Introduction
According to recent data, the number of worldwide
cancer cases is determined to increase by 75% over the next two
decades (1). In Korea, the
incidence of cancer cases has shown an annual increase of 3.3% from
1999 to 2010. Notably, colorectal cancer (CRC) incidence and
mortality has been increasing rapidly in Korea over the past few
decades. CRC is the third most common cancer with age-standardized
incidences of 48.6/100,000 individuals for men and 25.3/100,000
individuals for women in 2010; since then, these incidences have
increased by 5.9%/year in both genders (2). Surgery was initially seen as a
curative treatment and has now became a conventional therapy for
CRC. However, the recurrence rate is as high as 50% for patients
treated with conventional therapy and this is a major issue.
Chemotherapeutic regimens, such as FOLFOX [5-fluorouracil (FU) +
oxaliplatin + leucovorin] or FOLFIRI (5-FU + leucovorin +
irinotecan), neoadjuvant chemotherapy with bevacizumab, an antibody
that targets vascular endothelial growth factor, and/or cetuximab,
an antibody that targets epidermal growth factor receptor, have
been developed as a strategy to combat the recurrence of CRC
(3). In spite of these
combinations of multiple chemotherapeutic agents, patients develop
resistance to such treatments, thus novel strategies to replace or
complement current therapies are urgently required.
Apoptosis, the major form of cell suicide, is
critical to various physiological processes and the maintenance of
homeostasis in multicellular organisms. Hence, it is clear that the
dysregulation of apoptosis plays an important role in the
pathogenesis of several of human diseases, including cancer
(4). Over the years, accumulating
evidence clearly indicates that anticancer drugs are able to induce
apoptosis and that this process is involved in the mediation of
their cytotoxic effects. Furthermore, the selective regulation of
the apoptotic pathway in cancer cells, and not in normal cells has
been the goal of cancer researchers (5).
Over the past few decades, agents derived from
medicinal plants have gained a great deal of attention from
researchers and clinicians due to their safety, efficacy and
availability. In addition, secondary metabolites and structural
derivatives from natural sources have been applied towards treating
cancer for the past five decades. At least 40% of all available
anticancer drugs between 1940 and 2002 have originated from natural
sources or mimics of natural agents (6). Although a number of natural agents
have shown an ability to prevent and treat cancer, their molecular
mechanisms of action have been poorly defined. One such agent is
corosolic acid (CA), which was originally isolated from the fruits
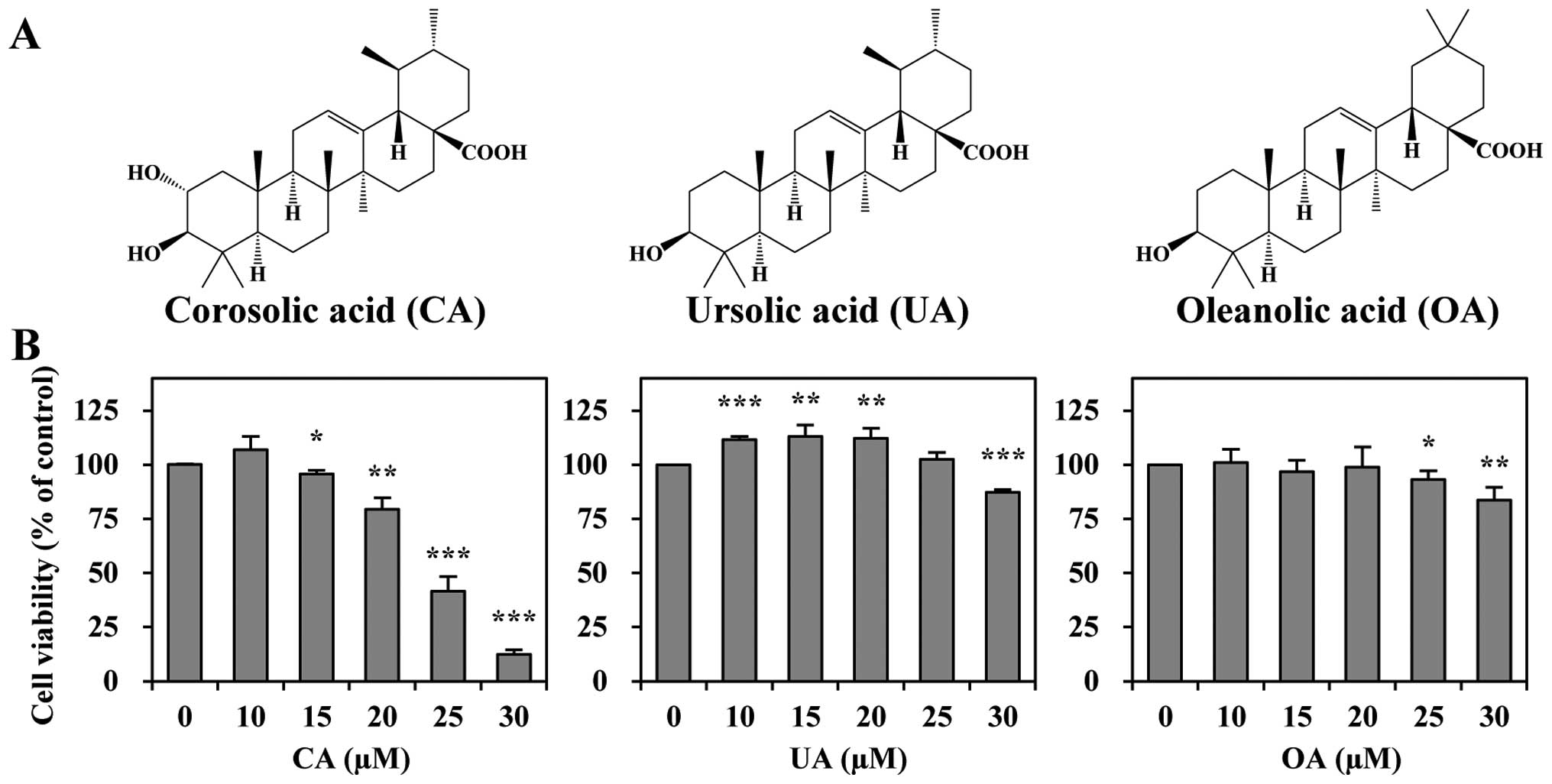
of Crataegus pinnatifida var. psilosa (7). CA (Fig. 1A) is an ursane-type pentacyclic
triterpene, which exists in abundance in the plant kingdom. It has
been found in various plants, including Schisandra chinensis
(8), Eriobotrya japonica
(known as loquat) (9),
Lagerstroemia speciosa L. (known as Banaba) (10), Orthosiphon stamineus
(11) and Weigela
subsessilis (12). CA has
been shown to exert numerous biological activities, such as
anti-diabetic (10,13), antioxidant (14), anti-atherosclerotic (15), cholesterol-reducing (16), anti-inflammatory (17,18) and anticancer activities (9,12,19–23). Previous studies have reported that
CA suppresses the proliferation of a wide variety of tumor cells,
including sarcoma (20),
glioblastoma (22), osteosarcoma
(21), leukemia (7,9),
as well as gastric (12,24), cervical (23) and lung cancer cells (19).
The mechanisms through which CA exerts these effects
are not yet fully understood. However, this triterpene has been
known to target numerous cell signaling molecules, such as nuclear
factor-κB (15), signal
transducer and activator of transcription-3 (22), AMP-activated protein
kinase-mammalian target of rapamycin (12), epidermal growth factor receptor
2/neu (24), protein kinase C
(7), GLUT4 (25), glycogen phosphorylase (26), phosphatidylinositol 3-kinase
(27), as well as caspases
(21).
Although CA has been shown to induce apoptosis, and
suppress cancer cell growth and metastasis in several human cancer
cell lines (7,20–22,24), its potential anticancer effects
and its mechanisms of action in CRC remain unelucidated. Therefore,
in the current study, we investigated whether CA induces apoptosis
in colon cancer cells. To our knowledge, our results indicate for
the first time that CA exerts anticancer effects through the
suppression of cell proliferation and the induction of apoptosis in
CRC.
Materials and methods
Chemicals
CA was purchased from LKT Laboratories (St. Paul,
MN, USA). Oleanolic acid (OA) was obtained from Cayman Chemical Co.
(Ann Arbor, MI, USA) and ursolic acid (UA) was from Sigma-Aldrich
(St. Louis, MO, USA). A 50 mM solution of the triterpenoids was
prepared in dimethyl sulfoxide and then diluted as needed in cell
culture medium. 3-(4,5-Dimethylthiazol-2-yl)-2,5-dipheny
tetrazolium bromide (MTT) was obtained from Amresco (Solon, OH,
USA). Various primary antibodies and z-VAD-FMK, a broad spectrum
caspase inhibitor, were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Mouse monoclonal antibody against
β-actin and Hoechst 33342 were obtained from Sigma-Aldrich.
RPMI-1640, fetal bovine serum (FBS), and penicillin-streptomycin
were purchased from HyClone (Logan, UT, USA).
Cell culture and cell viability
assay
The human CRC cell line, HCT116, which was obtained
from the American Type Culture Collection (Manassas, VA, USA), was
maintained at 37°C in a humidified condition of 95% air and 5%
CO2 in RPMI-1640 medium supplemented with 10% FBS and 1%
(v/v) penicillin-streptomycin. Cell viability was measured using
MTT, which is based on the conversion of MTT to MTT-formazan by
mitochondrial enzymes.
Nuclear staining with Hoechst 33342
The control and treated cells were washed with
phosphate-buffered saline (PBS) and stained with 4 μg/ml Hoechst
33342 for 20 min at room temperature. Subsequently, the cells were
washed with PBS and observed under a fluorescence microscope.
Western blot analysis
To determine the levels of protein expression, we
prepared cell extracts and performed western blot analysis. In
brief, cell extracts containing equal amounts of proteins were
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto PVDF membranes. The
membranes were probed with the desired primary antibodies and then
with horseradish peroxidase-conjugated secondary antibody. Signals
were detected by enhanced chemiluminescence (ECL) reagent (GE
Healthcare, Piscataway, NJ, USA).
Cell cycle analysis
In order to measure the number of cells in the
sub-G1 phase, cell cycle analysis was performed. The cells were
seeded in 6-well plates at 3×105 cells/well and allowed
to attach overnight. The cells were then treated with various
concentrations of CA for a period of 24 h, trypsinized, washed with
PBS and then fixed in 70% ethanol at −20°C overnight. Prior to
analyses, the cells were washed with PBS, suspended in cold
propidium iodide (PI; Sigma-Aldrich) solution (50 μ/ml in PBS), and
incubated at room temperature in the dark for 30 min. Flow
cytometry was then performed using a Cytomic FC500 flow cytometer
(Beckman Coulter, Istanbul, Turkey).
Annexin V/PI staining
In order to determine the number of apoptotic cells
following treatment with CA, and double staining with Annexin V and
PI was conducted using the BD Pharmingen FITC Annexin V Apoptosis
Detection kit (BD Biosciences, San Diego, CA, USA) according to the
manufacturer’s instructions. Flow cytometric analyses were
performed on a Cytomic FC500 flow cytometer (Beckman Coulter).
Caspase activity
To determine the effects of CA on caspase-mediated
cell death, a caspase activity assay using synthetic tetrapeptides
[Asp-Glu-Val-Asp (DEAD) for caspase-3; Ile-Glu-Thr-Asp (IETD) for
caspase-8; and Leu-Glu-His-Asp (LEHD) for caspase-9] labeled with
p-nitroaniline (pNA) as the substrate was conducted in accordance
with manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA).
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Statistical analysis was carried out with the use of a
two-tailed unpaired Student’s t-test. Values of
*P<0.05, **P<0.01 and
***P<0.001 were considered to indicate statistically
significant differences.
Results
We investigated the effects of CA on apoptosis and
on various regulatory genes involved in apoptosis and the cell
cycle in CRC cells. In addition, we also evaluated the role of
caspases in CA-induced apoptosis.
CA exerts a potent cytotoxic effect
compared to its structural derivatives
We investigated whether CA and its structural
analogues, UA and OA (Fig. 1A),
affect the viability of the human CRC cell line, HCT116. All three
triterpenes reduced the viability of the HCT116 cells; however, the
potency levels varied (Fig. 1B).
CA was the most effective in inhibiting cell growth (with an
IC50 of 24 μM), whereas UA and OA were less effective in
reducing cell viability. The order of potency of these triterpenes
was as follows; CA > UA > OA. These results suggest that CA
is the most potent anti-proliferative agent among the three
triterpenes examined.
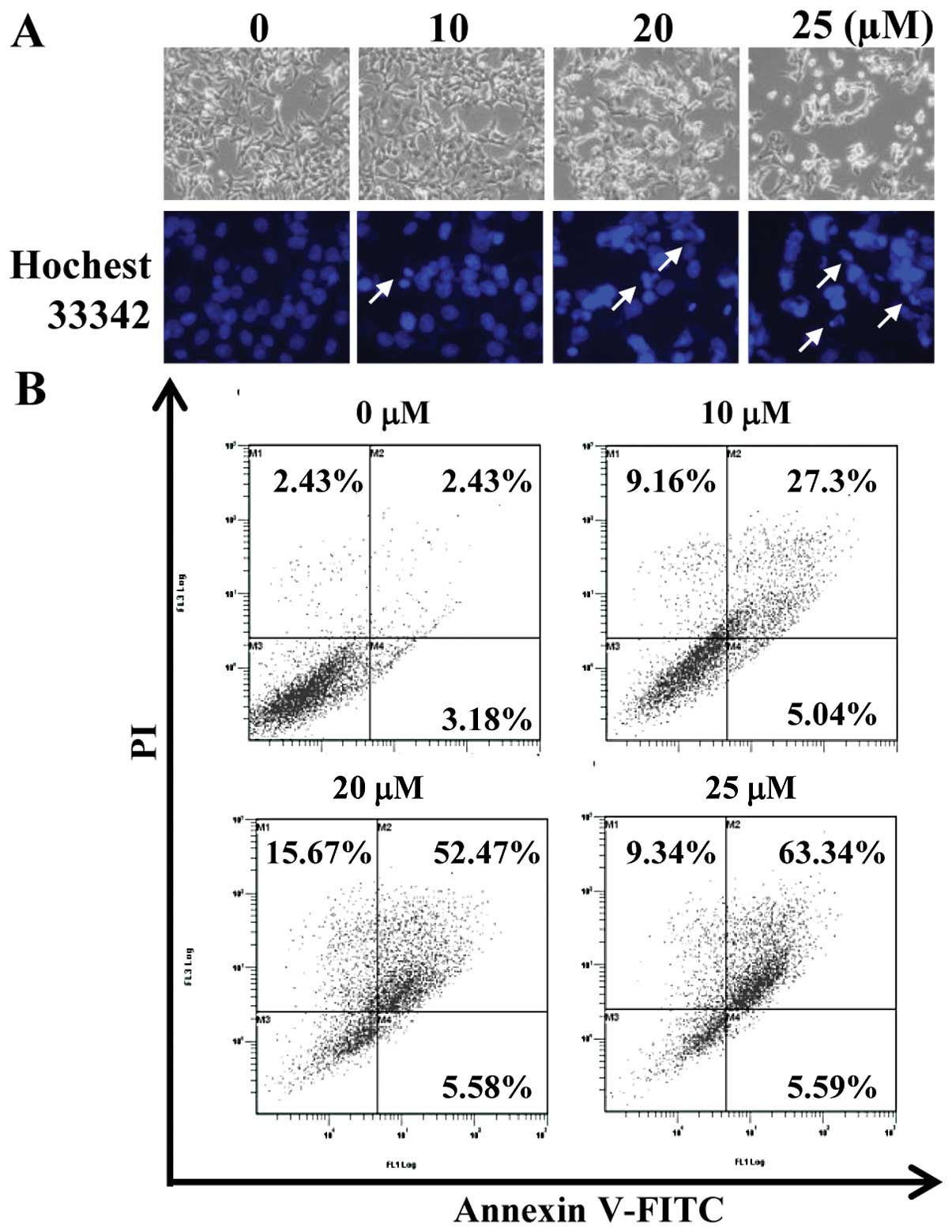
CA induces morphological changes and
chromatin condensation
Owing to the cytotoxic effects of CA, we further
investigated whether the growth inhibitory effects of CA are
associated with the induction of apoptosis. Following expose to CA
for 24 h, the morphological changes of the cells were observed
under a phase contrast light microscope. As shown in Fig. 2A (upper panel), CA altered the
shape of the cells from a polygonal to a small round shape. The
inhibition of cell growth was also detected in a
concentration-dependent manner. In order to determine whether CA
induces nuclear condensation, one of the characteristics of
apoptosis, Hoechst staining was conducted. The untreated cells
exhibited an intact nuclear structure, whereas nuclear condensation
was observed in the cells treated with CA in a
concentration-dependent manner (Fig.
2A, lower panel).
CA induces apoptosis in HCT116 cells
To confirm the effects of CA on apoptotic cell
death, we performed an Annexin V staining-based flow cytometric
analysis to assess the externalization of phosphatidylserine.
Treatment with various concentrations of CA resulted in a
concentration-dependent increase in both the early (Annexin
V+/PI−) and late apoptotic cell population
(Annexin V+/PI+) (Fig. 2B). The apoptotic indices were 5.6,
32.3, 58.1 and 68.9% at 0, 10, 20 and 25 μM CA, respectively.
Collectively, these results suggest that the growth inhibitory
effects of CA were due, at least in part, to the apoptosis of
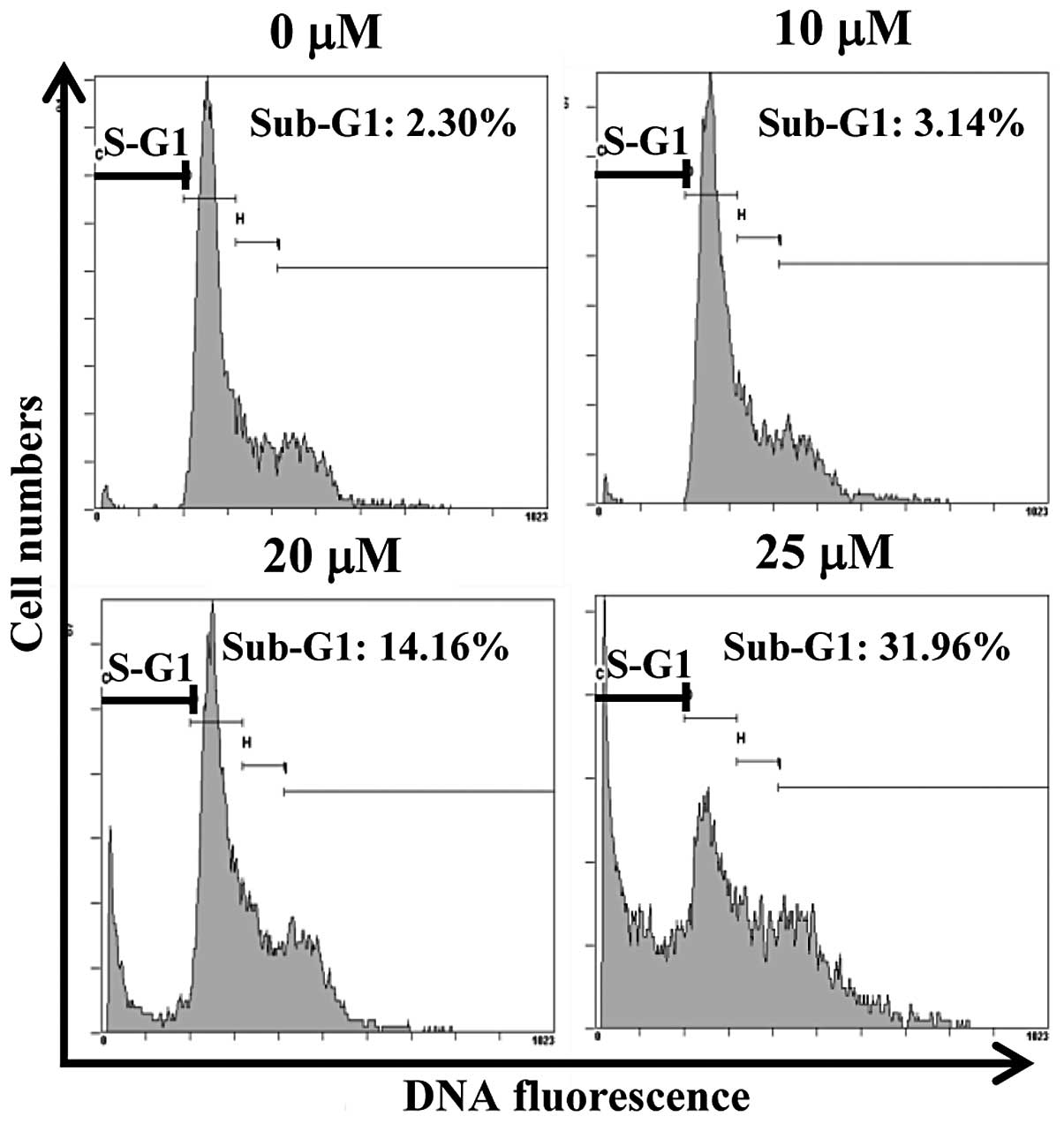
HCT116 cells. We found that CA markedly increased the sub-G1
hypodiploid cell population (genomic DNA fragmentation) (Fig. 3). Treatment with CA at 10, 20 and
25 μM for 24 h resulted in the accumulation of cells in the sub-G1
phase from 2.3% in the untreated control cells to 3.1, 14.2 and
32%, respectively. Overall, these results indicate that the
accumulation of the apoptotic population of colon cancer cells by
CA may be responsible for the CA-induced inhibition of cell
growth.
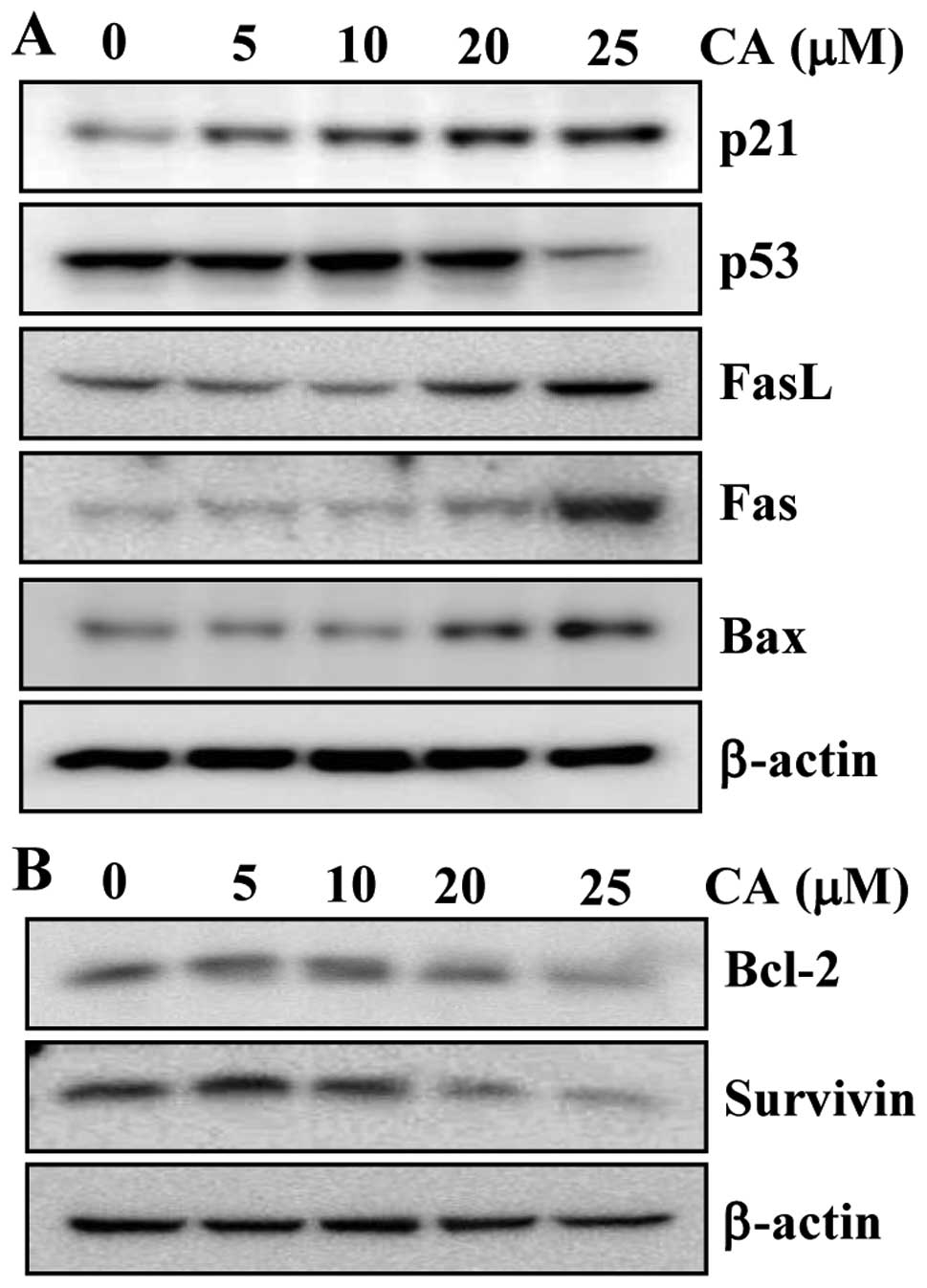
CA modulates the expression of
apoptosis-related proteins
Our results thus far indicated that CA is capable of
inducing apoptotic cell death. Therefore, we then investigated
whether CA alters the levels of proteins involved in apoptosis in
colon cancer cells. We first investigated whether CA affects the
expression of pro-apoptotic markers in human CRC cells. The
treatment of cells with CA upregulated the expression of p21, FasL,
Fas and Bax in a concentration-dependent manner (Fig. 4A). However, the level of the
pro-apoptotic protein, p53, was not altered significantly following
treatment with CA, and it even decreased at the highest
concentration of CA. This result suggests that CA induces apoptosis
through a p53-independent mechanism. We then examined whether CA
can modulate the expression of anti-apoptotic proteins. CA
suppressed the expression of the anti-apoptotic proteins, Bcl-2 and
survivin, in a concentration-dependent manner (Fig. 4B). The effects of CA on the
expression of anti-apoptotic proteins was more prominent at 20 and
25 μM.
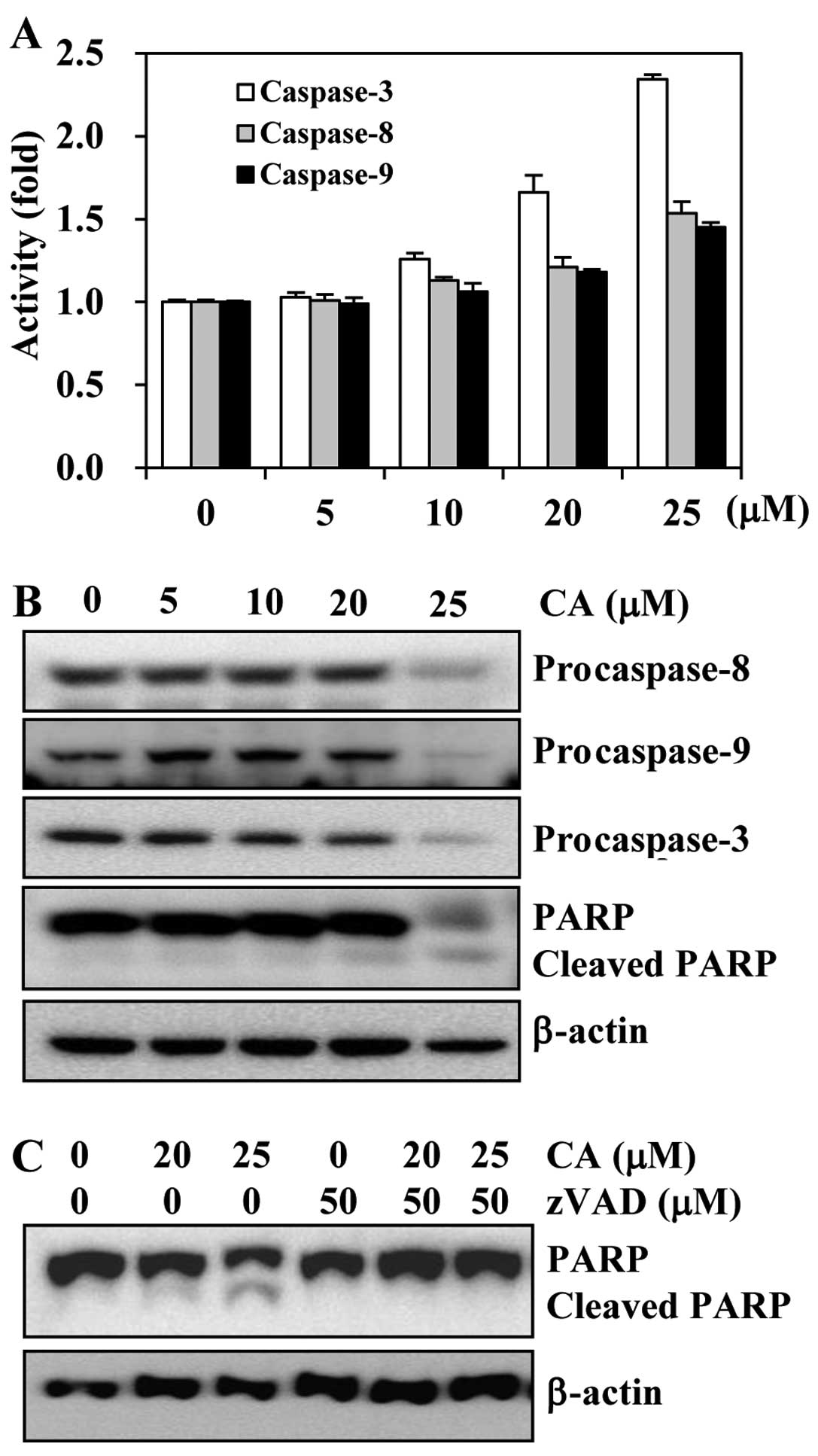
Activation of caspase-cascade is required
for the induction of apoptosis by CA
Since the activation of the caspase pathway has been
known to play a central role in the execution of apoptosis, we
investigated whether the apoptotic cell death induced by CA was
associated with the activation of caspases. First, we investigated
whether CA activates caspases in HCT116 cells. The cells were
treated with various concentrations of CA, and caspase activity was
determined by a colorimetric protease assay. The results revealed a
concentration-dependent increase in the activities of both
initiator caspases (caspase-8 and -9) and effector caspases
(caspase-3) (Fig. 5A). We also
observed that CA markedly induced the activation of caspase-3 in a
concentration-dependent manner, while the activities of caspase-8
and -9 were only slightly altered.
We then examined the effects of CA on the levels of
caspase-8, -9 and -3 and poly(ADP-ribose) polymerase (PARP) by
western blot analysis. Apoptosis is mediated through initiator
caspases, which lead to caspase-3 activation that precedes the
cleavage of PARP (5). CA induced
a decrease in the levels of procaspase-8, -9 and -3 and the
subsequent cleavage of PARP in HCT116 cells (Fig. 5B).
As the consecutive activation of caspases is
necessary for apoptosis to take place and since CA activated
caspases, we investigated whether the CA-induced apoptosis is
caspase-dependent. The HCT116 cells were pre-treated with
z-VAD-FMK, a pan-caspase inhibitor, prior to exposure of the cells
to CA and then determined apoptosis by analyzing the cleavage of
PARP. We found that CA induced the cleavage of PARP in a
concentration-dependent manner. However, pre-treatment of the cells
with z-VAD-FMK abolished the CA-induced cleavage of PARP (Fig. 5C). Overall, these results suggest
that the activation of caspases is involved in the induction of
apoptosis by CA.
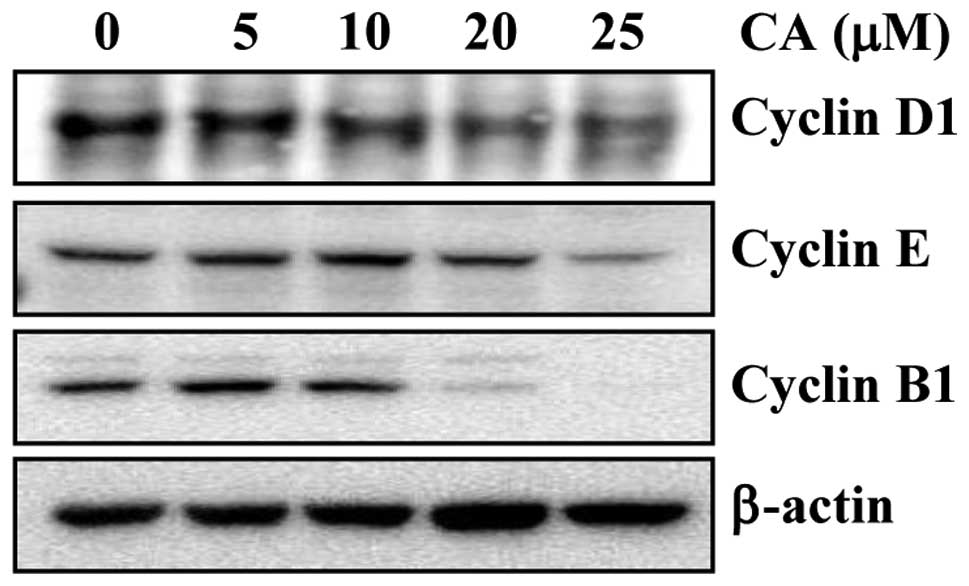
CA suppresses the expression of cell
cycle regulatory proteins
It is well-known that the induction of apoptosis may
be cell cycle-dependent (28).
Thus, previous reports have indicated that CA induces G0/G1 arrest
in human gastric cancer cells (24) and causes a late S phase or early
G2/M phase block in cervical cancer cells (23). Therefore, it is possible that CA
can cause apoptosis through the perturbation of the cell cycle. To
examine this possibility, we investigated the effects of CA on the
expression of the G1 regulators, cyclin D1 and cyclin E, and of the
G2/M regulator, cyclin B, in HCT116 cells. CA suppressed the
expression of cyclin D1, E and B1 in a concentration-dependent
manner (Fig. 6). Collectively,
these results indicate that the CA-induced downregulation of
cyclins is associated with apoptosis in colon cancer cells.
Discussion
Worldwide, almost one million individuals develop
CRC each year; thus, approximately 50% of these individuals can be
expected to die due to systemic disease within five years of
diagnosis (29). Although the
incidence of CRC has been slowly declining over the past few
decades, possibly due to improved screening and earlier detection
methods, high mortality rates emphasize the desperate need for
novel and improved therapies. In the present study, we aimed to
determine whether the natural agent, CA, induces apoptosis in human
CRC cells and to elucidate the possible mechanisms involved.
In present study, we compared the growth suppressive
effects of three triterpenes in HCT116 cells. These included ursane
types, such as CA and UA and oleanane types, such as OA (structures
are illustrated in Fig. 1A). We
found that the ursane-type triterpenes (CA and UA) had more potent
inhibitory effects on cell viability than the oleanane type (OA)
ones. In addition, CA was the most cytotoxic to HCT116 cells among
the three tested compounds. In agreement with these observations,
CA has been previously shown to exhibit the strongest
anti-proliferative activity in four different human leukemia cell
lines (9). In another study, CA
exhibited cytotoxic effects in both C6 rat glioma and human
epithelial carcinoma (A431) cells, whereas UA failed to show
cytotoxic effects in A431 cells at the concentration of 10–100 μM
(30). The authors suggested that
the position and number of hydroxyls on triterpenes are critical to
possess cytotoxicity, although the two agents harbor the same
ursane-type pentacyclic triterpene cores. Of note, the only
structural difference between UA and CA is a single additional
hydroxyl group on the A-ring of the ursane-12-en skeleton; thus,
this difference is responsible for the selective cytotoxicity
against C6 cells. Shifting the C-19 methyl to the C-20 position of
CA, decreases the cytotoxic activity of CA (see OA in Fig. 1B). How and which position of the
hydroxyl group of the A-ring in ursane-type triterpenes contributes
to selective cytotoxicity remains unclear; thus, further analysis
of the structure activity relationship is required.
The induction of apoptosis is considered a common
event for anticancer agents. In principle, two major different
activation pathways in apoptosis have been described; one involves
the disruption of mitochondrial membrane potential and the release
of cytochrome c. Released cytochrome c binds to
apoptotic protease activating factor 1 and then recruits
procaspase-9, resulting in the formation of an apoptosome complex
(known as the intrinsic pathway). The other pathway, also known as
the extrinsic pathway, initiates with death receptor ligation or
Fas/FasL interaction, resulting in the subsequent recruitment of
Fas-associated death domain protein (FADD). FADD then associates
with procaspase-8, resulting in the activation of caspase-8.
Finally, both caspase-9 and -8 activate caspase-3, leading to the
execution of apoptosis (31,32). In the present study, we found that
CA induced the activity of caspase-3, -8 and -9, indicating that
both the intrinsic and extrinsic pathways were activated. The
observed caspase cascade-mediated apoptosis-inducing properties of
CA are in agreement with those observed by others in osteosarcoma
(21), leukemia (9), as well as cervical (23) and lung cancer cells (19). We also observed the CA-mediated
cleavage of PARP, an indicator of caspase-3 activation; this was
abrogated when we employed the general caspase inhibitor,
z-VAD-FMK. These findings provide evidence that the apoptosis
induced by CA in HCT116 cells is mediated through caspase
activation. However, which caspase is primarily involved in
CA-induced apoptosis remains to be elucidated.
Other terpenoids, such as UA (33), saikosaponin D (34) and ginsenoside Rk1 (35), have been reported to induce
apoptosis through the activation of Fas/FasL. Upon activation by
the engagement with its ligand, FasL, Fas cleaves caspase-8 and
activates the caspase cascade, including caspase-3, leading to the
activation of the extrinsic pathway (36). The downregulation of Fas occurs
during tumor progression (37,38), and many cancer cells obtain a
survival strategy by decreasing their sensitivity to Fas-induced
apoptosis (39). However, the
role of FasL as a pro-or anti-apoptotic mediator has been largely
controversial in colon cancer cells (40). In the present study, we found that
CA upregulated the death receptor, Fas, and its ligand, FasL. In
addition, the activation of caspase-8 may have occurred due to the
upregulation of Fas that may ultimately have sensitized the colon
cancer cells to apoptosis by CA. Further research is required
however, to determine the exact effects of CA on the Fas/FasL
pathway. Our data suggest that CA induces apoptosis in HCT116
cells, at least in part, through the activation of the Fas/FasL
pathway.
We found that the growth inhibitory effects of CA
correlated with the downregulation of gene products, such as
survivin and Bcl-2, which are all known as anti-apoptotic proteins.
Survivin is a member of the inhibitor of apoptosis proteins, the
only endogenous proteins that inhibit the caspase cascade. The
Bcl-2 family protein, Bcl-2, is also involved in the loss of
caspase-9 activation, and therefore provides a survival strategy to
cancer cells from intrinsic pathway-mediated apoptosis. In
agreement with our observations, the suppression of survivin and
Bcl-2 expression by CA has been shown in a previous study (19).
A recent study indicated that the generation of
reactive oxygen species (ROS) by CA is a critical regulator of
caspase-mediated apoptosis in A549 lung cancer cells (19). Moreover, the exposure of cells to
the ROS scavenger, N-acetyl cysteine (NAC), prevented CA-induced
cytotoxicity, as well as caspase activation (19). In the present study, NAC failed to
prevent cell death induced by CA in the HCT116 cells (data not
shown). The precise reason for this difference is not clear;
however, the cell type and experimental conditions (i.e., dosage,
time and analytical methods) used may account for this
difference.
The majority of the anticancer properties assigned
to CA have been demonstrated in in vitro studies. Although
there are some reports on the anti-diabetic and antioxidant effects
of CA in animals, only one animal study revealing the anticancer
activities of CA has been reported. The oral administration of CA
has been shown to be effective in animal models of murine
osteosarcoma (20). The authors
demonstrated that the development of tumors and lung metastasis in
the animals was markedly suppressed by the oral administration of
CA (17.5 mg/kg). To date, to the best of our knowledge, no animal
studies have been conducted to assess the toxicity or
LD50 values of CA; however, in this study, we provide
strong evidence supporting the anticancer potential of CA.
Overall, the data from the present study provide
evidence that CA induces apoptosis through the activation of
caspases. This presents a rationale for the use of CA as an
anticancer agent. However, further studies using clinically
relevant animal models and human efficacy and safety studies are
required to explore the therapeutic potential of CA against
cancer.
Acknowledgements
This study was financially supported by the (2013
Post-Doc. Development Program) of Pusan National University, the
National Research Foundation of Korea (NRF) Grant Funded by the
Korea Government (MSIP) (no. 2009-0083538), and the R&D Program
of MKE/KEIT (10040391, Development of Functional Food Materials and
Device for Prevention of Aging-associated Muscle Function
Decrease). We thank the Aging Tissue Bank for providing research
information.
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): a population-based study. Lancet
Oncol. 13:790–801. 2012.PubMed/NCBI
|
|
2
|
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG
and Lee JS: Cancer statistics in Korea: incidence, mortality,
survival and prevalence in 2010. Cancer Res Treat. 45:1–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanwar SS, Poolla A and Majumdar AP:
Regulation of colon cancer recurrence and development of
therapeutic strategies. World J Gastrointest Pathophysiol. 3:1–9.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar
|
|
6
|
Newman DJ, Cragg GM and Snader KM: Natural
products as sources of new drugs over the period 1981–2002. J Nat
Prod. 66:1022–1037. 2003.PubMed/NCBI
|
|
7
|
Ahn KS, Hahm MS, Park EJ, Lee HK and Kim
IH: Corosolic acid isolated from the fruit of Crataegus
pinnatifida var. psilosa is a protein kinase C inhibitor
as well as a cytotoxic agent. Planta Med. 64:468–470.
1998.PubMed/NCBI
|
|
8
|
Li B, Meng X, Zhu L, Jiao X and Zhang J:
Application of high-speed counter-current chromatography for
isolation of triterpenes from Schisandra chinensis (Turcz.)
Baill and induction apoptosis mechanism of HSC-T6. Biomed Mater
Eng. 24:969–977. 2013.PubMed/NCBI
|
|
9
|
Uto T, Sakamoto A, Tung NH, et al:
Anti-proliferative activities and apoptosis induction by
triterpenes derived from Eriobotrya japonica in human
leukemia cell lines. Int J Mol Sci. 14:4106–4120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miura T, Takagi S and Ishida T: Management
of diabetes and its complications with banaba (Lagerstroemia
speciosa L.) and corosolic acid. Evid Based Complement Alternat
Med. 2012:8714952012.PubMed/NCBI
|
|
11
|
Caligiani A, Malavasi G, Palla G,
Marseglia A, Tognolini M and Bruni R: A simple GC-MS method for the
screening of betulinic, corosolic, maslinic, oleanolic and ursolic
acid contents in commercial botanicals used as food supplement
ingredients. Food Chem. 136:735–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee MS, Lee CM, Cha EY, et al: Activation
of AMP-activated protein kinase on human gastric cancer cells by
apoptosis induced by corosolic acid isolated from Weigela
subsessilis. Phytother Res. 24:1857–1861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rao AR, Veeresham C and Asres K: In vitro
and in vivo inhibitory activities of four Indian medicinal plant
extracts and their major components on rat aldose reductase and
generation of advanced glycation endproducts. Phytother Res.
27:753–760. 2013. View
Article : Google Scholar
|
|
14
|
Yin MC, Lin MC, Mong MC and Lin CY:
Bioavailability, distribution, and antioxidative effects of
selected triterpenes in mice. J Agric Food Chem. 60:7697–7701.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Yang J, Zhang Q, Chen LH and Wang
Q: Corosolic acid ameliorates atherosclerosis in apolipoprotein
E-deficient mice by regulating the nuclear factor-κB signaling
pathway and inhibiting monocyte chemoattractant protein-1
expression. Circ J. 76:995–1003. 2012.PubMed/NCBI
|
|
16
|
Takagi S, Miura T, Ishihara E, Ishida T
and Chinzei Y: Effect of corosolic acid on dietary
hypercholesterolemia and hepatic steatosis in KK-Ay diabetic mice.
Biomed Res. 31:213–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klein G, Kim J, Himmeldirk K, Cao Y and
Chen X: Antidiabetes and anti-obesity activity of Lagerstroemia
speciosa. Evid Based Complement Alternat Med. 4:401–407. 2007.
View Article : Google Scholar
|
|
18
|
Aguirre MC, Delporte C, Backhouse N, et
al: Topical anti-inflammatory activity of 2alpha-hydroxy
pentacyclic triterpene acids from the leaves of Ugni molinae.
Bioorg Med Chem. 14:5673–5677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nho KJ, Chun JM and Kim HK: Corosolic acid
induces apoptotic cell death in human lung adenocarcinoma A549
cells in vitro. Food Chem Toxicol. 56:8–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horlad H, Fujiwara Y, Takemura K, et al:
Corosolic acid impairs tumor development and lung metastasis by
inhibiting the immunosuppressive activity of myeloid-derived
suppressor cells. Mol Nutr Food Res. 57:1046–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai X, Zhang H, Tong D, et al: Corosolic
acid triggers mitochondria and caspase-dependent apoptotic cell
death in osteosarcoma MG-63 cells. Phytother Res. Feb 21–2011.(Epub
ahead of print).
|
|
22
|
Fujiwara Y, Komohara Y, Ikeda T and Takeya
M: Corosolic acid inhibits glioblastoma cell proliferation by
suppressing the activation of signal transducer and activator of
transcription-3 and nuclear factor-kappa B in tumor cells and
tumor-associated macrophages. Cancer Sci. 102:206–211. 2011.
View Article : Google Scholar
|
|
23
|
Xu Y, Ge R, Du J, et al: Corosolic acid
induces apoptosis through mitochondrial pathway and caspase
activation in human cervix adenocarcinoma HeLa cells. Cancer Lett.
284:229–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee MS, Cha EY, Thuong PT, Kim JY, Ahn MS
and Sul JY: Down-regulation of human epidermal growth factor
receptor 2/neu oncogene by corosolic acid induces cell cycle arrest
and apoptosis in NCI-N87 human gastric cancer cells. Biol Pharm
Bull. 33:931–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miura T, Itoh Y, Kaneko T, et al:
Corosolic acid induces GLUT4 translocation in genetically type 2
diabetic mice. Biol Pharm Bull. 27:1103–1105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wen X, Xia J, Cheng K, et al: Pentacyclic
triterpenes. Part 5: synthesis and SAR study of corosolic acid
derivatives as inhibitors of glycogen phosphorylases. Bioorg Med
Chem Lett. 17:5777–5782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi L, Zhang W, Zhou YY, et al: Corosolic
acid stimulates glucose uptake via enhancing insulin receptor
phosphorylation. Eur J Pharmacol. 584:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vermeulen K, Berneman ZN and Van
Bockstaele DR: Cell cycle and apoptosis. Cell Prolif. 36:165–175.
2003. View Article : Google Scholar
|
|
29
|
Weitz J, Koch M, Debus J, Höhler T, Galle
PR and Buchler MW: Colorectal cancer. Lancet. 365:153–165. 2005.
View Article : Google Scholar
|
|
30
|
Mazumder K, Tanaka K and Fukase K:
Cytotoxic activity of ursolic acid derivatives obtained by
isolation and oxidative derivatization. Molecules. 18:8929–8944.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang HY and Yang X: Proteases for cell
suicide: functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kischkel FC, Hellbardt S, Behrmann I, et
al: Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins
form a death-inducing signaling complex (DISC) with the receptor.
EMBO J. 14:5579–5588. 1995.PubMed/NCBI
|
|
33
|
Li Y, Xing D, Chen Q and Chen WR:
Enhancement of chemotherapeutic agent-induced apoptosis by
inhibition of NF-kappaB using ursolic acid. Int J Cancer.
127:462–473. 2010.PubMed/NCBI
|
|
34
|
Hsu YL, Kuo PL and Lin CC: The
proliferative inhibition and apoptotic mechanism of Saikosaponin D
in human non-small cell lung cancer A549 cells. Life Sci.
75:1231–1242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JS, Joo EJ, Chun J, et al: Induction
of apoptosis by ginsenoside Rk1 in SK-MEL-2-human melanoma. Arch
Pharm Res. 35:717–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizutani Y, Hongo F, Sato N, Ogawa O,
Yoshida O and Miki T: Significance of serum soluble Fas ligand in
patients with bladder carcinoma. Cancer. 92:287–293. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Osorio LM, Aguilar-Santelises M, De
Santiago A, Hachiya T, Mellstedt H and Jondal M: Increased serum
levels of soluble Fas in progressive B-CLL. Eur J Haematol.
66:342–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khong HT and Restifo NP: Natural selection
of tumor variants in the generation of ‘tumor escape’ phenotypes.
Nat Immunol. 3:999–1005. 2002.
|
|
40
|
Zhu Q, Liu JY, Yang CM, et al: Influence
of antitumor drugs on the expression of Fas system in SW480 colon
cancer cells. Eur J Gastroenterol Hepatol. 18:1071–1077. 2006.
View Article : Google Scholar : PubMed/NCBI
|