Mucin 5AC (MUC5AC), which
is one of the predominant mucins found in airway secretions, has
been implicated in pulmonary diseases that are associated with
mucus hypersecretion (23),
interleukin (IL)-8 and the tumor necrosis factor (TNF)-α protein.
These are pro-inflammatory mediators that are associated with the
pathophysiology of mucus hypersecretion in COPD (24–26). In this study, we analyzed these
factors to elucidate the effects of cold air and cigarette smoke on
mucus hypersecretion and the production of inflammatory factors.
Since the co-presence of cold air and cigarette smoke is common in
the lungs of patients with COPD, the synergistic effects of cold
air on mucus hypersecretion and the upregulation of inflammatory
factors that are induced by cigarette smoke inhalation were also
analyzed.
Materials and methods
Chemicals
Mouse monoclonal MUC5AC antibody and non-immune
mouse IgG were obtained from Chemicon (Temecula, CA, USA);
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and
fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG
were purchased from Beijing Biosynthesis Biotechnology Co., Ltd.
(Beijing, China); bovine serum albumin and mouse anti-β-actin
monoclonal antibody were from Sigma (St. Louis, MO, USA); TNF-α and
the IL-8 enzyme-linked immunosorbent assay (ELISA) kit were
obtained from Wuhan Boster Biological Technology (Wuhan, China);
the First-strand cDNA Synthesis kit was from Fermentas (Burlington,
ON, Canada); SYBR Premix EX Taq™ was purchased from Takara
Biotechnology (Dalian, China); rabbit anti-TRPM8(C-term) Polyclonal
Antibody was from Abgent (San Diego, CA, USA); ECL-Plus
chemiluminescence was obtained from Amersham Pharmacia Biotech
(Little Chalfont, Buckinghamshire, UK); and the rTRPM8 and 18S rRNA
primers were purchased from Shanghai Bioengineering Co., Ltd.
(Shanghai, China).
Treatment of animals
Pathogen-free male Sprague-Dawley (SD) rats (8 weeks
old, 260–280 g body weight) were purchased from the Laboratory
Animal Center of Chongqing Medical University (Chongqing, China)
[certificate: SCXK (YU) 2007-0001]. The SD rats were maintained
under optimal conditions for hygiene, temperature (20±2ºC) and
photoperiods (12 h light:12 h dark) and were provided food and
water ad libitum according to the institutional guidelines
for the care and use of laboratory animals. All animal procedures
were approved by the Ethics Committee of Chongqing Medical
University.
The first experiment was designed to determine the
effects of cold air on the ultrastructural changes in cilia and the
airway epithelial cell surface in SD rats. The rats were divided
into 2 groups: the control group and the group exposed to cold air.
There were 8 rats in each group. The control group was maintained
at a room temperature of 20±2ºC, and the group exposed to cold air
was maintained in a cold air therapeutic apparatus (Zimmer
Elektromedizin GmbH, Bayern, Germany), which provided cold air
stimulus to the rats through a breathing mask for 3 h daily at
−18ºC for 40 days.
The second experiment was designed to determine the
effects of cigarette smoke on TRPM8 expression and the role of cold
air in cigarette smoke-induced mucus hypersecretion. In this
experiment, the SD rats were divided into a control group, a
cigarette inhalation group, a group exposed to cold air and a group
exposed to cigarette inhalation plus cold air. Each group contained
6 rats. The rats in the cigarette inhalation group were exposed to
filtered mainstream smoke (Chongqing Cigarette Factory, Chongqing,
China) with 10 cigarettes/h for 6 h/day (morning, noon and evening)
over a period of 40 days using a smoking machine that was assembled
in our laboratory. The air flow rate was 1.5 l/min. The animals
were placed in a restraining box, and the smoke was delivered
cyclically. The rats in the group exposed to cigarette inhalation
plus cold air were co-treated with cigarette inhalation and cold
air. The procedure of cold air inhalation was the same as in the
first experiment. All the rats were sacrificed on day 40 using 2 ml
of 10% chloral hydrate anesthesia and samples were obtained for
analysis.
ELISA for the detection of MUC5AC protein
levels in bronchoalveolar lavage fluid (BALF)
At the end of the experiment, a thoracotomy was
performed and the left main bronchus was ligated proximally. BALF
was prepared by carefully instilling 2 ml of normal saline into the
right lung. The fluid was withdrawn and the process was repeated 3
times until 80% of the instilled fluid was collected. The BALF was
centrifuged at 3,000 rpm for 15 min, and the supernatant was stored
in a freezer at −70ºC. The total protein levels in BALF were
estimated by Bradford assay. Next, 100 mg of total protein were
incubated with bicarbonate-carbonate buffer at 40ºC in a 96-well
plate until dry. The plates were blocked with 2% bovine serum
albumin (Sigma) for 1 h at room temperature and were incubated with
a mouse monoclonal MUC5AC antibody (1:100) for 1 h. Subsequently,
HRP-conjugated goat anti-mouse IgG (1:10,000) was dispensed into
each well and incubated for 1 h. A color reaction was developed
with 3,3′-5,5′-tetramethylbenzidine peroxidase solution (Kirkegaard
and Perry Laboratories, Gaithersburg, MD, USA) and the reaction was
terminated with 1 M H2SO4. The absorbance was
read at 450 nm.
ELISA for the detection of IL-8 and TNF-α
protein levels in BALF
The TNF-α and IL-8 protein levels in BALF were
measured using an ELISA kit (Wuhan Boster Biological Technology)
according to the manufacturer’s instructions. Briefly, 100 μl of
assay diluent and 50 μl of sample were added to each well for a 2-h
incubation at room temperature. Subsequently, 200 μl of conjugate
were added to each well for another 2 h at room temperature, and
200 μl of substrate reaction solution were added to each well for a
30-min incubation. Finally, 50 μl of stop solution were added to
terminate the reaction. The absorbance was read at 450 nm.
Scanning electron microscopy (SEM) and
transmission electron microscopy (TEM)
Small fragments (1×1×2 mm) of bronchial tissue in
the right main bronchus were washed twice in phosphate-buffered
saline (PBS) and immediately fixed by immersion in 2.5%
glutaraldehyde in 0.15 M phosphate buffer (pH 7.2) at 4ºC for 24 h.
After fixation, the tissues were dehydrated through a graded series
of ethanol. After being air-dried at room temperature, the samples
were coated with platinum/palladium and analyzed under a scanning
electron microscope (JSM-6340F; Jeol, Ltd., Tokyo, Japan).
The tissue fragments were fixed in 2.5%
glutaraldehyde in 0.15 M phosphate buffer (pH 7.2) at room
temperature for 30 min and post-fixed with 1% osmium tetroxide in
the same buffer for 30 min at room temperature, following 2 5-min
washings with phosphate buffer. The samples were dehydrated in a
series of graded ethanol and embedded in Epon 812 (Nissin EM Co.,
Ltd., Tokyo, Japan). The samples were cured at 60ºC for 48 h and
sectioned on a Reichert ultramicrotome (70 nm; Leica, Wetzlar,
Germany). Following staining with uranyl acetate-lead citrate, the
samples were visualized in a Philips EM 400 (Philips, Eindhoven,
The Netherlands) TEM system.
In total, 50 cross-sections of cilia from each
specimen were observed. The ciliary ultrastructural anomalies were
counted under a magnification of ×50,000 by an observer who was
blinded to the experimental design, and the anomalies were
expressed as a percentage of the total number of cilia in the
fields of vision.
Real-time reverse transcription
polymerase chain reaction (qRT-PCR) for the detection of TRPM8 mRNA
in the bronchial tissues
The test specimens were obtained from the right main
bronchus. TRPM8 mRNA transcripts were measured by qRT-PCR. 18S rRNA
was selected as the endogenous control gene. Total RNA was isolated
from the tissues using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer’s instructions. In total, 5 μg
of total RNA were reverse transcribed into cDNA using the
first-strand cDNA synthesis kit according to the manufacturer’s
instructions. The PCR primers for rat TRPM8 and 18S rRNA were
designed according to the published cDNA sequences (Table I). The specificity of the PCR
primers was tested under normal PCR conditions, and the products of
the reaction were electrophoresed onto 2% agarose gels. Real-time
PCR was performed using SYBR Premix EX Taq™ in a Bio-Rad IQ5 PCR
System (Bio-Rad Laboratories, Hercules, CA, USA). PCR was performed
under the following conditions: denaturation at 94ºC for 15 min, 40
cycles of denaturation at 94ºC for 15 sec, annealing at 58ºC for 45
sec and extension at 72ºC for 1 min, followed by a final extension
at 72ºC for 5 min. Finally, the melting curve analysis was
performed to confirm that a single product was amplified and that
no primer dimers had interfered with the reaction. The comparative
Ct method (2−ΔΔCt) was used for the relative mRNA
quantification.
 | Table IPrimers used for real-time
RT-PCR. |
Table I
Primers used for real-time
RT-PCR.
| Gene | Sense (5′→3′) | Antisense
(5′→3′) | Product size
(bp) | GenBank accession
no. |
|---|
| rTRPM8 |
GCAGTGGTACATGAACGGAGT |
TGAAGAGTGAAGCCGGAATAC | 109 | NM_134371 |
| 18S rRNA |
CTTAGAGGGACAAGTGGCG |
GGACATCTAAGGGCATCACA | 71 | X01117 |
Western blot analysis for the detection
of TRPM8 protein expression
TRPM8 protein expression was measured by western
blot analysis. Briefly, the right main bronchus was washed 3 times
with ice-cold PBS. The tissues were resuspended in lysate buffer,
lysed on ice for 20 min and centrifuged at 12,000 rpm for 15 min at
4ºC. The protein content was determined by Bradford assay.
Equivalent amounts of protein (30 μg) from each sample were
separated on 10% SDS-PAGE gels and transferred onto a
polyvinylidene fluoride membrane (Sigma). The membrane was blocked
with 5% non-fat milk in Tris-buffered saline, incubated with a
rabbit anti-TRPM8 (C-term) polyclonal antibody (1:100) and a mouse
anti-β-actin monoclonal antibody (1:1,000) overnight at 4ºC,
followed by incubation with corresponding HRP-conjugated secondary
antibodies (1:2,000) overnight at 4ºC. Specific blots were
developed using ECL-Plus Chemiluminescence. The densitometric
quantification of the bands was performed using Quantity One
software (Bio-Rad Laboratories). The results were expressed as the
ratio of the expression of TRPM8 to β-actin.
Immunofluorescence for the detection of
MUC5AC expression in the bronchial tissue sections
The frozen sections (10–12 μm) from the right main
bronchus were placed at room temperature for 30 min and immersed in
cold acetone for 10 min. The sections were rinsed with PBS, and 3%
hydrogen peroxide was added for 5 min. After washing, the sections
were blocked using 1% BSA plus 1% normal goat serum and incubated
with a mouse monoclonal MUC5AC antibody (1:200) overnight at 4ºC.
Non-immune mouse IgG was used as a negative control. Following 3
10-min washes in PBS, the slides were incubated with an
FITC-conjugated secondary antibody (1:200) for 2 h at room
temperature. The samples were examined under a Leica inverted
TCS-SP2 confocal microscope (Leica, Heidelberg, Germany) that was
fitted with the appropriate fluorescence filters. The
quantification of the MUC5AC immunostaining in the bronchial
epithelium was performed using software equipped in the TCS-SP2
confocal microscope. The entire bronchial epithelium was selected
as a region of interest (ROI). The mean value was recorded and
analyzed. The measurement of TRPM8 expression was performed by an
observer who was unaware of which group the biopsy specimens were
obtained from.
Statistical analysis
The data are presented as the means ± SD, and data
analyses were performed using SPSS version 10.0 for Windows
software (SPSS Inc., Chicago, IL, USA). Differences were examined
for statistical significance using one-way ANOVA to compare TRPM8
expression between the different groups. The main effects of cold
air and smoking on the expression of MUC5AC and inflammatory
factors as well as the coordinated interaction between cold air and
smoking were analyzed by ANOVA with a factorial design. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
Effects of cold air stimuli on the
ultrastructure organization of cilia and the airway epithelial cell
surface
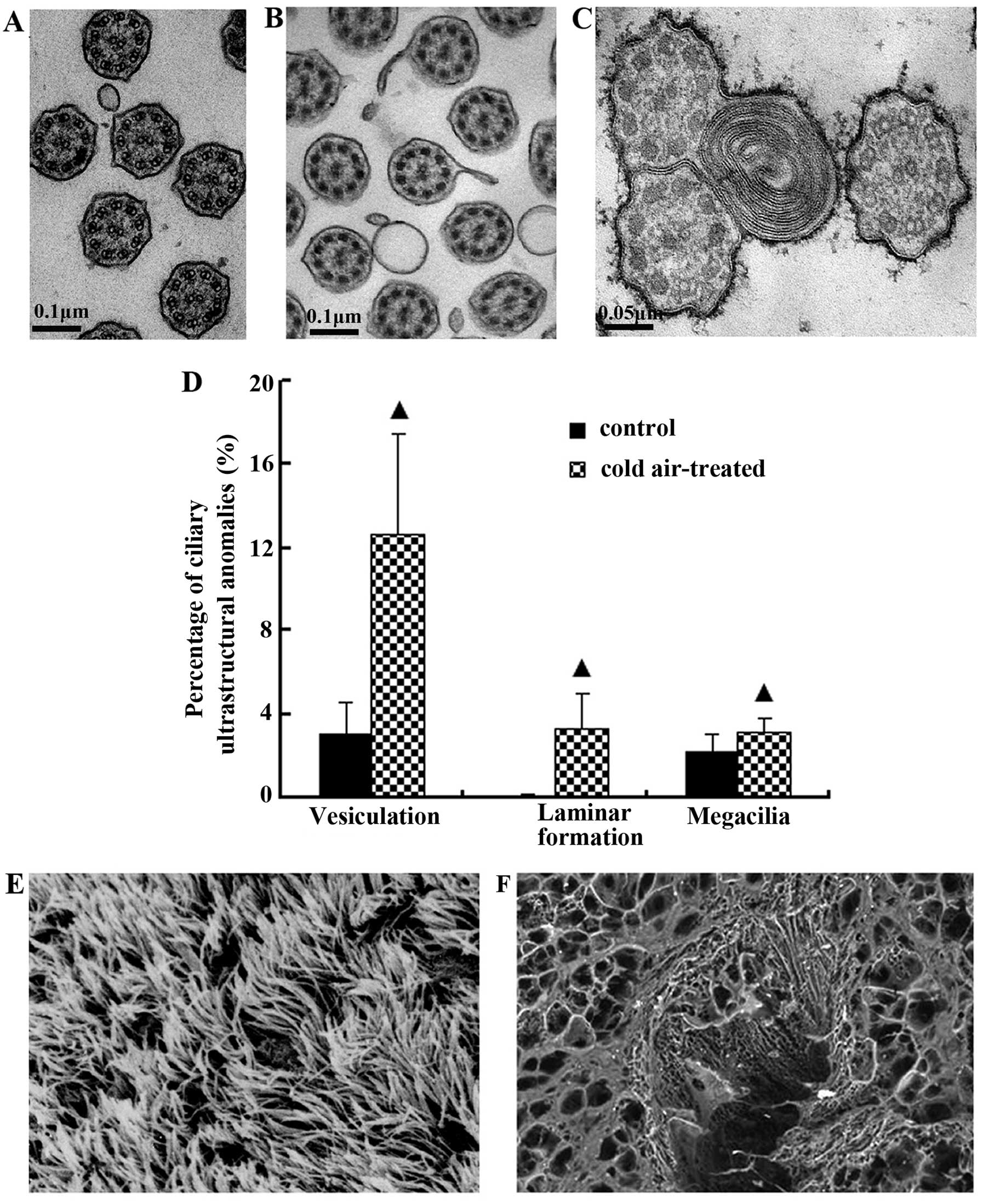
Normal ciliary axonema and ciliary membranes were
observed in the control group (Fig.
1A). The radial spokes from 2 central microtubules radiated to
9 peripheral pairs of microtubules were joined by a nexin link, and
the whole structure formed an axonema. Each pair comprised
microtubules A and B with 2 side arms that were arranged clockwise.
Following repeated cold air stimulation, the fromation of
diverticula and vesiculation (Fig.
1B), laminar formations in cilia and megacilia anomalies (3
axonemas constituted a compound cilium) (Fig. 1C) were significantly increased
when compared with the control group (P<0.05) (Fig. 1D).
The normal orientation of cilia was observed on the
epithelial surface in the control group (Fig. 1E). However, the bronchial ciliated
epithelium in the rats repeatedly exposed to cold air was covered
by varying degrees of accumulated mucus (Fig. 1F).
Effects of cigarette smoke on TRPM8
expression
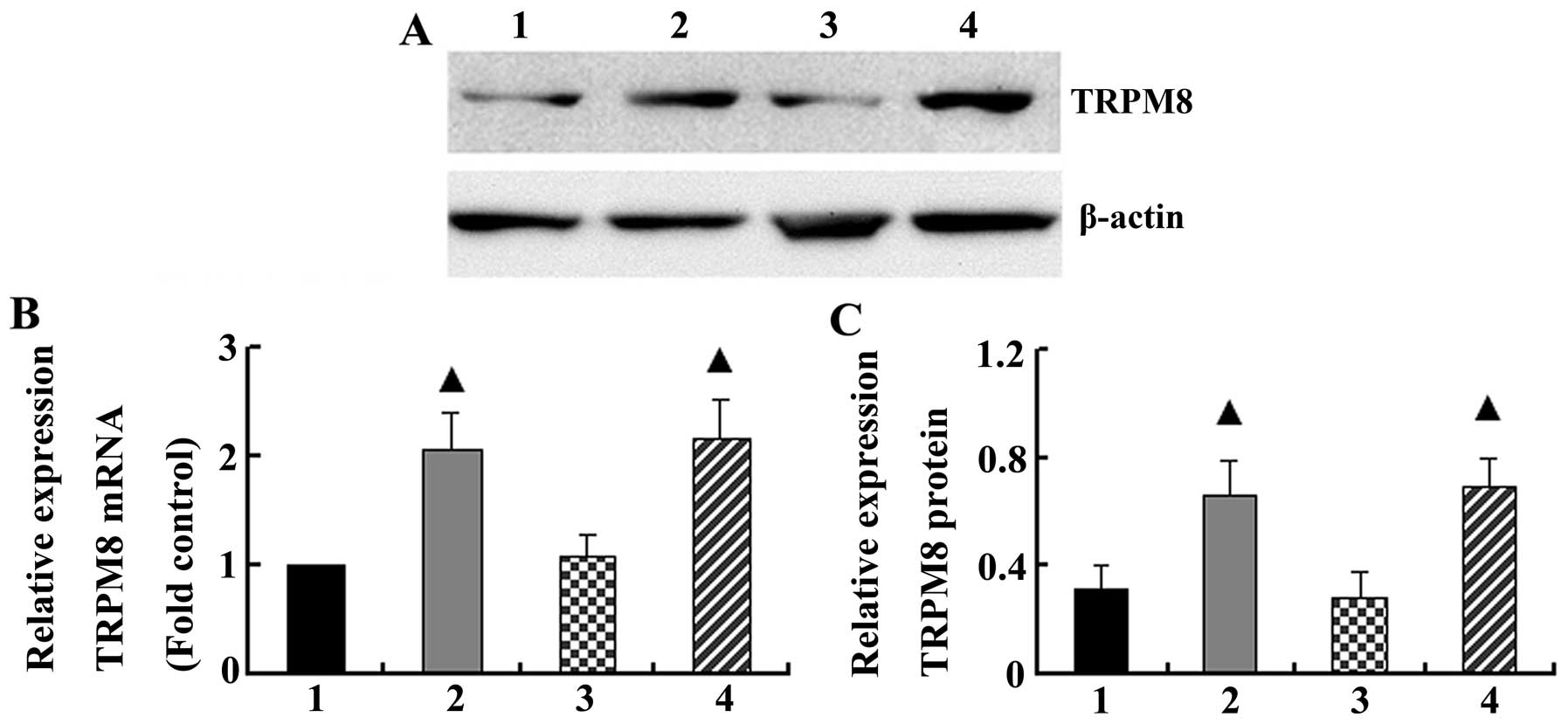
Real-time PCR and western blot analysis demonstrated
that cigarette smoke increased the basal mRNA and protein levels of
TRPM8 in the bronchial tissue (2.07±0.35 and 0.66±0.12,
respectively), whereas the mRNA and protein levels of TRPM8 were
1.0±0.00 and 0.31±0.09 in the control group, respectively (P=0.006
and P=0.00). However, cold air stimuli had no effect on TRPM8 mRNA
and protein expression in the bronchial tissue compared with the
control group (P>0.05). When the rats were exposed to cigarette
inhalation and cold air, the levels of TRPM8 mRNA and protein
(2.16±0.36 and 0.70±0.10, respectively) were similar to those from
the group exposed to cigarette smoke only (P=0.772 and P=0.640,
respectively) (Fig. 2).
Effects of cold air stimuli on the
cigarette smoke-induced intracellular synthesis and secretion of
MUC5AC protein
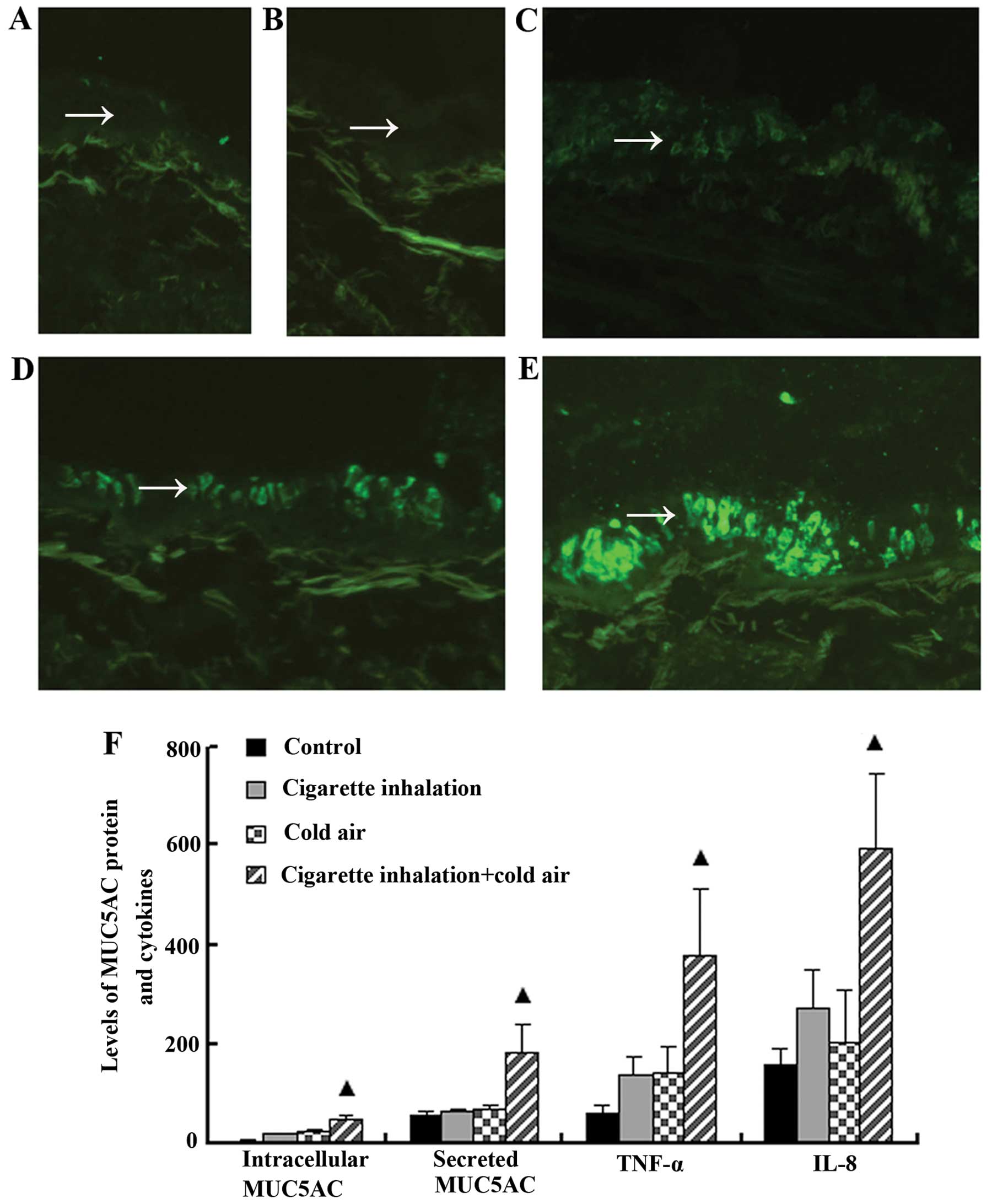
An immunofluorescence assay and ELISA revealed that
the relative levels of intracellular MUC5AC protein expressed in
goblet cells and MUC5AC protein secreted in BALF were increased in
the group exposed to cigarette smoke (17.74±2.92 and 66.08±3.86
μg/mg, respectively) compared with the control group, in which the
intracellular MUC5AC and secreted MUC5AC protein relative levels
were 3.40±1.00 and 56.74±7.83 μg/mg, respectively (P<0.001).
Significant increases in intracellular and secreted MUC5AC protein
(21.65±3.90 and 71.40±4.38 μg/mg, respectively) were observed in
the rats that were exposed to cold air compared with those in the
control group (P<0.01). The intracellular and secreted MUC5AC
protein levels were markedly increased (47.84±6.61 and 182.39±56.90
μg/mg, respectively; P<0.01) in the rats that were exposed to
cigarette smoke and cold air, and the coordinated interaction
between cold air and cigarette smoke was significant (F=12.33,
P=0.002; F=18.60, P=0.00) with 1.21- and 1.32-fold increases in the
total amounts of intracellular and secreted MUC5AC that were
induced by separate stimuli, respectively. Collectively, the
increased levels of MUC5AC in the rats that were exposed to
cigarette smoke and cold air suggested that cold air
synergistically increased MUC5AC mucin synthesis and the secretion
induced by cigarette smoke (Fig.
3).
Effects of cold air stimuli on the
cigarette smoke-induced production of inflammatory cytokines
The release of the TNF-α and IL-8 proteins in BALF
was increased following exposure to cigarette smoke (138.37±36.69
and 271.24±82.03 ng/l, respectively) compared with the control
group (58.81±17.48 and 156.48±35.56 ng/l, respectively;
P<0.001). Similarly, cold air induced a significant increase in
TNF-α and IL-8 protein levels in BALF (142.62±49.40 and
203.65±107.73 ng/l, respectively) compared with the control group
(P<0.001). Co-stimulation with cold air and cigarette smoke
resulted in a stronger synergistic increase in TNF-α and IL-8
levels (379.46±133.76 and 596.75±148.74 ng/l, respectively)
compared with stimulation by separate stimuli (1.35- and 1.25-fold
increases in the total amounts of TNF-α and IL-8 following
stimulation with cold air and cigarette smoke, respectively) and
the coordinated interaction between cold air and cigarette smoke
was significant (F=6.75, P=0.017; F=11.21, P=0.003). Overall, these
data indicate that co-exposure to cigarette smoke and cold air
induced the production of TNF-α and IL-8 in a synergistic manner
(Fig. 3).
Discussion
Cold air-induced COPD exacerbation is a well known
phenomenon that may elicit a series of respiratory pathological and
physiological changes, such as a reduction in lung function, an
increased frequency of exacerbation and morphological changes in
the airways (4,5,27).
In a recent study, we demonstrated that cold air that is
temporarily inhaled provokes robust excessive secretions of airway
mucus through the cold-mediated activation of the TRPM8 channel and
contributes to cold-induced COPD exacerbation (20). Airway secretions are cleared by
mucociliary clearance and other mechanisms, such as cough,
peristalsis, and two-phase gas-liquid flow. Mucociliary clearance
is a very complex process that involves several variables, such as
the structure, number, movement and co-ordination of cilia that are
present in the airways (28,29). Therefore, we in this study,
investigated whether the penetration of cold air into the lower
airway elicits ciliary ultrastructural anomalies and contributes to
mucus accumulation. We analyzed the ultrastructure organization of
the bronchial ciliary system in rats that were exposed to cold air
stimuli. Two types of ultrastructural anomalies were observed:
anomalies of the ciliary membrane and architectural ciliary
anomalies. Following repeated cold air stimulation, the formation
of diverticula and vesiculation were observed in the cilia.
Striking architectural ciliary anomalies were observed in the
cilia, such as 3 axonemas that constituted a compound cilium, which
are described as megacilia or compound cilia and laminar
formations.
In addition, we investigated the association between
repeated cold air stimulation and the amount of mucus on the
epithelial surface. We found that cilia on the epithelial surface
were covered by accumulated mucus following repeated cold air
stimulation. Previous studies have demonstrated that the ciliary
beat frequency decreased at low temperatures (30). Therefore, we speculated that the
ciliary ultrastructural anomalies that were induced by cold air
partly resulted in a lower ciliary beat frequency and defective
mucociliary clearance, which led to mucus accumulation on the
epithelial surface (28,31). Collectively, these results
indicate that ciliary anomalies and excessive MUC5AC secretion that
are induced by cold air stimuli may be the reason why the bronchial
ciliated epithelium was covered by accumulated mucus after the rats
were treated with repeated cold air stimulation.
Owing to the excessive accumulation of mucus in the
airways due to cold air, obstructive lung diseases may be more
common in cold areas; however, these results do not support this
hypothesis. The first-line defense against an inhaled insult that
impinges on and damages the epithelium is the production of mucus.
In a healthy subject, the production of mucus is an important
homeostatic defense mechanism to combat the onslaught of cold. Due
to the adaptation of TRPM8 to cold stimuli (32,33), cold air cannot evoke a continuous
cascade of MUC5AC secretion (20). Moreover, the cilia can undergo
morphological regeneration and functional restoration following a
mechanical injury (34,35). Thus, cold air can trigger
symptoms; however, it is unlikely to be a causal factor that
initiates respiratory diseases (1).
Previous studies have indicated that the TRPM8
receptor, which is expressed in human lung epithelial cells, plays
an essential and predominant role in mediating the respiratory
detection of cold stimuli (6,20).
Previous studies in our laboratory have demonstrated that the
upregulated expression of the TRPM8 channel in the bronchial
epithelial cells of subjects with COPD provokes an excessive
production of airway mucus in response to cold air and further
contributes to cold-induced COPD exacerbation (20). An intriguing issue is why COPD
patients present the state of upregulated expression of the TRPM8
channel in the bronchial epithelium. This issue was addressed by
determining the effects of cigarette smoke, the principal risk
factor for the development of COPD, on the basal expression of the
TRPM8 receptor in vivo. In this study, we demonstrate, using
animal models, that cigarette smoke upregulated the basal levels of
the TRPM8 channel in bronchial tissue and that cold air stimuli had
no effect on TRPM8 expression. This finding indicates that
cigarette smoke is a potential etiological factor for the elevated
expression of the TRPM8 channel. However, the detailed mechanisms
of cigarette smoke that are involved in this process require
further clarification. Cold air stimuli do not play a role in the
regulation of TRPM8 expression, but may exert their effects by
activating the TRPM8 channel (6).
In the present study, we demonstrate that cold air
induces the production of TNF-α and IL-8 in the airways, which is
in agreement with previous in vitro studies that
demonstrated that the activation of the TRPM8 variant in human lung
epithelial cells by cold exposure leads to increased expression
levels of several cytokine and chemokine genes, including IL-8 and
TNF-α (36). Moreover, these
results are consistent with those of previous studies (37–40), and suggest that cigarette smoke
has the potential to induce the production of IL-8 and TNF-α in the
airways. In this study, the concomitant presence of cigarette smoke
and cold air resulted in a synergistic enhancement of the
production of IL-8 and TNF-α. The underlying mechanisms of this
synergistic modulation may depend on the upregulated basal levels
of the TRPM8 channel in the bronchial epithelia that is induced by
cigarette smoke; this upregulated basal level of the TRPM8 channel
may be activated by cold stimuli and lead to signal amplification,
which causes further production of IL-8 and TNF-α. We demonstrated
that cigarette smoke and cold air promoted mucin synthesis and
mucus secretion. Cigarette smoke is a common agent that promotes
mucin synthesis and mucus secretion through a variety of
mechanisms, such as the epidermal growth factor receptor signaling
pathway (41), oxidant-dependent
mechanisms (42), goblet cell
hypertrophy and hyperplasia (43,44). However, the mechanisms responsible
for cold air-induced mucin synthesis and mucin secretion are
largely unknown. In our previous study, we demonstrated that cold
air provoked airway mucus hypersecretion through the TRPM8-mediated
influx of the Ca2+ signaling pathway (20). In addition, potential cold-related
products, including IL-8 and TNF-α, which are the inducers of mucin
gene expression, mucin synthesis and mucus secretion (24–26), act as secondary stimuli and may be
responsible for the upregulation of MUC5AC synthesis and secretion.
Therefore, the synergistic amplified production of MUC5AC that was
induced by the combination of cold air and cigarette smoke in our
study was due to the influx of Ca2+ through the
upregulated expression of the TRPM8 channel caused by cigarette
smoke and the synergistically enhanced expression of IL-8 and
TNF-α, which was provoked by the co-exposure of cold air and
cigarette smoke. The synergistic effect of cold air on cigarette
smoke-driven cytokine and MUC5AC upregulation may constitute an
amplification step that contributes to the severity and persistence
of mucus hyperproduction observed in COPD exacerbations that are
induced by cold air. Simultaneously, the ciliary ultrastructural
anomalies caused by cold air may further intensify the accumulation
of mucus.
Taken together, these results suggest that cigarette
smoke is a potential etiological factor for the elevated expression
of the TRPM8 channel, thus causing subjects with COPD to have
greater sensitivity to cold than healthy subjects; this greater
sensitivity is coupled with mucus hyperproduction and
hypersecretion in the airways when exposed to cold air. The present
study may provide a new rationale for therapies that target an
upregulated TRPM8 channel level in the treatment of cold
air-associated pathological mucus hyperproduction.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81370111 and 81270102),
the Chongqing Nature Science Foundation (no. KJ120301) and the
Scientific and Technological Research Program of Chongqing
Municipal Education Commission (no. cstc2012jjA10050). The authors
would also like to thank the editors of the American Journal
Experts, for professional English language editing of this
article.
References
|
1
|
Davis MS, Malayer JR, Vandeventer L, Royer
CM, McKenzie EC and Williamson KK: Cold weather exercise and airway
cytokine expression. J Appl Physiol. 98:2132–2136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koskela HO: Cold air-provoked respiratory
symptoms: the mechanisms and management. Int J Circumpolar Health.
66:91–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Larsson K, Tornling G, Gavhed D,
Muller-Suur C and Palmberg L: Inhalation of cold air increases the
number of inflammatory cells in the lungs in healthy subjects. Eur
Respir J. 12:825–830. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giesbrecht GG: The respiratory system in a
cold environment. Aviat Space Environ Med. 66:890–902.
1995.PubMed/NCBI
|
|
5
|
Donaldson GC, Seemungal T, Jeffries DJ and
Wedzicha JA: Effect of temperature on lung function and symptoms in
chronic obstructive pulmonary disease. Eur Respir J. 13:844–849.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabnis AS, Shadid M, Yost GS and Reilly
CA: Human lung epithelial cells express a functional cold-sensing
TRPM8 variant. Am J Respir Cell Mol Biol. 39:466–474. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Montell C: The TRP superfamily of cation
channels. Sci STKE. 2005:re32005.PubMed/NCBI
|
|
8
|
Song MY and Yuan JX: Introduction to TRP
channels: structure, function, and regulation. Adv Exp Med Biol.
661:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peier AM, Moqrich A, Hergarden AC, et al:
A TRP channel that senses cold stimuli and menthol. Cell.
108:705–715. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwan KY, Allchorne AJ, Vollrath MA,
Christensen AP, Zhang DS, Woolf CJ and Corey DP: TRPA1 contributes
to cold, mechanical, and chemical nociception but is not essential
for hair-cell transduction. Neuron. 50:277–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karashima Y, Talavera K, Everaerts W, et
al: TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl
Acad Sci USA. 106:1273–1278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colburn RW, Lubin ML, Stone DJ Jr, et al:
Attenuated cold sensitivity in TRPM8 null mice. Neuron. 54:379–386.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Zhang X and McNaughton PA:
Modulation of temperature-sensitive TRP channels. Semin Cell Dev
Biol. 17:638–645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nealen ML, Gold MS, Thut PD and Caterina
MJ: TRPM8 mRNA is expressed in a subset of cold-responsive
trigeminal neurons from rat. J Neurophysiol. 90:515–520. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Haute C, De Ridder D and Nilius B: TRP
channels in human prostate. Scientific World Journal. 10:1597–1611.
2010.
|
|
16
|
Li Q, Wang X, Yang Z, Wang B and Li S:
Menthol induces cell death via the TRPM8 channel in the human
bladder cancer cell line T24. Oncology. 77:335–341. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang XR, Lin MJ, McIntosh LS and Sham JS:
Functional expression of transient receptor potential melastatin-
(TRPM) and vanilloid-related (TRPV) channels in pulmonary arterial
and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol.
290:L1267–L1276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Voets T, Owsianik G and Nilius B: TRPM8.
Handb Exp Pharmacol. 179:329–344. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mälkiä A, Madrid R, Meseguer V, de la Peña
E, Valero M, Belmonte C and Viana F: Bidirectional shifts of TRPM8
channel gating by temperature and chemical agents modulate the cold
sensitivity of mammalian thermoreceptors. J Physiol. 581:155–174.
2007.PubMed/NCBI
|
|
20
|
Li M, Li Q, Yang G, Kolosov VP, Perelman
JM and Zhou XD: Cold temperature induces mucin hypersecretion from
normal human bronchial epithelial cells in vitro through a
transient receptor potential melastatin 8 (TRPM8)-mediated
mechanism. J Allergy Clin Immunol. 128:626–634. 2011. View Article : Google Scholar
|
|
21
|
Laniado-Laborín R: Smoking and chronic
obstructive pulmonary disease (COPD). Parallel epidemics of the 21
century. Int J Environ Res Public Health. 6:209–224.
2009.PubMed/NCBI
|
|
22
|
Taylor JD: COPD and the response of the
lung to tobacco smoke exposure. Pulm Pharmacol Ther. 23:376–383.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voynow JA, Gendler SJ and Rose MC:
Regulation of mucin genes in chronic inflammatory airway diseases.
Am J Respir Cell Mol Biol. 34:661–665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rogers DF: Physiology of airway mucus
secretion and pathophysiology of hypersecretion. Respir Care.
52:1134–1146. 2007.PubMed/NCBI
|
|
25
|
Wang IJ, Wu CY and Hu FR: Effect of
proinflammatory cytokines on the human MUC5AC promoter activity in
vitro and in vivo. Clin Ophthalmol. 1:71–77. 2007.PubMed/NCBI
|
|
26
|
Bautista MV, Chen Y, Ivanova VS, Rahimi
MK, Watson AM and Rose MC: IL-8 regulates mucin gene expression at
the posttranscriptional level in lung epithelial cells. J Immunol.
183:2159–2166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davis MS, Lockard AJ, Marlin DJ and Freed
AN: Airway cooling and mucosal injury during cold weather exercise.
Equine Vet J Suppl. 34:413–416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Houtmeyers E, Gosselink R, Gayan-Ramirez G
and Decramer M: Regulation of mucociliary clearance in health and
disease. Eur Respir J. 13:1177–1188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Armengot M, Milara J, Mata M, Carda C and
Cortijo J: Cilia motility and structure in primary and secondary
ciliary dyskinesia. Am J Rhinol Allergy. 24:175–180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith CM, Hirst RA, Bankart MJ, Jones DW,
Easton AJ, Andrew PW and O’Callaghan C: Cooling of cilia allows
functional analysis of the beat pattern for diagnostic testing.
Chest. 140:186–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Calderón-Garcidueñas L, Valencia-Salazar
G, Rodríguez-Alcaraz A, et al: Ultrastructural nasal pathology in
children chronically and sequentially exposed to air pollutants. Am
J Respir Cell Mol Biol. 24:132–138. 2001.PubMed/NCBI
|
|
32
|
Abe J, Hosokawa H, Sawada Y, Matsumura K
and Kobayashi S: Ca2+-dependent PKC activation mediates
menthol-induced desensitization of transient receptor potential M8.
Neurosci Lett. 397:140–144. 2006.
|
|
33
|
Daniels RL, Takashima Y and McKemy DD:
Activity of the neuronal cold sensor TRPM8 is regulated by
phospholipase C via the phospholipid phosphoinositol
4,5-bisphosphate. J Biol Chem. 284:1570–1582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baroody FM: Mucociliary transport in
chronic rhinosinusitis. Clin Allergy Immunol. 20:103–119. 2007.
|
|
35
|
Kim YM, Lee CH, Won TB, Kim SW, Kim JW,
Rhee CS and Min YG: Functional recovery of rabbit maxillary sinus
mucosa in two different experimental injury models. Laryngoscope.
118:541–545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sabnis AS, Reilly CA, Veranth JM and Yost
GS: Increased transcription of cytokine genes in human lung
epithelial cells through activation of a TRPM8 variant by cold
temperatures. Am J Physiol Lung Cell Mol Physiol. 295:L194–L200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arnson Y, Shoenfeld Y and Amital H:
Effects of tobacco smoke on immunity, inflammation and
autoimmunity. J Autoimmun. 34:J258–J265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adcock IM, Caramori G and Barnes PJ:
Chronic obstructive pulmonary disease and lung cancer: new
molecular insights. Respiration. 81:265–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mulligan RM, Atkinson C, Vertegel AA,
Reukov V and Schlosser RJ: Cigarette smoke extract stimulates
interleukin-8 production in human airway epithelium and is
attenuated by superoxide dismutase in vitro. Am J Rhinol Allergy.
23:e1–e4. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li YT, He B and Wang YZ: Exposure to
cigarette smoke upregulates AP-1 activity and induces TNF-alpha
overexpression in mouse lungs. Inhal Toxicol. 21:641–647. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takeyama K, Jung B, Shim JJ, et al:
Activation of epidermal growth factor receptors is responsible for
mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell
Mol Physiol. 280:L165–L172. 2001.PubMed/NCBI
|
|
42
|
Baginski TK, Dabbagh K, Satjawatcharaphong
C and Swinney DC: Cigarette smoke synergistically enhances
respiratory mucin induction by proinflammatory stimuli. Am J Respir
Cell Mol Biol. 35:165–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Innes AL, Woodruff PG, Ferrando RE,
Donnelly S, Dolganov GM, Lazarus SC and Fahy JV: Epithelial mucin
stores are increased in the large airways of smokers with airflow
obstruction. Chest. 130:1102–1108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haswell LE, Hewitt K, Thorne D, Richter A
and Gaça MD: Cigarette smoke total particulate matter increases
mucous secreting cell numbers in vitro: a potential model of goblet
cell hyperplasia. Toxicol In Vitro. 24:981–987. 2010. View Article : Google Scholar : PubMed/NCBI
|