Introduction
It has been well documented that diabetic patients
have an increased risk of atherosclerotic vascular disease. High
glucose (HG) may trigger many cellular events, such as oxidative
injury, enhanced mitogenicity, and vascular cell proliferation,
which are key processes in the development of atherosclerosis in
diabetic vascular complication (1). Diabetic vascular complications are
characterized by the progressive atherosclerotic lesion, and
vasculature alteration. The proliferation of vascular smooth muscle
cells (VSMC) in the blood vessel wall is a key event in diabetic
vascular complications and may play a crucial role in the
regulation of blood flow and pressure (2). Furthermore, differentiation and
migration of VSMC contribute to development of atherosclerosis in
native vessels as well as restenosis formation after vascular
injury (3–5).
Zipper-interacting protein kinase (ZIPK), a member
of the death-associated protein kinases (DAPKs) family, is isolated
from bladder smooth muscle as a 32-kDa phosphoprotein (6,7).
Similar to other DAPK family proteins, ZIPK is mainly involved in
the regulation of cell death including apoptosis and autophagy
(8–10), as well as mitotic processes
(11). In addition, several other
functions of ZIPK such as the regulation of smooth muscle
contraction have been identified. In smooth muscle, ZIPK was found
to phosphorylate light chain (LC20) at Ser19 and Thr18 in a
Ca2+/calmodulin-independent manner (12,13). In addition, ZIPK enhances STAT3
transcriptional activity (14),
while STAT3 plays a role in cell growth and apoptosis. However, a
physiological role for a ZIPK in HG-treated human aortic smooth
muscle cells (HASMCs) remains unclear.
The aim of this study, therefore, was to evaluate
the proliferation, apoptosis, migration as well as the cell cycle
progression of HG-treated HASMCs by overexpressing and knocking
down ZIPK, and explore the potential mechanisms involved.
Materials and methods
Cell culture and materials
HASMCs were cultured in Dulbecco’s modified Eagle’s
medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10%
fetal bovine serum (FBS) and 5% CO2 at 37°C. Cells
(293TN) were cultivated in DMEM (Gibco) with 10% of FBS. Human lung
fibroblast MRC-5 was maintained as a monolayer culture in nutrient
medium and Roswell Park Memorial Institute-1640 medium (RPMI-1640)
(Sigma-Aldrich, St. Louis, MO, USA). Cell culture reagents, TRIzol
reagent, Platinum SYBR-Green qPCR SuperMix -UDG plus ROX and
Lipofectamine 2000 were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). The real-time PCR Master mix kit and the
ReverTra Ace kit were purchased from Toyobo (Osaka, Japan).
Anti-intercellular adhesion molecule (ICAM)-1, anti-vascular cell
adhesion molecule (VCAM)-1, and anti-matrix metalloproteinase
(MMP)-2, anti-angio-associated migratory cell protein (AAMP) and
anti-human cell division cycle gene (Hcdc) 14A antibody were
purchased from Abcam (Cambridge, MA, USA).
Determination of apoptosis by flow
cytometry
To detect externalized phosphatidylserine (PS) as an
early indication of apoptosis, HASMCs (1×105/ml) were
incubated for 24 h at 37°C. The cells were then collected, washed
twice with FACS buffer (0.2% BSA/PBS), stained with Annexin V-FITC
and propidium iodide (PI) according to the manufacturer’s
instructions, and then analyzed with a Becton-Dickinson (BD)
(Franklin Lakes, NJ, USA) FACSCalibur flow cytometer and CellQuest
Pro software.
Determination of perturbations of cell
cycle
For the cell cycle analysis, the cells were grown in
6-well plates and transfected with plasmids. At 72 h after
transfection, the cells were collected, washed with ice-cold
phosphate-buffered saline (PBS), and fixed with 70% ethanol for at
least 1 h. The fixed cells were washed and stained with PI for 1 h
at room temperature. PI-stained nuclei were then analyzed using the
BD FACSCalibur system.
Migration assays
Matrigel-coated filter inserts (8 μm pore size) that
fit into 24-well migration chambers were obtained from
Becton-Dickinson. HASMCs were then plated on the upper chamber. The
lower chamber was filled with culture media. Cells in the chamber
were incubated for 24 h at 37°C and cells that invaded the lower
membrane surface were fixed with methanol and stained with
hematoxylin and eosin. The cells that passed through the Matrigel
and were located on the underside of the filter were counted.
Random fields were counted by light microscopy under a high-power
field (x400).
Cell proliferation assay
HASMCs were seeded at a density of 2×104
cells/well in 96-well culture plates and cultured in serum-free
DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, 100 g/ml streptomycin, 8 mM HEPES and 2 mM L-glutamine
at 37°C in an incubator with a humidified atmosphere of 95% air and
5% CO2. Proliferation of the cultured cells was assayed
using the Cell Counting kit-8 (CCK-8) (Dojindo Molecular
Technologies, Inc., Gaithersburg, MD, USA) according to the
manufacturer’s instructions (15,16). The rationale for the Cell Counting
assay using this specific kit is that the color-developing
substrate, WST-8, contained in CCK-8, is reduced by intracellular
dehydrogenase to water-soluble formazan, the amount of which can be
directly measured photometrically at 450 nm. The cell count has
been shown to be linearly proportional to the amount of formazan
generated.
Plasmid construction and lentivirus
production
Total RNA was extracted from MRC-5 cells using
TRIzol and cDNA was prepared by reverse transcription. The ZIPK
gene was amplified by PCR with the cDNA. The sequences of the
primers used were: 5′-GCTCTAGAGCCACCATGTCCACGTTCAGGCAG-3′ and
5′-CGGGATCCCTAGCGCAGCCCGCACTCC-3′. The PCR products were digested
by EcoRI and BamHI and a 1589 bp fragment containing
ZIPK gene was cloned into the lentiviral vector pCDH-green
fluorescent protein (GFP) Lentivector (System Biosciences, cat. no.
CD511A-1). The sequences of the resulting vector (PLV-ZIPK) were
verified by sequence analysis.
ZIPK was knocked down with an RNAi technique. The
sequence targeting human ZIPK mRNA was chosen based on the
full-length mRNA sequence in Genbank (accession no. NM_001348.1).
The target sequences of ZIPK (5′-GCCTGGAACATTCCTGGAT-3′) were
homologous to 806–835 nt of ZIPK mRNA, respectively. The
corresponding oligonucleotide templates of the shRNA were
chemically synthesized and cloned into the pSIH1-H1-copGFP shRNA
vector (System Biosciences, Mountain View, CA, USA) which was
digested by BamHI and EcoRI and purified by agarose
gel electrophoresis. An invalid RNAi sequence
(5′-GAAGCCAGATCCAGCTTCC-3′) was used as the negative control. The
correct vectors were constructed (designated as pLV-ZIPK and
pLV-NC) and confirmed by sequencing.
Virus packaging was performed in 293TN cells after
the co-transfection of PLV-ZIPK or PLV-shRNA or PLV-GFP control
vector (500 ng/μl, 4 μl) and Lentivirus Package plasmids mix (500
ng/μl, 20 μl) using Lipofectamine 2000. Viruses were harvested 72 h
after transfection, and the highly expressed lentivirus particles
were visualized by using green fluorescence. Five types of packaged
lentivirus termed by their contents were identified, including
pcDNA-ZIPK (ZIPK overexpression), pcDNA (control of pcDNA-ZIPK),
shRNA-ZIPK (ZIPK silencing), shRNA-scramzble (scrambled of
oligonucleotides as shRNA-ZIPK control) and shRNA (control of
shRNA-ZIPK).
Statistical analysis
Experiments were repeated at least three times. The
results were expressed as the mean ± SD and the data were analyzed
using one-way ANOVA followed by a Dunnett’s test or Student’s
t-test to determine any significant differences. P<0.05 was
considered to indicate statistical significance.
Results
Transfection efficiency of recombinant
lentivirus in HASMCs
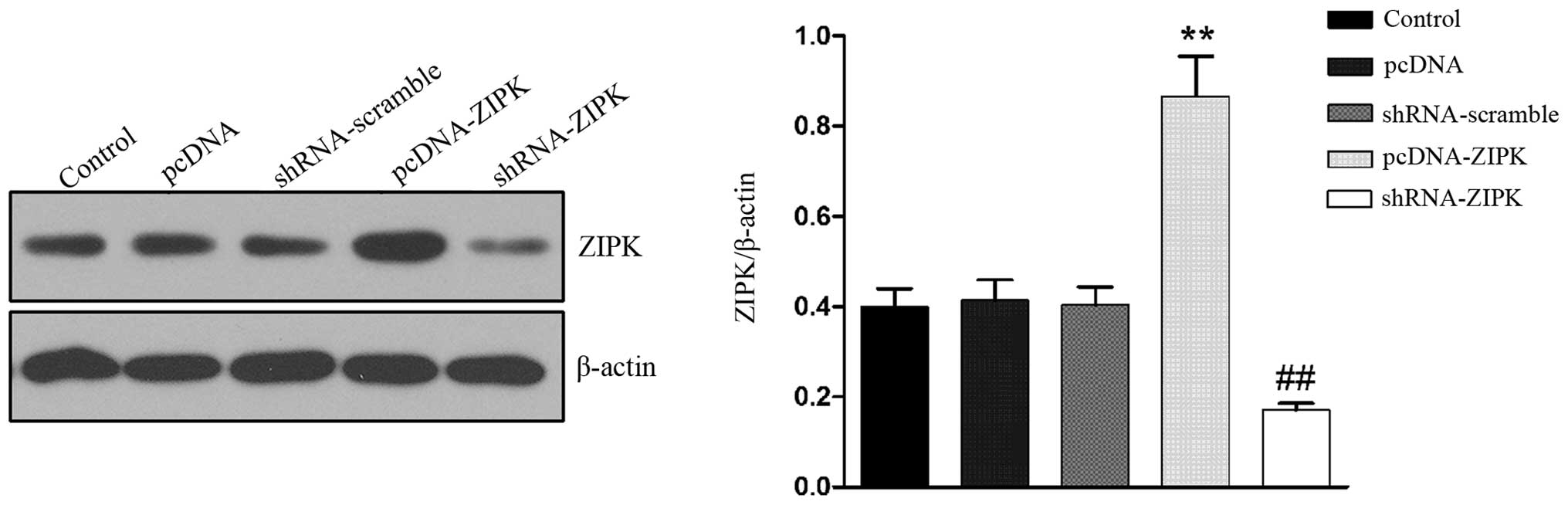
To elucidate the role of ZIPK on HASMCs treated with
HG, we overexpressed ZIPK by lentiviral infection and then knocked
down ZIPK by gene deletion using ZIPK shRNA. Western blotting was
performed to determine the expression of ZIPK. As shown in Fig. 1, the transfection of lentivirus
containing pcDNA-ZIPK in HASMCs upregulated ZIPK expression by
~2-fold, whereas transfection with lentivirus containing shRNA-ZIPK
significantly downregulated ZIPK expression.
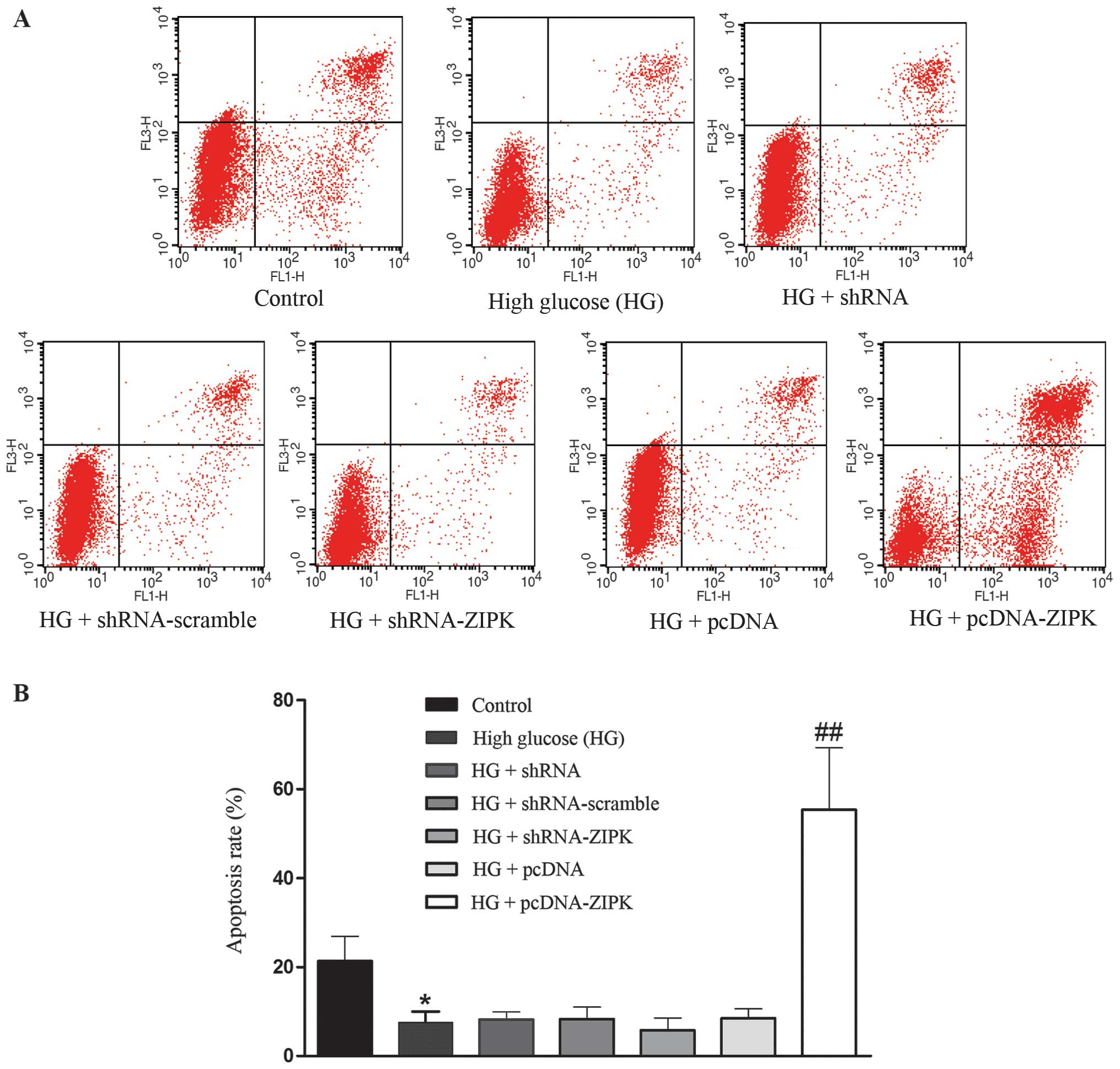
ZIPK induces apoptosis in HASMCs cultured
with HG
The percentage of apoptotic cells in HASMCs was
determined by Annexin V/PI staining with flow cytometric analysis.
We first investigated the apoptosis of HASMCs in response to
treatment with glucose at final concentrations of 25 mmol/l. As
shown in Fig. 2, ZIPK
overexpression significantly induced apoptosis in HG-treated
HASMCs, whereas non-infected control cells and cells infected with
shRNA-ZIPK or nonsense shRNA had no effect on the apoptosis of
HASMCs.
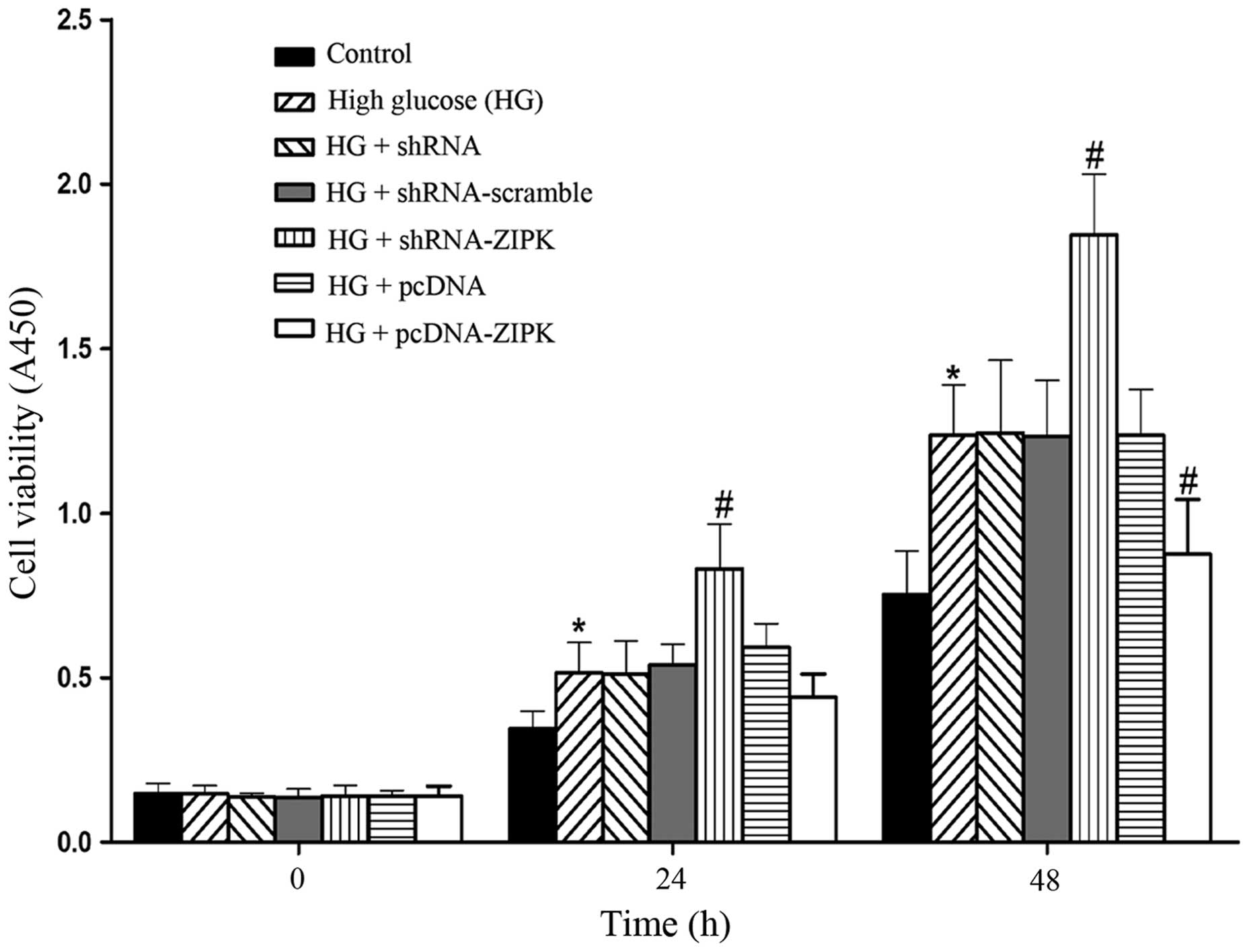
ZIPK inhibits the proliferation of HASMCs
cultured with HG
To determine the effect of ZIPK on HASMC
proliferation, we evaluated cell proliferation using the CCK-8. As
shown in Fig. 3, exposure of
HASMCs to HG stimulated cell proliferation, while shRNA-ZIPK
transfection further increased the pro-proliferative effect at 24-
and 48-h post-transfection. By contrast, an increase in the
proliferation rate stimulated by HG was markedly reduced as a
result of pre-treatment with pcDNA-ZIPK at 48-h post-transfection.
The results indicated the protective role of ZIPK in the
proliferation of HASMCs stimulated by HG.
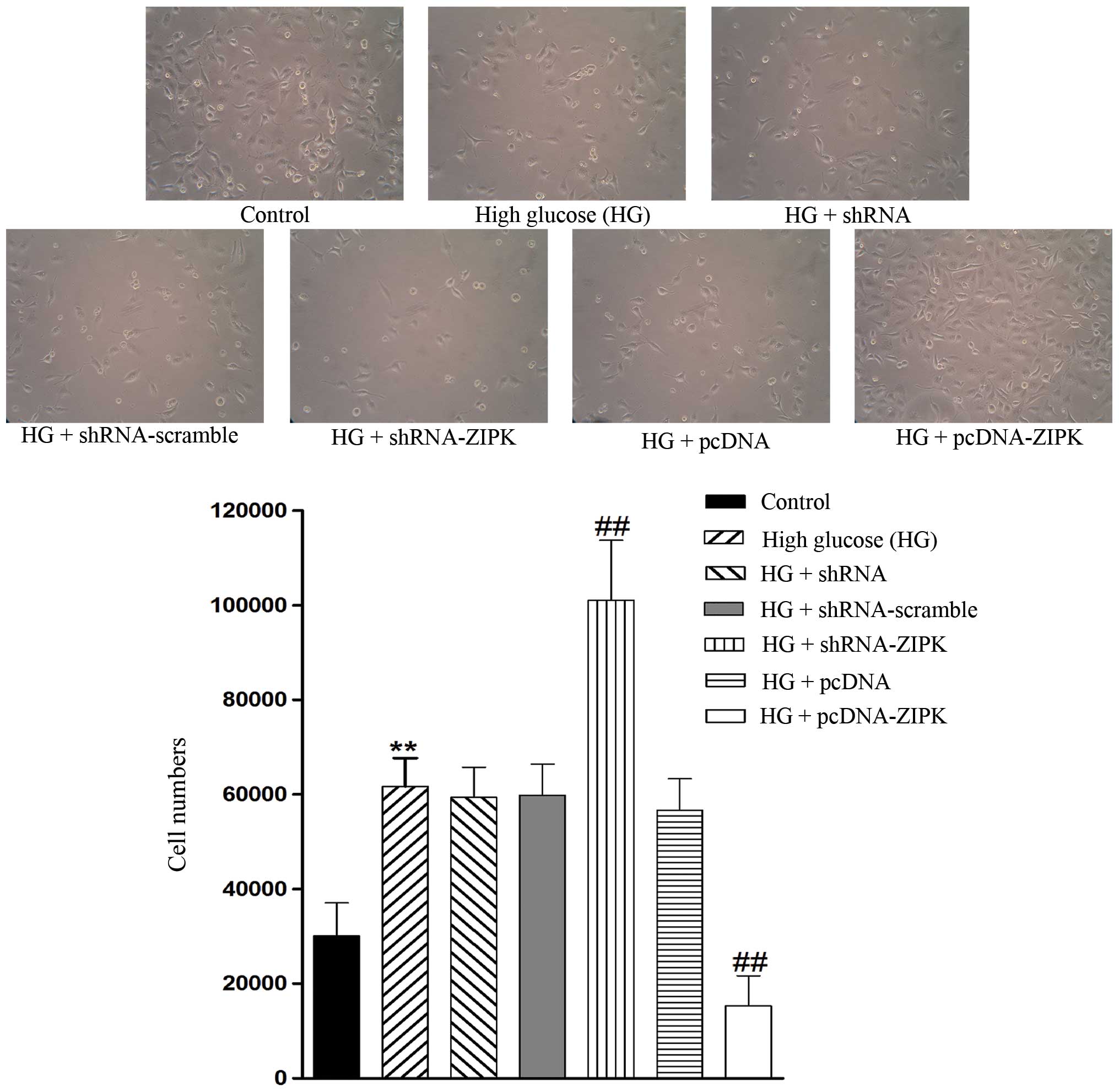
ZIPK decreases the migration ability of
HG-treated HASMCs
To delineate the role of ZIPK in migration, we
separately used lentivirus as a vector to downregulate and
upregulate ZIPK expression. As shown in Fig. 4, the migration of HASMCs was
promoted by treatment with HG as compared with the control, and
infection with shRNA-ZIPK further enhanced the migration rate,
whereas ZIPK overexpression markedly inhibited HG-treated HASMC
invasion. These experiments indicated that ZIPK may perform an
inhibitory role in the process of HASMC invasion.
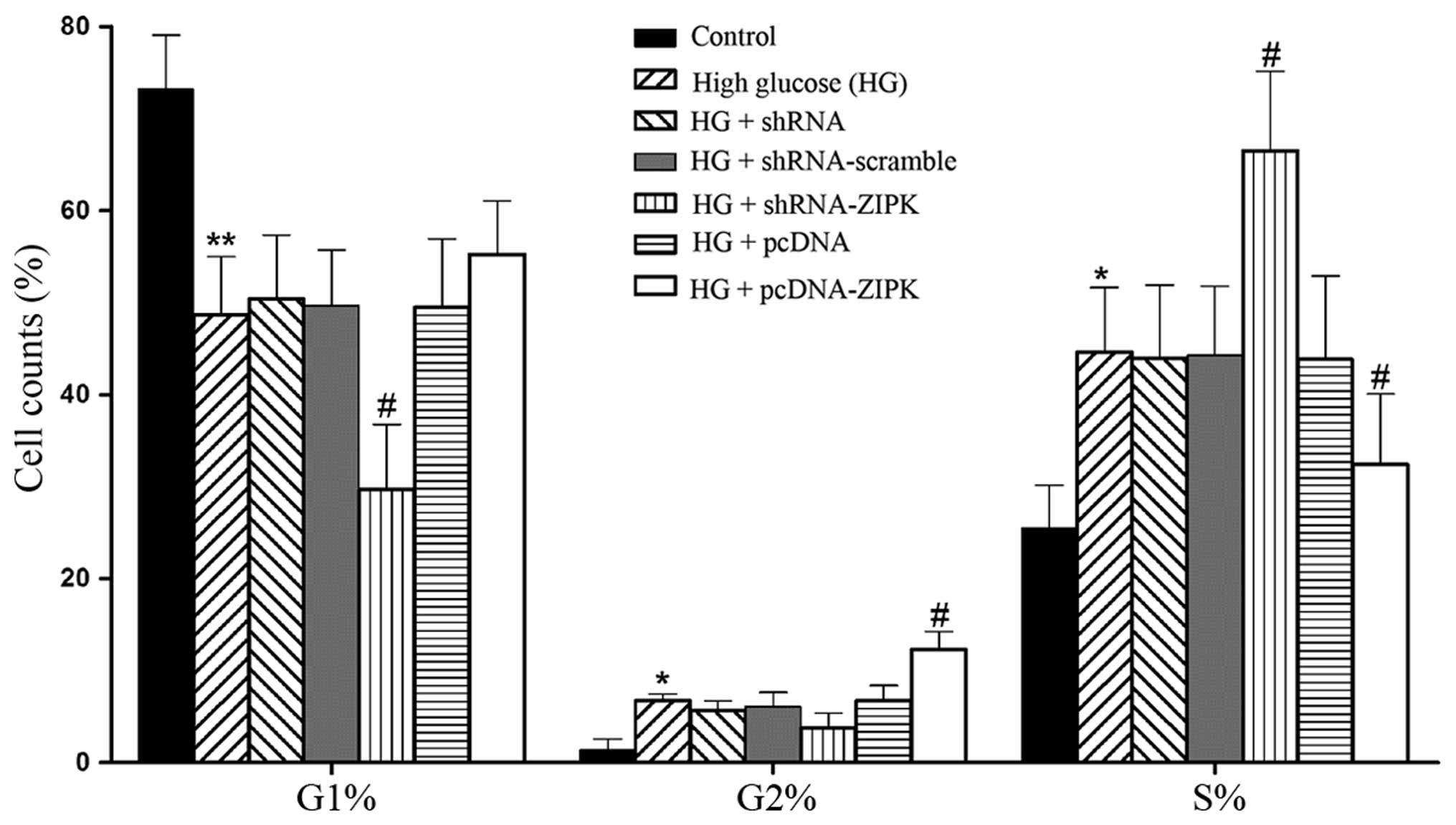
ZIPK participates in the mitotic
progression of HASMCs
Since ZIPK has been associated with the regulation
of mitotic processes (11), we
assessed the function of ZIPK in HASMCs on cell cycle with the HG
stimulation. Results are presented as diagrams of cell distribution
over the cell cycle phases of HASMCs. As shown in Fig. 5, HG treatment induced arrest in
the S phase and decreased the cell percentage in the G1 and G2
phase of the cell cycle as compared with the non-treated control.
Silencing of ZIPK expression markedly induced arrest in the S
phase, whereas HASMCs treated with pcDNA-ZIPK attenuated S-phase
retardation and retarded the cell cycle progression through an
increased G2 phase compared to the HG group. These data suggested
that the overexpression of ZIPK exerted a protective effect on cell
cycle disturbance of HASMCs stimulated by HG.
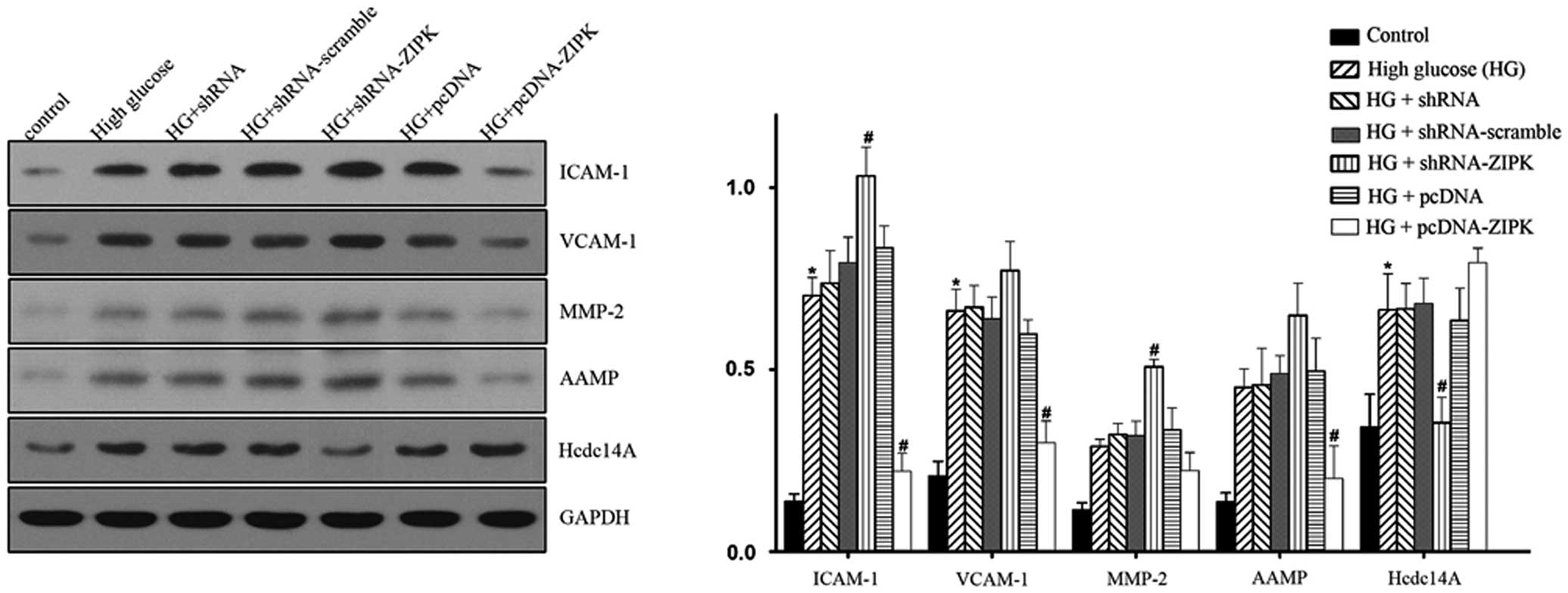
Modulations of cell cycle, apoptosis and
migration-related proteins by ZIPK in HG-treated HASMCs
To explain the potential mechanisms of ZIPK in
HASMCs, we examined the expression of several pivotal mediators of
the signaling pathway on cell apoptosis, migration, proliferation
and cell cycle, including ICAM-1, VCAM-1, MMP-2, AAMP and Hcdc14A
by western blotting. The results in Fig. 6 show that stimulation with HG
increased the levels of ICAM-1, VCAM-1 and Hcdc14A expression.
Infection of shRNA-ZIPK led to the upregulation of ICAM-1, VCAM-1,
MMP-2 and AAMP, while the expression of Hcdc14A was downregulated.
By contrast, ZIPK pre-treatment resulted in the downregulated
expression of ICAM-1, VCAM-1, MMP-2 and AAMP, and the upregulated
expression of Hcdc14A. The present investigation demonstrated that
gene transfer of ZIPK into HASMCs is a regulatory element in the
cell physiological process. However, the direct effect of ZIPK
remains to be determined.
Discussion
Patients with diabetes are at higher risk of
atherosclerotic disease than non-diabetic individuals with other
comparable risk factors. The proliferation and migration of HASMCs
play important roles in atherosclerosis during the processes
inherent to vascular disease (17,18). To the best of our knowledge, this
is the first study to focus on the effect of the overexpression and
knocking down of ZIPK by lentivirus in diverse cell processes,
including apoptosis, proliferation, migration and the cell cycle in
HASMCs treated with HG. ZIPK overexpression led to decreased
migration and cell growth, increased apoptosis and ameliorated cell
cycle disturbance in HG-treated HASMCs. Our results raise the
possibility that ZIPK is a novel potential therapeutic target for
the treatment of diabetic microvascular complication.
Findings of previous studies showed that full-length
ZIPK may induce diphosphorylation of LC20 and contribute to cell
motility and apoptosis (12,13). In addition, ZIPK plays a pivotal
role in the regulation of actin filament reorganization, and
control of Ca2+ sensitization to induce cell smooth
muscle contraction (13,19). Recent accumulating evidence
suggests that ZIPK plays a variety of roles in cell death as well
as cardiovascular pathophysiology. Cho et al demonstrated in
mesenteric arteries of spontaneously hypertensive rats (SHR) that
ZIPK modulated calyculin A-induced contraction by increasing
Ca2+-independent myosin LC (MLC) kinase activity
(20). Moreover, ZIPK has been
reported to promote ROS-dependent vascular inflammation and mediate
the development of hypertension in SHR (21).
To gain a better understanding of whether ZIPK
contributed to HASMC proliferation, apoptosis, migration as well as
cell cycle in an HG culture medium, we used two different
approaches: shRNA-ZIPK to downregulate and pcDNA-ZIPK to upregulate
ZIPK expression. The results showed that ZIPK overexpression
decreased HASMC proliferation and the migration rate. Moreover,
lentivirus-mediated ZIPK significantly induced HASMC apoptosis
treated by HG. The cell cycle experiments showed that inhibition of
the expression of ZIPK incurred arrest at the S phase, thereby
accelerating cell proliferation, while ZIPK overexpression reversed
the phenomenon of disturbing the cell cycle due to HG. Therefore,
the current data emphasized the possibility that recombinant
lentivirus-mediated ZIPK gene expression is an effective method of
inhibiting HASMC growth, restraining cell migration inducing
apoptosis as well as ameliorating cell cycle disorder in
vitro and identified ZIPK as an important component in cellular
physiological processes.
Increased proteolytic activity in the vessel wall
mediates the degradation of the extracellular matrix surrounding
the VSMC in response to HG injury. To clarify the potential
mechanisms of ZIPK in the proliferation, apoptosis, migration and
cell progression of HASMCs stimulated by HG, we examined several
key regulatory molecules: VCAM1 and ICAM-1 (22,23), together with AAMP proteins
(24), have been strongly
suggested to play an important role in cell migration; Hcdc14A is
shown to interact with interphase centrosomes and to regulate the
centrosome duplication cycle (25,26); MMP-2 caused cell proliferation
(27), while the activation of
MMP, particularly the gelatinases MMP-2, may contribute to the
pathogenesis of atherosclerosis by facilitating the migration of
VSMC (28,29). Our data have shown that ZIPK
overexpression resulted in the a decreased expression of ICAM-1,
VCAM-1, MMP-2 and AAMP, and an elevated expression of Hcdc14A.
Potential explanations for this decrease and increase in expression
may be : i) ZIPK may inhibit cell migration by the regulation of
multiple downstream signaling components including ICAM-1, VCAM-1,
AAMP and MMP-2; ii) ZIPK participated in cell cycle progression by
interacting with Hcdc14A and iii) ZIPK inhibited HASMC
proliferation presumably by the downregulation of MMP-2. However,
further mechanistic explorations to elucidate the signaling
mechanisms of ZIPK in HASMCs are required.
Of note, ZIPK overexpression upregulated the level
of Hcdc14A, which was consistent with results of a previous study
demonstrating that ZIPK physically interacted with Hcdc14A and
ZIPK-mediated phosphorylation was able to initiate the phosphatase
activity of Hcdc14A (data not shown). Hcdc14A phosphatase plays a
role in the regulation of the centrosome cycle, mitosis, and
cytokinesis and overproduction of Hcdc14A caused mitotic spindle
and chromosome segregation (30).
This finding may explain the reason for the overexpression of ZIPK
attenuating disturbance of the cell cycle.
In conclusion, our results demonstrate the
protective function of ZIPK in HASMCs under HG conditions and its
potential mechanisms. The results provide insight to a novel
therapeutic strategy for diabetic vascular complications.
Acknowledgements
This study was supported by the National Nature
Science Foundation for Young Scientists of China (no. 30900541) and
985 engineering advantage subject innovation platform (no.
985III).
References
|
1
|
Ruderman NB and Haudenschild C: Diabetes
as an atherogenic factor. Prog Cardiovasc Dis. 26:373–412. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Srivastava AK: High glucose-induced
activation of protein kinase signaling pathways in vascular smooth
muscle cells: a potential role in the pathogenesis of vascular
dysfunction in diabetes (review). Int J Mol Med. 9:85–89. 2002.
|
|
3
|
Blindt R, Krott N, Hanrath P, vom Dahl J,
van Eys G and Bosserhoff AK: Expression patterns of integrins on
quiescent and invasive smooth muscle cells and impact on cell
locomotion. J Mol Cell Cardiol. 34:1633–1644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross R: Cell biology of atherosclerosis.
Annu Rev Physiol. 57:791–804. 1995. View Article : Google Scholar
|
|
5
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawai T, Matsumoto M, Takeda K, Sanjo H
and Akira S: ZIP kinase, a novel serine/threonine kinase which
mediates apoptosis. Mol Cell Biol. 18:1642–1651. 1998.PubMed/NCBI
|
|
7
|
Kögel D, Plöttner O, Landsberg G,
Christian S and Scheidtmann KH: Cloning and characterization of
Dlk, a novel serine/threonine kinase that is tightly associated
with chromatin and phosphorylates core histones. Oncogene.
17:2645–2654. 1998.PubMed/NCBI
|
|
8
|
Brognard J, Zhang YW, Puto LA and Hunter
T: Cancer-associated loss-of-function mutations implicate DAPK3 as
a tumor-suppressing kinase. Cancer Res. 71:3152–3161. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mills JC, Stone NL, Erhardt J and Pittman
RN: Apoptotic membrane blebbing is regulated by myosin light chain
phosphorylation. J Cell Biol. 140:627–636. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coleman ML, Sahai EA, Yeo M, Bosch M,
Dewar A and Olson MF: Membrane blebbing during apoptosis results
from caspase-mediated activation of ROCK I. Nat Cell Biol.
3:339–345. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Engemann H, Heinzel V, Page G, Preuss U
and Scheidtmann KH: DAP-like kinase interacts with the rat homolog
of Schizosaccharomyces pombe CDC5 protein, a factor involved in
pre-mRNA splicing and required for G2/M phase transition. Nucleic
Acids Res. 30:1408–1417. 2002. View Article : Google Scholar
|
|
12
|
Murata-Hori M, Suizu F, Iwasaki T, Kikuchi
A and Hosoya H: ZIP kinase identified as a novel myosin regulatory
light chain kinase in HeLa cells. FEBS Lett. 451:81–84. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niiro N and Ikebe M: Zipper-interacting
protein kinase induces Ca(2+)-free smooth muscle contraction via
myosin light chain phosphorylation. J Biol Chem. 276:29567–29574.
2001.PubMed/NCBI
|
|
14
|
Sato N, Kawai T, Sugiyama K, Muromoto R,
Imoto S, Sekine Y, Ishida M, Akira S and Matsuda T: Physical and
functional interactions between STAT3 and ZIP kinase. Int Immunol.
17:1543–1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kakudo N, Shimotsuma A, Miyake S, Kushida
S and Kusumoto K: Bone tissue engineering using human
adipose-derived stem cells and honeycomb collagen scaffold. J
Biomed Mater Res A. 84:191–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kakudo N, Minakata T, Mitsui T, Kushida S,
Notodihardjo FZ and Kusumoto K: Proliferation-promoting effect of
platelet-rich plasma on human adipose-derived stem cells and human
dermal fibroblasts. Plast Reconstr Surg. 122:1352–1360. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasunari K, Kohno M, Kano H, Yokokawa K,
Minami M and Yoshikawa J: Mechanisms of action of troglitazone in
the prevention of high glucoseinduced migration and proliferation
of cultured coronary smooth muscle cells. Circ Res. 81:953–962.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suh SJ, Jin UH, Kim SH, Chang HW, Son JK,
Lee SH, Son KH and Kim CH: Ochnaflavone inhibits TNF-alpha-induced
human VSMC proliferation via regulation of cell cycle, ERK1/2, and
MMP-9. J Cell Biochem. 99:1298–1307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komatsu S and Ikebe M: ZIP kinase is
responsible for the phosphorylation of myosin II and necessary for
cell motility in mammalian fibroblasts. J Cell Biol. 165:243–254.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho YE, Ahn DS, Morgan KG and Lee YH:
Enhanced contractility and myosin phosphorylation induced by
Ca(2+)-independent MLCK activity in hypertensive rats. Cardiovasc
Res. 91:162–170. 2011.
|
|
21
|
Usui T, Okada M, Hara Y and Yamawaki H:
Death-associated protein kinase 3 mediates vascular inflammation
and development of hypertension in spontaneously hypertensive rats.
Hypertension. 60:1031–1039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piconi L, Quagliaro L, Da Ros R, Assaloni
R, Giugliano D, Esposito K, Szabó C and Ceriello A: Intermittent
high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6
expression in human umbilical endothelial cells in culture: the
role of poly(ADP-ribose) polymerase. J Thromb Haemost. 2:1453–1459.
2004. View Article : Google Scholar
|
|
23
|
Martinelli R, Gegg M, Longbottom R,
Adamson P, Turowski P and Greenwood J: ICAM-1-mediated endothelial
nitric oxide synthase activation via calcium and AMP-activated
protein kinase is required for transendothelial lymphocyte
migration. Mol Biol Cell. 20:995–1005. 2009. View Article : Google Scholar
|
|
24
|
Vogt F, Zernecke A, Beckner M, Krott N,
Bosserhoff AK, Hoffmann R, Zandvoort MA, Jahnke T, Kelm M, Weber C
and Blindt R: Blockade of angio-associated migratory cell protein
inhibits smooth muscle cell migration and neointima formation in
accelerated atherosclerosis. J Am Coll Cardiol. 52:302–311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mailand N, Lukas C, Kaiser BK, Jackson PK,
Bartek J and Lukas J: Deregulated human Cdc14A phosphatase disrupts
centrosome separation and chromosome segregation. Nat Cell Biol.
4:317–322. 2002. View
Article : Google Scholar
|
|
26
|
Kaiser BK, Zimmerman ZA, Charbonneau H and
Jackson PK: Disruption of centrosome structure, chromosome
segregation, and cytokinesis by misexpression of human Cdc14A
phosphatase. Mol Biol Cell. 13:2289–2300. 2002. View Article : Google Scholar
|
|
27
|
Galli A, Svegliati-Baroni G, Ceni E,
Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini
G, Benedetti A, Surrenti C and Casini A: Oxidative stress
stimulates proliferation and invasiveness of hepatic stellate cells
via a MMP2-mediated mechanism. Hepatology. 41:1074–1084. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shah PK: Role of inflammation and
metalloproteinases in plaque disruption and thrombosis. Vasc Med.
3:199–206. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tartakover-Matalon S, Cherepnin N, Kuchuk
M, Drucker L, Kenis I, Fishman A, Pomeranz M and Lishner M:
Impaired migration of trophoblast cells caused by simvastatin is
associated with decreased membrane IGF-I receptor, MMP2 activity
and HSP27 expression. Hum Reprod. 22:1161–1167. 2007. View Article : Google Scholar
|
|
30
|
Kaiser BK, Zimmerman ZA, Charbonneau H and
Jackson PK: Disruption of centrosome structure, chromosome
segregation, and cytokinesis by misexpression of human Cdc14A
phosphatase. Mol Biol Cell. 13:2289–2300. 2002. View Article : Google Scholar : PubMed/NCBI
|