Introduction
The mammalian target of rapamycin (mTOR) is an
essential serine/threonine kinase that belongs to the
phosphoinositide-3-OH kinase (PI3K)-related kinase family (1). It is an intracellular protein that
mediates cell growth, proliferation, migration and survival and
promotes angiogenesis in many types of cancer (2,3).
The inhibition of mTOR affects pathway-mediated transcription and
translation, leading to cell cycle arrest and anti-angiogenesis. As
regards the antitumor mechanisms of mTOR inhibitors, certain
studies have indicated that inhibitors of Akt and its downstream
target, mTOR signaling, have antitumor effects as the inhibition of
this pathway contributes to the initiation of autophagy (4–6).
mTOR nucleates two complexes, the mTOR complex 1
(mTORC1) and the mTOR complex 2 (mTORC2). mTORC1 is activated by
growth factors, nutrients and the cellular energy status. mTORC1
increases mRNA translation through the activation of ribosomal
p70S6 kinase (p70S6K), the inhibition of eIF4E-binding protein
(4EPB1) and autophagy [autophagy-related protein (Atg)13].
Autophagy has gained attention due to its
paradoxical roles in cell survival and cell death, particularly in
the pathogenesis and treatment of cancer (7). The regulation of autophagy is highly
complex and includes input from the cellular environment through
the PI3K/Akt/mTOR pathway (8).
Not surprisingly, there is an intricate relationship between
autophagy and apoptosis. Recent studies have indicated that
autophagy can function as a self-defense mechanism in cells that
are subjected to antitumor agents and that blocking autophagy can
trigger the activation of apoptosis (9–11).
Mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated protein kinase (ERK) is a key
molecule in intracellular signal transducing pathways that
transport extracellular stimuli from the cell surface to the nuclei
(12). A previous study
demonstrated that U0126, a MAPK inhibitor, blocks MAPK/ERK
signaling and decreases cell proliferation in osteosarcoma (OS) and
malignant fibrous histiocytoma (MFH) (13).
The MAPK and mTOR pathways have multiple
cross-connections that allow the coupling of cell-cycle activation
to the regulation of protein translation. Rapamycin and its analogs
activate the MAPK pathway in human cancer, which indicates that
there is a novel mTORC1-MAPK feedback loop (14). On the other hand, other studies
have indicated that the inhibition of the mTORC1 signaling pathway
promotes the initiation of autophagy (15), and the PI3K/Akt/mTOR and MAPK/ERK
pathway are two significant signaling pathways regulating autophagy
(16). Therefore, we hypothesized
that the combination of rapamycin and MAPK inhibitors may enhance
the growth inhibitory effect on tumor cells.
Soft tissue sarcomas account for only 1% of adult
malignancies, 60% of which are located in the extremities. MFH is
one of the most common soft tissue sarcomas in adults; it was first
described in 1963 by Ozzello et al (17) and then in 1964 by O’Brien and
Stout (18). In recent years,
drugs that target specific molecules have been developed as
treatments for human malignancies, including these sarcomas
(19). These drugs often
selectively inhibit specific molecules, such as growth factor
receptors or intracellular signaling proteins that are related to
tumor proliferation, migration and/or metastasis (20). In this study, we focused on the
mTOR and MAPK/ERK signaling pathways.
The aim of the present study was to examine the
effects of the mTOR inhibitor, rapamycin, on Nara-H cells (an
MFH-derived cell line). We examined whether rapamycin affects the
suppression of the phosphorylation of proteins in the mTOR pathway
and/or the induction of autophagy though the activation of MAPK/ERK
in Nara-H cells. Furthermore, we examined whether the combination
of rapamycin and a MAPK inhibitor induces apoptosis in Nara-H
cells.
Materials and methods
Chemical reagents
Rapamycin (CCI-779) was purchased from Calbiochem
(San Diego, CA, USA), dissolved in dimethyl sulfoxide (DMSO) and
stored at −20°C. The MEK inhibitor, U0126, was purchased from
Promega (Madison, WI, USA), dissolved in DMSO, and stored at room
temperature.
Cell lines and cell culture
The Nara-H cells were purchased from ScienStuff Co.
(Nara, Japan). The Nara-H cell line was established from a myxoid
MFH of the uterus by Kiyozuka et al (21). The cells were grown in Dulbecco’s
modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA)
containing 10% fetal bovine serum (FBS; Sigma-Aldrich) and 100 U/ml
penicillin. The cells were routinely maintained at 37°C in a
humidified 5% CO2 atmosphere, and cultures were used at
the mid-log phase.
In vitro proliferation assay
Cell proliferation was determined by the CellTiter
96® AQueous One Solution Cell Proliferation assay
(Promega). The cells were trypsinized and seeded at a density of
approximately 1×104 cells/well in 96-well cell culture
plates with 200 μl culture medium containing 10% FBS and incubated
for 48 h. Following this initial incubation, the growth medium was
replaced with medium containing 10% FBS and rapamycin at a
concentration of 0, 0.4, 2, 10 or 50 μM. After 24 and 48 h, the
medium was removed, the cells were washed with phosphate-buffered
saline (PBS), and fresh medium containing
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) reagent (100 μl medium plus 20 μl MTS regent/well) was added
to each well. In the experiments testing the combined effect of
rapamycin and U0126, the cells were treated with 40 μM rapamycin
and 50 μM U0126 for 24 h. In the experiments testing the effect of
rapamycin or U0126, the cells were treated with 40 μM rapamycin or
50 μM U0126 for 24 h. The optical density was measured at 490 nm
with an automatic microplate reader after 2 h of further incubation
following the addition of the MTS reagent. The absorbency is
directly proportional to the number of living cells. The percentage
viability of each well was calculated. At least three independent
experiments were performed.
Western blot analysis
The cells were trypsinized and seeded at a density
of approximately 6×105 cells/well in 6-well cell culture
plates in 2 ml culture medium with 10% FBS. After 48 h, the cells
were treated with 10% FBS containing rapamycin at the concentration
of 0, 0.4, 2, 10 or 50 μM for 24 h. In the experiments testing the
combined effect of rapamycin and U0126, the cells were treated with
40 μM rapamycin and 50 μM U0126 for 24 h. In the experiments
testing the effect of rapamycin or U0126, the cells were treated
with 40 μM rapamycin or 50 μM U0126 for 24 h. Following treatment,
the culture medium was replaced with lysis buffer (Cell Signalling
Technology, Beverly, MA, USA), and the cells were lysed on ice for
20 min. The cell lysates were spun at 15,000 × g using the Tabletop
Micro Refrigerated Centrifuge 3500 (Kubota Shoji Co. Ltd., Tokyo,
Japan) at 4°C for 30 min. The supernatant was then collected as the
total cellular protein extract. The protein concentrations were
determined using the Protein Assay Bicinchoninate kit (Nacalai
Tesque, Inc., Kyoto, Japan) and standardized with bovine serum
albumin. The samples of total cellular protein were loaded onto a
SDS polyacrylamide gel (10% or 12.5% commercial precast gel; Wako,
Tokyo, Japan), and the proteins were separated by SDS-PAGE under
reducing conditions. The separated proteins were
electrophoretically transferred onto nitrocellulose membranes (GE
Healthcare Bio-Sciences, Piscataway, NJ, USA). The membranes were
blocked for 90 min in blocking buffer that contained tris-buffered
saline (TBS-T) and EzBblock Chemi (Atto Co., Tokyo, Japan). The
membranes were then incubated overnight at 4°C with primary
antibodies (Table I) that were
diluted in the blocking buffer. The specific HRP-conjugated
secondary antibody incubations were performed overnight at 4°C with
gentle agitation. Bound antibodies were detected by using the ECL
plus western blotting detection system (GE Healthcare Bio-Sciences)
and the LAS-1000 plus image analyzer (Fujifilm Co., Tokyo, Japan).
Specific signals were quantified by densitometric analysis using
NIH ImageJ software.
 | Table IPrimary antibodies used in western
blot analysis. |
Table I
Primary antibodies used in western
blot analysis.
| Target | Source | Host | Dilution | Secondary
antibody | Gel (%) |
|---|
| MEK1/2 | Cell Signaling | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| Phospho-MEK1/2 | Cell Signaling | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| p44/42 MAPK
(ERK1/2) | Cell Signaling | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| Phospho-ERK1/2 | Chemicon | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| p70S6 | Cell Signaling | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| Phospho-p70S6 | Cell Signaling | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| 4EBP1 | Cell Signaling | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| Phospho-4EBP1 | Cell Signaling | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| Cleaved-PARP | BD Biosciences | Mouse | 1:1,000 | Anti-mouse | 10 |
|
Cleaved-caspase-3 | Cell Signaling | Rabbit | 1:1,000 | Anti-rabbit | 12.5 |
| Atg5-Atg12
complex | BML, Inc. | Mouse | 1:1,000 | Anti-mouse | 10 |
| p62/SQSTM1 | BML, Inc. | Rabbit | 1:1,000 | Anti-rabbit | 10 |
| LC-3 | BML, Inc. | Rabbit | 1:1,000 | Anti-rabbit | 12.5 |
| α-tubulin | Sigma | Mouse | 1:1,000 | Anti-mouse | 10 |
RNA interference
The cells were trypsinized and seeded at a density
of approximately 1×106 cells/well in 6-well cell culture
plates in 2 ml culture medium with 10% FBS. After 48 h, the cells
were washed with PBS and transfected with mTOR small interfering
RNA (siRNA; mTOR siRNA1 was purchased from Cell Signalling
Technology) using the X-tremeGENE siRNA Transfection Reagent (Roche
Applied Science, Penzberg, Germany) according to the manufacturer’s
instructions. Following transfection, the cells were incubated for
48 h before the extraction of total RNA.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cells using Isogen
(Nippon Gene, Tokyo, Japan). The RNA was then reverse-transcribed
into cDNA using High Capacity cDNA Reverse Transcription kits
(Applied Biosystems, Foster City, CA, USA) for RT-PCR. Quantitative
PCR was performed on an Eco™ Real-Time PCR System (Illumina, Inc.,
San Diego, CA, USA) using Power SYBR®-Green PCR Master
Mix (Applied Biosystems). The primers (mTOR, Atg5 and Beclin 1) for
quantitative PCR were synthesized and validated by Hokkaido System
Science Co., Ltd (Hokkaido, Japan). Atg5 is a gene product required
for the formation of autophagosomes (22). Beclin 1 is also known as Atg6,
which plays a key role in autophagy (23). All primers used in these
preparations are listed in Table
II.
 | Table IIGene-specific primers used for
RT-PCR. |
Table II
Gene-specific primers used for
RT-PCR.
| Primer | Nucleotide
(5′→3′) |
|---|
| mTOR forward | GGA GCT CCA GCA CTA
TGT CA |
| mTOR reverse | TTT CCT CTC ATT GGC
ATC TG |
| Atg5 forward | GCT TGG AGT AGG TTT
GGC TT |
| Atg5 reverse | CAA GTT GGA ATT CGT
CCA AA |
| Beclin 1
forward | CTC TGG CCA ATA AGA
TGG GT |
| Beclin 1
reverse | CGG CAG CTC CTT AGA
TTT GT |
Fluorescence microscopy images of cells
expressing pEGFP-LC3
The cells were trypsinized and seeded at a density
of approximately 1×106 cells/well on 25-mm circular
coverslips (Matsunami Glass Ind. Ltd., Osaka, Japan) in 2 ml
culture medium with 10% FBS overnight. The cells were then
transfected with the pEGFP-LC3 plasmid using X-tremeGENE HP DNA
Transfection Reagent (Roche Applied Science) and incubated for 24
h. The pEGFP-LC3 plasmid was purchased from Addgene (Cambridge, MA,
USA; Addgene database plasmid 21073), which was provided by Dr
Tamotsu Yoshimori (Osaka University, Osaka, Japan). The cells were
then washed with PBS and treated with rapamycin and/or U0126 for 24
h. Following treatment, the cells were imaged in an Attofluor cell
chamber (Molecular Probes/Invitrogen Life Technologies, Carlsbad,
CA, USA) on the thermo-controlled stage (Tokai Hit INU-ONI,
Shizuoka, Japan) of an inverted epifluorescence microscope (Carl
Zeiss LSM 700 confocal laser scanning microscope; Carl Zeiss Inc.,
Thornwood, NY, USA). Autophagy was evaluated by examining the
punctate forms (type II) of the autophagic marker, LC3, based on
pEGFP-LC3. Autophagy was quantitated by the percentage of
GFP-LC3-positive autophagic vacuoles or cells with LC3 punctate
dots.
Fluorescence microscopy images of
Annexin-V FITC-stained cells
The cells were trypsinized and seeded at a density
of approximately 1×106 cells/well on 25-mm circular
coverslips in 2 ml culture medium with 10% FBS for 48 h. The cells
were then washed with PBS and treated with rapamycin and/or U0126
for 24 h. Following treatment, the cells were incubated with
Annexin V-FITC and PI using an Annexin-V-Fluos Staining kit (Roche
Applied Science) for 15 min in a dark room. The cells then were
imaged in an Attofluor cell chamber on the thermo-controlled stage
of an inverted epifluorescence microscope, as described above.
Electron microscopy
The cells were trypsinized and seeded at a density
of approximately 1×104 cells/well on a
Lab-Tek® Chamber Slide (Nalge Nunc International,
Naperville, IL, USA) in 1 ml culture medium with 10% FBS for 48 h.
The cells were then treated with rapamycin and rapamycin plus U0126
for 24 h. For transmission electron microscopy, the treated cells
were washed and fixed with 2.5% glutaraldehyde in 0.1 M phosphate
buffer (pH 7.4) for 2 h and then post-fixed with 1% osmium
tetroxide in the same buffer for 2 h. They were dehydrated in a
graded series of ethanol and embedded in Epon 812. Ultrathin
sections were stained with 4% uranyl acetate and lead citrate and
observed under an electron microscope (JEM-1400; Jeol, Tokyo,
Japan) at 80 kV.
Statistical analysis
Statistical analyses for the cell proliferation
assay and quantitative PCR were performed using GraphPad Prism 5
software (GraphPad, San Diego, CA, USA) with one- or two-way ANOVA,
followed by post-hoc analysis. A value of p<0.05 was considered
to indicate a statistically significant difference.
Results
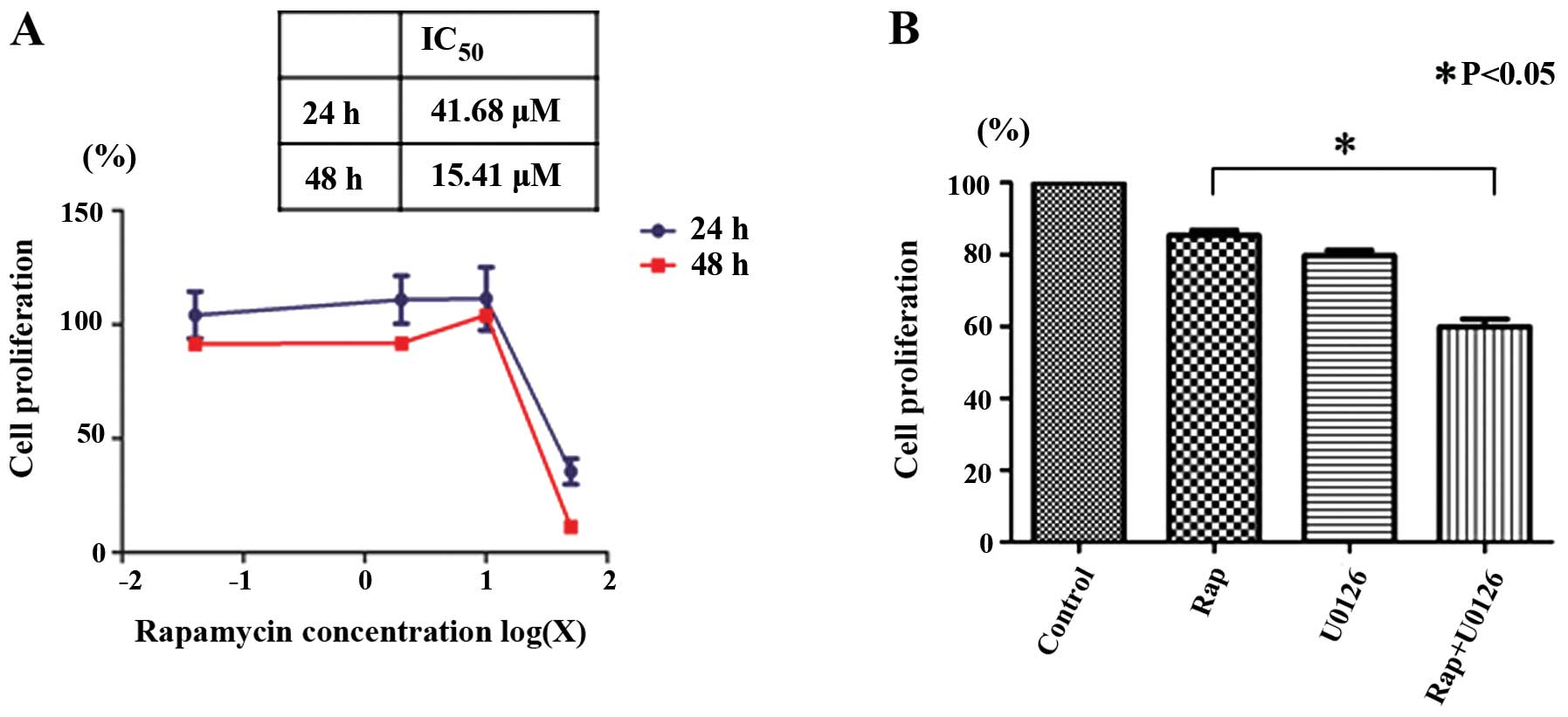
Rapamycin inhibits the proliferation of
Nara-H cells
We examined the effects of rapamycin on Nara-H cell
proliferation using the CellTiter 96® AQueous One
Solution Cell Proliferation assay to determine whether rapamycin
inhibits cell proliferation. Rapamycin inhibited Nara-H cell
proliferation in a dose- and time-dependent manner. The
IC50 for 24 h of rapamycin treatment in the Nara-H cells
was 41.68 μM (Fig. 1A).
Rapamycin-induced Nara-H cell death is
enhanced by U0126
We then examined the effects of rapamycin with or
without U0126 on Nara-H cell proliferation. Based on the
IC50 of rapamycin after 24 h, we examined the
proliferation of the Nara-H cells treated with 40 μM rapamycin (Rap
group), 50 μM U0126 (U0126 group), and 40 μM rapamycin plus 50 μM
U0126 (Rap + U0126 group) for 24 h. Cell proliferation was
significantly lower in the Rap + U0126 group than in the Rap group
(p<0.05) (Fig. 1B). These
results indicate that U0126 enhances the rapamycin-induced
suppression of Nara-H cell proliferation.
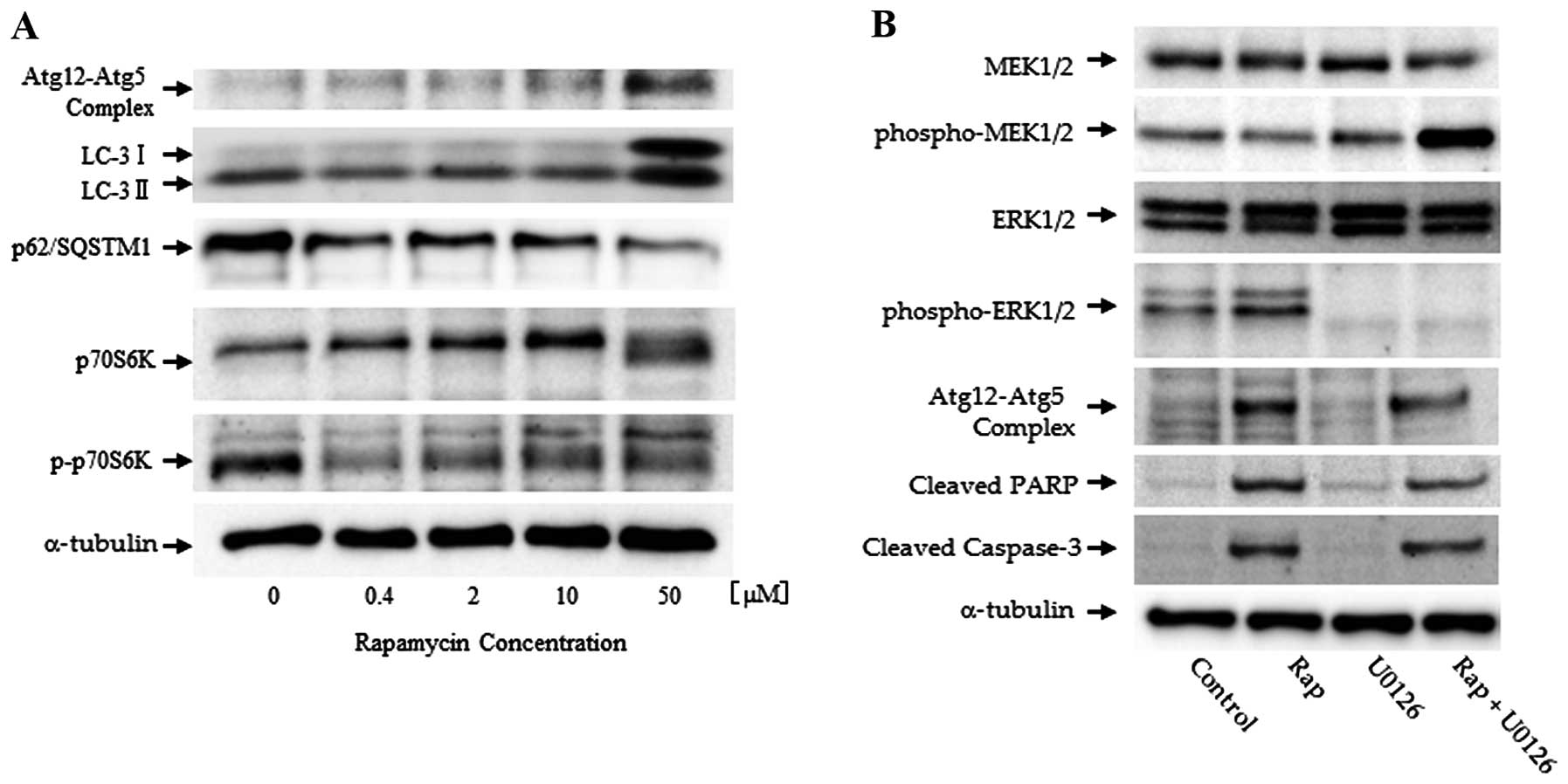
Western blot analysis
Western blot analysis demonstrated that treatment
with rapamycin induced the phosphorylation of p70S6K, one of the
key components in the mTOR pathway. Additionally, we examined the
expression of the Atg12-Atg5 autophagy-related gene complex,
p62/SQSTM1, and LC-3 in Nara-H cells exposed to various
concentrations of rapamycin (ranging from 0.4 to 50 μM) for 24 h
(Fig. 2A). Treatment with
rapamycin resulted in a dose-dependent decrease in the levels of
phospho-p70S6K, which is a downstream effector of mTOR. These
findings indicate that rapamycin affects the mTOR pathway by
inhibiting the phosphorylation of downstream effectors of mTOR.
LC-3II and Atg12-Atg5 complex expression was used as an autophagic
marker. p62/SQSTM1 is a polyubiquitin-binding protein, which is
degraded by autophagy (24).
Treatment with rapamycin resulted in a dose-dependent increase in
the expression of LC-3II and the Atg12-Atg5 complex in the Nara-H
cells. On the contrary, p62/SQSTM1 expression was decreased in a
dose-dependent manner (Fig.
2A).
Subsequently, the MAPK signaling pathway and the
expression of autophagic markers were evaluated. LC-3II expression
was increased by treatment with rapamycin. Simultaneously, the
phosphorylation of ERK1/2, which is one of the downstream effectors
of MAPK/ERK, was increased by rapamycin. In the cells treated with
rapamycin and rapamycin plus U0126, cleaved PARP and cleaved
caspase-3 were strongly expressed (Fig. 2B).
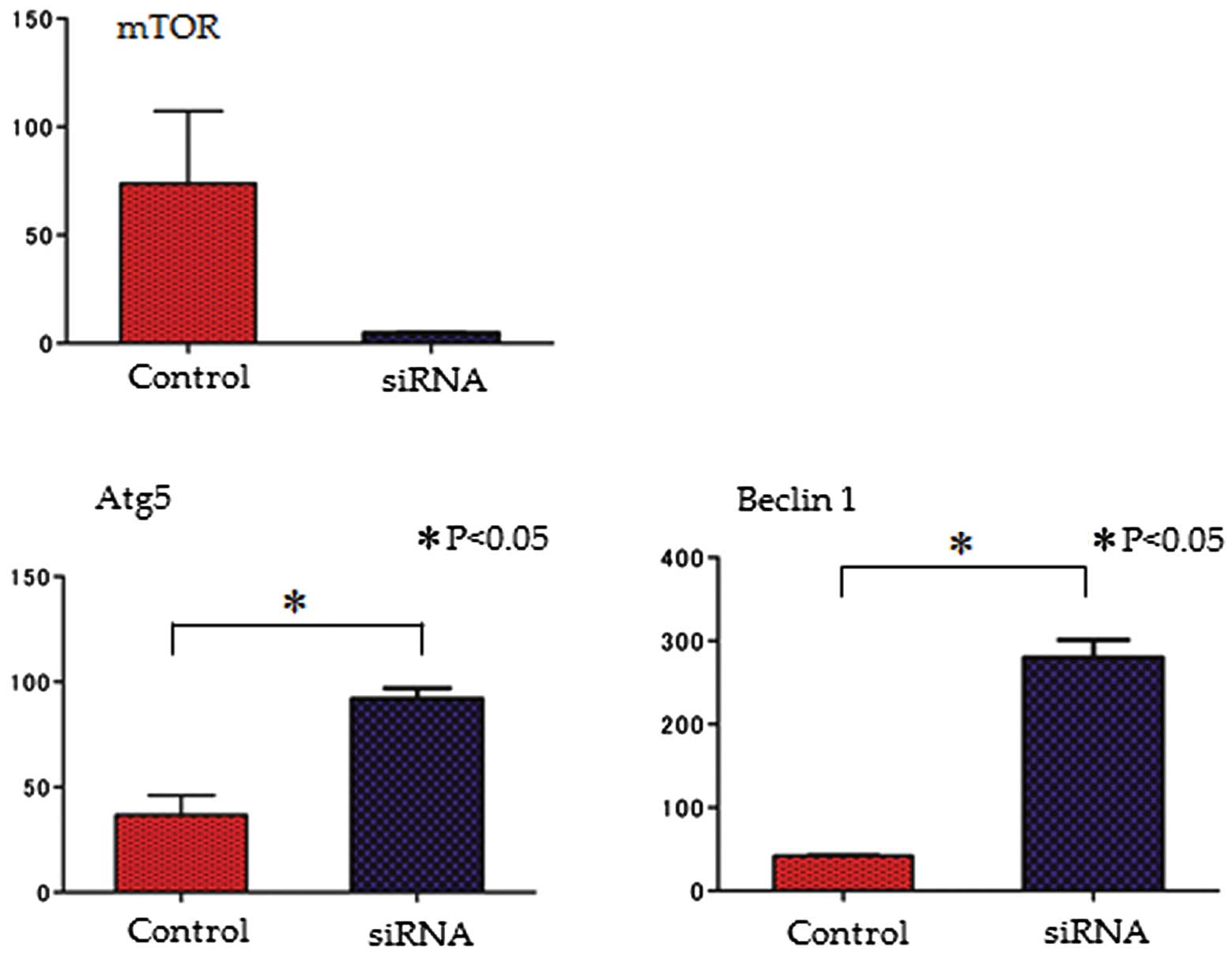
Quantitative PCR
To determine the effects of the knockdown of the
mTOR signaling pathway, the mRNA expression of mTOR, Atg5 and
Beclin 1 was evaluated following transfection with mTOR siRNA. The
expression of both autophagy-related genes (Atg5 and Beclin 1) was
increased by mTOR siRNA; on the contrary, the mRNA expression of
mTOR was decreased (Fig. 3).
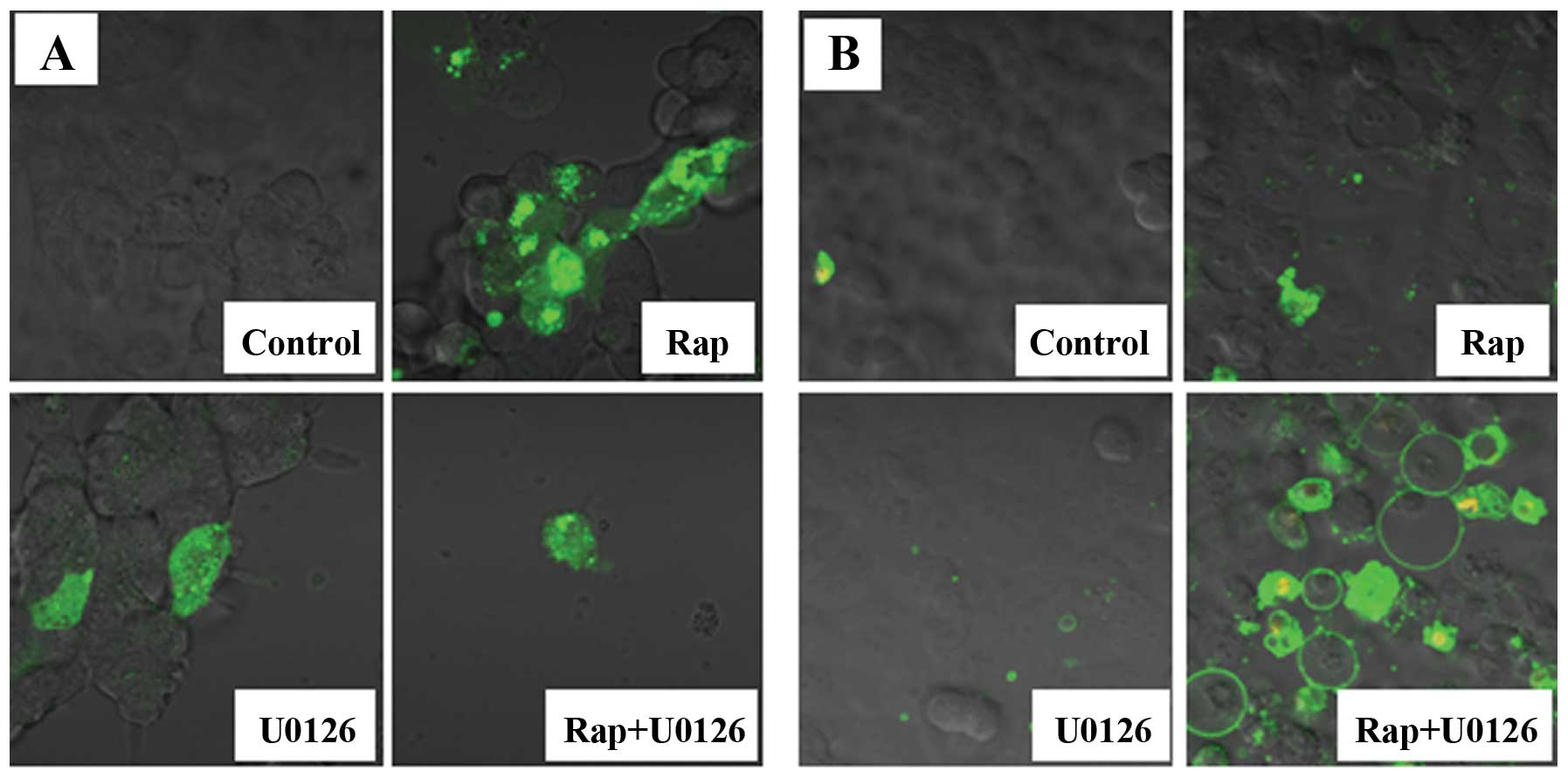
Fluorescence microscopy images
The expression of pEGFP-LC3 by fluorescence
microscopy, in which green fluorescent protein (GFP) is expressed
as a fusion protein at the amino terminus of LC3, was used to
evaluate autophagy. pEGFP-LC3 dot formation was markedly increased
in the Rap group (Fig. 4A).
We then used Annexin V-FITC and PI to detect the
apoptotic cells. Annexin V-FITC is a marker for early apoptosis,
and PI is a marker for late apoptosis and necrosis. We observed
several Annexin V-FITC-positive cells (early stage of apoptosis)
and high number of Annexin V-FITC plus PI-positive cells (late
stage of apoptosis) in the Rap + U0126 group (Fig. 4B). The number of apoptotic cells
was greatly increased in the Rap + U0126 group as compared to the
controls, the U0126 group or the Rap group.
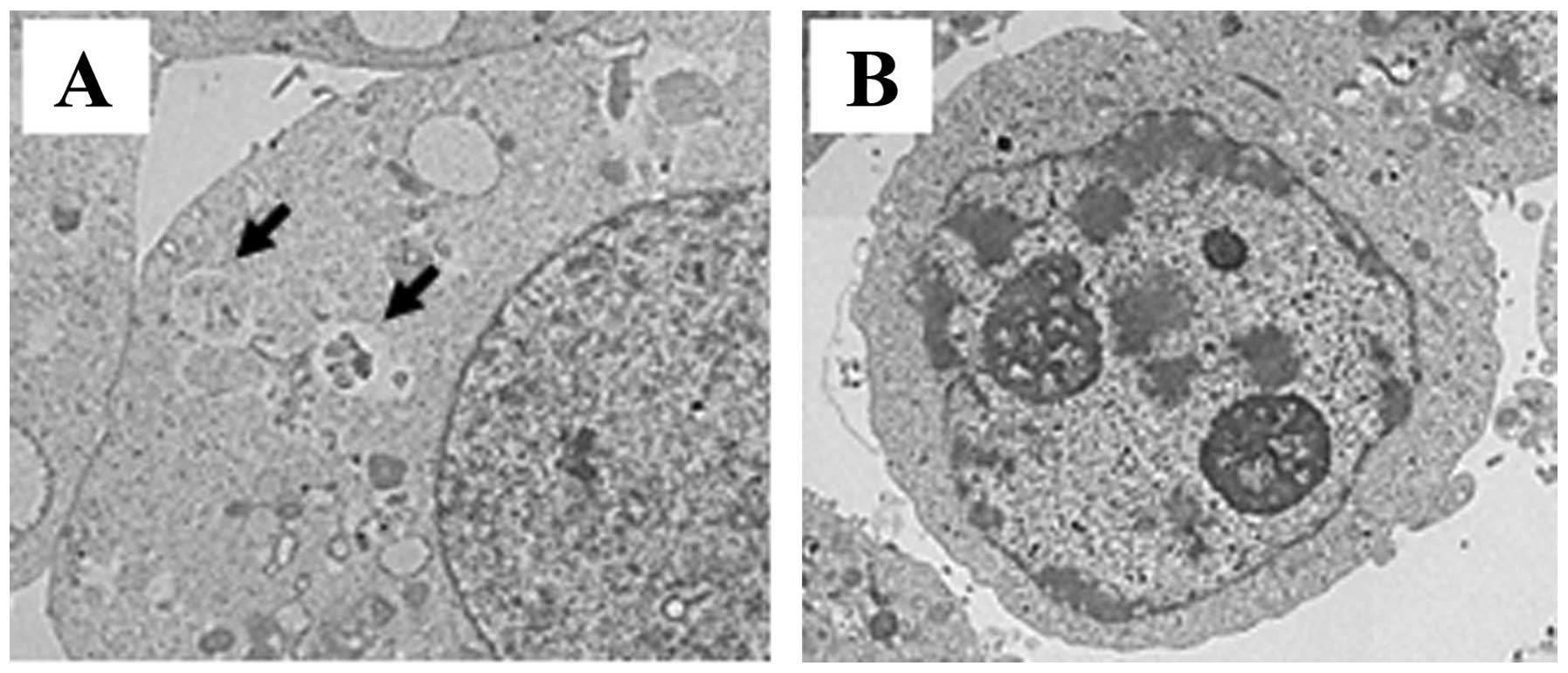
Electron microscopy
Electron microscopy revealed that the autophagosomes
were detected on the rapamycin-treated cells (Fig. 5A). Nuclear fragmentation and
chromatin condensation in the nucleus were detected in the Rap +
U0126 grou (Fig. 5B). Chromatin
condensation and oligonucleosomal DNA fragmentation are the nuclear
hallmarks of apoptosis (25).
Discussion
Soft tissue sarcomas
Soft tissue sarcomas, particularly high-grade
sarcomas, such as MFH, are clinically aggressive and frequently
metastasize to various organs. In the absence of effective systemic
chemotherapeutic treatments for aggressive sarcomas, targeted
therapies are being investigated and used as treatments. Targeted
drugs, including mTOR inhibitors, are currently being tested as
single agents or in combination with other agents, such as
autophagic inhibitors, for the treatment of sarcomas (26–28).
mTOR pathway
A variety of cell signaling events in the PI3K/Akt
pathway are mediated by mTOR, and this pathway plays a central role
in cell survival and proliferation in many types of cancer
(27). mTOR exerts its biological
functions as being part of two different protein complexes, mTORC1
and mTORC2. mTORC1 regulates autophagy, consisting of the mTOR
catalytic subunit, regulatory associated protein of mTOR (raptor),
G protein β-subunit-like protein (GβL, also known as mLST8) and
proline-rich Akt substrate of 40 kDa (PRAS40). mTORC2 consists of
mTOR, rapamycin-insensitive companion of mTOR (rictor), GβL,
SAPK-interacting protein 1 (SIN1) and protein observed with rictor
(PROTOR) and is not a direct regulator of autophagy (29,30). Rapamycin, a specific inhibitor of
mTORC1, has been shown to selectively and completely block the
mTORC1-dependent p70S6K phosphorylation and partially block 4EBP1
phosphorylation (31).
mTOR inhibition and induction of
autophagy
As previously demonstrated, the inhibition of the
Akt/mTOR pathway is consistently associated with triggering
autophagy in cancer cells (32).
Recent studies have also indicated that autophagy can function as a
self-defense mechanism in cells that are subjected to antitumor
agents and that blocking autophagy can trigger the activation of
apoptosis (9–11). Based on these findings, it has
been suggested that inhibitors of autophagy, such as
3-methyladenine (3-MA), may be an effective treatment for MFH as
they activate apoptosis. In our previous study, we demonstrated
that the combination of an mTOR inhibitor and an autophagic
inhibitor (temsirolimus and 3-MA, respectively) was an effective
treatment for Nara-H cells as this combination effectively
activated the apoptotic pathways (27). In the present study, our results
revealed that rapamycin suppressed the phosphorylation of p70S6K;
on the other hand, rapamycin increased the expression of the
Atg12-Atg5 complex and LC-3II. These results indicate that
suppression of the mTOR pathway is involved in the induction of
autophagy.
Rapamycin-induced autophagy and
activation of MAPK pathway
As a critical signaling pathway involved in
tumorigenesis, the MAPK/ERK signaling pathway is often activated in
numerous types of cancer cells. Accumulating evidence indicates
that the activation of ERK1/2 is associated with autophagy
(33). Saini et al
reported that there is a significant level of cross-talk between
the mTOR and MAPK/ERK pathways and that the inhibition of one
cascade activates the other (34). Thus, the inhibition of the
Raf/MEK/ERK pathway leads to increased activity in the
PI3K/AKT/mTOR pathway, possibly through the loss of feedback
inhibition. Similarly, blocking the PI3K/AKT/mTOR pathway leads to
increased activity in the MAPK/ERK pathway (34). In the present study, we examined
the expression and constitutive phosphorylation of molecules
involved in the MAPK/ERK signaling pathway in Nara-H cells. We
found that rapamycin indeed activated the ERK1/2 and Atg12-Atg5
complex. These results indicate that rapamycin induces autophagy
and activates the MAPK/ERK signaling pathway.
mTOR inhibitor and MAPK inhibitor
The present study demonstrates that apoptotic cell
death and strong anticancer efficacy result from the use of MAPK
inhibitors, inhibiting autophagy. Apoptotic cell death in the
Nara-H cells was illustrated by the expression of cleaved caspase-3
and PARP by western blot analysis (Fig. 2B) and by fluorescence microscopy
(Fig. 4B) and electron microscopy
(Fig. 5). The inhibition of
MEK1/2 by U0126 inhibited the expression of the Atg12-Atg5 complex,
thus enhancing the cytotoxic effects of rapamycin. The inhibition
of mTORC1 or mTORC2 by the transient or moderate activation of
MEK/ERK moderately enhanced Beclin 1 expression, resulting in
cytoprotective autophagy, whereas the inhibition of both mTORC1 and
mTORC2 by sustained MEK/ERK activation strongly induced Beclin 1
expression, leading to cytodestructive autophagy (35). Indeed, Beclin 1 gene expression in
the Nara-H cells was increased follwoing the knockdown of mTOR by
siRNA (Fig. 3B). Therefore, it is
thought that the combination of mTOR inhibitors and MAPK inhibitors
may result in strong antitumor effects (14).
In conclusion, the present study demonstrates that
rapamycin induces cytoprotective autophagy in Nara-H cells by
activating the MEK/ERK signaling pathway and that rapamycin-induced
apoptosis can be enhanced by MEK inhibitors. These results suggest
that self-protective mechanisms involving mTOR inhibitors in Nara-H
cells are prevented by the inhibition of the MEK/ERK pathway. In
other words, the MEK/ERK signaling pathway is activated by
rapamycin as a self-defense mechanism, and cytoprotective autophagy
is induced. The combination of an mTOR inhibitor (e.g., rapamycin)
and an MEK inhibitor (e.g., U0126) may offer effective treatment
for MFH, as this combination effectively activates apoptotic
pathways.
Acknowledgements
The authors thank Dr Tamotsu Yoshimori (Osaka
University, Osaka, Japan) for his gift of the pEGFP-LC3 plasmid and
Mr. Toshitaka Nakagawa (Division of Research Instrument and
Equipment, Kagawa University School of Medicine, Kagawa, Japan) for
providing technical assistance with fluorescence microscopy and
electron microscopy.
References
|
1
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar
|
|
2
|
Gridelli C, Maione P and Rossi A: The
potential role of mTOR inhibitors in non-small cell lung cancer.
Oncologist. 13:139–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mita MM and Tolcher AW: The role of mTOR
inhibitors for treatment of sarcomas. Curr Oncol Rep. 9:316–322.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KW, Mutter RW, Cao C, et al: Autophagy
for cancer therapy through inhibition of pro-apoptotic proteins and
mammalian target of rapamycin signaling. J Biol Chem.
281:36883–36890. 2006. View Article : Google Scholar
|
|
5
|
Hung JY, Hsu YL, Li CT, et al: 6-Shogaol,
an active constituent of dietary ginger, induces autophagy by
inhibiting the AKT/mTOR pathway in human non-small cell lung cancer
A549 cells. J Agric Food Chem. 57:9809–9816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan ML, Ooi JP, Ismail N, Moad AI and
Muhammad TS: Programmed cell death pathways and current antitumor
targets. Pharm Res. 26:1547–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amaravadi RK and Thompson CB: The roles of
therapy-induced autophagy and necrosis in cancer treatment. Clin
Cancer Res. 13:7271–7279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pattingre S, Espert L, Biard-Piechaczyk M
and Codogno P: Regulation of macroautophagy by mTOR and Beclin 1
complexes. Biochimie. 90:313–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu D, Yang Y, Liu Q and Wang J:
Inhibition of autophagy by 3-MA potentiates cisplatin-induced
apoptosis in esophageal squamous cell carcinoma cells. Med Oncol.
28:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanematsu S, Uehara N, Miki H, et al:
Autophagy inhibition enhances sulforaphane-induced apoptosis in
human breast cancer cells. Anticancer Res. 30:3381–3390.
2010.PubMed/NCBI
|
|
11
|
Ren Y, Huang F, Liu Y, Yang Y, Jiang Q and
Xu C: Autophagy inhibition through PI3K/Akt increases apoptosis by
sodium selenite in NB4 cells. BMB Rep. 42:599–604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito Y, Sasaki Y, Horimoto M, et al:
Activation of mitogen-activated protein kinases/extracellular
signal-regulated kinases in human hepatocellular carcinoma.
Hepatology. 27:951–958. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki K, Hitora T, Nakamura O, Kono R and
Yamamoto T: The role of MAPK pathway in bone and soft tissue
tumors. Anticancer Res. 31:549–553. 2011.PubMed/NCBI
|
|
14
|
Carracedo A, Ma L, Teruya-Feldstein J, et
al: Inhibition of mTORC1 leads to MAPK pathway activation through a
PI3K-dependent feedback loop in human cancer. J Clin Invest.
118:3065–3074. 2008.PubMed/NCBI
|
|
15
|
Huang S, Yang ZJ, Yu C and Sinicrope FA:
Inhibition of mTOR kinase by AZD8055 can antagonize
chemotherapy-induced cell death through autophagy induction and
down-regulation of p62/sequestosome 1. J Biol Chem.
286:40002–40012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto M, Suzuki SO and Himeno M:
Resveratrol-induced autophagy in human U373 glioma cells. Oncol
Lett. 1:489–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozzello L, Stout AP and Murray MR:
Cultural characteristics of malignant histiocytomas and fibrous
xanthomas. Cancer. 16:331–344. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O’Brien JE and Stout AP: Malignant fibrous
xanthomas. Cancer. 17:1445–1455. 1964.
|
|
19
|
Wardelmann E, Chemnitz JM and Wendtner CM:
Targeted therapy of soft tissue sarcomas. Onkologie. 35(Suppl 1):
21–27. 2012. View Article : Google Scholar
|
|
20
|
Arslan MA, Kutuk O and Basaga H: Protein
kinases as drug targets in cancer. Curr Cancer Drug Targets.
6:623–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiyozuka Y, Nakagawa H, Uemura Y, et al:
Novel cell lines established from a human myxoid malignant fibrous
histiocytoma arising in the uterus. Cancer Genet Cytogenet.
127:7–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yousefi S, Perozzo R, Schmid I, et al:
Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis.
Nat Cell Biol. 8:1124–1132. 2006. View
Article : Google Scholar
|
|
23
|
Perez-Carrion MD, Perez-Martinez FC,
Merino S, et al: Dendrimer-mediated siRNA delivery knocks down
Beclin 1 and potentiates NMDA-mediated toxicity in rat cortical
neurons. J Neurochem. 120:259–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pankiv S, Clausen TH, Lamark T, et al:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joselin AP, Schulze-Osthoff K and Schwerk
C: Loss of Acinus inhibits oligonucleosomal DNA fragmentation but
not chromatin condensation during apoptosis. J Biol Chem.
281:12475–12484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okuno S: Mammalian target of rapamycin
inhibitors in sarcomas. Curr Opin Oncol. 18:360–362. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura O, Hitora T, Akisue T, Kawamoto
T, Yamagami Y and Yamamoto T: Inhibition of induced autophagy
increases apoptosis of Nara-H cells. Int J Oncol. 39:1545–1552.
2011.
|
|
28
|
Li B, Takeda T, Tsuiji K, et al: Curcumin
induces cross-regulation between autophagy and apoptosis in uterine
leiomyosarcoma cells. Int J Gynecol Cancer. 23:803–808. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guertin DA and Sabatini DM: The
pharmacology of mTOR inhibition. Sci Signal. 2:pe242009.PubMed/NCBI
|
|
30
|
Yang Q and Guan KL: Expanding mTOR
signaling. Cell Res. 17:666–681. 2007. View Article : Google Scholar
|
|
31
|
Kim MS, Kuehn HS, Metcalfe DD and
Gilfillan AM: Activation and function of the mTORC1 pathway in mast
cells. J Immunol. 180:4586–4595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuo PL, Hsu YL and Cho CY: Plumbagin
induces G2-M arrest and autophagy by inhibiting the AKT/mammalian
target of rapamycin pathway in breast cancer cells. Mol Cancer
Ther. 5:3209–3221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han W, Sun J, Feng L, et al: Autophagy
inhibition enhances daunorubicin-induced apoptosis in K562 cells.
PLoS One. 6:e284912011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saini KS, Loi S, de Azambuja E, et al:
Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the
treatment of breast cancer. Cancer Treat Rev. 39:935–946. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Whiteman MW, Lian H, et al: A
non-canonical MEK/ERK signaling pathway regulates autophagy via
regulating Beclin 1. J Biol Chem. 284:21412–21424. 2009. View Article : Google Scholar : PubMed/NCBI
|