Introduction
Treatment with second-generation (atypical)
antipsychotics has been associated with weight gain and the
development of diabetes mellitus (1). Although atypical antipsychotic drugs
are of great benefit to a wide variety of individuals with
psychiatric disorders, particularly patients with schizophrenia
(2), clinical observations
indicate that these drugs can cause adverse metabolic effects
(3), including an increased risk
of obesity, diabetes and metabolic syndrome (4). However, the mechanisms underlying
this process remain unclear. Dopamine D2 receptors are of key
interest to the pathophysiology of schizophrenia (5), as all antipsychotics, typical as
well as atypical, appear to be dopamine D2 receptor antagonists
(6), particularly for
postsynaptic receptors (7).
Risperidone therapy is associated with modest weight gain (8). Claus et al (9) reported a mean weight gain of 2 kg
after 12 weeks of treatment with risperidone with a mean final dose
of 12 mg/day; similar gains were reported in the study by Owens
(10).
Body weight is determined by the balance between
energy intake and expenditure. Leptin and insulin are key hormones
in the regulation of energy balance and glucose homeostasis
(11,12). The actions of insulin are mediated
through the insulin receptor (IR), which belongs to the tyrosine
kinase receptor family. The binding of insulin to its receptor
leads to a rapid autophosphorylation of the receptor followed by
the tyrosine phosphorylation of IR substrate (IRS) proteins, which
induce the activation of downstream signaling cascades, including
phosphatidylinositol-3 kinase (PI3K) and protein kinase B/Akt
(PKB/Akt) (13). Leptin is a
major signaling molecule from the periphery that acts in the
hypothalamus to regulate energy homeostasis and body adiposity
(14,15). The effects of leptin involve the
long isoform of the leptin receptor in the hypothalamus (16), where it influences
pro-opiomelanocortin (POMC) neurons, which activate the secretion
of an anorexic neuropeptide (α-melanocyte-stimulating hormone), and
neuropeptide Y/agouti gene-related protein (NPY/AGRP) neurons,
which inhibit the expression of an orexic neuropeptide (NPY).
Leptin receptors belong to the cytokine receptor superfamily. The
binding of leptin to its receptor activates Janus kinase 2 (JAK2),
which, in turn, phosphorylates tyrosine residues in receptor tails,
leading to the recruitment and activation of signaling molecules
(16,17). Among these signaling molecules,
signal transducer and activator of transcription 3 (STAT3) directly
transmits the signals to the nucleus (18). The suppressor of cytokine
signaling (SOCS) family of proteins was first discovered in 1997
(19) and is also referred to as
JAK-binding proteins, signal transducer and activation of
transcription (STAT)-induced STAT inhibitors, or cytokine-inducible
Src homology-containing proteins family (20). SOCS proteins appear to be
inducible negative regulators of cytokine signaling through the
inhibition of the JAK/STAT pathway (20).
A key feature of human insulin and leptin resistance
involves a defect in the ability of insulin to stimulate PKB
phosphorylation and leptin-induced STAT3 phosphorylation. Thus, in
this study, we investigated whether risperidone exerts direct
biological effects on the insulin-induced PKB activation and
leptin-stimulated STAT3 phosphorylation in cultured SH-SY5Y cells.
Our results demonstrated that risperidone reduced both
insulin-mediated PKB activation and leptin-induced STAT3
phosphorylation. Furthermore, risperidone significantly increased
the mRNA levels of SOCS3 and SOCS6 in an extracellular
signal-related kinase (ERK)1/2-dependent manner. Taken together,
these results clearly indicate that the actions of risperidone are
mediated through the induction of SOCS3 and SOCS6 expression,
which, in turn, inhibit insulin-induced PKB phosphorylation.
Moreover, the induction of SOCS3 expression leads to a modulation
of leptin-stimulated STAT3 phosphorylation; this results in the
inhibition of the leptin and insulin signaling pathways. These
effects of risperidone may be responsible for the antipsychotic
drug-induced weight gain observed in patients with psychiatric
disorders.
Materials and methods
Antibodies and reagents
The following antibodies were used: anti-PKB,
anti-p-PKB (Ser473), anti-STAT3, anti-p-STAT3 (Tyr705), anti-ERK1/2
and anti-p-ERK1/2 antibodies were purchased from Cell Signaling
Technology (Danvers, MA, USA). Risperidone was obtained as a gift
from Johnson & Johnson (New Brunswick, NJ, USA). Insulin and
forskolin were from Sigma-Aldrich (St. Louis, MO, USA. Recombinant
human leptin was purchased from R&D Systems (Minneapolis, MN,
USA). The MEK inhibitor, U0126, and the cellular cyclic adenosine
3-monophosphate (cAMP)-dependent protein kinase (also known as
protein kinase A; PKA) inhibitor, H89, were obtained from
Calbiochem (La Jolla, CA, USA).
Cell culture
The human SH-SY5Y neuroblastoma cells line were used
for all the experiments. The cells were maintained at 37°C, 5%
CO2 in Dulbecco’s modified Eagle’s medium (DMEM),
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 g/ml streptomycin. For the treatment of the SH-SYSY cells
with risperidone and forskolin, the cells were starved for 24 h
followed by treatment of the cells with 100 nM risperidone or 10 μM
forskolin for the indicated periods of time (0, 2, 6, and 12 h).
The control sample was the untreated cells.
Western blot analysis
After the treatment of the cells with the different
reagents as described in the figure legends, the cells were washed
twice in ice-cold PBS and lysed at 4°C in the lysis buffer,
containing 50 mM Tris-HCl (pH 7.5), 25 mM NaF, 40 mM β-glycerol
phosphate (pH 7.5), 120 mM NaCl and 1% NP-40, 0.1 mM
phenylmethylsulfonyl fluoride, 0.1 mM sodium vanadate and 1 mM
benzamidine. The protein concentration of the samples was
determined by the Bradford protein assay with bovine serum albumin
as a standard. The cell lysates were analyzed by 10%
SDS-polyacrylamide gel electrophoresis and blotted onto
polyvinylidene difluoride membranes. After blocking with 5% skim
milk in Tris-buffered saline (TBS) containing 0.02% Tween-20, the
membranes were probed with the corresponding antibodies and
visualized by enhanced chemiluminescence, according to the
manufacturer’s instructions (GE Healthcare, Pittsburgh, PA,
USA).
Reverse transcription (RT)-PCR
Total cellular RNA was isolated from the SH-SY5Y
cells using PureHelix RNA Extraction Solution (NanoHelix Co., Ltd.,
Boston, MA, USA) following the manufacturer’s instructions. cDNA
was synthesized from 2 μg of RNA using oligo(dT) primer and a
first-strand cDNA synthesis kit (Promega, Madison, WI, USA). The
housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), was amplified as a control for RNA loading and variations
in cDNA synthesis efficiency. The following primer sets were
designed for the amplification of human SOCS1, SOCS3, SOCS6 and
SOCS7. The following primers were used: SOCS1 Forward, CAC GCA CTT
CCG CAC ATT and reverse, AGC AGC TCG AGG AGG CAG; SOCS3 forward,
GAG TAC CAC CTG AGT CTC CA and reverse, GAC CTC TCT CTC TTC CAC CT;
SOCS6, forward, AAA TGT CCT TTT CTC CGG TC and reverse, AAT TCA TTG
GCC CCC AAT AC; and SOCS7, forward, GCG GAA TTC ATG GGT GAT GTT and
reverse, TAT GGA TCC GC CTC ATT AGT AGC.
Statistical analysis
The quantification of the western blot analysis
results was carried out using the Tina version 2.1 program (Raytest
Isotopenmegerate). Briefly, the relative intensity (area density)
of the bands of interest was quantified using a densitometer. The
background value from a blank band was subtracted. The results were
calculated as the ratio change compared with the corresponding
control bands. Data are presented as the means ± SD of the 3
independent experiments. The results were analyzed using the
Student’s t-test (SPSS version 12.0 software, SPSS Inc., Chicago,
IL, USA). A value of p<0.05 was considered to indicate a
statistically significant difference, and p<0.01 a highly
significant difference compared with the corresponding control
values.
Results
Risperidone inhibits insulin-mediated PKB
activity in SH-SY5Y cells
Risperidone is an antipsychotic drug widely used to
treat patients with schizophrenia (21). Treatment with risperidone often
leads to an increase in body weight; however, the underlying
mechanisms remain unelucidated. Therefore, in this study, the
effects of risperidone on insulin signaling were investigated.
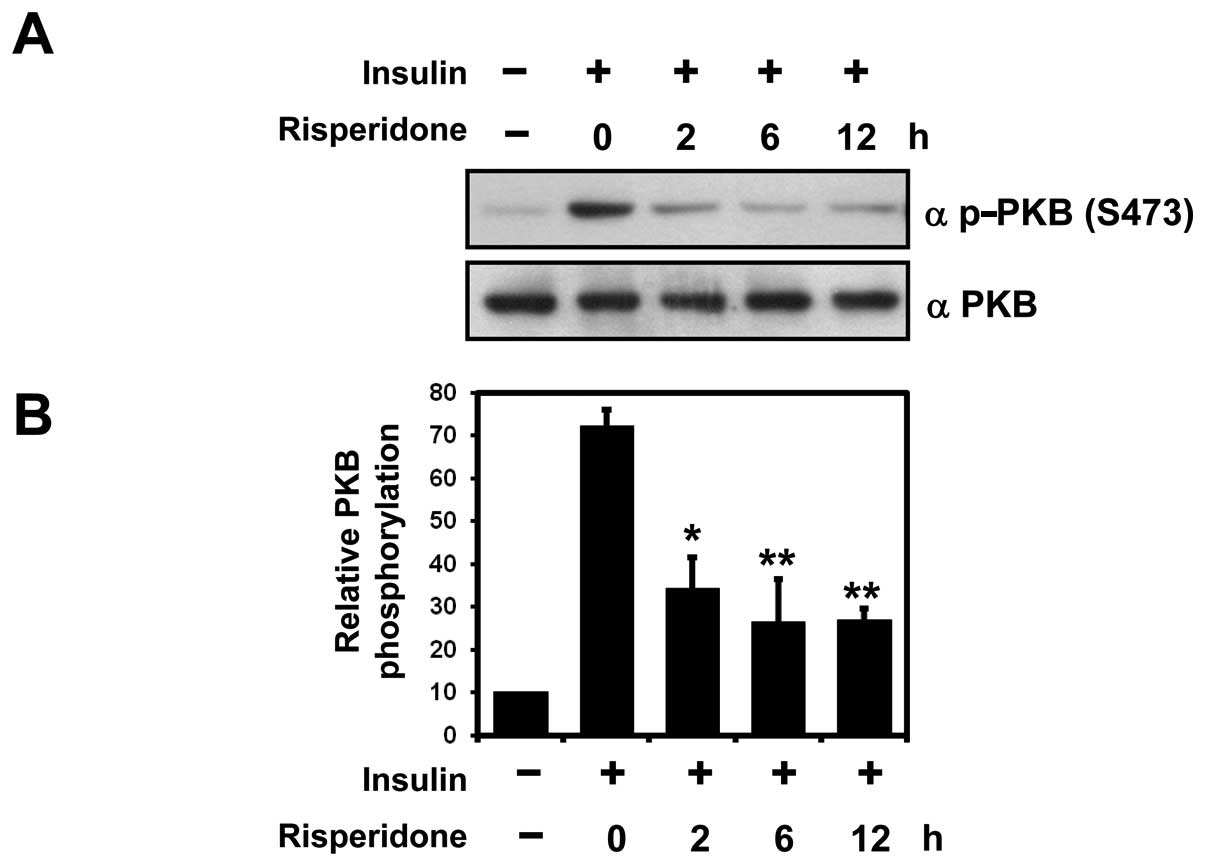
Western blot analyses utilizing an anti-phospho-PKB antibody
revealed that PKB phosphorylation on Ser473 was markedly inhibited
in the SH-SY5Y cells following treatment with risperidone,
beginning at 2 h post-treatment and continuing until 12 h
post-treatment (Fig. 1A).
Statistical analyses of the 3 independent experiments clearly
indicated that insulin-mediated PKB activation was inhibited by
risperidone in the SH-SY5Y cells (Fig. 1B).
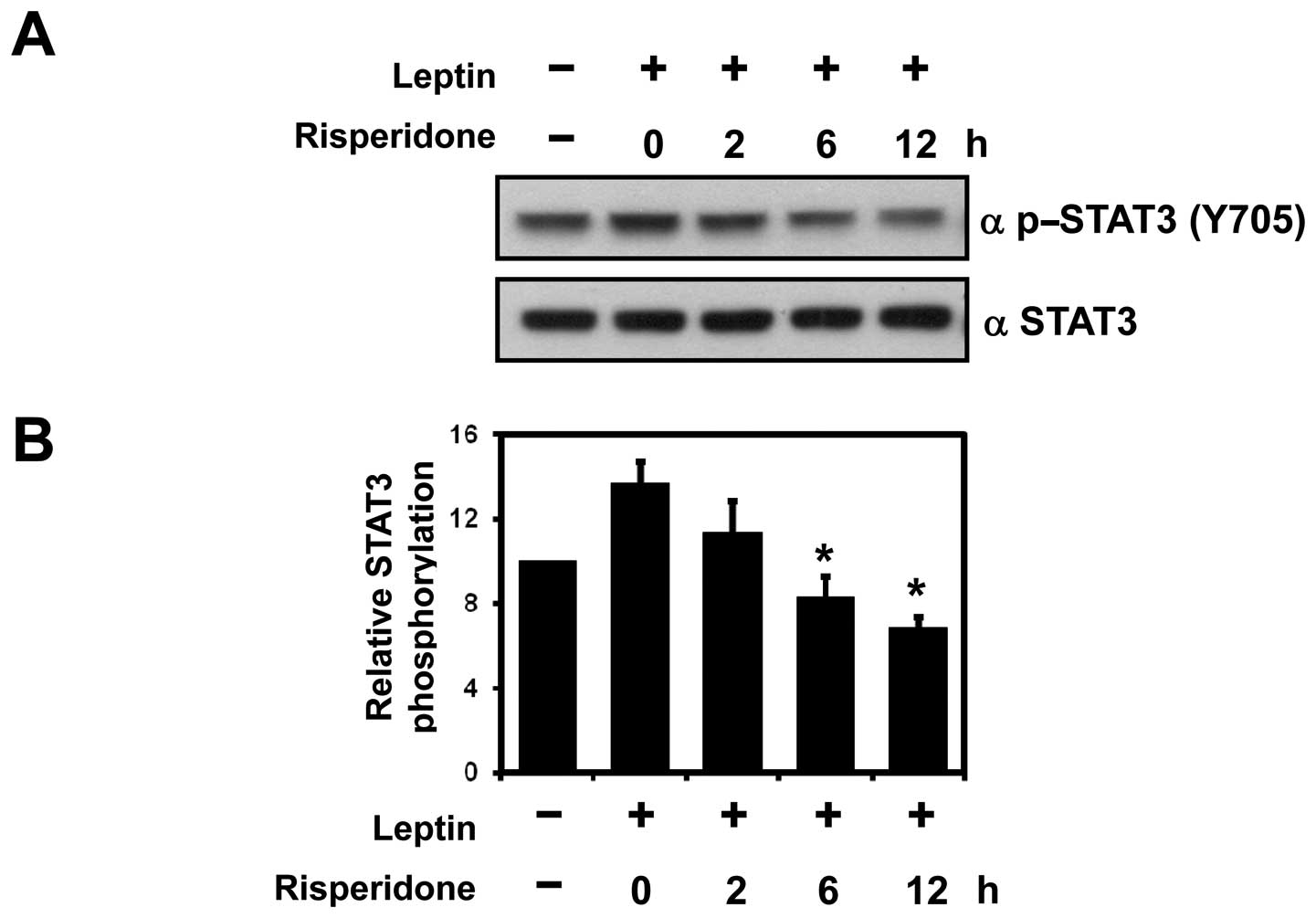
Leptin-mediated STAT3 activation is
blocked by risperidone in SH-SY5Y cells
The adipocyte-derived hormone, leptin, acts as a
satiety signal in hypothalamic nuclei and regulates energy
homeostasis and body weight (22,23). To evaluate the influence of
risperidone on the leptin signaling pathway, the SH-SY5Y cells were
pre-treated with 100 nM risperidone for the indicated periods of
time, followed by treatment with leptin for 30 min. The
leptin-induced STAT3 phosphorylation at Tyr705 was markedly
inhibited by risperidone, beginning at 2 h post-treatment and
continuing to 12 h post-treatment (Fig. 2A and B). This indicates that
risperidone inhibits the insulin and leptin signaling pathways and
may be an important mechanism underlying body weight gain in
schizophrenic patients following treatment with risperidone.
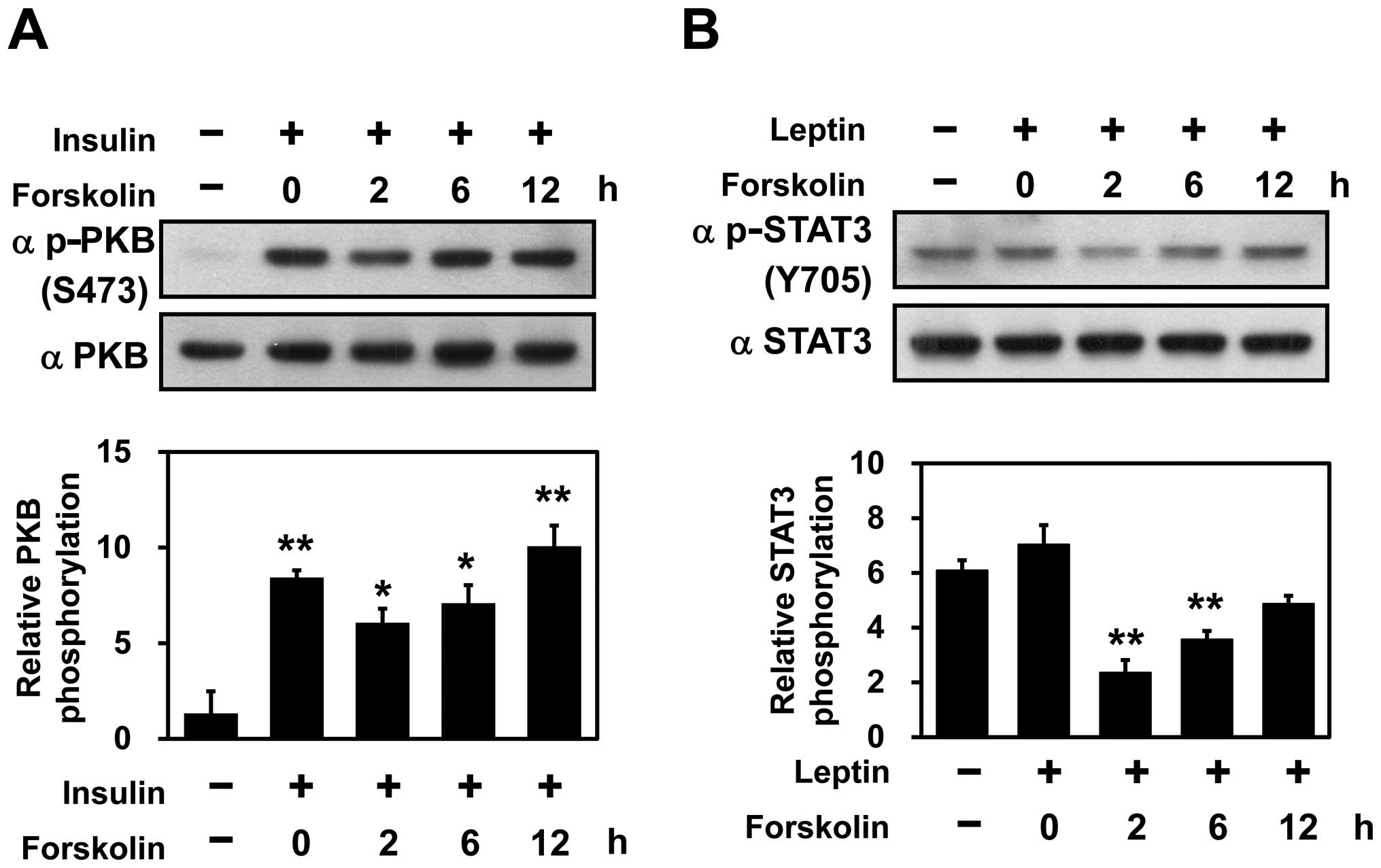
Adenylate cyclase activity is required
for the inhibition of PKB and STAT3 phosphorylation in SH-SY5Y
cells
Atypical antipsychotic drugs, including risperidone,
primarily antagonize dopamine D2 receptors, resulting in an
elevation of cellular cyclic adenosine 3-monophosphate (cAMP)
(24). To further evaluate the
effects of risperidone on insulin/leptin signaling, forskolin, an
activator of adenylate cyclase (25), was utilized. The SH-SY5Y cells
were pre-treated with 10 μM forskolin for the indicated periods of
time, followed by either insulin or leptin stimulation. Western
blot analyses with phospho-specific antibodies demonstrated that
the hormone-mediated phosphorylation of PKB and STAT3 was blocked
by pre-treatment with forskolin at 2 h, after which it recovered
slowly (Fig. 3A and B). This
suggests that the forskolin-mediated inhibition of PKB and STAT3
phosphorylation is regulated by adenylate cyclase.
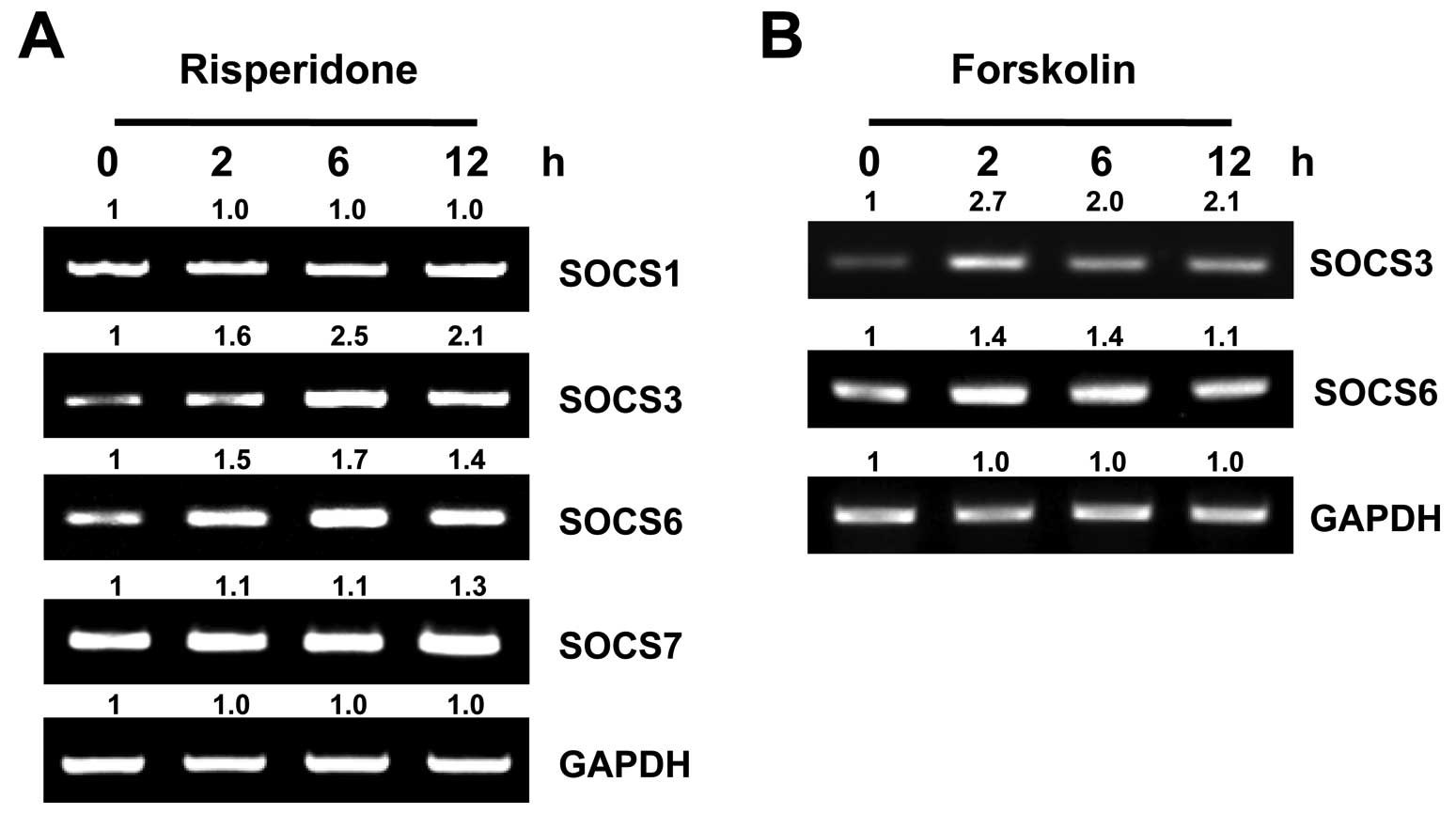
Upregulation of SOCS3 and SOC6 mRNA
levels following treatment with risperidone and forskolin in
SH-SY5Y cells
The elevation of intracellular cAMP-induced SOCS3
expression and the inhibition of STAT3 phosphorylation on the
tyrosine residue in endothelial cells have been described
previously (26). Furthermore,
SOCS3 and other SOCS proteins (SOCS1, SOCS6 and SOCS7) appear to
modulate insulin-mediated PKB signaling by several mechanisms
(27–30). Thus, in this study, the mRNA
levels of SOCS proteins were examined in the SH-SY5Y cells.
Following the administration of risperidone to the cells, the mRNA
levels of SOCS3 and SOCS6, but not those of SOCS1 and SOCS7,
gradually increased in time-dependent manner (Fig. 4A). To further confirm that this
action is dependent on adenylate cyclase activity, the cells were
treated with forskolin. The expression of SOCS3 and SOCS6 was
elevated at 2 h post-treatment and gradually decreased thereafter
(Fig. 4B). This indicates that
the risperidone-mediated induction of SOCS3 and SOCS6 is modulated
by adenylate cyclase.
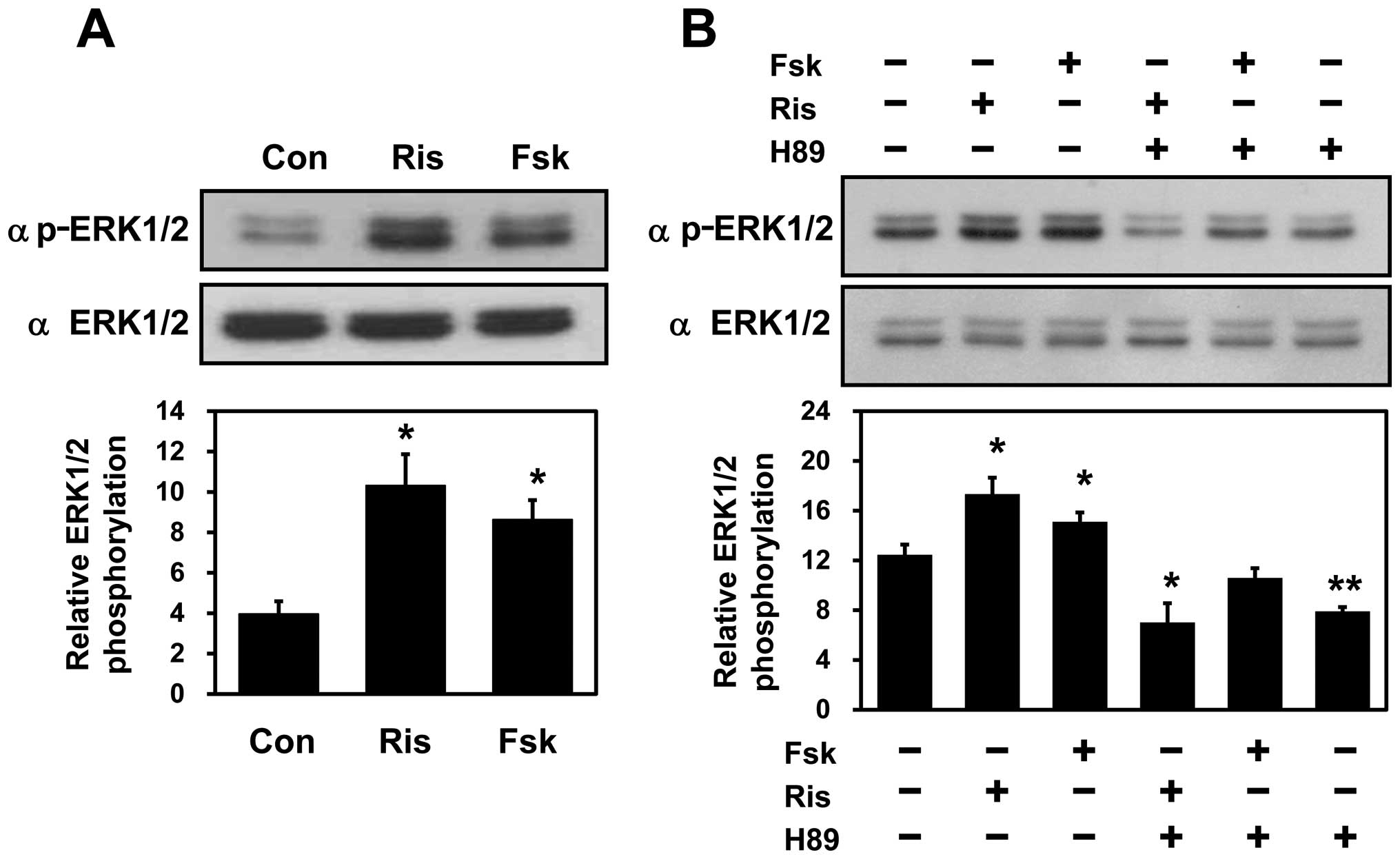
Risperidone-mediated ERK phosphorylation
is dependent on PKA activity
cAMP and PKA are evolutionary conserved molecules
with a well-established position in the complex network of signal
transduction pathways (31).
Intracellular cAMP can activate the mitogen-activated protein
kinase (MAPK)/ERK cascade through either Ras or Rap1 activation in
several cell types (32).
Therefore, the effects of either risperidone or forskolin on ERK1/2
activity in the SH-SY5Y cells were monitored. Western blot analyses
with phospho-specific anti-ERK antibodies revealed that ERK1/2
activation occurred in the risperidone- or forskolin-treated cells
(Fig. 5A), suggesting that
adenylate cyclase activity is important for risperidone-mediated
ERK1/2 activation. To further determine the possible involvement of
PKA in these events, H89, a specific PKA inhibitor, was utilized.
Risperidone- or forskolin-mediated ERK1/2 activation was completely
blocked in the SH-SY5Y cells treated with H89 (Fig. 5B), indicating that PKA activity is
required for risperidone- or forskolin-mediated ERK1/2
activation.
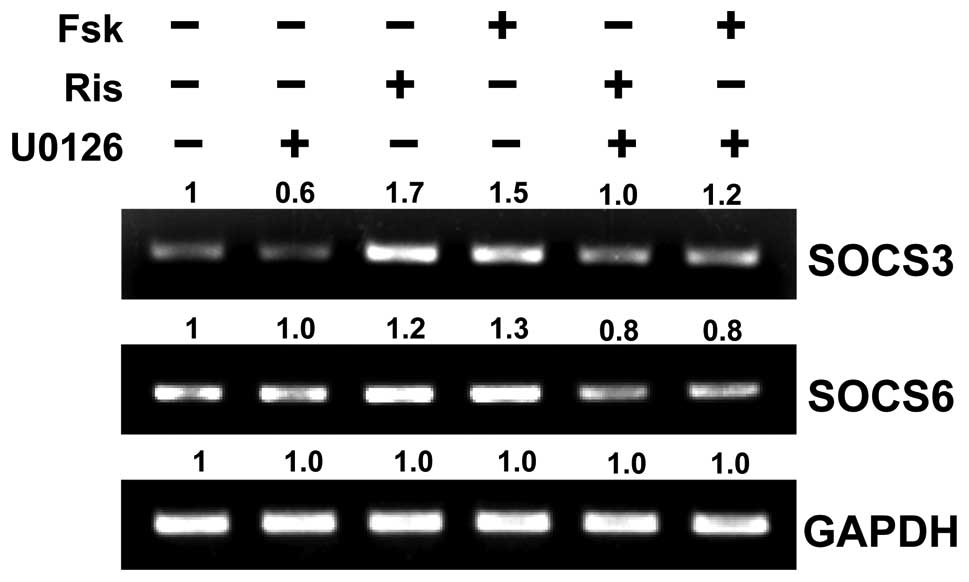
Upregulation of SOCS3 and SOCS6 by
treatment with risperidone is blocked by the MEK inhibitor,
U0126
Sands et al (26) reported that the elevation of
intracellular cAMP promotes the phosphorylation of ERK1/2, which,
in turn, leads to the induction of SOCS3 protein levels in vascular
endothelial cells. To further investigate the possibility that
cAMP-induced MAPK/ERK activation is required for SOCS3 induction,
the MEK inhibitor, U0126, was employed. RT-PCR using SOCS3- and
SOCS6-specific primers revealed that the risperidone- or
forskolin-mediated upregulation of SOCS3 and SOCS6 is dependent on
MEK1/2 activity (Fig. 6).
Discussion
As an important side-effect of antipsychotic
medication, weight gain can be a serious health issue in patients
with schizophrenia and other psychoses (8) and may have adverse implications with
adherence to long-term antipsychotic therapy. Excessive weight gain
may also lead to other adverse health effects, including type 2
diabetes, hyperlipidemia and cardiovascular disease (33). Risperidone and olanzapine, two
widely used atypical antipsychotics, have similar efficacy in the
treatment of patients with schizophrenia (34,35). However, weight gain occurs to
varying extents depending on the particular atypical antipsychotic
drug (34,35).
Risperidone is associated with modest weight gain
that is not related to the dose used. The majority of studies have
reported a mean weight gain of approximately 2–2.5 kg over
treatment periods ranging from 8 weeks to 1 year (9,10,36). The action of risperidone is
mediated through an inhibition of the post-synaptic dopamine D2
receptors (7). Insulin and leptin
are major peripheral signals acting in the hypothalamus to regulate
energy homoeostasis and body adiposity (11). IRs and (long isoform) leptin
receptors share a number of signaling cascades, such as PI3K/PKB
and JAK2/STAT3 (16,22). Studies have demonstrated that
atypical antipsychotic drugs exhibit inverse agonist activity at
dopamine D2 receptors (37) and
stimulate cAMP formation (24).
Furthermore, the inhibition of dopamine D2 receptors can activate
adenylyl cyclase, increase the levels of cAMP, and activate PKA
(38,39) and ERK; in addition, the cAMP
response element-binding protein (CREB) can be activated by
risperidone in a PKA-dependent manner (40).
To the best of our knowledge, this study provides
the first evidence that risperidone regulates both insulin and
leptin signaling in the human SH-SY5Y neuroblastoma cell line.
Treatment of the cells with risperidone markedly inhibited
insulin-induced PKB activation, as well as leptin-mediated STAT3
phosphorylation (Figs. 1 and
2). These events appear to occur
through adenylate cyclase downstream of the dopamine D2 receptor.
It has been reported that forskolin inhibits the interleukin
(IL)-6-stimulated phosphorylation of STAT3 in human aortic
endothelial cells (26).
Similarly, the current findings provide evidence that pre-treatment
with forskolin leads to an inhibition of insulin-induced PKB
phosphorylation and leptin-stimulated STAT3 phosphorylation at 2 h
post-treatment (Fig. 3A and B).
Moreover, the degree of inhibition by forskolin treatment of
insulin- and leptin-mediated signaling is less effective than
risperidone treatment, suggesting that the activation of adenylate
cyclase is part of risperidone-mediated signaling events.
Specific members of the SOCS protein family are
thought to play a role in the development of leptin and insulin
resistance (41). It has been
shown that SOCS3 is important in the development of leptin
resistance (42), and that the
inhibition of insulin is mediated by several SOCS proteins,
including SOCS1, SOCS3, SOCS6 and SOCS7 (27–30). It has been suggested that the
elevation of intracellular cAMP induces SOCS3 protein expression,
leading to the inhibition of leptin and the IL-6-stumulated
phosphorylation of STAT3 in human aortic endothelial cells
(26). In this study, we found
that cAMP-induced SOCS3 protein expression is mediated by ERK1/2.
Another study found that the elevation of intracellular SOCS3
levels block insulin signaling through the ubiquitin-mediated
degradation of IRS1 and IRS2 (43). Similarly, in this study, SOCS3
expression was enhanced by stimulation of the cells with
risperidone or forskolin (Fig. 4A and
B). SOCS6 expression was elevated, whereas the expression of
SOCS1 and SOCS7 was unaltered (Fig.
4A). The SOCS6 protein has been shown to be a key factor in the
inhibition of insulin-dependent PKB activation and IR-directed IRS1
phosphorylation (28).
cAMP-stimulated ERK signaling occurs through several mechanisms and
is a cell type-specific event (32), as cAMP can activate ERK1/2 in
either a PKA-dependent or PKA-independent manner (44–46). In the current system using SH-SY5Y
cells, the effects of risperidone on ERK1/2 were dependent on PKA
activity (Fig. 5B). These results
were confirmed by pre-treatment of the cells with the MEK
inhibitor, U0126, which clearly indicates that the
risperidone-induced SOCS3 and SOCS6 protein expression is dependent
on ERK1/2 activity (Fig. 6).
Thus, these data provide clear evidence that the action of
risperidone may be an important mechanism underlying the
attenuation of insulin-induced PKB phosphorylation and
leptin-stimulated STAT3 phosphorylation.
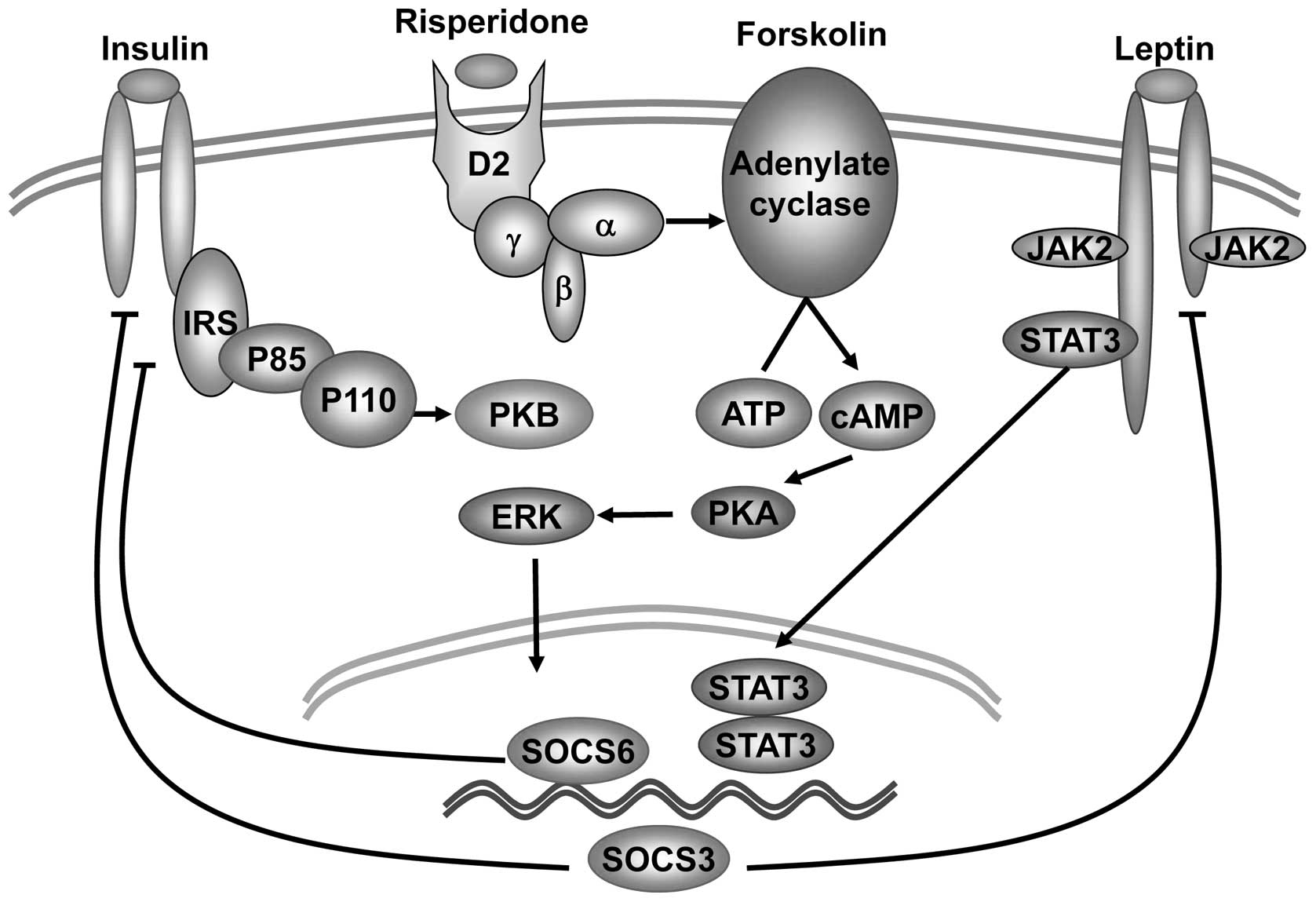
In conclusion, this study demonstrates that the
action of risperidone in the human SH-SY5Y neuroblastoma cell line
involves the induction of both SOCS3 and SOCS6 proteins. A possible
mechanism underlying risperidone-induced insulin and leptin
resistance is suggested (Fig. 7).
The administration of risperidone triggers the accumulation of
cAMP, which leads to the enhancement of the expression of both
SOCS3 and SOCS6 in a PKA/ERK1/2 signaling-dependent manner. SOCS3
and SOCS6 block insulin-stimulated PKB activity and SOCS3 inhibits
the leptin-stimulated phosphorylation of STAT3. Based on the
present data and previously published findings, it is possible to
delineate a tentative model in which risperidone, through the
cAMP/PKA/ERK pathway, induces the expression of SOCS3 and SOCS6
proteins. These findings suggest that this may be an important
mechanism underlying the risperidone-induced insulin and leptin
resistance, which leads to weight gain in schizophrenic
patients.
Acknowledgements
The present study was financially supported by the
National Research Foundation of Korea (NRF) grant funded by the
Korean Government (MEST) (nos. 2007-0054932, 2012R1A1A2004714 and
2012M3A9B6055302), and by a grant from the Korea Healthcare
technology R&D Project, Ministry for Health, Welfare and Family
Affairs, Republic of Korea (HI10C0573).
References
|
1
|
Lett TA, Wallace TJ, Chowdhury NI, Tiwari
AK, Kennedy JL and Muller DJ: Pharmacogenetics of
antipsychotic-induced weight gain: review and clinical
implications. Mol Psychiatry. 17:242–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Citrome L, Jaffe A, Levine J and
Lindenmayer JP: Dosing of quetiapine in schizophrenia: how clinical
practice differs from registration studies. J Clin Psychiatry.
66:1512–1516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melkersson KI, Dahl ML and Hulting AL:
Guidelines for prevention and treatment of adverse effects of
antipsychotic drugs on glucose-insulin homeostasis and lipid
metabolism. Psychopharmacology (Berl). 175:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scheen AJ and De Hert MA: Abnormal glucose
metabolism in patients treated with antipsychotics. Diabetes Metab.
33:169–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grace AA: Gating of information flow
within the limbic system and the pathophysiology of schizophrenia.
Brain Res Brain Res Rev. 31:330–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narendran R, Slifstein M, Guillin O, et
al: Dopamine (D2/3) receptor agonist positron emission tomography
radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in
vivo. Synapse. 60:485–495. 2006.
|
|
7
|
Masi G, Cosenza A, Mucci M and Brovedani
P: Open trial of risperidone in 24 young children with pervasive
developmental disorders. J Am Acad Child Adolesc Psychiatry.
40:1206–1214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nasrallah H: A review of the effect of
atypical antipsychotics on weight. Psychoneuroendocrinology.
28(Suppl 1): 83–96. 2003. View Article : Google Scholar
|
|
9
|
Claus A, Bollen J, De Cuyper H, et al:
Risperidone versus haloperidol in the treatment of chronic
schizophrenic inpatients: a multicentre double-blind comparative
study. Acta Psychiatr Scand. 85:295–305. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Owens DG: Extrapyramidal side effects and
tolerability of risperidone: a review. J Clin Psychiatry. 55:29–35.
1994.PubMed/NCBI
|
|
11
|
Morton GJ: Hypothalamic leptin regulation
of energy homeostasis and glucose metabolism. J Physiol.
583:437–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niswender KD, Baskin DG and Schwartz MW:
Insulin and its evolving partnership with leptin in the
hypothalamic control of energy homeostasis. Trends Endocrinol
Metab. 15:362–369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hemmings BA and Restuccia DF: PI3K-PKB/Akt
pathway. Cold Spring Harb Perspect Biol. 4:a0111892012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coppari R and Bjorbaek C: Leptin
revisited: its mechanism of action and potential for treating
diabetes. Nat Rev Drug Discov. 11:692–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Obici S, Feng Z, Morgan K, Stein D,
Karkanias G and Rossetti L: Central administration of oleic acid
inhibits glucose production and food intake. Diabetes. 51:271–275.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks
AS and Myers MG Jr: Regulation of Jak kinases by intracellular
leptin receptor sequences. J Biol Chem. 277:41547–41555. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Myers MG Jr: Leptin receptor signaling and
the regulation of mammalian physiology. Recent Prog Horm Res.
59:287–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
White DW, Kuropatwinski KK, Devos R,
Baumann H and Tartaglia LA: Leptin receptor (OB-R) signaling.
Cytoplasmic domain mutational analysis and evidence for receptor
homo-oligomerization. J Biol Chem. 272:4065–4071. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Starr R, Willson TA, Viney EM, Murray LJ,
Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA
and Hilton DJ: A family of cytokine-inducible inhibitors of
signalling. Nature. 387:917–921. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshimura A, Nishinakamura H, Matsumura Y
and Hanada T: Negative regulation of cytokine signaling and immune
responses by SOCS proteins. Arthritis Res Ther. 7:100–110. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyamoto S, Duncan GE, Marx CE and
Lieberman JA: Treatments for schizophrenia: a critical review of
pharmacology and mechanisms of action of antipsychotic drugs. Mol
Psychiatry. 10:79–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friedman JM and Halaas JL: Leptin and the
regulation of body weight in mammals. Nature. 395:763–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nilsson CL and Eriksson E: Haloperidol
increases prolactin release and cyclic AMP formation in vitro:
inverse agonism at dopamine D2 receptors? J Neural Transm Gen Sect.
92:213–220. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Insel PA and Ostrom RS: Forskolin as a
tool for examining adenylyl cyclase expression, regulation, and G
protein signaling. Cell Mol Neurobiol. 23:305–314. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sands WA, Woolson HD, Milne GR, Rutherford
C and Palmer TM: Exchange protein activated by cyclic AMP
(Epac)-mediated induction of suppressor of cytokine signaling 3
(SOCS-3) in vascular endothelial cells. Mol Cell Biol.
26:6333–6346. 2006. View Article : Google Scholar
|
|
27
|
Banks AS, Li J, McKeag L, et al: Deletion
of SOCS7 leads to enhanced insulin action and enlarged islets of
Langerhans. J Clin Invest. 115:2462–2471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mooney RA, Senn J, Cameron S, et al:
Suppressors of cytokine signaling-1 and -6 associate with and
inhibit the insulin receptor. A potential mechanism for
cytokine-mediated insulin resistance. J Biol Chem. 276:25889–25893.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Senn JJ, Klover PJ, Nowak IA, et al:
Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator
of interleukin-6-dependent insulin resistance in hepatocytes. J
Biol Chem. 278:13740–13746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueki K, Kondo T and Kahn CR: Suppressor of
cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance
through inhibition of tyrosine phosphorylation of insulin receptor
substrate proteins by discrete mechanisms. Mol Cell Biol.
24:5434–5446. 2004. View Article : Google Scholar
|
|
31
|
Wojtal KA, Hoekstra D and van Ijzendoorn
SC: cAMP-dependent protein kinase A and the dynamics of epithelial
cell surface domains: moving membranes to keep in shape. Bioessays.
30:146–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stork PJ and Schmitt JM: Crosstalk between
cAMP and MAP kinase signaling in the regulation of cell
proliferation. Trends Cell Biol. 12:258–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Metzger BE, Buchanan TA, Coustan DR, et
al: Summary and recommendations of the Fifth International
Workshop-Conference on Gestational Diabetes Mellitus. Diabetes
Care. 30(Suppl 2): S251–S260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lieberman JA, Stroup TS, McEvoy JP, et al:
Effectiveness of antipsychotic drugs in patients with chronic
schizophrenia. N Engl J Med. 353:1209–1223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tran PV, Hamilton SH, Kuntz AJ, et al:
Double-blind comparison of olanzapine versus risperidone in the
treatment of schizophrenia and other psychotic disorders. J Clin
Psychopharmacol. 17:407–418. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Csernansky JG, Miller JP, McKeel D and
Morris JC: Relationships among cerebrospinal fluid biomarkers in
dementia of the Alzheimer type. Alzheimer Dis Assoc Disord.
16:144–149. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akam E and Strange PG: Inverse agonist
properties of atypical antipsychotic drugs. Biochem Pharmacol.
67:2039–2045. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seeman P, Schwarz J, Chen JF, et al:
Psychosis pathways converge via D2high dopamine receptors. Synapse.
60:319–346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Albert PR, Neve KA, Bunzow JR and Civelli
O: Coupling of a cloned rat dopamine-D2 receptor to inhibition of
adenylyl cyclase and prolactin secretion. J Biol Chem.
265:2098–2104. 1990.PubMed/NCBI
|
|
40
|
Yang BH, Son H, Kim SH, Nam JH, Choi JH
and Lee JS: Phosphorylation of ERK and CREB in cultured hippocampal
neurons after haloperidol and risperidone administration.
Psychiatry Clin Neurosci. 58:262–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Howard JK and Flier JS: Attenuation of
leptin and insulin signaling by SOCS proteins. Trends Endocrinol
Metab. 17:365–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bjorbak C, Lavery HJ, Bates SH, et al:
SOCS3 mediates feedback inhibition of the leptin receptor via
Tyr985. J Biol Chem. 275:40649–40657. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rui L, Yuan M, Frantz D, Shoelson S and
White MF: SOCS-1 and SOCS-3 block insulin signaling by
ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem.
277:42394–42398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Laroche-Joubert N, Marsy S, Michelet S,
Imbert-Teboul M and Doucet A: Protein kinase A-independent
activation of ERK and H,K-ATPase by cAMP in native kidney cells:
role of Epac I. J Biol Chem. 277:18598–18604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yee WM and Worley PF: Rheb interacts with
Raf-1 kinase and may function to integrate growth factor- and
protein kinase A-dependent signals. Mol Cell Biol. 17:921–933.
1997.PubMed/NCBI
|
|
46
|
Richards JS: New signaling pathways for
hormones and cyclic adenosine 3′,5′-monophosphate action in
endocrine cells. Mol Endocrinol. 15:209–218. 2001.
|