Introduction
Gastric cancer (GC) is the fourth most commonly
diagnosed cancer with the second highest mortality rate worldwide
(1). Its incidence rate is the
highest in Eastern Asia (2).
Although substantial advances have been made in surgical techniques
and chemotherapy, which have improved the effectiveness of
treatment strategies for GC, the overall outcome for patients with
GC has not changed significantly over the past few decades
(3). Biotherapy, the fourth
treatment option after surgery, chemotherapy and radiotherapy,
includes gene therapy, which is a prospective new method that has
developed in recent years for malignant cancers. Some biological
prognostic markers which may aid in the understanding of disease
pathogenesis have been identified, such as Krüppel-like factor 8
(4), excision repair
cross-complementing gene 1 (ERCC1) (5) and Forkhead box protein M1 (FOXM1)
(6). However, compared to other
more extensively investigated cancers, such as breast, prostate and
colon carcinomas, the molecular mechanisms involved in the
pathologenesis of GC are poorly characterized, and the cure rate
for patients with GC remains low, while the morbidity rate remains
high (7). Hence, additional
studies are required in order to enhance our understanding of the
pathogenesis of GC and its biological features, along with newer
clinically relevant molecular markers that can be used in GC.
Heterotrimeric guanine nucleotide-binding proteins
(G proteins) are molecular switches that control signal
transduction, and their dysregulation can promote oncogenesis. Gαo,
encoded by guanine nucleotide-binding protein (G protein), alpha
activating activity polypeptide O (GNAO1), belongs to the family of
G protein alpha subunits (8) and
is abundantly expressed in neuronal tissue (9). Apart from the activation of second
messenger molecules, Gαo subunits can modulate the activity of
transcription factors and thereby regulate gene expression
(10–12). Moreover, recent studies have found
that Gαo subunit alterations play a potentially more significant
role in different types of cancer, such as breast cancer (13) and hepatocellular carcinoma (HCC)
(14). With the use of mismatch
repair detection (MRD) technology, a germline mutation at residue
R243 was found to be associated with breast cancer (13). The first somatic mutation of GNAO1
(Gαo) was described in breast cancer, and was proven to promote
oncogenic transformation possibly through signal transducer and
activator of transcription 3 (STAT3) signaling in vitro
(15).
However, to date, the expression and possible roles
of GNAO1 in GC have not been established. The aim of the present
study was to analyzed the expression of GNAO1 protein in GC tissues
and to determine the correlation between Gαo expression and the
clinicopathological parameters of patients with GC, as well as to
investigate the effects of GNAO1 protein expression on the
biological behavior of GC cells.
Materials and methods
Recruitment of patients with GC and
tissue specimens
Tissue samples from 70 unrelated patients with
primary GC, who were treated at Shanghai Huashan Hospital,
Shanghai, China were obtained at the time of surgical resection
between January and December 2005. The tumors and corresponding
adjacent tissues were obtained after first having received patient
informed consent under a protocol reviewed and approved by the
Human Research Review Committee of Huashan Hospital, Fudan
University, Shanghai, China. The tissues were fixed in paraffin for
pathological diagnosis and immunohistochemical staining.
Histopathological diagnosis of each neoplastic tissue was performed
according to the World Health Organization criteria by the
Department of Pathology, Huashan Hospital. Clinicopathological
staging was determined based on the TNM classification. All
patients with GC were confirmed by histological analysis, and tumor
samples were examined to ensure that tumor tissue was present in
>80% of the specimens. Follow-up data were collected until
December 2011 or until the patient became deceased, and the
occurrence of metastasis and/or local recurrence was recorded.
Immunohistochemistry
Immunohistochemical staining with rabbit anti-human
GNAO1 polyclonal antibody (Proteintech Group, Chicago, IL, USA) was
performed on 4-mm-thick sections of paraffin-embedded specimens.
Briefly, following deparaffination and hydration, the slides were
incubated in 0.3% H2O2 for 10 min in order to
block endogenous peroxidase before being boiled in citrate buffer
pH 6.0 for 15 min. The slides were then incubated with rabbit
anti-human GNAO1 polyclonal antibody (Proteintech Group) overnight
at 4°C in a moist chamber. Subsequently, the specimens were washed
3 times in PBS and treated with a biotinylated goat anti-rabbit
antibody at 37°C for 2 h. The specimens were washed another 3 times
in PBS and then treated with ABC reagent, followed by color
development in 3,3′-diaminobenzidine tetrahydrochloride (both from
Dako, Carpinteria, CA, USA). Finally, the slides were lightly
counterstained with haematoxylin (Baso Diagnostics Inc., Zhuhai,
China). All specimens were observed and photographed under a
microscope. Duplicate sections were immunostained without exposure
to primary antibodies and used as negative controls. The
immunostaining pattern of GNAO1 was characterized by cytoplasmic
staining of the carcinoma tissues. To quantify Gαo expression,
semi-quantitative scoring was performed for the evaluation of the
intensity (0, no staining; 1, weak staining; 2, strong staining)
and of the fraction of positively stained cancer cells (0, no
staining; 1, less than half; 2, more than half). The final score
was counted for each sample by multiplying the intensity and the
percentage of positively stained cells (0, no staining; 1, weak
staining; 2, moderate staining; 4, strong staining). Sections with
a score of 0 or 1 were classified as having a low expression of
Gαo, whereas those with a score of 2 or 4 were classified as having
a high expression or overexpression of Gαo. Samples were scored by
two independent researchers, neither of whom had knowledge or
information pertaining to the clinical status of the patients.
Immunohistochemical analysis and clinical data collection were
performed independently and in a blinded manner.
Cell culture
The human GC cell lines, MGC-803 and AGS, were
cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and
50 U/ml penicillin/streptomycin (Sigma, St. Louis, MO, USA) and
were incubated at 37°C in a humidified atmosphere with 5%
CO2. The medium was changed every 2–3 days.
Small interfering RNA (siRNA) and cell
transfection
Two pairs of siRNA for GNAO1 (siGNAO1-1 and
siGNAO1-2) were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China) and introduced into the cells using Lipofectamine
2000 (Invitrogen Life Technologies, Carlsbad, CA, USA) according to
the manufacturer’s instructions. In brief, the cells were seeded
and incubated to reach 80% confluence prior to transfection. Four
hours after transfection, the medium was replaced with full medium.
After 72 h, the cultured cells were split for protein extraction.
The total protein concentration in the cell lysates was determined
using the Enhanced BCA Protein Assay kit (Beyotime, Shanghai,
China). The GNAO1 siRNA sequences are as follows: 5′-CCCACUUCACAUUC
AAGAATT-3′ and 5′-GGGCAUCGAAUAUGGUGAUTT-3′. The negative control
siRNA (corresponding to non-silencing negative control siRNA)
sequence was: 5′-UUCUCCGAACGU GUCACGUTT-3′.
Cell proliferation assay
Cell proliferation was measured using the Cell
Counting kit (CCK)-8 and colony formation assay. CCK-8 assay was
performed using the CCK-8 kit (Beyotime). According to the
manufacturer’s instructions, 1–5×103 cells were seeded
into 96-well culture plates and allowed to attach for 24 h. The
cells were then transfected with the indicated concentrations of
the negative control or GNAO1 siRNA and incubated for 24, 48, 72 or
96 h. At the endpoint, 20 μl CCK-8 (5 g/l) were added for a further
4 h. A Multiscan GO spectrophotometer (Thermo Scientific, Waltham,
MA, USA) was used to measure the absorbance at 450 nm.
According to the colony formation assay, a total of
0.5–1×103 cells was plated in 6-well plates and
transfected with siRNA. Following incubation for an additional
10–14 days, the cells were fixed with methanol and stained with
0.1% crystal violet (Sigma). Colonies of >50 cells were manually
counted. The experiments were repeated at least 3 times.
Determination of apoptosis by flow
cytometric analysis
The AGS and MGC-803 cells were transfected with
siRNA targeting GNAO1 for 24 h. The cells were harvested and washed
twice with cold PBS and resuspended in binding buffer. After
Annexin V-FITC labeling and incubation for 10 min at room
temperature, the cell suspensions were immediately used in flow
cytometric analysis. All experiments were performed in
triplicate.
Western blot analysis
The cells were harvested and washed twice with
ice-cold PBS. Cell lysates were obtained using RIPA lysis buffer
supplemented with 1 mM PMSF (both from Beyotime), and were then
subjected to centrifugation at 12,000 × g for 5 min at 4°C. Total
protein concentrations in the supernatant were determined by the
bicinchoninic acid assay (BCA; Beyotime). In the current study,
experimental and control cells were used to examine the expression
of GNAO1, that of cleaved apoptosis-associated PARP, as well as
that of some pro-apoptotic proteins, including Bim and Puma. Total
protein extracts were separated on 10–12% SDS-PAGE (20–50 mg/lane),
and electrotransferred onto polyvinylidene fluoride membranes.
After the transfer, the membranes were blocked for 1 h at room
temperature with milk and then incubated overnight at 4°C with
anti-GNAO1 (1:500; Proteintech), anti-PARP (1:1,000), anti-Puma
(1:1,000) (they were both purchased from Cell Signaling Technology,
Danvers, MA, USA), anti-extracellular signal-regulated kinase 1 and
2 (ERK1/2; 1:1,000; Epitomics, Burlingame, CA, USA),
phospho-stress-activated protein kinase (SARK)/c-Jun N-terminal
kinase (JNK; 1:1,000), phospho-p38 mitogen-activated protein kinase
(MAPK; 1:1,000) (they were both purchased from Cell Signaling
Technology) or anti-β-tubulin (1:2,000; Epitomics) antibodies. The
membranes were then washed 3 times with TBST (Bioscience, Shanghai,
Inc., China) and incubated with anti-rabbit IgG, HRP-linked
antibody (1:2,000; Cell Signaling Technology) for 2 h at room
temperature. Washes were repeated another 3 times with TBST and
immunoreactivity was determined using the Chemiluminescence Imaging
LAS-3000 system (Fujifilm, Tokyo, Japan). Antibodies were diluted
using the SignalBoost™ Immunoreaction Enhancer kit (Calbiochem, La
Jolla, CA, USA), while β-tubulin served as an internal control.
Statistical analysis
Several clinicopathological parameters were
examined: age, gender, depth of invasion, nodal status, stage,
degree of differentiation, tumor size and rates of relapse. The
correlation between Gαo expression and each clinicopathological
characteristic was examined using the χ2 test. The
time-to-event endpoints for Gαo expression were plotted by the
Kaplan-Meier method, and the degree of significance was calculated
by the univariate log-rank test. Parameters that emerged as
significant (P<0.05) in univariate analysis were entered as
variables in the multivariate Cox regression model, and the hazard
ratio (HR) and independent prognostic impact were determined in a
stepwise backward manner. The data of from the cellular experiments
are expressed as the means ± SD. The independent samples t-test and
analysis of variance (ANOVA) were used to compare data between
different groups. All data were analyzed using SPSS software
version 13.0. A P-value of 0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological characteristics of
the recruited patients
The clinicopathological characteristics of the 70
patients with GC are presented in Table I. There were 50 males with a
median age of 73 years (range, 43–102) and 20 females with a median
age of 70 years (range, 45–85). The American Joint Committee on
Cancer (AJCC) staging system was used to stage the primary GC
samples; 11, 38, 17 and 4 patients were classified as having stage
I, II, III and IV tumors, respectively. The mean duration of
follow-up was 38.0 months (median, 35 months; range, 4–78
months).
 | Table IDemographic data and survival in
different stages of gastric cancer according to the AJCC
classification system. |
Table I
Demographic data and survival in
different stages of gastric cancer according to the AJCC
classification system.
| Variable | Stage I
n=11 | Stage II
n=38 | Stage III
n=17 | Stage IV
n=4 | Total
n=70 |
|---|
| Gender |
| Male | 9 | 26 | 14 | 1 | 50 |
| Female | 2 | 12 | 3 | 3 | 20 |
| Age (years)a | 68.4 (13.4) | 67.3 (13.3) | 78.76 (12.5) | 59.3 (11.0) | 69.8 (13.9) |
| Follow-up period
(months) | 55.2 (18.9) | 38.3 (22.0) | 33.0 (22.6) | 30.8 (22.0) | 38.0 (22.3) |
| Survival |
| Yes | 0 | 13 | 9 | 4 | 26 |
| No | 11 | 25 | 8 | 0 | 44 |
The expression panel of GNAO1 in GC
To characterize Gαo expression in human GC, 70
archival paraffin-embedded tissue sections were used for
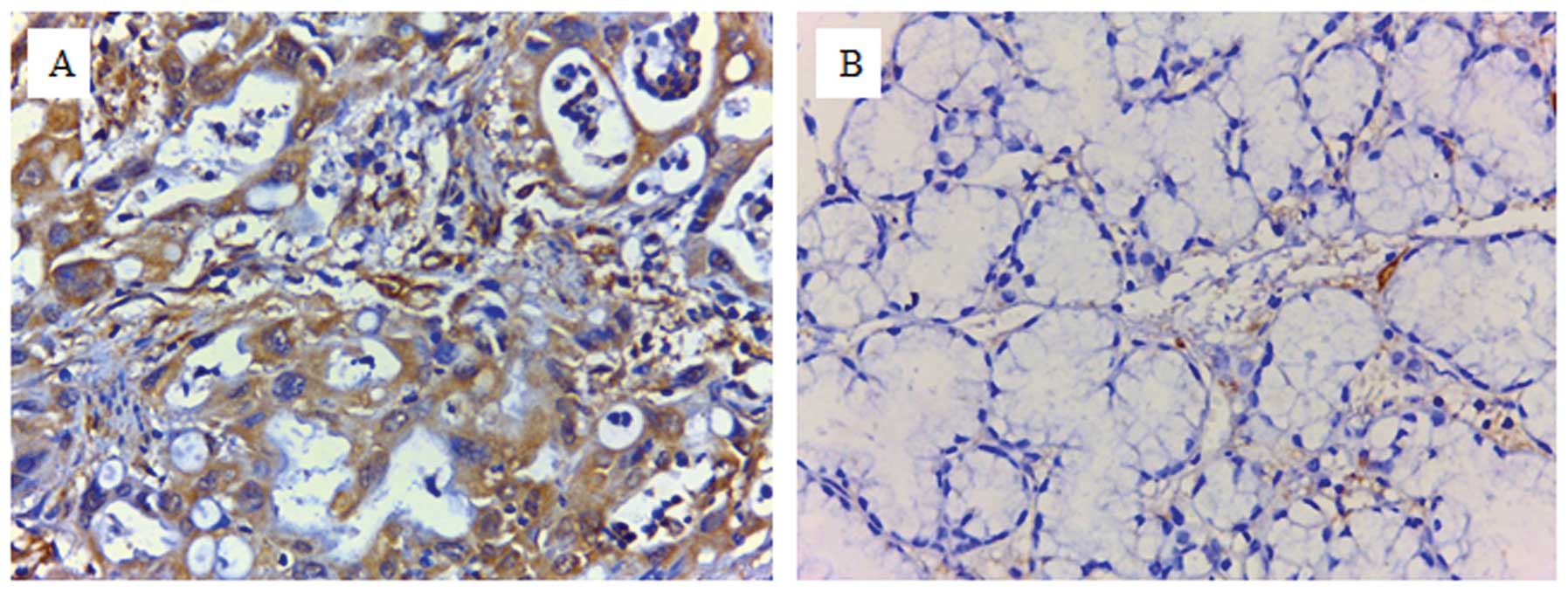
immunohistochemistry. Representative images of GNAO1-negative
(scores of 0 or 1) and -positive (scores of ≥2) expression are
presented in Fig. 1. The
cytoplasmic expression of Gαo (scores of ≥1) was clearly observed
in all GC tissues and in parts of the corresponding adjacent
tissues (7/26, 26.9%). Gαo was overexpressed (scores of 2 or 4) in
the cancer tissues from 44 of 70 patients (62.9%) and in none of
the corresponding adjacent tissues. It was further revealed that
the expression of Gαo correlated with tumor size (P=0.003), the
degree of histological differentiation (P=0.025) and TNM stage
(P=0.004). Of note, the Gαo staining index did not present any
statistically significant difference between tumors obtained from
patients with different age, gender, family history or lymph node
involvement (Table II).
 | Table IIGNAO1 expression in gastric cancer and
its correlation with clinicopathological parameters. |
Table II
GNAO1 expression in gastric cancer and
its correlation with clinicopathological parameters.
| | GNAO1 expression
score | |
|---|
| |
| |
|---|
| Variable | n | 0 or 1
n=26 | 2 or 4
n=44 | P-valuea |
|---|
| Age (years) | | | | |
| ≥66 | 21 | 10 | 11 | 0.359 |
| <66 | 49 | 16 | 33 | |
| Gender | | | | |
| Male | 50 | 18 | 32 | 0.969 |
| Female | 20 | 8 | 12 | |
| Tumor histology | | | | |
| Degree of
differentiation | | | | |
| Poor | 33 | 11 | 23 | 0.025 |
| Moderate | 6 | 0 | 6 | |
| Well | 0 | 0 | 0 | |
| Unknown | 31 | 15 | 16 | |
| Lymph node
involvement | | | | |
| Absent | 21 | 6 | 15 | 0.483 |
| Present | 49 | 20 | 29 | |
| Stage | | | | |
| I | 11 | 4 | 7 | 0.004 |
| II | 38 | 15 | 23 | |
| III | 17 | 5 | 12 | |
| IV | 4 | 2 | 2 | |
| Tumor size | | | | |
| ≥3 cm | 44 | 17 | 27 | 0.006 |
| <3 cm | 26 | 9 | 17 | |
Prognostic significance of GNAO1
expression in GC
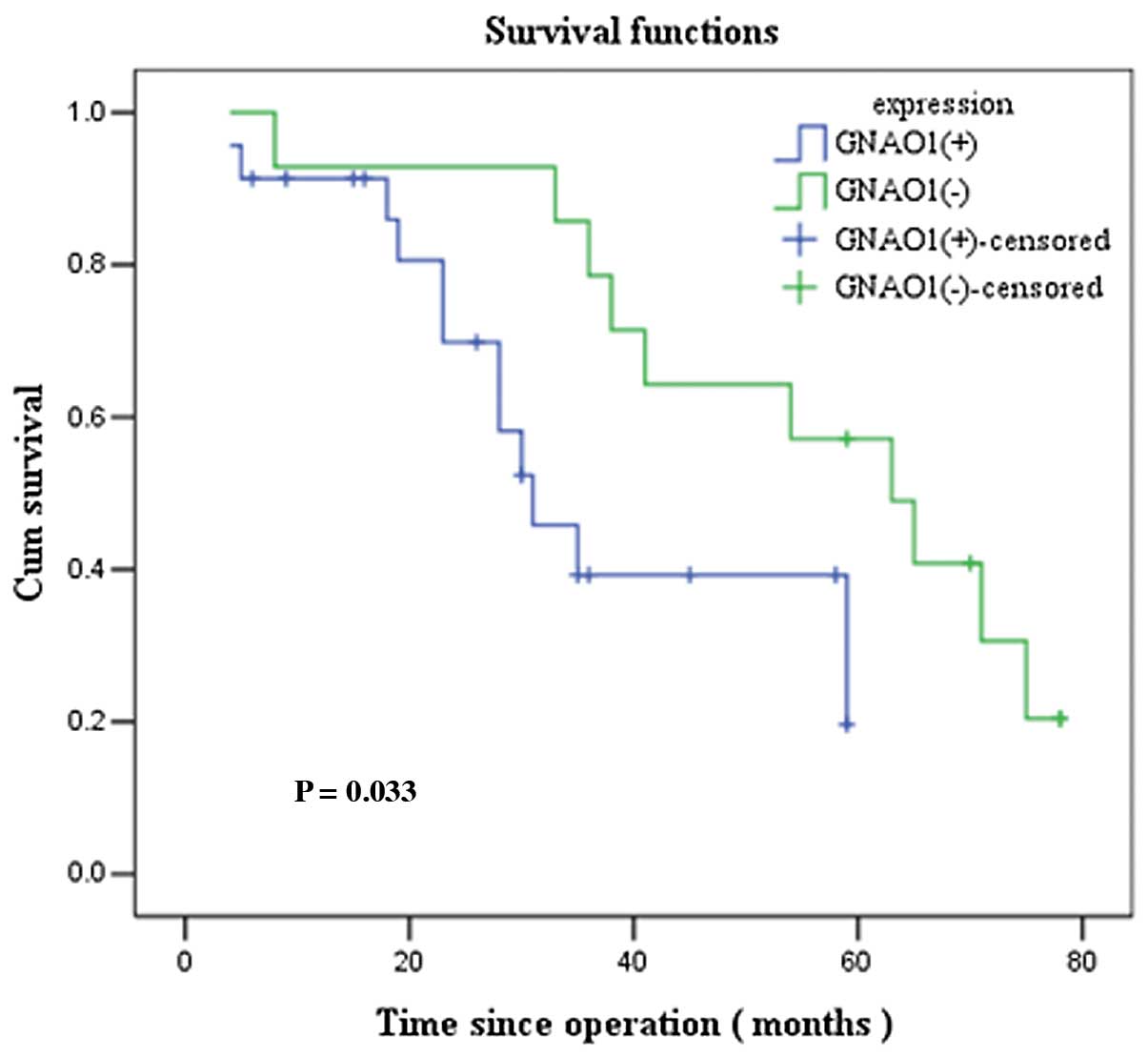
To evaluate whether GNAO1 expression can be used as
a prognostic factor, we performed survival analysis using the
Kaplan-Meier method. The overall survival curves in relation to
GNAO1 expression were statistically significant (P=0.033; Fig. 2). The mean survival was 55.0
months (median, 61 months) in the GNAO1-negative group and 27.7
months (median, 28 months) in the GNAO1-positive group. The
prognostic significance of GNAO1 expression in GC was further
confirmed by univariate survival analysis (P<0.05). After the
adjustment of other prognostic indicators by multivariate analysis,
GNAO1 remained an independent prognostic factor for patients with
GC (P=0.046) (Table III).
 | Table IIIUnivariate and multivariate analysis
of prognostic factors for survival in patients with gastric
cancer. |
Table III
Univariate and multivariate analysis
of prognostic factors for survival in patients with gastric
cancer.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | P-value | P-value | RR (95% CI) |
|---|
| Relapse |
| Yes (vs. no) | 0.039 | 0.139 | 0.535
(0.234–1.224) |
| TNM stage |
| Stage IV (vs. III
+ II + I) | 0.003 | 0.003 | 1.971
(1.255–3.096) |
| Tumor size |
| ≥3 cm (vs. <3
cm) | 0.038 | 0.186 | 0.471
(0.154–1.439) |
| GNAO1
expression |
| Positive (vs.
negative) | 0.045 | 0.046 | 2.766
(1.018–7.518) |
Roles of GNAO1 in GC cells
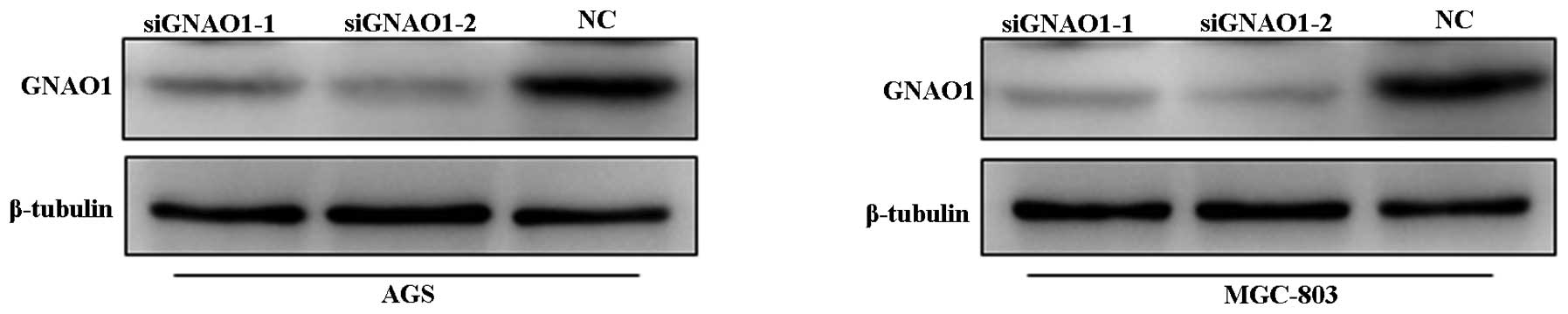
To examine the possible roles of GNAO1 in GC cells,
GNAO1 expression was knocked down by siRNA. The efficacy of GNAO1
siRNA was evaluated using a FAM fluorescent signal (data not shown)
and western blot analysis (Fig.
3). It was found that the expression of GNAO1 was almost
completely suppressed at the protein level when the AGS and MGC-803
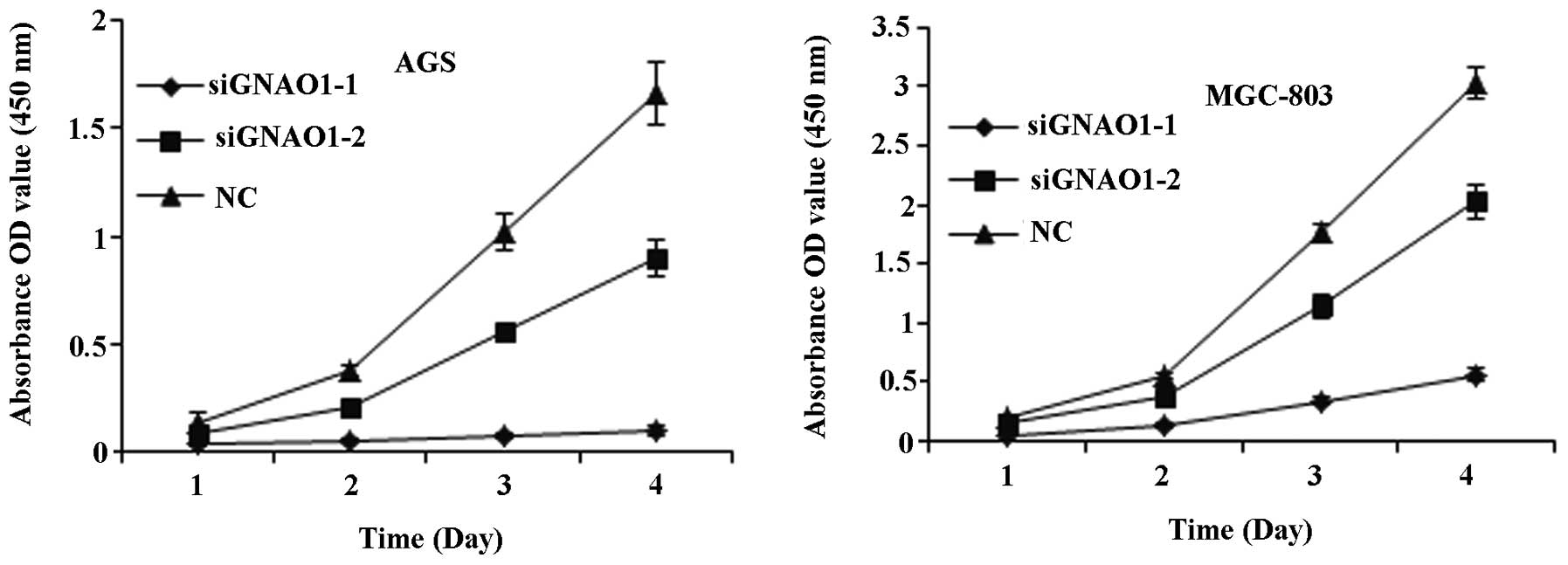
cells were transfected with GNAO1 siRNA. CCK-8 and colony formation
assays were conducted following transfection with siRNA and
revealed that GNAO1 siRNA significantly inhibited cell
proliferation (P<0.01) (Fig.
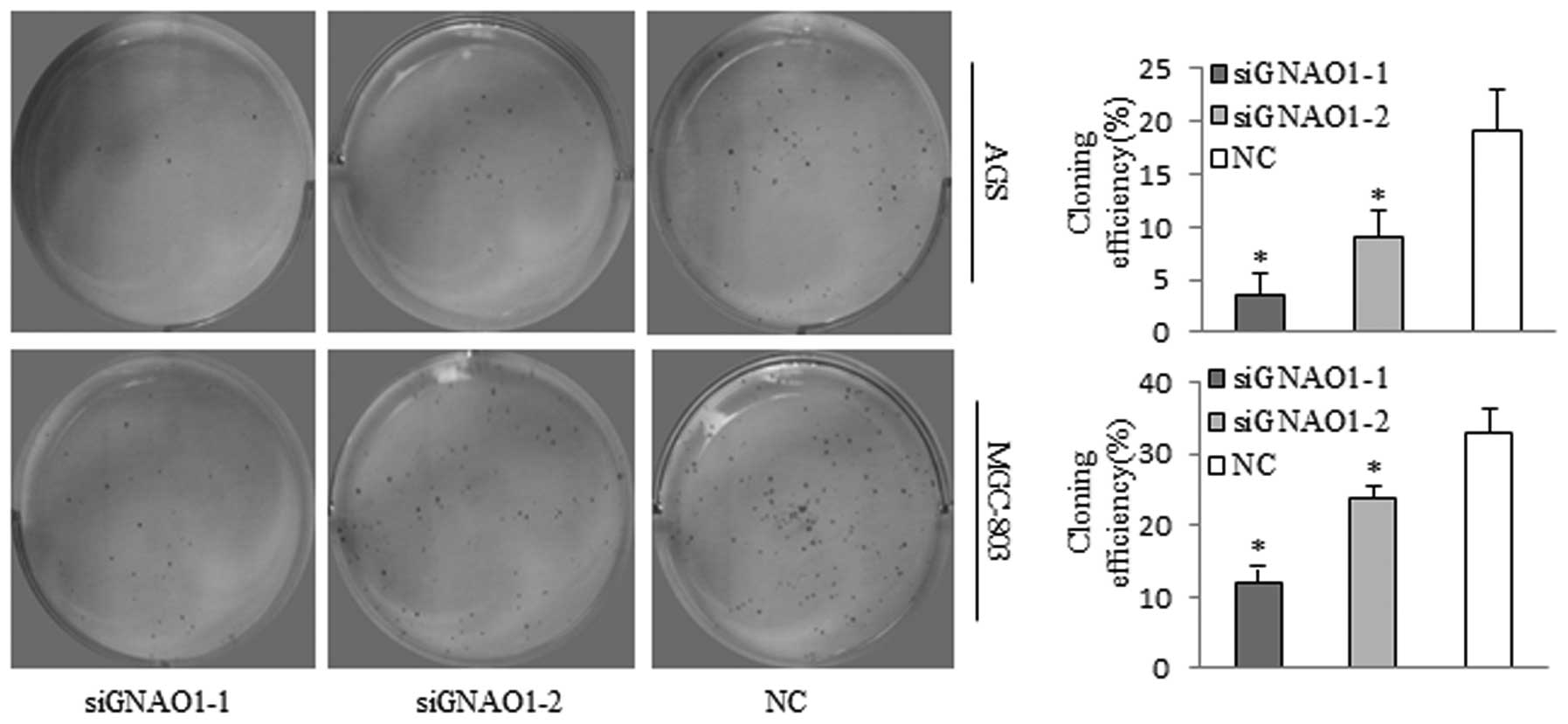
4) and the colony formation ability of the cells (P<0.05)
(Fig. 5).
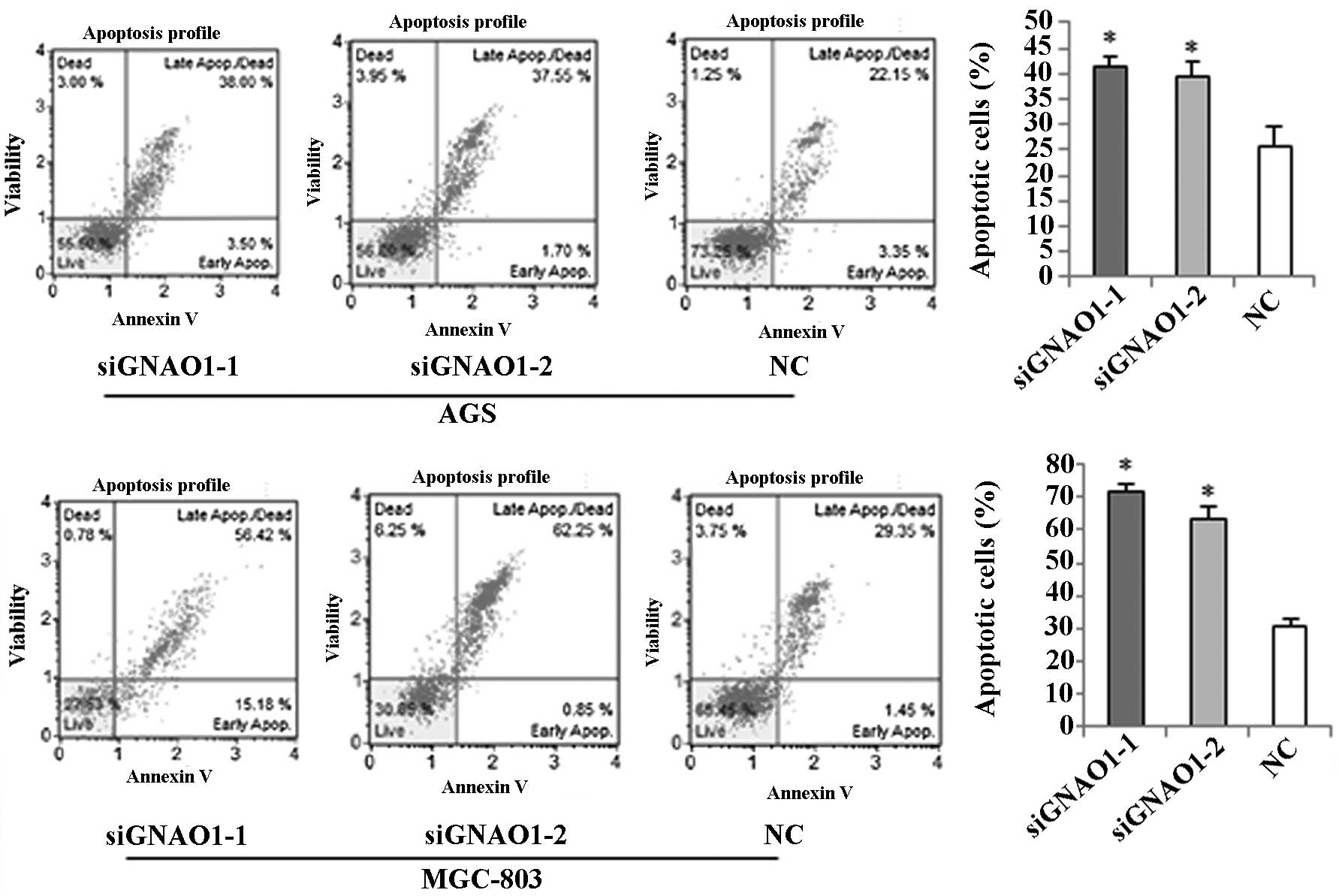
We also examined the effects of GNAO1 on the
apoptosis of GC cells by flow cytometry. Flow cytometric analysis
using Muse Annexin V and Dead Cell kit (Millipore) revealed that
the percentage of early, late and total apoptotic, as well as dead
cells markedly increased 48 h after transfection with GNAO1 siRNA
(Fig. 6).
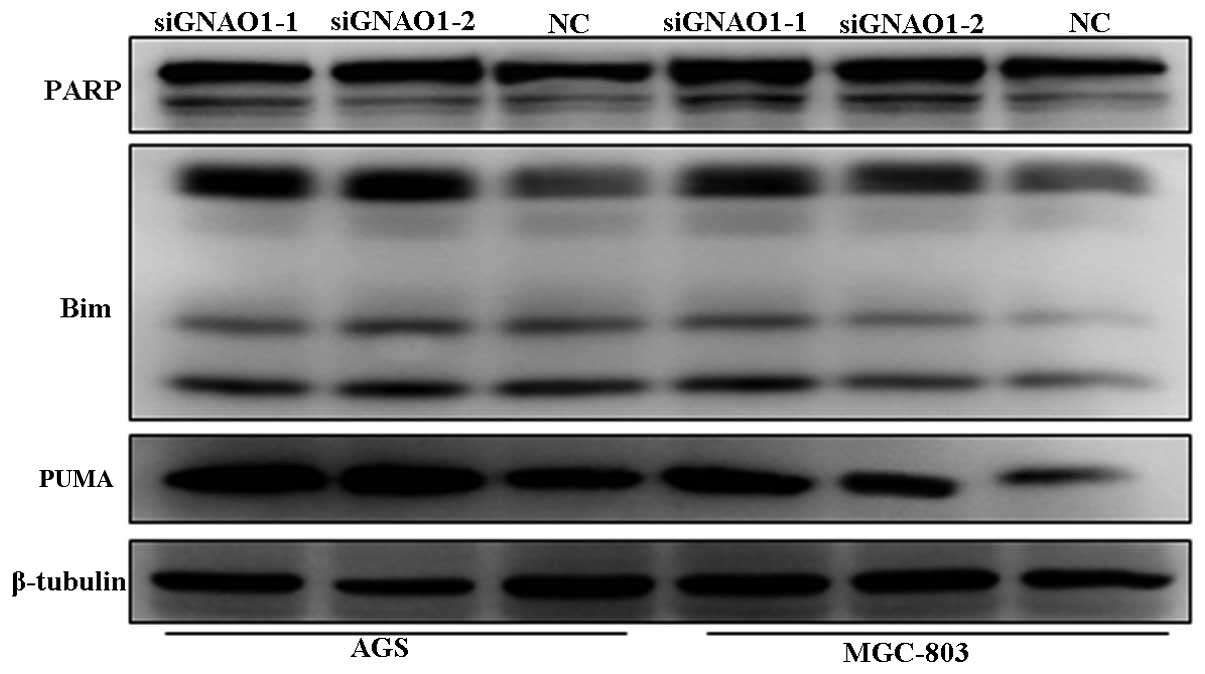
Induction of apoptosis-associated
mitochondrial events in GC cells by GNAO1 knockdown and the
involvement of the ERK signaling pathway
To confirm that GNAO1 participates in the regulation
of the proliferation and apoptosis of GC cells and to determine the
possible signaling pathways involved, we examined the expression of
the apoptosis-related protein, PARP, and that of the pro-apoptotic
proteins, Bim and Puma, as well as the activation of ERK1/2 and
phospho-SARK/JNK following transfection with siRNA. It was found
that the abundance of PARP, Puma and Bim was increased after GNAO1
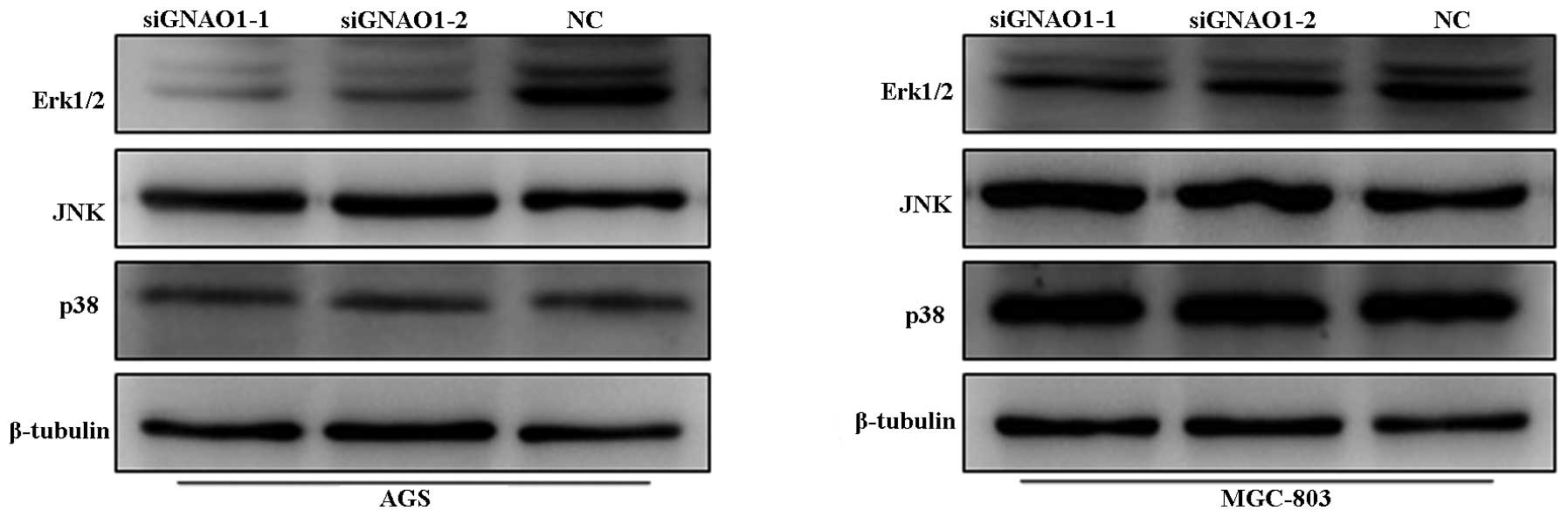
knockdown (Fig. 7). In addition,
the phosphorylation levels of ERK were markedly decreased in
response to treatment, while no significant changes were observed
in the phosphorylation levels of SARK/JNK and p38 (Fig. 8).
Discussion
GC remains a major public health issue worldwide
(16). Although it is curable if
detected early, the majority of patients are diagnosed at an
advanced stage and have a poor prognosis (17). The precise prediction of the risk
of recurrence would assist in minimizing the adverse effects of GC
and maximizing the therapeutic effects of treatment. Therefore,
greater knowledge of the molecular mechanisms underlying the
development of this deadly neoplasm is required if novel valuable
predictors of prognosis for patients with GC are to be developed.
It is well known that tumors are a result of uncontrolled cell
proliferation in different organs. The disruption of the balance
between cell proliferation and apoptosis is an important driving
force in the development of GC (18). Proliferation markers have been
widely used in order to diagnose and determine the behavior and
prognosis of benign and malignant tumors (19).
Based on the present study, we hypothesized that
GNAO1 may be a novel marker of proliferation for GC. Studies have
demonstrated that the GNAO1 gene is frequently mutated or absent in
many types of human primary carcinomas. However, few studies have
investigated the expression and biological function of the GNAO1
gene. To the best of our knowledge, there are no published reports
to date that discuss the prognostic significance of GNAO1 in human
cancer, including GC. In the current study, using samples from 70
patients with GC, we demonstrate that GNAO1 is overexpressed in GC
tissues and that its upregulation is inversely correlated with
patient survival. GNAO1 can also be used to predict poorer outcomes
for patients with GC. GNAO1 overexpression appears to be a useful
marker for predicting the outcome in patients with GC who have
undergone surgery to remove the tumor. Thus, patients with GC who
present with an overexpression of GNAO1 should perhaps be
followed-up more closely. Future studies with larger GC patient
groups are recommended to confirm the prognostic significance of
GNAO1 in this disease.
To further explain these observations, we also
examined the role of GNAO1 in vitro and concluded that GNAO1
may participate in the regulation of GC cell proliferation by
affecting cell apoptosis through the accumulation of apoptotic
proteins, including PARP, Puma and Bim, which may be partially
regulated by ERK inactivation.
It is well known that the cleavage of PARP is a
characteristic of apoptosis and that the BH3 domain-containing
proteins, Bim and Puma, can lead to apoptosis (20). The results of the present study
demonstrated the accumulation of Puma, Bim and PARP after the
knockdown of the GNAO1 gene (Fig.
7). In conjunction with the apoptotic effect of GNAO1 siRNA
demonstrated by flow cytometry, these findings indicate that GNAO1
expression inhibits the apoptosis of GC cells. It has recently been
shown that Gαo overexpression results in the increased
phosphorylation and hence in the activation of ERK1/2 but not in
that of p38 or JNK (21). MAPKs,
which consist of these 3 protein kinases (22,23), have been reported to regulate
diverse cellular functions, including embryogenesis, proliferation,
differentiation and apoptosis (24). In addition, previous studies have
indicated that the inhibition of the ERK pathway induces an
increase in Bim expression levels and activates the Puma promoter.
Consequently, it can be identified as the tunnel that interconnects
kinase signaling networks and the mitochondrion-dependent apoptotic
program (25–27). Thus, in this study, we
investigated the activity of ERK1/2, SARK/JNK and p38 MAPK after
GNAO1 silencing. Confirming our initial hypothesis, we found that
ERK activation may be involved in the downregulation of Bim and
Puma, as well as in the inhibition of the apoptosis of GC cells by
GNAO1.
To the best of our knowledge, in the current study
we demonstrate for the first time that GNAO1 overexpression in GC
is associated with tumor size, tumor differentiation, TNM stage and
poor prognosis. The present findings also demonstrate that the
knockdown of GNAO1 leads to reduced proliferation and promotes the
apoptosis of GC cells (MGC-803 and AGS). Thus, GNAO1 may serve as a
novel diagnostic and prognostic biomarker, as well as a potential
therapeutic target in GC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81125001 and 91129702),
the Ministry of Science and Technology of China (no. 2010CB732405)
and the Science and Technology Commission of Shanghai Municipality
(nos. 12JC1402000 and 12410705300).
References
|
1
|
Nakamura T, Nakatsu N, Yoshida Y, et al:
Identification of candidate genes determining chemosensitivity to
anti-cancer drugs of gastric cancer cell lines. Biol Pharm Bull.
32:1936–1939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji YB, Qu ZY and Zou X: Juglone-induced
apoptosis in human gastric cancer SGC-7901 cells via the
mitochondrial pathway. Exp Toxicol Pathol. 63:69–78. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jackson C, Cunningham D and Oliveira J:
Gastric cancer: ESMO clinical recommendations for diagnosis,
treatment and follow-up. Ann Oncol. 20(Suppl 4): 34–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang WF, Li J, Du LT, et al: Krüppel-like
factor 8 overexpression is correlated with angiogenesis and poor
prognosis in gastric cancer. World J Gastroenterol. 19:4309–4315.
2013.
|
|
5
|
Yamada Y, Boku N, Nishina T, et al: Impact
of excision repair cross-complementing gene 1 (ERCC1) on the
outcomes of patients with advanced gastric cancer: correlative
study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol.
24:2560–2565. 2013. View Article : Google Scholar
|
|
6
|
Li X, Qi W, Yao R, Tang D and Liang J:
Overexpressed transcription factor FOXM1 is a potential diagnostic
and adverse prognostic factor in postoperational gastric cancer
patients. Clin Transl Oncol. Jul 20–2013.(Epub ahead of print).
|
|
7
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
8
|
Neer EJ, Lok JM and Wolf LG: Purification
and properties of the inhibitory guanine nucleotide regulatory unit
of brain adenylate cyclase. J Biol Chem. 259:14222–14229.
1984.PubMed/NCBI
|
|
9
|
Gierschik P, Milligan G, Pines M, et al:
Use of specific antibodies to quantitate the guanine
nucleotide-binding protein Go in brain. Proc Natl Acad Sci U S A.
83:2258–2262. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corre I, Baumann H and Hermouet S:
Regulation by Gi2 proteins of v-fms-induced proliferation and
transformation via Src-kinase and STAT3. Oncogene. 18:6335–6342.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan X, Brass LF, Poncz M, Spitz F, Maire P
and Manning DR: The alpha subunits of Gz and Gi interact with the
eyes absent transcription cofactor Eya2, preventing its interaction
with the six class of homeodomain-containing proteins. J Biol Chem.
275:32129–32134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montminy M: Transcriptional regulation by
cyclic AMP. Annu Rev Biochem. 66:807–822. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kan Z, Jaiswal BS, Stinson J, et al:
Diverse somatic mutation patterns and pathway alterations in human
cancers. Nature. 466:869–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia D, Wei L, Guo W, et al: Genome-wide
copy number analyses identified novel cancer genes in
hepatocellular carcinoma. Hepatology. 54:1227–1236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garcia-Marcos M, Ghosh P and Farquhar MG:
Molecular basis of a novel oncogenic mutation in GNAO1. Oncogene.
30:2691–2696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
17
|
Wang XN and Liang H: Some problems in the
surgical treatment of gastric cancer. Chin J Cancer. 29:369–373.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: from dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Freeman A, Morris LS, Mills AD, et al:
Minichromosome maintenance proteins as biological markers of
dysplasia and malignancy. Clin Cancer Res. 5:2121–2132.
1999.PubMed/NCBI
|
|
20
|
Tait SW and Green DR: Mitochondria and
cell death: outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bratton MR, Antoon JW, Duong BN, et al:
Gαo potentiates estrogen receptor α activity via the ERK signaling
pathway. J Endocrinol. 214:45–54. 2012.
|
|
22
|
Baines CP and Molkentin JD: STRESS
signaling pathways that modulate cardiac myocyte apoptosis. J Mol
Cell Cardiol. 38:47–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan Y, Chen H, Qiao B, et al: Opposing
effects of ERK and p38 MAP kinases on HeLa cell apoptosis induced
by dipyrithione. Mol Cells. 23:30–38. 2007.PubMed/NCBI
|
|
24
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bean GR, Ganesan YT, Dong Y, et al: PUMA
and BIM are required for oncogene inactivation-induced apoptosis.
Sci Signal. 6:ra202013.PubMed/NCBI
|