Introduction
Ulcerative colitis (UC) is an inflammatory bowel
disease that is associated with an elevated risk of developing
colorectal cancer, and it is estimated that 10% of patients
suffering from UC for >10 years will develop colorectal cancer
(1,2). The underlying pathogenesis is not
fully known, but chronic inflammation distorts mucosal morphology
and induces dysplasia and subsequently cancer. Carcinomas occur
mainly after long-term illness of 8–10 years (3), and develop through low- and
high-degree dysplasia. It is reported that patients presenting only
one lesion of low-degree dysplasia may also harbour carcinomas
(4). The colonic mucosa of UC
patients may also harbour severe molecular abnormalities, such as
chromosomal instability (5) and
DNA aneuploidy. DNA aneuploidy may be present in dysplastic and
non-dysplastic mucosa of UC patients, and is reported to be
connected with the duration of disease (6–9).
It can be characterized as an independent risk factor for the
development of adenocarcinoma in UC (10,11). UC-patients who develop dysplasia
or adenocarcinomas are usually considered progressors, whereas
patients who do not develop these phenotypes during a lifetime of
UC are considered non-progressors. Since DNA-aneuploidy can be
considered an independent risk factor for malignancies in UC-colon
mucosa, we have also included this as a defining characteristic of
a UC-progressor case. The mechanisms behind what makes a UC-colon a
progressor or a non-progressor are not fully known, and it is
therefore of interest to examine the differences in molecular
features of the mucosa between these two types of UC-affected
colons. Molecular characteristics of dysplastic as well as
non-dysplastic lesions within a progressor that are not found in
non-progressors, could be a contribution to the understanding of
mechanisms behind carcinogenesis in UC colons, which could serve as
a marker for individuals at risk.
Telomerase is a ribonucleoprotein capable of
extending the telomeric sequence, generally known to be active in
germline cells and inactive in most somatic cells. The two main
subunits of telomerase are the catalytic subunit TERT-telomerase
reverse transcriptase (hTERT in humans), and TR (TER), the RNA
component (hTR or hTER in humans). In addition to hTERT and hTR, a
range of accessory proteins are also closely associated with the
complex (12). The level of hTERT
is generally assumed to be a limiting factor for assembly of the
telomerase complex, and it is reported that hTERT may also play a
role in cell proliferation, separate from its role in telomere
elongation (13). Telomerase
activity is frequently reported in cancer cells, and ~80–90% of all
solid tumours, including colorectal cancer, have reported
telomerase activity (14–16). Telomerase enables cancerous cells
to achieve replicative immortality, which is one of the hallmarks
of cancer (17). Telomerase
activity may therefore increase the lifespan of a cell, resulting
in an accumulation of genetic alterations in the cell that again
may contribute to the development of cancer (18). In UC mucosa, however, reports on
telomerase activity vary from reduced levels (19), levels not differing from non-UC
colons (20–22), to reports on elevated activity
(23,24). All these investigations used
versions of the Telomeric Repeat Amplification Protocol
(TRAP)-assay or PCR-ELISA, valid methods for measuring telomerase
activity in a sample by using tissue extracts. Due to the often
high levels of inflammation in a UC colon, elevated levels of
macrophages and neutrophils are present, and tissue extracts from
the colonic mucosa of UC patients may therefore comprise these cell
types. A TRAP-assay from colonic mucosal cells may therefore not
differentiate between telomerase activity in macrophages and
leucocytes in the tissue from that of the epithelial cells, making
results difficult to interpret. Notably, in a study on telomerase
activity in UC colonic mucosa, where mucosal cells had been
separated from stromal cells the results showed different levels of
activity in the two sets. Levels of telomerase activity were
reported as low in dysplastic mucosa, and a correlation between
telomerase activity and inflammation was detected. In this report,
DNA-status was not included (22). It has also been speculated as to
whether elevated levels of telomerase-activity in UC mucosa are a
direct result of enhanced cell proliferation in actively inflamed
colon tissue (24).
In the present study, we used immunohistochemistry
(IHC) to assess hTERT levels in UC material as it provides the
advantage of assessing the protein expression in specific cell
types within the tissue examined, thus allowing for the exclusion
of macrophages and neutrophils that would obfuscate hTERT level
data. We assessed hTERT protein levels using IHC in the colonic
mucosal cells of a set of progressor and non-progressor colons of
patients suffering from longstanding UC, to investigate whether any
differences in hTERT expression were related to progressor status,
mucosal dysplastic development or to DNA-ploidy status.
Materials and methods
Patients
Thirty patients suffering from longstanding UC were
included in this report. All the patients had suffered from UC for
>10 years prior to colectomy, and some patients had suffered as
long as 30 years. Patients also varied widely in age at the time
when symptoms first presented (from 10 to 60 years old). The 10
non-progressor patients included 5 males and 5 females. The
progressors included 17 males and 3 females. Use of this material
for research purposes received ethical approval from the Regional
Ethics Committee, REK S-06062.
UC colectomies: Progressors and
non-progressors
The colectomy specimens have previously been
described by Burum-Auensen et al (25). The colectomies (n=30) were grouped
into progressors and non-progressors, revealing 10 non-progressors
that presented no dysplastic lesions, and 20 progressors that all
presented at least one area of dysplasia/cancer. The majority of
cases also presented DNA aneuploidy.
At least eight sites from each colectomy were
examined, and within the progressors 83 non-dysplastic areas were
identified, 31 areas indefinite for dysplasia, 29 areas with
dysplasia and 8 adenocarcinomas. Since our analyses focused on
precancerous morphology changes, the adenocarcinomas were excluded.
A total of 18 non-dysplastic and 7 dysplastic areas revealed DNA
aneuploidy. The progressor lesions are shown in Table I. By definition the non-progressor
lesions were diploid and non-dysplastic.
 | Table ISummary of lesions in the progressor
colectomies (n=20) according to morphology and DNA-ploidy
status. |
Table I
Summary of lesions in the progressor
colectomies (n=20) according to morphology and DNA-ploidy
status.
| |
Colon
specimen # |
|---|
| |
|
|---|
| | 30 | 70 | 71 | 99 | 132 | 159 | 164 | 169 | 174 | 176 | 177 | 191 | 192 | 199 | 205 | 225 | 1514 | 1701 | 1729 | 1789 |
|---|
| Diploid | Non-dysplasia | 5 | 5 | 2 | 1 | 1 | 3 | 1 | 2 | 7 | 2 | 3 | 5 | 4 | 3 | 6 | 5 | 6 | 3 | 0 | 1 |
| Indefinite
dysplasia | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 2 | 1 | 0 | 1 | 5 | 2 | 1 | 1 | 2 | 2 | 0 |
| Dysplasia | 0 | 2 | 0 | 3 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 2 | 6 | 0 |
|
Adenocarcinomaa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Aneuploid | Non-dysplasia | 1 | 0 | 0 | 0 | 0 | 3 | 1 | 1 | 1 | 4 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Indefinite
dysplasia | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Dysplasia | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Adenocarcinomaa | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
hTERT IHC
Tissue microarrays (TMAs) from eight sites within
each colon were made using a Beecher tissue microarrayer as
described previously (25). Core
size was 0.6 mm. All cores were previously evaluated by an
experienced pathologist (OPFC). At least two tissue cores from each
mucosal region were sampled. Two tonsillar sections were used as
positive controls. Sections (4 μm) were exposed to 0.5%
H2O2 solution for 10 min, followed by antigen
retrieval in the citrate buffer at pH 6.0. Incubation of TMAs with
the primary antibody against telomerase [mouse monoclonal ab5181,
dilution (1:500); Abcam, Cambridge, UK], was performed for 1 h at
room temperature. Staining was performed using a Ventana Nexes
machine using Ventana Iview DAB detection kit (Ventana Medical
Systems, Tucson, AZ, USA) according to the manufacturer’s
instructions. Sections stained with Tris-buffered saline (TBS)
instead of primary antibody served as negative staining controls.
hTERT protein expression was defined for each sample as the
percentage of positive cells out of 1,200 randomly selected mucosal
epithelial cells. Only cells with nuclear staining were counted as
positive for hTERT-expression. hTERT-staining of a non-UC control
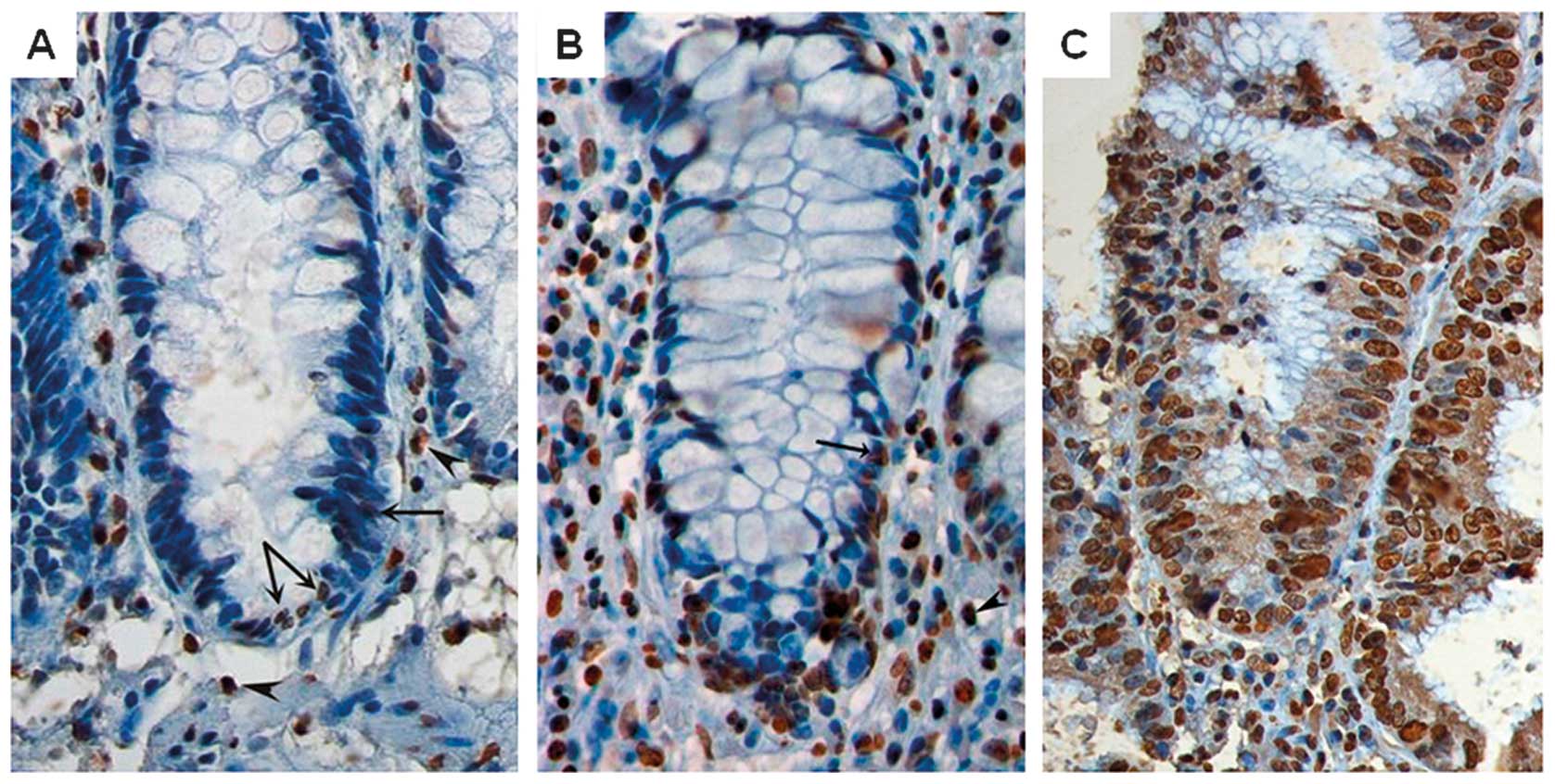
sample and progressor lesions with high and low hTERT levels are
presented in Fig. 1. The antibody
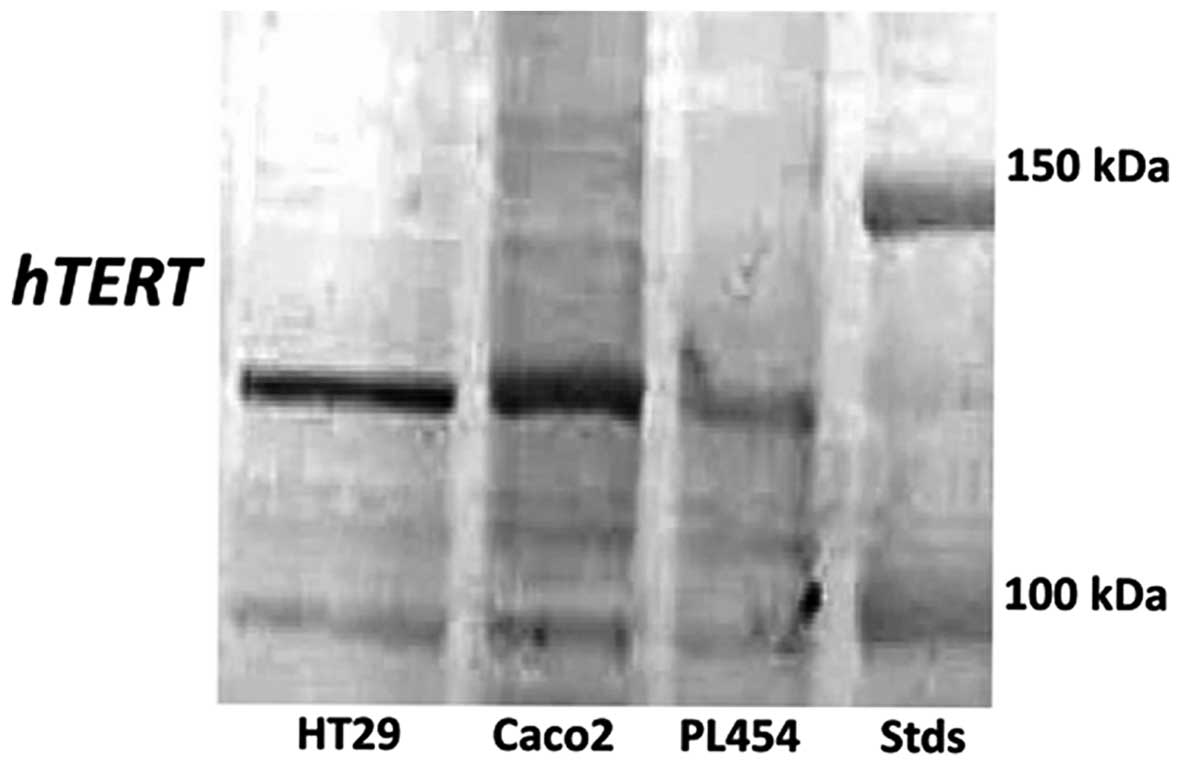
was tested for specificity using several human cancer cell lines,
and a single band of 127 kDa was detected (Fig. 2).
We have previously presented the immunostaining for
Ki67 for this material, showing significantly elevated levels of
Ki67 in UC colons compared to non-UC controls (25).
Statistical analysis
As each patient included in this study contributed
with more than one biopsy, we evaluated the levels of protein
expression in relation to the morphologic parameters, as well as
the association analyses of protein levels for hTERT and Ki67, by
using a multilevel model that compensates for patient differences.
The linear mixed model (LMM), with restricted maximum likelihood
(REML) estimations and a Bonferroni post-hoc test was performed.
Tests were performed in PASW® statistics 18 (Chicago,
IL, USA). All tests were two-sided and a p-level of 0.05 denoted
significance.
Results
TMA evaluation
TMAs do not consistently exhibit full colonic crypts
as observed in whole sections, but since we found the expression of
hTERT in our study to be evenly distributed throughout the colonic
mucosa we concluded that hTERT protein levels could be estimated
reliably (data not shown).
As Ki67 protein expression is linked to the growth
fraction in UC-colonic mucosa we did not consider TMAs as reliable
in assessing Ki67 expression related to dysplastic development.
The assessment of Ki67 protein expression was
performed within the same tissue cores as for hTERT protein
assessments, thus evaluation of the association between hTERT and
Ki67 was considered to be reliable.
Levels of hTERT in the colonic mucosa of
progressors vs. non-progressors
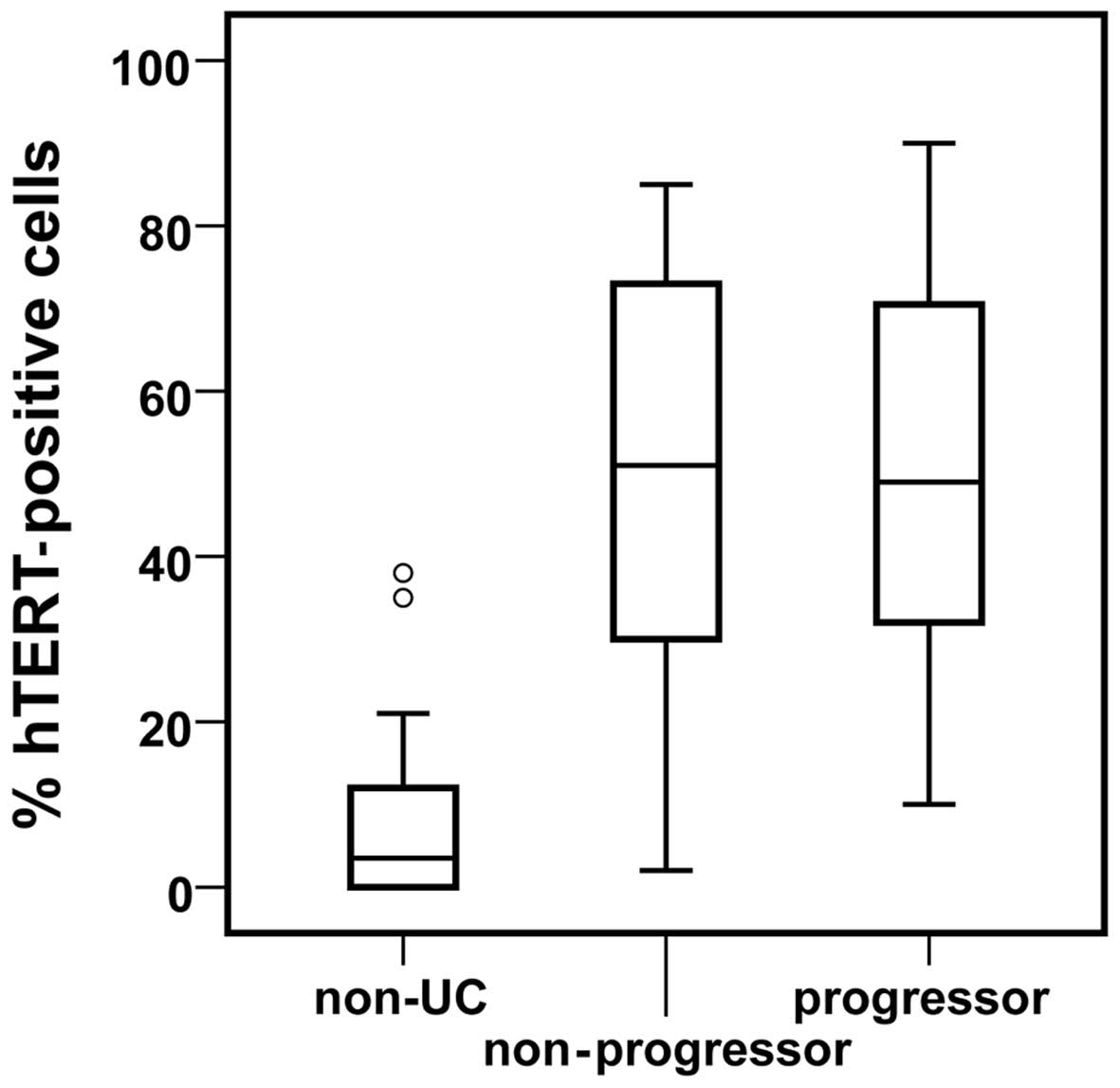
Levels of hTERT were significantly elevated
(p<0.001) in the colonic mucosa of progressors and
non-progressors, compared to non-UC controls (Fig. 3). No difference was observed
comparing progressor and non-progressor colectomies. Statistically
elevated levels of Ki67 in overall UC colons compared to non-UC
controls have been previously presented (25).
Levels of hTERT within the colonic mucosa
of progressor colectomies
The progressors were divided according to age at
onset, as it has been recently shown that progressors with late
onset of UC (> 50 years old) differed in telomere biology from
progressors with early onset of UC (<50 years old) (26). The results yielded no statistical
difference in the protein levels of hTERT when comparing late and
early onset UC (p=0.2).
No statistically significant difference in the
levels of hTERT expression was detected between diploid lesions and
lesions presenting aneuploidy, without correcting for differences
in mucosal morphology (p=0.12). No significant differences in hTERT
levels were identified between non-dysplastic lesions, lesions
indefinite for dysplasia and dysplastic lesions without correcting
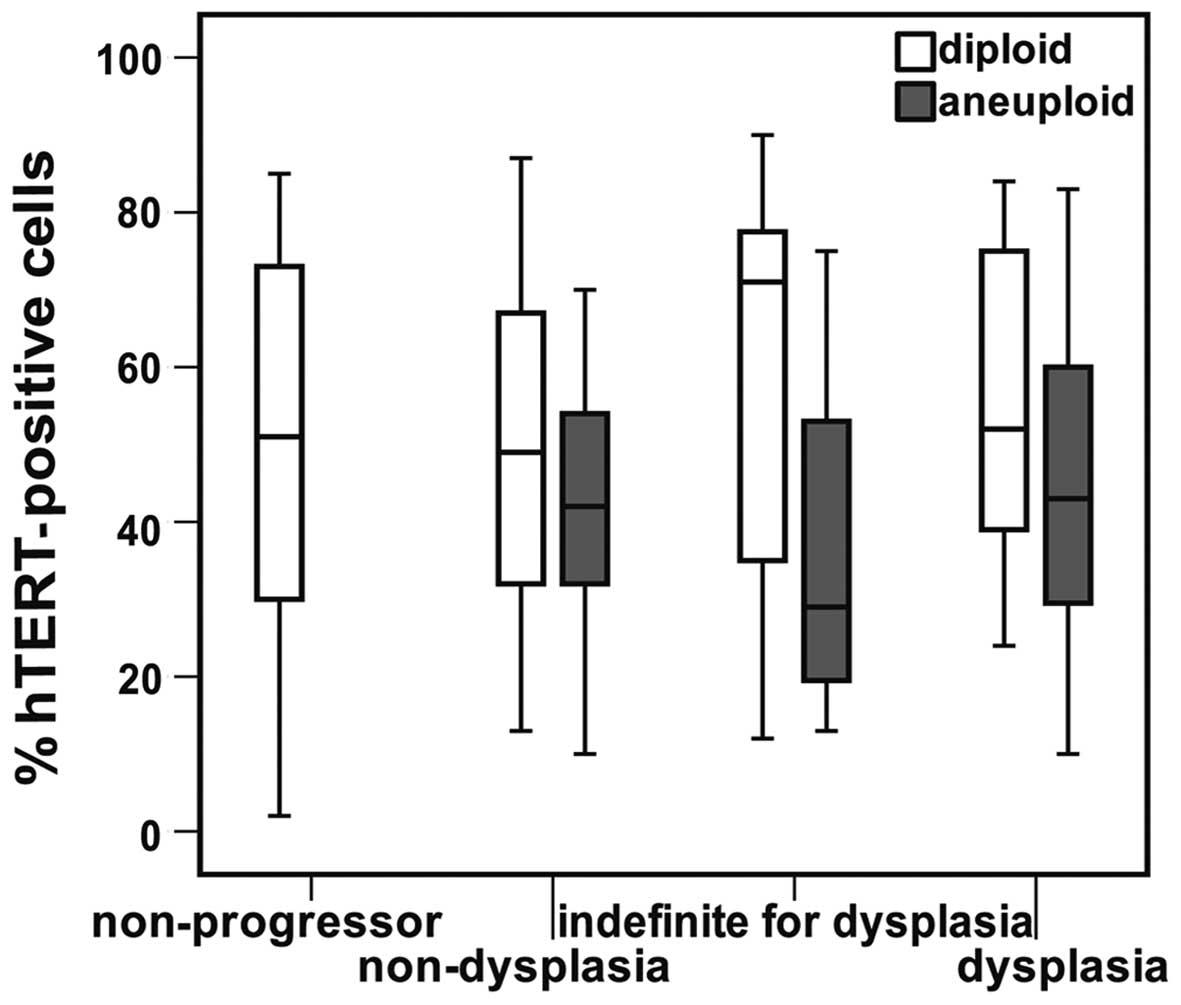
for DNA-ploidy status (p=0.14). However, when stratifying for
mucosal morphology and comparing hTERT protein levels within
diploid lesions with those harbouring aneuploid populations, we
found that the aneuploid lesions tended to have less hTERT
expression than the diploid counterparts (Fig. 4).
Within the non-dysplastic aneuploid and diploid
lesions of the progressor colons the hTERT levels differed to a
statistically significant extent (p=0.037) when using LMM
accounting for the differences between the patients. The p-values
detected using LMM for DNA-ploidy status within each morphologic
group are presented in Table II.
By ignoring patient differences, and using a t-test, a
statistically significant difference was found between hTERT levels
stratified for DNA-ploidy status within the lesions scored as
indefinite for dysplasia. Diploid lesions had higher levels of
hTERT than aneuploid lesions.
 | Table IILMM test p-values for hTERT protein
levels stratified for DNA-ploidy status within different
morphologic stages from progressors. |
Table II
LMM test p-values for hTERT protein
levels stratified for DNA-ploidy status within different
morphologic stages from progressors.
| Morphology | p-value |
|---|
| Non-dysplasia | 0.037 |
| Indefinite for
dysplasia | 0.374 |
| Dysplasia | 0.565 |
Associations between protein levels of
hTERT and Ki67 in the colonic mucosa of progressors and
non-progressors
An association analysis between hTERT and Ki67
revealed statistically significant results within the
non-progressors (p=0.047) when using LMM, compensating for patient
variation. No association was detected within the different lesions
of the progressors (Table III).
No association was detected between the protein levels of hTERT and
Ki67 within the progressors when stratifying for DNA-ploidy
status.
 | Table IIIP-values generated from LMM analyses
for the association between hTERT and Ki67 protein expression in
UC-morphology. |
Table III
P-values generated from LMM analyses
for the association between hTERT and Ki67 protein expression in
UC-morphology.
| Morphology | p-value |
|---|
| Non-progressor | 0.047 |
| Non-dysplasia | 0.097 |
| Indefinite for
dysplasia | 0.102 |
| Dysplasia | 0.731 |
All analyses were also performed excluding all cases
harbouring adenocarcinomas. This did not alter the results to any
statistically significant degree.
Discussion
In the present study, we found significantly raised
levels of hTERT protein in the mucosa of both progressor and
non-progressor UC colectomies compared to non-UC control samples
(p<0.001), but no significant difference was detected between
hTERT levels in the progressor and non-progressor colectomies. UC
is reportedly a disease of accelerated aging of colonic mucosa
(27), with an elevated cell
division rate as documented by Greco et al (28). The fact that we detected similar
hTERT levels in progressor and non-progressor colectomies is
consistent with those studies, as the patients had suffered from UC
for >10 years. Elevated levels of hTERT were found in mildly
active UC in the mucosa of patients suffering from UC on average 6
years (29).
For examination of possible differences in the
levels of hTERT within the progressors we stratified the areas of
the 20 progressor colectomies by morphological characteristics, and
compared diploid areas with areas containing aneuploid clones,
using LMM. This comparison showed a pattern of lower hTERT
expression in aneuploid lesions within areas of similar morphology.
Within the non-dysplastic lesions this difference was statistically
significant (p=0.037). If each lesion was included in the analysis
as independent data entries (Student’s t-test), we found a
significant difference between hTERT levels in diploid and
aneuploid lesions indefinite for dysplasia. However, the protein
levels of hTERT vary between patients, a fact that potentially
affects our statistical findings, creating false positives. Several
of the aneuploid lesions of indefinite dysplastic morphology were
found within the same colon (Table
I), and this could skew our results. We therefore found the
p-values yielded by the LMM analysis controlling for patient
variations to be valid. The hTERT-protein expression in diploid,
non-dysplastic lesions did not differ from the levels found in the
non-progressors, which are all non-dysplastic and diploid. This
shows that when the confounding factor of differences in mucosal
morphology is accounted for, reduced levels of hTERT are linked to
DNA aneuploidy and possibly also associated with its development.
Increased levels of hTERT may enhance the proliferative activity of
the inflamed tissue harbouring increased levels of reactive oxygen
species (ROS), and possibly contribute to the development of
dysplasia and cancer. It has been demonstrated that UC colons have
enhanced cell proliferation (24)
and elevated levels of ROS (30).
Both these agents are reported to facilitate telomere shortening.
Too short or even missing telomeres can induce
breakage-fusion-bridge (BFB) cycles, which again can lead to
chromosomal instability (5) and
DNA aneuploidy (31,32). Elevated levels of BFB were shown
in UC-progressor colons, but DNA-ploidy status of the lesions
examined was not investigated (31). Activation of telomerase can
prevent BFB-cycles by adding telomeric sequences to short telomeres
or broken chromosome ends (33).
Our results showing less hTERT present in lesions that contain
aneuploid cell populations are consistent with these results.
UC progressors have been shown to differ in mean
telomere length depending on the patients’ age at disease onset,
where early onset (<50 years of age when diagnosed) harboured
shorter telomeres than those observed in UC progressors with later
UC onset (26). All our patients
had suffered from active colitis for >10 years at the time of
colectomy and all had presented extensive colitis. Only two
progressors were diagnosed with UC after the age of 50, and these
did not differ in hTERT expression from the patients diagnosed at
an earlier age.
It is possible that any differences in hTERT levels
of the colonic mucosa between the progressors and non-progressors
were levelled out by continuous impairment of the colonic mucosa
due to inflammation and regeneration. Telomeres in UC colonic
mucosa are reported to shorten more rapidly than in non-UC mucosa
(27,31), and activation of telomerase might
be a response to this attrition. This could indicate that hTERT
expression is not a biomarker for differentiating a progressor
colon from a non-progressor colon prior to colectomy.
A study of four colectomies from patients suffering
from UC for >20 years revealed a regional correlation between
dysplasia and telomerase activity measured by a version of the
ELISA. One patient did not present dysplasia or telomerase activity
(23). However, the ELISA method
used in the study detected assembled telomerase enzyme complexes,
and it was suggested that lack of detected telomerase activity in
some of the samples could be due to degradation of the RNA
component of the holoenzyme prior to sampling (23). IHC of hTERT may omit tissue-based
problems such as partial degradation, as it is based on visual
examination of stained formalin or alcohol-fixed, paraffin-embedded
tissue.
IHC facilitates the investigation of protein
expression in specific cell types within a tissue. This feature can
prove valuable when examining UC colons, where a high percentage of
leucocytes are generally present in the mucosa. Examining hTERT
protein expression by IHC allowed us to assess the differences in
the extent of hTERT expression in the colonic mucosal cells,
without the confounding contributions from mucosal leukocytes. In a
report examining coronary plaques, neutrophils were found to have
elevated levels of telomerase activity (34). This is confirmed in our study by
the presence of hTERT-positive leucocytes in the lamina propria
(Fig. 1).
However, immunohistochemical detection of hTERT has
proven to be a difficult task, as some antibodies can also bind to
other proteins not associated with telomerase activity (35), antibodies that are not
commercially available, or those that are commercially available
but have not been proven to be specific (i.e., non-specific
cytoplasmic rather than specific nuclear staining). As new
antibodies binding to hTERT have become available and tested for
binding specificity, reports of hTERT-expression have emerged.
Elevated levels of hTERT in precancerous lesions have been
identified in gastric tissue (36), and colonic adenocarcinomas
(37). The hTERT protein levels
of colonic mucosa may provide insight into the transition from
normal-looking mucosal morphology towards a possible colorectal
cancer, as normal colonic mucosa has low hTERT levels, whereas
colorectal cancers have high levels of hTERT (38). In our study the nuclear hTERT
staining was very distinct. The monoclonal hTERT antibody used was
specific, as confirmed by western blotting of several human cancer
cell lines that showed a single band at 127 kDa as expected
(Fig. 2). We have previously
shown, that progressors harboured significantly more ultra-short
telomeres compared to non-progressor colons, and that the
difference remained statistically significant when we compared the
diploid, non-dysplastic progressor lesions to the non-progressors.
In terms of mean telomere length, no difference was found between
progressors and non-progressors (39). Thus, an association between mean
telomere length and hTERT protein levels seems to exist, whereas no
association was observed between the amount of ultra-short
telomeres and levels of hTERT in longstanding UC.
In a previous study, our group showed that the
proliferation marker Ki67 was significantly elevated in UC colons
compared to non-UC control samples, thus confirming that
proliferation is enhanced in UC colonic mucosa (25). Also, protein expression of Ki67
has been shown to increase with advancing degree of growth fraction
due to the developing stage of dysplasia in the colonic mucosa of
the UC colon (40). We found that
hTERT expression was significantly associated with the expression
of Ki67 within the non-progressor lesions. Within the progressors
this association was lost, even when diploid, non-dysplastic
lesions were examined separately. Together with our findings of a
borderline significant p-value for association between hTERT and
Ki67 within progressor non-dysplasia, and no significance detected
within the increasing levels of distorted morphology (Table III), it seems the association
between proliferation and hTERT protein expression is lost during
the development of dysplasia. The lack of difference in hTERT
protein levels between progressors and non-progressors, together
with elevated amounts of ultra-short telomeres identified in the
progressor lesions and the hTERT/Ki67association found only within
non-progressors leads to the hypothesis that the positive
association between hTERT and Ki67 in the non-progressors may be a
protective agent against shortening of the cells telomeres.
In conclusion, we have shown that the protein levels
of hTERT were significantly elevated in the mucosa of progressors
and non-progressor UC colons compared to non-UC control samples. In
the progressor colons, aneuploid non-dysplastic lesions had a
significantly lower expression of hTERT than the diploid
non-dysplastic lesions, and diploid, non-dysplastic lesions did not
differ from the non-progressors with regard to expression of hTERT
protein in the colonic mucosal cells, thus low levels of hTERT
associated with aneuploidy. We also found that within the
non-progressors there was an association of hTERT expression and
expression of the proliferation marker Ki67. No association of
hTERT/Ki67 protein expression was detected in the progressors, even
when only diploid non-dysplastic lesions were examined, indicating
that the association of the two proteins may act as a protective
mechanism against the development of progressor characteristics
within a UC colon.
Acknowledgements
The authors would like to thank Thu Hong Thy Nguyen
for helping with western blotting. This study was made possible by
the generous funding from South-Eastern Norway Regional Health
Authority and by Stiftelsen UNI. These organisations had no role in
collecting, analysing or interpreting the data or in writing the
report.
References
|
1
|
Macdougall IP: The Cancer risk in
ulcerative colitis. Lancet. 2:655–658. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: a meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathy C, Schneider K, Chen YY, Varma M,
Terdiman JP and Mahadevan U: Gross versus microscopic pancolitis
and the occurrence of neoplasia in ulcerative colitis. Inflamm
Bowel Dis. 9:351–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gorfine SR, Bauer JJ, Harris MT and Kreel
I: Dysplasia complicating chronic ulcerative colitis: is immediate
colectomy warranted? Dis Colon Rectum. 43:1575–1581. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stenoien DL, Sen S, Mancini MA and
Brinkley BR: Dynamic association of a tumor amplified kinase,
Aurora-A, with the centrosome and mitotic spindle. Cell Motil
Cytoskeleton. 55:134–146. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammarberg C, Slezak P and Tribukait B:
Early detection of malignancy in ulcerative colitis. A
flow-cytometric DNA study. Cancer. 53:291–295. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyer KF, Nause SL, Freitag-Wolf S, et al:
Aneuploidy characterizes adjacent non-malignant mucosa of
ulcerative colitis-associated but not sporadic colorectal
carcinomas: a matched-pair analysis. Scand J Gastroenterol.
48:679–687. 2013. View Article : Google Scholar
|
|
8
|
Fozard JB, Quirke P, Dixon MF, Giles GR
and Bird CC: DNA aneuploidy in ulcerative colitis. Gut.
27:1414–1418. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meling GI, Clausen OP, Bergan A,
Schjølberg A and Rognum TO: Flow cytometric DNA ploidy pattern in
dysplastic mucosa, and in primary and metastatic carcinomas in
patients with longstanding ulcerative colitis. Br J Cancer.
64:339–344. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gerling M, Nousiainen K, Hautaniemi S, et
al: Aneuploidy-associated gene expression signatures characterize
malignant transformation in ulcerative colitis. Inflamm Bowel Dis.
19:691–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerling M, Meyer KF, Fuchs K, et al: High
frequency of aneuploidy defines ulcerative colitis-associated
carcinomas: a comparative prognostic study to sporadic colorectal
carcinomas. Ann Surg. Jun:42010.(Epub ahead of print).
|
|
12
|
Sauerwald A, Sandin S, Cristofari G,
Scheres SH, Lingner J and Rhodes D: Structure of active dimeric
human telomerase. Nat Struct Mol Biol. 20:454–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mukherjee S, Firpo EJ, Wang Y and Roberts
JM: Separation of telomerase functions by reverse genetics. Proc
Natl Acad Sci USA. 108:E1363–E1371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chadeneau C, Hay K, Hirte HW, Gallinger S
and Bacchetti S: Telomerase activity associated with acquisition of
malignancy in human colorectal cancer. Cancer Res. 55:2533–2536.
1995.PubMed/NCBI
|
|
16
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baykal A, Rosen D, Zhou C, Liu J and Sahin
AA: Telomerase in breast cancer: a critical evaluation. Adv Anat
Pathol. 11:262–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Usselmann B, Newbold M, Morris AG and
Nwokolo CU: Deficiency of colonic telomerase in ulcerative colitis.
Am J Gastroenterol. 96:1106–1112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Engelhardt M, Drullinsky P, Guillem J and
Moore MA: Telomerase and telomere length in the development and
progression of premalignant lesions to colorectal cancer. Clin
Cancer Res. 3:1931–1941. 1997.PubMed/NCBI
|
|
21
|
Kleideiter E, Friedrich U, Möhring A, et
al: Telomerase activity in chronic inflammatory bowel disease. Dig
Dis Sci. 48:2328–2332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Risques RA, Lai LA, Himmetoglu C, et al:
Ulcerative colitis-associated colorectal cancer arises in a field
of short telomeres, senescence, and inflammation. Cancer Res.
71:1669–1679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holzmann K, Klump B, Weis-Klemm M, et al:
Telomerase activity in long-standing ulcerative colitis. Anticancer
Res. 20:3951–3955. 2000.PubMed/NCBI
|
|
24
|
Myung SJ, Yang SK, Chang HS, et al:
Clinical usefulness of telomerase for the detection of colon cancer
in ulcerative colitis patients. J Gastroenterol Hepatol.
20:1578–1583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burum-Auensen E, De Angelis PM, Schjølberg
AR, Røislien J, Andersen SN and Clausen OP: Spindle proteins Aurora
A and BUB1B, but not Mad2, are aberrantly expressed in dysplastic
mucosa of patients with longstanding ulcerative colitis. J Clin
Pathol. 60:1403–1408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salk JJ, Bansal A, Lai LA, et al: Clonal
expansions and short telomeres are associated with neoplasia in
early-onset, but not late-onset, ulcerative colitis. Inflamm Bowel
Dis. 19:2593–2602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Risques RA, Lai LA, Brentnall TA, et al:
Ulcerative colitis is a disease of accelerated colon aging:
evidence from telomere attrition and DNA damage. Gastroenterology.
135:410–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Greco V, Lauro G, Fabbrini A and Torsoli
A: Histochemistry of the colonic epithelial mucins in normal
subjects and in patients with ulcerative colitis. A qualitative and
histophotometric investigation. Gut. 8:491–496. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sipos F, Galamb O, Herszényi L, et al:
Elevated insulin-like growth factor 1 receptor, hepatocyte growth
factor receptor and telomerase protein expression in mild
ulcerative colitis. Scand J Gastroenterol. 43:289–298. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roessner A, Kuester D, Malfertheiner P and
Schneider-Stock R: Oxidative stress in ulcerative
colitis-associated carcinogenesis. Pathol Res Pract. 204:511–524.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O’Sullivan JN, Bronner MP, Brentnall TA,
et al: Chromosomal instability in ulcerative colitis is related to
telomere shortening. Nat Genet. 32:280–284. 2002.PubMed/NCBI
|
|
32
|
Lo AW, Sabatier L, Fouladi B, Pottier L,
Ricoul M and Murnane JP: DNA amplification by
breakage/fusion/bridge cycles initiated by spontaneous telomere
loss in a human cancer cell line. Neoplasia. 4:531–538. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hackett JA and Greider CW: Balancing
instability: dual roles for telomerase and telomere dysfunction in
tumorigenesis. Oncogene. 21:619–626. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Narducci ML, Grasselli A, Biasucci LM, et
al: High telomerase activity in neutrophils from unstable coronary
plaques. J Am Coll Cardiol. 50:2369–2374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu YL, Dudognon C, Nguyen E, et al:
Immunodetection of human telomerase reverse-transcriptase (hTERT)
re-appraised: nucleolin and telomerase cross paths. J Cell Sci.
119:2797–2806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duarte MC, Babeto E, Leite KRM, et al:
Expression of TERT in precancerous gastric lesions compared to
gastric cancer. Braz J Med Biol Res. 44:100–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Simsek BC, Pehlivan S and Karaoglu A:
Human telomerase reverse transcriptase expression in colorectal
tumors: correlations with immunohistochemical expression and
clinicopathologic features. Ann Diagn Pathol. 14:413–417. 2010.
View Article : Google Scholar
|
|
38
|
Hiyama E, Hiyama K, Yokoyama T and Shay
JW: Immunohistochemical detection of telomerase (hTERT) protein in
human cancer tissues and a subset of cells in normal tissues.
Neoplasia. 3:17–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Friis-Ottessen M, Bendix L, Kølvraa S,
Norheim-Andersen S, De Angelis PM and Clausen OP: Telomere
shortening correlates to dysplasia but not to DNA aneuploidy in
longstanding ulcerative colitis. BMC Gastroenterol. 14:82014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andersen SN, Rognum TO, Bakka A and
Clausen OP: Ki-67: a useful marker for the evaluation of dysplasia
in ulcerative colitis. Mol Pathol. 51:327–332. 1998. View Article : Google Scholar : PubMed/NCBI
|