Introduction
Diabetic retinopathy (DR) is a major cause of
blindness in developed countries, and oxidative stress caused by
hyperglycemia is one of the most common causes of diabetic
microangiopathy (1). Currently,
the specific cause of diabetic microangiopathy is not completely
understood. Recently, a unifying hypothesis has been suggested
whereby the production of mitochondrial reactive oxygen species
(ROS) in response to chronic hyperglycemia may be the key initiator
of four pathogenic pathways: the polyol pathway, increased
formation of advanced glycation end-products, activation of protein
kinase C, and the hexosamine pathway (1–3).
These studies have emphasized the important role of increased
mitochondrion ROS production in diabetes complications, including
retinopathy. Therefore, mitochondrial ROS may serve as an important
target for DR treatment. We previously found (4) that uncoupling protein 2 (UCP2) has a
negative regulatory role in ROS generation; however, the exact
mechanism remains to be determined.
UCPs are mitochondrial transporters present in the
inner membrane of mitochondria. They belong to the family of anion
mitochondrial carriers. Five different UCPs have already been
identified: UCP1-4 and UCP5, or brain mitochondrial carrier protein
1 (BMCP1). These proteins are expressed in different tissues and
play different roles in cellular metabolism. Brain-specific UCP4
and UCP5 have been suggested to play a role in apoptosis in the
brain (5,6). Immunocytochemistry of UCP expression
in endothelial cells and pericytes showed that UCP1 and UCP2
expression was positive in both types of cells, while UCP3 was
negatively expressed (7). UCP2
was initially described by Fleury et al (8). It shares 59% identity to UCP1 and is
expressed most abundantly in mitochondria from the spleen, certain
regions of the brain, the pancreas, lungs, stomach, intestine,
white adipose tissue, thymocytes, kidney, cardiomyocytes,
macrophages and mast cells, but not in muscle, heart, liver, or
brown adipose tissue (8–18). Expression of UCP2 has been shown
to result in a decrease in mitochondrial superoxide (19–24). UCP2 has been shown to have a
protective effect in the brain, preventing acute damage produced by
ischemia (25–28); in the circulatory system,
preventing atherosclerotic plaques (29); and in cardiomyocytes, where its
overexpression is anti-apoptotic (30). Findings of previous studies have
shown that UCP2 plays a role in macrophages and pancreatic β cells
by affecting the killing capacity and glucose-induced insulin
secretion, respectively (13,19,22,31,32).
Considering that the overexpression of UCP2 can
inhibit vascular damage in DR and induce pathological changes
associated with vascular injury and nerve-tissue degeneration, we
aimed to determine whether UCP2 was able to reduce high
glucose-induced endothelial cell apoptosis and protect blood
vessels from damage. In this study, we investigated whether UCP2
inhibits high glucose-induced apoptosis of human umbilical vein
endothelial cells (HUVECs) to provide experimental evidence for the
application of UCP2 as a new protective factor of DR.
Materials and methods
Construction and identification of the
UCP2 expression plasmid
The primers targeting the human UCP2 gene were
synthesized based on a cDNA library (GenePharma, Shanghai, China),
and the sequences were as follows: UCP2 forward,
5′-GGAGATACCAAAGCACCGTCAATG-3′ and reverse,
5′-AGCACAGTTGACAATGGCATTACG-3′; hACTB forward,
5′-CCCTGGCACCCAGCAC-3′ and reverse, 5′-GCC GATCCACACGGAGTAC-3′.
Each of these fragments was amplified, digested with
AgeI and then ligated into the pGC-FU-3FLAG vector for 15
min at 23 and 42°C. The recombinant was transformed into competent
Escherichia coli cells treated with calcium chloride and
incubated for 16 h at 37°C.
PCR conditions were as follows: 94°C for 5 min, 30
cycles at 94°C for 30 sec, 55°C for 30 sec, 72°C for 2 min and then
72°C for 10 min. Positive clones, as confirmed by PCR, were chosen
for sequencing. The plasmids with the correct sequence were
transfected into 293T cells, and expression of the protein was
observed under a fluorescence microscope (Nikon, Tokyo, Japan).
Western blotting was performed to detect the expression of the
protein. The virus titer was determined by quantitative PCR
(qPCR).
Cell culture and lentivirus
transduction
HUVECs were obtained from the ScienCell Research
Laboratories (San Diego, CA, USA). The cells were cultured in
Endothelial Cell Medium (ECM; ScienCell Research Laboratories,
Carlsbad, CA, USA) with 5% (v/v) fetal bovine serum (FBS) at 37°C
in 5% (v/v) CO2 and 95% humidity. When they reached
confluence, the cells were maintained in 1% (v/v) fetal calf serum
and exposed to normal glucose (NG, 5.5 mmol/l) or high glucose (HG,
30 mmol/l) for 3–7 days, during which the medium was changed every
2 days. When the HUVECs were ~50% confluent in fresh serum-free
medium, they were transiently transfected with control lentivirus
or UCP2-overexpression lentivirus at a multiplicity of infection
(MOI) of 100. The cells were cultured in ECM with 5% (v/v) FBS
after infection for 4 h and then selected using 200 μm/ml
puromycin. The stable overexpressing lines were established when
>90% of the transfected cells were found to strongly express GFP
under a fluorescent microscope. The HUVECs were divided into four
groups: NG, HG, high glucose + the lentiviral-negative vector
control group (NC), and high glucose +
UCP2+/+-transfected group (UCP2).
RNA isolation and RT-qPCR
Total RNA was isolated from HUVECs using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. RNA extract (2 μl) was
reverse-transcribed into cDNA in a total reaction volume of 20
μl using a RevertAid™ First Strand cDNA Synthesis kit
(Fermentas, Burlington, ON, Canada). qPCR was performed using IQ
Supermix (Bio-Rad, Hercules, CA, USA), with 20 μl reaction
mixtures containing 1 μl cDNA, 8 μl sterilized water,
10 μl SYBR-Green real-time PCR Master Mix (Takara Bio Inc.,
Shiga, Japan), and 1 μl of primer. The UCP2, cytochrome
c, caspase-3 and Bcl-2 amplification signals were normalized
to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression and
evaluated using the equation: fold-change = 2−ΔΔct. The
primer sequences used in this study were: hUCP2 forward,
5′-GGCTGGAGGTGGTCGGAG-3′ and reverse, 5′-CAGAAGTGAAGTGGCAAGGGAG-3′;
cytochrome c forward 5′-TTTATTATGAAGTGTTCCCAGT GCC-3′ and
reverse, 5′-CTCCCCAGATGATGCCTTTG-3′; caspase-3 forward,
5′-CAGCCGCCAATAAGAACAAAG-3′ and reverse, 5′-CCGCCTCACAATAGCACCC-3′;
Bcl-2 forward, 5′-TACCTGAACCGGCACCTG-3′ and reverse,
5′-GCCGTACAGTTCCACAAAGG-3′; hGAPDH forward,
5′-GGGTGTGAACCATGAGAAGTATG-3′ and reverse,
5′-GATGGCATGGACTGTGGTCAT-3′.
Protein extraction and western blot
analysis
HUVECs were washed three times with ice-cold
phosphate-buffered saline (PBS, 4°C, pH 7.4) for 5 min at room
temperature and prepared using a protein extraction kit and a
protease inhibitor kit (Pierce, Rockford, IL, USA). The supernatant
was collected and the protein content of each lysate was determined
using a BCA Protein Assay kit (Tianlai Shengwu Jishu, Tianlai,
China) according to the manufacturer’s instructions. Equal amounts
(15 μl) of protein were electrophoresed on a 10% (w/v)
sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred
onto a 0.22 μm PVDF membrane (Millipore, Billerica, MA,
USA). The primary antibodies used to probe the membranes included
anti-UCP2 (1:500; cat. no. ab7973), anti-Bcl-2 (1:500; cat. no.
ab7973) (both from Abcam, Cambridge, UK), anti-cytochrome c
(1:200; cat no. 1896-1; Epitomics, Burlingame, CA, USA),
anti-caspase-3 (1:500; cat. no. ab44976; Abcam) and anti-GAPDH
(1:3000; cat. no. KM9002; Sungene Biotech, Tianjin, China). The
membranes were washed and incubated with peroxidase-conjugated
secondary antibodies (rabbit 1:5,000; rat 1:2,000; Sungene
Biotech). Enhanced chemiluminescence western blotting detection
reagents (Pierce) were used to detect UCP2, Bcl-2, cytochrome
c and caspase-3 protein levels. Experiments were performed
in triplicate.
Flow cytometry
According to the manufacturer’s instructions, the
Annexin-V FITC apoptosis detection kit (Beyotime, Shanghai, China)
was used to measure apoptosis in NG, HG, NC and UCP2 cells. Cells
(1×105) were plated in a volume of 1 ml into each well
of a 6-well plate. After various incubation periods, cells were
trypsinized, and the cells and culture medium were collected.
Following centrifugation at 1,000 × g for 10 min, the supernatant
was discarded. The cells were resuspended in 1 ml PBS, and
transferred to an Eppendorf tube. Annexin-V FITC was added and
mixed on ice and left in the dark for 15 min. Cell apoptosis was
detected by flow cytometry. Experiments were performed in
triplicate.
Immunofluorescence and confocal
microscopy
Cells cultured on glass coverslips were fixed for 15
min at 4°C in PBS containing 4% (w/v) paraformaldehyde. The
fixative was removed, and cells were either permeabilized with 0.5%
(v/v) Triton X-100 in PBS for 6–7 min or were not permeabilized.
Cells were then blocked with 4% (w/v) bovine serum albumin in PBS
for 30 min at room temperature. The non-permeabilized and
permeabilized cells were treated with Bcl-2, cytochrome c,
caspase-3 monoclonal antibody (1:100, 1:300 and 1:300,
respectively) and/or rabbit polyclonal antibody for different
organelle markers (2–5 μg/ml) overnight at 4°C. After
removing the unbound primary antibodies and washing them five times
with PBS containing 0.5% (v/v) Triton X-100 (PBST), the cells were
incubated with Oregon Green-conjugated (excitation/emission
wavelength 496/524 nm) or Rhodamine Red-conjugated
(excitation/emission wavelength, 570/590 nm) anti-rabbit secondary
antibodies for 40 min at room temperature. The cells were washed
and then stained with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI)
nuclear dye for 10 min at 37°C. After the cells were washed five
times with PBST, coverslips were mounted onto glass slides and
sealed with quenching agent and glycerol. Confocal images were
acquired using an Olympus Fluoview 1000 microscope (Olympus, Center
Valley, PA, USA). Experiments were performed in triplicate.
Cell viability
We performed the CCK8 assay to monitor cell
proliferation. Each group of cells was washed, counted, and seeded
at a density of 4×104 cells/ml in a 96-well plate. CCK8
solution was added 3 h before incubation in the various treatments
was completed. Cell viability was determined with a
spectrophotometer at an absorbance of 450 nm. Experiments were
performed in triplicate.
Statistical analysis
SPSS 17.0 was used to analyze experimental data.
Experimental findings were presented as the mean ± standard
deviation. One-way ANOVA followed by the Student-Newman-Keuls test
was used to compare the effect of treatment on the various
parameters. Non-parametric data were analyzed using Chi-square test
or the Fisher’s exact method. P<0.05 was considered to indicate
statistical significance.
Results
Identification of recombinant plasmid
Plenti6.3/V5 DEST
The positive clones were identified after PCR
amplification, and the size of the PCR product was 911 bp. The
detected sequence was identical to the known UCP2 sequence in
GenBank (Gene ID NM 003355.2). Western blotting revealed a 32 kDa
band in cell extracts, which was in accordance with the expected
size of the UCP2-Flag protein (32 kDa). These results indicated
that the UCP2 recombinant plasmid was successfully expressed in
HUVECs and suggested that these cells were successfully transduced
with lentivirus.
Overexpression of UCP2 by stable
transfection of Plenti6.3/V5 DEST
Total mRNA and protein extracts were prepared from
untransfected controls (NG and HG), lentiviral-negative vector
controls (NC) and UCP2-transfected (UCP2) HUVECs. The UCP2 mRNA and
protein expression levels in these cells were determined by RT-qPCR
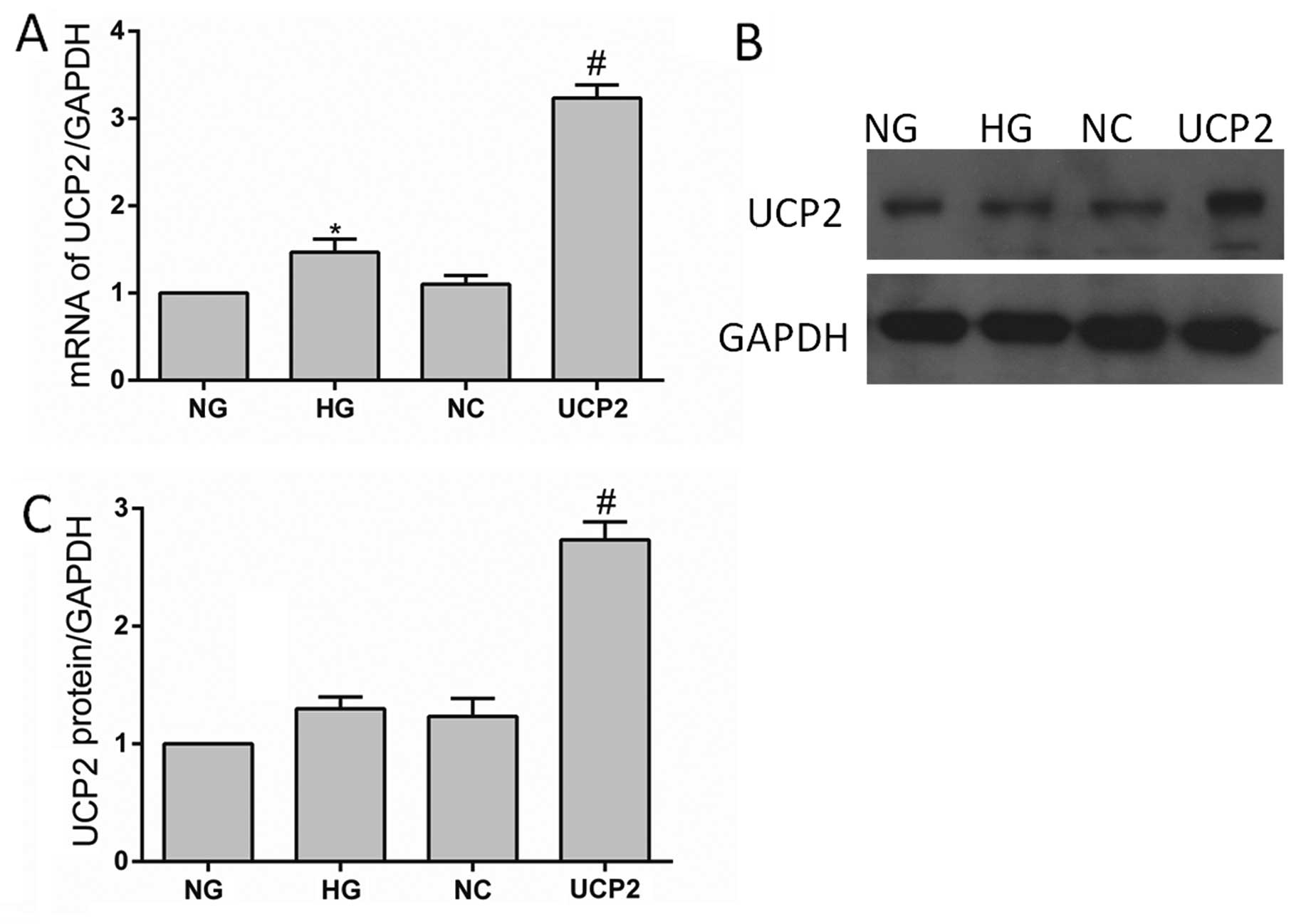
and western blot assays, respectively. RT-qPCR demonstrated that
the mRNA levels of UCP2 protein expression in the transfectants
containing Plenti6.3/V5 DEST were increased 3-fold when compared
with the untreated control HUVECs, which was consistent with the
increase of UCP2 protein expression (Fig. 1). There was no significant
difference between the cells transfected with the control
lentiviral-negative vector and the untransfected cells (p>0.05).
These results indicated that the stable transfection of
Plenti6.3/V5 DEST upregulated UCP2 expression in HUVECs.
UCP2 promoted HUVEC cell proliferation at
HG concentrations
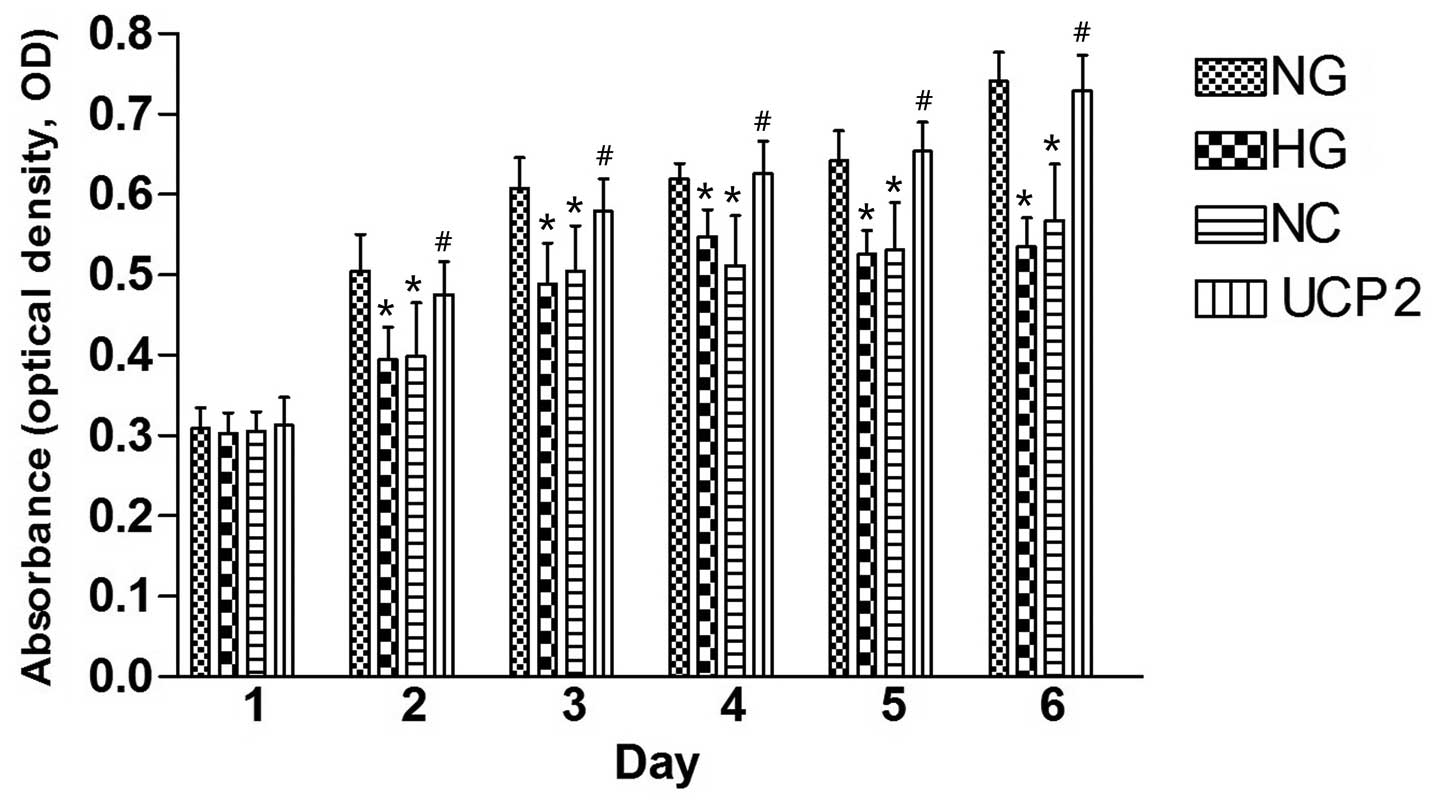
The CCK8 assay (Fig.
2) revealed a gradual increase in HUVEC cell proliferation from
day one to six in the four cell lines post-infection. On day one
post-infection, there was no statistically significant difference
in the proliferation of HUVECs among the four cell lines by ANOVA
(p>0.05). On day two post-infection, NG cells showed
significantly greater HUVEC cell proliferation than the HG and NC
cells (p<0.05), and UCP2 cells showed significantly greater
HUVEC cell proliferation as compared to NC cells (p<0.05).
However, there was no statistically significant difference in HUVEC
cell proliferation between the HG and NC cells (p>0.05). The
difference between cell lines over the six successive days was
statistically significant by ANOVA.
UCP2 attenuated high glucose-induced
apoptosis in HUVECs
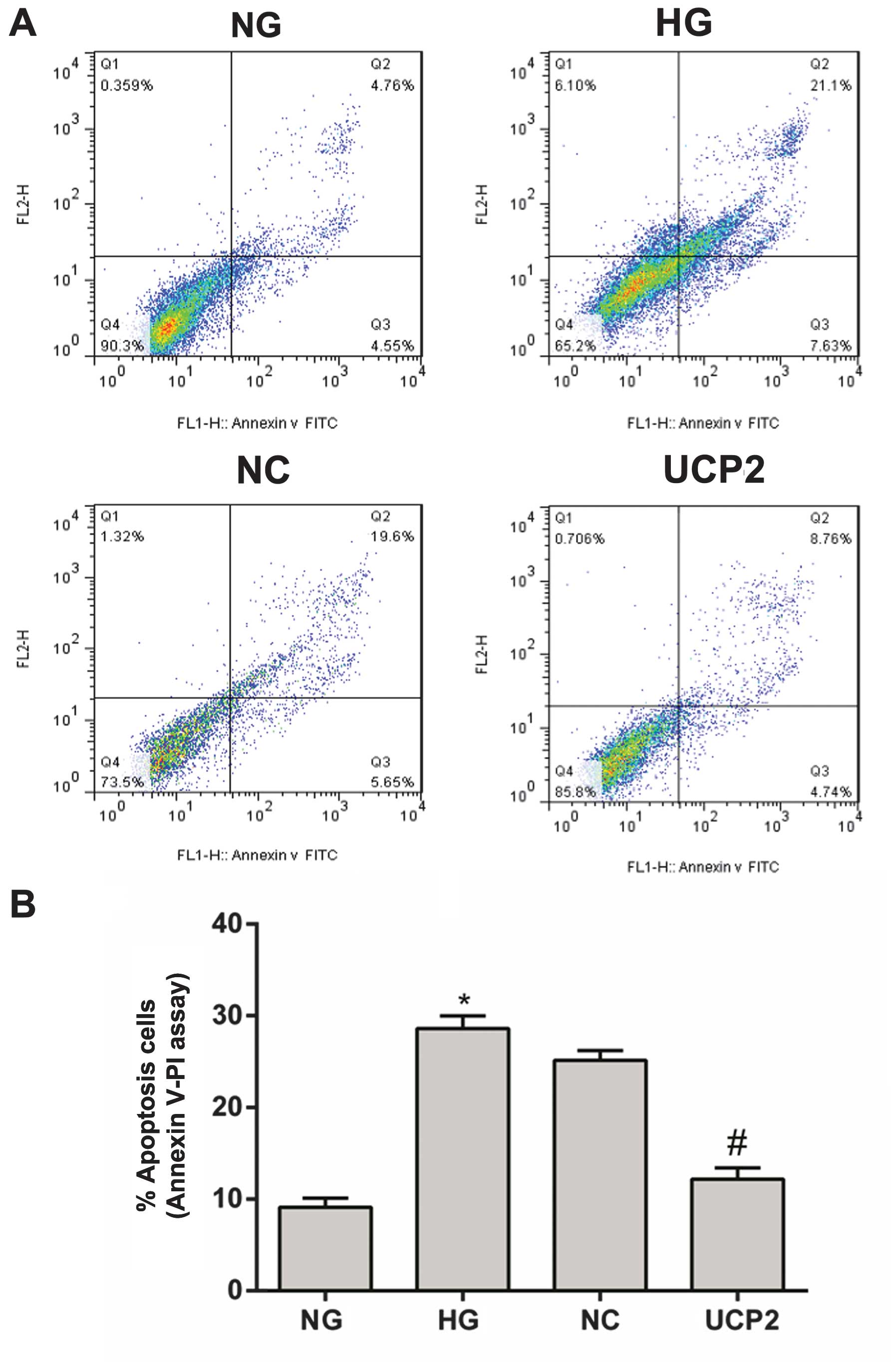
With Annexin V FITC labeling, cell apoptosis was
analyzed using flow cytometry on day three post-infection. The
apoptotic rate in the NG group was 9.31% (Fig. 3A). This apoptotic rate was
significantly different when compared with the HG, NC or UCP2 group
(p<0.05), with the apoptotic rates being 28.73, 25.25 and 13.5%,
respectively (Fig. 3A). There was
no significant difference in the apoptotic rate between the HG and
NC groups (p>0.05) (Fig.
3).
Differing effects of UCP2 on
apoptosis-related protein expression
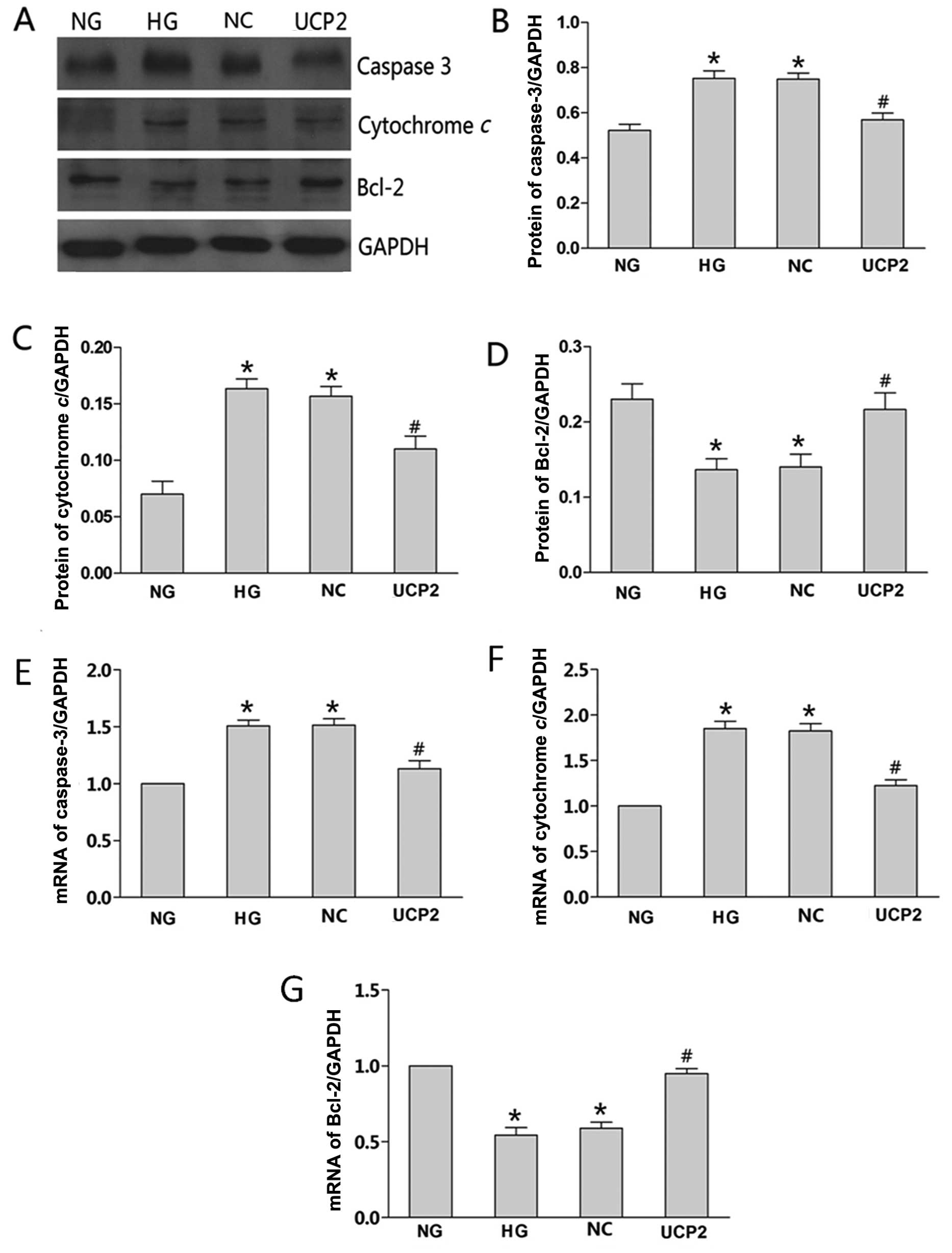
We investigated whether the overexpression of UCP2
induced by Plenti6.3/V5 DEST affected apoptosis-associated gene
expression, such as caspase-3, cytochrome c and Bcl-2. mRNA
and protein levels of the three proteins in the NG-, HG-, NC- and
UCP2-transfected cells were determined by RT-qPCR and western
blotting, respectively. The expression trend of the three proteins
was found to be consistent with the flow cytometric results. When
compared with the NG group, caspase-3 and cytochrome c
expression in the HG or NC group was significantly greater than
that in the NG group (p<0.05), while the anti-apoptotic Bcl-2
expression in the HG or NC group was significantly lower than that
in the NG group (p<0.05). The protein levels of caspase-3,
cytochrome c and Bcl-2 showed no significant changes in the
HG and NC groups (p>0.05). Caspase-3 and cytochrome c
expression in the UCP2 group was significantly lower than that in
the NC group (p<0.05), while the anti-apoptotic Bcl-2 expression
in the UCP2 group was significantly greater than that in the NC
group (p<0.05) (Fig. 4A–D). We
also analyzed the levels of the three proteins in the conditioning
media by RT-qPCR. The results were consistent with our western blot
analysis (Fig. 4E–G).
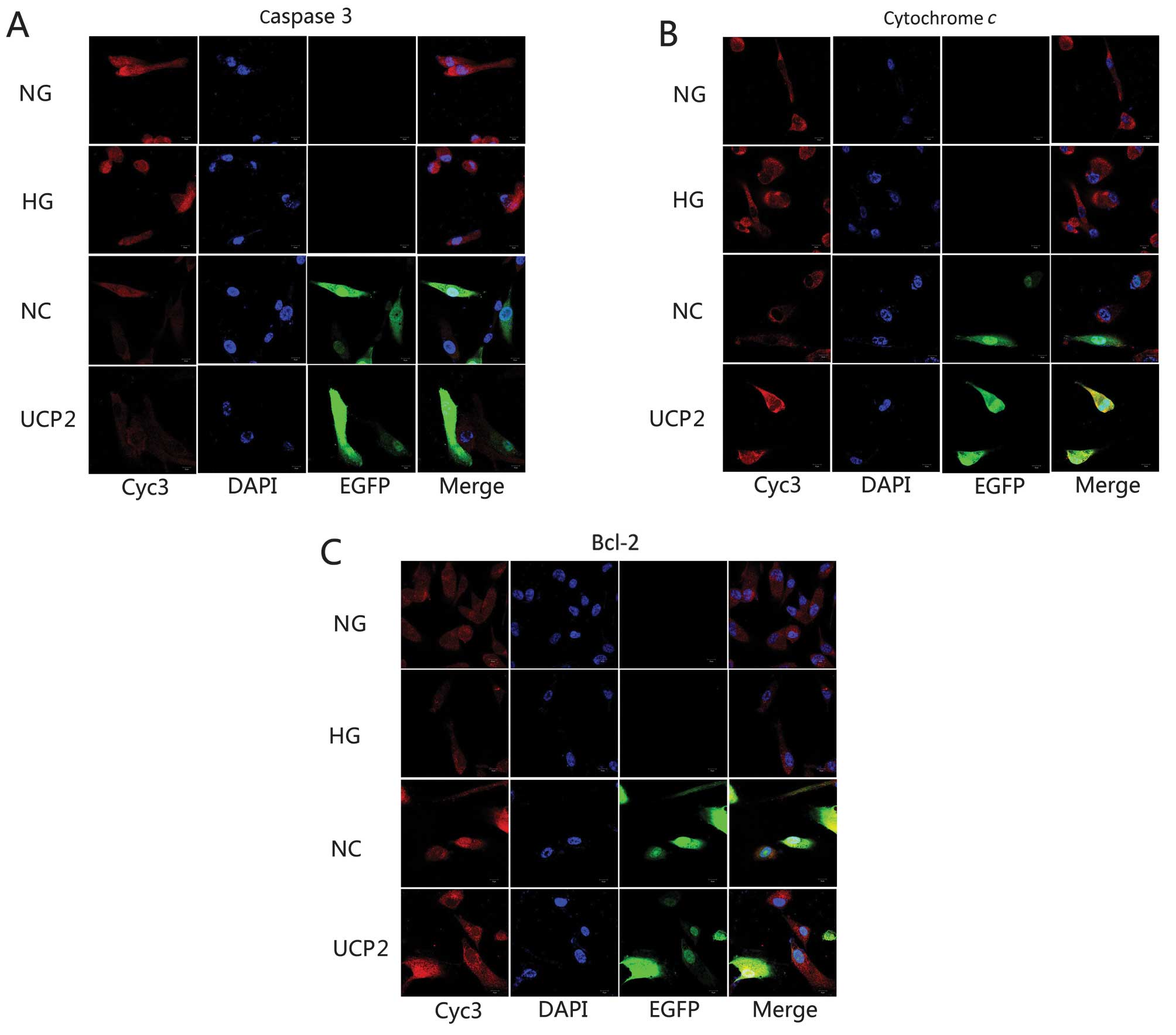
Localization of apoptosis-related
proteins in HUVECs
HUVECs were either labeled against the primary
antibodies for caspase-3, cytochrome c or Bcl-2 and nuclei
were labeled with blue fluorescent DAPI. Lentivirus-transfected
cells were labeled with green fluorescent EGFP. Laser confocal
scanning microscopy was employed to observe alterations in the
localization of the three proteins. The localized region appeared
yellow or orange-yellow. All three proteins were distributed
throughout HUVECs, with the fluorescent density in the cytoplasmic
area being relatively strong and aggregating in the nuclear
periphery. Fluorescence in the nucleus was not well distributed and
clustered in aggregates in the plasmosome. When compared with the
NG group, caspase-3 and cytochrome c density in the HG or NC
group was significantly stronger than that in the NG group, while
the anti-apoptotic Bcl-2 density in the HG or NC group was
significantly weaker than that in the NG group. The protein
densities of caspase-3, cytochrome c and Bcl-2 showed no
significant changes in the HG and NC groups. Caspase-3 and
cytochrome c densities in the UCP2 group were significantly
weaker than those in the NC group (p<0.05) while the
anti-apoptotic Bcl-2 density in the UCP2 group was significantly
stronger than that in the NC group (Fig. 5). The density trend of the three
proteins was found to be consistent with western blotting and
RT-qPCR.
Discussion
In comparison to hUCP1, hUCP2 was found to have a
greater effect on mitochondrial membrane potential when expressed
in yeast. It was found to have properties consistent with a role in
diabetes and obesity. Sayeed et al (33) found that UCP2 silencing in poorly
differentiated breast tumor cells rapidly led to the induction of
apoptosis and cell differentiation. However, whether UCP2 plays a
role in apoptosis induced by HG remains unclear. In this study, we
found that UCP2 promoted HUVEC proliferation and attenuated high
glucose-induced apoptosis. Beltramo et al (34) found that apoptosis of endothelial
cells and pericytes increased in the presence of high levels of
glucose after 3 days of culture. In previous studies (35–37) it has been reported that cultured
retinal pericytes exposed to high levels of glucose (25–30 mM) for
a period of ≥7 days show a higher rate of apoptosis than cells
grown at 5.5 mM glucose. Moreover, Cui et al (7) found that cells cultured in HG for
24–25 (23 mM) days and 45–46 (30 mM) days, resulted in an increased
rate of apoptosis in pericytes and endothelial cells when compared
with cells exposed to low glucose concentrations (5 mM). There were
no significant differences between the rates of apoptosis in the 23
and 30 mM glucose groups. Our results were consistent with these
studies. Compared with the NG group, the apoptotic rate was
significantly increased in the HG and NC groups, while the
apoptotic rate in the UCP2 group was significantly decreased. There
was no significant difference in the rate of apoptosis between the
HG and NC groups. Thus, we concluded that UCP2 attenuates high
glucose-induced apoptosis in HUVECs.
Evidence suggests that UCP2 is related to cell
proliferation. Elorza et al (38) found that UCP2 deficiency results
in a significant decrease in cell proliferation at the
erythropoietin-dependent phase of erythropoiesis. By contrast,
Pecqueur et al (39) found
that UCP2−/− cells exhibit enhanced proliferation
associated with a metabolic switch from fatty acid oxidation to
glucose metabolism. At present, the results are controversial.
Therefore, to solve this problem, we tested UCP2+/+
HUVEC cell proliferation following HG treatment. Our results were
consistent with those of Pecqueur et al (39). On day one post-infection, there
was no statistically significant difference in the proliferation of
HUVECs among the four cell lines. On day two post-infection, NG
cells showed significantly greater HUVEC cell proliferation than
the HG and NC cells, and UCP2 cells showed significantly greater
HUVEC cell proliferation than the NC cells. However, there was no
statistically significant difference in HUVEC cell proliferation
between the HG and NC cells. Thus, UCP2 promoted HUVEC
proliferation.
Apoptosis is a programmed cell death process that
serves to remove abnormally proliferative cells, and UCP2 has been
previously associated with apoptotic and proliferative activity in
poorly differentiated breast tumor cells. However, various cell
types and rat species have been shown to use different mechanisms
of UCP2 to influence both apoptotic and proliferative activity. For
example, Sayeed et al (33) showed that the negative regulation
of UCP2 by TGFβ signaling rapidly leads to the induction of
apoptosis and cell differentiation. In addition, proliferation
signaling pathways such as Rb/E2F, c-Myc, and Ras are characterized
by their ability to activate nuclear transcription factors located
in the cytosol (40). However,
Elorza et al (38) showed
that UCP2 modulates cell proliferation through the MAPK/ERK pathway
during erythropoiesis. Furthermore, Pecqueur et al (39) demonstrated that UCP2 controls
proliferation by promoting fatty acid oxidation and limiting
glycolysis-derived pyruvate utilization.
To investigate the effect of UCP2 on cell apoptosis
and proliferation, we focused on the expression of the
apoptosis-related proteins caspase-3, cytochrome c and
Bcl-2, which determine cell fate at the mitochondrial level.
Specifically, western blotting and RT-qPCR were used to detect
expression levels of the three proteins. Compared with the NG
group, caspase-3 and cytochrome c expression in the HG or NC
group was significantly increased, while the anti-apoptotic Bcl-2
expression in the HG or NC group was significantly decreased.
Moreover, protein levels of caspase-3, cytochrome c and
Bcl-2 showed no significant changes in the HG and NC groups.
Notably, caspase-3 and cytochrome c expression in the UCP2
group was significantly lower than that in the NC group, while the
anti-apoptotic Bcl-2 expression in the UCP2 group was significantly
higher than that in the NC group. Therefore, UCP2 promotes cell
proliferation and inhibits HG-induced apoptosis in HUVECs via the
Bcl-2 up- and downregulation of caspase-3 and cytochrome
c.
In conclusion, to the best of our knowledge, these
are the first results showing that UCP2 promotes cell proliferation
and inhibits HG-induced apoptosis in HUVECs via Bcl-2 up- and
downregulation of caspase-3 and cytochrome c in vitro.
Future efforts should focus on whether these effects are also
present in vivo.
Acknowledgements
This study was supported by grants from the Research
Fund for the National Nature Science Funding of China (nos.
30930097, 81273424 and 81170862), Major National Science and
Technology projects during the 12th Five-Year Plan
(2011ZX09302-007-02).
References
|
1
|
Forbes JM, Coughlan MT and Cooper ME:
Oxidative stress as a major culprit in kidney disease in diabetes.
Diabetes. 57:1446–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brownlee M: The pathobiology of diabetic
complications: a unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng Z, Chen H, Ke G, Fan Y, Zou H, et
al: Protective effect of perindopril on diabetic retinopathy is
associated with decreased vascular endothelial growth
factor-to-pigment epithelium-derived factor ratio: involvement of a
mitochondria-reactive oxygen species pathway. Diabetes. 58:954–964.
2009. View Article : Google Scholar
|
|
5
|
Mattson MP and Kroemer G: Mitochondria in
cell death: novel targets for neuroprotection and cardioprotection.
Trends Mol Med. 9:196–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mattson MP and Liu D: Mitochondrial
potassium channels and uncoupling proteins in synaptic plasticity
and neuronal cell death. Biochem Biophys Res Commun. 304:539–549.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui Y, Xu X, Bi H, Wu J, et al: Expression
modification of uncoupling proteins and MnSOD in retinal
endothelial cells and pericytes induced by high glucose: the role
of reactive oxygen species in diabetic retinopathy. Exp Eye Res.
83:807–816. 2001. View Article : Google Scholar
|
|
8
|
Fleury C, Neverova M, Collins S, Raimbault
S, et al: Uncoupling protein-2: a novel gene linked to obesity and
hyperinsulinemia. Nat Genet. 15:269–272. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pecqueur C, Couplan E, Bouillaud F and
Ricquier D: Genetic and physiological analysis of the role of
uncoupling proteins in human energy homeostasis. J Mol Med.
79:48–56. 2001. View Article : Google Scholar
|
|
10
|
Pecqueur C, Alves-Guerra MC, Gelly C,
Levi-Meyrueis C, Couplan E, et al: Uncoupling protein 2, in vivo
distribution, induction upon oxidative stress, and evidence for
translational regulation. J Biol Chem. 276:8705–8712. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Couplan E, del Mar Gonzalez-Barroso M,
Alves-Guerra MC, Ricquier D, Goubern M and Bouillaud F: No evidence
for a basal, retinoic, or superoxide-induced uncoupling activity of
the uncoupling protein 2 present in spleen or lung mitochondria. J
Biol Chem. 277:26268–26275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang CY, Baffy G, Perret P, Krauss S,
Peroni O, et al: Uncoupling protein-2 negatively regulates insulin
secretion and is a major link between obesity, beta cell
dysfunction, and type 2 diabetes. Cell. 105:745–755. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krauss S, Brand MD and Buttgereit F:
Signaling takes a breath - new quantitative perspectives on
bioenergetics and signal transduction. Immunity. 15:497–502. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krauss S, Zhang CY, Scorrano L, Dalgaard
LT, St-Pierre J, et al: Superoxide-mediated activation of
uncoupling protein 2 causes pancreatic beta cell dysfunction. J
Clin Invest. 112:1831–1842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turner JD, Gaspers LD, Wang G and Thomas
AP: Uncoupling protein-2 modulates myocardial
excitation-contraction coupling. Circ Res. 106:730–738. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horvath TL, Warden CH, Hajos M, Lombardi
A, Goglia F, et al: Brain uncoupling protein 2: uncoupled neuronal
mitochondria predict thermal synapses in homeostatic centers. J
Neurosci. 19:10417–10427. 1999.PubMed/NCBI
|
|
17
|
Liu Y, Chen L, Xu X, Vicaut E and Sercombe
R: Both ischemic preconditioning and ghrelin administration protect
hippocampus from ischemia/reperfusion and upregulate uncoupling
protein-2. BMC Physiol. 9:172009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Della-Morte D, Dave KR, DeFazio RA, Bao
YC, Raval AP, et al: Resveratrol pretreatment protects rat brain
from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2
pathway. Neuroscience. 159:993–1002. 2009. View Article : Google Scholar
|
|
19
|
Arsenijevic D, Onuma H, Pecqueur C,
Ricquier D, et al: Disruption of the uncoupling protein-2 gene in
mice reveals a role in immunity and reactive oxygen species
production. Nat Genet. 26:435–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nègre-Salvayre A, Hirtz C, Carrera G,
Casteilla L, et al: A role for uncoupling protein-2 as a regulator
of mitochondrial hydrogen peroxide generation. FASEB J. 11:809–815.
1997.PubMed/NCBI
|
|
21
|
Li LX, Skorpen F, Egeberg K, Jørgensen IH
and Grill V: Uncoupling protein-2 participates in cellular defense
against oxidative stress in clonal beta-cells. Biochem Biophys Res
Commun. 282:273–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai Y, Onuma H, Bai X, Medvedev AV,
Collins S, et al: Persistent nuclear factor-kappa B activation in
Ucp2−/− mice leads to enhanced nitric oxide and inflammatory
cytokine production. J Biol Chem. 280:19062–19069. 2005.
|
|
23
|
Nishio K, Qiao S and Yamashita H:
Characterization of the differential expression of uncoupling
protein 2 and ROS production in differentiated mouse
macrophage-cells (Mm1) and the progenitor cells (M1). J Mol Histol.
36:35–44. 2005. View Article : Google Scholar
|
|
24
|
Brand MD, Affourtit C, Esteves TC, Parker
N, et al: Mitochondrial superoxide: production, biological effects,
and activation of uncoupling proteins. Free Radic Biol Med.
37:755–767. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Conti B, Sugama S, Lucero J, Bartfai T, et
al: Uncoupling protein 2 protects dopaminergic neurons from acute
1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem.
93:493–501. 2005. View Article : Google Scholar
|
|
26
|
Mattiasson G, Shamloo M, Gido G, Wieloch
T, et al: Uncoupling protein-2 prevents neuronal death and
diminishes brain dysfunction after stroke and brain trauma. Nat
Med. 9:1062–1068. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paradis E, Clavel S, Bouillaud F, Ricquier
D and Richard D: Uncoupling protein 2: a novel player in
neuroprotection. Trends Mol Med. 9:522–525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richard D, Clavel S, Huang Q, Sanchis D
and Ricquier D: Uncoupling protein 2 in the brain: distribution and
function. Biochem Soc Trans. 29:812–817. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blanc J, Alves-Guerra MC, Esposito B,
Rousset S, Mallat Z, et al: Protective role of uncoupling protein 2
in atherosclerosis. Circulation. 107:388–390. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teshima Y, Akao M, Jones SP and Marbán E:
Uncoupling protein-2 overexpression inhibits mitochondrial death
pathway in cardiomyocytes. Circ Res. 93:192–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Polonsky KS and Semenkovich CF: The
pancreatic beta cell heats up: UCP2 and insulin secretion in
diabetes. Cell. 105:705–707. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suh YH, Kim SY, Lee HY, Song DK, et al:
Overexpression of short heterodimer partner recovers impaired
glucose-stimulated insulin secretion of pancreatic beta-cells
overexpressing UCP2. J Endocrinol. 183:133–144. 2004. View Article : Google Scholar
|
|
33
|
Sayeed A, Meng Z, Luciani G, Dairkee SH,
et al: Negative regulation of UCP2 by TGFβ signaling characterizes
low and intermediate-grade primary breast cancer. Cell Death Dis.
1:e532010.
|
|
34
|
Beltramo E, Berrone E, Buttiglieri S and
Porta M: Thiamine and benfotiamine prevent increased apoptosis in
endothelial cells and pericytes cultured in high glucose. Diabetes
Metab Res Rev. 20:330–336. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Liu X, He Z, Yanoff M, Jian B and Ye
X: Expression of apoptosis regulatory genes by retinal pericytes
after rapid glucose reduction. Invest Ophthalmol Vis Sci.
39:1535–1543. 1998.PubMed/NCBI
|
|
36
|
Naruse K, Nakamura J, Hamada Y, Nakayama
M, Chava S, Komori T, Kato K, Kasuya Y, Miwa K and Hotta N: Aldose
reductase inhibition prevents glucose-induced apoptosis in cultured
bovine retinal microvascular pericytes. Exp Eye Res. 71:309–315.
2000. View Article : Google Scholar
|
|
37
|
Romeo G, Liu WH, Asnaghi V, Kern TS and
Lorenzi M: Activation of nuclear factor-kappaB induced by diabetes
and high glucose regulates a proapoptotic program in retinal
pericytes. Diabetes. 51:2241–2248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elorza A, Hyde B, Mikkola HK, Collins S
and Shirihai OS: UCP2 modulates cell proliferation through the
MAPK/ERK pathway during erythropoiesis and has no effect on heme
biosynthesis. J Biol Chem. 283:30461–30470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pecqueur C, Bui T, Gelly C, Tompson CB, et
al: Uncoupling protein-2 controls proliferation by promoting fatty
acid oxidation and limiting glycolysis-derived pyruvate
utilization. FASEB J. 22:9–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sears RC and Nevins JR: Signaling networks
that link cell proliferation and cell fate. J Biol Chem.
277:11617–11620. 2002. View Article : Google Scholar : PubMed/NCBI
|