Introduction
Chronic airway disorders, including chronic
obstructive pulmonary disease, cystic fibrosis and asthma, are
associated with persistent pulmonary inflammation, airway
remodeling, goblet cell metaplasia and collagen deposition
(1), which significantly
contribute to morbidity and mortality worldwide. The incidence and
severity of chronic lung diseases are constantly increasing.
Chronic lung diseases affect from 100 to 150 million individuals
worldwide and are associated with a significantly high mortality
rate (2). The pathogenesis of
asthma was originally thought to be a T helper 2 (Th2)-mediated
disease, although proinflammatory cytokines also play significant
and distinct roles in the pathogenesis of asthma. Th2 cytokines,
such as interleukin (IL)-4, IL-5, IL-9 and IL-13 induce changes in
the airways and lung parenchyma, which in turn lead to airway
eosinophilia, epithelial cell proliferation with goblet cell
hyperplasia, increased mucus secretion, smooth muscle hyperplasia,
subepithelial fibrosis, immunoglobulin E (IgE) secretion and an
increased production of chemokines that attract T cells,
eosinophils, neutrophils and mast cells (3). Th2 cytokines, such as IL-5-activate
eosinophils (4,5), whose migration in turn induces
eosinophilic airway inflammation. IL-4 directly promotes the
characteristics of asthma, such as Ig isotype switching in B cells
to primarily produce IgE and IgG1 (6,7).
IL-13 production has been observed in the skin and gingival
fibroblasts (8,9). Transforming growth factor β1
(TGF-β1) and IL-13 activate lung fibroblasts in patients with
asthma (10). In the asthmatic
patient, eosinophils produce profibrotic and proangiogenic
cytokines, such as TGF-β1 and vascular endothelial growth factor
(VEGF) (11). TGF-β1 expression
is increased in patients with asthma, and this increase seems to
correlate with disease severity and the degree of peribronchial
fibrosis (12).
VEGF has been shown to play a critical role in
increasing Th2 cell-mediated inflammation and TGF-β1 production
(13), which is consistent with
the finding that the inhibition of VEGF expression attenuates
peribronchial fibrosis by blocking TGF-β1 production (14). The recruitment and lung
infiltration of eosinophils are controlled by chemokines and
adhesion molecules. Vascular cell adhesion molecule 1 (VCAM-1) is
expressed on activated endothelial cells and contributes to the
firm adhesion of eosinophils to vascular endothelial cells
(15). Intercellular adhesion
molecule 1 (ICAM-1) is an endothelial transmembrane protein that
facilitates the endothelial transmigration of eosinophils into
different pathological environments (16).
Bangpungtongseong-san (BPTS) is a major traditional
herbal medicine widely used in the treatment of obesity (17). BPTS has been reported to inhibit
atherosclerosis (18), obesity
(19), hypertension (20) and allergic rhinitis (21). However, to the best of our
knowledge, the effects of BPTS on airway inflammation and lung
fibrosis in chronic asthma have not yet been described.
Glucocorticoids are anti-inflammatory medications and are often
used as maintenance therapy in patients with acute and chronic
asthma, despite the fact that some of them are steroid resistant.
There is a great need for a more effective treatment for chronic
asthma, with fewer undesired side-effects. In the present study, we
examined whether BPTS exerts inhibitory effects on airway
inflammation and pulmonary fibrosis in a mouse model of chronic
asthma induced by repeated ovalbumin (OVA) challenge.
Materials and methods
Preparation of BPTS extract
BPTS was prepared in our laboratory from a mixture
of chopped crude herbs purchased from Omniherb (Yeongcheon, Korea)
and HMAX (www.HMAX.co.kr; Chungbuk, Korea). BPTS was
prepared from a mixture of herbs (shown in Table I) and was extracted in distilled
water at 100°C for 2 h. The extract was evaporated to dryness and
freeze-dried (yield, 22.21%). The composition of BPTS was analyzed
using high-performance liquid chromatography (HPLC) as previously
described (22). The chemical
standards used to identify and quantify the compounds in the BPTS
were geniposide, liquiritin, baicalin and glycyrrhizin.
 | Table ICrude components contained in
Bangpungtongseong-san (BPTS) extract. |
Table I
Crude components contained in
Bangpungtongseong-san (BPTS) extract.
| Scientific name | Amount (g) | Company of
purchase | Source |
|---|
| Talcum | 6.375 | HMAX | China |
| Glycyrrhiza
uralensis | 4.5 | HMAX | China |
| Gypsum | 2.625 | HMAX | China |
| Scutellaria
baicalensis | 2.625 | HMAX | Jeongseon, Korea |
| Platycodon
grandiflorum | 2.625 | Omniherb | Yeongcheon,
Korea |
| Ledebouriella
seseloides | 1.6875 | HMAX | China |
| Cnidium
officinale | 1.6875 | Omniherb | Yeongcheon,
Korea |
| Angelica
gigas | 1.6875 | Omniherb | Pyeongchang,
Korea |
| Paeonia
lactiflora | 1.6875 | Omniherb | Hwasun, Korea |
| Rheum
undulatum | 1.6875 | HMAX | China |
| Ephedra
sinica | 1.6875 | HMAX | China |
| Mentha
pulegium | 1.6875 | Omniherb | China |
| Forsythia
koreana | .6875 | HMAX | China |
| Erigeron
canadensis | 1.6875 | HMAX | China |
| Schizonepeta
tenuifolia | 1.3125 | Omniherb | China |
| Atractylodes
japonica | 1.31 | HMAX | China |
| Gardenia
jasminoides | 1.3 | Omniherb | Muju, Korea |
| Zingiber
officinale | 6.25 | Omniherb | Yeongcheon,
Korea |
| Total | 44.125 | | |
Animals and environmental conditions
Specific pathogen-free female BALB/c mice (7 weeks
old) were purchased from Orient Co. (Seoul, Korea) and maintained
in an animal facility under standard laboratory conditions for 1
week before the experiments were conducted. The animals were
provided with water and standard chow ad libitum. All the
experimental procedures were carried out in accordance with the
National Institutes of Health (NIH) Guidelines for the Care and Use
of Laboratory Animals and were approved by the Animal Care and Use
Committee of Chungnam National University, Daejeon, Korea. The
animals were cared for in accordance with the National Animal
Welfare Law of Korea.
Experimental protocol
Specific pathogen-free female BALB/c mice (n=35)
were sensitized on days 0 and 14 by an intraperitoneal injection of
20 μg of OVA, emulsified with 2 mg of aluminum hydroxide in 200 μl
of phosphate-buffered saline (PBS) (pH 7.4). On day 21, the mice
received an airway challenge with OVA [1% (w/v)] for 1 h with the
use of an ultrasonic nebulizer (NE-U12; Omron Corp., Tokyo, Japan);
this step was repeated 3 times per week, for 4 weeks. Each day
during the 4 weeks of the airway challenge, BPTS was freshly
prepared in PBS and administered by gavage at 50 mg/kg or 100 mg/kg
of body weight. The mice were divided into 5 groups as follows (n=7
per group): PBS/PBS, normal control mice treated with PBS only;
OVA/PBS; OVA-sensitized/challenged mice treated orally with PBS;
OVA/montelukast (Mon), OVA-sensitized/challenged mice treated
orally with montelukast (30 mg/kg); OVA/BPTS-50,
OVA-sensitized/challenged mice treated orally with BPTS (50 mg/kg);
and OVA/BPTS-100, OVA-sensitized/challenged mice treated orally
with BPTS (100 mg/kg).
At the end of the 4 weeks of OVA challenge,
bronchoalveolar lavage fluid (BALF) samples were obtained for
analysis, as previously described (22). In brief, the mice were sacrificed
48 h after the final challenge by an intraperitoneal injection of
pentobarbital (50 mg/kg; Hanlim Pharmaceutical Co., Seoul, Korea),
and a tracheostomy was performed. To obtain BALF, ice-cold PBS (0.5
ml) was infused into the lungs 3 times and withdrawn each time via
tracheal cannulation (total volume 1.5 ml). Total inflammatory cell
numbers were determined by counting cells in at least 5 squares of
a hemocytometer, after having excluded the dead cells by Trypan
blue staining. To determine the differential cell count, 100 μl of
BALF were centrifuged (200 × g, 4°C, 10 min) onto slides using a
Cytospin unit (Hanil Science Industrial, Seoul, Korea). The slides
were dried, and the cells were fixed and stained using
Diff-Quik® staining reagent (B4132-1A; IMEB Inc., San
Marcos, CA, USA) according to the manufacturer’s instructions. The
supernatant obtained from the BALF Cytospin was stored at −70°C for
biochemical analysis.
Determination of total cell, eosinophil,
lymphocyte, neutrophil and macrophage cell counts in BALF
Differential cell counting was performed as
previously described (22).
Measurement of cytokine and chemokine
levels in BALF
The levels of IL-4, IL-13, IL-33, tumor necrosis
factor-α (TNF-α) and eotaxin in BALF were measured using
enzyme-linked immunosorbent assay (ELISA) kits according to the
manufacturer’s instructions (BioSource International, Camarillo,
CA, USA) as previously described (22).
Measurement of TGF-β1 and VEGF expression
levels in lung tissue
c and VEGF expression levels in BALF were measured
using the respective ELISA kits according to the manufacturers’
instructions (TGF-β1, R&D Systems, Inc., Minneapolis, MN, USA;
and VEGF, Immuno-Biological Laboratories Co., Ltd., Minneapolis,
MN, USA). Total protein concentration was measured with a kit assay
(Bio-Rad, Hercules, CA, USA). The results are expressed as pg/mg
protein.
Measurement of total and OVA-specific IgE
expression levels in BALF and plasma
Serum was collected via centrifugation (200 × g, 10
min) and stored at −70°C. Total and OVA-specific IgE levels were
measured using ELISA as previously described (22).
Immunoblot analysis
Equal amounts of total lung protein (30 μg) were
heated at 100°C for 5 min, loaded onto 8% sodium dodecyl sulfate
polyacrylamide gels and electrophoresed. The proteins were then
transferred onto nitrocellulose membranes (at 100 V for 2 h), and
the membranes were blocked for 1 h with Tris-buffered saline
containing 0.05% Tween-20 (TBST) plus 5% skim milk. The blocked
membranes were incubated with primary antibodies against VEGF,
VCAM-1, ICAM-1, TGF-β1 and Smad3 (all at a 1:1,000 dilution; Abcam,
Cambridge, MA, USA) and anti-β-actin antibody (1:1,000 dilution;
Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at
4°C. The membranes were washed three times with TBST and then
incubated with a horseradish peroxidase-conjugated secondary
antibody (1:3,000 dilution; Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA) for 1 h at room temperature. The
membranes were washed another 3 times with TBST and developed using
the enhanced chemiluminescence kit (Amersham Pharmacia Biotech,
Uppsala, Sweden) according to the manufacturer’s instructions. For
quantitative analysis, densitometric band values were determined
using ChemiDoc (Bio-Rad).
Histological analysis
For histological examination, before the lungs were
removed, the left lungs were filled intratracheally with a fixative
(0.8% formalin, 4% acetic acid) using a ligature around the
trachea. Tissues were embedded in paraffin, sectioned at 4 μm
thickness, and stained with hematoxylin and eosin (H&E)
solution (MHS-16 and HT110-1-32 respectively; Sigma, St. Louis, MO,
USA) and periodic acid-Schiff (PAS) (IMEB Inc.) in order to
estimate inflammation and mucus production, respectively. To
evaluate peribronchial fibrosis, the sections were stained with
Masson’s trichrome.
For immunohistochemistry, the paraffin sections were
deparaffinized, dehydrated, washed in PBS with 0.3% Triton X-100
and pre-incubated for 10 min at room temperature with 10% goat
serum to block non-specific staining. The slides were then
incubated with primary mouse TGF-β1 antibodies (1:100 dilution,
Abcam) overnight at 4°C. The following day, the primary antibodies
were removed, the sections were washed and incubated with
biotinylated secondary antibody at 37°C for 1 h, and then incubated
with an avidin-biotin-peroxidase complex (Vector Laboratories Inc.,
Burlingame, CA, USA) at room temperature for 1 h. After the excess
complex was removed, the sections were washed with PBS and
incubated with 0.05% diaminobenzidine (1:200; Millipore, Billerica,
MA, USA) for a further 10 min. The sections were counterstained,
rinsed in PBS to terminate the reaction, and protected with cover
slips for microscopic examination.
Statistical analysis
Data are presented as the means ± SD. Statistical
significance was determined using analysis of variance followed by
a multiple comparison test with Bonferroni adjustment. Values of
p<0.05 or <0.01 were considered to indicate statistically
significant differences.
Results
BPTS reduces the number of inflammatory
cells in BALF
The effects of BPTS were investigated on various
cell types present in BALF. The repeated challenge with OVA causes
infiltration predominantly by neutrophils. As shown in Fig. 1, the number of neutrophils,
lymphocytes, macrophages, eosinophils and total cells in BALF 2
decreased significantly in a dose-dependent manner following
treatment with BPTS.
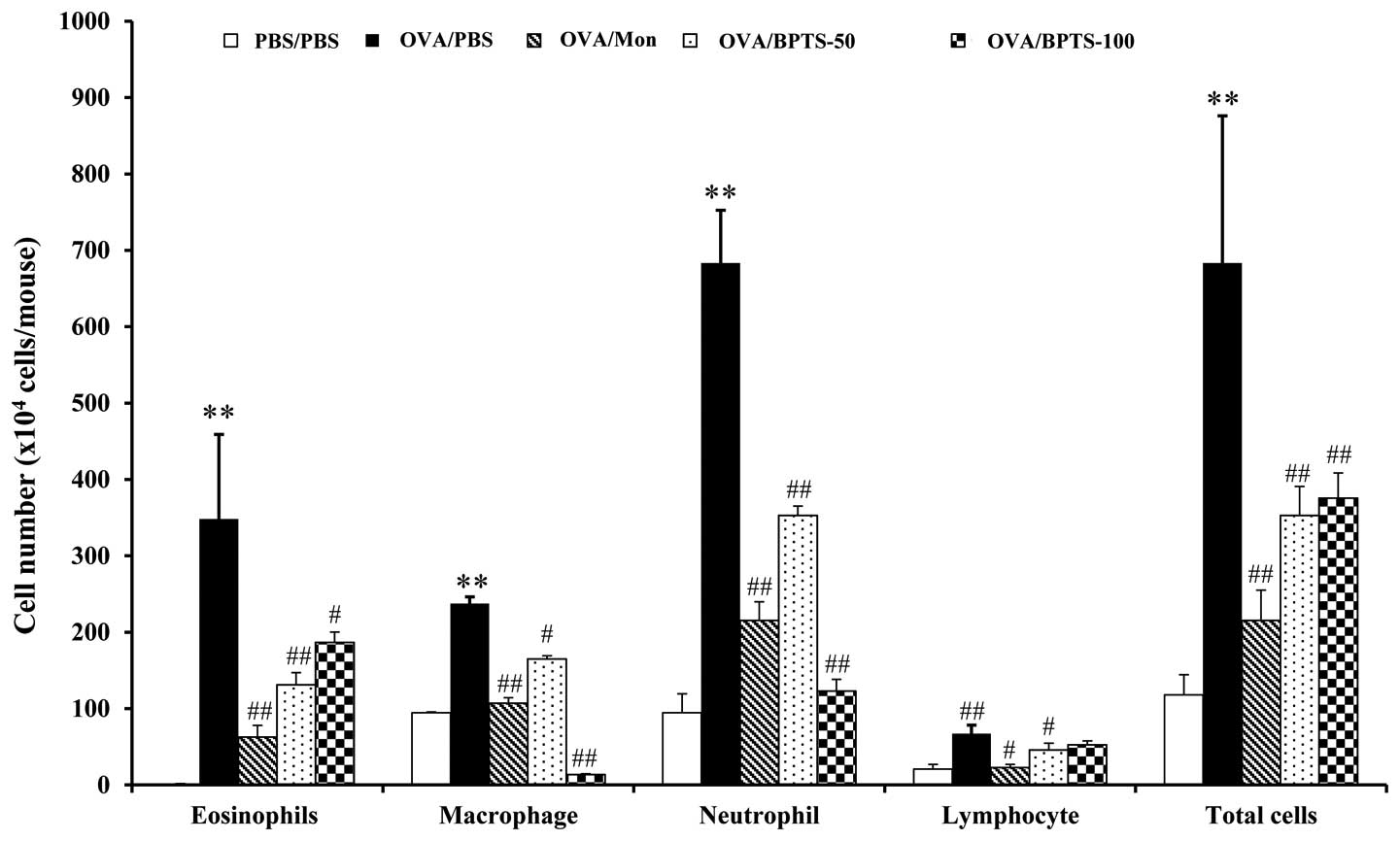 | Figure 1Bangpungtongseong-san water extract
(BPTS) inhibits the recruitment of inflammatory cells in
bronchoalveolar lavage fluid (BALF) of mice 48 h after the final
ovalbumin (OVA) challenge. Cells were isolated by centrifugation
and stained with Diff-Quik stain reagent. With the use of light
microscopy after having counted cells in at least 5 squares of a
hemocytometer and having excluded dead cells with the use of Trypan
blue, all inflammatory cells were calculated. Phosphate-buffered
saline (PBS)/PBS, normal control mice treated with PBS only;
OVA/PBS, OVA-sensitized/challenged mice treated orally with PBS;
OVA/montelukast (Mon), OVA-sensitized/challenged mice montelukast
(30 mg/kg); OVA/BPTS-50, OVA-sensitized/challenged mice treated
orally with BPTS (50 mg/kg); OVA/BPTS-100,
OVA-sensitized/challenged mice treated orally with BPTS (100
mg/kg). Values are expressed as the means ± SEM (n=7/group).
**Significantly different from PBS/PBS, P<0.01;
#,##significantly different from OVA/PBS, P<0.05 and
<0.01, respectively. |
BPTS decreases the levels of IL-4, IL-13
and eotaxin in BALF
The levels of IL-4, IL-13 and eotaxin in BALF were
significantly higher in the OVA-sensitized/challenged mice
(OVA/PBS) compared with the PBS/PBS mice (Fig. 2). The BPTS-treated mice presented
significantly lower levels of these 3 compounds compared with the
OVA/PBS mice. Similar to the Th2-type cytokine levels, the eotaxin
levels increased in the OVA/PBS group and decreased in a
dose-dependent manner in the BPTS-treated group (Fig. 2C).
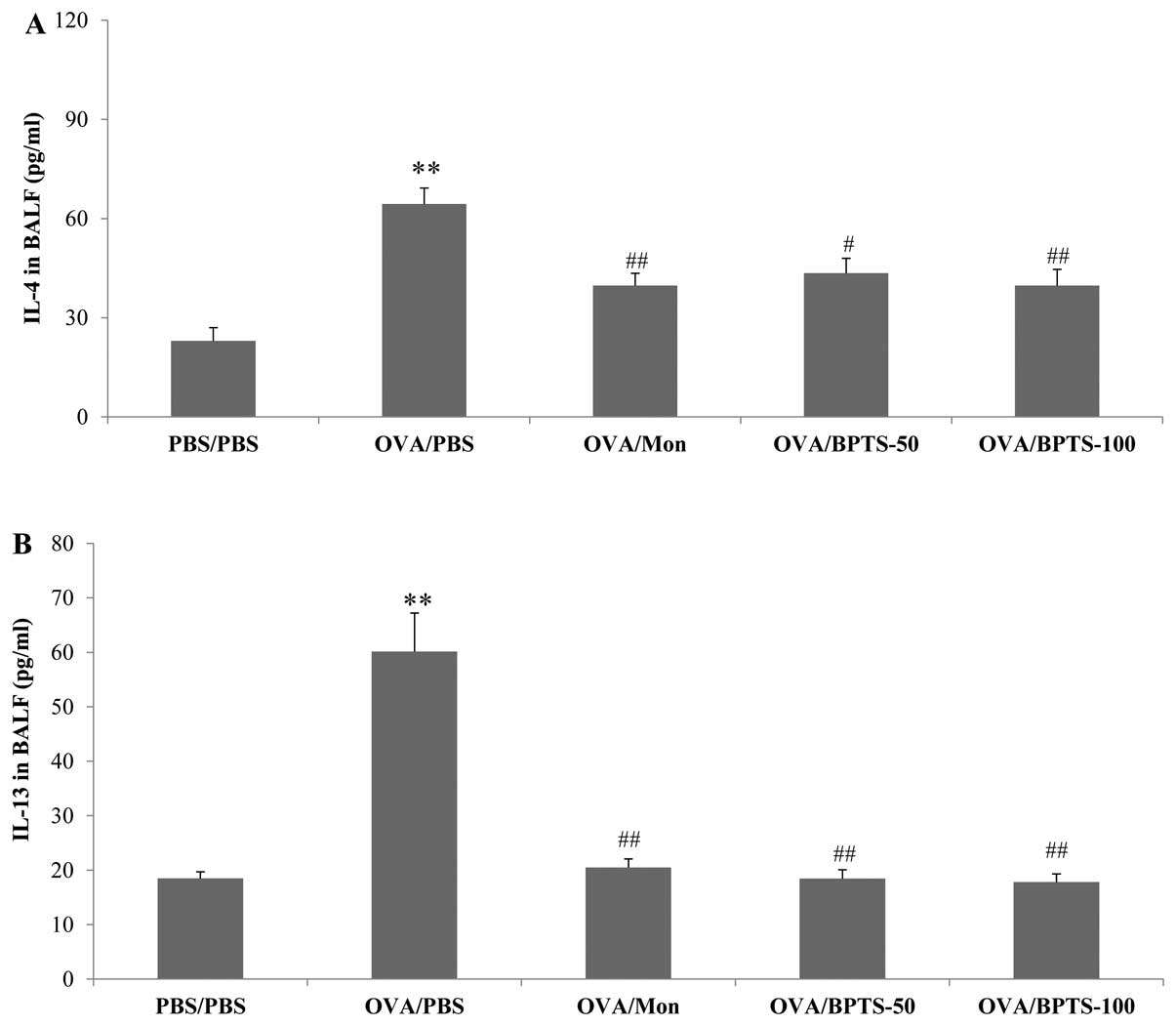 | Figure 2Bangpungtongseong-san water extract
(BPTS) reduces the levels of (A) IL-4, (B) IL-13, and (C) eotaxin
in bronchoalveolar lavage fluid (BALF) of mice 48 h after the final
OVA challenge. Phosphate-buffered saline (PBS)/PBS, normal control
mice treated with PBS only; OVA/PBS, OVA-sensitized/challenged mice
treated orally with PBS; OVA/montelukast (Mon),
OVA-sensitized/challenged mice treated orally with montelukast (30
mg/kg); OVA/BPTS-50, OVA-sensitized/challenged mice treated orally
with BPTS (50 mg/kg); OVA/BPTS-100, OVA-sensitized/challenged mice
treated orally with BPTS (100 mg/kg). Values are expressed as the
means ± SEM (n=7/group). **Significantly different from
PBS/PBS, P<0.01; #,##significantly different from
OVA/PBS, P<0.05 and <0.01, respectively |
BPTS decreases the expression levels of
total IgE and OVA-specific IgE in BALF and plasma
The expression levels of total IgE and OVA-specific
IgE in BALF and plasma were much higher in the OVA/PBS mice than in
the PBS/PBS mice (Table II). The
BPTS-treated mice had significantly lower levels of total IgE and
OVA-specific IgE in BALF and plasma compared with the OVA/PBS
mice.
 | Table IILevels of total IgE and ovalbumin
(OVA)-specific IgE in bronchoalveolar lavage fluid (BALF) and
plasma. |
Table II
Levels of total IgE and ovalbumin
(OVA)-specific IgE in bronchoalveolar lavage fluid (BALF) and
plasma.
| BALF | Plasma |
|---|
|
|
|---|
| Group | Total IgE
(ng/ml) | OVA-specific IgE
(ng/ml) |
|---|
| PBS/PBS | 1.50±1.11 | <0 |
| OVA/PBS | 32.55±17.94a |
204.91±37.72a |
| OVA/Mon | 14.42±4.30c |
101.30±46.93c |
| OVA/BPTS-50 | 15.49±4.99c |
115.21±44.78c |
| OVA/BPTS-100 | 19.65±5.87b | 185.22±22.99 |
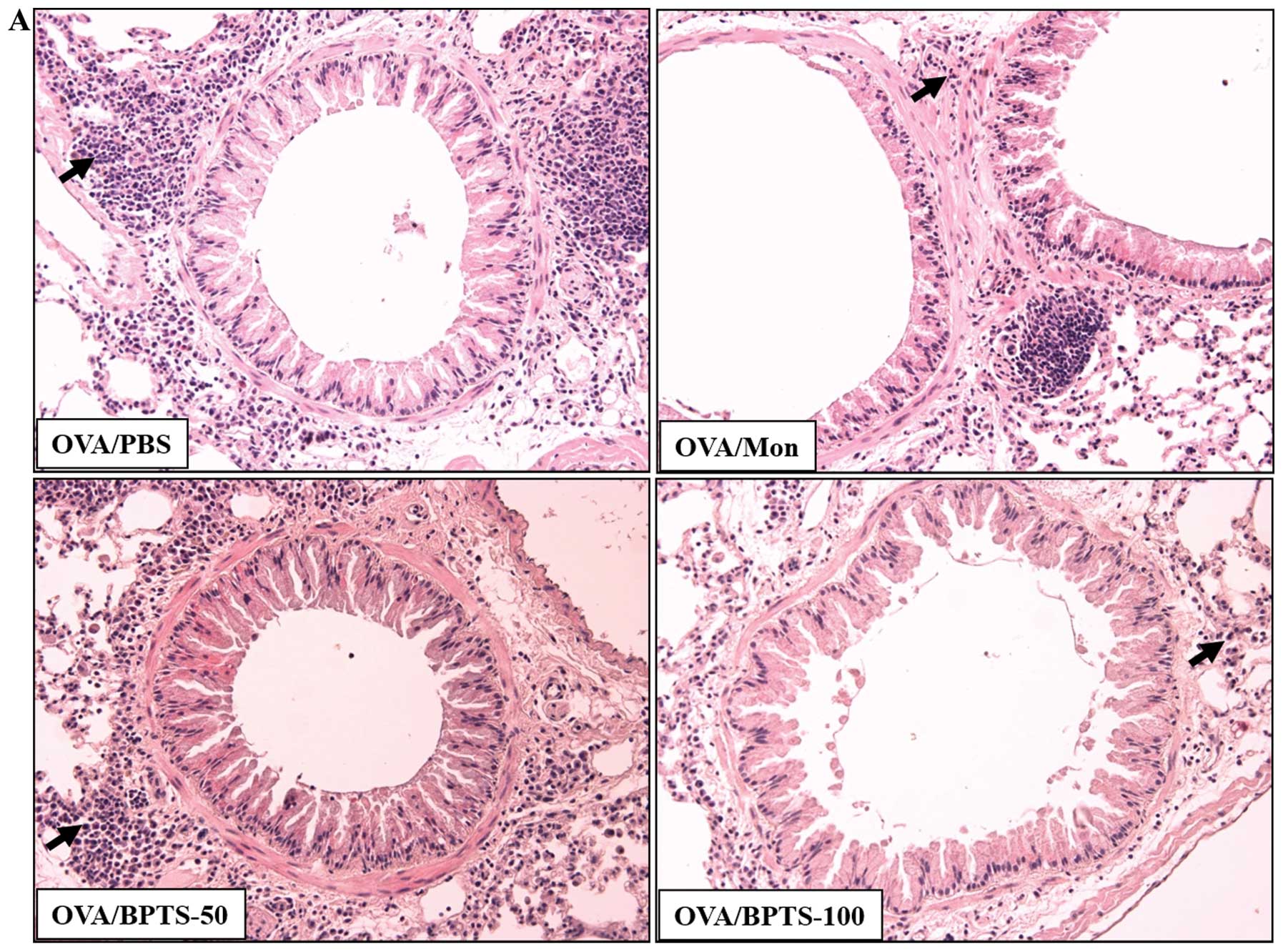
BPTS decreases inflammatory infiltration,
mucus hypersecretion and peribronchial collagen deposition
Histological analysis of the lung sections of the
OVA/PBS mice showed dense inflammatory cell infiltration into the
perivascular and peribronchial regions, as well as increased mucus
secretion (Fig. 3A and B). The
OVA/PBS mice had significantly greater subepithelial and
interstitial fibrosis compared with the PBS/PBS mice, as assessed
by Masson’s trichrome staining (Fig.
3C). Collagen levels in lung tissue, as assessed by ELISA, were
significantly higher in the OVA/PBS mice (Fig. 3D). The OVA-challenged mice treated
with BPTS presented significantly lower levels of inflammatory cell
infiltration, mucus hypersecretion and peribronchial fibrosis
(including collagen levels) compared with the OVA/PBS mice.
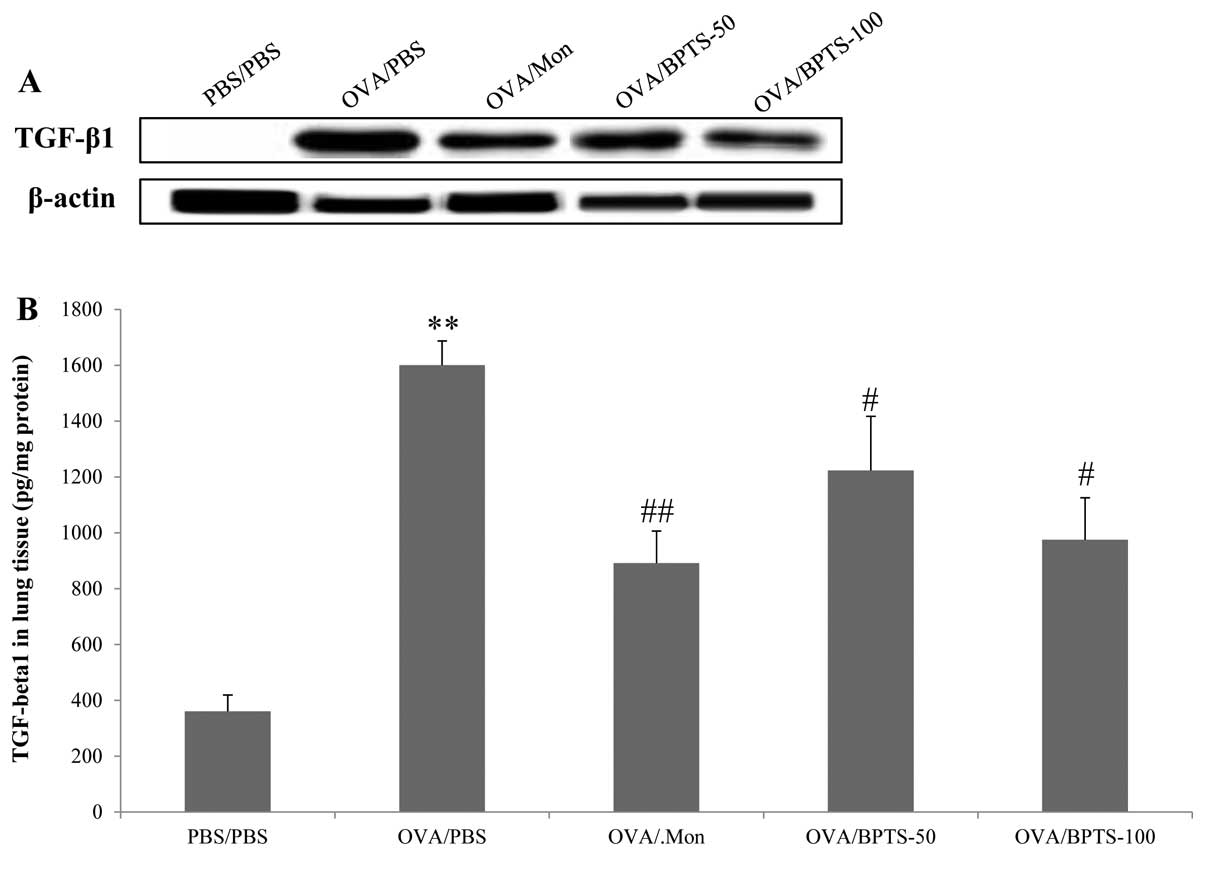
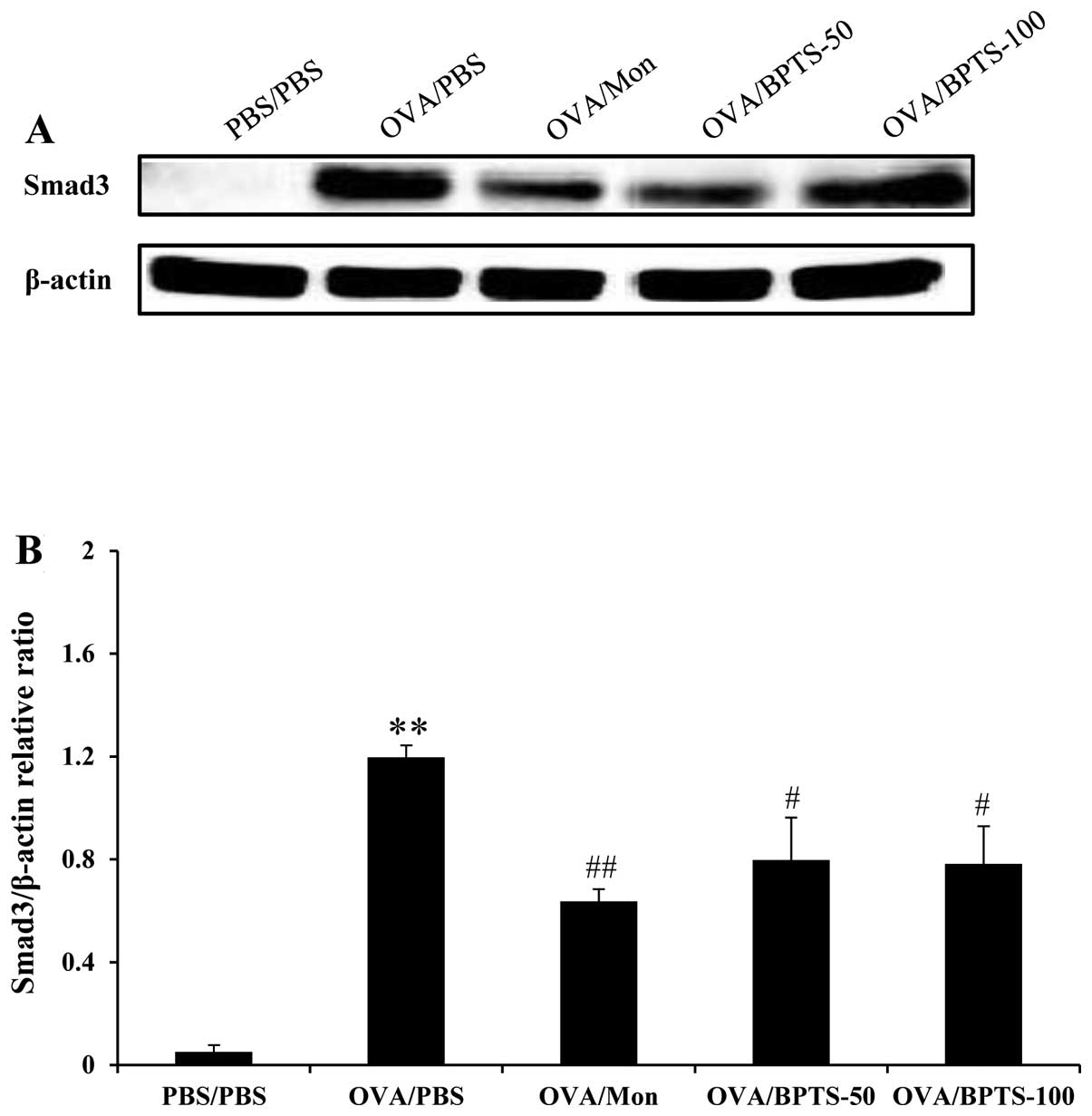
BPTS decreases TGF-β1 and Smad3
expression levels in lung tissue
To identify the possible mechanisms underlying the
inhibitory effects of BPTS on pulmonary fibrosis, TGF-β1 and Smad3
expression levels were examined in relation to the fibrotic lung
response of OVA/PBS mice. Western blot analysis revealed that
TGF-β1 (Fig. 4A) and Smad3
(Fig. 5A and B) protein
expression levels were significantly higher in the lung tissues of
OVA/PBS mice compared with those of PBS/PBS mice, after they had
undergone the final OVA challenge. BPTS treatment led to a
significant attenuation of the increased TGF-β1 expression levels
compared with the OVA-challenged mice treated with PBS (Fig. 4A). These results were consistent
with those obtained by ELISA analysis of the lung tissue (Fig. 4B) and immunohistochemistry
(Fig. 4C).
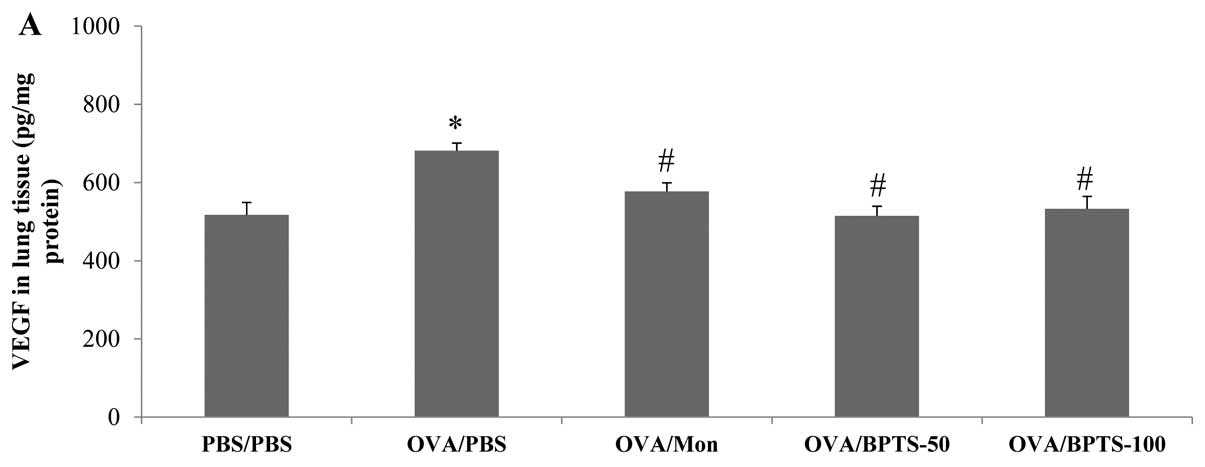
BPTS decreases the expression levels of
VEGF in BALF and lung tissue
As shown in Fig.
6A, the VEGF expression levels in the lung tissue were
significantly higher in the OVA/PBS mice compared with the PBS/PBS
mice. The level of VEGF was significantly lower in the BPTS-treated
mice compared with the OVA/PBS mice. Consistent with these results,
the immunoblot analysis results (Fig.
6B and C) and immunohistochemistry (Fig. 6D) of the lung sections revealed
that VEGF was more abundant in the epithelial cells and
inflammatory cells of the OVA/PBS mice compared with those of the
PBS/PBS mice. The BPTS-treated mice showed a reduced VEGF
expression compared with the untreated OVA-challenged mice.
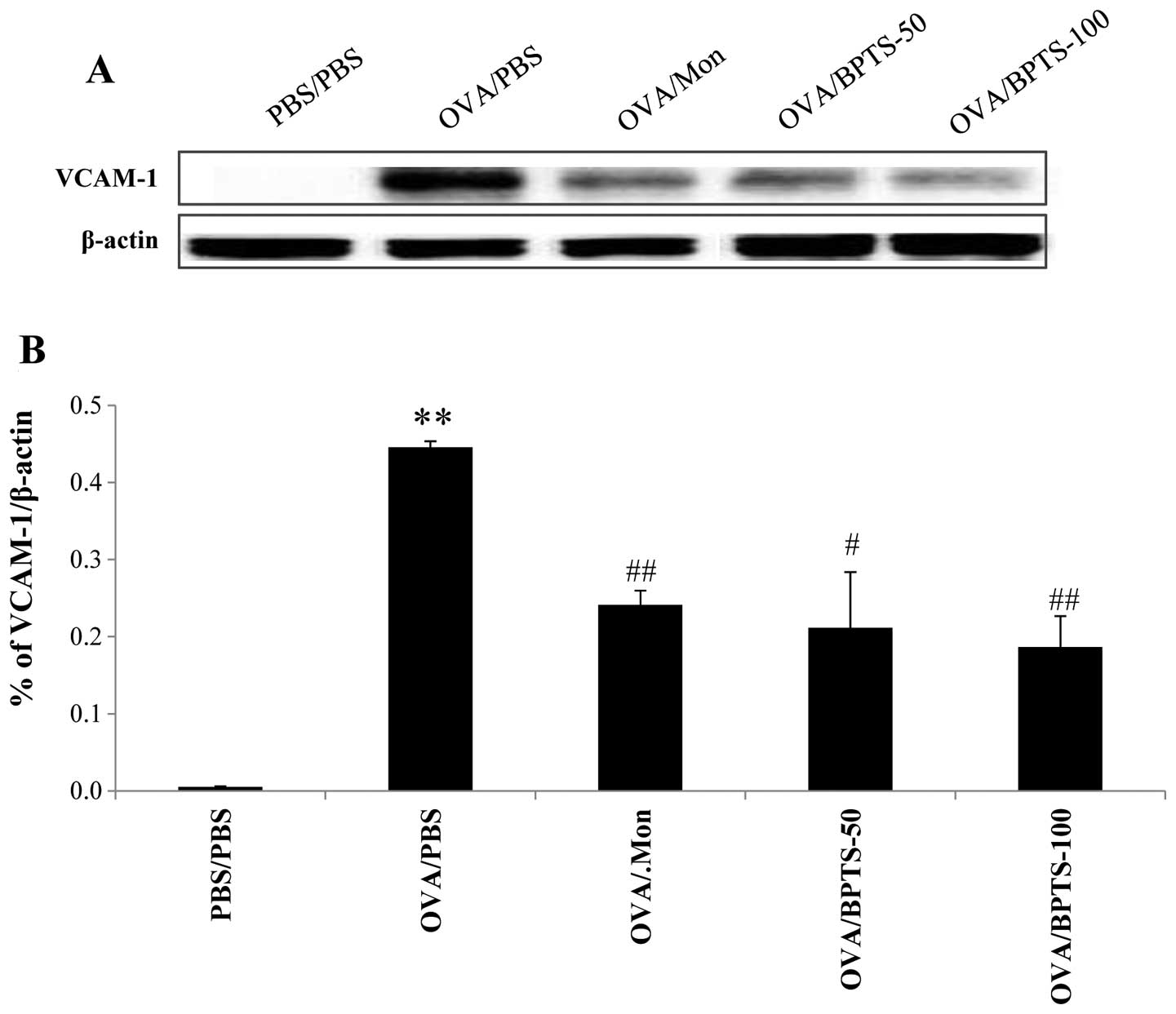
BPTS decreases the expression levels of
VCAM-1 and ICAM-1 in lung tissue
To determine whether BPTS protects against asthmatic
effects through VEGF, we investigated the expression of adhesion
molecules related to inflammatory cell infiltration in the lungs.
As shown in Fig. 7, the
expression levels of ICAM-1 and VCAM-1 in the lung tissue were
significantly higher in the OVA/PBS mice compared with the PBS/PBS
mice. BPTS treatment reduced the expression of VCAM-1 (Fig. 7A and B) and ICAM-1 (Fig. 7C and D) compared with control the
OVA/PBS mice.
Discussion
In the present study, we evaluated the effects of
BPTS in a murine model of experimental chronic OVA-induced asthma.
We measured the levels of Th2 cytokines, chemokines and IgE, and
assessed the histopathological changes following treatment with
BPTS in OVA-challenged mice. To determine whether BPTS acts through
an anti-fibrotic mechanism, immunoblot analysis and
immunohistochemistry were performed to measure the abundance of
adhesion molecules (VCAM-1 and ICAM-1), VEGF, TGF-β1 and Smad3
proteins in lung tissue. Chronic exposure of the OVA-challenged
mice to BPTS reduced airway inflammatory cell infiltration, as well
as the levels of cytokines, chemokines and IgE, collagen
deposition, and the expression levels of VEGF, adhesion molecules,
TGF-β1 and Smad3.
Chronic asthma is characterized by airway
inflammation, remodeling of the airways and hyperresponsiveness to
environmental stimuli. Chronic asthma is accompanied by thickening
of the bronchial walls, epithelial damage, subepithelial fibrosis,
increased deposition of extracellular matrix proteins, and
increased activity of various cytokines and growth factors, all of
which contribute to airway remodeling. Airway inflammation is a
hallmark characteristic of chronic asthma and is closely associated
with Th2 cell activation and the release of cytokines, including
IL-4, IL-5 and IL-13, which in turn accelerate the airway
inflammatory cell infiltration (23). At elevated levels, Th2 cytokines
can stimulate the production of growth factors, including VEGF and
TGF-β1, thereby resulting in pulmonary fibrosis (24,25).
In our study, the administration of BPTS was found
to reduce airway inflammatory cell infiltration and attenuate mucus
hypersecretion by decreasing the Th2 cytokine and IgE expression
levels. Histopathological analysis supported these findings,
showing that BPTS attenuated both inflammatory cell infiltration in
the peribronchial and perivascular regions, as well as the
associated mucus production. According to these results, the
administration of BPTS may suppress an ongoing Th2 response in
chronic asthma.
In the current study, OVA-challenged mice displayed
the pathophysiological features of chronic asthma, including airway
inflammation, bronchial wall remodeling, elevated levels of Th2
cytokines, increased collagen concentration and increased levels of
VEGF and TGF-β1. BPTS reduced the concentration of both IL-4 and
IL-13 in BALF. These Th2 cytokines stimulate bronchial epithelia to
secrete TGF-β1, by passing the need for inflammatory cells
(26). The Th2 cytokine level
reduction is closely linked to the expression levels of growth
factors, such as VEGF and TGF-β1, which are considered to be
important in the pathophysiological process of chronic asthma.
Bronchial wall remodeling, a fundamental factor in the development
of asthma, results from abnormal differentiation patterns of
bronchial cells, in particular fibroblasts (27,28). Cellular responses induce IgE
switching, which leads to mucus hypersecretion from goblet cells
(22). As shown by the results of
the current study, the administration of BPTS reduced airway
inflammatory cell infiltration and attenuated mucus hypersecretion
by decreasing Th2 cytokine and IgE expression levels. Inflammatory
cell infiltration and mucus hypersecretion are regulated by the
elevated production levels of proinflammatory cytokines, in
particular TGF-β, in regions of epithelial damage, as reflected in
BALF from asthmatics (29). Our
finding that BPTS reduced VEGF, TGF-β1 and Smad3 expression levels
in the lung tissue are consistent with their roles in chronic
asthma.
TGF-β1 is an important fibrogenic and
immunomodulatory factor that is considered to play a pivotal role
in the pathogenesis of airway remodeling (30). Cytokine TGF-β1 concentration is
elevated in asthma, and this increase is thought to be involved in
the airway pathology associated with asthma (31). The release of TGF-β1 is regulated
by eosinophils and is stimulated by environmental factors, such as
allergens and cigarette smoke (32). Therefore, inhibiting the
production of TGF-β1 or blocking its bioactivity in order to
suppress airway collagen formation is considered a potential
treatment strategy for preventing airway remodeling(12,33).
In asthma, the increased vascularization of the
airway walls correlates with an increased VEGF expression in the
airways (34). Lee et al
(24) reported direct evidence
for the involvement of VEGF in lung tissue remodeling. VEGF is a
multifunctional cytokine required for endothelial cell survival
that has potent actions on vascular endothelial cells, including
increased vascular permeability, the induction of endothelial cell
mitogenesis and increased cell migration (35,36).
Consistent with previous studies, the present study
showed that OVA challenge increased TGF-β1 and VEGF expression
levels and decreased the airway migration of eosinophils. These
data suggest that TGF-β1 inhibition is regulated by the suppression
of eosinophil migration. Thus, treatment with BPTS may prevent
pulmonary fibrosis in lung tissue by inhibiting collagen
deposition. These observations are consistent with our findings
that VEGF, TGF-β1 and Smad3 protein expression levels were
upregulated, and pulmonary fibrosis was increased in OVA-challenged
mice. By contrast, the administration of BPTS significantly blocked
the pulmonary fibrosis induced in OVA-challenged mice by reducing
VEGF, TGF-β1, and Smad3 expression levels in lung tissue. These
results suggest that BPTS attenuates pulmonary fibrosis induced by
repeated OVA challenge, at least partly by inhibiting VEGF and
TGF-β1-Smad3 signaling.
In conclusion, the administration of BPTS reduces
airway inflammation and collagen deposition by reducing Th2
cytokine release and inhibiting the expression of VEGF, TGF-β1 and
Smad3 in lung tissue from OVA-challenged mice. These data suggest
that BPTS effectively protects against chronic asthma symptoms,
such as inflammatory cell infiltration, mucus-secreting goblet cell
hyperplasia and collagen deposition in the airways, which are
induced by repeated OVA challenge.
Acknowledgements
This research was part of a project (The Evidence
Based Medicine for Herbal Formula) funded by the Basic Herbal
Medicine Research Group in the Korea Institute of Oriental
Medicine.
References
|
1
|
Busse WW and Lemanske RF Jr: Asthma. N
Engl J Med. 344:350–362. 2001. View Article : Google Scholar
|
|
2
|
Rincon M and Irvin CG: Role of IL-6 in
asthma and other inflammatory pulmonary diseases. Int J Biol Sci.
8:1281–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finkelman FD, Holmes J, Urban JF Jr, Paul
WE and Katona IM: T help requirements for the generation of an in
vivo IgE response: a late acting form of T cell help other than
IL-4 is required for IgE but not for IgG1 production. J Immunol.
142:403–408. 1989.PubMed/NCBI
|
|
4
|
Adachi T, Motojima S, Hirata A, Fukuda T
and Makino S: Eosinophil viability-enhancing activity in sputum
from patients with bronchial asthma, Contributions of interleukin-5
and granulocyte/macrophage colony-stimulating factor. Am J Respir
Crit Care Med. 151:618–623. 1995.
|
|
5
|
Sur S, Kita H, Gleich GJ, Chenier TC and
Hunt LW: Eosinophil recruitment is associated with IL-5, but not
with RANTES, twenty-four hours after allergen challenge. J Allergy
Clin Immunol. 97:1272–1278. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oettgen HC and Geha RS: IgE in asthma and
atopy: cellular and molecular connections. J Clin Invest.
104:829–835. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nelms K, Keegan AD, Zamorano J, Ryan JJ
and Paul WE: The IL-4 receptor: signaling mechanisms and biologic
functions. Annu Rev Immunol. 17:701–738. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zurita-Salinas CS, Palacios-Boix A, Yáñez
A, González F and Alcocer-Varela J: Contamination with Mycoplasma
spp. induces interleukin-13 expression by human skin fibroblasts in
culture. FEMS Immunol Med Microbiol. 15:123–128. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Botero JE, Contreras A and Parra B:
Profiling of inflammatory cytokines produced by gingival
fibroblasts after human cytomegalovirus infection. Oral Microbiol
Immunol. 23:291–298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilson MS and Wynn TA: Pulmonary fibrosis:
pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mauviel A: Transforming growth factor
beta: a key mediator of fibrosis. Meth Mol Med. 117:69–80.
2005.PubMed/NCBI
|
|
12
|
Minshall EM, Leung DY, Martin RJ, Song YL,
Cameron L, Ernst P and Hamid Q: Eosinophil-associated TGF-beta1
mRNA expression and airways fibrosis in bronchial asthma. Am J
Respir Cell Mol Biol. 17:326–333. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vasquez-Pinto LM, Nantel F, Sirois P and
Jancar S: Bradykinin B(1) receptor antagonist R954 inhibits
eosinophil activation/proliferation/migration and increases
TGF-beta and VEGF in a murine model of asthma. Neuropeptides.
44:107–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee KS, Park SJ, Kim SR, Min KH, Lee KY,
Choe YH, Hong SH, Lee YR, Kim JS, Hong SJ and Lee YC: Inhibition of
VEGF blocks TGF-β1 production through a PI3K/Akt signaling pathway.
Eur Respir J. 31:523–531. 2008.
|
|
15
|
Foster CA: VCAM-1/α 4-integrin adhesion
pathway: therapeutic target for allergic inflammatory disorders. J
Allergy Clin Immunol. 98:S270–S277. 1996.
|
|
16
|
Zhu X, Subbaraman R, Sano H, Jacobs B,
Sano A, Boetticher E, Muñoz NM and Leff AR: A surrogate method for
assessment of β2-integrin-dependent adhesion of human eosinophils
to ICAM-1. J Immunol Methods. 240:157–164. 2000.
|
|
17
|
Shimada T, Kudo T, Akase T and Aburada M:
Preventive effects of Bofutsushosan on obesity and various
metabolic disorders. Biol Pharm Bull. 31:1362–1367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohno K, Chung HJ, Maruyama I and Tani T:
Bofutsushosan, a traditional Chinese formulation, prevents intimal
thickening and vascular smooth muscle cell proliferation induced by
balloon endothelial denudation in rats. Biol Pharm Bull.
28:2162–2165. 2005. View Article : Google Scholar
|
|
19
|
Morimoto Y, Sakata M, Ohno A, Maegawa T
and Tajima S: Effects of Byakko-ka-ninjin-to, Bofu-tsusho-san and
Gorei-san on blood glucose level, water intake and urine volume in
KKAy mice. Yakugaku Zasshi. 122:163–168. 2002.(In Japanese).
|
|
20
|
Kim HJ, Yoon KM, Im EY, Byun JS, Kim DJ
and Kwak MA: Three case report of Bangpungtongsung-san effect on
improvement of hypertension patients. KJOPP. 23:740–743. 2009.
|
|
21
|
Kim HJ, Park OS, Kim KS, Cha JH and Kim
YB: The effect of Bangpungtongsung-San on model of allergic
rhinitis. JKOOD. 19:21–30. 2006.
|
|
22
|
Lee MY, Shin IS, Lim HS, Seo CS, Ha H and
Shin HK: Kochia scoparia fruit attenuates allergic airway
inflammation in ovalbumin (OVA)-induced murine asthma model. Inhal
Toxicol. 23:938–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuk JE, Lee MY, Kwon OK, Cai XF, Jang HY,
Oh SR, Lee HK and Ahn KS: Effects of astilbic acid on airway
hyperresponsiveness and inflammation in a mouse model of allergic
asthma. Int Immunopharmacol. 11:266–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee G, Link H, Baluk P, Homer RJ, Chapoval
S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM and Elias JA:
Vascular endothelial growth factor (VEGF) induces remodeling and
enhances TH2-mediated sensitization and inflammation in the lung.
Nat Med. 10:1095–1103. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chaudhary NI, Roth GJ, Hilberg F,
Müller-Quernheim J, Prasse A, Zissel G, Schnapp A and Park JE:
Inhibition of PDGF, VEGF and FGF signaling attenuates fibrosis. Eur
Respir J. 29:976–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Richter A, Puddicombe SM, Lordan JL,
Bucchieri F, Wilson SJ, Djukanovic R, Dent G, Holgate ST and Davies
DE: The contribution of interleukin (IL)-4 and IL-13 to the
epithelial-mesenchymal trophic unit in asthma. Am J Respir Cell Mol
Biol. 25:385–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sumi Y and Hamid Q: Airway remodeling in
asthma. Allergol Int. 56:341–348. 2007. View Article : Google Scholar
|
|
28
|
Westergren-Thorsson G, Larsen K, Nihlberg
K, Andersson-Sjöland A, Hallgren O, Marko-Varga G and Bjermer L:
Pathological airway remodelling in inflammation. Clin Respir J.
4:1–8. 2010. View Article : Google Scholar
|
|
29
|
Howell JE and McAnulty RJ: TGF-β: its role
in asthma and therapeutic potential. Current Drug Targets.
7:547–565. 2006.
|
|
30
|
Halwani R, Al-Muhsen S, Al-Jahdali H and
Hamid Q: Role of transforming growth factor-β in airway remodeling
in asthma. Am J Respir Cell Mol Biol. 44:127–133. 2011.
|
|
31
|
Salib RJ and Howarth PH: Transforming
growth factor-β in allergic inflammatory disease of the upper
airways: friend or foe? Clin Exp Allergy. 39:1128–1135. 2009.
|
|
32
|
Baek KJ, Cho JY, Rosenthal P, Alexander
LE, Nizet V and Broide DH: Hypoxia potentiates allergen induction
of HIF-1α, chemokines, airway inflammation, TGF-β1, and airway
remodeling in a mouse model. Clin Immunol. 147:27–37.
2013.PubMed/NCBI
|
|
33
|
Yamaguchi M, Niimi A, Matsumoto H, Ueda T,
Takemura M, Matsuoka H, Jinnai M, Otsukda K, Oguma T, Takeda T, Ito
I, Chin K and Mishima M: Sputum levels of transforming growth
factor-β1 in asthma: relation to clinical and computed tomography
findings. J Investig Allergol Clin Immunol. 18:202–206. 2008.
|
|
34
|
Hoshino M, Takahashi M and Aoike N:
Expression of vascular endothelial growth factor, basic fibroblast
growth factor, and angiogenin immunoreactivity in asthmatic airways
and its relationship to angiogenesis. J Allergy Clin Immunol.
107:295–301. 2001. View Article : Google Scholar
|
|
35
|
Chavakis E and Dimmeler S: Regulation of
endothelial cell survival and apoptosis during angiogenesis.
Arterioscler Thromb Vasc Biol. 22:887–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferrara N, Gerber HP and Le Couter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|