Introduction
Myelodysplastic syndrome (MDS) encompasses a diverse
group of neoplastic bone marrow disorders, characterized by
abnormal cellular morphology and defects in the normal
differentiation and proliferation of hematopoietic precursors.
Various subtypes of MDS have a risk of transformation to acute
myeloid leukemia (AML) (1,2).
Recently, the chromosome aberrations in MDS have been elucidated;
interstitial deletion of chromosome 5 is the most common
cytogenetic abnormality in MDS (3). The del(5q) occurs either in
isolation or together with another karyotypic abnormality (called
complex karyotype). Isolated deletion of a segment of the long arm
of chromosome 5q has been associated with a relatively favorable
prognosis and a perceived low risk of progression to AML. In
contrast, the complex karyotype has been associated with a poor
prognosis and high risk of AML (4). In addition, a common deletion region
(CDR) exists in the region of 5q31-32 (5). Research on the relationship between
the CDR gene and cell biology can facilitate further understand of
the pathogenesis of MDS/AML.
Secreted protein acidic and rich in cysteine (SPARC)
has been described as a counter-adhesive, matricellular protein
exhibiting a diversity of biological functions associated with
morphogenesis, remodeling, cellular migration and proliferation
(6). The expression of SPARC is
varied in different types of cancers, and its role in tumorigenesis
appears complex and is not well defined (7). For example, SPARC expression is high
in breast and clorectal cancer (8,9),
but low in prostate and lung cancer (10,11). In addition, haploinsufficiency of
SPARC in the 5q- syndrome increases the adhesion of hematopoietic
stem cells to supporting stromal cells and provides a clonal
advantage (12). In 5q- syndrome,
elevated SPARC expression inhibits the growth of tumor cells, while
its low expression leads to tumor development. Therefore,
lenalidomide has been used to treat patients with the 5q- syndrome
via elevating SPARC expression (13).
MDS primarily affects the elderly and the prevalence
is increasing; a majority of subtypes of MDS transform to AML
(14). However, it is not clear
whether SPARC plays a crucial role in MDS/AML. Therefore, exploring
the function of genes in chromosome 5q, particularly in the CDR has
important clinical value in transformed MDS/AML. Our study shed
light on the pathogenesis of MDS/AML, and investigating the
function of SPARC in transformed MDS/AML may provide new clues in
the treatment and research of the transformation of MDS into
AML.
In the present study, firstly we detected the
expression of SPARC in SKM-1 cells. SKM-1 is a cell line derived
from a male patient with AML transformed from MDS (15). We detected SPARC expression in
SKM-1 cells by immunofluorescence. Then we employed
lentiviral-mediated RNA interference to knock down SPARC in SKM-1
cells in order to determine its effects on the proliferation,
apoptosis, and the expression of p53 and apoptosis-related genes in
MDS/AML.
Materials and methods
Cell culture
The MDS/AML cell line SKM-1 was kindly provided by
Professor Jianfeng Zhou at Tongji Medical College, Huazhong
University of Science and Technology and was cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (Gibco BRL, Grand
Island, NY, USA) in a humid atmosphere at 37°C with 5%
CO2.
Immunofluorescence
SKM-1 cells (106 cells/ml) were incubated
with polysorbate at 37°C for 1 h, washed with PBS three times and
then incubated with 0.2% Triton for 1 h. Next the cells were
incubated with the primary antibody (mouse anti-human SPARC
monoclonal antibody; 1:1000; Abcam, USA) overnight, washed with PBS
three times and incubated with a fluorescent secondary antibody
(green fluorescent antibody; 1:1000; 2 h). After washing with PBS,
the cells were observed under a fluorescence microscope.
Construction of the SPARC recombinant
lentivirus
A lentiviral vector containing a CMV-driven GFP
reporter and a U6 promoter upstream of the cloning sites
(AgeI and EcoRI) was used for cloning small hairpin
RNAs (shRNAs). The target sequence for SPARC was
5′-CCAGGTGGAAGTAGGAGAATT-3′, and the NC-GFP-LV sequence was
5′-TTCTCCGAACGTGTCACGT-3′. SPARC cDNA was amplified by reverse
transcription-polymerase chain reaction (RT-PCR) and subcloned into
a lentiviral vector RNAi-LV to construct a recombinant lentiviral
vector named SPARC-RNAi-LV. According to the manual for packaging
of the retrovirus in 293 cells, Lipofectamine 2000, pHelper 1.0 and
pHelper 2.0 (Jikai Co., Shanghai, China) were used. The
supernatants were then collected for determination of the viral
titer.
RNA interference
The cells were cultured in 6-well plates
(106 cells/ml) and infected with the lentivirus at a
multiplicity of infection (MOI) of 100 for 10 h. The medium was
replaced with basic medium. After 4 days, the cells were observed
under a fluorescence microscope to evaluate the infection
efficiency.
RT-PCR
Total RNA of each group was extracted from the cells
using RNAiso Plus (Takara Biotechnology, Dalian, China) and used
for cDNA synthesis. Each well (25 μl reaction volume) contained
12.5 μl Taq (Takara Biotechnology), 1 μl of each primer (10
μmol/l), 2 μl cDNA template (50 ng/μl) and 8.5 μl ddH2O.
PCR primers are listed in Table
I. The cycling parameters were as follows: 97°C for 5 min, then
30 cycles of 97°C for 1 min, 56°C for 30 sec, and 72°C for 30 sec,
and a final extension at 72°C for 7 min. All primers were designed
using Primer 5 software and synthesized by Takara Biotechnology.
RT-PCR results were analyzed using Quantity One software (Bio-Rad,
Hercules, CA, USA).
 | Table IRT-PCR primers. |
Table I
RT-PCR primers.
| Genes | Forward and reverse
primers | Product length
(bp) |
|---|
| SPARC | F:
5′-ACCTGTCACTGTCTTGTACCCTTGT-3′
R: 5′-CGGCGTTTGGAGTGGTAGAA-3′ | 300 |
| Actin | F:
5′-GTGATCTTGGACTTGATATTGGTG-3′
R: 5′-GTCCATACCCAAGGCATCCTG-3′ | 594 |
| P53 | F:
5′-CCTCCTCAGCATCTTATCCGA-3′
R: 5′-GTGCTCGCTTAGTGCTCCCT-3′ | 312 |
| Caspase-3 | F:
5′-GGTTCTGGAGGATTTGGTGATG-3′
R: 5′-GACGCCGCAACTTCTCACAG-3′ | 320 |
| Caspase-9 | F:
5′-TGTGGTGGGGAGCAGAAAGA-3′
R: 5′-TTCACCGAAACAGCATTAGCG-3′ | 323 |
| Fas | F:
5′-CAATTCTGCCATAAGCCCTGT-3′
R: 5′-CTTGGTGTTGCTGGTGAGTGT-3′ | 324 |
Real-time PCR
Quantitative real-time PCR was performed using an
ABI PRISM 7500 real-time PCR system (Applied Biosystems, Foster
City, CA, USA). The total reaction system was 25 μl: SYBR Premix Ex
Taq II (12.5 μl), 1 μl of each primer (10 μmol/l) and 2 μl cDNA
template (50 ng/μl), and ddH2O (8.5 μl). The primers
were designed using Primer 5 software and synthesized by Takara
Biotechnology, and are listed in Table II.
 | Table IIReal-time PCR primers used in this
study. |
Table II
Real-time PCR primers used in this
study.
| Genes | Forward and reverse
primers | Product length
(bp) |
|---|
| SPARC | F:
5′-GGCCTGGATCTTTCTCCTT-3′
R: 5′-CCCACAGATACCTCACCTC-3′ | 126 |
| Actin | F:
5′-CCACGAAACTACCTTCAACTAA-3′
R: 5′-GTGATCTCCTTCTGCATCCTGT-3′ | 132 |
| P53 | F:
5′-CTTTGAGGTGCGTGTTTGTG-3′
R: 5′-GTTGGGCAGTGCTCGCTTAG-3′ | 124 |
| Caspase-3 | F:
5′-GGCATTGAGACAGACAGTGGTG-3′
R: 5′-GGCACAAAGCGACTGGATGA-3′ | 153 |
| Caspase-9 | F:
5′-GGTTCTGGAGGATTTGGTGATG-3′
R: 5′-GACGCCGCAACTTCTCACAG-3′ | 184 |
| Fas | F:
5′-ATCTGGACCCTCCTACCTCTGG-3′
R: 5′-GATGCAGGCCTTCCAAGTTCT-3′ | 151 |
Western blot analysis
Cells were lysed in 100 μl RIPA buffer supplemented
with 1 μl PMSF, and the protein concentration of the lysate was
determined using a BCA protein assay kit (Beyotime, Beijing,
China). A total of 50 μg of protein per lane was separated by
SDS-PAGE and transferred to PVDF membranes. The membranes were
blocked with 5% skimmed milk for 2 h, and then incubated overnight
at 4°C with the primary antibodies (mouse anti-human or rabbit
anti-human monoclonal antibody; 1:1000; Abcam) for SPARC, p53,
caspase-9, caspase-3 and Fas, followed by incubation with
HRP-conjugated goat anti-rabbit or HRP-conjugated goat anti-mouse
(1:1000) for 1 h at 37°C. Membranes were washed four times with
TBST and developed using the ECL method. Band intensity was
analyzed using Quantity One software.
Cell proliferation assay
Cell proliferation was determined using an MTS
assay. Cells were seeded at 500 cells/well into a 96-well plate.
For the MTS assay, 20 μl of MTS (Promega, Madison, WI, USA) was
added to each well and incubated at 37°C at 95% humidity and 5%
CO2 for 1 h. The optical density at 490 nm was read with
an enzyme immunoassay instrument (Bio-Rad).
Annexin V and 7-AAD assay of
apoptosis
Cells were collected (106 cells/ml) and
washed twice with PBS, suspended in 200 μl binding buffer, 1 μl
Annexin V-PE and 5 μl 7-AAD (KeyGen Biotech, Shanghai, China) for
15 min in the dark. The apoptotic cells were determined by flow
cytometry with CellQuest software (BD Biosciences, San Jose, CA,
USA).
Cell cycle distribution
Cells were collected and fixed with 70% anhydrous
ethanol at 4°C overnight, and then incubated with RNase for 1 h at
37°C, followed by incubation with 100 μg/ml propidium iodide (PI)
at room temperature for 30 min. The cell cycle profiles were
analyzed using Multicycle software (USA).
Statistical analysis
All results are expressed as means ± SE and were
analyzed by GraphPad Prism 5 software. Each experiment was repeated
three times. Comparison among groups was analyzed by one-way ANOVA.
A P-value of <0.05 was considered to indicate a statistically
significant result.
Results
Knockdown of SPARC by lentiviral-mediated
RNAi in SKM-1 cells
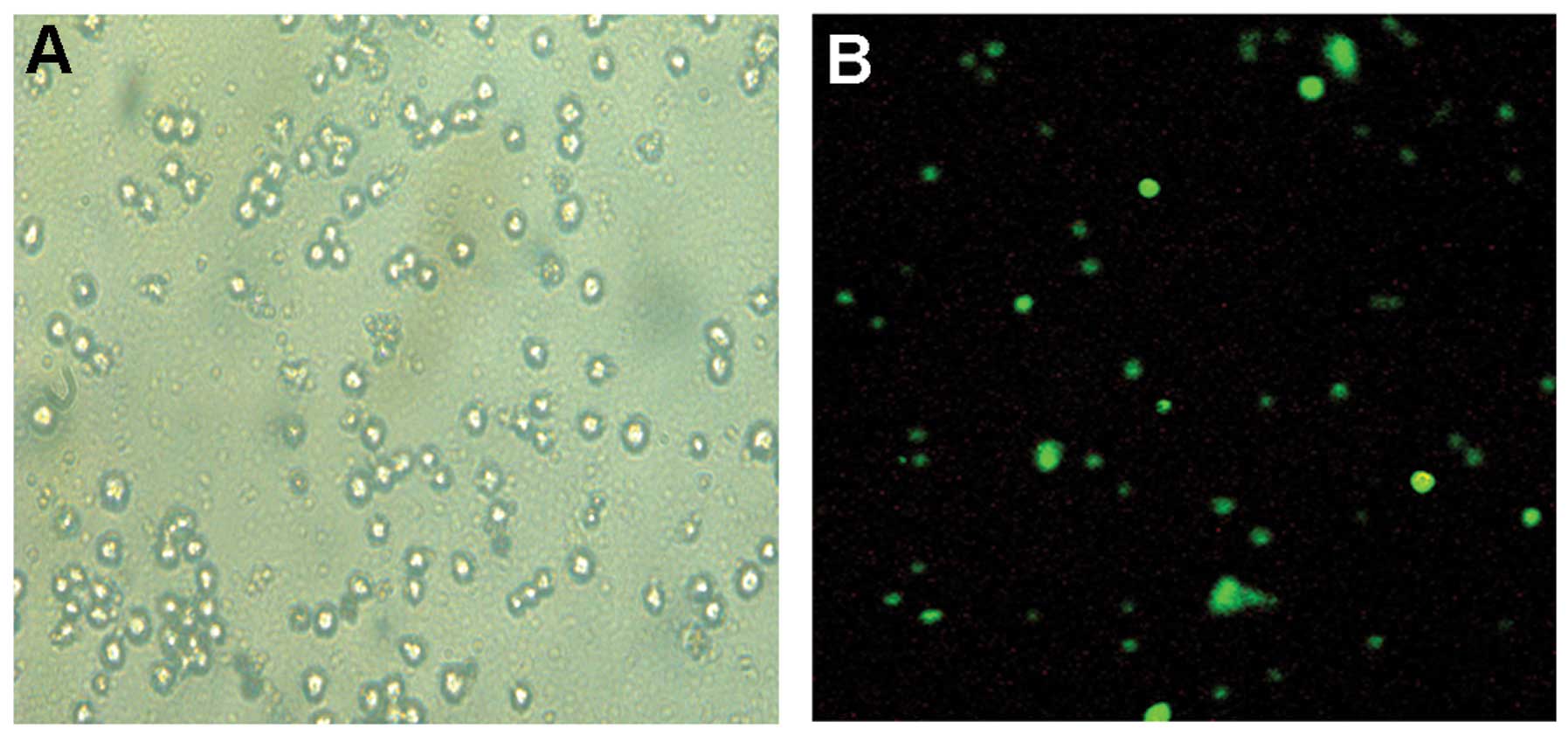
The immunofluorescence analysis demonstrated that
SPARC was abundantly expressed in the SKM-1 cells (Fig. 1). Next we constructed the SPARC
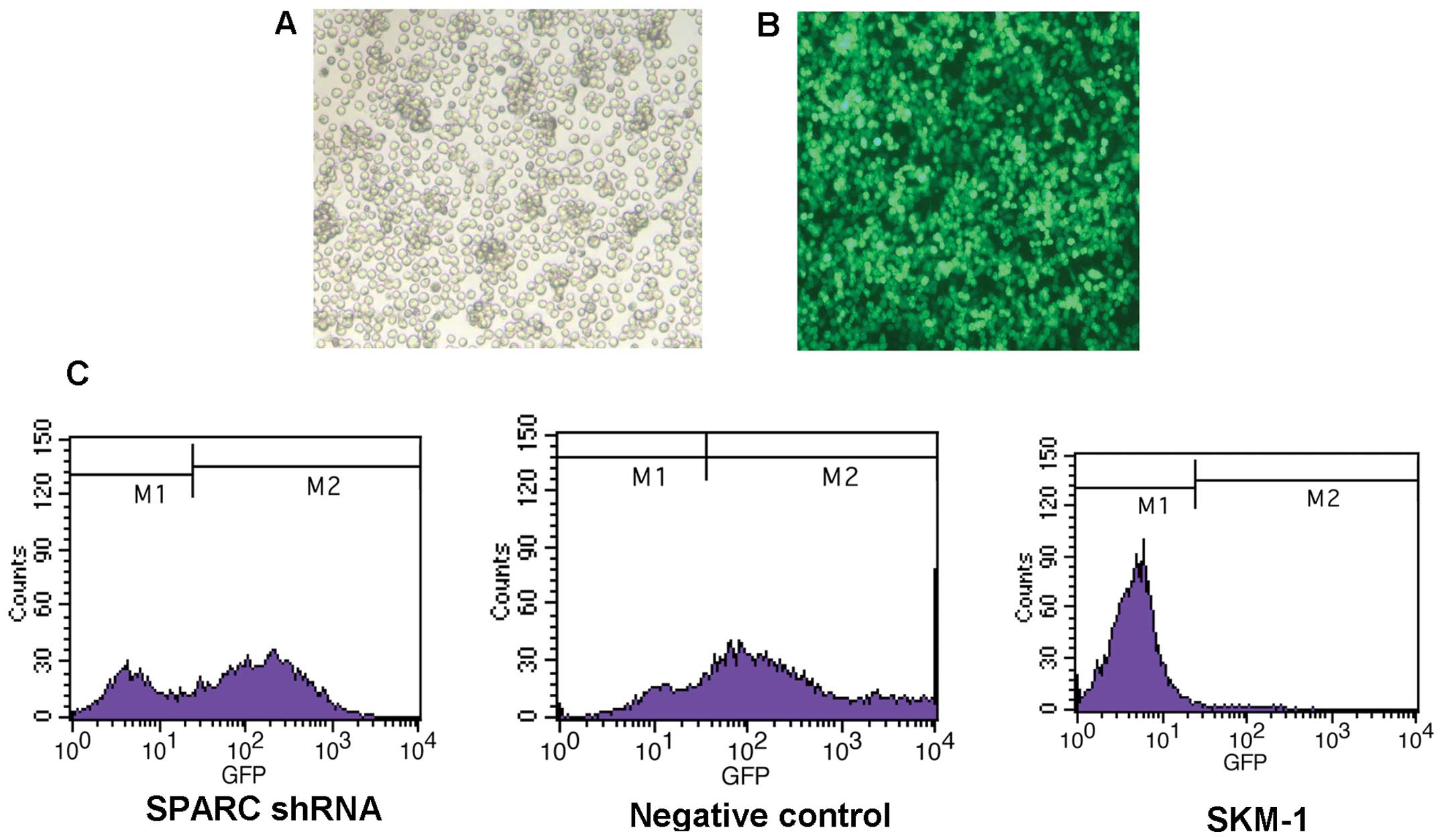
shRNA lentiviral vector and infected the SKM-1 cells with SPARC
shRNA. Following infection, we found that >60% of cells were
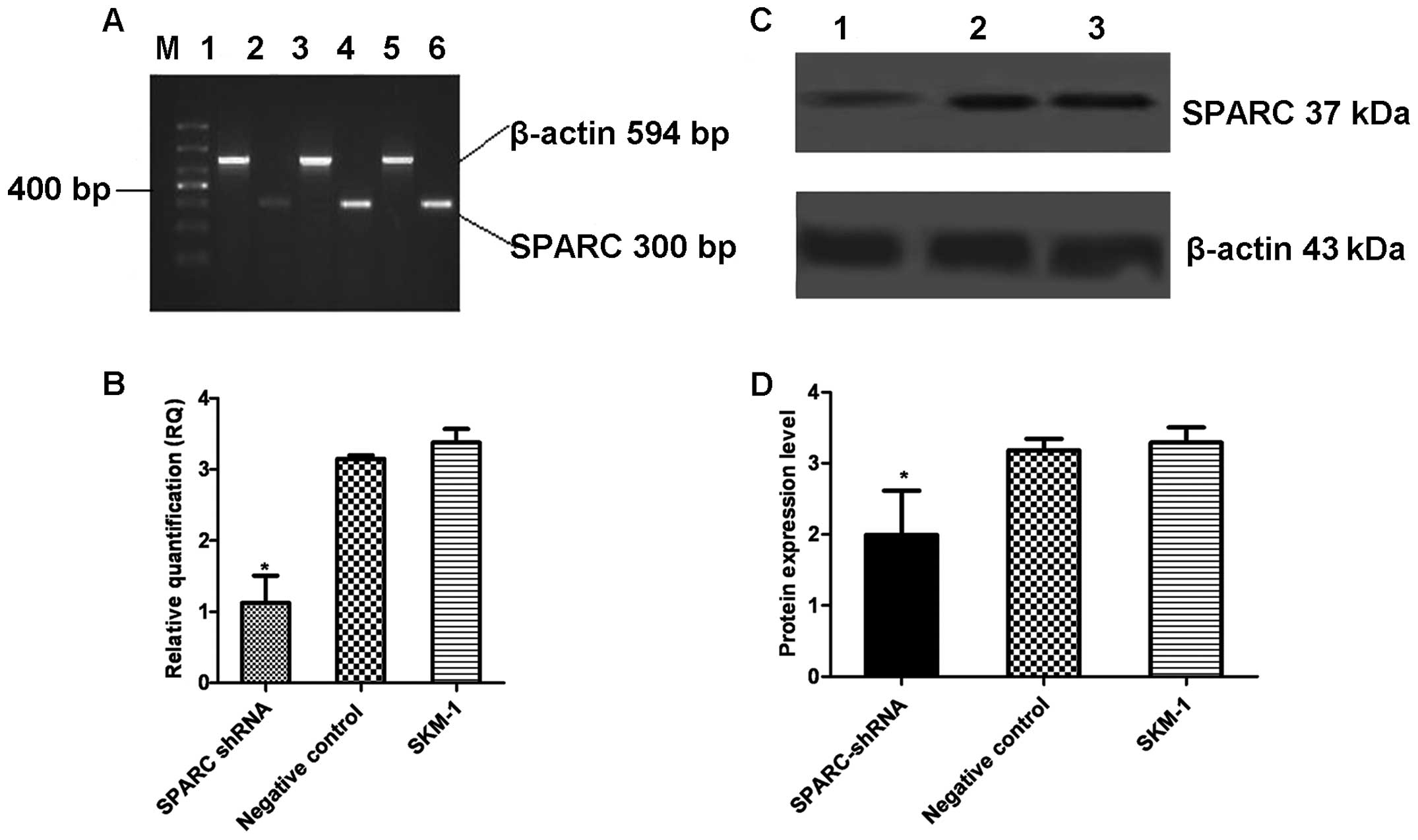
GFP-positive, indicating high infection efficiency (Fig. 2). RT-PCR, real-time PCR and
western blot analysis showed that SPARC shRNA significantly reduced
the expression of SPARC at both the mRNA and protein levels
(Fig. 3).
Knockdown of SPARC inhibits MDS/AML cell
proliferation
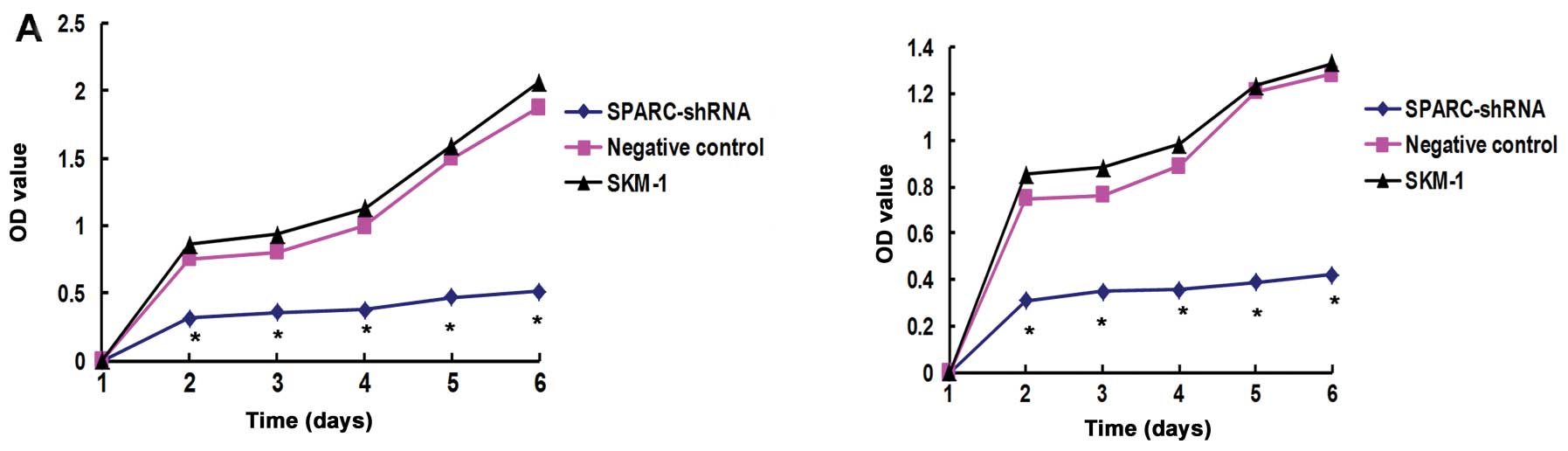
To evaluate the effects of SPARC knockdown on the
proliferation of SKM-1 cells, we performed an MTS assay. After 2
days, the OD value of cells treated with SPARC shRNA decreased to
0.5–0.7 when compared to the OD values in the negative control and
SKM-1 cells. The results showed that knockdown of SPARC inhibited
the proliferation of SKM-1 cells (Fig. 4A).
Knockdown of SPARC induces cell cycle
arrest at the G1/G0 phase and apoptosis in MDS/AML cells
To elucidate the mechanism by which knockdown of
SPARC inhibits the proliferation of MDS/AML cells, we performed
flow cytometric analysis to evaluate the cell cycle. As shown in
Fig. 4D, 40–50% of SKM-1 cells
infected with SPARC shRNA were arrested at the G1 or G1/G0 phase
(P<0.05) compared with 10–20% in the negative control and SKM-1
cells (Fig. 4E). In addition, we
performed flow cytometry analysis to evaluate apoptosis and found
that ~20% cells were induced to apoptosis after infection with
SPARC shRNA. The percentage of apoptotic cells was much higher in
the SPARC shRNA-infected cells than these percentages in the other
two groups (P<0.05) (Fig. 4B and
C). Taken together, these data indicate that knockdown of SPARC
inhibits the proliferation of MDS/AML cells by inducing cell cycle
arrest and apoptosis.
Knockdown of SPARC leads to upregulation
of expression of apoptotic factors in MDS/AML cells
To understand the molecular mechanism by which SPARC
regulates apoptosis, we examined the expression of apoptotic
factors such as p53, caspase-3, caspase-9 and Fas in MDS/AML cells
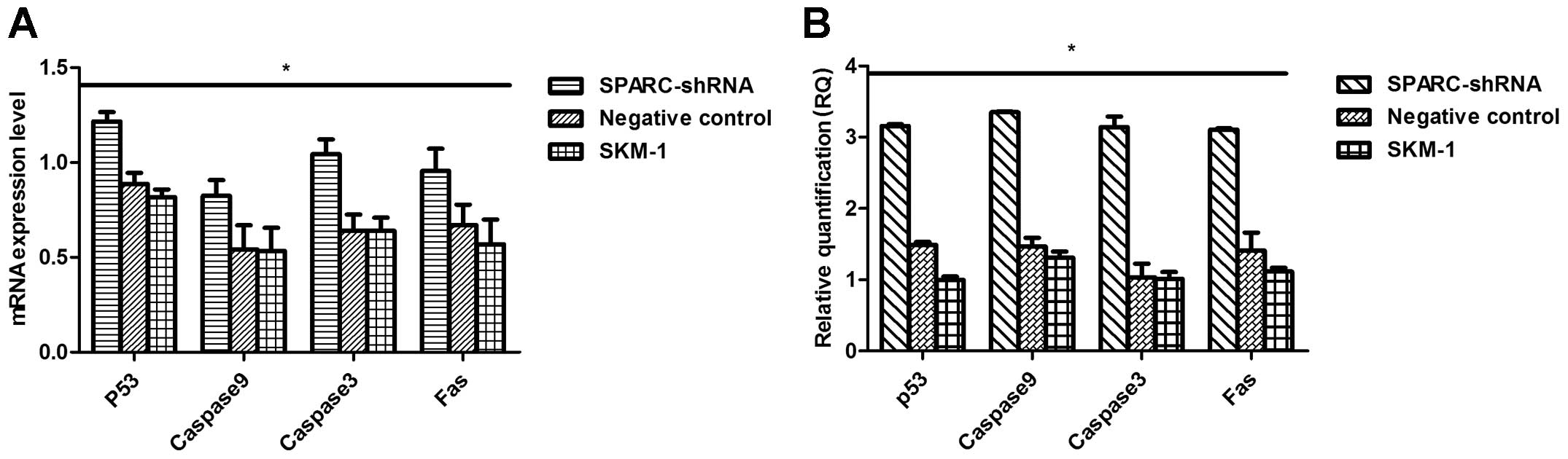
with SPARC knockdown. RT-PCR, real-time PCR and western blot
analysis showed that the expression of p53, caspase-3, caspase-9
and Fas at both the mRNA and protein levels was obviously higher in
the SPARC shRNA-infected cells than levels in the other two groups
(Fig. 5). These results suggest
that SPARC inhibits MDS/AML cell apoptosis by downregulating the
expression of p53, caspase-3, caspase-9 and Fas.
Discussion
In the present study, we aimed to investigate the
role of SPARC in the transformation of MDS into AML. Thus we
selected a human MDS/AML cell line, SKM-1, with complex abnormal
karyotype including del(9q), i(17q) and t(17p) (16,17). Following knockdown of SPARC, a
del(5q)-like model which is an ideal model for this study was
obtained. First, abundant expression of SPARC in SKM-1 cells was
confirmed by immunofluorescence. Then we used lentiviral-mediated
shRNA to knockdown the expression of SPARC in SKM-1 cells.
In order to better understand the function of SPARC
in SKM-1 cells, we analyzed cell proliferation and apoptosis. By
MTS assay, knockdown of SPARC suppressed MDS/AML cell
proliferation. Flow cytometric analysis indicated that knockdown of
SPARC increased the number of cells in the G1/G0 phase, while
decreasing the number of cells in the S phase, revealing G1/G0
phase arrest. In addition, we found increased apoptosis in the
MDS/AML cells after knockdown of SPARC. SPARC is a potential
tumor-suppressor gene in 5q- syndrome, and is thought to have
anti-adhesive effects, thereby promoting apoptosis. It also
regulates cellular migration and proliferation. Based on the
results of the MTS assay and flow cytometry in our study, decreased
expression of SPARC pointed to a contrary conclusion. SPARC was
found to be differentially expressed in tumors and its surrounding
stroma in various types of cancers in comparison to normal tissue.
To understand the mechanism of SPARC in the proliferation and
apoptosis of transformed MDS/AML, we detected the expression of
p53, caspase-3, caspase-9 and Fas in the SPARC shRNA-infected cells
and in the other groups.
Recently, expression of SPARC was correlated with
p53 in MDS. The haploinsufficiency of SPARC induced the increased
expression of p53 in the 5q- syndrome (18). p53 is a well-known tumor
suppressor that impedes the cell cycle at the G1-S checkpoint
(19). To understand the
association between p53 and SPARC which regulates the proliferation
and apoptosis of MDS/AML cells, we detected the expression of p53
in SPARC shRNA-infected cells, negative control cells and SKM-1
cells. We found that knockdown of SPARC increased the expression of
p53. Thus, we believe that cell growth regulation by SPARC is
related to the expression of p53 in MDS/AML. These results are
generally in agreement with previous studies concerning ovarian
cancer (20), glioma (21), gastric cancer (22) and melanoma cells (23) and the relationship of p53.
Furthermore, since SPARC knockdown induced cell
apoptosis and reduced proliferation, while p53 expression was
increased, we assumed that the intrinsic pathway of apoptosis
should be regulated by apoptosis-related genes. Therefore, we
detected the expression of caspase-9, caspase-3 and Fas which are
common apoptosis-related genes. Upon apoptosome formation,
caspase-9 becomes catalytically active and acts on downstream
target caspase-3 (24,25). In the present study, we found that
knockdown of SPARC increased the expression of caspase-9 and
caspase-3. These results suggest that knockdown of SPARC activates
caspase-9 and the downstream molecule, caspase-3. In addition, Fas
is an upstream factor in the apoptosis pathway (26). We found that its expression was
increased in SKM-1 cells following SPARC knockdown. Programmed cell
death, apoptosis, is vital for normal development and cell
homeostasis. In brief, SPARC plays an important role in MDS/AML
cell proliferation by controlling cell-related apoptosis genes, but
whether these signaling pathway genes have a relationship with
transformed MDS/AML, and whether these increases are contributing
factors to the transformation of MDS into AML require further
study. These results suggest that the regulation of the expression
of these apoptosis-related proteins may be used to prevent the
transformation of MDS to AML and cell proliferation.
In conclusion, we present evidence that SPARC
regulates SKM-1 cell proliferation and apoptosis, which is
associated with the expression of p53. Knockdown of SPARC inhibits
cell proliferation, induces cell cycle arrest and apoptosis and
activates the p53 pathway. These data suggest that SPARC may act as
an oncogene in transformed MDS/AML while it acts as a
tumor-suppressor in 5q- syndrome. In combination with the results
of our previous study (27), 5q-
syndrome has a favorable prognosis and this may be related to the
relative genetic stability as evidenced by an absence of other
cytogenetic abnormalities and a failure to find other
leukemia-associated mutations in the 5q- syndrome group in contrast
to other MDS patients with the del(5q). Thus, we conclude that
high-risk MDS with del(5q) may possess a totally different
pathophysiology with the 5q- syndrome which may explain the reason
for the transformation to AML resulting in poor prognosis. These
findings may be of pathogenetic importance in the MDS
transformation into AML and suggest that SPARC is a potential
therapeutic target for MDS/AML.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30971277 and 81250034) and
the Chongqing Natural Science Foundation (grant no. CSTC
2009BB5070).
References
|
1
|
Nimer SD: Myelodysplastic syndromes.
Blood. 111:4841–4851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghariani I, Braham N, Hassine M and Kortas
M: Myelodysplastic syndrome classification. Ann Biol Clin.
71:139–144. 2013.(In French).
|
|
3
|
Jädersten M and Hellström-Lindberg E: New
clues to the molecular pathogenesis of myelodysplastic syndromes.
Exp Cell Res. 316:1390–1396. 2010.PubMed/NCBI
|
|
4
|
Davids MS and Steensma DP: The molecular
pathogenesis of myelodysplastic syndromes. Cancer Biol Ther.
10:309–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giagounidis AA, Germing U, Wainscoat JS,
et al: The 5q- syndrome. Hematology. 9:271–277. 2004.
|
|
6
|
Swaminathan SS, Oh DJ, Kang MH, et al:
Secreted protein acidic and rich in cysteine (SPARC)-null mice
exhibit more uniform outflow. Invest Ophthalmol Vis Sci.
54:2035–2047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rotllant J, Liu D, Yan YL, et al: Sparc
(osteonectin) functions in morphogenesis of the pharyngeal skeleton
and inner ear. Matrix Biol. 27:561–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsiao YH, Lien HC, Hwa HL, et al: SPARC
(osteonectin) in breast tumors of different histologic types and
its role in the outcome of invasive ductal carcinoma. Breast J.
16:305–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiese AH, Auer J, Lassmann S, et al:
Identification of gene signatures for invasive colorectal tumor
cells. Cancer Detect Prev. 31:282–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Said N, Frierson HF Jr, Chernauskas D, et
al: The role of SPARC in the TRAMP model of prostate carcinogenesis
and progression. Oncogene. 28:3487–3498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isler SG, Ludwig CU, Chiquet-Ehrismann R,
et al: Evidence for transcriptional repression of SPARC-like 1, a
gene downregulated in human lung tumors. Int J Oncol. 25:1073–1079.
2004.PubMed/NCBI
|
|
12
|
Tohyama K: 5q- syndrome, MDS with isolated
del(5q). Nihon Rinsho. 2:362–366. 2012.(In Japanese).
|
|
13
|
Duong VH, Komrokji RS and List AF:
Efficacy and safety of lenalidomide in patients with
myelodysplastic syndrome with chromosome 5q deletion. Ther Adv
Hematol. 3:105–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Usmani SZ, Sawyer J, Rosenthal A, et al:
Risk factors for MDS and acute leukemia following total therapy 2
and 3 for multiple myeloma. Blood. 121:4753–4757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakagawa T, Matozaki S, Murayama T, et al:
Establishment of a leukaemic cell line from a patient with
acquisition of chromosomal abnormalities during disease progression
in myelodysplastic syndrome. Br J Haematol. 85:469–476. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakagawa T and Matozaki S: The SKM-1
leukemic cell line established from a patient with progression to
myelomonocytic leukemia in myelodysplastic syndrome (MDS) -
contribution to better understanding of MDS. Leuk Lymphoma.
17:335–339. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimura S, Kuramoto K, Homan J, et al:
Antiproliferative and antitumor effects of azacitidine against the
human myelodysplastic syndrome cell line SKM-1. Anticancer Res.
32:795–798. 2012.PubMed/NCBI
|
|
18
|
Barlow JL, Drynan LF, Hewett DR, et al: A
p53-dependent mechanism underlies macrocytic anemia in a mouse
model of human 5q- syndrome. Nat Med. 16:59–66. 2010. View Article : Google Scholar
|
|
19
|
Liebermann DA, Hoffman B and Vesely D: p53
induced growth arrest versus apoptosis and its modulation by
survival cytokines. Cell Cycle. 6:166–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yiu GK, Chan WY, Ng SW, et al: SPARC
(secreted protein acidic and rich in cysteine) induces apoptosis in
ovarian cancer cells. Am J Pathol. 159:609–622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seno T, Harada H, Kohno S, et al:
Downregulation of SPARC expression inhibits cell migration and
invasion in malignant gliomas. Int J Oncol. 34:707–715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin J, Chen G, Liu Y, et al:
Downregulation of SPARC expression decreases gastric cancer
cellular invasion and survival. J Exp Clin Cancer Res. 29:592010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Horie K, Tsuchihara M and Nakatsura T:
Silencing of secreted protein acidic and rich in cysteine inhibits
the growth of human melanoma cells with G arrest induction. Cancer
Sci. 101:913–919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khalil H, Peltzer N, Walicki J, et al:
Caspase-3 protects stressed organs against cell death. Mol Cell
Biol. 32:4523–4533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, et al: Caspase-9, caspase-3 and caspase-7 have distinct
roles during intrinsic apoptosis. BMC Cell Biol. 14:322013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Wang Y, Wang Y, et al: Distinct
different sensitivity of Treg and Th17 cells to Fas-mediated
apoptosis signaling in patients with acute coronary syndrome. Int J
Clin Exp Pathol. 6:297–307. 2013.PubMed/NCBI
|
|
27
|
Wang L, Fidler C, Nadig N, et al:
Genome-wide analysis of copy number changes and loss of
heterozygosity in myelodysplastic syndrome with del(5q) using
high-density single nucleotide polymorphism arrays. Haematologica.
93:994–1000. 2008. View Article : Google Scholar
|