Introduction
Protein phosphorylation and dephosphorylation at
serine (Ser)/threonine (Thr) residues is a central biochemical
reaction that is regulated by Ser/Thr kinase and phosphatase
activities. While much attention has been paid to the roles of
different kinases, little is known about the role of phosphatases
(1). The Ser/Thr protein
phosphatase PP1 and its non-enzymatic co-factor, growth arrest and
DNA damage-inducible protein 34 (GADD34), can mediate the
dephosphorylation of phosphorylated eukaryotic translation
initiation factor 2 subunit α (p-eIF2α), which is a key factor in
the regulation of protein synthesis (2,3).
Therefore, the Ser/Thr protein phosphatase PP1 is considered an
important cellular target of protein synthesis.
In 2005, Boyce et al reported that a small
molecule, named salubrinal, selectively inhibited the
PP1/GADD34-mediated dephosphorylation of p-eIF2α, thereby
protecting 36% of PC12 cells from endoplasmic reticulum (ER)
stress-induced apoptosis, with cell viability increasing from
approximately 40 to 76% (4). In a
previous study by our group, we confirmed the ability of salubrinal
to protect cardiomyocytes from apoptosis (5). However, salubrinal is still far from
being the ideal cardioprotective therapeutic agent due to its
side-effects, poor solubility and high effective concentration.
Apart from salubrinal, very few substances that can potentially
protect cardiomyocytes are known.
A structure-activity relationship (SAR) study on
salubrinal was previously carried out by our group (6) in search of potent cardioprotective
agents derived from this molecule. We demonstrated that the
trichloromethyl and cinnamide groups of salubrinal are
indispensable for its activity, whereas the quinoline ring terminus
is a key site for modification. We therefore modified the quinoline
ring terminus, as well as the thiourea group, and synthesized a
total of 95 molecules. Through screenings with cell viability
assays, we focused on the small molecule, PP1–12. In the present
study, we evaluated the ability of this molecule to protect
neonatal cardiomyocytes in vitro from apoptosis induced by
tunicamycin (TM) and ischemia/reperfusion (I/R) injury. In
addition, we aimed to determine its effects on myocardial I/R
injury in rat hearts in vivo.
Materials and methods
Cell culture and treatments
Neonatal cardiomyocytes were isolated from the
ventricles of the hearts of 1-day-old Wistar rats (Academy of
Military Medical Sciences, Beijing, China, License no.
SCXK-2007-004). Briefly, the rats were sacrificed and the hearts
excised. Following homogenization with a scalpel, the hearts were
treated with 0.125% trypsin and 0.1% collagenase II (4:1) at 37°C
for 10 min; 4–5 rounds of digestion were performed. Isolated cells
were collected by centrifugation and resuspended in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum
(FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. The cultures
were enriched with myocytes by pre-plating for 90 min to deplete
the non-myocyte population. Non-attached cells were plated onto
plastic culture dishes at the appropriate cell density. The cells
were cultured at 37°C in 95% air-5% CO2 for 24 h, and
then half of the DMEM medium containing 10% FBS was replaced with
0.1% 5-bromo-2-deoxyuridine (BrdU). The culture medium was changed
every 48 h. After 4 days, the culture medium was changed to fresh
DMEM containing 1% FBS and the cells were cultured for another 24
h. Subsequently, the cells were treated with TM. The medium was
changed to fresh DMEM containing 1% FBS 1 h prior to exposure to
reagents, in order to obtain consistent ER stress response data.
All reagents were obtained from Gibco-BRL (Gaithersburg, MD, USA),
unless otherwise stated.
Cell viability assay
Adenosine triphosphate (ATP) bioluminescence was
used as a marker of cell proliferation and viability. The presence
of ATP was estimated using the CellTiter-Glo®
Luminescent Cell Viability Assay kit (Promega Corp., Madison, WI,
USA) following the manufacturer’s instructions. Briefly, purified
neonatal rat cardiomyocytes were inoculated into 96-well plates
(104/well) and treated with various concentrations of TM
dissolved in DMSO (Sigma, St. Louis, MO, USA). Various
concentrations of PP1–12 (0.01, 0.03, 0.1, 0.3, 1, 3, 10 μmol/l)
were added 30 min prior to treatment with TM. Subsequently, a
volume of CellTiter-Glo reagent equal to the volume of the cell
culture medium present in each well was added, and the contents
were mixed for 2 min in an orbital shaker to induce cell lysis. The
wells were incubated at room temperature for 10 min to stabilize
the luminescent signal.
Immunocytochemistry
Purified neonatal rat cardiomyocytes
(104/well) were inoculated into 96-well assay plates
(black plates with clear bottom). Various concentrations of PP1–12
(0.1, 0.3, 1 μmol/l) were added 30 min prior to treatment with TM.
Cells were fixed in 4% paraformaldehyde for 15 min at room
temperature, rinsed with PBS, and incubated with blocking buffer
(1X PBS, 0.3% Triton X-100) supplemented with 5% serum from the
same species as the secondary antibody, for 1 h at room
temperature. Then, blocking solution was removed, primary mouse
monoclonal antibody against C/EBP homologous protein (CHOP)/growth
arrest and DNA damage-inducible protein 153 (GADD153) from Cell
Signaling Technology (1:1,600 dilution) was added, and the cells
were incubated overnight at 4°C. Subsequently, 3 μM goat anti-mouse
IgG (H&L) secondary antibody conjugated with fluorochromes
Hoechst 33258 (nuclear dye) or DyLight™ 594 (1:500; Thermo Fisher
Scientific, Waltham, MA, USA) were added, followed by incubation
for 1 h at room temperature in the dark. The secondary antibody was
aspirated and the wells were washed 3 times with PBS. The cells
were then scanned on an In Cell Analyzer 2000 high-content
screening (HCS) reader (General Electric, Atlanta, GA, USA).
Multiparametric analysis of
apoptosis
Cellomics® Multiparameter Apoptosis kits
were purchased from Thermo Fisher Scientific. Multiparametric
analysis of apoptosis was carried out using Hoechst dye, Alexa
Fluor 488 conjugated to phalloidin and MitoTracker, as previously
described (7). Purified neonatal
rat cardiomyocytes (104/well) were inoculated into
96-well assay plates (black plates with clear bottom). Various
concentrations of PP1–12 (0.3, 1, 3 μmol/l) were added 30 min prior
to treatment with TM. At 30 min before the end of the incubation
period, we added MitoTracker/Hoechst solution followed by
incubation for an additional 30 min at 37°C. Fixation solution was
added without removing the medium and the cells were incubated
under a fume hood at room temperature for 10 min. After rinsing
with PBS, the cells were permeabilized with permeabilization buffer
and incubated for 15 min. The cells were then incubated with Alexa
Fluor 488 phalloidin solution for 30 min at room temperature in the
dark. The solution was aspirated and the cells were washed 3 times
with PBS. The data were evaluated on an In Cell Analyzer 2000 HCS
reader.
Rat models of I/R injury
Adult male Sprague-Dawley rats weighing 200–250 g
were obtained from the Charles River Company of China (License no.
SCXK-2011-0004). All experiments were performed in accordance with
the ethical regulations of the Institutional Animal Care and Use of
Laboratory Animals of the Chinese PLA General Hospital. Fifty rats
were randomly divided into 5 experimental groups: i) the
sham-operated group; ii) the vehicle-treated group (I/R + DMSO);
iii) the I/R + S1 group (I/R +1 mg/kg/day PP1–12); iv) the I/R + S3
group (I/R +3 mg/kg/day PP1–12); and v) the I/R + S10 group (I/R +
10 mg/kg/day PP1–12). PP1–12 was dissolved in DMSO and administered
by intraperitoneal injection once a day for 3 consecutive days. The
final injection was administered 30 min prior to the coronary
occlusion. The rat model of I/R injury was established by ligation
of the left anterior descending coronary artery (LAD). The heart
was exposed via a left thoracotomy and the left coronary artery was
ligated 2–3 mm from its original location between the conus of the
pulmonary artery and the left atrium, using a 6-0 silk suture. An
inflatable balloon of fixed atmosphere was placed between the
artery and the silk suture. The coronary artery was occluded to
induce ischemia for 30 min, followed by deflation of the balloon to
allow coronary reperfusion for 2 h. In the sham-operated group, the
suture around the coronary artery was not tied.
Western blot analysis
In vitro, purified neonatal cardiomyocytes
(106/well) were inoculated into 6-well plates and
treated with various concentrations of PP1–12 (0.1, 0.3, 1 μmol/l)
30 min prior to treatment with TM. In vivo, the rats were
sacrificed and the hearts excised and cut into into 50-mg sections
and frozen in liquid nitrogen. Primary cells and tissue scraps were
lysed in whole-cell lysis buffer [62.5 mM Tris-HCl (pH 6.8 at
25°C), 2% w/v SDS, 10% glycerol, 50 mM DTT]. The homogenates were
heated at 100°C for 10 min, and centrifuged at 12,000 × g for 10
min at 4°C. Tissue extracts (50 μg of protein) were homogenized in
lysis buffer (50 mM Tris-HCL, pH 7.5, 150 mM NaCl, 1% Nonidet P-40,
0.5% sodium deoxycholate) with protease inhibitors and a
phosphatase inhibitor. Following centrifugation at 13,000 × g, the
supernatants were boiled for 5 min in Laemmli loading buffer. The
supernatants were used as protein samples. We used BCA to determine
the protein concentration of each sample. Cellular proteins were
separated by electrophoresis on 10% SDS polyacrylamide gels and
transferred onto PVDF membranes. After blocking (in 1X TBS, 0.1%
Tween-20 with 5% w/v non-fat dry milk), the membranes were
incubated overnight in gentle agitation mode at 4°C with the
following antibodies: rat monoclonal anti-caspase-12 and anti-GAPDH
(1:200 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), and rabbit polyclonal anti-phospho-eIF2α, anti-eIF-2α and
anti-β-actin (1:200 dilution; Cell Signaling Technology). This was
followed by incubation with the appropriate horseradish
peroxidase-conjugated secondary antibodies (1:5,000 dilution;
Beijing Zhongshan Golden Bridge Biotechnology Co., Beijing, China)
for 1 h at room temperature. The film was scanned and analyzed with
an imaging densitometer (AlphaImager FluorChem 5500, Alpha Innotech
Corp., San Leandro, CA, USA).
Terminal deoxynucleotidyl transferase
dUTP nick end-labeling (TUNEL) assay
Hearts fixed in 10% formalin were embedded in
paraffin and sectioned into 4-μm-thick slices. To detect apoptotic
cells, TUNEL labeling was performed using an In Situ Apoptosis
Detection kit (Roche Diagnostics GmbH, Basel, Switzerland). To
analyze the number of apoptotic cells in the infarcted hearts,
digital images were acqired at ×400 magnification, and 5 random
fields from each sample were quantified. We counted the number of
TUNEL-positive cardiomyocytes and hematoxylin-stained nuclei in the
entire section of each sample.
2,3,5-Triphenyltetrazolium chloride (TTC)
staining
The hearts were rapidly excised, placed in a 0.9%
saline solution, and cut transversally from the apex to the base
into 5 slices of equal thickness (2.0 mm). The slices were
incubated for 10 min in phosphate-buffered 1% TTC at 37°C, then
fixed in 10% formalin solution. The area that was not stained by
TTC was the area of infarction, and was assessed by a blinded
observer using computer-assisted planimetry (NIH Image 1.57
software, available at: http://rsb.info.nih.gov/nih-image). The percentage of
infarction was expressed as the ratio of the infarct size (IS, or
area of infraction) to the ventricle size (VS).
Statistical analysis
Each experiment was performed in a minimum of 3
different cultures and was repeated at least 3 times. The presented
data are a compilation of these repetitions, apart from the western
blot images, which are from one representative of the 3 replicate
experiments. Values are shown as the means ± standard deviation
(SD). Statistical analyses were performed by a one-way analysis of
variance (ANOVA). Values of P<0.05 were considered to indicate
statistically significant differences.
Results
In a previous study, through the screening of 95
compounds designed as inhibitors of Ser/Thr protein phosphatase
PP1, we identified a small molecule with a low half maximal
effective concentration (EC50) and almost zero toxicity
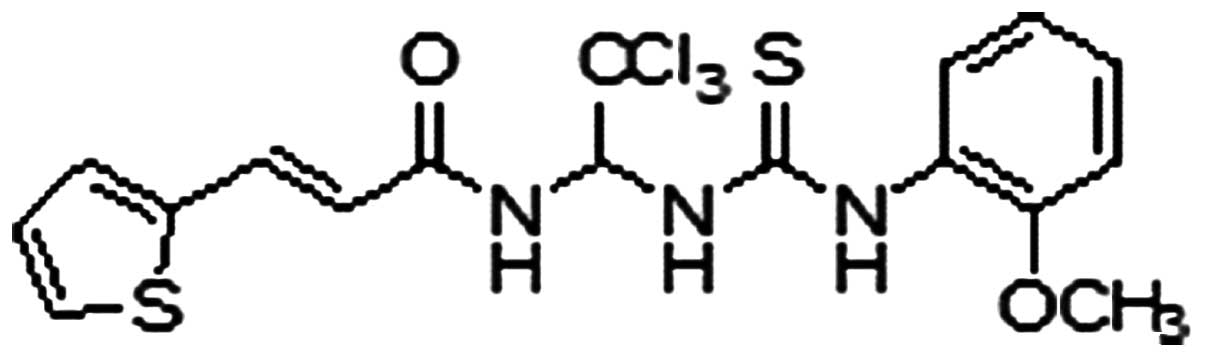
(6). The chemical structure of
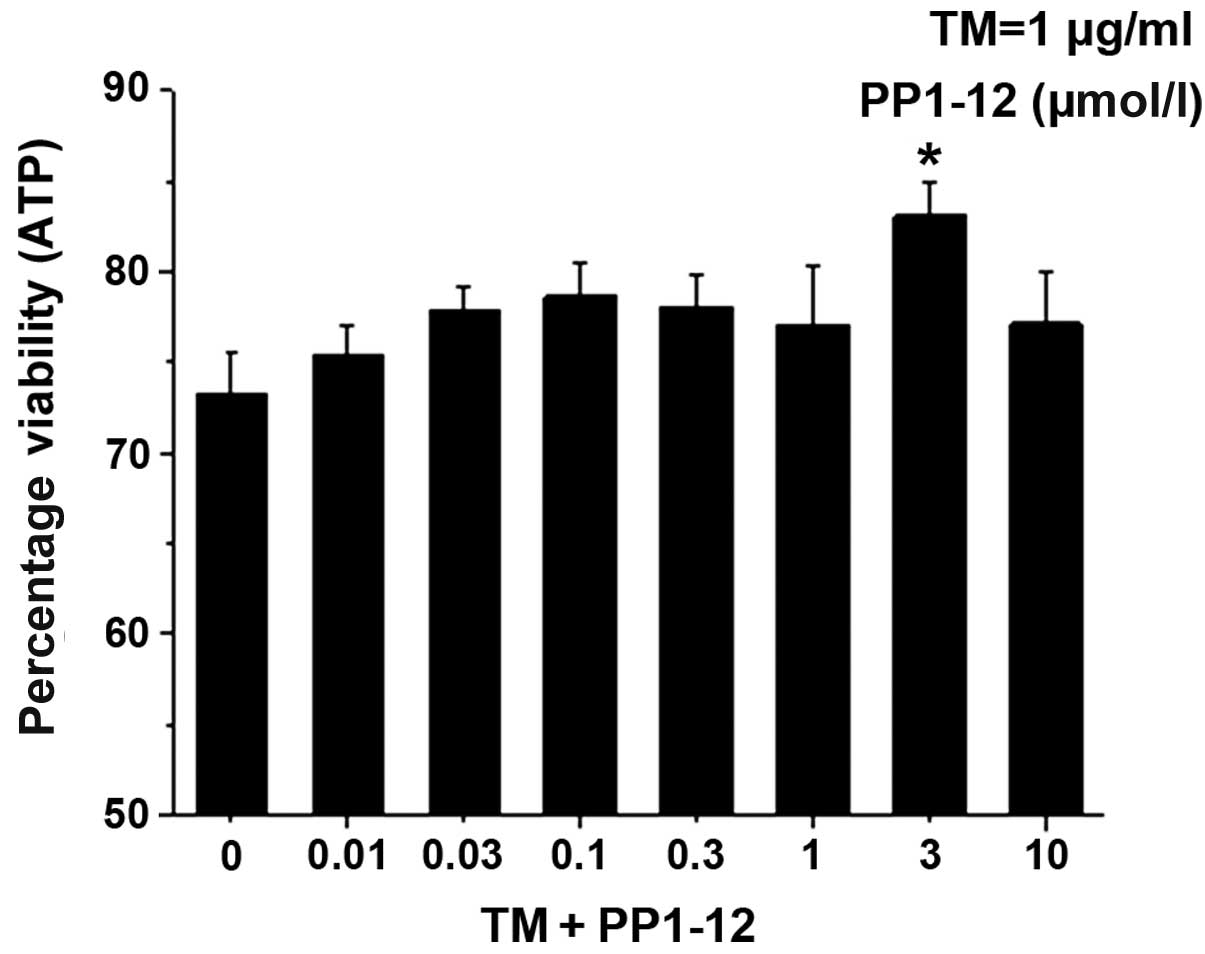
this compound, named PP1–12, is shown in Fig. 1. PP1–12 protected the viability of
the cells treated with the protein glycosylation inhibitor, TM, in
a dose-dependent manner, with 3 μmol/l assessed as the most
effective concentration, through the calculations of the ATP
content (Fig. 2).
TM inhibits protein glycosylation in the ER, thereby
inducing ER stress. Apoptosis is induced by severe and prolonged ER
stress (8). PP1–12 was designed
as an inhibitor of Ser/Thr protein phosphatase PP1, targeting the
phosphatase activity of eIF2α and thereby, reducing ER-related
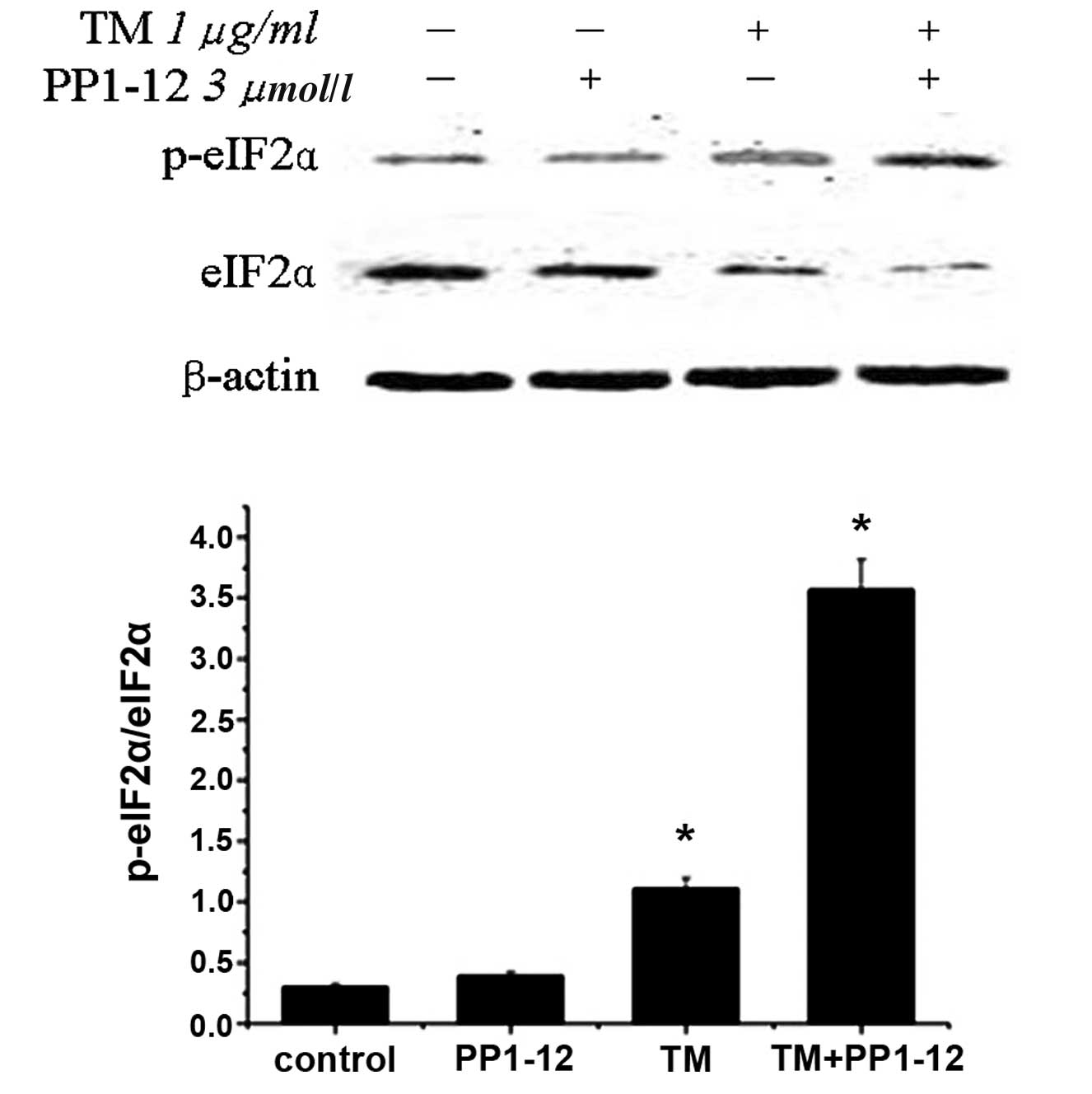
apoptosis. In this study, the level of eIF2α phosphorylation was
detected by western blot analysis. Notably, p-eIF-2α/eIF-2α was
slightly increased in the TM-treated cells. As expected, the
p-eIF-2α/eIF-2α level considerably increased with PP1–12
pre-treatment (Fig. 3). PP1–12,
as a protein phosphatase inhibitor, is known to induce the robust
phosphorylation of eIF2α (2,6).
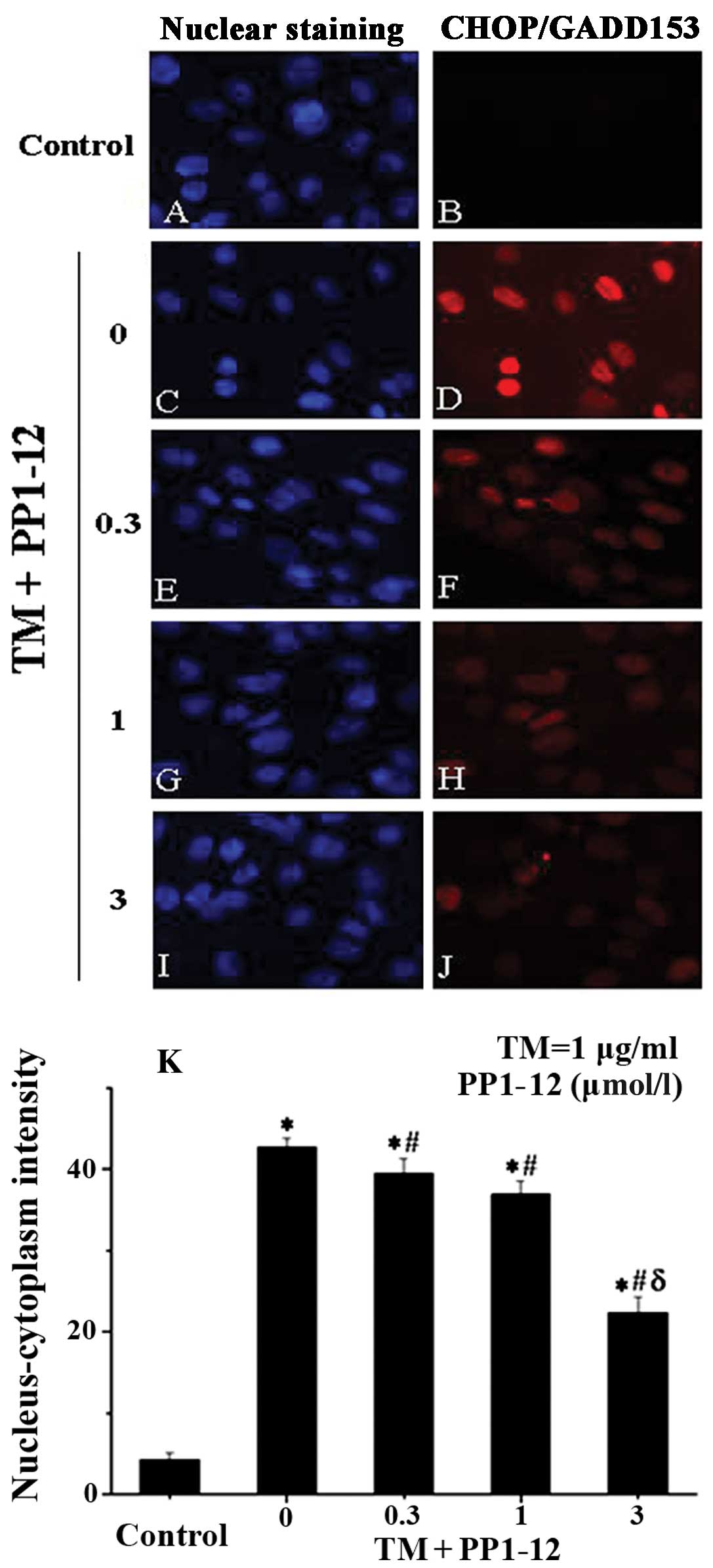
The CHOP protein is specific to ER stress-induced apoptosis, with
its expression induced by severe and prolonged ER stress. To
examine the association betweem PP1–12, ER stress and apoptosis, we
examined CHOP expression by immunocytochemistry (9), as previously described. We observed
that CHOP was present at relatively high levels in the TM-treated
cells, while its relative expression (nucleus/cytoplasm-detected
intensity) was reduced with PP1–12 pre-treatment in a
dose-dependent manner (Fig. 4),
decreasing from 40% in the TM-treated cells to 20% in the cells
treated with TM + 3 μmol/l PP1–12.
We then examined the anti-apoptotic effects of
PP1–12 by the multiparametric analysis of apoptosis. The cells
displayed significant nuclear condensation, a decrease in
mitochondrial membrane potential and an increase in actin
cytoskeleton content following exposure to TM (1 μg/ml) for 36 h,
as compared to the control group, treated with DMSO. Apoptosis was
inhibited by PP1–12 (0.3, 1 and 3 μmol/l), as shown by a reduction
in nuclear condensation (Fig. 5A, D,
G, J and M), but also, by the increase in mitochondrial
membrane potential (Fig. 5B, E, H, K
and N) and the decrease in actin cytoskeleton content (Fig. 5C, F, I, L and O); these changes
were significant at a concentration of 3 μmol/l PP1–12 (Fig. 5P).
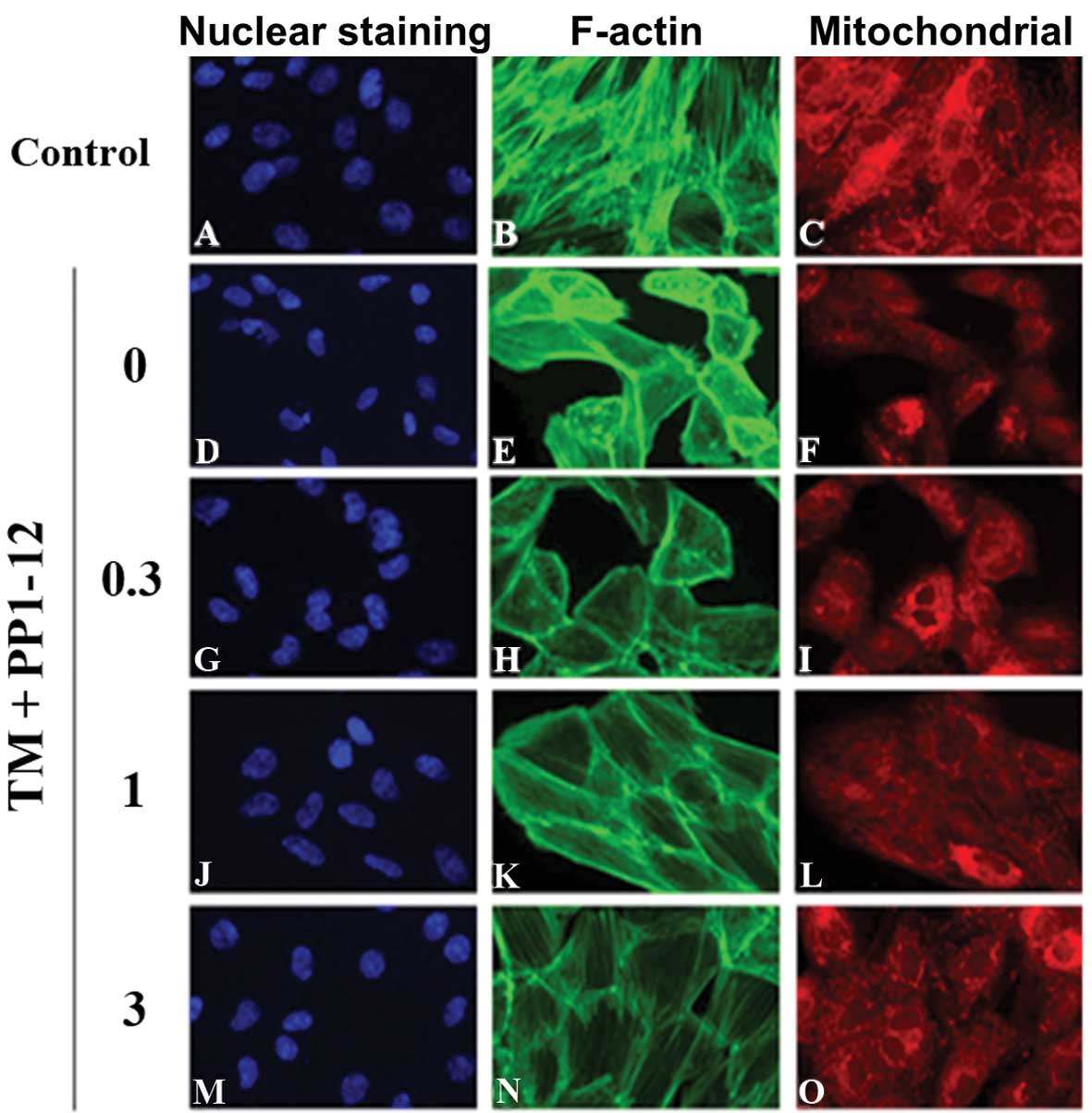 | Figure 5PP1–12 reduces apoptosis in
cardiomyocytes treated with tunicamycin (TM) for 36 h, as assessed
by high-content analysis (HCA). (A, D, G, J and M) Nuclear staining
with Hoechst. (B, E, H, K and N) Staining for F-actin. (C, F, I, L
and O) Mitochondrial staining. (P) F-actin content and
mitochondrial mass/potential ratio. *P<0.001 for the
comparison between the TM group treated with DMSO (0. μM PP1–12)
and the control group (DMSO); #P<0.001 for the
comparison between the TM group treated with 3 μmol/l PP1–12 and
the TM + DMSO group. |
To examine whether the addition of PP1–12 has a
protective effect on the heart under pathological stress, we
selected a widely used model of I/R. We induced ischemia for 30 min
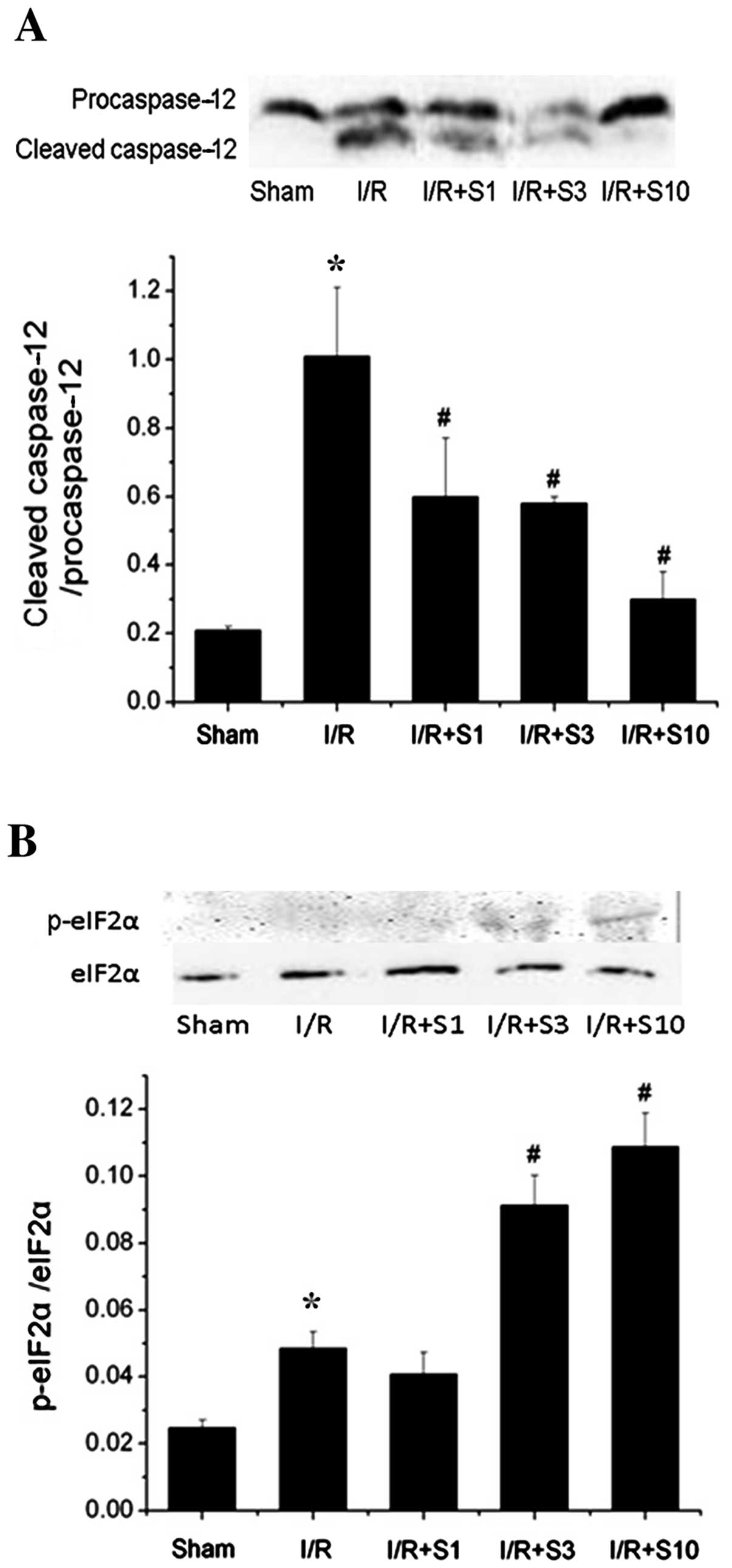
and then reperfusion injury for 2 h. The expression of p-eIF2α and
cleaved caspase-12 (the ER stress-specific apoptotic protein) was
detected by western blot analysis. Caspase-12 was activated by I/R,
as demonstrated by an increase in the level of the cleaved
caspase-12/caspase-12 ratio. The activation of caspase-12 was
largely reverted by PP1–12. A decrease by up to 63.47% was observed
upon PP1–12 pre-treatment (10 mg/kg/day) compared to the I/R
(vehicle-treated) group (Fig.
6A). Similar to the in vitro assays, the level of
p-eIF2α was slightly increased in the I/R (vehicle-treated, I/R +
DMSO) group compared to the control (sham-operated) group, and
there was a significant increase in p-eIF2α expression (Fig. 6B) in the rats pre-treated with
PP1–12 (10 mg/kg/day).
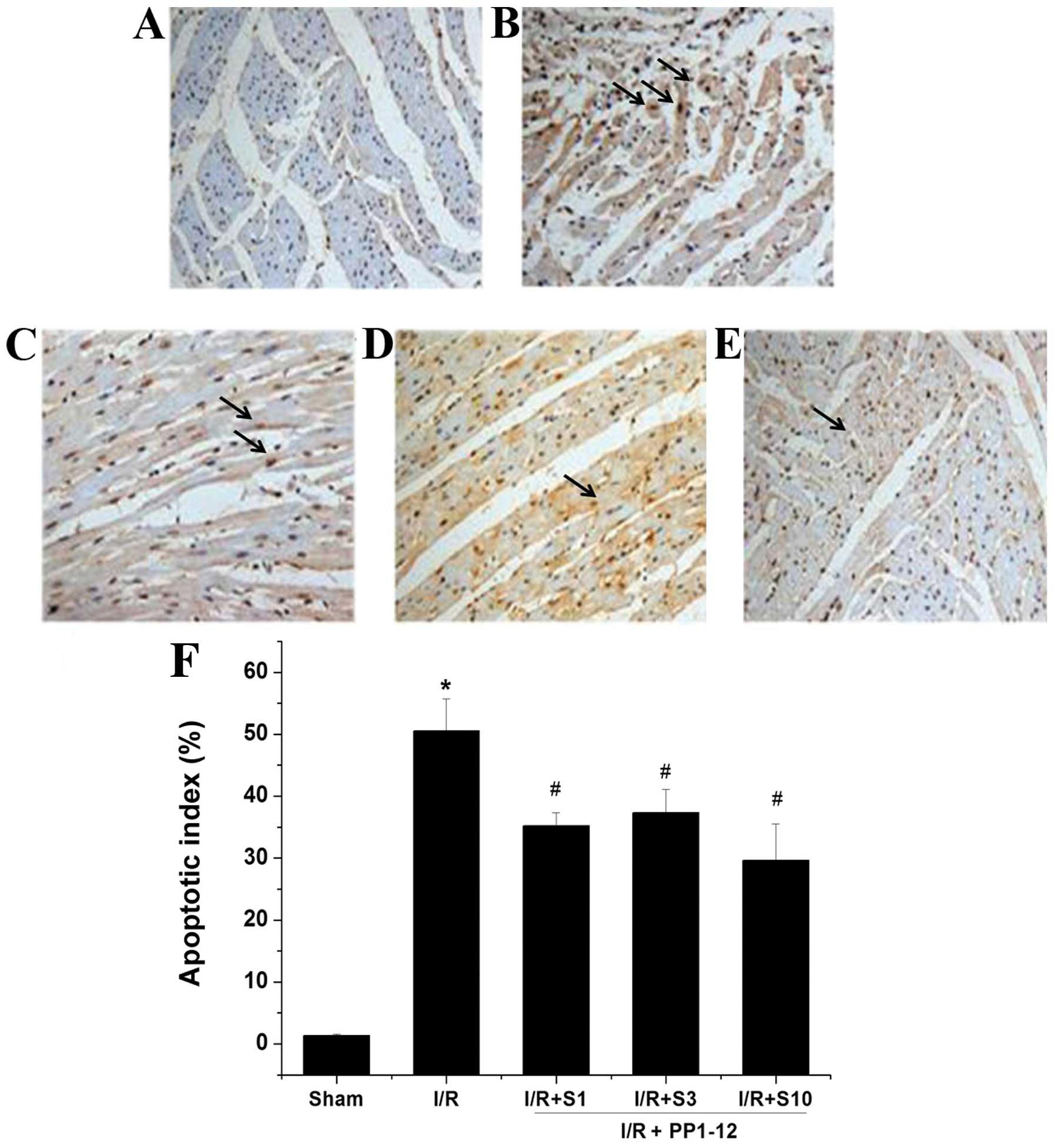
TUNEL is a method widely used for the detection of
apoptosis, whereby apoptotic cells are distinguished by their
color. The number of TUNEL-positive cells was significantly
increased in the hearts of rats in the I/R (vehicle-treated) group
compared with the sham-operated group (Fig. 7B). Treatment with PP1–12 markedly
and significantly reduced the number of TUNEL-positive cells. Among
the doses of PP1–12 tested, that of 10 mg/kg/day had the most
pronounced effect. At this dose, the number of TUNEL-positive cells
was reduced by almost 10% compared to treatment with the vehicle
(I/R group). No TUNEL-positive cells were observed in the
sham-operated group (Fig.
7A).
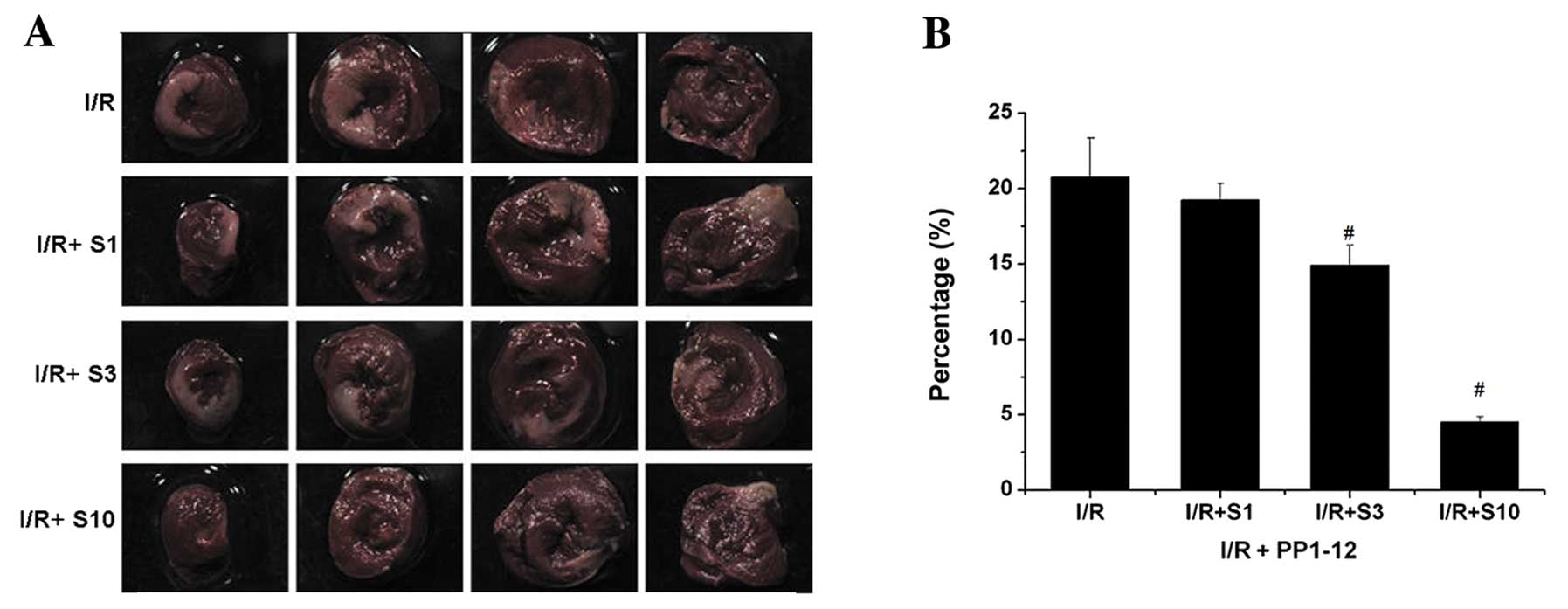
The area of infarction is a vital index of
myocardial cardiac injury. I/R can induce heart infarction by
inducing cell apoptosis. The infarct size was measured using TTC
cell staining and was expressed as the percentage ratio of the
infarct size to the ventricle size (IS/VS). In the vehicle-treated
group (I/R + DMSO), the percentage of IS/VS was approximately
20.738%. Following the administration of PP1–12, the I/R + S1, I/R
+ S3 and I/R + S10 groups (treated with 1, 3 and 10 mg/kg/day
PPI-12, respectively) showed a decrease in the myocardial infarct
size of 19.231, 14.917 (P<0.05) and 4.518% (P<0.05),
respectively, as compared to the vehicle-treated group (Fig. 8).
Discussion
Our study provides evidence on the anti-apoptotic
effects of the inhibitor of the Ser/Thr protein phosphatase PP1,
PP1–12, on cardiomyocytes, through the upregulation of p-eIF2α.
PP1, the major isotype of Ser/Thr protein
phosphatases in cardiomyocytes, is a critical negative regulator of
Ca2+ cycling and contractility. PP1 is a holoenzyme
comprising catalytic and regulatory subunits (10). The inhibition of PP1 has been
shown to enhance cardiac function and delay the progression of
heart failure (11–13). Notably, previous studies have
focused on the inhibition of the catalytic activity of PP1. In
2005, Boyce et al (4)
found that salubrinal, a selective inhibitor of the regulatory
subunit of PP1, protects PC-12 cells from apoptosis. They further
demonstrated that salubrinal inhibits targeting, by PP1, of the
eIF2α protein, which normally results in its dephosphorylation and
allows proteostasis. In 2012, we demonstrated for the first time
that salubrinal has a similar protective effect on cardiomyocytes
(5). There are a number of
studies focusing on protein phosphorylation, and all highlight the
important effects of protein phosphorylation on protein expression.
Protein phosphorylation can be regulated by the balance between
kinase and phosphatase activities. While more attention has been
paid to the roles of different kinases, less is known about the
role of phosphatases (14). We
previously synthesized a number of inhibitors of Ser/Thr protein
phosphatase PP1 based on salubrinal, of which 24 displayed
protective effects on cardiomyocytes (6). Viability assays revealed that, of
these molecules, PP1–12 protects cardiomyocytes from TM-induced
death. We thus focused on PP1–12, since it further displayed
reduced EC50 and low toxicy.
Cardiomyocytes are cells with no proliferative
ability. Therefore, the maintenance of their population through the
prevention of apoptosis is crucial. Accumulating evidence suggests
that ER stress-mediated cell apoptosis is one of the leading causes
of cardiomyocyte death. TM is one of the most widely used compounds
for triggering ER stress through the inhibition of protein
glycosylation (14). ER stress in
abnormal cardiomyocytes induces the so-called unfolded protein
response (15), which involves
p-eIF2α-mediated inhibition of protein translation, thereby
protecting cells from ER sress. The dephosphorylation of p-eIF2α is
regulated by a complex containing the Ser/Thr phosphatase PP1 and
its co-factor, GADD34 (16). The
apoptotic pathway is induced by severe and prolonged ER stress
(17,8). PP1–12 was designed as an inhibitor
of the PP1/GADD34 complex to mediate the dephosphorylation of
p-eIF2α. As expected, western blot analysis revealed an increase in
the p-eIF-2α/eIF-2α ratio upon PP1–12 pre-treatment. Of note, TM
alone also induced a slight increase in this ratio, which possibly
reflects a self-protective mechanism of cardiomyocytes against ER
stress. CHOP is a key transcription factor acting downstream of
p-eIF2α, whose expression is closely associated with the induction
of ER-induced apoptosis (18).
Our immunohistochemical analyses suggested that TM induces, while
PP1–12 inhibits, CHOP transcription. This result further indicated
that PP1–12 may reduce ER stress-related apoptosis. High-content
screening (HCS), which allows fluorescence-based multiparametric
analyses, has rapidly become a widely used method for the detection
of apoptosis. The most obvious advantage of using this method is
that it allows an image-based analysis. Moreover, HCS combined with
a microplate reader, allows the analysis of a larger number of
samples, entails the reduced consumption of reagents, and lower
heterogeneity. In our study, HCS analysis revealed that PP1–12
protects cardiomyocytes from apoptosis, as shown by the reduction
in nuclear condensation, the increase in mitochondrial membrane
potential and the decrease in actin cytoskeleton content. Taken
together, our data suggest that the protective effects of PP1–12
are at least partly mediated by a resistance to apoptosis. This
result is consistent with the results of our previous study on the
effects of salubrinal on cardiomyocytes (6).
In 2013, Oh et al demonstrated that decoy
peptides target protein phosphatase 1 and inhibit the
dephosphorylation of phospholamban in cardiomyocytes (19). In addition, Hanana et al
pointed out that the activation of ERK by a potent inhibitor of
protein phosphatase exerts a cell survival effect (20). Together with our results, these
data demonstrate that PP1 may be a protective agent in
cardiomyocyte injury.
A number of studies have demonstrated that ER stress
response is activated in heart tissue exposed to prolonged and
acute stress. I/R is a type of acute stress that can activate ER
stress (21). In our model of
I/R, p-eIF2α, as well as cleaved caspase-12 were activated.
Caspase-12 is an apoptosis-specific protein, which can be cleaved
only in ER stress-related apoptosis (22). In our study, PP1–12 significantly
increased the expression of p-eIF2α and reduced the level of
cleaved caspase-12. This result indicates that PP1–12 inhibits ER
stress-related apoptosis. Thus, we concluded that PP1–12 may have
an anti-apoptotic effect. Our hypothesis was further supported by
the decrease in the number of TUNEL-positive cells pre-treated with
PP1–12. The area of infarction is a vital index of heart injury in
models of I/R (23). In our
study, the infarct size decreased with the increasing
concentrations of PP1–12. This indicates that PP1–12 exerts a
protective effect in the rat model of I/R. Although the
anti-apoptotic effects of PP1–12 may explain the protection of the
cells in heart tissue, the underlying mechanisms which render
PP1–12 a more potent protective agent compared to other inhibitors
of Ser/Thr protein phosphatase PP1 remain unknown.
In conclusion, the data presented in this study
demonstrate that PP1–12 protects cardiomyocytes from apoptosis.
Thus, PP1–12, as an inhibitor of Ser/Thr protein phosphatase PP1,
may thus represent a promising therapeutic agent for the control of
heart disease. In the future, drugs that target Ser/Thr protein
phosphatase PP1 may therefore complement the currently used
clinical treatments.
Acknowledgements
This study was funded by the Ministry of Science
Foundation of the Chinese People’s Liberation Army, 12th Five-Year
Plan (no. BWS12J048) and the Major International Science and
Technology Cooperation Project (no. 2013DFA31170).
References
|
1
|
Moscardó A, Vallés J, Piñón M, et al:
Regulation of cytosolic PlA2 activity by PP1/PP2A serine/threonine
phosphatases in human platelets. Platelets. 17:405–415.
2006.PubMed/NCBI
|
|
2
|
Novoa I, Zeng H, Harding HP and Ron D:
Feedback inhibition of the unfolded protein response by
GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol.
153:1011–1022. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan T, Luo BL, Wei TH, et al: Salubrinal
protects against cigarette smoke extract-induced HBEpC apoptosis
likely via regulating the activity of PERK-eIF2alpha signaling
pathway. Arch Med Res. 43:522–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyce M, Bryant K, Jousse CJ, et al: A
selective inhibitor of eIF2alpha dephosphorylation protects cells
from ER stress. Science. 307:935–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu CL, Li X, Hu GL, et al: Salubrinal
protects against tunicamycin and hypoxia induced cardiomyocyte
apoptosis via the PERK-eIF2alpha signaling pathway. J Geriatr
Cardiol. 9:258–268. 2012.PubMed/NCBI
|
|
6
|
Liu J, He KL, Li X, et al: SAR, cardiac
myocytes protection activity and 3D-QSAR studies of salubrinal and
its potent derivatives. Curr Med Chem. 19:6072–6079. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gasparetto M, Gentry T, Sebti S, et al:
Identification of compounds that enhance the anti-lymphoma activity
of rituximab using flow cytometric high-content screening. J
Immunol Methods. 292:59–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faitova J, Krekac D, Hrstka R and Vojtesek
B: Endoplasmic reticulum stress and apoptosis. Cell Mol Biol Lett.
11:488–505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chatterjee J and Kohn M: Targeting the
untargetable: recent advances in the selective chemical modulation
of protein phosphatase-1 activity. Curr Opin Chem Biol. 17:361–368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pathak A, Monte F, Zhao W, et al:
Enhancement of cardiac function and suppression of heart failure
progression by inhibition of protein phosphatase 1. Circ Res.
96:756–766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicolaou P, Hajjar RJ and Kranias EG: Role
of protein phosphatase-1 inhibitor-1 in cardiac physiology and
pathophysiology. J Mol Cell Cardiol. 47:365–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamada M, Ikeda Y, Yano M, et al:
Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery
ameliorates heart failure progression in genetic cardiomyopathy.
FASEB J. 20:1197–1199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaya H, Utsumi T, Shimoke K, et al:
Nicotine suppresses tunicamycin-induced, but not
thapsigargin-induced, expression of GRP78 during ER stress-mediated
apoptosis in PC12 cells. J Biochem. 144:251–257. 2008. View Article : Google Scholar
|
|
15
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou W, Brush MH, Choy MS and Shenolikar
S: Association with endoplasmic reticulum promotes proteasomal
degradation of GADD34 protein. J Biol Chem. 286:21687–21696. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen G, Gong M, Yan M and Zhang X:
Sevoflurane induces endoplasmic reticulum stress mediated apoptosis
in hippocampal neurons of aging rats. PLoS One. 8:e578702013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Zhang C, Hong Z, et al: C/EBP
homologous protein (CHOP) mediates neuronal apoptosis in rats with
spinal cord injury. Exp Ther Med. 5:107–111. 2013.PubMed/NCBI
|
|
19
|
Oh JG, Kim J, Jang SP, et al: Decoy
peptides targeted to protein phosphatase 1 inhibit
dephosphorylation of phospholamban in cardiomyocytes. J Mol Cell
Cardiol. 56:63–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanana H, Talarmin H, Pennec JP, Droguet
M, Morel J and Dorange G: Effect of okadaic acid on cultured clam
heart cells: involvement of MAP kinase pathways. Biol Open.
1:1192–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang B, Fan P, Xu A, et al: Improved
functional recovery to I/R injury in hearts from lipocalin-2
deficiency mice: restoration of mitochondrial function and
phospholipids remodeling. Am J Transl Res. 4:60–71. 2012.PubMed/NCBI
|
|
22
|
Nakagawa T, Zhu H, Morishima N, et al:
Caspase-12 mediates endoplasmic reticulum-specific apoptosis and
cytotoxicity by amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vidavalur R, Swarnakar S, Thirunavukkarasu
M, et al: Ex vivo and in vivo approaches to study mechanisms of
cardioprotection targeting ischemia/reperfusion (i/r) injury:
useful techniques for cardiovascular drug discovery. Curr Drug
Discov Technol. 5:269–278. 2008. View Article : Google Scholar : PubMed/NCBI
|