1. Introduction
The thymus is the primary site for T-cell
lymphopoiesis, providing a coordinated environment for critical
factors to induce and support lineage commitment, differentiation
and survival of thymus-seeding cells. The function of the thymus
includes the maturation and selection of antigen-specific T cells
and selective release of these cells to the periphery. The
maturation process consists of the development of T-cell antigen
receptors with high diversity that are capable of binding to
different antigens, while the selection process consists of
eliminating those cells that support receptors that can bind to
antigens of the organism itself.
T-cell differentiation is a highly complex process
that includes progressive acquisition of different membrane markers
and rearrangement of the genes coding for the antigen-specific
T-cell receptor (TCR). This diversity of markers is generated by
re-arrangement of the TCR genes from a set of germ-line genes
(1–3). It has been found that the selection
of the T-cell repertoire and rearrangement of TCR genes are not
purely automatic genetically programmed mechanisms; instead, they
are stepwise processes, controlled and probably induced by a
variety of stromal cells in different regions of the organ. This
insight has led to the concept of the thymus microenvironment, a
three-dimensional network composed of several distinct cell types,
such as epithelial cells, macrophages, dendritic cells and
fibroblasts, as well as extracellular matrix (ECM) elements
(4,5). Evidence from molecular biology and
immunohistochemical findings have revealed that there are several
different microenvironments in the thymus, each responsible for a
specific step of the stepwise process of T-cell development and
differentiation (6–8). Within the microenvironment,
different types of intercellular communications occur and probably
play a role in T-cell differentiation (9,10).
Furthermore, an increasing amount of experimental data have
indicated the presence of an intrinsic thymus innervation that
actively participates to modulate the development and maturation of
T cells in the thymus microenvironment (11,12).
2. Genesis of the thymus
microenvironment
The thymus parenchyma is characterized by a
succession of different microenvironments that offer specific
assistance to several T-cell maturation steps. This complex
cellular architecture of the thymus parenchyma has its origin in
the heterogeneous embryonal genesis of the organ. The long-held
understanding that the thymus develops in the embryo from the
endoderm of the third pharyngeal pouch, and from the ectoderm of
the branchial clefts and related mesenchyma, which derive from
pharyngeal arch (13,14), has been challenged. In their
study, Gordon et al (15)
showed through labelling experiments that ectoderm may not be
involved in thymus genesis, and that endoderm cells alone seem to
be sufficient to generate a complete thymic microenvironment.
Recently, several studies have provided more support for the
‘endodermic-centric’ model of thymus organogenesis (16).
In the human foetus at about the 9th week of
embryonal life, the thymus primordium begins to be invaded by
lymphoid stem cells, and following this invasion the epithelial
stroma becomes reticulated. During the 9th to 12th week of
embryonal life, the surface of the thymus primordium becomes
indented by mesenchymal septae, which begin to separate thymus
parenchyma into pseudolobuli. In the 17th week of embryonal life,
these septae reach the cortico-medullary junction. At the 12th week
of embryonal life, the differentiation of cortex and medulla
becomes evident, and is completed at approximately the end of the
4th fetal month. From that point on, the thymus delivers thymocytes
to the peripheral lymphoid organs and is to be considered fully
functional. In the postnatal thymus, extended perivascular spaces
with argyrophilic fibers, containing capillaries, arterial venous
blood vessels and lymphatic vessels, sprouting from these septae,
are predominantly found between the cortex and medulla (17).
The thymus continues to grow between birth and
puberty and then begins its atrophic phase, when the organ is
primarily replaced with fat (a phenomenon known as ‘organ
involution’). The atrophy is accompanied by increased circulating
levels of sex hormones. Notably, it has been observed that chemical
or physical castration of an adult subject results in the thymus
increasing in size and activity (18). During this organ involution, the
thymus becomes progressively smaller, and its microanatomy changes
significantly with a loss of thymic epithelial cells (TEC) and a
decrease of lymphopoiesis (17,19). However, remnants of thymic
epithelial tissue with a cortical lymphocyte population are
preserved, and for the entire life of the subject the thymus acts
as a site of T-cell differentiation and maturation, with only
partial reduction of endocrine physiology (17).
The macroscopic structure of the mature thymus organ
is divided into lobules that have an outer part known as the cortex
and an inner core known as the medulla, separated by the
cortico-medullary border. T-cell maturation and development occur
along a cortico-medullary cell gradient, in which immature T-cells
acquire step by step a particular expression of the membrane
factor, following exposure to different signals regulated by a
complex interaction with the thymic microenvironment. Furthermore
the T-cell maturation steps, along cortical thickness, have
evidenced a different expression of pro-thymocyte membrane proteins
that lead to subdivision of the cortex into the outer and deep
cortex (20,21). An important site inside the thymus
parenchyma is the perivascular site, through which immature T cells
enter the thymus (cortical perivascular site) or exit the thymus
when mature (medullar perivascular sites). In particular, the
perivascular spaces at the cortico-medullary junction are narrowly
interdigitated with the thymic epithelial tissue and are the site
of exchange between the thymic epithelial region and the periphery.
Both lymphatic stem cells as well as precursors of macrophages,
presumably monocytes, invade the thymus and settle in the
presumptive cortex and medulla of the fetal thymus (22–24).
The different regions into which the thymus
parenchyma is divided show unusual cytological characteristics with
complex humoral and paracrine relationships between stromal cells
and developing pro-thymocytes.
The thymic epithelium is the major component of the
thymic microenvironment and plays important and multifaceted roles
in early events of T-cell differentiation (25,26) in at least two distinct ways: i)
through secretion of a variety of polypeptides and thymic hormones
and ii) through cell-to-cell contacts, including those occurring
through classical adhesion molecules (10,25,27). However, the submorphological
aspects are not only different in the TEC types, but there are also
distinctions in the antigenic pattern (10,25). Van de Wijngaert et al
(28), studying sections obtained
from healthy donors, classified epithelial cells into six types,
according to their ultrastructural appearance and location in the
human thymus. More recently, transmission electron microscope
observations have confirmed that there are four functional subtypes
of medullar reticulo-epithelial (RE) cells; according to their
morphological appearance, these subtypes are termed
undifferentiated, squamous, villous and cystic (26).
The structure of the ECM is also important in
defining the properties of thymus microenvironments as it affects
the reciprocal relationships between cells and tissue organization
(29–31). The ECM of the thymus is a
well-organized macromolecular system containing collagen (types I
and IV) and other glycoproteins (fibronectin, laminin), with a
variable composition in different compartments of the organ
(32). Of note, although
chemokines and ECM proteins can drive thymocyte migration, a
combined role of these molecules likely concurs for the resulting
migration patterns of thymocytes in their various differentiation
stages. Among ECM moieties, there are proteins with opposing
functions, such as laminin or fibronectin vs. galectin-3, which
promote, respectively, adhesion and de-adhesion of thymocytes to
the thymic microenvironment (33). Thus, the physiological migration
of thymocytes should be conceived as a resulting vector of
multiple, simultaneous or sequential stimuli, involving chemokines,
adhesive and de-adhesive ECM proteins (31). Moreover, these interactions may be
physiologically regulated in situ by matrix
metalloproteinases (MMPs) (34,35) and hormonally regulated (9,36).
3. Endocrine function of the thymus
microenvironment
Thymus physiology is pleiotropically influenced by
hormones, but it also expresses endocrine functions, secreting
several humoral factors, such as thymopoietin and the thymosins,
which are involved in the maturation of prothymocytes and assist
mature thymocytes in their peripheral tissue colonizing (9,37).
The thymic hormones are produced by the RE cellular
network of the thymic stromal cellular microenvironment. The thymic
hormones have different influences on T-cell maturation:
thymopoietin induces the phenotypic differentiation of T
precursors; β-3 and β-4 thymosins induce maturation of
prothymocytes; α-7 thymosin is involved in the generation of
suppressor T lymphocytes; and α-1 thymosin influences the early and
late stages of T maturation (37,38). The intrathymic
compartmentalization of secretory cells is also noteworthy, in that
it corresponds to various stages of T-lymphocyte differentiation
(39).
Thymic RE cells also produce numerous cytokines,
including IL-1, -3, -6, -7 and G-CSF, M-CSF, which likely are
important during the various stages of thymocyte activation and
differentiation (26,29,40).
In the RE cells of the thymus we have a unique
phenomenon of molecular biology, because there is the presence of
pituitary hormones, such as growth hormone, prolactin,
adrenocorticotopic hormone and thyroid-stimulating hormone, as well
as secretion of neuropeptides, production of a number of
interleukins and growth factors, and expression of the receptors
for all these substances (9,41).
4. Anatomy of thymus innervation
The anatomical innervation of the thymus is complex,
due to the numerous nerve pathways that supply it. The human thymus
is innervated by nerve fibers originating from postganglionic cell
bodies located in the superior cervical and stellate ganglion
(formed by the fusion of the third cervical ganglion with the first
thoracic ganglion) of the sympathetic chain (42). These nerve fibers reach the thymus
through the perivascular plexus, leading the sympathetic component
of visceral innervation. In addition, the thymus is reached by
fibers running into the vagal branches of the superior and inferior
(or recurrent) laryngeal nerve. There is debate regarding whether
these fibers are parasympathetic (12,43–46) or not (47,48). All these nerve fibers form a rich
perivascular plexus that branches along parenchymal vessels, thus
becoming the access way for nerve fibers. In this case, the
sympathetic and parasympathetic component may be mixed and cause
some enigmatic responses (49).
Sympathetic and parasympathetic nerve fibers enter the thymus along
with blood vessels and branch into the cortex, the capsular and
septal system, the cortico-medullary junction and the medulla
(11,50) (Fig.
1A).
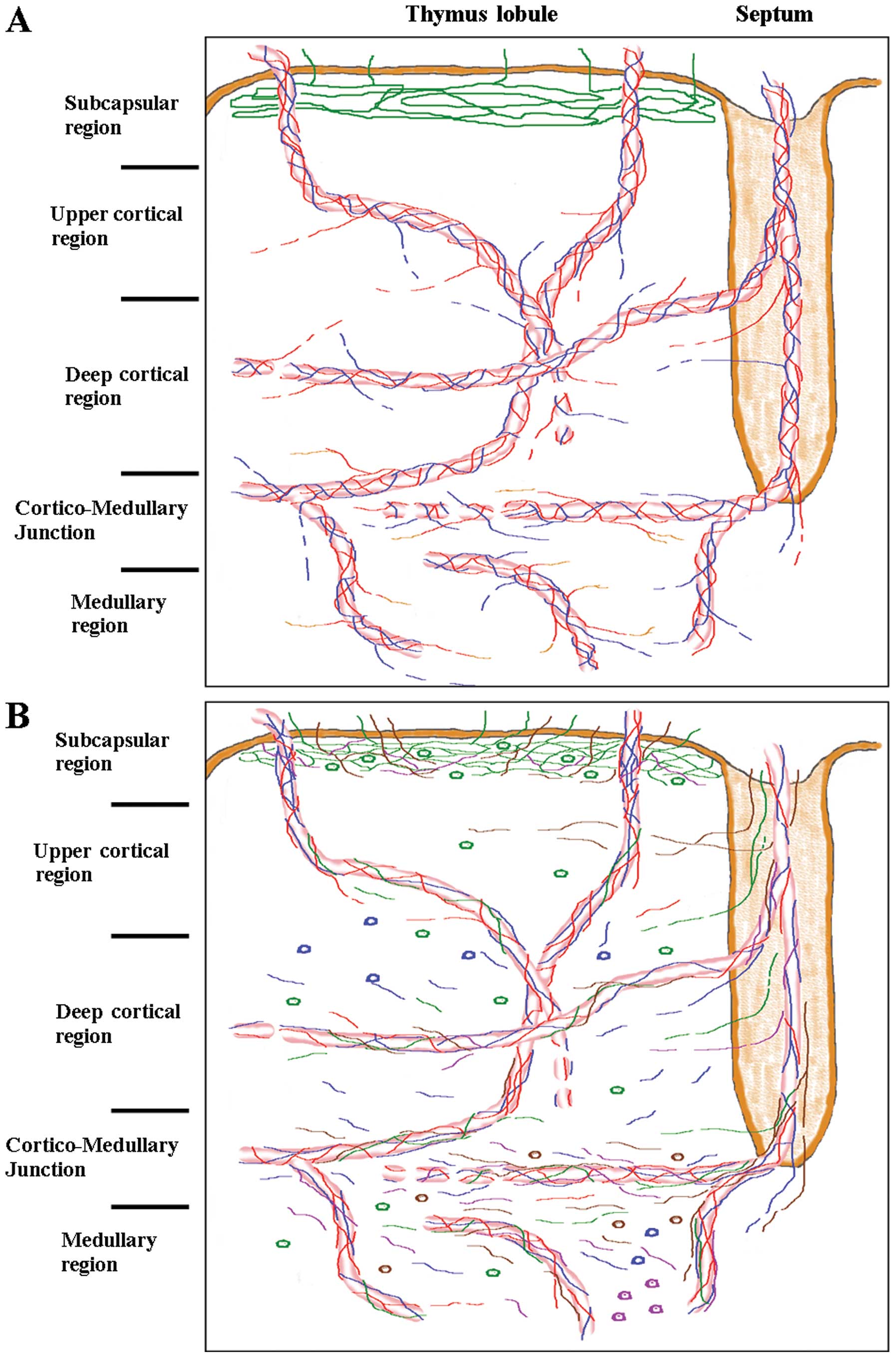 | Figure 1(A) Graphic drawing of intrinsic
innervation pattern of the thymus lobule. Green line, phrenic nerve
fibers; red line, sympathetic nerve fibers; blue line,
parasympathetic nerve fibers; orange line, dopaminergic-sympathetic
nerve fibers. (B) Graphic drawing of neuropeptides pattern of the
thymus lobule. Green line, SP+ nerve fibers; red line,
neuropeptide Y-containing (NPY+) nerve fibers; blue
line, VIP+ nerve fibers; violet line,
neurotensin-immunopositive (NT+) nerve fibers; brown
line, CGRP+ nerve fibers; green cells, SP+;
blue cells, VIP+; violet cells, NT+; brown
cells, CGRP+. |
The sympathetic noradrenergic
innervation
The sympathetic noradrenergic innervation of the
thymus has been identified as tyrosine hydroxylase (TH)
immunohistochemical-positive nerve fibers (51). In the subcapsular area around
vasculature, noradrenergic nerve profiles are predominantly limited
to the cortex with a slightly higher density near the
cortico-medullary junction. At this level, noradrenergic fibers of
septal origin run in venous sinuses, and from that point they reach
the thymic cortex and medulla (50,52).
Several direct interactions have been described
between noradrenergic nerve fibers and thymus parenchymal cells
such as mast cells (53,54), cortico-medullary macrophages
(53), and TEC of deep cortex and
medulla (55). Even if
conventional electron microscopy was unable to identify specific
contacts between these cells and sympathetic neuroeffector
junctions, it has been supposed that a diffusible release of
neurotransmitters may influence the physiology of these cells
(56). The strict association of
noradrenergic fibers with thymus stromal cells and TEC strongly
supports the hypothesis that the secretory activity of epithelial
cells is subject to neuroimmuomodulation (57). Previous studies have demonstrated
that in the thymus, the release of noradrenaline from sympathetic
nerve terminals is of axonal and vesicular origin (51,58).
Noradrenaline released from nerve terminals seems to
have a direct role in the maturation of thymocytes, mediated
through the activation of β-adrenoceptors; it has been observed
that noradrenaline inhibits the proliferation of thymocytes and
promotes their differentiation in vitro (39,57,59). Noradrenergic nerve fibers run in
close contact with TEC, and TEC expresses β-adrenoceptors (55,56). The same type of TECs form the
blood-thymus barrier in the outer thymic cortex, and may be
targeted by noradrenaline released from perivascular nerves to
regulate the entry trafficking of proto-thymocytes. Furthermore,
some types of TECs are also the main cells responsible for
performing thymus microenvironment, in which the relationships
between TEC and noradrenergic fibers may directly affect the
maturation and differentiation of thymocytes to T-lymphocytes
(57,60,61).
Other sympathetic catecholamines have been found in
thymus parenchyma as well (11,54,58,62). In particular, it has been observed
that catecholamines influence the synthesis of cytokines, which are
known to affect the T-cell proliferative/differentiative program
(63).
It should be noted that direct production of
noradrenaline (64), dopamine
(65,66) and other catecholamines has been
found in thymocytes and stromal cells, which act in an
autocrine/paracrine manner to regulate several immune functions
such as differentiation, apoptosis and cytokine production
(39,63).
The parasympathetic innervation
The parasympathetic innervation of the thymus has
been demonstrated by acetylcholinesterase (AChE) or choline
acetyltransferase (ChAT) histochemistry (44). The two methods have yielded some
variable results, and therefore there is still doubt as to whether
there is parasympathetic innervation of the thymus (47,48). However, several authors have
delineated the presence of parasympathetic fibers, consisting
primarily of non-myelinated C-fibers (12,43,67,68). They pass from the capsular region
to the cortex and reach the medulla running in association with
blood vessels (12,68,69). In vivo studies of the
effects of efferent vagal innervations of rat thymus have shown
that nicotinic receptors mediate mechanisms responsible for
lymphocyte release from the thymus (70).
The neurotransmitter acetylcholine is involved in
the mutual interplay between developing T cells and thymic
epithelium, and thereby may influence the generation of T-cell
repertoires (71,72). Moreover, cholinergic agonists may
influence T-cell maturation, negatively affecting thymocyte
apoptosis (nicotinic effect) (73). Thymic acetylcholine is mainly
derived from thymocytes themselves, and its production and release
depend on the activation of thymic lymphocytes (71,72). The occurrence of acetylcholine
secretion by cells of thymus parenchyma renders cholinergic
innervation unclear.
Thus, sympathetic noradrenergic and parasympathetic
cholinergic nerve fibers innervate the vasculature and parenchymal
fields of the thymus: the noradrenergic innervation is prominent in
the subcapsular area, the deep cortex and the connective tissue of
the organ (capsule, interlobular septae) while cholinergic nerve
endings are observed in the cortex and abundantly at the
corticomedullary junction.
Another minor component of the thymus nerve supply
is the phrenic nerve, a somatic nerve originating in the cervical
plexus (74). The phrenic nerve
accesses the intraparenchymal subcapsular region directly through
the connectival capsula of thymus lobules, forming a rich
subcapsular intraparenchymal plexus without being involved in the
perivascular plexus. Phrenic nerve branching has not been found in
other regions of thymic parenchyma outside the subcapsular region
(12).
The strict association between nerve fibers and
blood vessels strongly supports the hypothesis that autonomous
innervation is also involved in controlling T-cell trafficking,
i.e., both entry of T cells into the thymus and their exit from the
thymus as mature T cells.
The dopaminergic system in the
thymus
Although modulation of immune responses by the
noradrenergic system and its main neurotransmitter, noradrenaline,
as well as adrenaline, has been extensively investigated (75), little information is available
concerning modulation by the other catecholamines such as dopamine.
It was previously shown that the dopaminergic system is essential
in the intrathymic microenvironment for modulating and coordinating
the homeostasis of thymocyte survival or death by apoptosis
(76,77).
Experimental findings have indicated that the thymus
expresses a dopaminergic system characterized by the presence of
dopamine, vesicular monoamine transporters and the five subtypes of
dopamine receptors (66,78). In particular, in the rat thymus,
the dopamine system was found to be expressed in the
cortico-medullary junction and in the medulla, but not in the
thymic cortex (12). However, DAT
immune reaction was also found in the wall of arteries located in
the septa of connective tissue as well as in the medulla, with a
reticular localization and an apparent negative reaction of
thymocytes (78). Other authors
have identified dopamine synthesis and storage in dendritic cells,
indicating a functional role of dopamine in cell-cell communication
in the thymic microenvironment as well (65).
Following specific denervation (12), the persistence of D1 and D2
receptor expression in the thymus medulla was shown, while dopamine
and DAT disappeared with the loss of sympathetic innervation,
indicating their specific location on nerve fibers. These findings
suggest that dopamine originates from thymus parenchymal cells and
from sympathetic neuroeffector plexuses, and is released in the
lymphoid microenvironment, emphasizing the importance of a
paracrine/secretory loop of dopamine within the thymus
microenvironment (65,79).
Dopamine receptors have been identified in
peripheral mature lymphocytes (80). High doses of dopamine in
vitro may inhibit mitogen-induced proliferation and promote
apoptosis in lymphocytes (81–83). Data from in vivo studies
have provided conflicting results, with evidence for both
stimulatory and inhibitory effects of dopamine on lymphocyte
function (84–86). However, previous studies have
offered a detailed focus on the action of dopamine in lymphocytes.
In human T cells, during TCR activation, D4 receptor was observed
to be associated with lymphocyte quiescence by upregulating the
transcription factor KLF2 (87).
Following cytofluorimetric analysis, Mignini et al (77), found a modulated expression of
dopaminergic markers on maturing thymocytes. In particular, during
the lineage selection of thymocytes, the acquisition of the
CD4+ or CD8+ identity of thymocytes is
accompanied by particular modulation of dopaminergic system
expression on the two cells (65,77,79).
Taken together, these findings indicate that the
dopaminergic system is widely involved during the last steps of
lymphocyte maturation in the thymus parenchyma.
5. Regulatory peptides
Regulatory peptides have been shown to be involved
in the regulation of immune processes in several animal and human
studies, and a role for neuropeptides in T-cell development has
been investigated (9,88–90). In the human thymus, neuropeptides
such as neuropeptide Y (NPY), vasoactive intestinal polypeptide
(VIP), substance P (SP), calcitonin gene-related peptide (CGRP),
and neurotensin (NT) have been described. These neuropeptides have
been observed to be present in cells and nerve fibers, indicating a
complex scenario in which neuropeptides seem to function as a
secretory system and a modulator system of neurotransmission
(11,90) (Fig.
1B).
NPY
Neuropeptide Y-containing (NPY+) nerve
fibers have a distribution similar to that of TH-immunoreactive
nerve fibers (53,69,91,92). Chemical denervation of
noradrenergic nerve fibers with 6-hydroxydopamine (6-OHDA) depletes
NPY in the rat thymus, suggesting that NPY is co-localized with
noradrenaline in sympathetic nerves (4,58).
Nerve fibers containing NPY extend from the subcapsular and septal
plexuses into superficial and deep cortical regions, the CM-J, and
medulla, mainly detected in relation to the vasculature, while only
a few NPY+ fibers were observed in the parenchyma of the
same zone. The densest innervation occurs at the corticomedullary
junction (90).
NPY+ fibers were observed to arborize
between thymocytes and associated cells such as mast cells or
macrophages (93). This intimate
contact suggests a role for NPY in the development of thymocyte
modulation (94,95). In particular, the preferential
localization of NPY fibers in perivascular spaces suggests that the
effects of NPY in human thymus may be marked by compartment
specificity, and may indicate a focalised role for NPY in thymocyte
migration (90).
VIP
Several findings support the hypothesis that VIP may
modulate thymocyte development. It is known that VIP distribution
is linked to key zones in the thymus lobule, and thus it is the
primary signalling factor in modulating thymic responses to
different stimuli (96). The
distribution of VIP-positive fibers is similar to that of TH- and
NPY-containing nerve fibers, although they are expressed with lower
density (90,97). In all the thymus compartments, VIP
immunoreactivity was observed with a varicose profile in
perivascular sites and with a profile of very fine free fibers in
parenchymal sites. A wide reticular pattern of VIP+
fiber staining was observed in the subcapsular zone, branching
along the perivascular sites, and not in the parenchyma. In the
septal zone, some VIP+ fibers left vessels to enter the
deep cortex. In the deep cortex, the CM-J zone, and in the medulla,
VIP+ fibers were present adjacent to blood vessels and
free fibers in intraparenchymal sites. In thymus cortex and
medulla, VIP immunoreactivity was also observed in cells, often in
proximity to VIP+ fibers, whereas no VIP+
cell localization was observed in the subcortical, septal or CM-J
zones (90). The presence of
VIP+ fibers and cells in the cortex lobular zone may be
the neuroanatomical basis for the modulatory effect of VIP observed
on the proliferation/apoptosis balance on
CD4+CD8+ double-positive thymocytes (88,98). Similarly, the presence of
VIP+ fibers and cells in CM-J and medulla may be the
neuroanatomical basis for the VIP downregulation of lymphocyte
mobility from the thymus.
Functional findings have shown that VIP primarily
affects three important aspects of thymocyte function: cytokine
production, mobility and apoptosis. Through apoptosis, VIP affects
T-cell development, participating in the generation and maturation
of immune cells and in thymic response to stressful conditions.
Immunomodulatory activity of VIP is mediated, at least in part, by
the production of cytokines which are known to control intrathymic
T-cell development (99).
In the human thymus, NPY and VIP are the main
peptidergic components in terms of diffusion, localized in
cholinergic and adrenergic fibers, respectively (90).
SP
SP+ fibers were found to be associated
with the vasculature and others were found freely branching in the
parenchyma. In the subcapsular zone, SP+ fibers were
observed as parenchymal fibers only, and were not associated with
perivascular spaces. Instead, they were detected as the only
neuropeptide-positive fibers occurring in the subcapsular zone
parenchyma. In deeper cortical zones, rare SP+ fibers
were associated with perivascular sites. However, free
SP+ fibers were distributed in parenchyma, mainly as
branching of septal SP+ fibers. At the corticomedullary
junction, SP+ fibers were found branching in association
with the vasculature, with few SP+ fibers running
towards the medullary zone which received only a sparse parenchymal
innervation of SP+ fibers (53,90,91). A close association was observed
between free SP-immunoreactive fibers and macrophages at the
corticomedullary junction, and between these nerve fibers and
macrophages and mast cells along the capsule, interlobular septum
and cortex (53,100,101). SP+ cells exhibited a
cell layer in subcapsular localization, and scattered cells were
observed in the cortex and medulla. A close association was
observed between free SP-immunoreactive fibers and SP-positive
cells (90,101).
The parenchymal, but not perivascular, distribution
of SP+ cells in the subcapsular zone, and the occurrence
of the well-defined subcortical localization of SP+
cells, suggest an important role for this neuropeptide in the
proliferation and first step of maturation of double-negative
(CD4−CD8−) thymocytes (102). Mignini et al (90) have noted a similar distributive
pattern of phrenic nerve fibers and SP+ fibers in the
subcapsular zone of human thymus, an observation that suggests the
potential co-localization of the two systems.
CGRP
CGRP-immunoreactive nerve fibers with vascular and
parenchymal localization (53,91,93) were detected. Similar to
SP+ fibers, these nerve fibers travel along the capsule
and interlobular septa and enter the thymic cortex from the septum.
At the cortical level, these peptidergic fibers branch around
immature thymocytes in cortical parenchyma or enter the medulla
(92,100,101). SP-/CGRP-immunoreactive nerve
fibers were also found adjacent to mast cells in the capsule,
interlobular septum, and at the corticomedullary junction (53,100,103).
Sparse cells positive for CGRP were noted during the
late embryonic period at the cortico-medullary boundary and in the
medulla. The number of these cells increases in adulthood and is
reduced in the thymus of aged mice (104). Thymic lymphocytes synthesize
CGRP, which probably acts as a paracrine mediator in immune cells;
intimate contact with macrophages and mast cells was observed
(105).
Functional and pharmacological analyses have shown
that CGRP mediates different immune responses of thymocytes via
distinct receptor subtypes (106,107).
CGRP affects the proliferation of CD4-positive T
cells, suggesting that the neuropeptide is an inhibitor of the
proliferation of virgin mature T cells while they remain in the
thymus (106). Cytometric
analysis revealed that in cultures the majority of thymocytes
undergoing CGRP-induced apoptosis belong to the CD4/CD8- positive
(double-positive/DP) cell types (107,108). Endogenous CGRP may serve as a
natural inhibitor of inappropriate induction of mature,
antigen-sensitive cells in the thymus, as well as play a role in
investigations on thymocyte.
CGRP and SP are likely fundamental physiological
regulatory substances that are crucial in the differentiation,
maturation and proliferation of thymocytes and are likely to be
involved in the promotion of apoptosis in the thymus (102,107,108).
NT
Unlike observations in experimental animals
(53,109) the occurrence of
NT-immunopositive (NT+) fibers was detected in several
zones of human thymus lobule, as free fine fibers. NT+
fibers was detected in the CM-J and medullary zones. Furthermore,
NT+ cells were detected in the medullary zone of thymus
lobule only and their appearance seems to be compatible with TEC
morphology (90).
The neuropeptide NT is known to be involved in the
modulation of dopamine signalling in the brain (110). NT has been found to be expressed
in human TEC (111) and it has
been reported to modulate cell functions of both innate and
adaptive immunity (112,113). The prevalent localization of NT
on the medullary zone, where the thymus dopaminergic system has
been specifically localized (12,66), may support this hypothesis,
indicating that NT has a functional role in the thymus medullary
environment (90). These findings
lead us to hypothesize the potential involvement of NT in thymus
dopaminergic signalling.
6. Conclusions
In the last three decades, strong evidence has been
gathered for a functional link between the nervous and immune
systems, opening a new area of investigation: the neuroimmune
modulation (10,11,58).
The rich nerve fiber network in the thymus
parenchyma forms a complex scenario where T-lymphocyte maturation
is under strict control. The large amount of data collected thus
far indicates that there is step by step neuronal control of
lymphocyte maturation in and release from the thymus. In addition,
neurotransmitters involved in signal transmission are widely
modulated by neuropeptide co-secretion, adding further complexity
to the nervous system control on the thymus microenvironment and
lymphocyte maturation. Furthermore, immune cells synthesize, store
and release neurotransmitters and neuropeptides, performing a
secretory/paracrine activity that contributes to regulate the
maturation and function of immune cells. It is not of secondary
importance that thymus parenchymal cells are able to secrete
endocrine factor and are responsive to different circulating
endocrine factors.
Alteration of these complex relationships has been
associated with several diseases affecting the physiological
function of the immune system, such as myastenia gravis, multiple
sclerosis and functional alteration in aging (45,90,114).
The implications of these findings for immune
system function merit particular attention, especially in the field
of neurological disease, in which neurotransmitter signalling shows
altered responses. Similarly, further study is needed to examine
the therapeutic uses of drugs that affect neurotransmitters,
especially with regard to their side-effects on immune
function.
Investigations into neuroimmune modulation may lead
to improved understanding of the physiological and pathological
mechanisms involved in the immune system.
References
|
1
|
Benoit C and Mathis D: T-lymphocyte
differentiation and biology. Fundamental Immunology. Paul W: 4th
edition. Lippincott-Raven; Philadelphia, PA: pp. 367–409. 1999
|
|
2
|
Haks MC, Oostervegel MA, Blom B, Spits HM
and Kruisbeek A: Cell-fate decision in early T-cell development:
regulation by cytokine receptors and the pre-TCR. Semin Immunol.
11:23–37. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Werlen G, Hausmann B, Naeher D and Palmer
E: Signaling life and death in the thymus: timing is everything.
Science. 299:1859–1863. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kendall MD and Al-Shawaf AA: Innervation
of rat thymus gland. Brain Behav Immun. 5:9–28. 1991. View Article : Google Scholar
|
|
5
|
Boyd RL, Tucek CL, Godfrey DI, Izon DJ,
Wilson TJ, Davidson NJ, Bean AG, Ladyman HM, Ritter MA and Hugo P:
The thymic microenvironment. Immunol Today. 14:445–59. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Res P and Spits H: Developmental stages in
the human thymus. Semin Immunol. 11:39–46. 1999. View Article : Google Scholar
|
|
7
|
Anderson G, Harman BC, Hare KJ and
Jenkinson EJ: Microenvironmental regulation of T-cell development
in the thymus. Semin Immunol. 12:457–464. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anderson G and Jenkinson EJ: Lymphostromal
interactions in thymic development and function. Nature Rev
Immunol. 1:31–40. 2001. View Article : Google Scholar
|
|
9
|
Savino W and Dardenne M: Neuroendocrine
control of thymus physiology. Endocr Rev. 21:412–443. 2000.
|
|
10
|
Rezzani R, Bonomini F and Rodella LF:
Histochemical and molecular overview of the thymus as site for
T-cells development. Prog Histochem Cytochem. 43:73–120. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mignini F, Streccioni V and Amenta F:
Autonomic innervation of immune organs and neuroimmune modulation.
Auton Autacoid Pharmacol. 23:1–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mignini F, Sabbatini M, D’Andrea V and
Cavallotti C: Intrinsic innervation and dopaminergic markers after
experimental denervation in rat thymus. Eur J Histochem.
54:e172010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bockman DE: Development of the thymus.
Microsc Res Tech. 38:209–215. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sultana DA, Tomita S, Hamada M, Iwanaga Y,
Kitahama Y, Khang NV, Hirai S, Ohigashi I, Nitta S, Amagai T,
Takahashi S and Takahama Y: Gene expression profile of the third
pharyngeal pouch reveals role of mesenchymal MafB in embryonic
thymus development. Blood. 113:2976–2987. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gordon J, Wilson VA, Blair NF, Sheridan J,
Farley A, Wilson L, Manley NR and Blackburn CC: Functional evidence
for a single endodermal origin for the thymic epithelium. Nat
Immunol. 5:546–53. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordon J and Manley NR: Mechanisms of
thymus organogenesis and morphogenesis. Development. 138:3865–3878.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taub DD and Longo DL: Insight into thymic
aging and regeneration. Immunol Rev. 205:72–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sutherland JS, Goldberg GL, Hammett MV,
Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA,
Chidgey AP and Boyd RL: Activation of thymic regeneration in mice
and humans following androgen blockade. J Immunol. 175:2741–2753.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cavallotti C, D’Andrea V, Tonnarini G,
Cavallotti C and Bruzzone P: Age-related changes in the human
thymus studied with scanning electron microscopy. Micros Res Tech.
71:573–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petrie HT: Role of thymic organ structure
and stromal composition in steady-state postnatal T-cell
production. Immunol Rev. 189:8–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Germain RN: T-cell development and the
CD4-CD8 lineage decision. Nat Rev Immunol. 2:309–322. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson M, Anderson SK and Farr AG:
Thymic vasculature: organizer of the medullary epithelial
compartment? Int Immunol. 12:1105–1110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pearse G: Normal structure, function and
histology of the thymus. Toxicol Pathol. 34:504–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petrie HT and Zúñiga-Pflücker JC: Zoned
out: functional mapping of stromal signaling microenvironments in
the thymus. Ann Rev Immunol. 25:649–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gray DH, Ueno T, Chidgey AP, Malin M,
Goldberg GL, Takahama Y and Boyd RL: Controlling the thymic
microenvironment. Curr Op Immunol. 17:137–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bodey B: Thymic reticulo-epithelial cells:
key cells of neuroendocrine regulation. Expert Opin Biol Ther.
7:939–949. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patel DD and Haynes BF: Cell adhesion
molecules involved in intrathymic T-cell development. Semin
Immunol. 5:282–292. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van de Wijngaert FP, Kendall MD, Schuurman
HJ, Rademakers LH and Kater L: Heterogeneity of epithelial cells in
the human thymus. An ultrastructural study. Cell Tissue Res.
237:227–237. 1984.PubMed/NCBI
|
|
29
|
Le PT and Singer KH: Human thymic
epithelial cells: adhesion molecules and cytokine production. Int J
Clin Lab Res. 23:56–60. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prockop SE and Petrie HT: Regulation of
thymus size by competition for stromal niches among early T-cell
progenitors. J Immunol. 173:1604–1611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Savino W, Mendes-da-Cruz DA, Smaniotto S,
Silva-Monteiro E and Villa-Verde DM: Molecular mechanisms governing
thymocyte migration: combined role of chemokines and extracellular
matrix. J Leukoc Biol. 75:951–961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Savino W, Dalmau SR and Dealmeida VC: Role
of extracellular matrix-mediated interactions in thymocyte
migration. Dev Immunol. 7:279–291. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Villa-Verde DM, Silva-Monteiro E,
Jasiulionis M, Farias-de-Oliveira DA, Brentani RR, Savino W and
Chammas R: Galectin-3 modulates carbohydrate-dependent thymocyte
interactions with the thymic microenvironment. Eur J Immunol.
32:1434–1444. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aoudjit F, Masure S, Opdenakker G,
Potworoski EF and St-Pierre Y: Gelatinase B (MMP-9), but not its
inhibitor (TIMP-1), dictates the growth rate of experimental thymic
lymphoma. Int J Cancer. 82:743–747. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilkinson B, Owen JJ and Jenkinson EJ:
Factors regulating stem cell recruitment to the fetal thymus. J
Immunol. 162:3873–3881. 1999.PubMed/NCBI
|
|
36
|
Lannes-Vieira J, Dardenne M and Savino W:
Extracellular matrix components of the mouse thymus
micrenvironment: ontogenetic studies and modulation by
glucocorticoid hormones. J Histochem Cytochem. 39:1539–1546. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Siemion I, Kluczyk A and Cebrat M: The
peptide molecular links between the central nervous and the immune
systems. Amino Acids. 29:161–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lunin SM and Novoselova EG: Thymus
hormones as prospective anti-inflammatory agents. Exper Opin Ther
Targets. 14:775–786. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leposavić G, Pilipović I and Perišić M:
Cellular and nerve fibre catecholaminergic thymic network: steroid
hormone dependent activity. Physiol Res. 60(Suppl 1): S71–S82.
2011.PubMed/NCBI
|
|
40
|
Artico M, Cavallotti C, Cameroni M and
Cavallotti D: Interleukin 1β as simulator of the rat thymus.
Cytokine. 15:261–265. 2001.
|
|
41
|
Berczi I, Quintanar-Stephano A and Kovacs
K: Neuroimmune regulation in immunecompetence, acute illness and
healing. Ann NY Acad Sci. 1153:220–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tollefson L and Bulloch K: Dual-label
retrograde transport: CNS innervation of the mouse thymus distinct
from other mediastinum viscera. J Neurosci Res. 25:20–28. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mićić M, Leposavić G, Ugresić N, Bogojević
M and Isaković K: Parasympathetic innervation of the rat thymus
during first life period: histochemical and biochemical study.
Thymus. 19:173–182. 1992.PubMed/NCBI
|
|
44
|
Cavallotti D, Artico M, Iannetti G and
Cavallotti C: Quantification of acetylcholinesterase-positive
structures in human thymus during development and aging. Neurochem
Int. 36:75–82. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Artico M, Cavallotti C, Tranquilli Leali
FM, Falconi M and Cavallotti D: Effect of interferon on human
thymus microenvironment. Immunol Lett. 85:19–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zimring JC, Kapp LM, Yamada M, Wess J and
Kapp JA: Regulation of CD8+ cytolytic T lymphocyte
differentiation by a cholinergic pathway. J Neuroimmunol.
164:66–75. 2005.
|
|
47
|
Nance DM and Sanders VM: Autonomic
innervation and regulation of the immune system (1987–2007). Brain
Behav Immun. 21:736–745. 2007.
|
|
48
|
Trotter RN, Stornetta RL, Guyenet PG and
Roberts MR: Transneuronal mapping of the CNS network controlling
sympathetic outflow to the rat thymus. Auton Neurosci. 131:9–20.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Besedovsky HO and del Rey A:
Immune-neuro-endocrine interactions: facts and hypotheses. Endocr
Rev. 17:64–102. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Al-Shawaf AA, Kendal MD and Cowen T:
Identification of neural profiles containing vasoactive intestinal
polypeptide, acetylcholinesterase and catecholamines in the rat
thymus. J Anat. 174:131–143. 1991.PubMed/NCBI
|
|
51
|
Vizi ES and Elenkov IJ: Nonsynaptic
noradrenaline release in neuro-immune responses. Acta Biol Hung.
53:229–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cavallotti C, Artico N and Cavallotti D:
Occurrence of adrenergic nerve fibers and noradrenaline in thymus
gland of juvenile and aged rats. Immunol Lett. 70:53–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Müller S and Weihe E: Interrelation of
peptidergic innervation with mast cells and ED1-positive cells in
rat thymus. Brain Behav Immun. 5:55–72. 1991.PubMed/NCBI
|
|
54
|
Artico M, Cavallotti C and Cavallotti D:
Adrenergic nerve and mast cells: correlation in rat thymus. Immunol
Lett. 84:69–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Leposavić G, Mićić M, Ugresić N, Bogojević
M and Isaković K: Components of sympathetic innervation of the rat
thymus during late fetal and postnatal development:
histofluorescence and biochemical study. Sympathetic innervation of
the rat thymus. Thymus. 19:77–87. 1992.
|
|
56
|
Vizi ES, Orsó E, Osipenko ON, Hasko G and
Elenkov IJ: Neurochemical, electrophysiological and
immunocytochemical evidence for a noradrenergic link between the
sympathetic nervous system and thymocytes. Neuroscience.
68:1263–1276. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Leposavić G, Pilipović I, Radojević K,
Pešić V, Perišić M and Kosec D: Cathecolamines as immunomodulators:
a role for adrenoceptor-mediated mechanisms in fine tuning of
T-cell development. Autonom Neurosci. 144:1–12. 2008.PubMed/NCBI
|
|
58
|
Elenkov IJ, Wilder RL, Chrousos GP and
Vizi ES: The sympathetic nerve-an integrative interface between two
supersystems: the brain and the immune system. Pharmacol Rev.
52:595–638. 2000.PubMed/NCBI
|
|
59
|
Madden KS and Felten DL: β-adrenoceptor
blockade alters thymocyte differentiation in aged mice. Cell Mol
Biol. 47:189–196. 2001.
|
|
60
|
Kavelaars A: Regulated expression of α-1
adrenergic receptors in the immune system. Brain Behav Immun.
16:799–807. 2002.
|
|
61
|
Pešić V, Kosec D, Radojević K, Pilipović
I, Perišić M, Vidić-Danković B and Leposavić G: Expression of
α1-adrenoceptors on thymic cells and their role in fine tuning of
thymopoiesis. J Neuroimmunol. 214:55–66. 2009.
|
|
62
|
Cavallotti D, Artico M, Iannetti G and
Cavallotti C: Occurrence of adrenergic nerve fibers in human thymus
during immune response. Neurochem Int. 40:211–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wrona D: Neural-immune interactions: an
integrative view of the bidirectional relationship between the
brain and the immune systems. J Neuroimmunol. 172:38–58. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Leposavić G, Radojević K, Vidić-Danković
B, Kosec D, Pilipović I and Perišić M: Early postnatal castration
affects thymic and thymocyte noradrenaline levels and
β-adrenoceptor-mediated influence on the thymopoiesis in adult
rats. J Neuroimmunol. 182:100–115. 2007.PubMed/NCBI
|
|
65
|
Nakano K, Higashi T, Takagi R, Hashimoto
K, Tanaka Y and Matsushita S: Dopamine released by dendritic cells
polarizes Th2 differentiation. Int Immunol. 21:645–654. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mignini F, Tomassoni D, Traini E and
Amenta F: Dopamine, vesicular transporters and dopamine receptor
expression and localization in rat thymus and spleen. J
Neuroimmunol. 206:5–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nijima A, Hori T, Katafuchi T and Ichijo
T: The effect of interleukin-1β on the efferent activity of the
vagus nerve to the thymus. J Auton Nerv Syst. 54:137–144. 1995.
|
|
68
|
Dovas A, Lucchi ML, Bortoloami R, Grandis
A, Palladino AR, Banelli E, Caretta M, Magni F and Paolocci N:
Collaterals of recurrent laringeal nerve fibres innervate the
thymus: a fluorescent tracer and HRP investigation of efferent
vagal neurons in the rat brainstem. Brain Res. 809:141–148. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mitchell B, Kendall M, Adam E and
Schumacher U: Innervation of the thymus in normal and bone marrow
reconstituted severe combined immunodeficient (SCID) mice. J
Neuroimmunol. 75:19–27. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Antonica A, Ayroldi E, Magni F and
Paolocci N: Lymphocyte traffic changes induced by monolateral vagal
denervation in mouse thymus and peripheral lymphoid organs. J
Neuroimmunol. 64:115–122. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rinner I, Kawashima K and Schauenstein K:
Rat lymphocytes produce and secrete acetylcholine in dependence of
differentiation and activation. J Neuroimmunol. 81:31–37. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rinner I, Globerson A, Kawashima K,
Korsatko W and Schauenstein K: A possible role for acetylcholine in
the dialogue between thymocytes and thymic stroma.
Neuroimmunomodulation. 6:51–55. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yamada T, Murayama T and Nomura Y:
Muscarinic acetylcholine receptors on rat thymocytes: their
possible involvement in DNA fragmentation. Jpn J Pharmacol.
73:311–316. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kendall MD: Functional anatomy of the
thymic microenvironment. J Anat. 177:1–29. 1991.PubMed/NCBI
|
|
75
|
Kohm AP and Sanders VM: Norepinephrine and
β2-adrenergic receptor stimulation regulate CD4+ T and B
lymphocytes function in vitro and in vivo. Pharmacol Rev.
53:487–525. 2001.
|
|
76
|
Sarkar C, Basu B, Chakroborty D, Dasgupta
PS and Basu S: The immunoregulatory role of dopamine: an update.
Brain Behav Immun. 24:525–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mignini F, Sabbatini M, Capacchietti M,
Amantini C, Bianchi E, Artico M and Tammaro A: T-cell
subpopulations express a different pattern of dopaminergic markers
in intra- and extra- thymic compartments. J Biol Regul Homeost
Agents. 27:463–475. 2013.PubMed/NCBI
|
|
78
|
Mignini F, Traini E, Tomassoni D and
Amenta F: Dopamine plasma membrane transporter (DAT) in rat thymus
and spleen: an an immunochemical and immunohistochemical study.
Autonom Autocoid Pharmacol. 26:183–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cosentino M, Fietta AM, Ferrari M, Rasini
E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F and
Lecchini S: Human CD4+ CD25+ regulatory
T-cells selectively express tyrosine hydroxylase and contain
endogeneous catecholamines subserving an autocrine/paracrine
inhibitory functional loop. Blood. 109:632–642. 2007.
|
|
80
|
Oberbek R: Catecholamines: physiological
immunomodulators during health and illness. Curr Med Chem.
13:1979–1989. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Caronti B, Calderaro C, Passarelli F,
Palladini G and Pontieri FE: Dopamine receptor mRNAs in the rat
lymphocytes. Life Sci. 62:1919–1925. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kavelaars A, Cobelens PM, Teunis MA and
Heijnen CJ: Changes in innate and acquired immuno response in mice
with targeted deletion of the dopamine transporter gene. J
Neuroimmunol. 161:162–168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Olanow CW: The pathogenesis of cell death
in Parkinson’s disease-2007. Mov Disord. 17(Suppl): S335–S342.
2007.
|
|
84
|
Cook-Mills JM, Cohen RL, Perlman RL and
Chambers DA: Inhibition of lymphocyte activation by catecholamines:
evidence for a non-classical mechanism of catecholamine action.
Immunology. 85:544–549. 1995.PubMed/NCBI
|
|
85
|
Tsao CW, Lin YS and Cheng JT: Effect of
dopamine on immune cell proliferation in mice. Life Sci.
61:PL361–PL371. 1997.PubMed/NCBI
|
|
86
|
Ilani T, Strous RD and Fuchs S:
Dopaminergic regulation of immune cells via D3 dopamine receptor: a
pathway mediated by activated T-cells. FASEB J. 18:1600–1602.
2004.PubMed/NCBI
|
|
87
|
Sarkar C, Das S, Chakroborty D, Chowdhury
UR, Basu B, Dasgupta PS and Basu S: Cutting Edge: stimulation of
dopamine D4 receptors induce T-cell quiescence by upregulating
Kruppel-like factor-2 expression through inhibition of ERK1/ERK2
phosphorylation. J Immunol. 177:7525–7529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Trejter M, Warchol JB, De Caro R,
Brelinska R, Nussdorfer GG and Malendowcz LK: Studies on the
involvement of endogenous neuropeptides in the control of thymocyte
proliferation in the rat. Histol Histopathol. 16:155–158.
2001.PubMed/NCBI
|
|
89
|
Silva AB and Palmer DB: Evidence of
conserved neuroendocrine interactions in the thymus: intrathymic
expression of neuropeptides in mammalian and non-mammalian
vertebrates. Neuroimmunomodulation. 18:264–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mignini F, Sabbatini M, D’Andrea V and
Cavallotti C: Neuropeptides of human thymus in normal and
pathological conditions. Peptides. 32:920–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bellinger DL, Lorton D, Romano TD,
Olschowka JA, Felten SY and Felten DL: Neuropeptide innervation of
lymphoid organs. Ann NY Acad Sci. 594:17–33. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Krantz A, Kendall MD and von Gaudecker B:
Studies on rat and human thymus to demonstrate immunoreactivity of
calcitonin gene-related peptide, tyrosine hydroxylase and
neuropeptide Y. J Anat. 191:441–450. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Weihe E, Nohr D, Michel S, Müller S,
Zentel HJ, Fink T and Krekel J: Molecular anatomy of the
neuro-immune connection. Int J Neurosci. 59:1–23. 1991. View Article : Google Scholar
|
|
94
|
De la Fuente M, Del Rio M, Victor VM and
Medina S: Neuropeptide Y effects on murine natural killer activity:
changes with ageing and cAMP involvement. Regul Pept. 101:73–79.
2001.PubMed/NCBI
|
|
95
|
Bedoui S, Kawamura N, Straub RH, Pabst R,
Yamamura T and von Hörsten S: Relevance of neuropeptide Y for the
neuroimmune crosstalk. J Neuroimmunol. 134:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Delgado M, Martinez C, Leceta J and
Gomariz RP: Vasoactive intestinal peptide in thymus: synthesis,
receptors and biological actions. Neuroimmunomodulation. 6:97–107.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bellinger DL, Lorton D, Horn L, Brouxhon
S, Felten SY and Felten DL: Vasoactive intestinal polypeptide (VIP)
innervation of rat spleen, thymus and lymph nodes. Peptides.
18:1139–1149. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Delgado M, Garrido E, Martinez C, Leceta J
and Gomariz RP: Vasoactive intestinal peptide and pituitary
adenylate cyclase-activating polypeptides (PACAP27 and PACAP38)
protect CD4+CD8+ thymocytes from
glucocorticoid-induced apoptosis. Blood. 87:5152–5161.
1996.PubMed/NCBI
|
|
99
|
Ganea D, Gonzales-Rey E and Delgado M: A
novel mechanism for immunosuppression: from neuropeptides to
regulatory T cells. J Neuroimmune Pharmacol. 1:400–409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lorton D, Bellinger DL, Felten SY and
Felten DL: Substance P innervation of the rat thymus. Peptides.
11:1269–1275. 1990. View Article : Google Scholar
|
|
101
|
Jurjus AR, More N and Walsh RJ:
Distribution of substance P positive cells and nerve fibers in the
rat thymus. J Neuroimmunol. 90:143–148. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Piantelli M, Maggiano N, Larocca LM, Ricci
R, Ranelletti FO, Lauriola L and Capelli A:
Neuropeptide-immunoreactive cells in human thymus. Brain Behav
Immun. 4:189–197. 1990. View Article : Google Scholar
|
|
103
|
Bulloch K, Radojcic T, Yu R, Hausman J,
Lenhard L and Baird S: The distribution and function of calcitonin
gene-related peptide in the mouse thymus and spleen. Psycho Neuro
Endocrin Immunol. 4:186–194. 1991.
|
|
104
|
Bulloch K, McEwen BS, Diwa A, Radojcic T,
Hausman J and Baird S: The role of calcitonin gene-related peptide
in the mouse thymus revisited. Ann NY Acad Sci. 25:129–136. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Xing L, Guo J and Wang X: Induction and
expression of β-calcitonin gene-related peptide in rat T
lymphocytes and its significance. J Immunol. 165:4359–4366.
2000.
|
|
106
|
Bulloch K, McEwen BS, Diwa A and Baird S:
Relationship between dehydroepiandrosterone and calcitonin
gene-related peptide in the mouse thymus. Am J Physiol.
268:E168–E173. 1995.PubMed/NCBI
|
|
107
|
Bulloch K, McEwen BS, Nordberg J, Diwa A
and Baird S: Selective regulation of T-cell development and
function by calcitonin gene-related peptide in thymus and spleen.
An example of differential regional regulation of immunity by the
neuroendocrine system. Ann NY Acad Sci. 840:551–562. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sakuta H, Inaba K and Muramatsu S:
Calcitonin gene-related peptide enhances apoptosis of thymocytes. J
Neuroimmunol. 67:103–109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Polak JM and Bloom SR: The central and
peripheral distribution of neurotensin. Ann NY Acad Sci. 400:75–93.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Katsanos GS, Anogianaki A, Castellani ML,
Ciampoli C, De Amicis D, Orso C, Pollice R, Vecchiet J, Tetè S,
Salini V, Caraffa A, Patruno A, Shaik YB, Kempuraj D, Doyle R,
Antinolfi PL, Cerulli G, Conti CM, Fulcheri M, Neri G and Sabatino
G: Biology of neurotensin: revisited study. Int J Immunopathol
Pharmacol. 21:255–259. 2008.PubMed/NCBI
|
|
111
|
Vanneste Y, Thome AN, Vandersmissen E,
Charlet C, Franchimont D, Martens H, Lhiaubet AM, Schimpff RM,
Rostène W and Geenen V: Identification of neurotensin-related
peptides in human thymic epithelial cell membranes and relationship
with major histocompatibility complex class I molecules. J
Neuroimmunol. 76:161–166. 1997. View Article : Google Scholar
|
|
112
|
Lhiaubet AM, Avard C and Schimpff RM:
Apparent functionality but impractical quantification of
neurotensin receptors on human peripheral lymphocytes. Hormone Res.
49:233–239. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ramez M, Bagot M, Nikolova M, Boumsell L,
Vita N, Chalon P, Caput D, Ferrara P and Bensussan A: Functional
characterization of neurotensin receptors in human cutaneous T cell
lymphoma malignant lymphocytes. J Invest Dermatol. 117:687–693.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hannestad J, Monjil DF, Díaz-Esnal B, Cobo
J and Vega JA: Age-dependent changes in the nervous and endocrine
control of the thymus. Micros Res Tech. 63:94–101. 2004. View Article : Google Scholar : PubMed/NCBI
|















