Introduction
Renal cell carcinoma (RCC) is the most common renal
parenchyma tumor, accounting for approximately 3% of adult
malignant tumors, presenting the highest morbidity and mortality
rate in tumors of the urinary system, second to bladder cancer
(1). Clear cell RCC (ccRCC)
represents >75% of all RCC cases and is the most aggressive of
all known RCC subtypes, presenting a complex biological behavior
which differs significantly between individuals (2). The post-operative recurrence rate of
ccRCC reaches up to 20–40% (3,4).
Once metastatic disease develops, the 5-year survival rate for
patients with ccRCC decreases from 60% to <5% (5). Thus, it is mandatory to elucidate
the mechanisms involved in the pathogenesis of RCC in order to
discover novel therapeutic targets.
Wnts are secreted signaling proteins that influence
multiple processes ranging from stem cell control to cell
differentiation and polarity (6).
The Wnt signaling pathway is involved in the occurrence and
development of a variety of carcinomas, such as hepatocellular
(7) and ovarian cancer (8), melanoma (9), medulloblastoma (10) and RCC (11). The Wnt/β-catenin signaling pathway
is also known as the canonical Wnt pathway. Genetic and
embryological studies have revealed that β-catenin is a component
of the Wnt signaling pathway and that it exhibits signaling
functions (12,13). When β-catenin accumulates, it can
combine with the transcription factor, T-cell factor/lymphoid
enhancer factor (TCF/LEF), in the nucleus, and then activate the
transcription of the signaling target gene of Wnt (14). Dickkopf (DKK) family proteins are
among 5 Wnt antagonist families, with DKK1-DKK4 being the 4 main
members (15). DKK4 blocks signal
transduction by binding to the Wnt co-receptor (16). As a negative feedback regulator,
DKK4 plays a crucial role in regulating the Wnt/β-catenin signaling
pathway; it inhibits the proliferation and invasion of colon cancer
cells by blocking the canonical Wnt pathway (17). DKK4 inhibits the TCF/LEF
transcription complex, and regulates the Wnt/β-catenin pathway
(18). It has been proven that
the canonical Wnt pathway is activated in ccRCC tissues, and that
patients with high β-catenin expression levels have bad prognosis
(19,20). However, there are limited studies
on the effect of DKK4 on the proliferation and invasion of ccRCC
cells.
Germ line mutations of the Von Hippel-Lindau (VHL)
gene lead to the development of a variety of tumors, including
ccRCC. An early event during the evolution of ccRCC is the loss of
function of the VHL gene (21).
Gene deletion, mutation and methylation are common causes of
abnormal VHL expression, and approximately 50% of all ccRCC cases
present with these abnormalities (22). The VHL gene functions in several
pathways associated with carcinogenesis, most notably the
hypoxia-inducible pathway (23).
The abnormal expression of VHL induces hypoxic conditions and
activates the hypoxia-inducible factor (HIF), a transcription
factor. Thus, VHL participates in a number of pathways, including
β-catenin-mediated signaling pathways (19).
Considering the fact that both VHL and DKK4 function
as inhibitors of the Wnt/β-catenin signaling pathway, in the
current study, we thus aimed to investigated the interaction
between DKK4 and VHL and the Wnt/β-catenin pathway in ccRCC. We
also examined the expression of DKK4 in human renal cell lines and
tumor tissues. In addition, stable DKK4-transfected cell lines were
established in order to examine the effects of DKK4 on cell
proliferation and metastasis in vitro. Finally, we
investigated the expression of DKK4 to determine its clinical
relevance in the prognosis of 30 patients with RCC in order to
further elucidate the role of DKK4 in ccRCC.
Materials and methods
Clinical samples
A total of 30 patients (18 males vs 12 females;
average age, 59±7.8 years) with pathologically confirmed ccRCC were
enrolled in this study (Tenth Peoples’ Hospital of Tongji
University, Shanghai, China). The patients were classified
according to the Fuhrman nuclear grading system and were staged
according to the tumor-lymph node-metastasis (TNM) classification
system (Table I), in which T
refers to the size of the original (primary) tumor and whether it
has invaded nearby tissue, N refers to the degree of spread to
regional lymph nodes, and M refers to distant metastasis (spread of
cancer from one part of the body to another). All patients had not
undergone any prior radiotherapy, chemotherapy or immunotherapy.
Samples were obtained from tumor and adjacent normal kidney tissues
from the patients with ccRCC after obtaining written informed
consent. The study was approved by the Ethics Committee of the
Tenth Peoples’ Hospital of Tongji University.
 | Table ICorrelation between dickkofp-4 (DKK4)
expression and clinicopathological parameters (n=30). |
Table I
Correlation between dickkofp-4 (DKK4)
expression and clinicopathological parameters (n=30).
| No. of patients
(%) |
|---|
|
|
|---|
| Parameters | High expression
(n=19) | Low expression
(n=11) | P-value |
|---|
| Age (years) | | | |
| Mean ± SD | 64±8.3 | 59±7.8 | 0.047a |
| Gender | | | |
| Male, n=18 | 10 (55.6) | 8 (45.4) | |
| Female, n=12 | 9 (75.0) | 3 (25.0) | 0.25 |
| Fuhrman grade | | | |
| 1, n=7 | 6 (85.7) | 1 (24.3) | Reference |
| 2, n=20 | 11 (55.0) | 9 (45.0) | 0.15 |
| 3, n=3 | 2 (66.7) | 1 (33.3) | 0.55 |
| Pathological
stage | | | |
| I, n=5 | 4 (80.0) | 1 (20.0) | |
| II, n=20 | 11 (55.0) | 9 (45.0) | |
| I+II, n=25 | 15 (60) | 10 (40) | Reference |
| III, n=2 | 1 (50) | 1 (50) | |
| IV, n=3 | 2 (66.7) | 1 (33.3) | |
| III + IV, n=5 | 3 (60.0) | 2 (40.0) | 1.00 |
| Pathological tumor
classification | | | |
| pT1, n=15 | 10 (66.7) | 5 (33.3) | |
| pT2, n=10 | 4 (40.0) | 6 (60.0) | |
| pT1 + pT2,
n=25 | 14 (56.0) | 11 (44.0) | Reference |
| pT3, n=4 | 3 (75.0) | 1 (25.0) | |
| pT4, n=1 | 1 (100.0) | 0 (0.0) | |
| pT3 + pT4,
n=5 | 4 (80.0) | 1 (20.0) | 0.33 |
| Lymph node
metastasis | | | |
| Negative,
n=27 | 17 (63) | 10 (37) | Reference |
| Positive, n=3 | 2 (66.7) | 1 (33.3) | 0.90 |
| Distant
metastasis | | | |
| Negative,
n=26 | 17 (65.4) | 9 (36.6) | Reference |
| Positive, n=4 | 2 (50) | 2 (50) | 0.57 |
| Overall
survival | | | |
| Alive, n=22 | 16 (72.7) | 6 (27.3) | Reference |
| Deceased, n=8 | 3 (37.5) | 5 (62.5) | 0.08 |
| Recurrence | | | |
| No, n=21 | 14 (66.7) | 7 (33.3) | Reference |
| Yes, n=9 | 5 (55.6) | 4 (44.4) | 0.58 |
Cell culture
The RCC cell lines, A-498 and 786-O, as well as
RCC4(−) and T3-14(+), were purchased from the Cell Institute,
Shanghai Branch of Chinese Academy of Sciences. The cell lines were
cultured in 50 ml Roswell Park Memorial Institute (RPMI)-1640
medium (Gibco-BRL, Carlsbad, CA, USA) and supplemented with 10%
fetal bovine serum (FBS) (HyClone, Logan, UT, USA) at 37°C with 5%
CO2.
Plasmid construction and DNA
transfection
After having constructed pCDH-DKK4, a plasmid that
contained the human full-length combinational DNA fragment of DKK4,
the 786-O and A-498 cells were transfected with it, using the
GeneAmp PCR System 9700 (Perkin-Elmer, Waltham, MA, USA) in order
to create stable cell lines that overexpressed DKK4. Transfected
cells were selected by culturing them with G418 (150 μg/ml) for 2
months. Empty vector transfectants were used as controls. Single
colonies of stable transfectants were isolated for further analysis
based on their DKK4 expression levels. The top 2 stable clones of
786-O cells that had the highest DKK4 mRNA expression levels
compared to those of the controls were selected, and named DKK4
clones I and II, respectively. DKK4 expression levels in the cells
were confirmed by quantitative reverse transcriptase-polymerase
chain reaction (qRT-PCR) before carrying out the following
experiments. All experiments were performed in triplicate.
Cell viability assay
Stably transfected 786-O cells with high expression
levels of DKK4 mRNA were maintained for 1 week in medium
supplemented with 150 μg/ml G418. One week later, cell viability
was detected by MTS assay (GMS10043 V.A, Genmed Scientifics, Inc.,
Arlington, MA, USA) according to the manufacturer’s instructions.
Trypan blue (0.04%, Sigma, St. Louis, MO, USA) exclusion was also
used to identify viable cells with intact plasma membranes. To
verify the effect of DKK4 on renal cancer cells, we also performed
similar MTS assays using the A-498 cell line.
Cell invasion assay
The invasion ability of the stably transfected 786-O
and A-498 cells with high expression levels of DKK4 was examined by
Transwell assay (Corning, Inc., Lowell, MA, USA). Transwell
chambers (Corning) were used according to the manufacturer’s
instructions. In brief, the infected cells were harvested,
resuspended in serum-free medium and subsequently transferred to
hydrated Matrigel chambers (50 μl/well). The chambers were then
incubated for 48 h in culture medium which was added to the lower
chambers prior to examination. The cells on the upper surface of
the filter were scraped and washed away, while the invaded cells on
the lower surface were fixed and stained with 0.04% trypan blue for
2 h. Finally, the invaded cells were counted under a microscope and
the relative number was thus calculated.
Apoptosis assay
Stably transfected DKK4 clone I and clone II cells,
as well as the control cells were washed twice in
phosphate-buffered saline (PBS) and their density was adjusted to
1×106/ml. The cells were stained in the dark for 15 min
with 5 μl of Annexin V and 5 μl of propidium iodide (PI)
(Invitrogen Life Technologies, Carlsbad, CA, USA) at room
temperature, according to the manufacturer’s instructions (Biosea,
Beijing, China). The apoptotic cells were detected and measured on
a flow cytometer (Perkin-Elmer) and examined under an inverted
fluorescence microscope (ChongQing Optical & Electrical
Instrument Co., ChongQing, China).
TCF/LEF
The TCF/LEF assay with TCF-reporter plasmids
(TOPFlash, which contains a wild-type TCF binding site; and
FOPFlash, which contains a mutant TCF binding site; Millipore,
Billerica, MA, USA) were used to monitor the activity of the
Wnt/β-catenin signal transduction pathway. The cells were
co-transfected with pRL-TK (Promega Corp., Madison, WI, USA)
Renilla luciferase to normalize the transfection efficiency. The
changes in the activity of TCF/LEF before and after transfection
with DKK4 were detected by the analysis of luciferase activity.
RT-PCR analysis
Total mRNA was extracted from formalin-fixed,
paraffin-embedded human renal cancer tissues and matched adjacent
normal tissues, as well as from RCC4(−) and T3-14(+) cells using
TRIzol reagent (Invitrogen Life Technologies) in order for reverse
transcription to be performed with the use of an RT reagent kit
(Takara Bio, Inc., Shiga, Japan). The real-time experiments were
conducted on an iQ5 Multicolor Real-Time PCR Detection System
(Bio-Rad, Hercules, CA, USA) using the SYBR®-Green
real-time PCR kit (Takara Bio, Inc.). The PCR reactions consisted
of 10 min at 95°C followed by 50 cycles at 95°C for 15 sec, at 60°C
for 60 sec. The data were calculated using the 2−ΔΔCt
method normalized to actin, the individual internal control. The
primers used were as follows: DKK4 forward,
5′-TGGCACACATGCAGAAGGAACAAC-3′ and reverse,
5′-AATGACGAGCACAGCAAAGTCCAG-3′; actin forward,
5′-ACCCAGCACAATGAAGATCA-3′ and reverse, 5′-CGATCCACACGGAGTACTTG-3′;
cyclin D1 forward, 5′-AGAAGCTGTGCATCTACACCGACA-3′ and reverse,
5′-TGATCTGTTTGTTCTCCTCCGCCT-3′; c-myc forward,
5′-TGCAGCTGCTTAGACGCTGGATTT-3′ and reverse,
5′-TGTTGGTGAAGCTAACGTTGAGGG-3′.
Western blot analysis
Nuclear protein and cytoplasmic protein were
extracted separately with the use of western blotting luminol
reagent, ProteoJET™ cytoplasmic and nuclear protein kits (Santa
Cruz Biotechnology, Santa Cruz, CA, USA). Protein quantification
was performed using the Coomassie brilliant blue G250 kit
(Beyotime, Jiangsu, China). For western blot analysis, a total of
20 μg of cell protein lysate was loaded per lane. Samples were
resolved in 12% precise protein gels (Pierce, Courtaboeuf, France)
and transferred onto polyvinylidene fluoride (PVDF) membranes
(Amersham Biosciences, Fairfield, CT, USA). The membranes were
immersed in a solution of 0.3% skim milk, Tris-buffered saline
(TBS) and 0.1% Tween-20 for 1 h and then probed with primary
polyclonal and monoclonal antibodies against DKK4, actin, cyclin
D1, c-myc and β-catenin (all purchased from Epitomics, Burlingame,
CA, USA). The blots were washed in TBS and incubated with secondary
antibodies. Proteins were enhanced by chemiluminescence (ECL, Santa
Cruz) for visualization. The reported protein expression levels
were expressed relative to the β-catenin levels.
Analysis of in vivo tumor growth
A total of 16 female nude mice (strain BALB/c nude;
Shanghai experimental Animal Center, Chinese Academy of Sciences),
aged 4 to 5 weeks received subcutaneous injections of empty
vector-transfected A-498 cells (group a, n=4) or A-498 cells stably
transfected with DKK4 (group b, n=4), and empty vector-transfected
786-O cells (group c, n=4) or 786-O cells stably transfected with
DKK4 (group d, n=4), in the left armpit at a volume of 200 μl.
Tumor size was measured using calipers once a week for 35 days, and
was calculated on the basis of width (x) and length (y) according
to the following formula: x2y/2, where x<y. After the
mice were sacrificed, the tumors were resected and weighed. RNA and
protein were extracted from the tumor tissues for the determination
of the expression levels of DKK4 mRNA and protein.
Statistical analysis
All experiments were performed in triplicate and all
observed differences between the treated and control cells were
analyzed by the Student’s t-test. The data are presented as the
means ± SD of 3 independent experiments. Correlation analyses were
conducted using non-parametric tests and values of p<0.05 were
considered to indicate statistically significant differences.
Results
DKK4 mRNA expression levels in renal
cancer tissues and adjacent normal tissues
RT-PCR was performed to examine the expression of
DKK4 in renal cancer tissues and adjacent normal kidney tissues. As
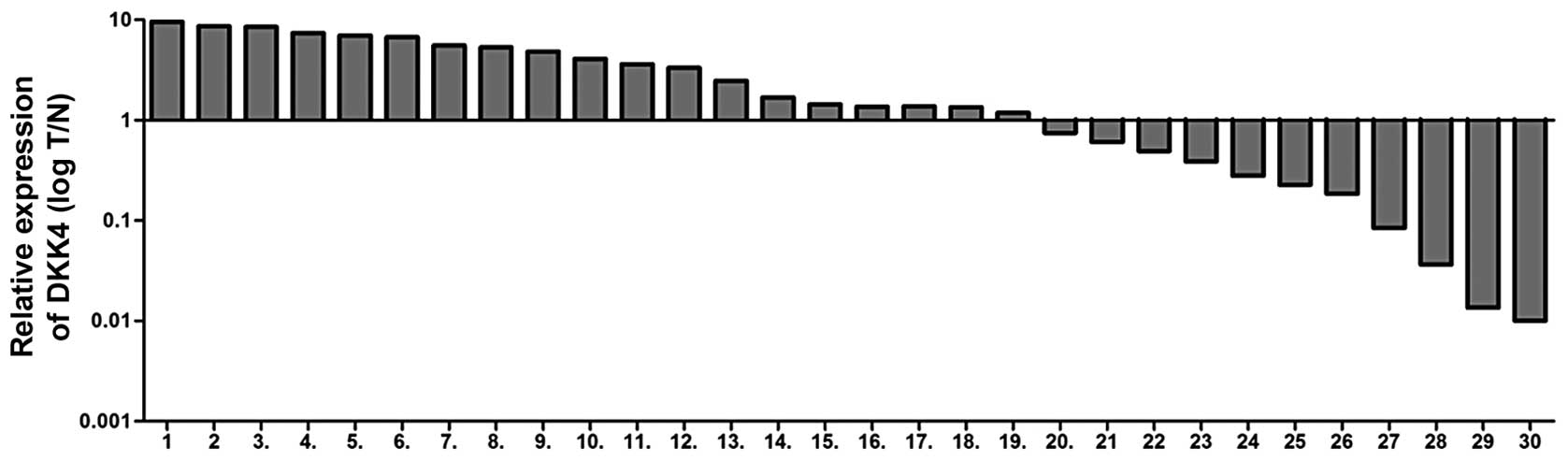
shown in Fig. 1, the mRNA
expression levels of DKK4, in 30 paired tissues, were increased in
19 ccRCC tissues (63.3%) compared to the adjacent tissues. The
correlation between DKK4 expression levels and clinicophathological
characteristics was also investigated, including gender, Fuhrman
grade, pathological tumor classification (pT), pathological lymph
node status (pN), pathological metastasis status (pM) and outcomes
(survival and recurrence). There was no significant association
observed between DKK4 expression levels and clinicophathological
parameters (p>0.05) apart from the age of the patients (Table I).
Cell viability, invasion and apoptosis of
cells with high DKK4 expression
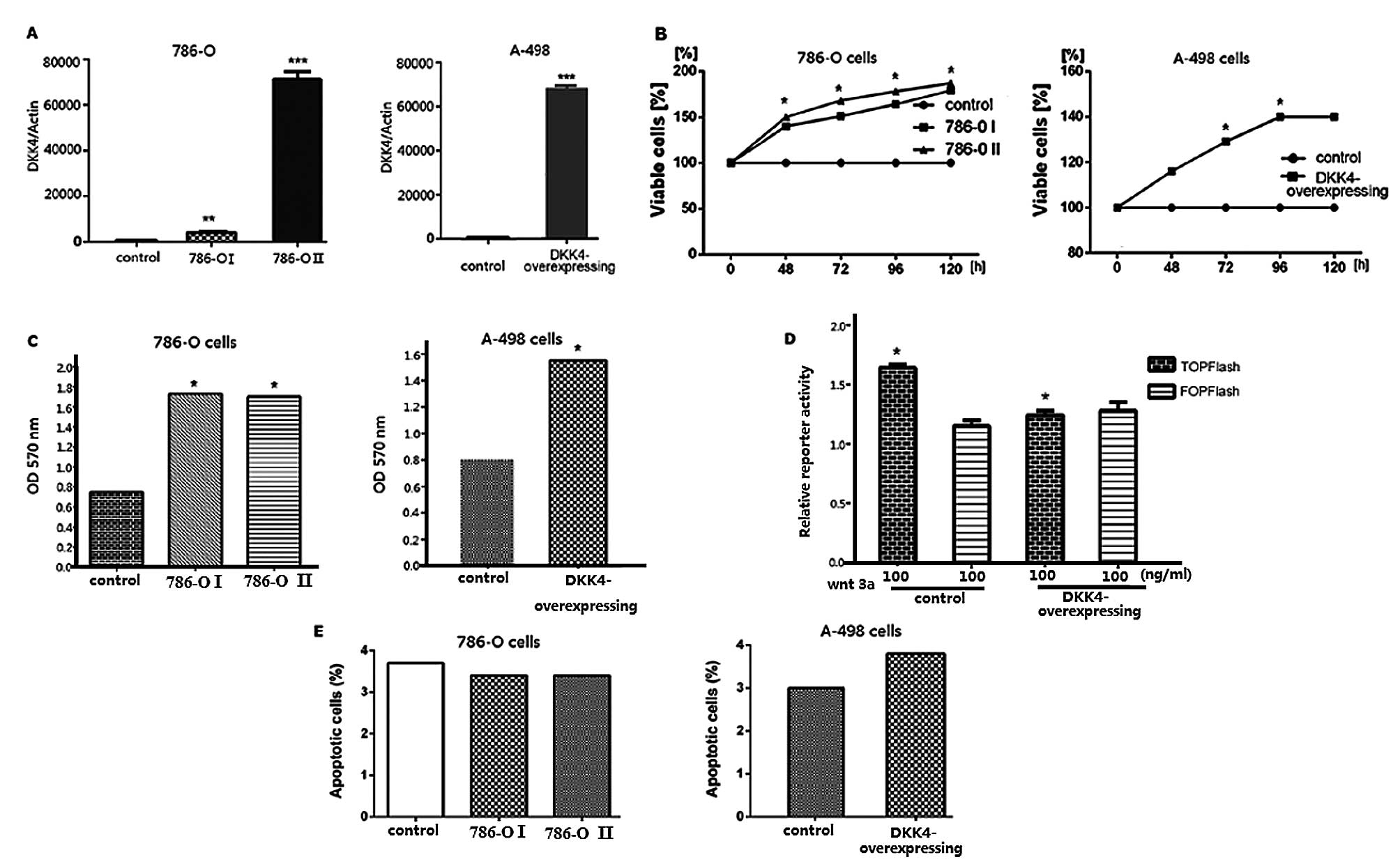
Following transfection of the 786-O and A-498 cells
with the pCDH-DKK4 expression plasmid, DKK4 expression levels were
confirmed by qRT-PCR. DKK4 overexpression was observed in both the
786-O and A-498 cells (Fig. 2A).
Analyses of cell viability (MTS) and cell invasion, as well as
TCF/LEF reporter and apoptosis assays were performed using stably
transfected 786-O and A-498 cells with high expression levels of
DKK4. The enhanced growth of the transfected 786-O and A-768 cells
was observed by MTS assay (Fig.
2B). DKK4 promoted the in vitro invasion ability of the
786-O and A-768 cells (Fig. 2C).
The relative TCF/LEF activity was significantly decreased in the
786-O and A-768 cells co-transfected with the TOPFlash reporter
plasmid compared with the controls (Fig. 2D). No significant differences were
observed between the 2 cell lines and the control cells as regards
the number of apoptotic cells (Fig.
2E).
In vivo experiments
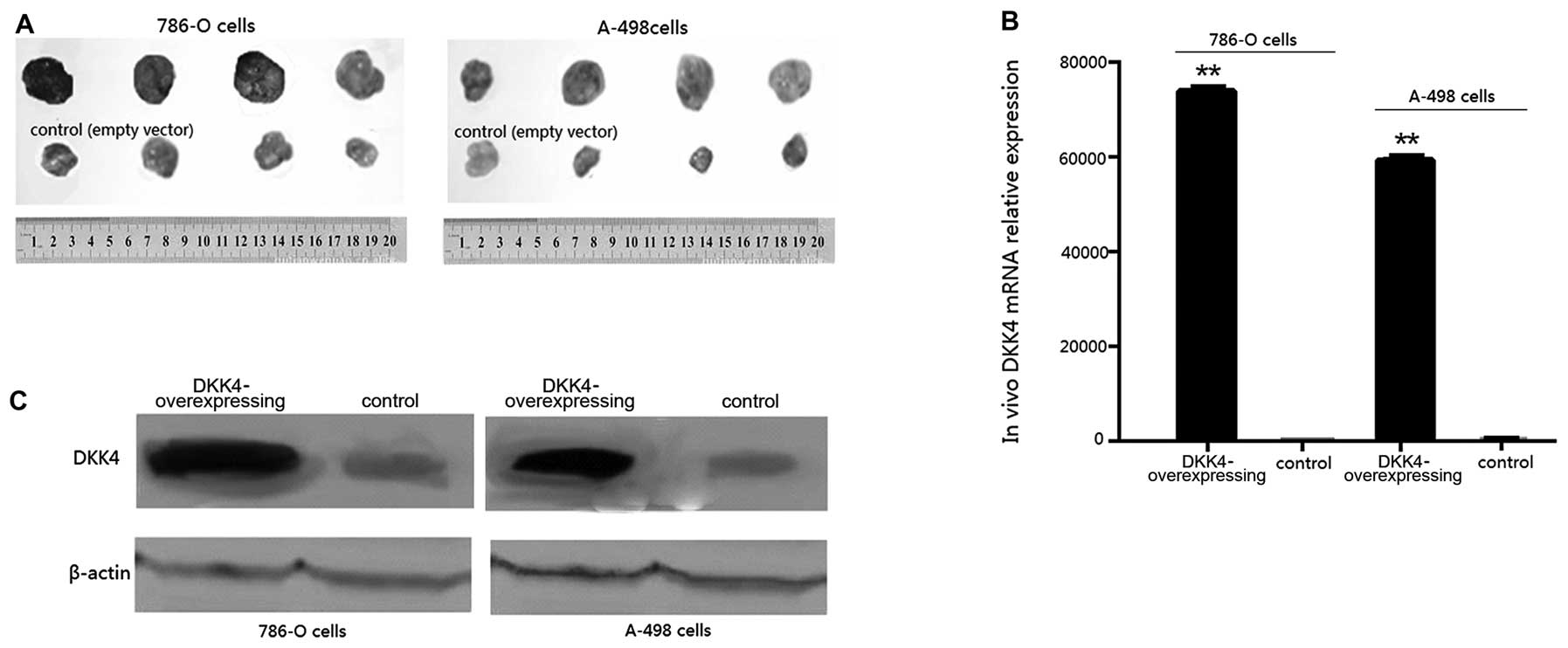
Tumors developed in all the mice. Tumor volumes in
the mice overexpressing DKK4 were larger at 2 weeks, and
significantly larger at 6 weeks than those of the control mice at
the respective time points (p<0.05, Fig. 3A). The final tumor weights of
groups a, b, c and d were 2.173±0.097, 3.014±0.201, 2.51±0.217 and
3.673±0.142 g, respectively. The results from qRT-PCR (Fig. 3B) and western blot analysis
(Fig. 3C) confirmed the
overexpression of DKK4 in the tumors formed from 786-O and A-498
transplanted cells transfected with pCDH-DKK4 compared with those
formed from cells transfected with the empty vector
(p<0.001).
VHL protein expression and DKK4
expression in ccRCC tissues
VHL protein expression in another 50 ccRcc tissues
was detected by western blot analysis. VHL protein was negatively
expressed in 60% of the tissues (30/50) which were classified as
the VHL(−) group, while the remaining 20 tissues were classified as
the VHL(+) group. DKK4 expression levels in all 50 tissues was
detected by qRT-PCR, and DKK4 was overexpressed in 68% (34/50) of
the tissues. The DKK4 overexpression ratio in the VHL(−) group was
higher than that in the VHL(+) group (50% vs. 18%), and the
difference was statistically significant (p < 0.05). In
addition, the overexpression of DKK4 correlated with the expression
of the VHL gene (r=0.403, p<0.05). DKK4 overexpression also
correlated with VHL gene expression (r=0.403, p<0.05).
Detection of β-catenin, cyclin D1 and
c-myc expression
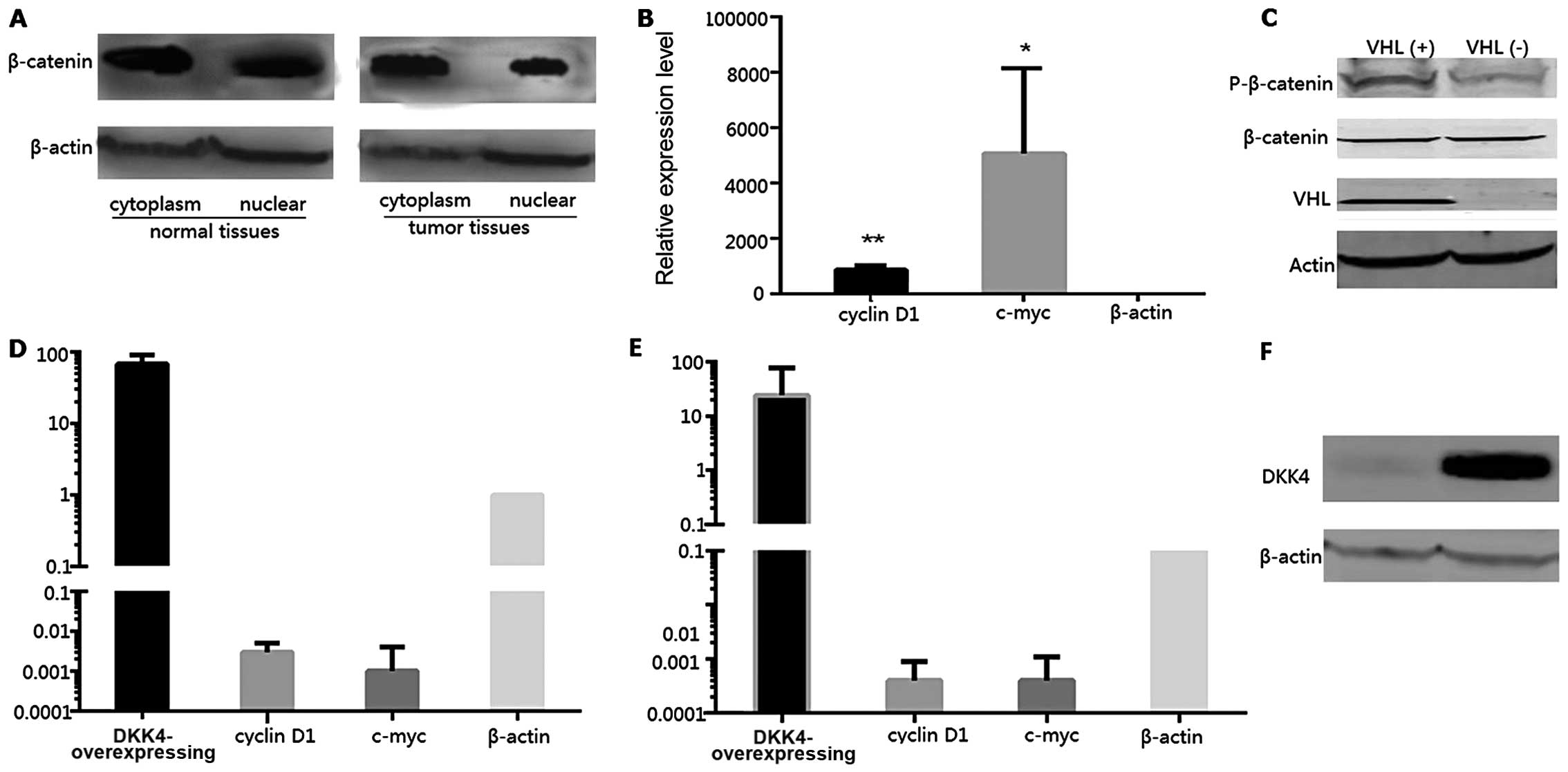
The protein expression levels of β-catenin in the
cytoplasm and nucleus were compared between the tumor and normal
tissues. The expression levels of β-catenin in the nucleus of the
tumor cells were decreased compared with those in the nucleus of
normal cells; however, the differences in the expression levels in
the cytoplasm of tumor and normal tissues were not statistically
significant (Fig. 4A). Thus, the
β-catenin expression levels of the downstream genes, cyclin D1 and
c-myc, were examined in the VHL(+) and VHL(−) groups by qRT-PCR.
The decreased expression levels of these genes in the VHL(−)
tissues were also found to be statistically significant (Fig. 4B, p<0.05). The expression
levels of phosphorylated β-catenin (p-β-catenin) presented a sharp
increase in the VHL(+) tissues; however, there was no notable
difference observed between the total β-catenin expression levels
in the VHL(+) and VHL(−) tissues (Fig. 4C). DKK4, cyclin D1, c-myc and
β-catenin expression levels were also detected in RCC4(−) and
T3-14(+) cells by qRT-PCR and western blot analysis. The DKK4
expression levels in the RCC4(−) cells were 67.555-fold higher
compared with those in the T3-14(+) cells, while the cyclin D1 and
c-myc expression levels were markedly decreased in the RCC4(−)
cells (Fig. 4D). In addition we
also determined the cyclin D1 and c-myc expression levels in human
embryonic kidney (HEK)293 cells transfected with the plasmid
vector, pCDH-DKK4. DKK4 was highly expressed in the HEK293 cells
(Fig. 4F), and the expression
levels of cyclin D1 and c-myc were significantly decreased
(Fig. 4E).
Discussion
DKK4 expression was determined in 30 cases of ccRCC,
and it was found to be highly increased in 63.3% of the ccRCC
tissues compared with the adjacent normal tissues (19/30). No
correlation was observed between DKK4 expression and
clinicopathological parameters, such as Fuhrman grade, pathological
stage, lymph node and distant metastasis, survival and recurrence.
The overexpression of DKK4 indicated that its activation is
involved in ccRCC. In 1999 it was reported that DKK4 regulates the
Wnt/β-catenin signaling pathway negatively (26), but since then, few studies have
investigated the effects of DKK4 on tumorigenesis and the
devolopment of ccRCC. In the current study, cells that expressed
high levels of DKK4 were found to have enhanced cell viability and
a greater invasion potential. Matsui et al (24) reported that DKK2 and DKK4 were
upregulated in colorectal cancer, and that DKK4 expression levels
correlated with nuclear β-catenin levels. However, Fatima et
al (25), demonstrated, with
functional assays, that DKK4 overexpression inhibited cell
proliferation, reduced colony formation and suppressed cell
migration in hepatocellular carcinoma. The DKK family is a family
of secretory glycoproteins, consisting of two structuraly conserved
cysteine-rich domains and each domain contains 10 cysteine residues
(26). The amino-terminal domain
(Cyst-1) is domain-specific, and the carboxy-terminal domain
(Cyst-2) is highly conserved. Cysteine residues on Cyst-2 have the
typical cysteine pattern of the colipase domain and can therefore
combine colipase tightly, which participates in fat hydrolysis
(27). Thus, the structure of the
DKK family may be associated with the Wnt/β-catenin signaling
pathway (28). By detecting cells
transfected with LEF-1 and β-catenin, DKK4 is selected as a
potential target gene of the non-canonical Wnt signaling pathway
(Wnt/PCP), and also participates in cell movements during the
morphogenesis period (29). In
fact, apart from the Wnt/β-catenin pathway, Wnt can be activated by
other pathways dependent on Jun N-terminal kinase (JNK). JNK
activation induces apoptosis, and JNK activation by DKK3 has been
reported to be involved in the Wnt pathway regulation in prostate
and breast cancer (30,31). However, the effects of DKK4 on the
Wnt/JNK pathway in ccRCC and the coresponding mechanisms require
further verification.
The results of the current study demonstrated that
the canonical Wnt/β-catenin was inhibited, leading to the reduced
expression of β-catenin and its downstream genes, cyclin D1 and
c-myc, as well as a decrease in TCF/LEF activity. However, in the
study by Fatima et al (25), the expression levels of DKK4
β-catenin and cyclin D1 were decreased in hepatoma carcinoma cells,
and β-catenin was accumulated in the cytoplasm. Similar results
have been observed in colorectal cancer (32). Under the activation of
Wnt/β-catenin signaling pathway, DKK4 overexpression can inhibit
the activities of β-catenin/TCF-4 effectively, as well as cell
cycle proliferation and conversion. DKK4 may play different roles
in tumorigenesis and in the development of different types of
tumor. Further studies are required in order to elucidate the
underlying mechanisms.
In vivo studies offer a more realistic
microenvironment for the growth of tumor cells. In this study,
cells overexpressing DKK4 were implanted into BALB/c nude mice, in
order to investigate the effects of DKK4 activation on the
oncogenicity of ccRCC. The neoplasm incidence rate was 100%, and
the tumor weights of the mice implanted with cells overexpressing
DKK4 were significantly higher than those of the control mice
implanted with an empty vector. DKK4 is considered to inhibit the
development of tumors, as an antagonist of the Wnt pathway and has
also been reported to be able to inhibit the proliferation of tumor
cells in hepatic carcinomas (25). However, the issue of whether DKK4
acts as a tumor inhibitor or promoter remains to be resolved.
VHL is believed to induce the oxygen-deficient
environment in cells, as it activates many HIF-mediated signaling
pathways (11,33). The abnormal expression of VHL
promotes the migration of β-catenin from the cytoplasm into the
nucleus, as well as its combination with TCF and LEF, and the
activation of cyclin D1 and c-myc, and consequently tumorigenesis
(19,34). The combination of β-catenin with
TCF/LEF in the nucleus activates downstream genes (35). In our study, in a total of 50
cases of ccRCC, in the VHL(−) group, DKK4 was found to be
overexpressed in 50% of the specimens, contrary to the VHL(+)
group, where only an 18% rate presented a significant difference
(p<0.05). The high expression of DKK4 correlated with the
expression of VHL (r=0.403; p<0.05). As a positive cell cycle
factor, cyclin D1 is associated with uncontrollable cell
proliferation, as a tumorigenesis factor (36). The overexpression of cyclin D1
induces system disorders in the normal cell cycle (37). When c-myc, another well known
nuclear oncogene, is regulated by carcinogenic factors, it escapes
the normal regulatory cell cycle check, leading to its continuous
overexpression and resulting in the alteration of normal cells ubto
tumor cells (38). Thus, the
abnormal expression of c-myc may be a basis for tumorigenesis.
Recent studies have been conducted on miRNAs in
ccRCC. It has been reported that miR-1826 is upregulated in VHL(+)
ccRCC cells compared with VHL-inactivated 786-O and A-498 cell
lines. miR-1826 has also been found responsible for the decreased
expression levels of β-catenin and the survival of its downstream
genes (39). VHL expression can
also be inhibited by miR-23, but β-catenin/TCF-4 transcription
activity, as well as the proliferation and invasion of tumor cells
can be enhanced. Nevertheless, once the VHL gene is transduced all
effects are abolished (40). The
oncogenic activity of miRNAs related or not with high expression
levels of DKK4, as well as the effects of VHL on DKK4 require
further investigation.
DKK4 blocks the canonical Wnt/β-catenin pathway in
ccRCC; however, its effects on the biological behavior of ccRCC may
also be due to other activated pathways. Further studies are
required to fully elucidate the roles of DKK4 in ccRCC
tumorigenesis and tumor progression.
References
|
1
|
Ljungberg B, Hanbury DC, Kuczyk MA, et al:
Renal cell carcinoma guideline. Eur Urol. 51:1502–1510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Wondergem B and Dykema K: A
comprehensive study of progressive cytogenetic alterations in clear
cell renal cell carcinoma and a new model for ccRCC tumorigenesis
and progression. Adv Bioinformatics. 2010:4283252010. View Article : Google Scholar
|
|
3
|
Lam JS, Leppert JT, Figlin RA and
Belldegrun AS: Surveillance following radical or partial
nephrectomy for renal cell carcinoma. Curr Urol Rep. 6:7–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Y, Dai Y, Yang J, et al: Microarray
analysis of microRNA expression in renal clear cell carcinoma. Eur
J Surg Oncol. 35:1119–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Figlin RA, Pierce WC, Kaboo R, et al:
Treatment of metastatic renal cell carcinoma with nephrectomy,
interleukin-2 and cytokine-primed or CD8 (+) selected tumor
infiltrating lymphocytes from primary tumor. J Urol. 158:740–745.
1997.
|
|
6
|
Nelson WJ and Nusse R: Convergence of Wnt,
β-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
|
|
7
|
Capurro MI, Xiang YY, Lobe C and Filmus J:
Glypican-3 promotes the growth of hepatocellular carcinoma by
stimulating canonical Wnt signaling. Cancer Res. 65:6245–6254.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rask K, Nilsson A, Brännström M, et al:
Wnt-signalling pathway in ovarian epithelial tumours: increased
expression of β-catenin and GSK3β. Br J Cancer. 89:1298–1304.
2003.
|
|
9
|
Chien AJ, Moore EC, Lonsdorf AS, et al:
Activated Wnt/β-catenin signaling in melanoma is associated with
decreased proliferation in patient tumors and a murine melanoma
model. Proc Natl Acad Sci USA. 106:1193–1198. 2009.
|
|
10
|
Gilbertson RJ: Medulloblastoma: signalling
a change in treatment. Lancet Oncol. 5:209–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banumathy G and Cairns P: Signaling
pathways in renal cell carcinoma. Cancer Biol Ther. 10:658–664.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang D, Hawke D, Zheng Y, et al:
Phosphorylation of β-catenin by AKT promotes β-catenin
transcriptional activity. J Biol Chem. 282:11221–11229. 2007.
|
|
14
|
Jho EH, Zhang T, Domon C, Joo CK, Freund
JN and Costantini F: Wnt/β-catenin/Tcf signaling induces the
transcription of Axin2, a negative regulator of the signaling
pathway. Mol Cell Biol. 22:1172–1183. 2002.
|
|
15
|
Hirata H, Hinoda Y, Majid S, et al:
DICKKOPF-4 activates the noncanonical c-Jun-NH2 kinase signaling
pathway while inhibiting the Wnt-canonical pathway in human renal
cell carcinoma. Cancer. 117:1649–1660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfaff E, Becker S, Günther A and
Königshoff M: Dickkopf proteins influence lung epithelial cell
proliferation in idiopathic pulmonary fibrosis. Eur Respir J.
37:79–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pendás-Franco N, García J, Peña C, et al:
DICKKOPF-4 is induced by TCF/β-catenin and upregulated in human
colon cancer, promotes tumour cell invasion and angiogenesis and is
repressed by 1α, 25-dihydroxyvitamin D3. Oncogene. 27:4467–4477.
2008.PubMed/NCBI
|
|
18
|
Katoh Y and Katoh M: Comparative genomics
on DKK2 and DKK4 orthologs. Int J Mol Med. 16:477–481.
2005.PubMed/NCBI
|
|
19
|
Peruzzi B, Athauda G and Bottaro DP: The
von Hippel-Lindau tumor suppressor gene product represses oncogenic
β-catenin signaling in renal carcinoma cells. Proc Natl Acad Sci
USA. 103:14531–14536. 2006.PubMed/NCBI
|
|
20
|
Kim YS, Kang YK, Kim JB, Han S, Kim KI and
Paik SR: β-catenin expression and mutational analysis in renal cell
carcinomas. Pathol Int. 50:725–730. 2000.
|
|
21
|
Tanimoto K, Makino Y, Pereira T and
Poellinger L: Mechanism of regulation of the hypoxia-inducible
factor-1α by the von Hippel-Lindau tumor suppressor protein. EMBO
J. 19:4298–4309. 2000.
|
|
22
|
Ma X, Yang K, Lindblad P, Egevad L and
Hemminki K: VHL gene alterations in renal cell carcinoma patients:
novel hotspot or founder mutations and linkage disequilibrium.
Oncogene. 20:5393–5400. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel PH, Chadalavada RS, Chaganti R and
Motzer RJ: Targeting von Hippel-Lindau pathway in renal cell
carcinoma. Clin Cancer Res. 12:7215–7220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsui A, Yamaguchi T, Maekawa S, et al:
DICKKOPF-4 and -2 genes are upregulated in human colorectal cancer.
Cancer Sci. 100:1923–1930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fatima S, Lee N, Tsang F, et al: Dickkopf
4 (DKK4) acts on Wnt/β-catenin pathway by influencing β-catenin in
hepatocellular carcinoma. Oncogene. 31:4233–4244. 2012.
|
|
26
|
Krupnik VE, Sharp JD, Jiang C, et al:
Functional and structural diversity of the human Dickkopf gene
family. Gene. 238:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aravind L and Koonin EV: A colipase fold
in the carboxy-terminal domain of the Wnt antagonists-the
Dickkopfs. Curr Biol. 8:R477–R478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Tilbeurgh H, Bezzine S, Cambillau C,
Verger R and Carrière F: Colipase: structure and interaction with
pancreatic lipase. Biochim Biophys Acta. 1441:173–184.
1999.PubMed/NCBI
|
|
29
|
Bazzi H, Fantauzzo KA, Richardson GD,
Jahoda CA and Christiano AM: The Wnt inhibitor, Dickkopf 4, is
induced by canonical Wnt signaling during ectodermal appendage
morphogenesis. Dev Biol. 305:498–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Veeck J, Bektas N, Hartmann A, et al: Wnt
signalling in human breast cancer: expression of the putative Wnt
inhibitor Dickkopf-3 (DKK3) is frequently suppressed by promoter
hypermethylation in mammary tumours. Breast Cancer Res. 10:R822008.
View Article : Google Scholar
|
|
31
|
Zenzmaier C, Untergasser G, Hermann M,
Dirnhofer S, Sampson N and Berger P: Dysregulation of Dkk-3
expression in benign and malignant prostatic tissue. Prostate.
68:540–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baehs S, Herbst A, Thieme SE, et al:
Dickkopf-4 is frequently down-regulated and inhibits growth of
colorectal cancer cells. Cancer Lett. 276:152–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morais C, Johnson DW and Gobe G: The
VHL-HIF signaling in renal cell carcinoma: promises and pitfalls.
Emerging Research and Treatments in Renal Cell Carcinoma. Amato RJ:
InTech Publishers; Croatia: pp. 57–82. 2011
|
|
34
|
Linehan WM, Rubin JS and Bottaro DP: VHL
loss of function and its impact on oncogenic signaling networks in
clear cell renal cell carcinoma. Int J Biochem Cell Biol.
41:753–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mao B, Wu W, Davidson G, et al: Kremen
proteins are Dickkopf receptors that regulate Wnt/beta-catenin
signalling. Nature. 417:664–667. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baldin V, Lukas J, Marcote M, Pagano M and
Draetta G: Cyclin D1 is a nuclear protein required for cell cycle
progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu JF, Shao JC, Wang DB, Qin R and Zhang
H: Expression and significance of cell cycle regulators in gastric
carcinoma. Ai Zheng. 24:175–179. 2005.PubMed/NCBI
|
|
38
|
Spencer CA and Groudine M: Control of
c-myc regulation in normal and neoplastic cells. Adv Cancer Res.
56:1–48. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirata H, Hinoda Y, Ueno K, Nakajima K,
Ishii N and Dahiya R: MicroRNA-1826 directly targets β-catenin
(CTNNB1) and MEK1 (MAP2K1) in VHL-inactivated renal cancer.
Carcinogenesis. 33:501–508. 2012.PubMed/NCBI
|
|
40
|
Chen L, Han L, Zhang K, et al: VHL
regulates the effects of miR-23b on glioma survival and invasion
via suppression of HIF-1α/VEGF and β-catenin/Tcf-4 signaling. Neuro
Oncol. 14:1026–1036. 2012.PubMed/NCBI
|