Introduction
Reactive oxygen species (ROS) [e.g., superoxide
anions, hydroxyl radicals, and hydrogen peroxide
(H2O2)] can harm cellular proteins, lipids
and DNA (1,2). Long-term effects of oxidative damage
to the skin include premature aging, cancer and inflammation
(3–5).
Heme oxygenase-1 (HO-1, one of three heme oxygenase
isoforms) is an extensively studied phase II enzyme that is
essential for the rate-limiting step of heme catabolism. Heme
catabolism leads to the formation of carbon monoxide, ferrous iron
(Fe2+) and biliverdin, which is converted into bilirubin
by biliverdin reductase (6). The
end products of heme catabolism exert antioxidant effects by
neutralizing intracellular ROS (7,8).
Thus, HO-1 demonstrates potent cytoprotective properties against
oxidative damage (9–11), and the induction of its expression
increases cellular resistance to oxidative stress.
HO-1 expression is primarily regulated at the
transcriptional level and is induced by the transcription factor
nuclear factor erythroid 2-related factor 2 (Nrf2) (12,13). Under basal conditions, Nrf2 is
constitutively docked to Kelch-like ECH-associated protein 1
(Keap1) in the cytoplasm, culminating in the ubiquitination and
proteosomal degradation of Nrf2 (14). However, Keap1 is oxidized under
conditions of electrophilic stress, which blocks Nrf2 degradation
and allows Nrf2 phosphorylation, nuclear translocation, and
association with the antioxidant response element (ARE) within the
HO-1 gene promoter region. The net result of ARE binding by Nrf2 is
the activation of multiple antioxidant genes, including HO-1
(15).
Similar to Keap1, extracellular signal-regulated
kinase (ERK) and protein kinase B (PKB, Akt) are involved in the
transduction of various signals from the cell surface to the
nucleus. Both of their signaling pathways are associated with the
modulation of ARE-driven gene expression via Nrf2 activation
(16,17).
The polyphenolic compound 7,8-dihydroxyflavone (DHF)
is a member of the flavonoid family with antioxidant properties
(18–21).
In a previous study, we reported that DHF protected
cells against oxidative stress-induced genotoxicity by scavenging
intracellular ROS and enhancing Akt activity (22).
In the present study, we investigated the
cytoprotective mechanisms of DHF against oxidative stress-induced
cell damage in human keratinocytes with respect to its stimulatory
effects on HO-1 expression and activity. We also investigated the
molecular mechanisms involved in DHF-mediated cytoprotection and
HO-1 induction, with a particular focus on ERK- and Akt-Nrf2
signaling cascades.
Materials and methods
Materials
[3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] bromide
(MTT) and zinc protoporphyrin (ZnPP) were purchased from Sigma
Chemical Co. (St. Louis, MO, USA). Both U0126 and LY294002 were
provided by Calbiochem (San Diego, CA, USA). Primary antibodies
against HO-1, ERK, phospho ERK, Nrf2, and β-actin were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
primary Akt and phospho Akt (Ser 473) antibodies were purchased
from Cell Signaling Technology (Beverly, MA, USA). Rabbit
polyclonal antibody specific for phospho Nrf2 was purchased from
Epitomics, Inc. (Burlingame, CA, USA). Any other chemicals and
reagents were of analytical grade.
Cell culture
Human keratinocytes (HaCaT cells) were obtained from
Amore Pacific (Gyeonggi-do, Republic of Korea). Cells were cultured
in Dulbecco’s modified Eagle’s medium containing 10%
heat-inactivated fetal calf serum, streptomycin (100 μg/ml), and
penicillin (100 U/ml). The cells were maintained at 37°C in an
incubator with a humidified atmosphere of 5% CO2.
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
Cells (3×106 cells/ml) were seeded into
100-mm culture dish. Sixteen hours later, the cells were treated
with DHF at concentrations of 0.5, 1, 5, 10, or 20 μg/ml. The cells
were collected at the indicated times and washed twice with
phosphate-buffered saline (PBS). Total RNA was isolated from the
cells using TRIzol® reagent (Gibco-BRL, Grand Island,
NY, USA). RT-PCR conditions for HO-1 and the housekeeping gene,
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), were as follows:
35 cycles of 94°C for 45 sec; 53°C for 45 sec; and 72°C for 60 sec.
The primer pairs (Bionics, Seoul, Republic of Korea) used were:
HO-1, forward: 5′-GAGAATGCTGAGTTCATG-3′ and reverse:
5′-ATGTTGAGCAGGAAGGC-3′; GAPDH, forward:
5′-GTGGGCCGCCCTAGGCACCAGG-3′ and reverse:
5′-GGAGGAAGAGGATGCGGCAGTG-3′. Amplified products were resolved on
1% agarose gels, stained with ethidium bromide, and images were
captured under ultraviolet light.
HO-1 activity assay
HO-1 enzyme activity was measured as previously
described (23). Briefly, cells
were homogenized in 0.5 ml ice-cold 0.25 M sucrose solution
containing 50 mM potassium phosphate buffer (pH 7.4). Homogenates
were centrifuged at 200 × g for 10 min. Supernatants were
centrifuged at 9000 × g for 20 min, and then centrifuged at 15,000
× g for 60 min. The resulting pellet (cell lysate) was resuspended
in 50 mM potassium phosphate buffer (pH 7.4), and the amount of
protein in the cell lysate was determined.
A reaction mixture (200 μl) containing 500 μg/ml
cell lysate, 0.2 mM hemin, 0.5 mg/ml rat liver cytosol as the
source of biliverdin reductase, 0.2 mM MgCl2, 2 mM
glucose-6-phosphate, 1 U/ml glucose-6-phosphate dehydrogenase, 1 mM
NADPH, and 50 mM potassium phosphate buffer (pH 7.4) was incubated
at 37°C for 1 h. The reaction was stopped with chloroform (0.5 ml).
Following extraction, the chloroform layer was measured
spectrophotometrically. Bilirubin formation from the hemin
substrate was calculated according to the difference in absorption
at 464 and 530 nm.
Western blot analysis
Cells (3×106 cells/ml) were seeded into a
100-mm culture dish. Sixteen hours later, the cells were treated
with DHF (5 μg/ml), collected at the indicated times and washed
twice with PBS. The collected cells were lysed on ice for 30 min in
100 μl lysis buffer [120 mM NaCl, 40 mM Tris (pH 8.0), and 0.1% NP
40] and centrifuged at 13,000 × g for 15 min. Supernatants were
collected from the lysates, and protein concentrations of the
supernatants were determined. Aliquots of the lysates (40 μg
protein) were boiled for 5 min and electrophoresed on 10% sodium
dodecyl sulfate polyacrylamide gels. Electrophoresed proteins were
transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA,
USA), which were incubated with primary antibodies. Membranes were
then incubated with horseradish peroxidase-conjugated secondary
antibodies (Pierce, Rockland, IL, USA) and images were captured
with LAS-3000 (Fujifilm Corp., Tokyo, Japan). Protein bands were
detected using an enhanced chemiluminescence Western blot detection
kit (Amersham, Little Chalfont, UK).
Preparation of nuclear extracts
The cells were collected at the indicated times and
lysed on ice in 1 ml lysis buffer [10 mM Tris-HCl (pH 7.9), 10 mM
NaCl, 3 mM MgCl2, and 1% NP-40] for 4 min. Following
centrifugation at 3,000 × g for 10 min, the pellets were
resuspended in 50 μl extraction buffer [20 mM HEPES (pH 7.9), 20%
glycerol, 1.5 mM MgCl2, 300 mM NaCl, 0.2 mM EDTA, 1 mM
DTT, and 1 mM PMSF], incubated on ice for 30 min, and centrifuged
at 13,000 × g for 5 min. Supernatants were collected and stored at
−70°C after measurement of the protein concentration.
Immunocytochemistry
Cells were plated on coverslips, fixed with 4%
paraformaldehyde for 30 min, and permeabilized with PBS containing
0.1% Triton X-100 for 2.5 min. The cells were then treated with
blocking medium (PBS containing 3% bovine serum albumin) for 1 h
and incubated with a primary anti-Nrf2 antibody diluted in blocking
medium for 2 h. The immunoreacted primary antibody was detected by
incubating cells with a fluorescein isothiocyanate
(FITC)-conjugated secondary antibody (1:500 dilution) (Jackson
ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h. After
washing with PBS, stained cells were mounted onto microscope slides
in mounting medium containing DAPI to label nuclei (Vector,
Burlingame, CA, USA). Images were collected using a Zeiss confocal
microscope and Zeiss LSM 510 software.
Chromatin immunoprecipitation (ChIP)
The ChIP assay was performed with a Simple ChIP™
enzymatic chromatin IP kit (Cell Signaling Technology, Danvers, MA,
USA) according to the manufacturer’s instructions, with slight
modifications. Briefly, cellular proteins were crosslinked by the
addition of 1% formaldehyde. Prepared chromatin was digested using
a nuclease for 12 min at 37°C. A primary anti-Nrf2 antibody and
normal rabbit IgG were added to the chromatin digest, and the
mixture was incubated with constant rotation overnight at 4°C. To
capture the immunoprecipitated complexes, ChIP-grade protein G
magnetic beads were added. The beads were washed, and the
immunoprecipitate was eluted with ChIP elution buffer. Crosslinking
was reversed by incubating the eluent at 65°C for 30 min, followed
by the addition of proteinase K and incubation at 65°C for 2 h. The
immunoprecipitated DNA fragments were purified on spin columns. DNA
recovered from the immunoprecipitated complexes was subjected to 35
cycles of PCR. The primers for the HO-1 gene promoter were:
forward: 5′-CCAGAAAGTGGGCATCAGCT-3′ and reverse:
5′-GTCACATTTATGCTCGGCGG-3′. PCR products were resolved on 1%
agarose gels, and DNA bands were visualized using ethidium bromide
staining and images were captured under ultraviolet light.
Cell viability assay
The effect of DHF on cell viability was determined
using the MTT assay, which is based on the reduction of a
tetrazolium salt by a mitochondrial dehydrogenase in viable cells
(24). Cells were seeded in a
96-well plate at a density of 3×105 cells/ml and treated
for 2 h with 5 μg/ml DHF, 10 μM ZnPP (an inhibitor of HO-1), 10 nM
U0126 (an inhibitor of ERK kinase), and 5 μM LY294002 (an inhibitor
of PI3K), followed by 1 mM H2O2 or 30
mJ/cm2 UVB for 1 h. After incubation for 24 h at 37°C,
50 μl MTT stock solution (2 mg/ml) was added to each well to
achieve a total reaction volume of 250 μl. After incubation for 2.5
h, the supernatants were aspirated. The formazan crystals in each
well were dissolved in 150 μl dimethyl sulfoxide, and the
absorbance at 540 nm was read on a scanning multi-well
spectrophotometer.
Statistical analysis
Values are given as the mean ± standard error of the
mean (SEM). The results were subjected to an analysis of variance
(ANOVA) followed by the Tukey’s test to analyze differences between
conditions. P<0.05 was considered to indicate statistical
significance.
Results
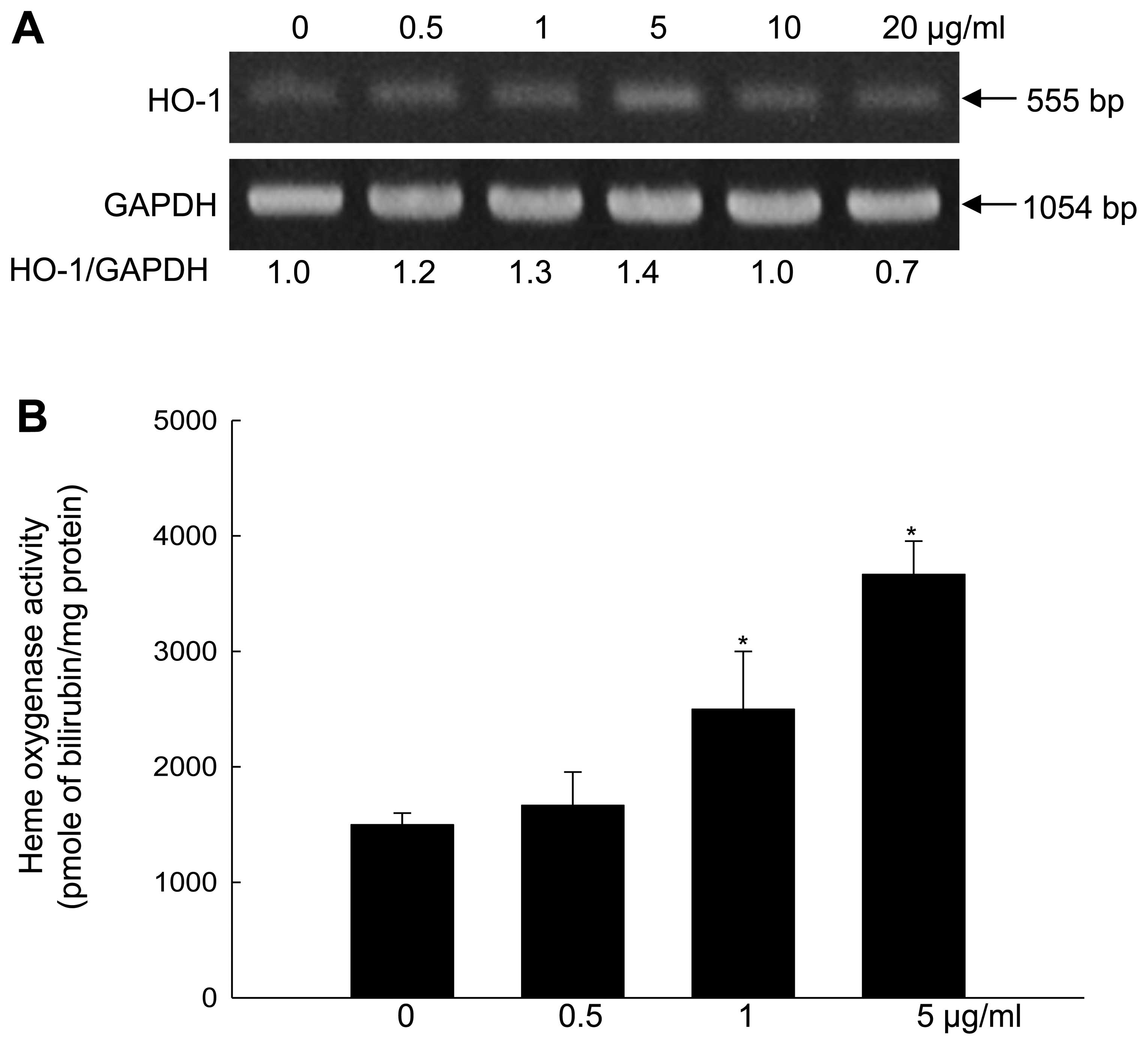
DHF induces HO-1 mRNA levels and
activity
DHF at concentrations of 0.5, 1 and 5 mg/ml
dose-dependently increased HO-1 mRNA levels in HaCaT cells, while
DHF at concentrations of 10 and 20 mg/ml slightly decreased mRNA
levels relative to the level induced by 5 mg/ml DHF (Fig. 1A). Furthermore, DHF (0.5, 1, and 5
mg/ml) dose-dependently increased HO-1 activity, with a clear
effect at 5 mg/ml (Fig. 1B). When
cell viability was assessed by the MTT assay, DHF did not show any
cytotoxicity at 0.5, 1, or 5 mg/ml (data not shown). From these
results, 5 mg/ml DHF was selected as the optimal concentration for
subsequent experiments. DHF (5 mg/ml) enhanced HO-1 mRNA and
protein expression in a time-dependent manner (Fig. 1C and D). The time course for the
elevated HO-1 expression was closely correlated with that for the
increased HO-1 activity (Fig.
1E).
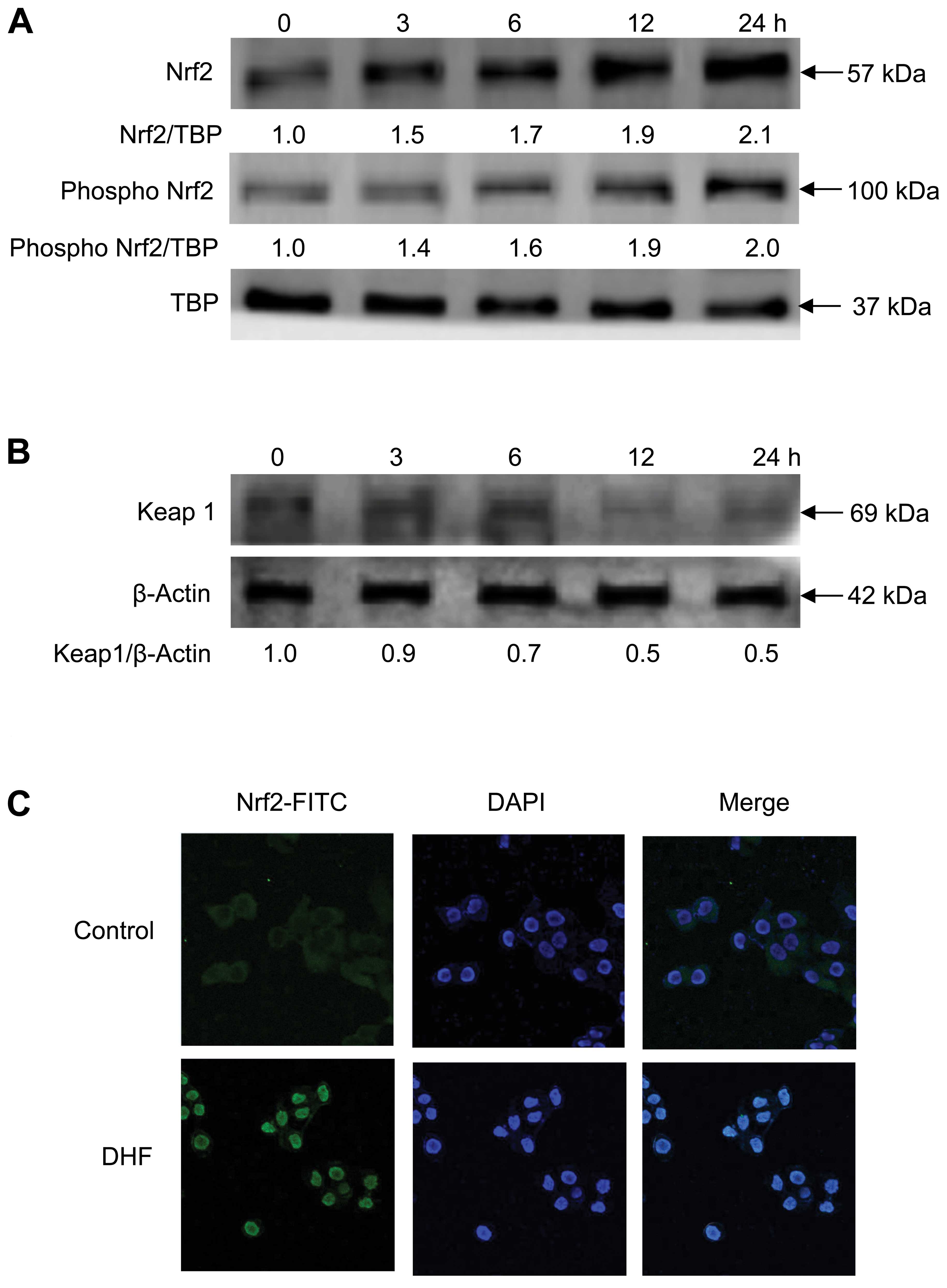
DHF induces Nrf2 expression, nuclear
translocation and binding to ARE
Nrf2 is a redox-sensitive basic-leucine zipper
transcription factor. Its activity is blocked by Keap1, a negative
regulator of Nrf2 that forms a complex with the transcription
factor in the cytoplasm and prevents its phosphorylation and
translocation into the nucleus (14). Protein levels of total nuclear and
phosphorylated Nrf2 (phospho Nrf2) were increased in a
time-dependent manner by DHF treatment of HaCaT cells (Fig. 2A), whereas Keap1 expression was
decreased (Fig. 2B). DHF markedly
increased the translocation of Nrf2 from the cytosol into the
nucleus (Fig. 2C). Moreover, Nrf2
binding to the ARE sequence in the HO-1 promoter region was
markedly higher in DHF-treated cells than in untreated cells, as
assessed by the ChIP assay (Fig.
2D).
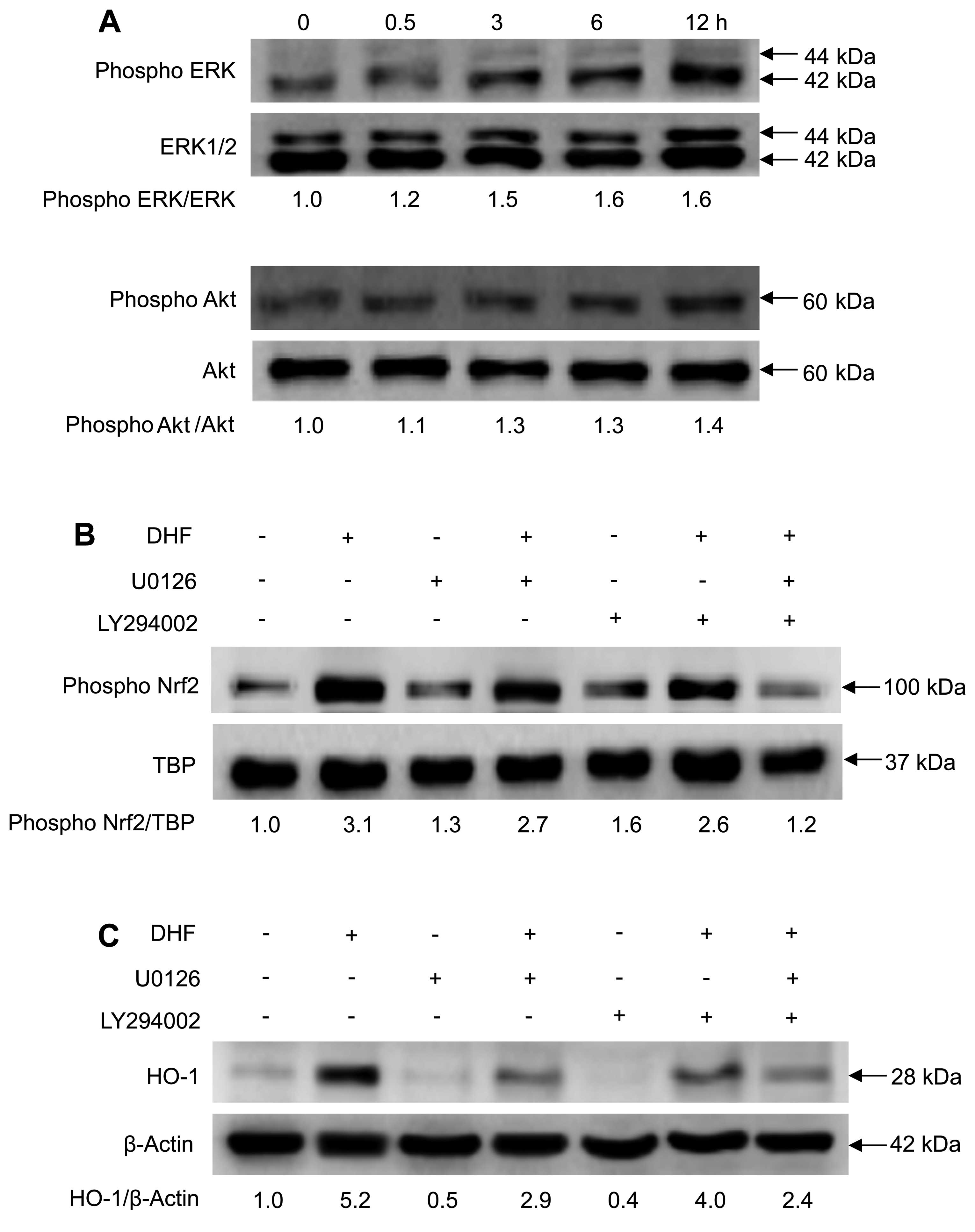
DHF activates Nrf2 and increases HO-1
expression via phosphorylation of ERK and Akt
To investigate the upstream signaling pathways
involved in DHF-enhanced Nrf2 activation and HO-1 expression, we
examined the phosphorylation of ERK and Akt, which are important
signaling enzymes that are involved in cellular protection against
oxidative stress (16,25). DHF treatment of keratinocytes
increased the phosphorylation of ERK and Akt in a time-dependent
manner (Fig. 3A). Moreover,
selective inhibitors of ERK (U0126) and Akt (LY294002, which blocks
PI3K upstream of Akt) reduced the levels of DHF-induced
phospho-Nrf2 (Fig. 3B) and HO-1
(Fig. 3C). These results suggest
that DHF increased the levels of phospho-Nrf2 and HO-1 via ERK- and
PI3K/Akt-dependent pathways.
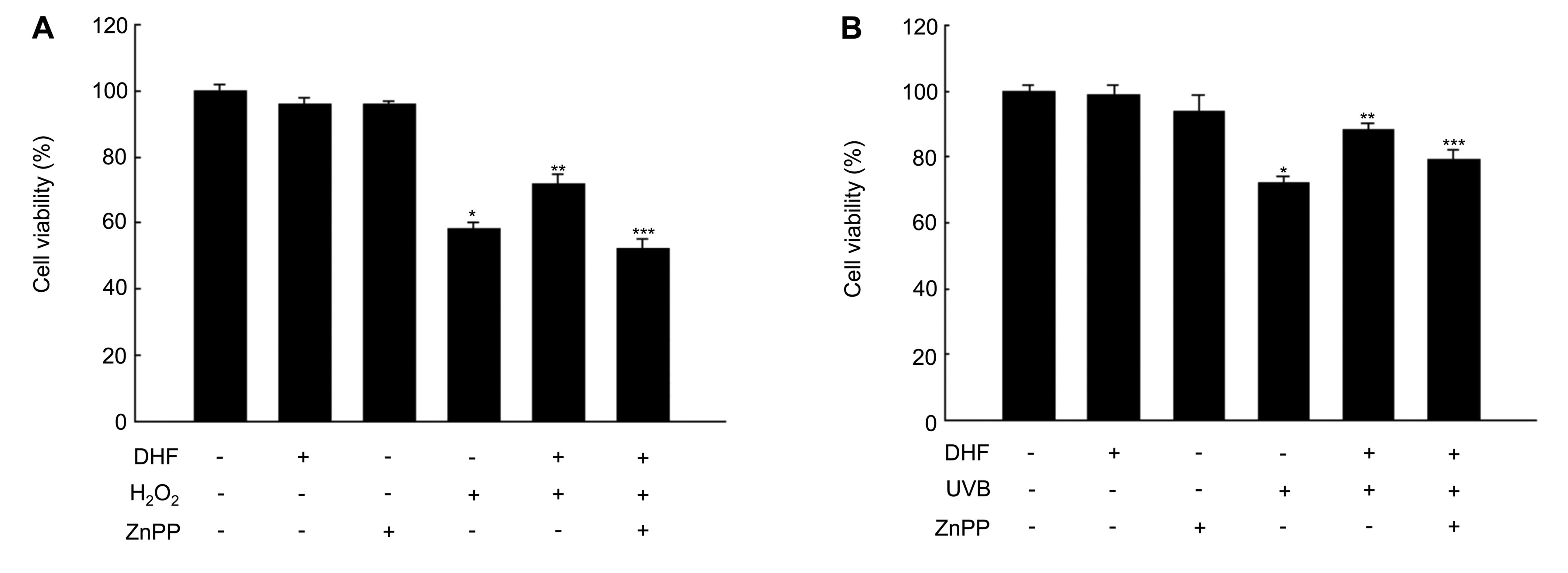
Involvement of HO-1 and ERK and Akt
pathways in DHF-induced cytoprotection against oxidative
stress
To determine whether DHF-enhanced HO-1 activity
confers cytoprotection to keratinocytes against oxidative stress,
HaCaT cells were pre-treated with the HO-1 inhibitor ZnPP and then
exposed to H2O2 or UVB radiation. Cell
viability was significantly reduced by H2O2
or UVB exposure, but not by pre-treatment with DHF or ZnPP alone
(Fig. 4A and B). Pre-treatment
with DHF prevented H2O2 and UVB-induced
cytotoxicity. ZnPP attenuated the protective effect of DHF against
H2O2 and UVB-induced cellular damage, as
assessed by reduced cell viability in the MTT assay (Fig. 4A and B). Therefore, the
cytoprotective effect of DHF is likely to be mediated through HO-1
induction.
We also examined whether DHF can mitigate oxidative
damage to cells through the activation of ERK and Akt. U0126 and
LY294002 reduced the protective effect of DHF against
H2O2 and UVB exposure (Fig. 4C and D), suggesting that ERK and
Akt signaling pathways are also involved in DHF-mediated
cytoprotection.
Discussion
The epidermis and its associated cells comprise a
critical defense mechanism against oxidative damage. Keratinocytes
in particular are constantly exposed to environmental pro-oxidants.
Such exposure leads to harmful effects on the skin (e.g., premature
aging and cancer) in the absence of appropriate protective cell
responses.
The current study has demonstrated that DHF, a
naturally occurring antioxidant, activated HO-1 expression by
targeting the ARE sequence within the HO-1 gene promoter region.
The DHF-mediated activation of HO-1 was regulated through the
stimulation of Nrf2 in an ERK and PI3K/Akt-dependent manner.
Findings of previous studies have demonstrated the involvement of
ERK and PI3K/Akt in the activation of HO-1 expression and
Nrf2-mediated signal transduction (25–27). For example, ERK phosphorylates
Nrf2, triggering dissociation of the Keap1-Nrf2 complex and
translocation of Nrf2 into the nucleus, where it forms a
heterodimer with a small Maf protein (16,28–30). In the present study, DHF treatment
increased the levels of phospho-ERK and -PI3K/Akt. In addition,
specific inhibitors of the ERK and PI3K/Akt pathways suppressed the
nuclear translocation of Nrf2, suggesting that Nrf2 is a direct
downstream target of ERK and PI3K/Akt. Thus, ERK and PI3K/Akt may
be crucial components of the cellular signaling network responsible
for the activation of Nrf2 and the transcriptional regulation of
HO-1 gene expression in keratinocytes.
Similar to DHF, several compounds isolated from
natural products induce HO-1 expression via activation of Nrf2
downstream of ERK and/or PI3K/Akt. For instance, eckol (found in
brown algae) and (-)-epigallocatechin gallate (found in green tea)
increase HO-1 levels by activating ERK, PI3K/Akt and Nrf2 (31,32). In addition, baicalein (originally
isolated from the root of Scutellaria baicalensis) prevents
oxidative damage in PC12 cells by stimulating the PI3K/Akt/Nrf2
pathway in order to upregulate HO-1 expression (33). Furthermore, DHP exhibited the most
potent protective effects as compared with other flavonoids in the
neuroprotective area (34).
Under normal physiological conditions, Nrf2 is
sequestered in the cytoplasm by Keap1. Following the oxidation of
specific cysteine residues within Keap1 by electrophilic agents or
ROS, Nrf2 dissociates from its cytoplasmic docking protein and
translocates into the nucleus. There, it binds to the promoter
regions of several phase II detoxifying enzymes or antioxidant
enzymes including HO-1, thereby activating their transcription
(35).However, because the
half-life of Nrf2 is very short (<10 min), these mechanisms
necessitate the stabilization of the Nrf2 protein (36,37).
In our system, DHF treatment decreased Keap1
expression and increased the level and nuclear translocation of
Nrf2. These results suggest that DHF may retard Keap1-mediated Nrf2
degradation. Similarly, baicalein increases Keap1 degradation by
the proteasome system and prevents the Keap1-mediated inactivation
of Nrf2 (33). We also showed
that ERK and PI3K inhibitors decreased the DHF-induced accumulation
of phospho-Nrf2 through the inhibition of ERK and Akt
phosphorylation. This suggests that ERK and PI3K/Akt phosphorylate
Nrf2 and facilitate its release from the Keap1-Nrf2 complex,
allowing activated Nrf2 to translocate into the nucleus.
In summary, the present results suggest that DHF
protects HaCaT cells against oxidative stress-induced cell damage.
DHF exerts its protective functions by i) activating ERK and
PI3K/Akt, ii) facilitating the translocation of Nrf2 into the
nucleus and its subsequent binding to ARE and iii) upregulating
HO-1 expression.
Acknowledgements
This study was supported by a grant from the Korean
Ministry of Knowledge and Economy (R0000445).
References
|
1
|
Ismail NS, Pravda EA, Li D, Shih SC and
Dallabrida SM: Angiopoietin-1 reduces H(2)O(2)-induced increases in
reactive oxygen species and oxidative damage to skin cells. J
Invest Dermatol. 130:1307–1317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okayama Y: Oxidative stress in allergic
and inflammatory skin diseases. Curr Drug Targets Inflamm Allergy.
4:517–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Black HS: Potential involvement of free
radical reactions in ultraviolet light-mediated cutaneous damage.
Photochem Photobiol. 46:213–221. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cerutti PA: Prooxidant states and tumor
promotion. Science. 227:375–381. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harman D: Free radical theory of aging: an
update: increasing the functional life span. Ann NY Acad Sci.
1067:10–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryter SW, Alam J and Choi AM:
Hemeoxygenase-1/carbon monoxide: from basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tenhunen R, Marver HS and Schmid R: The
enzymatic conversion of heme to bilirubin by microsomal heme
oxygenase. Proc Natl Acad Sci USA. 61:748–755. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maines MD: Heme oxygenase: function,
multiplicity, regulatory mechanisms, and clinical applications.
FASEB J. 2:2557–2568. 1988.PubMed/NCBI
|
|
9
|
Ryter SW and Choi AM: Heme oxygenase-1:
molecular mechanisms of gene expression in oxygen-related stress.
Antioxid Redox Signal. 4:625–632. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Otterbein LE, Soares MP, Yamashita K and
Bach FH: Heme oxygenase-1: unleashing the protective properties of
heme. Trends Immunol. 24:449–455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li MH, Cha YN and Surh YJ: Peroxynitrite
induces HO-1 expression via PI3K/Akt-dependent activation of
NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med.
41:1079–1091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khor TO, Huang MT, Kwon KH, Chan JY, Reddy
BS and Kong AN: Nrf2-deficient mice have an increased
susceptibility to dextran sulfate sodium-induced colitis. Cancer
Res. 66:11580–11584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alam J and Cook JL: Transcriptional
regulation of the heme oxygenase-1 gene via the stress response
element pathway. Curr Pharm Des. 9:2499–2511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu S, Khor TO, Cheung KL, Li W, Wu TY,
Huang Y, Foster BA, Kan YW and Kong AN: Nrf2 expression is
regulated by epigenetic mechanisms in prostate cancer of TRAMP
mice. PLoS One. 5:e85792010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li MH, Jang JH, Na HK, Cha YN and Surh YJ:
Carbon monoxide produced by heme oxygenase-1 in response to
nitrosative stress induces expression of glutamate-cysteine ligase
in PC12 cells via activation of phosphatidylinositol 3-kinase and
Nrf2 signaling. J Biol Chem. 282:28577–28586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu R, Chen C, Mo YY, Hebbar V, Owuor ED,
Tan TH and Kong AN: Activation of mitogen-activated protein kinase
pathways induces antioxidant response element-mediated gene
expression via a Nrf2-dependent mechanism. J Biol Chem.
275:39907–39913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva MM, Santos MR, Caroco G, Rocha R,
Justino G and Mira L: Structure-antioxidant activity relationships
of flavonoids: a re-examination. Free Radic Res. 36:1219–1227.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melidou M, Riganakos K and Galaris D:
Protection against nuclear DNA damage offered by flavonoids in
cells exposed to hydrogen peroxide: the role of iron chelation.
Free Radic Biol Med. 39:1591–1600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Williams RJ, Spencer JP and Rice-Evans C:
Flavonoids: antioxidants or signaling molecules? Free Radic Biol
Med. 36:838–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moridani MY, Pourahmad J, Bui H, Siraki A
and O’Brien PJ: Dietary flavonoid iron complexes as cytoprotective
superoxide radical scavengers. Free Radic Biol Med. 34:243–253.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang R, Kang KA, Piao MJ, Ko DO, Wang ZH,
Chang WY, You HJ, Lee IK, Kim BJ, Kang SS and Hyun JW: Preventive
effect of 7,8-dihydroxyflavone against oxidative stress-induced
genotoxicity. Biol Pharm Bull. 32:166–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kutty RK and Maines MO: Oxidation of heme
c derivatives by purified heme oxygenase. Evidence for the presence
of one molecular species of heme oxygenase in the rat liver. J Biol
Chem. 257:9944–9952. 1982.PubMed/NCBI
|
|
24
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mtchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
25
|
Cullinan SB and Diehl JA: PERK-dependent
activation of Nrf2 contributes to redox homeostasis and cell
survival following endoplasmic reticulum stress. J Biol Chem.
279:20108–20117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bloom DA and Jaiswal AK: Phosphorylation
of Nrf2 at Ser40 by protein kinase C in response to antioxidants
leads to the release of Nrf2 from INrf2, but is not required for
Nrf2 stabilization/accumulation in the nucleus and transcriptional
activation of antioxidant response element-mediated NAD(P)H:
quinone oxidoreductase-1 gene expression. J Biol Chem.
278:44675–44682. 2003.
|
|
27
|
Hwang YP and Jeong HG: Ginsenoside Rb1
protects against 6-hydroxydopamine-induced oxidative stress by
increasing heme oxygenase-1 expression through an estrogen
receptor-related PI3K/Akt/Nrf2-dependent pathway in human
dopaminergic cell. Toxicol Appl Pharmacol. 242:18–28. 2010.
View Article : Google Scholar
|
|
28
|
Chan K, Han XD and Kan YW: An important
function of Nrf2 in combating oxidative stress: detoxification of
acetaminophen. Proc Natl Acad Sci USA. 98:4611–4616. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim YC, Masutani H, Yamaguchi Y, Itoh K,
Yamamoto M and Yodoi J: Hemin-induced activation of the thioredoxin
gene by Nrf2. A differential regulation of the antioxidant
responsive element by a switch of its binding factors. J Biol Chem.
276:18399–18406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwak MK, Itoh K, Yamamoto M and Kensler
TW: Enhanced expression of the transcription factor Nrf2 by cancer
chemopreventive agents: role of antioxidant response element-like
sequences in the nrf2 promoter. Mol Cell Biol. 22:2883–2892. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Na HK, Kim EH, Jung JH, Lee HH, Hyun JW
and Surh YJ: (-)-Epigallocatechin gallate induces Nrf2-mediated
antioxidant enzyme expression via activation of PI3K and ERK in
human mammary epithelial cells. Arch Biochem Biophys. 476:171–177.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY,
Kang MY, Lee SJ, Lee NH, Surh YJ and Hyun JW: Up-regulation of
Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin
compound, through activation of Erk and PI3K/Akt. Int J Biochem
Cell Biol. 42:297–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Cui W, Li G, Yuan S, Xu D, Hoi
MP, Lin Z, Dou J, Han Y and Lee SM: Baicalein protects against
6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1
and involving PKCα and PI3K/AKT signaling pathways. J Agric Food
Chem. 60:8171–8182. 2012.PubMed/NCBI
|
|
34
|
Jang SW, Liu X, Yepes M, Shepherd KR,
Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE and Ye K: A
selective TrkB agonist with potent neurotrophic activities by
7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 107:2687–2692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jain AK, Mahajan S and Jaiswal AK:
Phosphorylation and dephosphorylation of tyrosine 141 regulate
stability and degradation of INrf2: a novel mechanism in Nrf2
activation. J Biol Chem. 283:17712–17720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee OH, Jain AK, Papusha V and Jaiswal AK:
An auto-regulatory loop between stress sensors INrf2 and Nrf2
controls their cellular abundance. J Biol Chem. 282:36412–36420.
2007. View Article : Google Scholar : PubMed/NCBI
|