Introduction
Diabetes mellitus (DM), as a state of chronic
hyperglycemia, and Parkinson’s disease (PD) are diseases which
consist a global health threat. In recent years, there have been
increasing data indicating that hyperglycemia is associated with an
increased risk of developing PD (1,2).
However, the mechanisms underlying the association between
hyperglycemia and PD have not yet been elucidated.
Deng and Rajput (6) were the first to report, in 2001,
that 1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (ADTIQ),
a salsolinol-like compound, is found at highly concentrated levels
in the brains of patients who suffer from PD (3). When the brains of deceased patients
with PD were compared to those of normal subjects, it was found
that patients with PD presented elevated levels of ADTIQ in all the
examined brain areas. This finding indicated that elevated ADTIQ
expression levels may be one of the mechanisms involved in the
increased risk patients with diabetes have of developing PD
(4).
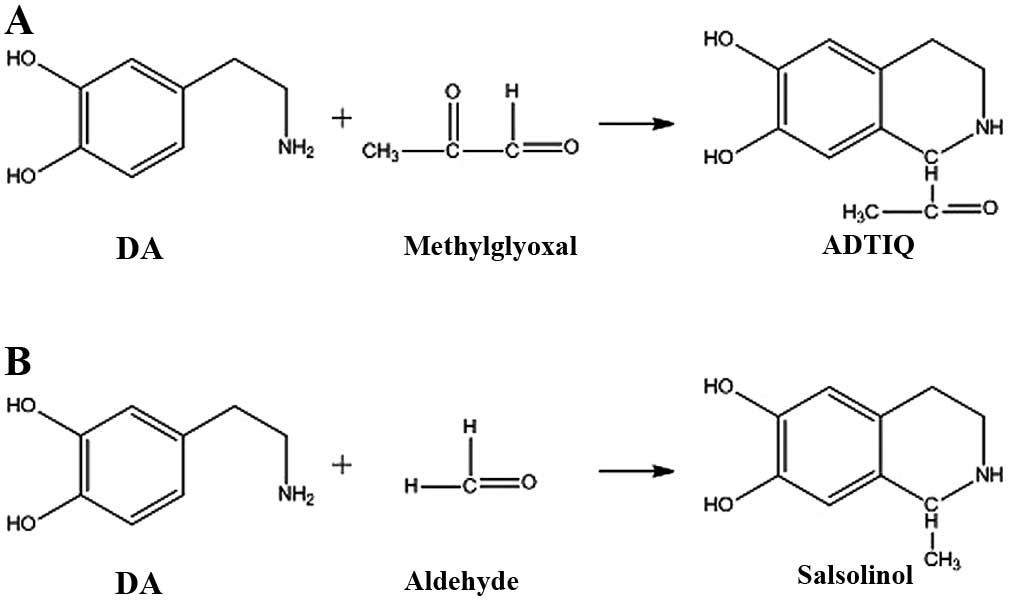
ADTIQ is an endogenous product acquired by a
reaction between methylglyoxal (MG) and dopamine (DA). The reactive
α-keto-aldehyde MG is the most important carbonyl, formed
endogenously as a byproduct of the glycolytic pathway and formed
either by the degradation of triosephosphates, or non-enzymatically
by sugar fragmentation reactions (5). MG is gradually accumulated under
hyperglycemic conditions, which may induce oxidative stress
(6).
The neurotoxin,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), is known to
deplete striatal DA and to cause neuronal degeneration of the
nigrostriatal pathway when administered to humans. The reason
behind this, is that MPTP may induce oxidative stress and
mitochondrial dysfunction, which can lead to PD (7). Catechol isoquinolines (CIQs) are
considered to be naturally occurring MPTP-like neurotoxins.
Salsolinol is demonstrated to be formed from DA and acetaldehyde by
an (R)-salsolinol synthase-mediated condensation, N-methylated into
(R)-N-methylsalsolinol by a neutral (R)-salsolinol
N-methyltransferase, and then oxidized into an ion of
6,7-dihydroxy-1,2-dimethyl-isoqiolinium, an analogue of
1-methyl-4-phenylpyridinium (MPP+). The toxicity of
N-methylsalsolinol and its oxidation product for dopaminergic
neurons has previously been examined in vivo and in
vitro (8,9).
The chemical structure of ADTIQ is very similar to
that of salsolinol (Fig. 1).
Therefore, in the current study, we hypothesize that ADTIQ may be
another endogenous neurotoxin with a possibly negative effect on
the nervous system, which may cause damage to the peripheral,
automatic and central nervous systems, and may ultimately lead to
PD.
In the present study, ADTIQ levels and those of its
precursor, MG, were examined in a cell model of hyperglycemia and a
rat model of diabetes. Proteomics was used to analyze the proteins
involved, also analyzing their role in glucose metabolism.
Materials and methods
Cell line
The parental SH-SY5Y human neuroblastoma cell line
was provided by Professor Wei-Hong Song (University of British
Columbia).
Animals
The study complied with the ‘Guide for the Care and
use of Laboratory Animals’ published by the US National Institutes
of Health (NIH publication no. 85-23, revised in 1985) and all
animal experiments were approved by the Institutional Animal
Research Advisory Committee of the Beijing Institute of Technology.
Wistar rats (male, 180–200 g body weight, 6–8 weeks old) and their
granular food were provided by the Institute of Laboratory Animal
Science, Chinese Academy of Medical Sciences (Beijing, China). The
animals were maintained under a 12-h light/dark cycle at
approximately 24±1ºC and were allowed free access to food and
water.
Cell culture and establishment of the rat
model of diabetes
The SH-SY5Y cells were grown under standard
conditions in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 2 mM L-glutamine and 10% fetal calf serum at 37ºC
(5% CO2).
A total of 40 Wistar rats were randomly divided into
2 groups, a control group and a diabetes model group (10 rats in
the control group and 30 rats in the model group). Baseline blood
glucose levels of all animals were measured after 12 h of fasting.
Diabetes was induced by a single intraperitoneal injection of
prepared streptozotocin (60 mg/kg body weight; Sigma, St. Louis,
MO, USA) dissolved in sterile saline (0.85% NaCl). The control rats
received an equal volume of the vehicle (normal saline).
Non-fasting blood glucose levels were quantified a week later with
the use of a commercially available glucometer.
Streptozotocin-injected rats whose initial blood glucose levels
were <300 mg/dl were considered as non-diabetic. Non-fasting
blood glucose levels were monitored every 7th day during the course
of the study and were examined again right before all the rats were
anesthetized. The model group was fed for 18 weeks in order to
imitate chronic hyperglycemia induced by long-term injury to the
nervous system.
Measurement of free hydroxyl radicals in
SH-SY5Y cells by high-performance liquid chromatography
(HPLC)-electrochemical detector (ECD) assay
The SH-SY5Y cells were exposed to 60 mM glucose
(10). After 48 h, the cells were
harvested and 200 μl 10 mM ortho-oxybenzoic were added to the
cells. The cells were lysed by sonication (50 cycles of 10 sec),
centrifuged at 16,000 × g (4ºC, 10 min) following incubation with
0.4 M PCA (1:2/v:v) for 30 min, and passed through a 0.22-μm filter
membrane prior to analysis. All these samples were analyzed by
HPLC-ECD using a CoulArray Electrochemical Detector (Model 5600A;
ESA, USA) under the following conditions: i) the organic phase
consisted of 10% methanol diluted in water (solvent A); ii) the
balanced solution contained 35 mM citric acid, 45 mM sodium
acetate, 0.13 mM Na2EDTA, 0.2 mM SHS water-solution, pH
4.0 (solvent B); while iii) for the stationary phase, Alltima
C18 (4.6×150 mm, 5 μm) analytical column was used. The
column had a flow rate of 1 ml/min, it reached a temperature of
30ºC, while its electric potential was: −50, 50, 300, 450, 650,
780, 900 mV. The volume of the sample used was 40 μl.
Detection of MG and ADTIQ in
hyperglycemic cells and rats with diabetes by liquid chromatography
(LC)-mass spectrometry (MS)
LC-MS for the detection of MG and ADTIQ was carried
out as previously described (11,12). The prepared cells and brain
tissues were lysed by sonication (50 cycles of 10 sec) and
centrifuged at 16,000 × g (4ºC, 10 min) following incubation with
0.4 M PCA (1:2/v:v) for 30 min. The supernatant was passed through
a 0.22-μm filter membrane. O-phenylenediamine (2 mM) was added to
the samples followed by incubation for 1 h at 37ºC. As MG cannot be
retained on C18 columns, an indirect derivatization
method was used to measure MG levels in the rat brains. MG reacted
with o-phenylenediamine and the product, 2-methylquinoxaline
(2-MQ), was detected as a measure of MG content. Tissue samples
were separated into 2 groups, one for derivatization and another
for no derivatization.
LC was performed with the discovery F5-SH column
(4.6×250 mm, 5 μm). The mobile phase consisted of 32% methanol and
68% formic acid (pH 3.49), while the flow rate was 0.7 ml/min and
the UV detection wave length 315 nm. The volume of the sample
analyzed was 50 μl. Mass spectrum analysis was performed under the
following conditions: mode electrospray ionization (ESI), positive
ion mode; MS scanning; nebulizer pressure, 28 psi; drying gas flow
rate, 8 l/min; scanning range, 135–225 m/z; and extracted ion
chromatography (EIC), 145 (2-MQ) and 208 (ADTIQ).
Protein extraction and trypsin
digestion
The control and model rat brain specimens were
prepared and lysed by sonication (50 cycles of 10 sec) in 300 μl
lysis buffer [8 M urea, 4%
3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonic acid
(CHAPS), 10 mM dithiothreitol (DTT) and 0.09 g solid urea]. The
homogenates were left at room temperature for 1 h after having been
centrifuged at 20,000 × g for 60 min at 4ºC, and the supernatants
were then collected as protein extracts. Protein concentration was
determined by the Lowry method after dialysis. Proteins (500 μg)
were freeze-dried at −56ºC under a vacuum, as previously described
(13,14).
Desiccated proteins were dissolved in 200 μl buffer
solution (8 M urea, 10 mM DTT and 50 mM
NH4HCO3) and incubated at 37ºC for 4 h.
Subsequently, 5 μl of iodoacetamide (1 M) were added in order for
the alkylation reaction to occur in the dark for 1 h. The urea
concentration was diluted in 500 μl NH4HCO3
(50 mM). Porcine trypsin (Promega, Madison, WI, USA) was added in a
final enzyme to protein ratio of 1:20. Digestion was conducted at
37ºC for 18 h.
Peptide detection and quantification by
LC-ESI-time-of-flight (TOF) MS
The quantification of the peptide mixtures in
limited amounts of rat brain tissues was carried out by
LC-ESI-TOF-MS (Agilent 6210 Time-of-Flight LC/MS System; Agilent
Technologies, Inc., Santa Clara, CA, USA). Samples were separated
by a C18 column (4.6×250 mm, 5 μm) in acetonitrile-0.1%
formic acid (5:95) (mobile phase) with a flow rate of 0.8 μl/min
for online enrichment. They were then analyzed by a Zorbax
C18 column (0.5×150 mm, 5 μm). The peptides were
separated and analyzed completely within 70 min. The ESI positive
scanning range was 300–1,800 m/z.
Analysis of peptides by LC-MS/MS
The peptide mixtures were separated by
chromatography and analyzed by ion trap mass spectrometry (Agilent
1100 series LC/MSD mass spectrometer; Agilent Technologies, Inc.)
with the reversed-phase (RP) C18 column (4.6×250 mm, 5
μm), at a flow rate of a 0.8 ml/min. Samples of proteolytic digests
(80 μl) were injected into the column and run with a linear
gradient of 95% solvent A (0.1% formic acid) to 100% solvent B
(acetonitrile) for a time period of 70 min and post run for another
10 min (14).
Bioinformatics analysis
MassHunter and MassProfiler, analysis software
provided by Agilent Technologies, Inc. were used in order to
analyze the TOF data. Significantly differentially expressed
peptides were selected from the mass data. The same samples were
analyzed by LC-TOF before they were validated by LC-MS/MS. The m/z
value of significantly differentially expressed peptides was
preferentially selected for LC-MS/MS analysis.
Trypsin cleaves proteins at the C terminus of lysine
and arginine residues, thereby generating a peptide mass
fingerprint (PMF) that can be used to search databases. The MS/MS
data were searched using the MASCOT tool (http://www.matrixscience.com; Matrix Science, London,
UK). The threshold parameter values for mass accuracy were 150 ppm
and one miscleavage was allowed. The search was performed against
rat protein sequences. PMFs from our samples were compared to
corresponding fingerprints from NCBInr (http://ncbi.nih.gov/National Center for Biotechnical
Information, Bethesda, MD, USA), MSDB
(csc-fserve.hh.med.ic.ac.uk/msdb.html/Proteonomics Department,
Hammersmith Campus, Imperial College, London, UK) and EnsemblC
(http://www.ensembl.org/Sanger Centre,
Hinxton, UK) databases in order to find matches with the virtual
tryptic protein masses. Gene Ontology entries were retrieved based
on the IPI data files from Swiss-Prot. Gene Ontology terms were
mapped to Ontoglyph terms (http://61.50.138.118/GOfact/) to provide a
coarse-grained classification of gene function (15).
Detection of protein content by western
blot analysis
Brain homogenate expression levels of
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and adenosine
triphosphate (ATP) synthase were determined by western blot
analysis. First, the proteins were separated by SDS-PAGE, then
electrotransferred (electrodes were attached and the power supply
was set to 100 V at constant voltage for 1 h at 4ºC). Finally,
immunodetection was carried out as follows: the membranes were
stained, blocked and washed, and then subjected to first antibody
and second antibody and detection for the target protein.
Statistical analysis
SPSS 13.0 software (SPSS, Chicago, IL, USA) was used
for statistical analysis. Values are expressed as the means ±
standard deviation (SD). Data were analyzed using one-way ANOVA,
followed by a Student’s two-tailed paired t-test for comparisons
between 2 groups. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Measurement of free hydroxyl radicals in
hyperglycemic SH-SY5Y cells
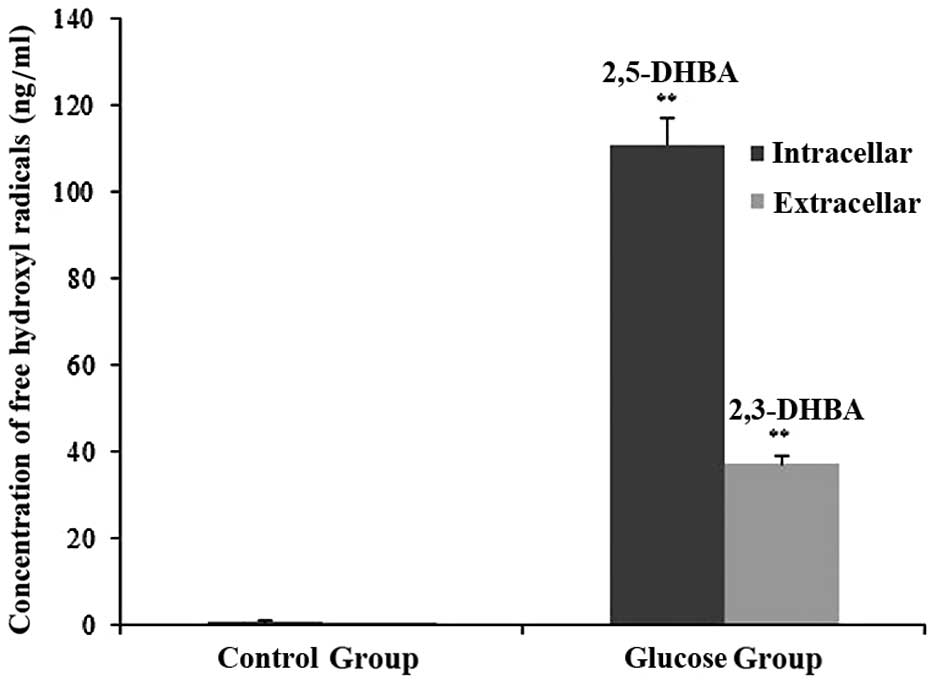
Salicylic acid hydroxylation is a specific scavenger
for free hydroxyl radicals. Thus, the hydroxylation products,
2,3-dihydroxybenzoic acid (2,3-DHBA) and 2,5-dihydroxybenzoic acid
(2,5-DHBA), were measured in order to indirectly evaluate the
degree of oxidative stress (16).
Free hydroxyl radicals in the SH-SY5Y cells treated with or without
glucose were measured by HPLC-ECD assay. No 2,3-DHBA or 2,5-DHBA
expression was detected in the control cells. However, there was a
large quantity of free hydroxyl radicals in the hyperglycemic
cells. Compared with the cells in the control group, an elevated
level of glucose markedly promoted free hydroxyl radical formation
in the hyperglycemic cells (p<0.01) (Fig. 2).
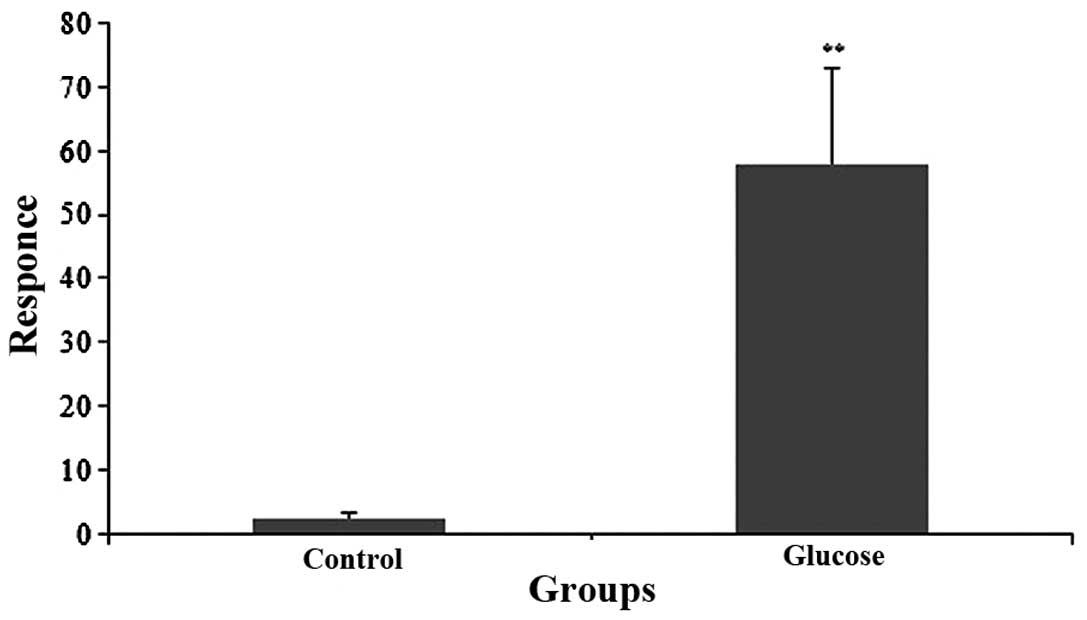
Measurement of ADTIQ levels in the cell
model of hyperglycemia
Only minimal amounts of ADTIQ were detected in the
control group, contrary to the large amount that was detected in
the cells treated with a final concentration of 60 mM glucose for 2
h. Compared with the control group, we observed a significant
increase in the expression levels of ADTIQ in the neuronal SH-SY5Y
cells cultured under hyperglycemic conditions following a 2-h
incubation period (p<0.01) (Fig.
3).
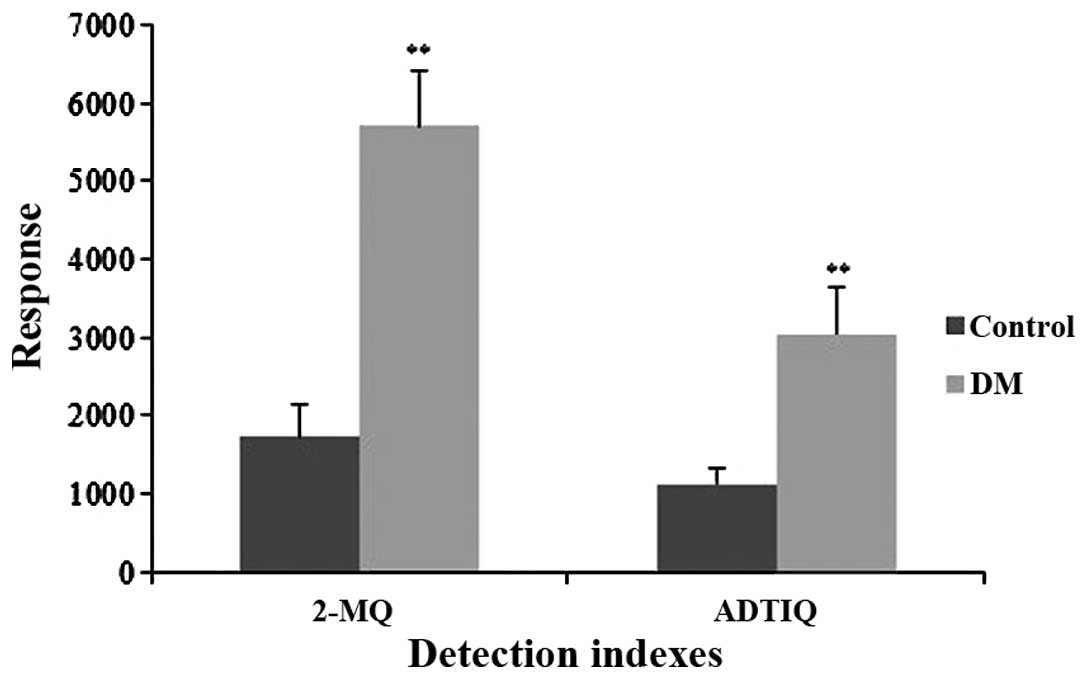
Measurement of MG and ADTIQ levels in the
brains of rats with diabetes
The abundance of MG can be indirectly determined by
measuring the levels of 2-MQ (17). LC/MS analysis was conducted to
determine whether ADTIQ accumulated in the brains of rats with
diabetes. The results indicated that the brains of rats with
diabetes rat had significantly higher levels of ADTIQ (p<0.01)
compared with the control group (Fig.
4).
Measurement of relative protein levels in
rat brains
A proteomics approach was used to search for
differentially expressed proteins in the brains of rats with
diabetes (Materials and methods). The results of this proteomics
and bioinformatics analysis revealed that, in comparison to the
control group, the expression levels of 7 key enzymes from the
glycolytic pathway were significantly increased in the brains of
rats with diabetes, while the levels of ATP synthase, an enzyme
from the oxidative phosphorylation pathway, and of those superoxide
dismutase (SOD) were significantly decreased. Compared with the
control group, the diabetes group had a higher rate of glycolysis
and a lower rate of oxidative phosphorylation (Table I).
 | Table IExpression profiles of nine proteins
in the brains of the control rats and rats with diabetes. |
Table I
Expression profiles of nine proteins
in the brains of the control rats and rats with diabetes.
| No. | Protein
annotation | Database accession
no. | Sequence
coverage | Control group
(%) | Diabetes group
(%) |
|---|
| 1 | Hexokinase | 1BG3A | 12 | 0.21±0.05 | 0.51±0.03 |
| 2 |
Fructose-bisphosphate aldolase | ADRTA | 7 | 0.33±0.01 | 0.72±0.01 |
| 3 | Triosephosphate
isomerase | TPIS_RAT | 6 | 0.27±0.08 | 0.77±0.02 |
| 4 |
Glyceraldehyde-3-phosphate
dehydrogenase | DERTG | 16 | 0.16±0.03 | 0.54±0.04 |
| 5 | Phosphoglycerate
mutase, brain form | PMGB | 14 | 0.14±0.04 | 0.38±0.04 |
| 6 | Enolase | ENOA | 9 | 0.22±0.02 | 0.42±0.01 |
| 7 | Pyruvate
kinase | KPY1 | 7 | 0.25±0.05 | 0.57±0.01 |
| 8 | SOD | DSRTN | 9 | 0.55±0.02 | 0.21±0.01 |
| 9 | ATP synthase | ATPB_RAT | 9 | 0.71±0.02 | 0.27±0.03 |
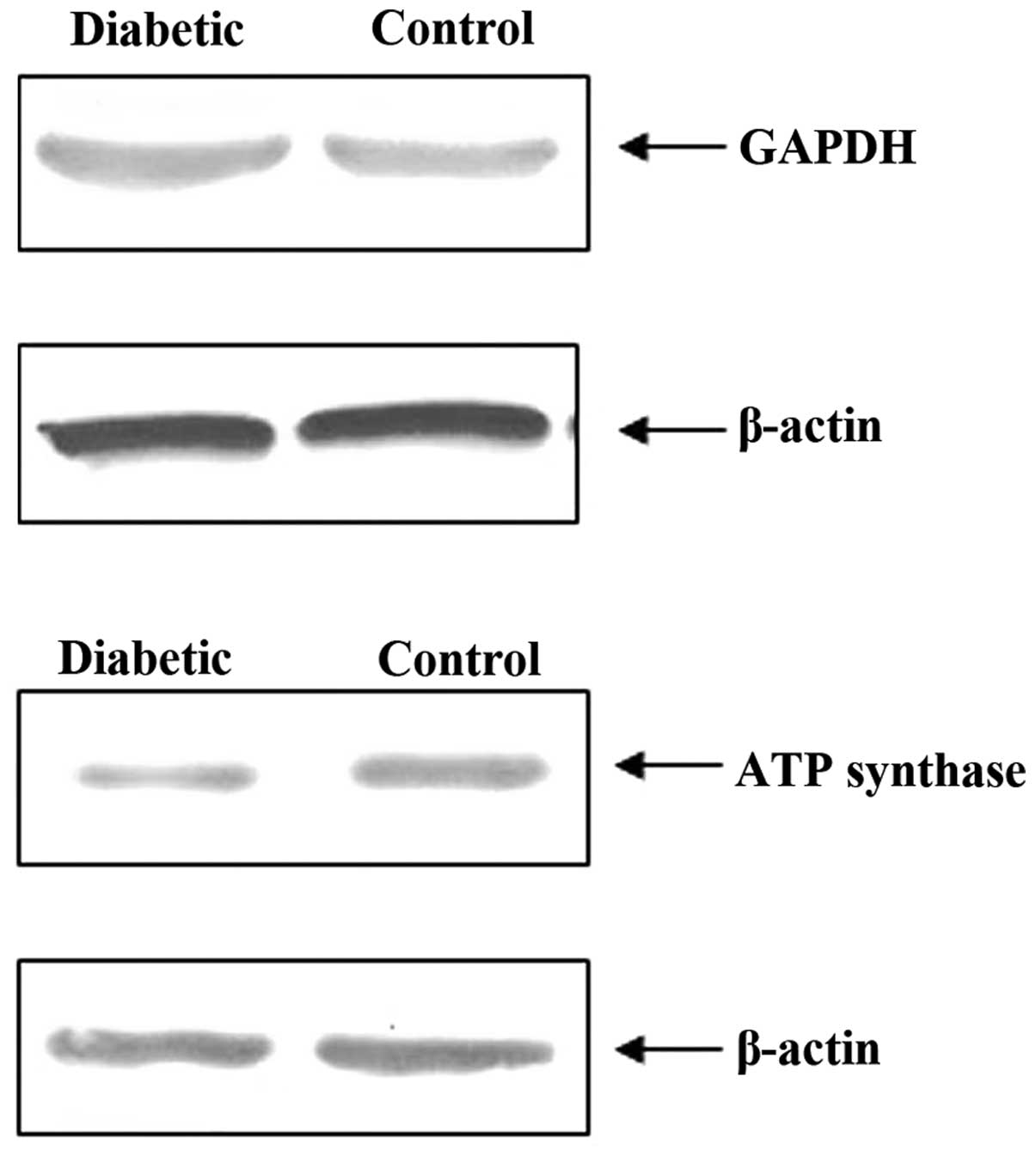
Two proteins, GAPDH and ATP synthase, were selected
to be semi-quantified by western blot analysis in order to verify
the reliability of the proteomics results. It was shown that,
compared with the control group, the GAPDH content in the rats with
diabetes was increased while the ATP synthase content was
decreased. These results were consistent with the those from
proteomics analysis. Thus, the comparative proteomics analysis
proved to be a feasible and reliable approach for studying relative
protein levels (Fig. 5).
Discussion
The pathological process of PD is complex, involving
a number of different factors, such as mitochondrial dysfunction,
abnormal protein aggregation, familial inheritance and
excitotoxicity (18). A previous
study demonstrated that CIQs, such as salsolinol and NM-salsolinol
are promising endogenous neurotoxins that may lead to the
development of PD (19). Thus, a
‘vicious’ cycle may be induced by CIQs. ADTIQ is structurally
similar to salsolinol, and has been suggested to be a neurotoxin
(20,21).
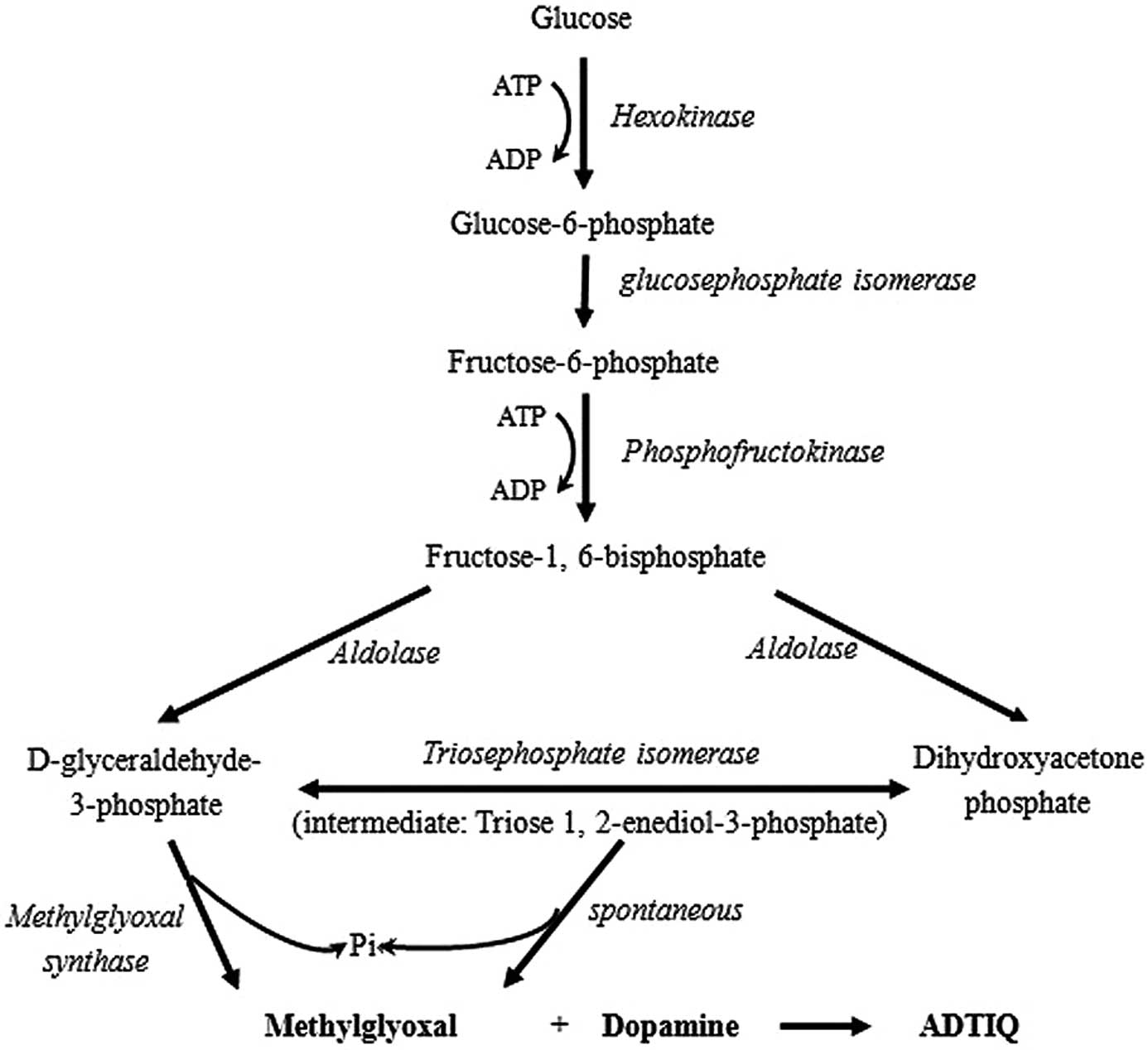
Glycolysis is a crucial process in DM and the most
extensively investigated metabolic pathway (22). MG is an important byproduct in
this pathway and its production is increased due to hyperglycemia.
The formation of dihydroxyacetone phosphate and
D-glyceraldehyde-3-phosphate by fructose-1, 6-bisphosphate is
catalyzed by aldolase. Triosephosphate isomerase catalyzes the
aldoketose isomerization of these triosephosphates and eventually
only D-glyceraldehyde-3-phosphate follows the glycolytic pathway,
by being converted into 1,3-bisphosphoglycerate in a reaction
catalyzed by D-glyceraldehyde-3-phosphate dehydrogenase (23). However, triose phosphates are
unstable molecules and L-elimination reactions of the phosphoryl
group from the common 1,2-enediolate of both trioses may occur,
leading to MG formation (24,25). This is a non-enzymatic,
parametabolic reaction and therefore MG occurrence is an
unavoidable consequence of glycolytic metabolism (26). MG can also be formed from the
leakage of the 1,2-enediolate intermediate in the active center of
triosephosphate isomerase in a paracatalytic reaction (27). Consequently, ADTIQ is produced
when DA reacts with MG (Fig.
6).
As previously demonstrated, reactive oxygen species
(ROS) induced by high glucose levels are involved in vivo in
both death receptor- and mitochondrial-dependent apoptosis of the
nervous system (28).
Antioxidants may be a therapeutic option for preventing
cardiovascular damage in patients with DM. ROS has been shown to be
involved in collagen-induced platelet activation and aggregation
(29).
In the SH-SY5Y cell model of hyperglycemia, a large
amount of free radicals was generated during the course of
treatment with high glucose, consistent with the observation that
ROS generation and elimination systems were imbalanced under
hyperglycemic conditions. Intracellular ROS and hydroxyl radical
accumulation induced lipid peroxidation (LPO) (30); thus, lipid molecules were consumed
uninterruptedly, decreasing the amount of unsaturated fatty acid
and affecting the fluidity of biological membranes (31). MG and other aldehydes react with
proteins and enzymes, which cause them to lose their biological
activity and lead to an abnormal metabolism. Specifically, MG
reacts with DA to form ADTIQ, which confirms the hypothesis that
CIQs induce a ‘vicious’ cycle of oxidative stress.
In the present study, compared with the control
group, the ADTIQ levels in the brains of rats with diabetes were
found to be significantly elevated (p<0.01). We also
demonstrated that the levels of 7 key enzymes from the glycolytic
pathway were increased significantly in the brains of rats with
diabetes. More precisely, the expression levels of
fructose-bisphosphate aldolase and triosephosphate isomerase were
higher, suggesting that hyperglycemia enhanced the concentration of
dihydroxyacetone phosphate and D-glyceraldehyde-3-phosphate, thus
leading to an increase in MG and ADTIQ levels. When ADTIQ
accumulated and reached a critical level, it damaged neurons and
induced oxidative stress and apoptosis. Our results also indicated
that SOD and ATP synthase protein levels were significantly reduced
in the rats with diabetes. SOD is a potent antioxidant enzyme which
exerts its effects by scavenging ROS. There are data suggesting
that elevated glucose levels serve as a causal link between the
mitochondrial hyperglycemia-induced overproduction of superoxide
and each of the three major pathways responsible for hyperglycemic
vascular damage caused to endothelial cells (32,33). It has previously been demonstrated
that the C-peptide has a preventative effect on neuronal
hippocampal apoptosis in type 1 diabetes, although it does not have
any effect on oxidative stress (34,35). These results indicate that
hyperglycemic conditions may reduce the activity of SOD; however,
the mechanisms involved remain unclear. The decreased levels of ATP
synthase suggest that hyperglycemic conditions may induce
mitochondrial dysfunction and thus lead to the apoptosis of
neuronal cells. However, it is evident that multiple factors are
involved in the progression from diabetes to PD.
In conclusion, the present study demonstrates that
ADTIQ may be a type of endogenous neurotoxin and is found in the
brains of rats with diabetes. We also provide evidence in support
of the existence of a ‘vicious’ cycle of oxidative stress. The
accumulation of ADTIQ in diabetes be an important factor in the
connection between diabetes and PD.
Acknowledgements
The authors wish to acknowledge the financial
support provided by the Ministry of Science and Technology of the
People’s Republic of China (grant nos. 2012YQ040140, 2009BAK59B01,
02 and 03) and the National Natural Science Foundation of China
(grant no. 81202996).
Abbreviations:
|
ADTIQ
|
1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline
|
|
ATP
|
adenosine triphosphate
|
|
CHAPS
|
3-[(3-cholamido- propyl)
dimethylammonio]-1-propanesulfonic acid
|
|
CIQs
|
catechol isoquinolines
|
|
DA
|
dopamine
|
|
2,3-DHBA
|
2,3-dihydroxybenzoic acid
|
|
2,5-DHBA
|
2,5-dihydroxybenzoic acid
|
|
DM
|
diabetes mellitus
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
DTT
|
dithiothreitol
|
|
ECD
|
electrochemical detector
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
LPO
|
lipid peroxidation
|
|
MG
|
methylglyoxal
|
|
MPP+
|
1-methyl-4-phenylpyridinium
|
|
MPTP
|
1-methyl-4-phenyl-1,2,3,6-tetra-
hydropyridine
|
|
RP
|
reversed-phase
|
|
2-MQ
|
2-methylquinoxaline
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium
bromide
|
|
PD
|
Parkinson’s disease
|
|
PMFs
|
peptide mass fingerprints
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Xu Q, Park Y, Huang X, Hollenbeck A, Blair
A, Schatzkin A and Chen H: Diabetes and Risk of Parkinson’s
Disease. Diabetes Care. 34:910–915. 2011.
|
|
2
|
Schernhammer E, Hansen J, Rugbjerg K,
Wermuth L and Ritz B: Diabetes and the risk of developing
Parkinson’s disease in Denmark. Diabetes Care. 34:1102–1108.
2011.
|
|
3
|
Arvanitakis Z, Wilson RS, Schneider JA,
Bienias JL, Evans DA and Bennett DA: Diabetes mellitus and
progression of rigidity and gait disturbance in older persons.
Neurology. 63:996–1001. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng YL, Zhang YQ, Li YJ, Xiao SY, Song
DW, Qing H, Li Q and Rajput AH: Occurrence and distribution of
salsolinol-like compound,
1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (ADTIQ) in
parkinsonian brains. J Neural Transm. 119:435–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ristow M: Neurodegenerative disorders
associated with diabetes mellitus. J Mol Med (Berl). 82:510–529.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng YL and Rajput AM: 126th Annual
Meeting, American Neurological Association: Abstracts: Plenary
Session: Epilepsy. Ann Neurol. 50(Suppl 1): S19–S22. 2001.
View Article : Google Scholar
|
|
7
|
Haik GM Jr, Lo TW and Thornalley PJ:
Methylglyoxal concentration and glyoxalase activities in the human
lens. Exp Eye Res. 59:497–500. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu W, Wang D, Zheng J, An Y, Wang Q,
Zhang W, Jin L, Gao H and Lin L: Effect of (R)-salsolinol and
N-methyl-(R)-salsolinol on the balance impairment between dopamine
and acetylcholine in rat brain: involvement in pathogenesis of
Parkinson disease. Clin Chem. 54:705–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Xiao S, Wang L, Wang H, Zhu Y, Li
Y and Deng Y: Absolute quantification of semicarbazide-sensitive
amine oxidase in human umbilical artery by single-reaction
monitoring with electrospray tandem mass spectrometry. Anal Bioanal
Chem. 397:709–715. 2010. View Article : Google Scholar
|
|
10
|
Wang R, Qing H, Liu XQ, Zheng XL and Deng
YL: Iron contributes to the formation of catechol isoquinolines and
oxidative toxicity induced by overdose dopamine in dopaminergic
SH-SY5Y cells. Neurosci Bull. 24:125–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song DW, Du HQ, Wang L, Hu GF and Deng YL:
Analysis of catechol quinoline substance in corpus striatum and
hippocampus from brains of diabetic rat models by HPLC-MS.
Chemistry. 11:1049–1052. 2010.
|
|
12
|
Song DW, Hu GF, Zhou Y, Wang HB, Wang L,
Zhu Y and Deng YL: Proteomic analysis of proteins related to
Parkinson’s disease in the corpus striatum and hippocampus of
diabetes rat model. Chemistry. 6:430–434. 2008.
|
|
13
|
Kuhla B, Loske C, Garcia De Arriba S,
Schinzel R, Huber J and Münch G: Differential effects of ‘Advanced
glycation endproducts’ and beta-amyloid peptide on glucose
utilization and ATP levels in the neuronal cell line SH-SY5Y. J
Neural Transm. 11:427–439. 2004.
|
|
14
|
Emdadul Haque M, Asanuma M, Higashi Y,
Miyazaki I, Tanaka K and Ogawa N: Apoptosis-inducing neurotoxicity
of dopamine and its metabolites via reactive quinone generation in
neuroblastoma cells. Biochim Biophys Acta. 1619:39–52.
2003.PubMed/NCBI
|
|
15
|
Collins MO, Yu L, Coba MP, Husi H,
Campuzano I, Blackstock WP, Choudhary JS and Grant SGN: Proteomic
analysis of in vivo phosphorylated synaptic proteins. J Biol Chem.
280:5972–5982. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song DW, Li Q, Luan YJ, Niu LY, Qing H and
Deng YL: Comparative proteomic analysis of neural stem cells
between differentiating and undifferentiating to dopaminergic
neuron. Complex Medical Engineering (CME). In: IEEE/ICME
International Conference, 1817–1823; 2007
|
|
17
|
Diez L, Livertoux MH, Stark AA,
Wellman-Rousseau M and Leroy P: High-performance liquid
chromatographic assay of hydroxyl free radical using salicylic acid
hydroxylation during in vitro experiments involving thiols. J
Chromatogr B Biomed Sci Appl. 763:185–193. 2001. View Article : Google Scholar
|
|
18
|
Ferger B, Spratt C, Earl CD, Teismann P,
Oertel WH and Kuschinsky K: Effects of nicotine on hydroxyl free
radical formation in vitro and on MPTP-induced neurotoxicity in
vivo. Naunyn Schmiedebergs Arch Pharmacol. 358:351–359. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Copeland RL Jr, Das JR, Kanaan YM, Taylor
RE and Tizabi Y: Antiapoptotic effects of nicotine in its
protection against salsolinol-induced cytotoxicity. Neurotox Res.
12:61–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi H, Maruyama W, Akao Y, Takahashi T,
Iwasa K, Youdim MB and Naoi M: N-Propargylamine protects SH-SY5Y
cells from apoptosis induced by an endogenous neurotoxin,
N-methyl(R)salsolinol, through stabilization of mitochondrial
membrane and induction of anti-apoptotic Bcl-2. J Neural Transm.
113:21–32. 2006. View Article : Google Scholar
|
|
21
|
Maruyama W, Akao Y, Youdim MB, Davis BA
and Naoi M: Transfection-enforced Bcl-2 overexpression and an
anti-Parkinson drug, rasagiline, prevent nuclear accumulation of
glyceraldehyde-3-phosphate dehydrogenase induced by an endogenous
dopaminergic neurotoxin, N-methyl(R)salsolinol. J Neurochem.
78:727–735. 2001. View Article : Google Scholar
|
|
22
|
Wanpen S, Kooncumchoo P, Shavali S,
Govitrapong P and Ebadi M: Salsolinol, an endogenous neurotoxin,
activates JNK and NF-kappaB signaling pathways in human
neuroblastoma cells. Neurochem Res. 32:443–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kheradpezhouh M, Shavali S and Ebadi M:
Salsolinol causing parkinsonism activates endoplasmic
reticulum-stress signaling pathways in human dopaminergic SK-N-SH
cells. Neurosignals. 12:315–324. 2003. View Article : Google Scholar
|
|
24
|
Thornalley PJ, Jahan I and Ng R:
Suppression of the accumulation of triosephosphates and increased
formation of methylglyoxal in human red blood cells during
hyperglycaemia by thiamine in vitro. J Biochem. 129:543–549. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dmitriev LF and Dugin SF: Aldehydes and
disturbance of carbohydrate metabolism: some consequences and
possible approaches to its normalization. Arch Physiol Biochem.
113:87–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheu KF, Ho HT, Nolan LD, Markovitz P,
Richard JP, Utter MF and Frey PA: Stereochemical course of
thiophosphoryl group transfer catalyzed by mitochondrial
phosphoenolpyruvate carboxykinase. Biochemistry. 23:1779–1783.
1984. View Article : Google Scholar
|
|
27
|
Richard JP: Mechanism for the formation of
methylglyoxal from triosephosphates. Biochem Soc Trans. 21:549–553.
1993.PubMed/NCBI
|
|
28
|
Han YC, Randell E, Vasdev S, Gill V, Gadag
V, Newhook LA, Grant M and Hagerty D: Plasma methylglyoxal and
glyoxal are elevated and related to early membrane alteration in
young, complication-free patients with Type 1 diabetes. Mol Cell
Biochem. 305:123–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
MacDonald MJ, Chaplen FWR, Triplett CK,
Gong Q and Drought H: Stimulation of insulin release by
glyceraldehyde may not be similar to glucose. Arch Biochem Biophys.
447:118–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caccese D, Pratico D, Ghiselli A, Natoli
S, Pignatelli P, Sanguigni V, Luliano L and Violi F: Superoxide
anion and hydroxyl radical release by collagen-induced platelet
aggregation - role of arachidonic acid metabolism. Thromb Haemost.
83:485–490. 2000.PubMed/NCBI
|
|
31
|
Shi HL and Liu KJ: Effects of glucose
concentration on redox status in rat primary cortical neurons under
hypoxia. Neurosci Lett. 410:57–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adam W, Kurz A and Saha-Möller CR:
Peroxidase-catalyzed oxidative damage of DNA and 2′-deoxyguanosine
by model compounds of lipid hydroperoxides: involvement of peroxyl
radicals. Chem Res Toxicol. 13:1199–1207. 2000.
|
|
33
|
Hong JH, Kim MJ, Park MR, Kwag OG, Lee IS,
Byun BH, Lee SC, Lee KB and Rhee SJ: Effects of vitamin E on
oxidative stress and membrane fluidity in brain of
streptozotocin-induced diabetic rats. Clin Chim Acta. 340:107–115.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sima AAF and Li ZG: The effect of
C-peptide on cognitive dysfunction and hippocampal apoptosis in
type 1 diabetic rats. Diabetes. 54:1497–1505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stevens MJ, Zhang W, Li F and Sima AA:
C-peptide corrects endoneurial blood flow but not oxidative stress
in type 1 BB/Wor rats. Am J Physiol Endocrinol Metab.
287:E497–E505. 2004. View Article : Google Scholar : PubMed/NCBI
|