Introduction
The underground parts of Nardostachys
chinensis (N. chinensis), which belongs to genus
Valerianaceae, have been used in traditional Korean medicine for
centuries to elicit stomachic, anti-arrhythmic and sedative effects
(1). This plant is known to be
rich in sesquiterpenoids (2),
which have been shown to exhibit antimalarial, antinociceptive
(3) and cytotoxic activities
(4). Nardosinone, isolated from
N. chinensis has been shown to act as an enhancer of nerve
growth factor in neuronal cells (5) and to possess anti-inflammatory
properties in lipopolysaccharide (LPS)-stimulated macrophages
(6).
HL-60 cells differentiate into either
macrophage/monocytic (7,8) or granulocytic lineages (9), depending on the type of stimuli. The
induction of the differentiation of HL-60 cells has been shown to
be associated with the activation of various protein kinases,
including isoforms of protein kinase C (PKC) and mitogen-activated
protein kinases (MAPKs) (10–13). MAPKs are serine/threonine kinases
that are involved in the regulation of a variety of cellular
responses, such as cell proliferation, differentiation and
apoptosis (14,15). Based on structural differences,
they are classified into three subfamilies: extracellular
signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and
p38 MAPK kinase. The ERK signaling pathway is mainly activated by
mitogens and growth factors, and plays a major role in the
regulation of cell growth, survival and differentiation (16,17). By contrast, the JNK and p38 MAPK
pathways are activated in response to chemicals and environmental
stress, and are frequently associated with the induction of
apoptosis (17,18). In the leukemic cell line, HL-60,
the activation of MAPKs has been shown to be involved in monocytic
and granulocytic differentiation (19–23). Specifically, ERK activation is
required for the differentiation of leukemic cells into monocytes
and granulocytes (19–21). While JNK activation is associated
with monocytic differentiation (22,23), p38 MAPK activation is involved in
the inhibition of monocytic differentiation (23).
PKC is considered a potential target for the
development of novel anticancer therapeutic agents. PKC plays a key
role in the regulation of the response of hematopoietic cells to
physiological and pathological inducers of proliferation and
differentiation (24,25). The 12 PKC isoforms identified to
date are classified into three distinct groups on the basis of the
presence of functional domains and subsequent differences in their
regulation: i) the conventional isozymes (cPKCs: α, βI, βII and γ)
are diacylglycerol- and calcium-dependent; ii) the novel isozymes
(nPKCs: δ, σ, η, θ and μ) are diacylglycerol-dependent and
calcium-independent; and iii) the atypical PKC isozymes (aPKCs: ξ,
ι and λ) are diacylglycerol- and calcium-independent (26). Of the PKC isoforms, the
calcium-dependent PKC isozymes are most abundantly expressed in
leukemic cells and have been implicated in the induction of the
differentiation of HL-60 cells (27).
Since PKC and MAPKs have been implicated in the
induction of the differentiation of leukemic cells, in this study,
we investigated whether N. chinensis extract induces the
differentiation of HL-60 cells through the activation of PKC and
MAPKs.
Materials and methods
Reagents and antibodies
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), nitrotetrazolium blue chloride (NBT), phorbol 12-myristate
13-acetate (PMA), protease inhibitor cocktail, and anti-β-actin
were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO,
USA). 2′-Amino-3′-methoxyflavone (PD98059),
4-(4-fluorophenyl)-2-(4-methylsulfonylphenyl)-5-(4-pyridyl)
1H-imidazole (SB203580), bisindolylmaleimide (GF 109203X),
chelerythrine chloride and
1-(5-isoquinolinesulfonyl)-2-methylpiperazine dihydrochloride (H-7)
were purchased from Calbiochem (La Jolla, CA, USA). Methanol,
acetonitrile and water of high-performance liquid chromatography
(HPLC) grade were obtained from Fisher Scientific Co. (Pittsburgh,
PA, USA). Nardosinone was purchased from Sichuan Weikeqi Biological
Technology Co., Ltd. (Chengdu, Sichuan, China) at >98% purity.
RPMI-1640, fetal bovine serum (FBS) and antibiotic-antimycotic
solution were purchased from Gibco (Grand Island, NY, USA).
RPE-conjugated anti-CD11b and FITC-conjugated anti-CD14 antibodies
were purchased from Dako (Glostrup, Denmark). Anti-ERK,
anti-phosphorylated (p)-ERK, anti-p38, anti-p-p38, anti-JNK and
anti-p-JNK antibodies were purchased from Cell Signaling Technology
Inc. (Beverly, MA, USA). Anti-PKCα, anti-PKCβI, anti-PKCβII and
anti-PKC γ antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA).
Preparation of N. chinensis
extract
The dried roots and stems of N. chinensis
were purchased from Nonglim-Saengyak Co., Ltd. (Seoul, Korea).
N. chinensis was identified by Professor B.-H. Jeon and
Professor G.-S. Lee, two of the authors. A voucher specimen
(DP-2012-NC) has been deposited at the Department of Pathology,
College of Korean Medicine, Wonkwang University, Iksan, Korea. An
aqueous extract was prepared by boiling 100 g of N.
chinensis with one liter of distilled water for 2 h, and then
centrifuged at 2,000 rpm for 15 min to remove the insoluble
ingredients. The supernatant was filtered through Whatman filter
paper no. 4 in a Büchner funnel under a vacuum and stored at −20ºC
overnight. The frozen extract was freeze-dried in a vacuum chamber.
The yield of the extract was 12.82% (w/w). The freeze-dried powder
was then dissolved in PBS (pH 7.4) at a concentration of 40 mg/ml
and stored at −20ºC; it was diluted in cell culture medium prior to
use.
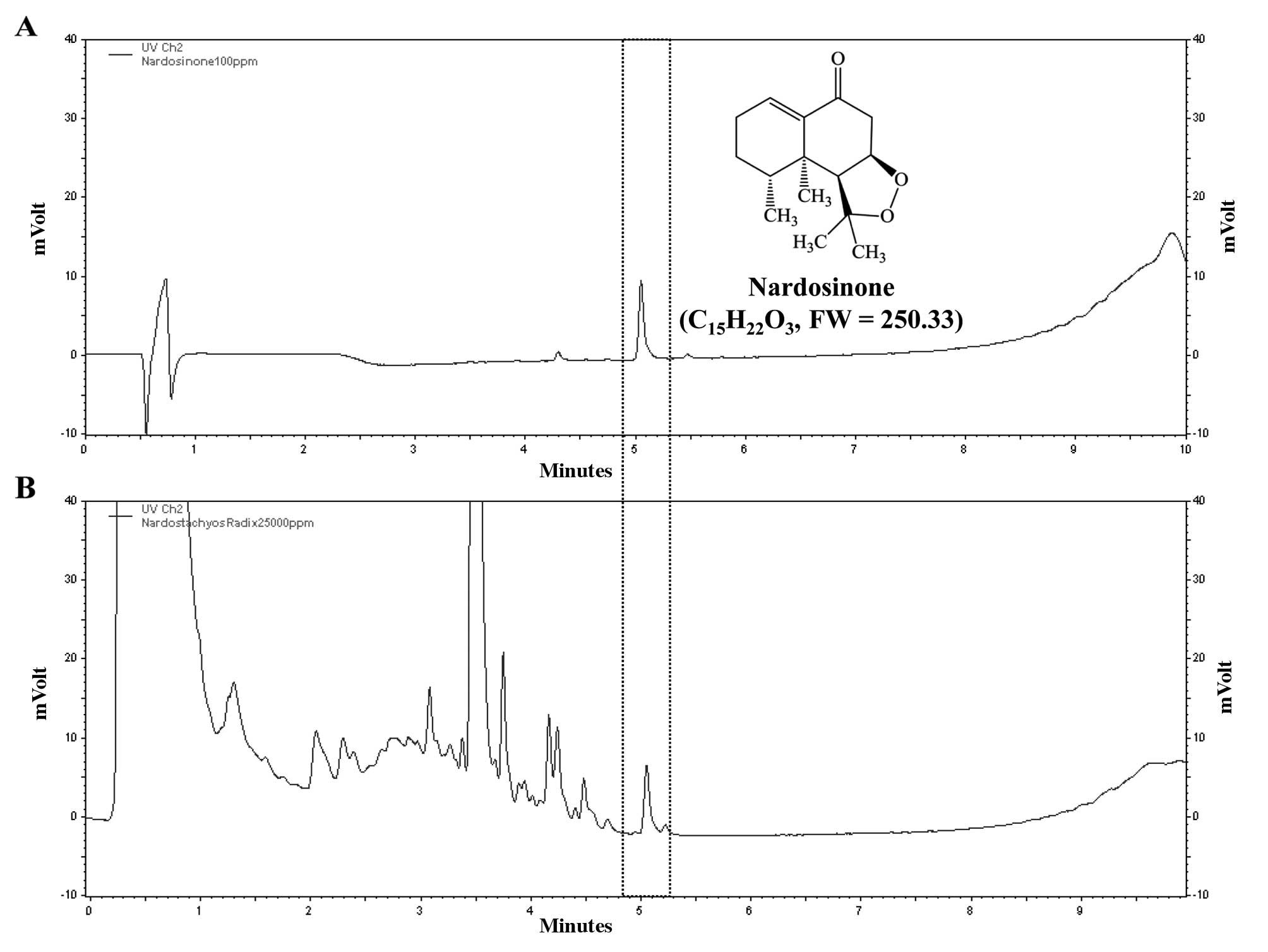
HPLC analysis of N. chinensis
extract
The aqueous extract of N. chinensis was
analyzed by liquid chromatography (LC) using a SmartLC sytsemt
(LC800series; GL Sciences, Tokyo, Japan) equipped with an Inertsil
ODS-4 column (2.1×50 mm, 2 μm ID) at 35ºC. The mobile phase
consisted of water (solution A) and acetonitrile (solution B). A
gradient of the mobile phase was used as follows: 10% solution B
for 1 min, 10–90% solution B for 1–9 min. The final concentration
of nardosinone dissolved in methanol was 0.1 mg/ml and that of the
N. chinensis extract dissolved in water was 25 mg/ml. The
flow rate was set to 0.4 ml/min and the injection volume was 1 μl.
The wavelength for the detection of nardosinone was 280 nm. The
acquired data were processed using EZChrom Elite software version
3.3.2 SP1 (Agilent Technologies, Santa Clara, CA, USA). The
nardosinone peak was detected at 5.051 min retention time and the
water extract of N. chinensis had the same retention time
(Fig. 1).
Cell culture
HL-60 cells were obtained from the American Type
Culture Collection (ATCC; Rockville, MD, USA) and cultured in
RPMI-1640 medium supplemented with 10% FBS and an
antibiotic-antimycotic solution at 1:100 dilution, at 37ºC in a
humidified 95% air and 5% CO2 incubator.
Determination of cell viability
Exponentially growing cells were seeded into 24-well
plates (1×105 cells/well) in duplicate. The cells were
treated with increasing concentrations of N. chinensis
extract for 24, 48 and 72 h. Following treatment, each well was
incubated with 100 μl of 5 mg/ml MTT for 4 h. Water-insoluble
MTT-formazan crystals were solubilized by the addition of an equal
volume of solubilization solution (10% SDS, 0.01 N HCl) and
overnight incubation in a humidified atmosphere of 5%
CO2 at 37ºC. The amount of formazan was determined at
570 nm using a SpectraMax 250 Microplate spectrophotometer
(Molecular Devices, Sunnyvale, CA, USA). The relative percentage of
viable cells was calculated using the following equation: % cell
viability = [mean optical density (OD) of treated cells/mean OD of
control cells] ×100.
NBT reduction assay
HL-60 cells (1×106 cells/60-mm dish) were
cultured in RPMI-1640 medium containing N. chinensis extract
and 10% FBS for 72 h, and then the NBT reducing activity of the
cells was determined by the method described in the study by
Sakashita et al (28) with
a slight modification. Briefly, the cells were harvested by
centrifugation and suspended in 200 μl of 2 mg/ml NBT solution.
Following the addition of 2 μl of 100 μg/ml PMA solution, the cell
suspension was incubated at 37ºC for 20 min, and 200 μl of 1 N HCl
were added at 4ºC to terminate the reaction. Following
centrifugation, 600 μl of DMSO were added to the cell pellets to
solubilize the formazan crystals. The amount of formazan was
determined at 560 nm using a SpectraMax 250 Microplate
spectrophotometer.
Flow cytometric analysis
The HL-60 cells treated with N. chinensis
extract were harvested, washed twice with ice-cold PBS (pH 7.4),
and then suspended in 100 μl of PBS containing 0.25% BSA. After the
addition of 10 μl of RPE-conjugated anti-CD11b or FITC-conjugated
anti-CD14 antibodies, the cells were incubated in the dark at 4ºC
for 30 min, washed twice with PBS containing 0.25% BSA and fixed in
500 μl of PBS containing 1% formaldehyde. The levels of antibody
binding to the cells were quantified using fluorescence-activated
cell sorting (FACS) technology on a FACSCalibur flow cytometer (BD
Biosciences, San Diego, CA, USA).
Western blot analysis
Cells were washed with ice-cold PBS (pH 7.4), gently
resuspended in ice-cold lysis buffer (50 mM Tris-HCl at pH 7.4, 150
mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and 1%
protease inhibitor cocktail), and incubated on ice for 30 min. Cell
lysates were centrifuged for 10 min at 14,000 rpm at 4ºC, and the
protein concentration was determined by Bradford assay. Samples
containing 40 μg of total protein were resolved in a SDS-PAGE gel,
and transferred onto a nitrocellulose membrane for 3 h at 40 V. The
membrane was probed with primary antibodies and immunoreactivity
was detected using HRP-conjugated goat anti-rabbit IgG or rabbit
anti-mouse secondary antibodies. Immunoreactive bands were
visualized using the SuperSignal West Pico Chemiluminescent
substrate by Thermo Fisher Scientific Inc. (Waltham, MA, USA) and
were quantified using the Molecular Imager ChemiDoc XRS system
(Bio-Rad, Hercules, CA, USA).
PKC activity assay
PKC activity was measured using a PKC Kinase
Activity Non-Radioactive Assay kit (Stressgen Biotechnologies
Corp., Victoria, BC, Canada) as follows: the cells were washed with
ice-cold PBS (pH 7.4), and lysed on ice for 5 min in sample
preparation lysis buffer (20 mM MOPS, 50 mM β-glycerolphosphate, 50
mM NaF, 1 mM sodium vanadate, 5 mM EGTA, 2 mM EDTA, 1% NP-40, 1 mM
dithiothreitol and 1% protease inhibitor cocktail), and centrifuged
at 13,000 rpm for 15 min at 4ºC. The protein concentration was
determined by Bradford assay. Lysed protein extracts (500 ng) were
diluted into 30 μl of the dilution buffer and loaded on 96-well
plates coated with a PKC substrate peptide. The PKC assay was
initiated by the addition of 10 μl of adenosine triphosphate (ATP;
1 mg/ml) to each well at 30ºC, and the incorporation of phosphate
into the substrate peptide was measured as per the manufacturer’s
instructions after 1 h. The wells were then washed twice with
antibody dilution buffer, and 40 μl of phospho-specific substrate
antibodies were added to each well followed by incubation for 1 h.
Each well was subsequently washed four times for 10 min with
washing buffer and a 1:1,000 dilution of anti-rabbit IgG
HRP-conjugated antibody in the kit’s dilution buffer, and incubated
for 30 min. The wells were washed four times, and 60 μl of
tetramethylbenzidine substrate was added and the wells were
incubated for 30 min. The HRP reaction was quenched with the
addition of 20 μl of acid stop solution and the absorbance of each
well was measured at 450 nm using a SpectraMax 250 Microplate
spectrophotometer. The relative percentage of PKC activity was
calculated using the following equation: % PKC activity = (mean OD
of treated cells/mean OD of control cells) ×100.
Statistical analysis
Statistical analysis was performed using Microsoft
Office Excel 2010 (Microsoft, Redmond, WA, USA). The data are
expressed as the means ± standard deviation (SD). Statistically
significant differences between groups were assessed by the
Student’s t-test.
Results
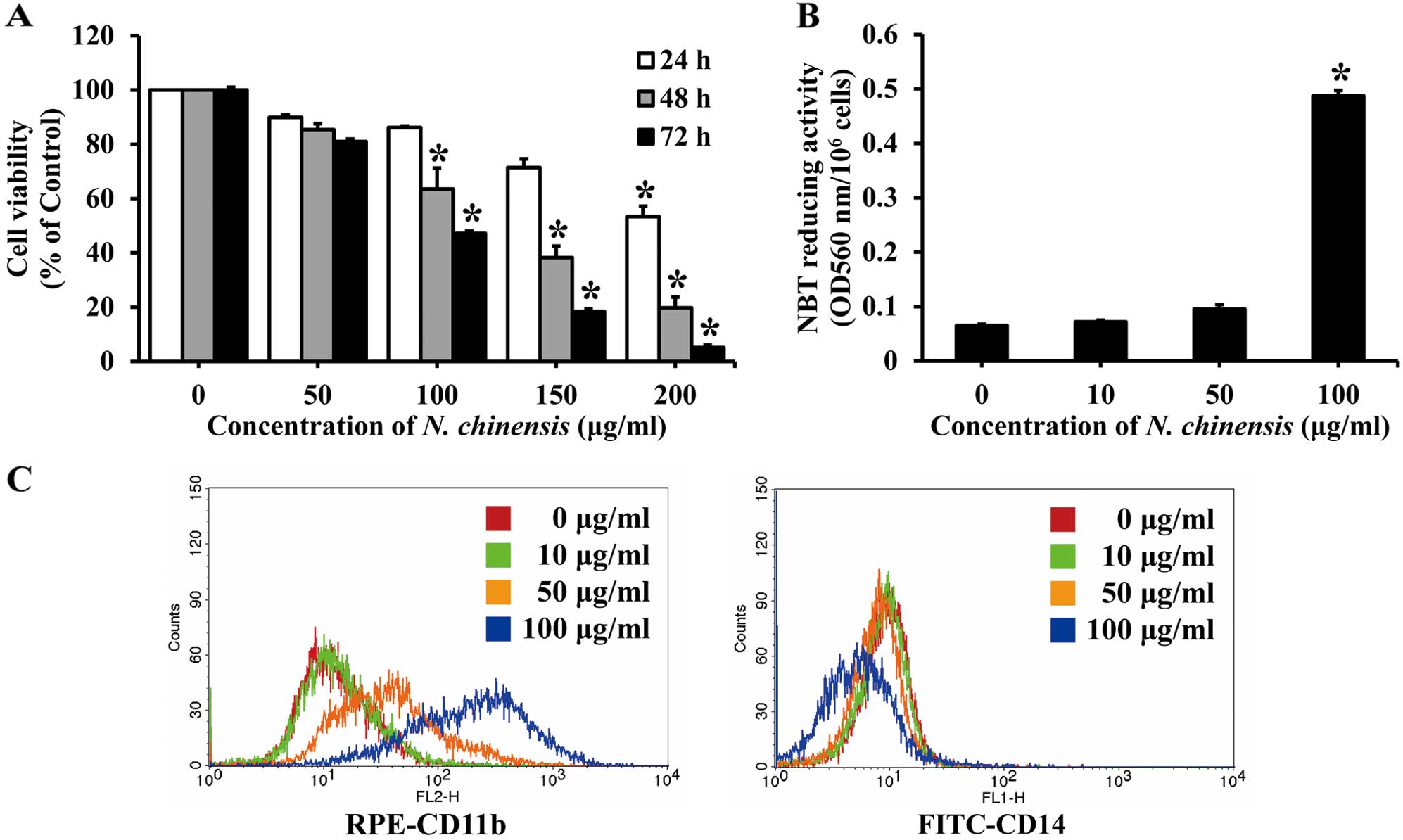
Effects of N. chinensis extract on the
proliferation and differentiation of HL-60 cells
The effects of N. chinensis extract on cell
proliferation were evaluated by MTT assay. The HL-60 cells were
treated with a series of concentrations (50–200 μg/ml) of the
extract for 24, 48 or 72 h. Treatment with the extract decreased
the proliferation of HL-60 cells in a dose- and time-dependent
manner. After 72 h, 100 μg/ml of the extract inhibited cell
proliferation by approximately 50% (Fig. 2A).
To determine the effects of N. chinensis
extract on the differentiation of HL-60 cells, the cells were
treated with various concentrations of the extract for 72 h, and
then assessed for their NBT reducing activity, which is a marker of
the degree of cell differentiation. NBT reducing activity increased
in a dose-dependent manner, with an increase of approximately
7.5-fold observed with 100 μg/ml of the N. chinensis extract
(Fig. 2B).
To further confirm that the differentiation of HL-60
cells was induced by the extract, the expression of cell surface
markers (i.e., CD11b and CD14) was assessed. CD11b expression
(detected with a FITC-conjugated antibody) is a marker of
granulocytic and monocytic differentiation, while CD14 expression
(detected with a RPE-conjugated antibody) is a specific marker of
monocytic differentiation. The HL-60 cells were incubated with 100
μg/ml of N. chinensis extract for 72 h, and the relative
levels of the two cell surface markers were then measured by flow
cytometry. The number of CD11b-positive HL-60 cells was increased
following treatment with the N. chinensis extract in a
dose-dependent manner, whereas that of CD14-positive cells was not
significantly increased (Fig.
2C). These results indicate that the extract induces the
differentiation of HL-60 cells into granulocytes.
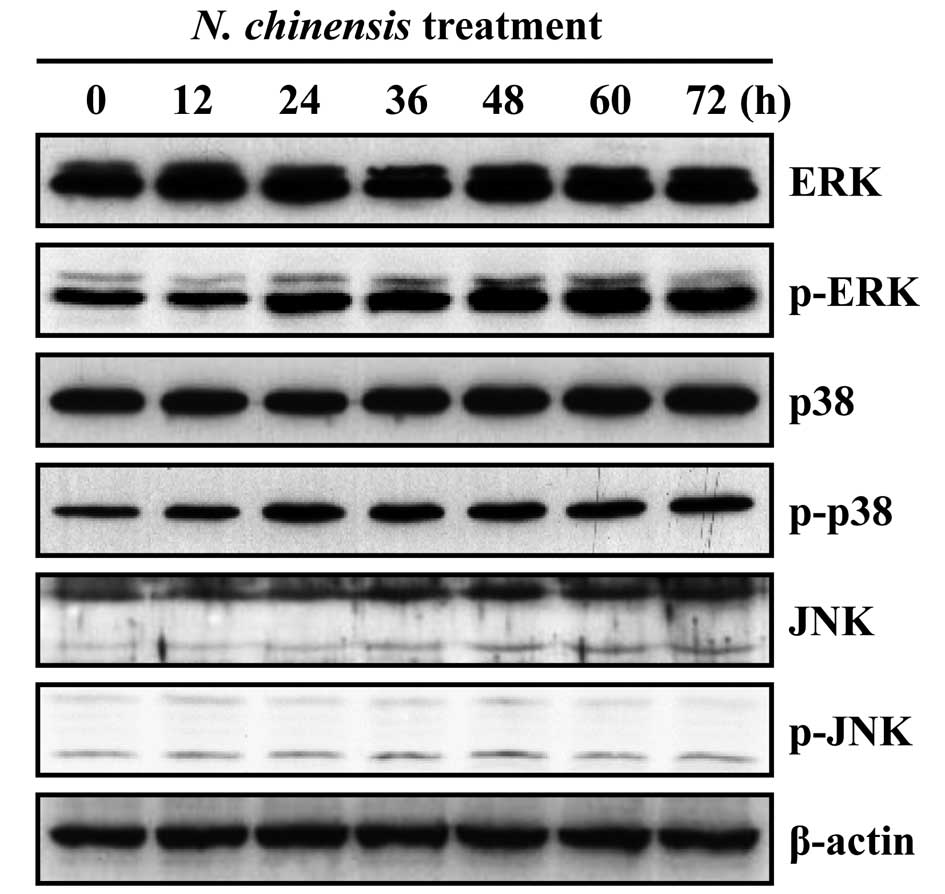
Effects of N. chinensis extract on the
activation of MAPKs in HL-60 cells
MAPK signaling pathways have been shown to play an
important role in the regulation of differentiation (19–23). To determine the involvement of
MAPKs in the granulocytic differentiation of HL-60 cells induced by
the N. chinensis extract, we examined the effects of the
extract on the activation of ERK, p38 MAPK and JNK. The extract
induced the time-dependent activation of ERK and p38 MAPK, whereas
the activation of JNK was unaffected (Fig. 3). To confirm whether the
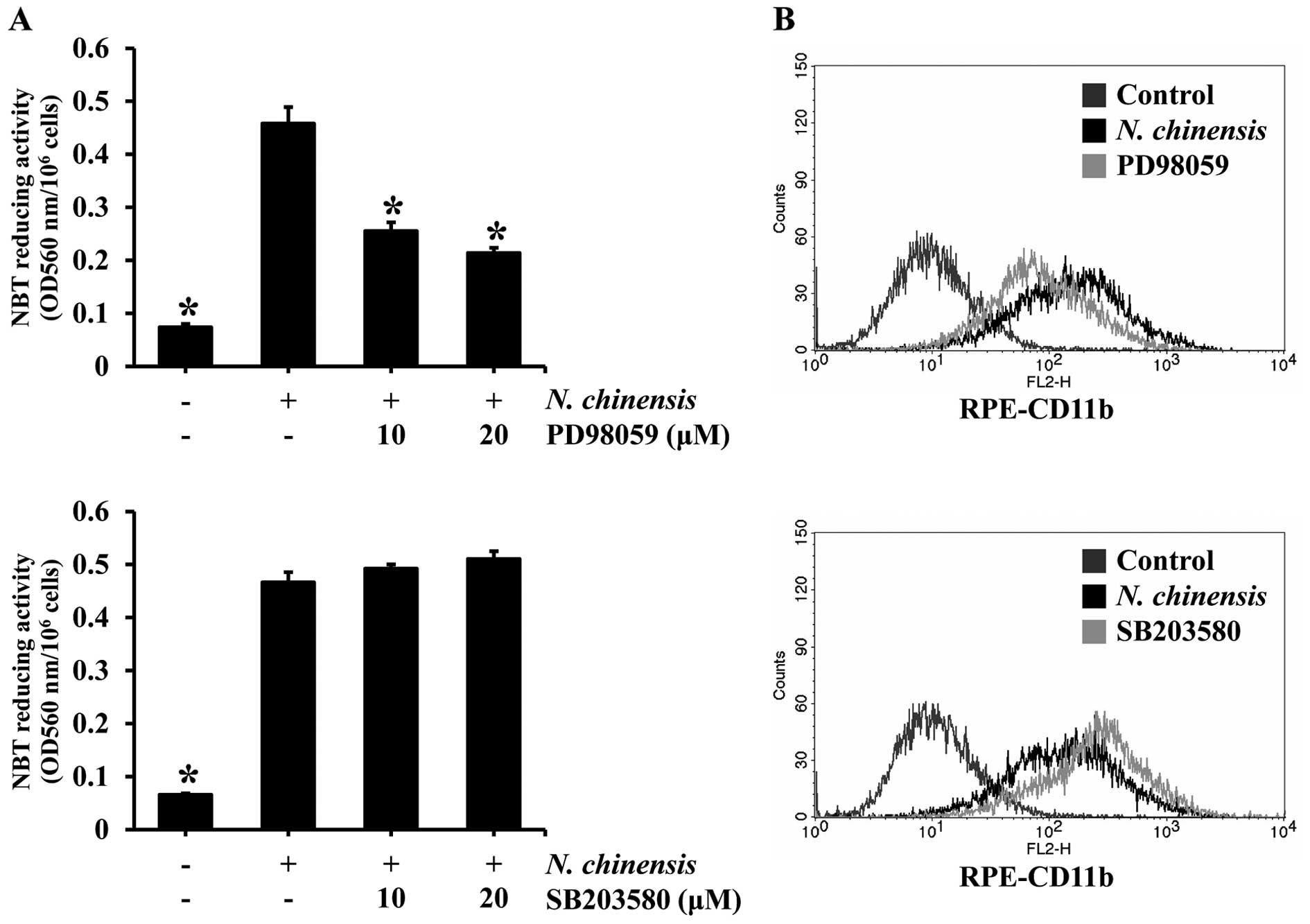
activation of ERK and p38 MAPK is involved in the induction of the
differentiation of HL-60 cells by the extract, we examined the
effects of inhibitors of ERK and p38 MAPK on N.
chinensis-induced differentiation. The HL-60 cells were
pre-treated with PD98059 (an ERK inhibitor) or SB203580 (a p38 MAPK
inhibitor) prior to treatment with N. chinensis extract, and
then the degree of cell differentiation was assessed by NBT
reducing activity assay and the expression of the granulocytic
differentiation surface marker, CD11b, was assessed by flow
cytometry (Fig. 4). The ERK
inhibitor significantly reduced the NBT reducing activity, as well
as CD11b expression in the HL-60 cells treated with the extract.
However, the p38 MAPK inhibitor did not have such an effect,
suggesting that ERK, but not p38 MAPK, is involved in the induction
of the differentiation of HL-60 cells by the N. chinensis
extract.
Effects of N. chinensis extract on the
activation of PKC in HL-60 cells
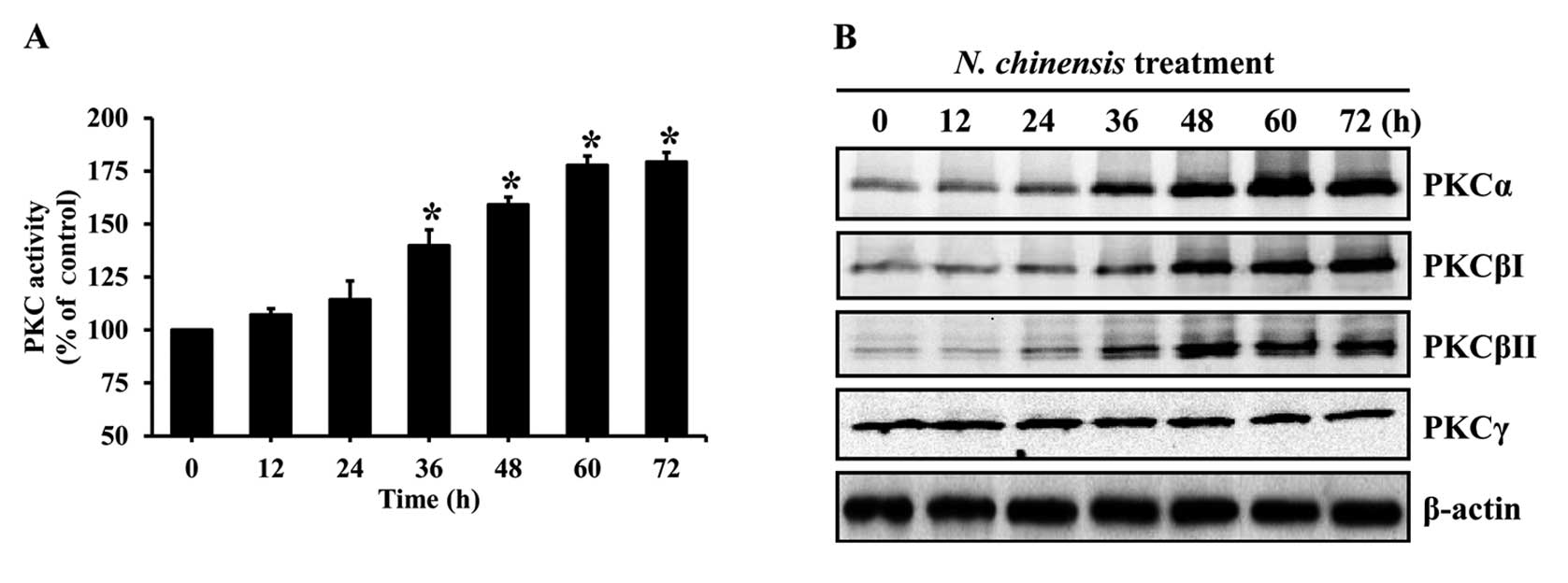
The activation of PKC is required for the
differentiation of HL-60 cells (27). To evaluate the involvement of PKC
in the differentiation of HL-60 cells induced by the N.
chinensis extract, the cells were treated with the extract for
various periods of time, and the activity of PKC was evaluated. The
extract significantly increased PKC activity in a time-dependent
manner (Fig. 5A). In addition, to
examine changes in conventional PKC isoforms in the N.
chinensis-treated HL-60 cells, the protein levels of PKC
isoforms were determined by western blot analysis. N.
chinensis extract markedly increased the protein levels of
PKCα, PKCβI and PKCβII in the HL-60 cells, whereas the protein
level of PKCγ remained constant (Fig.
5B).
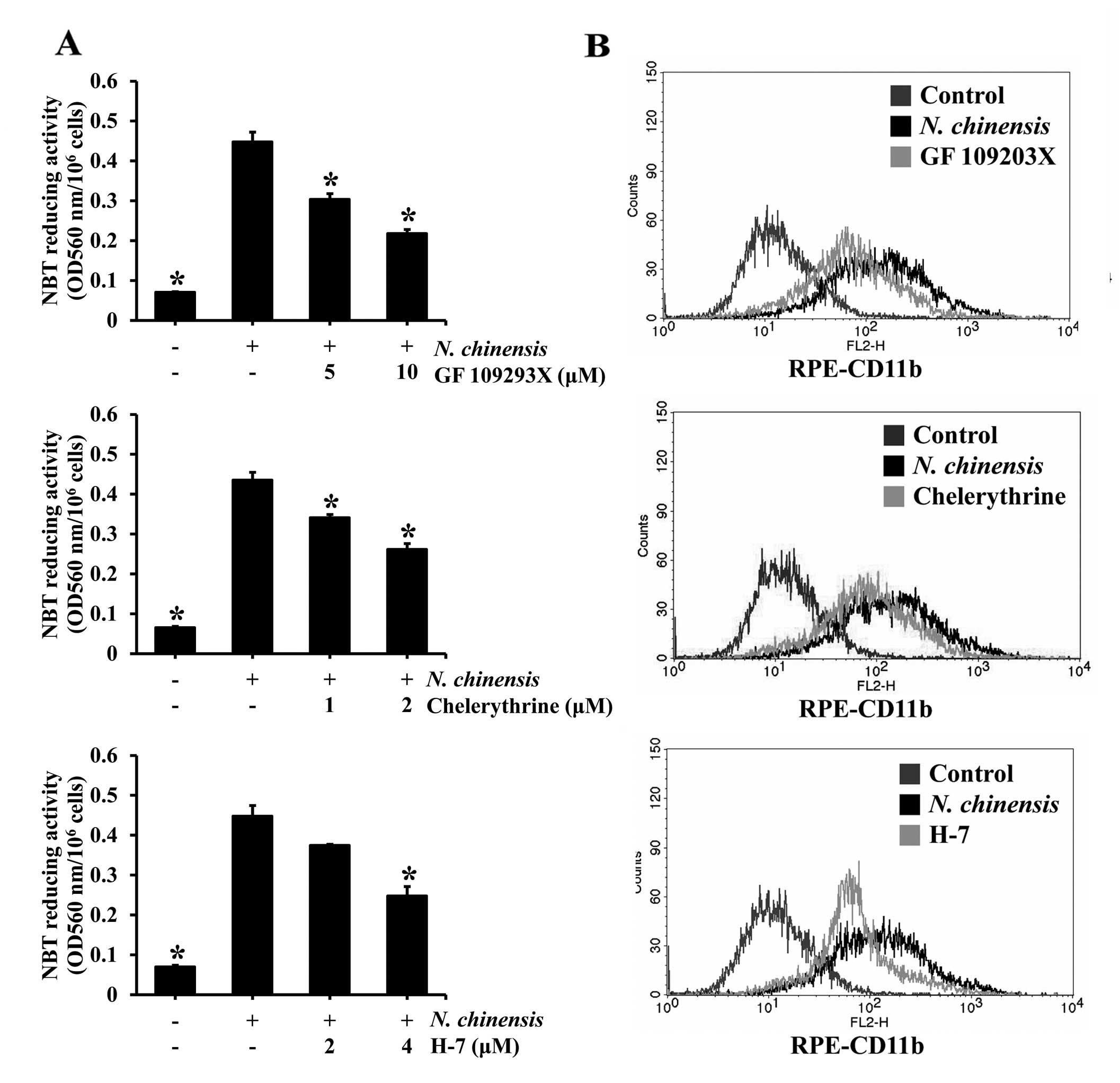
To determine whether PKC activation is involved in
the differentiation of HL-60 cells induced by the N.
chinensis extract, the cells were pre-treated with PKC
inhibitors (GF 109293X, chelerythrine and H-7) and then exposed to
to the N. chinensis extract for 72 h. All three PKC
inhibitors tested inhibited the N. chinensis extract-induced
differentiation of HL-60 cells in a dose-dependent manner, and
significantly in the majority of cases (Fig. 6A). To further confirm the effects
of the PKC inhibitors, the expression of the cell surface marker,
CD11b, following treatment with the inhibitors was determined by
flow cytometry. All three inhibitors reduced CD11b expression,
indicating that the granulocytic differentiation of the HL-60 cells
was inhibited by the PKC inhibitors (Fig. 6B). These results suggest that the
PKC pathway is involved in the induction, by the N.
chinensis extract, of the granulocytic differentiation of HL-60
cells.
Involvement of the PKC-dependent ERK
pathway in the granulocytic differentiation of HL-60 cells induced
by the N. chinensis extract
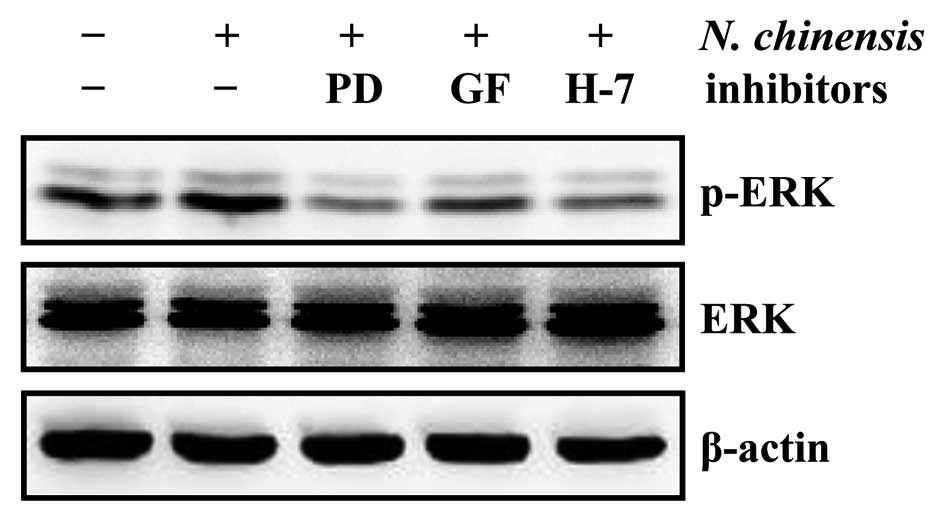
PKC has been shown to be an upstream element in the
MAPK signaling pathway, regulating the differentiation of HL-60
cells (12,29). To determine the involvement of the
PKC/ERK pathway in the granulocytic differentiation of HL-60 cells
induced by the N. chinensis extract, we examined the protein
levels of ERK and p-ERK following treatment of the cells with the
extract in the absence or presence of PD98059, GF 109293X and H-7
(Fig. 7). Our results already
indicated that the specific ERK inhibitor, PD98059, which inhibits
the ERK pathway by preventing the activation of ERK by c-Raf,
inhibited the differentiation of HL-60 cells into granulocytes
induced by the N. chinensis extract (Fig. 4). All three PKC inhibitors reduced
the levels of p-ERK in the N. chinensis-treated HL-60 cells
(Fig. 7), suggesting that the
N. chinensis extract-induced granulocytic differentiation of
HL-60 cells is mediated by the PKC-dependent ERK signaling
pathway.
Discussion
The present study demonstrates that the medicinal
plant, N. chinensis, induces the differentiation of HL-60
promyelocytic leukemic cells into granulocytes through the
activation of the PKC-dependent ERK signaling pathway.
Experiments were carried out using HL-60 cells,
which were previously suggested to constitute an excellent model
system to study the mechanisms of cell differentiation (12). HL-60 cells differentiate into
macrophage/monocyte or granulocytic lineages, induced by chemicals
or changes in culture conditions. Generally, the treatment of HL-60
cells with DMSO or all-trans retinoic acid (ATRA) leads to
granulocytic differentiation, while monocytic differentiation can
be induced by chemicals, such as PMA, 1,25-dihydroxyvitamin
D3, or sodium butyrate (8,9,30,31).
As is already known, the induction of the
differentiation of HL-60 cells requires the activation of a variety
of signal transduction pathways, such as PKC (24,25) and MAPKs (19–23). Previous studies have reported that
ERK, but not JNK or p38 MAPK activation is involved in ATRA-induced
granulocytic differentiation (19,20). By contrast, JNK activation has
been shown to be associated with monocytic differentiation induced
by 1,25-dihydroxyvitamin D3 (22), while ERK activation only plays a
transient role (21). Of note,
p38 MAPK inhibitors have been shown to activate JNK while inducing
monocytic differentiation (23).
It is most likely that in both the ATRA-induced granulocytic
differentiation and 1,25-dihydroxyvitamin D3-induced
monocytic differentiation of HL-60 cells, the ERK pathway is
involved. In our study, the activation of ERK and p38 MAPK was
observed when the HL-60 cells differentiated into granulocytes
following treatment with the N. chinensis extract; however,
the activation of JNK was not observed. The induction of the
differentiation of HL-60 cells by the N. chinensis extract
was significantly reduced only by an ERK inhibitor, but not a p38
MAPK inhibitor, suggesting that the ERK pathway plays an important
role in the induction of the granulocytic differentiation of HL-60
cells by the N. chinensis extract. It is possible that the
p38 MAPK and JNK pathways are not required for the induction of the
granulocytic differentiation of HL-60 cells, at least under our
experimental conditions.
PKC has been shown to be one of the upstream
elements in the MAPK signaling pathway, involved in the
differentiation of HL-60 cells (12,29); the key role of PKC in promoting
the differentiation of HL-60 cells is now generally accepted. As
expected, PKC inhibitors, such as GF 109203X, chelerythrine and
H-7, block cell differentiation (12,13,32). In our study, N. chinensis
extract increased PKC activity and the protein levels of PKCα,
PKCβI and PKCβII in the HL-60 cells. The inhibition of PKC resulted
in a significant decrease in the N. chinensis-induced
differentiation of HL-60 cells. Most importantly, we demonstrated
that the inhibition of PKC reduced ERK activation, which was
induced by the N. chinensis extract in HL-60 cells. These
results suggest that the activation of the PKC-dependent ERK
signaling pathway is involved in the induction of the granulocytic
differentiation of HL-60 cells by N. chinensis extract.
In conclusion, the data from the present study
demonstrate that N. chinensis extract induces PKC
activation, as shown by the significantly increased protein levels
of PKCα, PKCβI, and PKCβII, as well as the activation of ERK, thus
inducing the granulocytic differentiation of HL-60 cells. PKC
inhibitors significantly inhibited the N. chinensis-induced
ERK activation in HL-60 cells. Overall, N. chinensis extract
induces granulocytic differentiation through the activation of the
PKC-dependent ERK signaling pathway in HL-60 cells. Our results
suggest that N. chinensis extract can be used in the
treatment of leukemic diseases.
Acknowledgements
This study was supported by Wonkwang University in
2012.
References
|
1
|
Seo BI, Lee JH, Choi HY, Kwon DY and Boo
YM: Korean Medicine Herbology. Younglim Press; Seoul: pp. 496–497.
2008
|
|
2
|
Xiao PG, Li DP and Yang SL: Modern Chinese
Materia Medica. Chemical Industry Press; Beijing: pp. 2522002
|
|
3
|
Takaya Y, Takeuji Y, Akasaka M, et al:
Novel guaiane endoperoxides, nardoguaianone A–D, from
Nardostachys chinensis roots and their antinociceptive and
antimalarial activities. Tetrahedron. 56:7683–7678. 2000.
|
|
4
|
Itokawa H, Masuyama K, Morita H and Takeya
K: Cytotoxic sesquiterpenes from Nardostachys chinensis.
Chem Pharm Bull (Tokyo). 41:1183–1184. 1993. View Article : Google Scholar
|
|
5
|
Li P, Matsunaga K, Yamamoto K, Yoshikawa
R, Kawashima K and Ohizumi Y: Nardosinone, a novel enhancer of
nerve growth factor in neurite outgrowth from PC12D cells. Neurosci
Lett. 273:53–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang JS, Lee SA, Hong SS, et al:
Inhibitory constituents of Nardostachys chinensis on nitric
oxide production in RAW 264.7 macrophages. Bioorg Med Chem Lett.
22:706–708. 2012.
|
|
7
|
Huberman E and Callaham MF: Induction of
terminal differentiation in human promyelocytic leukemia cells by
tumor-promoting agents. Proc Natl Acad Sci USA. 76:1293–1297. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rovera G, Santoli D and Damsky C: Human
promyelocytic leukemia cells in culture differentiate into
macrophage-like cells when treated with a phorbol diester. Proc
Natl Acad Sci USA. 76:2779–2783. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collins SJ, Ruscetti FW, Gallagher RE and
Gallo RC: Terminal differentiation of human promyelocytic leukemia
cells induced by dimethyl sulfoxide and other polar compounds. Proc
Natl Acad Sci USA. 75:2458–2462. 1978. View Article : Google Scholar
|
|
10
|
Pan Q, Granger J, O’Connell TD, Somerman
MJ and Simpson RU: Promotion of HL-60 cell differentiation by
1,25-dihydroxyvitamin D3 regulation of protein kinase C levels and
activity. Biochem Pharmacol. 54:909–915. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kharbanda S, Saleem A, Emoto Y, Stone R,
Rapp U and Kufe D: Activation of Raf-1 and mitogen-activated
protein kinases during monocytic differentiation of human myeloid
leukemia cells. J Biol Chem. 269:872–878. 1994.PubMed/NCBI
|
|
12
|
Kim SH, Oh SM and Kim TS: Induction of
human leukemia HL-60 cell differentiation via a PKC/ERK pathway by
helenalin, a pseudoguainolide sesquiterpene lactone. Eur J
Pharmacol. 511:89–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SH, Kim HJ and Kim TS: Differential
involvement of protein kinase C in human promyelocytic leukemia
cell differentiation enhanced by artemisinin. Eur J Pharmacol.
482:67–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cross TG, Scheel-Toellner D, Henriquez NV,
Deacon E, Salmon M and Lord JM: Serine/threonine protein kinases
and apoptosis. Exp Cell Res. 256:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
16
|
Cobb MH: MAP kinase pathways. Prog Biophys
Mol Biol. 71:479–500. 1999. View Article : Google Scholar
|
|
17
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yen A, Roberson MS, Varvayanis S and Lee
AT: Retinoic acid induced mitogen-activated protein
(MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent
MAP kinase activation needed to elicit HL-60 cell differentiation
and growth arrest. Cancer Res. 58:3163–3172. 1998.
|
|
20
|
Yen A, Roberson MS and Varvayanis S:
Retinoic acid selectively activates the ERK2 but not JNK/SAPK or
p38 MAP kinases when inducing myeloid differentiation. In Vitro
Cell Dev Biol Anim. 35:527–532. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X and Studzinski GP: Activation of
extracellular signal-regulated kinases (ERKs) defines the first
phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60
cells. J Cell Biochem. 80:471–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Rao J and Studzinski GP:
Inhibition of p38 MAP kinase activity up-regulates multiple MAP
kinase pathways and potentiates 1,25-dihydroxyvitamin D(3)-induced
differentiation of human leukemia HL60 cells. Exp Cell Res.
258:425–437. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X and Studzinski GP: Inhibition of
p38MAP kinase potentiates the JNK/SAPK pathway and AP-1 activity in
monocytic but not in macrophage or granulocytic differentiation of
HL60 cells. J Cell Biochem. 82:68–77. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caponigro F, French RC and Kaye SB:
Protein kinase C: a worthwhile target for anticancer drugs?
Anticancer Drugs. 8:26–33. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa M and Shirakawa S: The
expression and possible roles of protein kinase C in haematopoietic
cells. Leuk Lymphoma. 8:201–211. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Quest AF: Regulation of protein kinase C:
a tale of lipids and proteins. Enzyme Protein. 49:231–261.
1996.PubMed/NCBI
|
|
27
|
Komada F, Nishikawa M, Uemura Y, Morita K,
Hidaka H and Shirakawa S: Expression of three major protein kinase
C isozymes in various types of human leukemic cells. Cancer Res.
51:4271–4278. 1991.PubMed/NCBI
|
|
28
|
Sakashita A, Nakamaki T, Tsuruoka N, Honma
Y and Hozumi M: Granulocyte colony-stimulating factor, not
granulocyte-macrophage colony-stimulating factor, co-operates with
retinoic acid on the induction of functional
N-formyl-methionyl-phenylalanine receptors in HL-60 cells.
Leukemia. 5:26–31. 1991.
|
|
29
|
Marcinkowska E, Wiedlocha A and
Radzikowski C: 1,25-Dihydroxyvitamin D3 induced activation and
subsequent nuclear translocation of MAPK is upstream regulated by
PKC in HL-60 cells. Biochem Biophys Res Commun. 241:419–426. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Breitman TR, Selonick SE and Collins SJ:
Induction of differentiation of the human promyelocytic leukemia
cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA.
77:2936–2940. 1980. View Article : Google Scholar
|
|
31
|
Tanaka H, Abe E, Miyaura C, Shiina Y and
Suda T: 1 alpha,25-dihydroxyvitamin D3 induces differentiation of
human promyelocytic leukemia cells (HL-60) into
monocyte-macrophages, but not into granulocytes. Biochem Biophys
Res Commun. 117:86–92. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang SN, Lee MH, Kim KM, Cho D and Kim TS:
Induction of human promyelocytic leukemia HL-60 cell
differentiation into monocytes by silibinin: involvement of protein
kinase C. Biochem Pharmacol. 61:1487–1495. 2001. View Article : Google Scholar : PubMed/NCBI
|