Introduction
Human skeletal muscle is often injured physically or
chemically and changes occur with aging and disease. The
age-related loss of muscle mass, strength and quality, referred to
as sarcopenia, is a common feature of aging that is characterized
by a decline in both the number and size of muscle fibers (1–3).
With these age-related changes, the estimated rate of muscle loss
is 1–2% per year after the age of 50 years (4,5).
The loss of muscle mass due to apoptosis with normal aging has been
investigated in a number of studies (6,7).
Apoptosis, a process of individual cell death
regulated by the activation of specific genes, is an important
regulatory process that occurs during normal development and in the
progression of specific diseases (8). Although apoptosis may occur via
several mechanisms (9,10), one mechanism involves external
factors that bind to membrane death receptors outside the cell, and
another involves internal cellular events that lead to the release
of specific cell death molecules from the mitochondria (11–13). In the mitochondrial-mediated
pathway, in response to cellular stress or DNA damage, p53 can
induce apoptosis by regulating the proteins of the Bcl-2 family and
by translocating to the mitochondria and activating apoptotic
signaling directly (14–16). In addition, caspase-3 can activate
caspase-activated DNase, leading to DNA fragmentation and cell
death (17). Caspase-independent
mechanisms also exist, such as the release of apoptosis-inducing
factor (AIF) and endonuclease G (EndoG) from the mitochondria,
inducing large-scale DNA fragmentation (18–21).
The release of apoptosis-inducing factors by the
mitochondria, nuclear translocation and DNA agmentation associated
with AIF have been demonstrated in several systems and cell types.
AIF is translocated to the nucleus after being released from the
mitochondria, inducing DNA fragmentation (22–24). AIF is found in several human
tissues, including cardiac and skeletal muscle (25,26). In addition, the calcium-dependent
proteinase (calpain) system is present in every vertebrate cell. At
least 3 calpains exist in humans: calpain-1 (μ-calpain), calpain-2
(m-calpain) and calpain-3 (n-calpain, p94) (27,28). A number of studies have
demonstrated that the AIF and EndoG pro-apoptotic factors, which
are released from the mitochondria by calpain activity, are
upregulated in sarcopenic muscle (29–32). However, whether this causes the
apoptosis that occurs with the normal aging process in human muscle
is not known. Previously, we reported the age-dependent induction
of AIF in the human semitendinosus skeletal muscle (33). The aim of the present study was to
investigate the general pattern of skeletal muscle apoptosis,
particularly in the human gracilis skeletal muscle with extended
age (up to 50 years old). We examined the expression of
apoptosis-related factors to elucidate the key players associated
with the aging-related process in muscles from 10- and 50-year-old
individuals.
Materials and methods
Muscle sampling
Samples of gracilis skeletal muscle were collected
from individuals of different ages (10, 20, 30, 40 and 50 years
old) who underwent anterior cruciate ligament reconstruction with
gracilis. Muscles were harvested from individuals of 10 to 50 years
of age and 8 samples were analyzed in each age group. All the human
subjects were healthy with no muscle-related clinical conditions.
Muscle tissues were prepared from the musculotendinous junction,
mounted, immediately frozen in liquid nitrogen and stored at −80ºC
for immunohistochemical and biochemical analyses. Ethical consent
was obtained from the Kyung Hee Medical Center Institutional Review
Board.
Histological analysis
Different human gracilis skeletal muscle tissues
(n=8 per age group) were fixed in 4% paraformaldehyde, embedded in
optimum cutting temperature (OCT) compound, divided into
15-μm-thick sections and stained with hematoxylin and eosin
(H&E).
In situ terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL)
TUNEL assays were performed to detect DNA strand
breaks using a commercial kit following the instructions provided
by the manufacturer (Chemicon International, Temecula, CA, USA).
Briefly, 15-μm-thick sections of skeletal muscle (n=8 per age
group) were mounted onto Silane-coated glass slides. The dehydrated
sections were treated with 20 μg/ml DNase-free proteinase K
(Sigma-Aldrich Corp., St. Louis, MO, USA) to retrieve antigenic
epitopes, followed by 3% H2O2 to quench
endogenous peroxidase activity. Free 3′-OH termini were labeled
with digoxigenin-dUTP for 1 h at 37ºC utilizing a terminal
deoxynucleotidyl transferase reaction mixture. The incorporated
digoxigenin-conjugated nucleotides were detected using a
horseradish peroxidase-conjugated anti-digoxigenin antibody and
3,3′-diaminobenzidine. The dehydrated sections were cleared in
xylene, mounted with Canada balsam and enclosed with
coverslips.
Immunohistochemistry
Sections (15-μm-thick) of frozen muscle tissue (n=8
per age group) were mounted onto Silane-coated glass slides and
fixed in 4% paraformaldehyde (Sigma-Aldrich Corp.) for 1 h at 4ºC
and endogenous peroxidase activity was blocked by the immersion of
the sections in 3% H2O2 in 100% methanol for
15 min. All samples were incubated in 10% normal donkey serum (NDS)
in phosphate-buffered saline (PBS) for 1 h at room temperature and
were incubated with antibodies AIF (diluted 1:100; Cell Signaling
Technology, Inc., Danvers, MA, USA). Immunohistochemical procedures
used antibodies from several sources to establish antibody
specificity and confirm immunostaining and protein expression.
Primary antibody binding was visualized using Cy3-labeled donkey
anti-rabbit antibody (1:500; Jackson ImmunoResearch Inc., West
Grove, PA, USA). After staining, the sections were mounted with
mounting medium with DAPI.
Extraction of total RNA and reverse
transcriptase PCR
Frozen gracilis skeletal muscle was homogenized on
ice in 1 ml of ice-cold TRIzol reagent (Invitrogen Corp., Carlsbad,
CA, USA). First-strand cDNA synthesis with 5 μg of total RNA was
performed using MMLV reverse transcriptase and oligo(dT) primers
for 1 h at 42ºC. Subsequently, the PCR amplification was performed
by a modified method originally described in the study by Saiki
et al (34). Total RNA was
solubilized in RNase-free H2O and quantified twice by
measuring the optical density (OD) at 260 nm. cDNA was synthesized
from 2 g of total RNA, and reverse transcription (Promega Corp.,
Madison, WI, USA) was performed at 42ºC for 1 h following
incubation at 95ºC for 5 min. cDNA amplification was carried out
according to the following procedure: 95ºC for 1 min, 56ºC
(β-actin), 58ºC (AIF, caspase-3, Bacl-2, Bax and calpain-1) for 1
min, 72ºC for 1 min. Twenty-six to 40 cycles were run, and the
reaction was prolonged for 10 min at 72ºC. The sequences of the
primers used for PCR were as follows: AIF forward, 5′-AGACGATCCCAAA
TAATGCAG-3′ and reverse, 5′-TAGCTCTAGGTGAG TCTTGG-3′; caspase-3
forward, 5′-CGAAATTCAAA GGATGGCTCCTGGTT-3′ and reverse, 5′-CGGTTAA
CCCGGGTAAGAAATGTGCAT-3′; Bcl-2 forward, 5′-GCA
CGCTGGGAGAAAGGGTACGAT-3′ and reverse, 5′-CACA
TCTCCAGCATCCCACTCGTA-3′; Bax forward, 5′-TGCC TCAGGATGCGTCCACCAA-3′
and reverse, 5′-CGGC AATCATCCTCTGCATGCTCCAT-3′; calpain-1 forward,
5′-CATGGTGCTGACCAAGATGAAGGAGAT-3′ and reverse,
5′-GCGCAGCCGCCTCACGGCTCCCAGCCT GTT-3′; and β-actin forward,
5′-TCATGAGTGTGACG TTGACATCCGT-3′ and reverse, 5′-CCTAGAAGCATTT
GCGGTGCACGATG-3′. The PCR products were separated on 1.5% agarose
gels, visualized by ethidium bromide staining using the i-MAX gel
image analysis system (CoreBioSystem, Seoul, Korea), and analyzed
using Alpha Ease™ FC software (Alpha Innotech Corp., San Leandro,
CA, USA).
Western blot analysis
Western blot analyses were performed to detect AIF
and caspase-3 expression on muscle tissue (n=8). Muscle samples
were placed in loading buffer, boiled for 5 min and centrifuged.
Following quantification, the supernatants were loaded on a 10%
sodium dodecylsulfate-polyacrylamide gel and subjected to
electrophoresis. The fractionated proteins were transferred onto a
polyvinylidene fluoride (PVDF) membranes (Milipore, Billerica, MA,
USA), and the membranes, after blocking in 10% non-fat dry milk in
TPBS buffer for 1 h at room temperature, were incubated with the
primary antibodies to AIF (diluted 1:1,000), caspase-3 (diluted
1:1,000) and β-tubulin (diluted 1:1,000) (all from Cell Signaling
Technology, Inc.) and then for 2 h with horseradish
peroxidase-conjugated secondary antibodies (1:500; Jackson
ImmunoResearch Inc.). After intervening washes, the membranes were
developed with the ECL western blotting detection system (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and the resulting
chemilumnescence was exposed to film (Agfa HealthCare, Greenville,
SC, USA). A tonsil served as a positive control.
Statistical analysis
Statistical analysis was performed using GraphPrism
4.0.3 software (GraphPad Software, Inc., San Diego, CA, USA). All
data are presented as the means ± standard deviation (SD) and a
Student’s t-test was used to compare group means. AIF antibody and
TUNEL assay were observed under a light microscope at magnification
(×400). Images were captured using a Zeiss fluorescent microscope
and myofibers were counted and measured using Axiovision 4 software
(Carl Zeiss MicroImaging GmbH, Jena, Germany).
Results
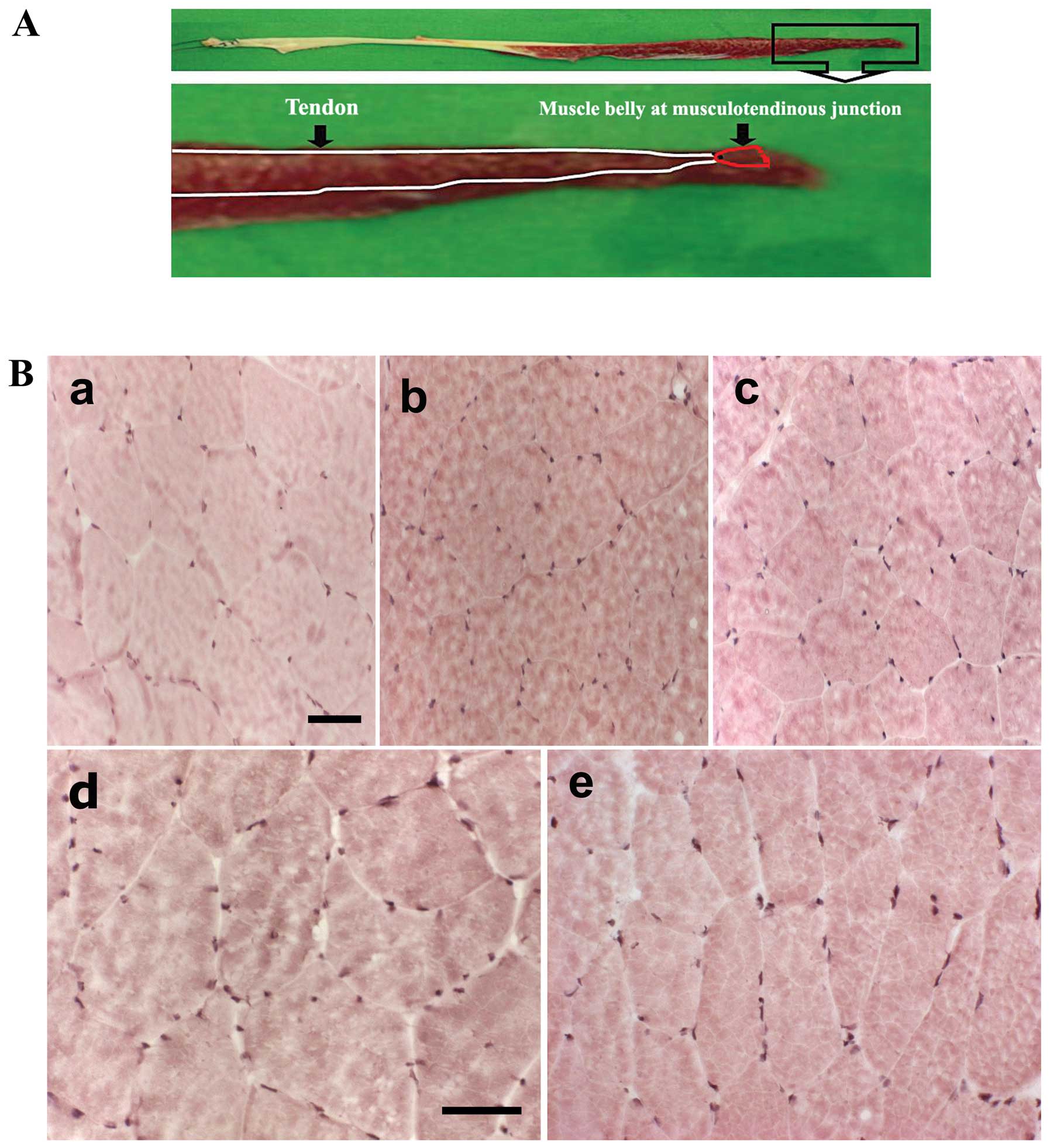
Muscle sampling and histological
analysis
Samples of gracilis skeletal muscle were collected
from the musculotendinous junction of different individuals
(Fig. 1A). We used H&E
staining to examine the morphological changes induced by apoptosis
from aging in human gracilis skeletal muscle (Fig. 1B). The detection of large numbers
of nuclei is a distinct feature of necrosis. With H&E staining,
none of the tissues showed evidence of necrosis.
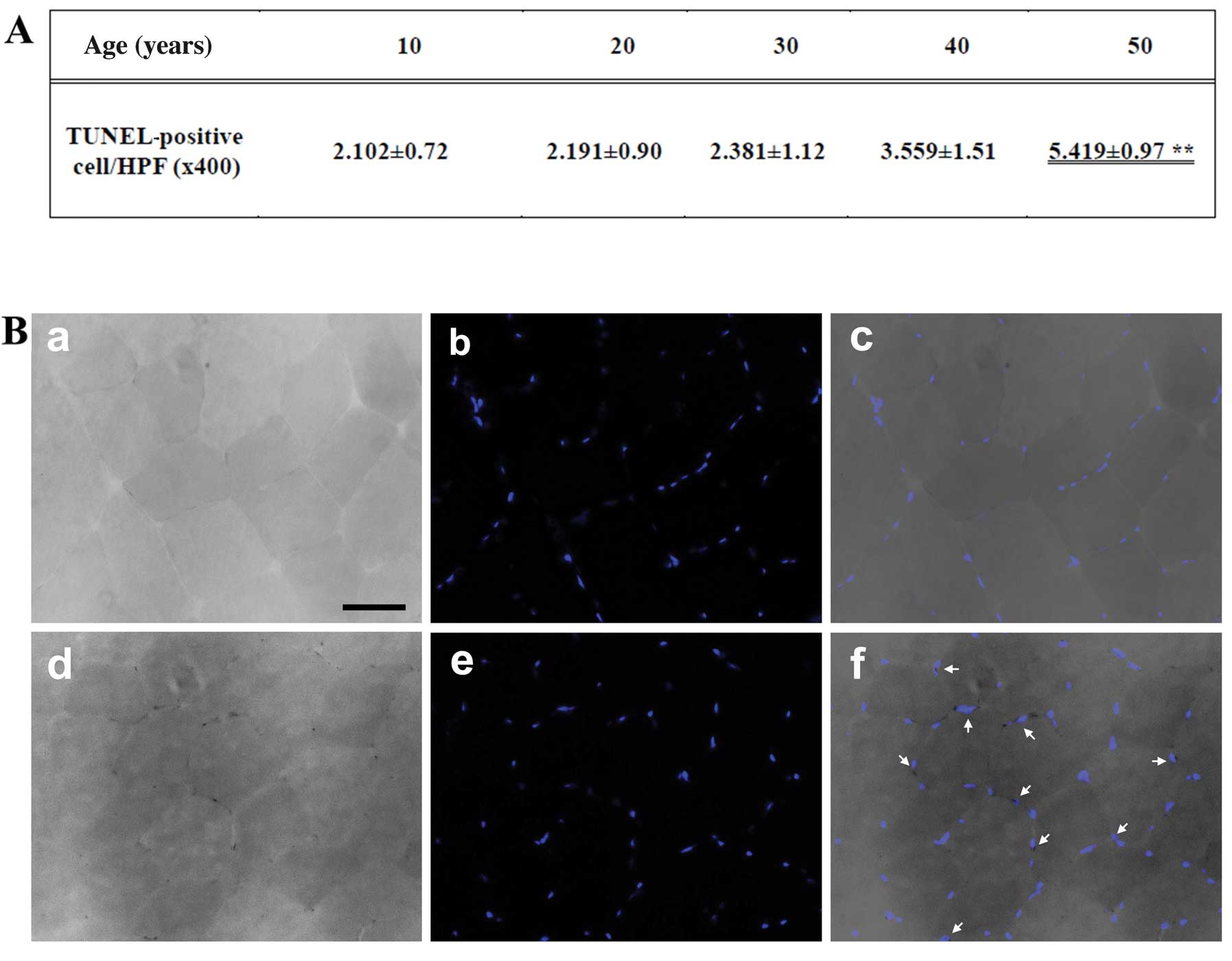
Detection of apoptosis
We performed TUNEL staining of the gracilis muscle
sections. The size (mm2) of each area containing
TUNEL-positive cells and the distance from the border of the
infarction core were measured with a microruler under ×200
magnification. The number of apoptotic cells was counted in 3
high-power fields (HPF; ×400) under a light microscope, and the
mean was recorded as cells/HPF (Fig.
2A). The number of TUNEL-positive nuclei increased with age,
and was significantly higher in the muscles of the individuals of
50 years of age than the muscles of those who were 10 years of age
(Fig. 2B).
Changes in mRNA expression of
apoptosis-related factors
Since caspase-3 and AIF play pivotal roles in
apoptosis, we investigated whether their activity is increased in
gracilis muscle with age. The caspase-3 mRNA levels tended to
increase with age; however, these changes were not significant
(Fig. 3A and B). On the other
hand, the gracilis muscle in individuals who were 10 and 50 years
of age was found to have elevated levels of Bax (130±8%) and the
relative mRNA expression level of Bcl-2 (76±8%) in the gracilis
muscle declined significantly with age (Fig. 3C and D). We investigated whether a
caspase-independent mechanism is involved in the increased
apoptosis in the muscles of 50-year-olds. Our results revealed that
the relative AIF mRNA level increased with age, and was 100±10,
118±11, 130±10, 139±11 and 160±11% in the individuals who were 10,
20, 30, 40 and 50 years of age, respectively. The expression of AIF
significantly correlated with the mRNA expression of calpain-1 in
muscle, which was 100±11, 95±10, 125±12, 121±11 and 164±11%, in the
individuals who were 10, 20, 30, 40 and 50 years of age,
respectively (Fig. 3E and F).
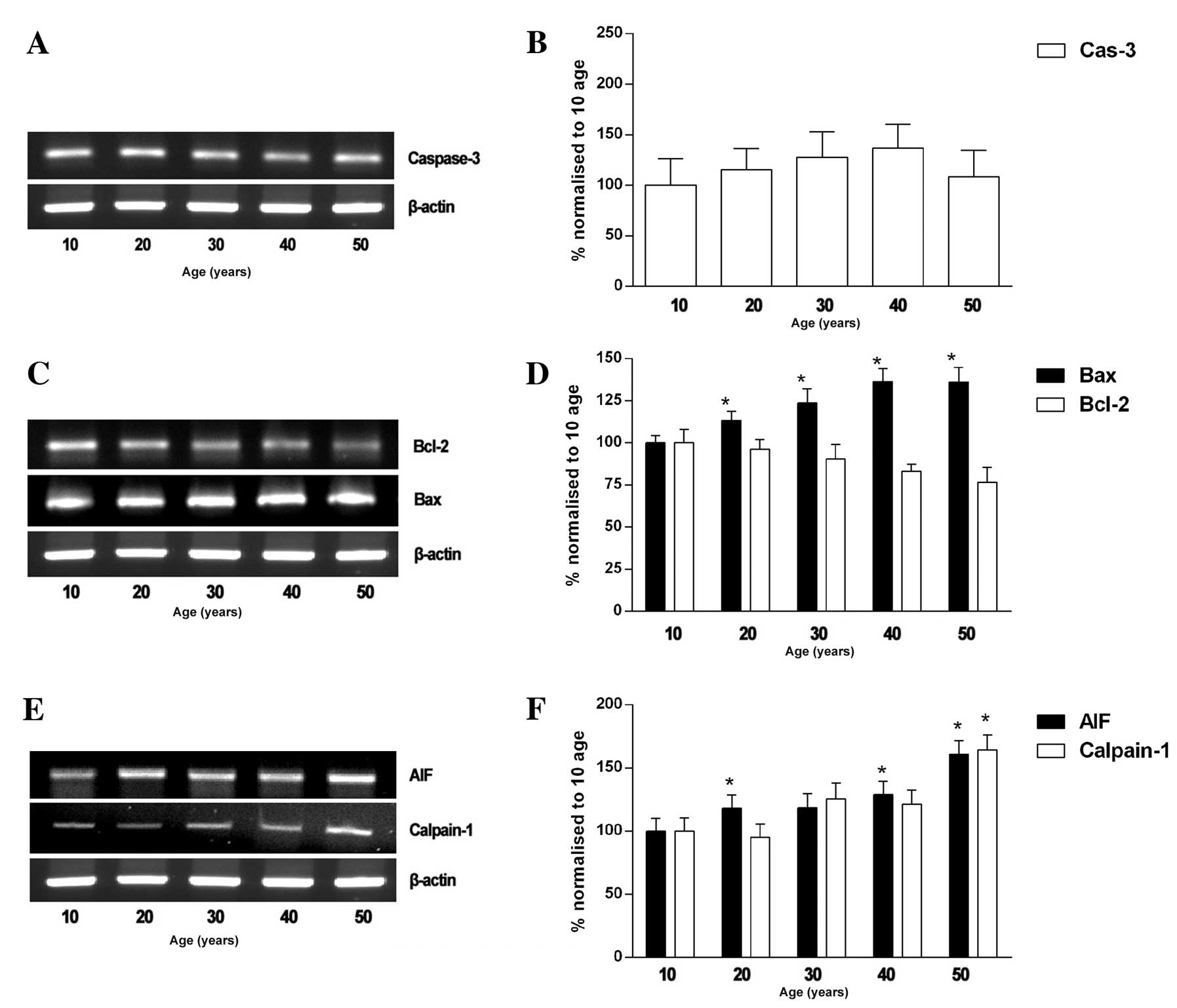 | Figure 3mRNA expression levels of AIF,
calpain-1, Bcl-2, Bax and caspase-3 in human gracilis skeletal
muscle of individuals of 10 to 50 years of age (n=8). (A–C)
Representative immunoblots of AIF, calpain-1, Bcl-2, Bax and
caspase-3. (D–F) Quantitative analysis of AIF, calpain-1, Bcl-2,
Bax and caspase-3. Quantification of polymerase chain reaction
(PCR) signals obtained using a densitometric analysis of the signal
product optical density (OD). The bands were quantified by
normalization to those from the individuals of 10 years of age.
*P<0.05, normalized to muscles of individuals of 10
years of age. Cas-3, caspase-3. |
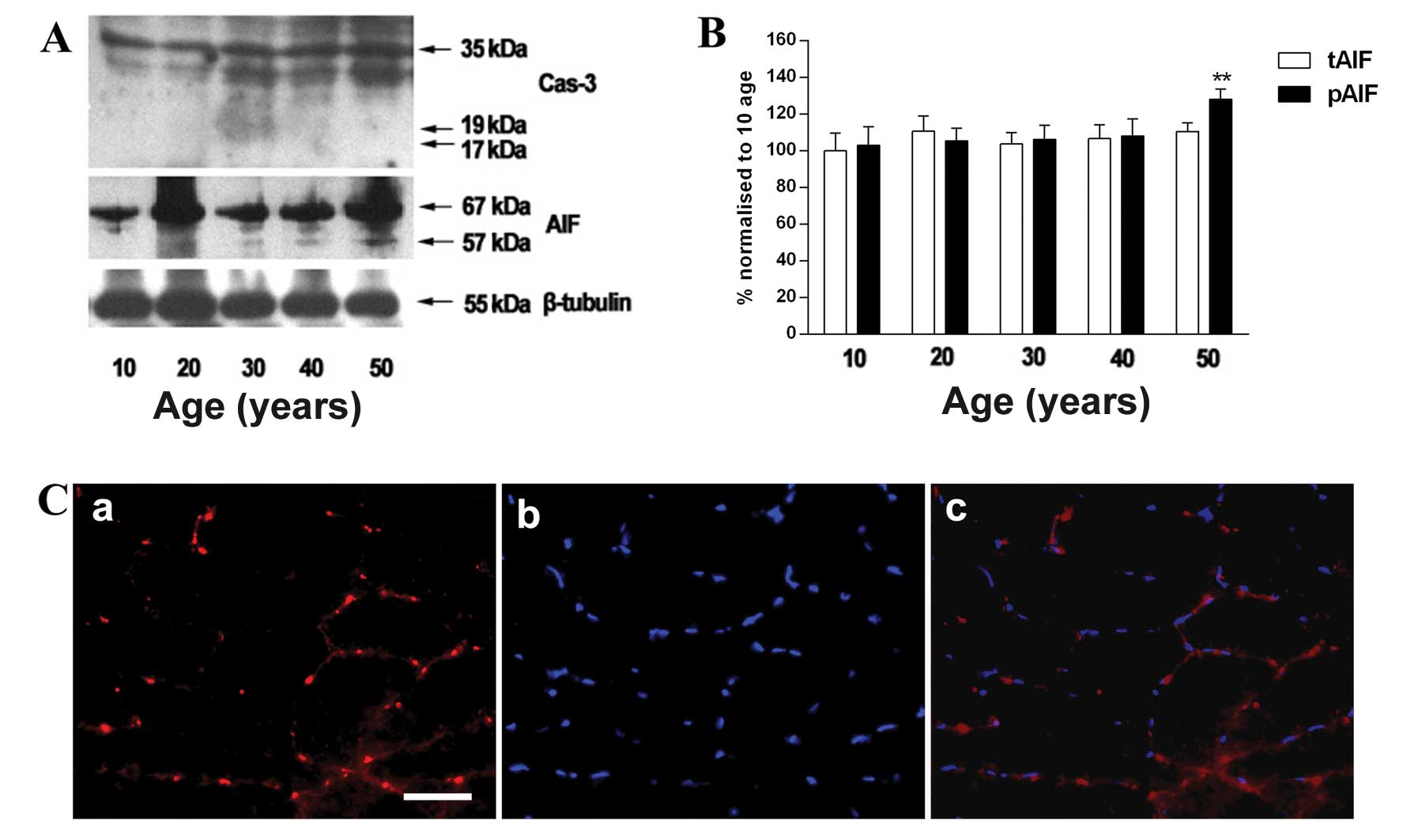
Changes in expression of AIF in gracilis
muscle of individuals of 50 years of age
The relative expression of AIF was higher in the
individuals who were 50 years of age than those who were 10 years
of age (Fig. 4B), while the
protein level of cleaved caspase-3 was not observed in the gracilis
muscle (Fig. 4A). In addition,
AIF was stained with a specific anti-AIF antibody and merged with
DAPI staining to determine whether AIF co-localizes in the nuclei
(Fig. 4C).
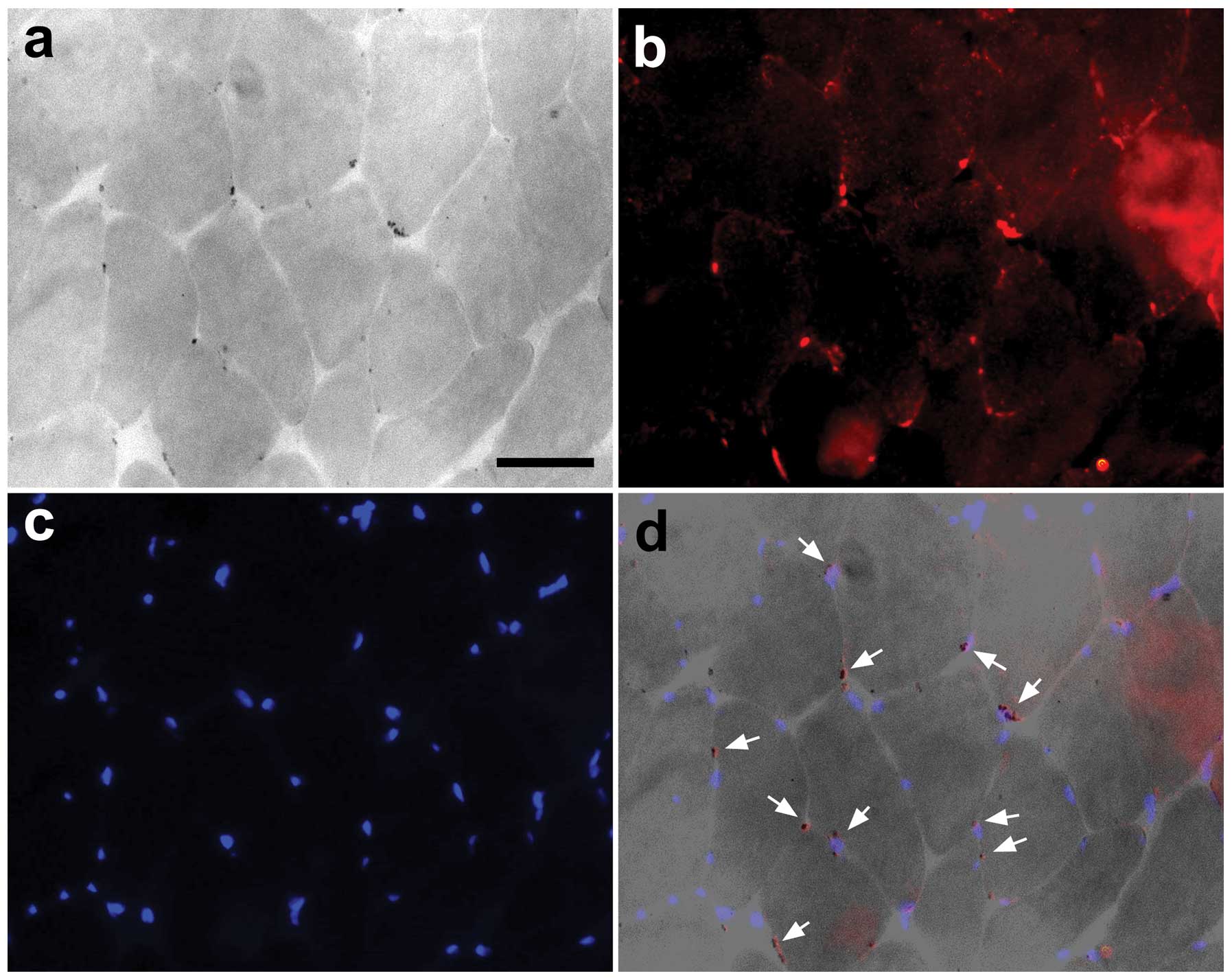
Association between the number of
AIF-positive cells and apoptotic nuclei
The expression of AIF and TUNEL indicated that the
muscle nuclei were undergoing apoptotic changes (Fig. 5).
Discussion
Although increased apoptosis in skeletal muscle
occurs under several pathophysiological conditions (22,35,36), whether apoptosis occurs during
normal aging is not clear. In this study, we examined skeletal
muscle samples from individuals between 10 and 50 years of age, as
well as from middle-aged individuals, when degeneration begins and
self-renewal activity occurs for the maintenance of skeletal muscle
cells. We demonstrated that apoptotic DNA fragmentation increased
progressively with age in the human gracilis muscle. These results
are in accordance with those presented in the study by Strasser
et al (37), who found an
increased incidence of apoptosis using a TUNEL assay in human
rhabdosphincter skeletal muscle with age. In addition, we have
previously reported the age-dependent induction of AIF and a
significant increase in DNA fragmentation in the human
semitendinosus skeletal muscle (33).
Caspase-3 is a central mediator of cell death as a
number of apoptotic signaling pathways converge at this point
(9,23). Studies have shown that caspase-3
mRNA and protein levels are elevated in the muscles of individuals
of 50 years of age, although the protein level of cleaved caspase-3
was not separated in these muscles. This could be explained by the
fact that in skeletal muscle, cytochrome c initiates the
caspase-dependent apoptotic pathway, whereas AIF resides in the
mitochondrion and upon stimulation, translocates to the nucleus to
induce DNA fragmentation in a caspase-independent manner (29,30,38,39). AIF is a principal mediator of cell
death as apoptotic signaling pathways converge at this point. In
this study, we observed an increase in the mRNA level of AIF
measured by RT-PCR. The muscle of individuals of 50 years of age
had a higher AIF mRNA expression compared with the controls, with
increased total and cleaved AIF levels in the gracilis muscle.
Consistent with the mRNA data, the AIF protein content was higher
in the muscles of individuals of 50 years of age than in those who
were 10 years of age. Furthermore, we found that the level of AIF
immunoreactivity increased with age, and a positive correlation was
observed between AIF and DNA fragmentation. The relevance of
caspase-independent apoptosis to age-related muscle changes was
supported by the positive staining for AIF in the nucleus and the
extent of apoptotic DNA fragmentation.
Bcl-2 and Bax are important apoptotic regulatory
proteins that respectively inhibit and promote mitochondrial
apoptogenic protein release (40,41). In addition, AIF is released from
the mitochondria in response to increased levels of Bax or
decreased levels of Bcl-2 (42).
We observed an age-related increase in Bax and a decrease in Bcl-2
levels in skeletal muscle. Similarly, as shown in a previous study,
a significant increase in Bax levels occurred in the gastrocnemius
and soleus muscles of old sedentary rats, concomitant with the
reduced expression of Bcl-2 in the rat soleus muscle (43). As previously demonstrated, the
releae of AIF is mediated by the direct proteolysis of the protein
by calpain-1 (32). In addition,
the AIF and EndoG pro-apoptotic factors, which are released from
the mitochondria by calpain, are expressed in muscle in the
elderly. Therefore, one of the most remarkable changes that we
observed in the skeletal muscle of middle-aged individuals was the
increased calpain-1 levels. Depending on the relative levels of
anti-apoptotic and apoptotic factors released from the mitochondria
(44), the caspase-independent
pathway involving AIF is activated, triggering apoptosis directly
(10).
In conclusion, our study demonstrates that increased
apoptosis occurs in human gracilis skeletal muscle in individuals
of 50 years of age compared to those who are 10 years of age,
confirming the age-related increase in apoptosis in skeletal
muscle. The involvement of apoptotic pathways in the aging process
was suggested by the selective changes in the expression levels of
the apoptosis regulatory proteins, Bax, Bcl-2, AIF and calpain-1.
This indicates the correlation between the expression of AIF and
apoptosis in individuals between 10 and 50 years of age human
gracilis skeletal muscle.
References
|
1
|
Nikolić M, Bajek S, Bobinac D, Vranić TS
and Jerković R: Aging of human skeletal muscles. Coll Antropol.
29:67–70. 2005.
|
|
2
|
Marcell TJ: Sarcopenia: causes,
consequences, and preventions. J Gerontol A Biol Sci Med Sci.
58:M911–M916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roubenoff R: Sarcopenia and its
implications for the elderly. Eur J Clin Nutr. 54:S40–S47. 2000.
View Article : Google Scholar
|
|
4
|
Hughes VA, Frontera WR, Roubenoff R, Evans
WJ and Singh MA: Longitudinal changes in body composition in older
men and women: role of body weight change and physical activity. Am
J Clin Nutr. 76:473–481. 2002.PubMed/NCBI
|
|
5
|
Lexell J: Human aging, muscle mass, and
fiber type composition. J Gerontol A Biol Sci Med Sci. 50:11–16.
1995.
|
|
6
|
Dirks A and Leeuwenburgh C: Apoptosis in
skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol.
282:R519–R527. 2002.PubMed/NCBI
|
|
7
|
Zhang Y and Herman B: Ageing and
apoptosis. Mech Ageing Dev. 123:245–260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: the significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar
|
|
9
|
Mayer B and Oberbauer R: Mitochondrial
regulation of apoptosis. News Physiol Sci. 18:89–94. 2003.
|
|
10
|
Primeau AJ, Adhihetty PJ and Hood DA:
Apoptosis in heart and skeletal muscle. Can J Appl Physiol.
27:349–395. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai J, Yang J and Jones DP: Mitochondrial
control of apoptosis: the role of cytochrome c. Biochim
Biophys Acta. 1366:139–149. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Green D and Kroemer G: The central
executioners of apoptosis: caspases or mitochondria? Trends Cell
Biol. 8:267–271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holloszy JO, Chen M, Cartee GD and Young
JC: Skeletal muscle atrophy in old rats: differential changes in
the three fiber types. Mech Ageing Dev. 60:199–213. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsujimoto Y: Cell death regulation by the
Bcl-2 protein family in the mitochondria. J Cell Physiol.
195:158–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baker SJ and Reddy EP: Modulation of life
and death by the TNF receptor superfamily. Oncogene. 17:3261–3270.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Candé C, Cohen I, Daugas E, Ravagnan L,
Larochette N, Zamzami N and Kroemer G: Apoptosis-inducing factor
(AIF): a novel caspase-independent death effector released from
mitochondria. Biochimie. 84:215–222. 2002.PubMed/NCBI
|
|
19
|
Candé C, Vahsen N, Garrido C and Kroemer
G: Apoptosis-inducing factor (AIF): caspase-independent after all.
Cell Death Differ. 11:591–595. 2004.PubMed/NCBI
|
|
20
|
Li LY, Luo X and Wang X: Endonuclease G is
an apoptotic DNase when released from mitochondria. Nature.
412:95–99. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Loo G, Schotte P, van Gurp M, Demol H,
Hoorelbeke B, Gevaert K, Rodriguez I, Ruiz-Carrillo A,
Vandekerckhove J, Declercq W, Beyaert R and Vandenabeele P:
Endonuclease G: a mitochondrial protein released in apoptosis and
involved in caspase-independent DNA degradation. Cell Death Differ.
8:1136–1142. 2001.PubMed/NCBI
|
|
22
|
Adams V, Gielen S, Hambrecht R and Schuler
G: Apoptosis in skeletal muscle. Front Biosci. 6:D1–D11. 2001.
|
|
23
|
Pollack M and Leeuwenburgh C: Apoptosis
and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci.
56:B475–B482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joza N, Susin SA, Daugas E, Stanford WL,
Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF,
Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW,
Zúñiga-Pflücker JC, Kroemer G and Penninger JM: Essential role of
the mitochondrial apoptosis-inducing factor in programmed cell
death. Nature. 410:549–554. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Daugas E, Nochy D, Ravagnan L, Loeffler M,
Susin SA, Zamzami N and Kroemer G: Apoptosis inducing dactor (AIF)
a ubiquitous mitochondrial oxidoreductase involved in apoptosis.
FEBS Lett. 476:118–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daugas E, Susin SA, Zamzami N, Ferri KF,
Irinopoulou T, Larochette N, Prévost MC, Leber B, Andrews D,
Penninger J and Kroemer G: Mitochondrio-nuclear translocation of
AIF in apoptosis and necrosis. FASEB J. 14:729–739. 2000.PubMed/NCBI
|
|
27
|
Suzuki K, Imajoh S, Emori Y, Kawasaki H,
Minami Y and Ohno S: Calcium-activated neutral protease and its
endogenous inhibitor. Activation at the cell membrane and
biological function. FEBS Lett. 220:271–277. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueyama H, Kumamoto T, Fujimoto S, Murakami
T and Tsuda T: Expression of three calpain isoform genes in human
skeletal muscles. J NeurolSci. 155:163–169. 1998.PubMed/NCBI
|
|
29
|
Dargelos E, Poussard S, Brulé C, Daury L
and Cottin P: Calcium-dependent proteolytic system and muscle
dysfunctions: a possible role of calpains in sarcopenia. Biochimie.
90:359–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dirks AJ and Leeuwenburgh C: Aging and
lifelong calorie restriction result in adaptations of skeletal
muscle apoptosis repressor, apoptosis-inducing factor, X-linked
inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol
Med. 36:27–39. 2004. View Article : Google Scholar
|
|
31
|
Norberg E, Gogvadze V, Ott M, Horn M,
Uhlén P, Orrenius S and Zhivotovsky B: An increase in intracellular
Ca2+is required for the activation of mitochondrial
calpain to release AIF during cell death. Cell Death Differ.
15:1857–1864. 2008.
|
|
32
|
Polster BM, Basañez G, Etxebarria A,
Hardwick JM and Nicholls DG: Calpain I induces cleavage and release
of apoptosis-inducing factor from isolated mitochondria. J Biol
Chem. 280:6447–6454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park SY, Kim HY, Lee JH, Yoon KH, Chang MS
and Park SK: Age-dependent induction of apoptosis-inducing factor
(AIF) in the human semitendinosus skeletal muscle. Cell Mol Biol
Lett. 15:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saiki RK, Bugawan TL, Horn GT, Mullis KB
and Erlich HA: Analysis of enzymatically amplified beta-globin and
HLA-DQ alpha DNA with allele-specific digonucleotide probes.
Nature. 324:163–166. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Borisov AB and Carlson BM: Cell death in
denervated skeletal muscle is distinct from classical apoptosis.
Anat Rec. 258:305–318. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sandri M, Carraro U, Podhorska-Okolov M,
Rizzi C, Arslan P, Monti D and Franceschi C: Apoptosis, DNA damage
and ubiquitin expression in normal and mdx muscle fibers
after exercise. FEBS Lett. 373:291–295. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Strasser H, Tiefenthaler M, Steinlechner
M, Eder I, Bartsch G and Konwalinka G: Age-dependent apoptosis and
loss of rhabdosphincter cells. J Urol. 164:1781–1785. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alway SE, Degens H, Krishnamurthy G and
Smith CA: Potential role for Id myogenic repressors in apoptosis
and attenuation of hypertrophy in muscles of aged rats. Am J
Physiol Cell Physiol. 283:C66–C76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger
JM and Kroemer G: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bidère N, Lorenzo HK, Carmona S, Laforge
M, Harper F, Dumont C and Senik A: Cathepsin D triggers Bax
activation, resulting in selective apoptosis-inducing factor (AIF)
relocation in T lymphocytes entering the early commitment phase to
apoptosis. J Biol Chem. 278:31401–31411. 2003.PubMed/NCBI
|
|
41
|
Susin SA, Zamzami N, Castedo M, Hursch T,
Marchetti P, Macho A, Daugas E, Geuskens M and Kroemer G: Bcl-2
inhibits the mitochondrial release of an apoptogenic protease. J
Exp Med. 184:1331–1341. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria:
a primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997.
|
|
43
|
Song W, Kwak HB and Lawler JM: Exercise
training attenuates age-induced changes in apoptotic signaling in
rat skeletal muscle. Antioxid Redox Signal. 8:517–528. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen M, Won DJ, Krajewski S and Gottlieb
RA: Calpain and mitochondria in ischemia/reperfusion injury. J Biol
Chem. 277:29181–29186. 2002. View Article : Google Scholar : PubMed/NCBI
|