Irritable bowel syndrome (IBS) is a chronic
functional gastrointestinal disorder with a worldwide prevalence of
10–20% (1–15). The diagnosis of IBS is based
mainly on the assessment of the symptoms, which are abdominal
discomfort/pain, altered bowel habits and abdominal
bloating/distension (1,4). Patients with IBS can be subdivided
into four subtypes according to the Rome III criteria and based on
the stool pattern: diarrhea-predominant (IBS-D),
constipation-predominant (IBS-C), mixed diarrhea and constipation
(IBS-M) and unclassified IBS (U-IBS) (16,17).
IBS is usually diagnosed in younger patients (i.e.,
<50 years of age) and is more common in women than in men
(3–6,8,9,11,
12,14,15,18,19). Although IBS is not known to be
associated with the development of serious disease or with excess
mortality, it considerably reduces the quality of life of patients
(1,19–21). In addition to the increased
morbidity caused by IBS, this condition represents an economic
burden to society as a result of the overconsumption of healthcare
resources by and low productivity of IBS patients (22).
IBS patients often associate their symptoms with
specific food items, such as milk and milk products, wheat
products, caffeine, certain meats, cabbage, onion, peas/beans, hot
spices, fried foods and smoked foodstuffs (23–25). However, surveys of the diets of
IBS patients have failed to detect any differences in diet
composition between IBS patients and the community as regards the
intake of energy, carbohydrates, proteins and fats (26–32). However, a study on food
intolerance and IBS found that 62% of the subjects had either
limited or excluded food items from their daily intake, and 12% of
these subjects had made such drastic changes in their diet that
nutritional deficiencies could be foreseen in the long term
(33).
Certain studies have found IBS patients to be
intolerant to various alcoholic beverages and generally have a low
alcohol consumption (23,29). However, other studies found that
the alcohol intake in patients with IBS was the same as or higher
than that in the background population (30,31). The common belief among IBS
patients is that lactose is the main cause of their symptoms, and
consequently, they often reduce their intake of milk and milk
products (29,31,34,35). Milk and other dairy products are
the most important dietary source of calcium, vitamin B2
(riboflavin) and phosphorus in the Western world (36). Thus, while IBS patients consume
more products that are alternatives to milk, such as soy, rice and
oat milk, they have a low daily intake of calcium, vitamin B2 and
phosphorus (29).
IBS patients have a lower consumption of foods known
to be rich in fermentable oligo-, di- and monosaccharides, and
polyols (FODMAPs), such as spaghetti, pasta, rice, millet, couscous
and buns than healthy controls (29). Moreover, IBS patients have lower
consumptions of certain vegetables (raw vegetables, raw broccoli,
paprika, onion, leeks, garlic, cabbage, tomatoes, mushrooms and
green beans) (29). On the other
hand, they consume more FODMAP-rich fruits and vegetables, such as
grapes, pears, peaches, peas, mango, plums and melon (29).
The importance of dietary factors and the
associations between diet and symptoms in IBS have been discussed
in the literature (23,37–41). The aim of this review was to shed
light on the possible interaction between dietary intake and gut
hormones, and the importance of diet management in reducing the
symptoms and improving the quality of life of IBS patients.
The effect of diet on IBS symptoms may be attributed
to the interaction between poorly absorbed carbohydrates/fiber and
the intestinal bacterial flora, or between ingested nutrients and
the gut neuroendocrine system, and food allergy or intolerance.
Certain short-chain carbohydrates (FODMAPs) are
poorly absorbed, resulting in a significant proportion of them
reaching the distal small bowel and colon (42,43), where they provide a substrate for
bacterial fermentation. This results in the production of gas, with
the consequent distension of the large intestine and increased
intraluminal pressure. FODMAPs include fructose, lactose, sugar
alcohols (sorbitol, maltitol, mannitol, xylitol and ismalt),
fructans and galactans. Fructose and lactose are present in apples,
pears, watermelon, honey, fruit juices, dried fruits, as well as
milk and milk products. Polyols are used in low-calorie food
products. Galactans and fructans are present in wheat, rye, garlic,
onions, legumes, cabbage, artichokes, leeks, asparagus, lentils,
inulin, soy, Brussels sprouts and broccoli (39,40,44). A low intake of FODMAPs has been
found to reduce the gastrointestinal symptoms in patients with IBS
(42,43,45,46).
Increasing the intake of dietary fiber is a standard
recommendation for patients with IBS (47). However, in clinical practice,
increased fiber intake in these patients has been shown to increase
the symptoms of abdominal pain, bloating and distension. The
examination of the effects of fiber intake on IBS symptoms has
revealed that increased fiber intake does not improve symptoms
compared with a placebo or a low-fiber diet (47). However, it has been reported that
the intake of soluble fiber is effective in improving overall IBS
symptoms relative to consuming insoluble fiber (47–50).
The effects of FODMAPs and fiber on IBS symptoms are
strongly associated with the intestinal flora. The dominance of
Clostridium spp. in the intestinal flora, which break down
FODMAPs and fiber, results in gas production, with a consequent
increase in the distension of the large intestine, causing
abdominal discomfort or pain. Food supplements with beneficial
bacteria, such as Lactobacillus spp. and
Bifidobacterium spp. would result in a greater tolerance to
both FODMAPs and fiber, since these bacteria do not produce gas on
fermenting carbohydrates. It has been reported that the intestinal
flora of IBS patients comprise fewer Lactobacillus spp. and
Bifidobacterium spp. than the flora of healthy individuals
(51,52).
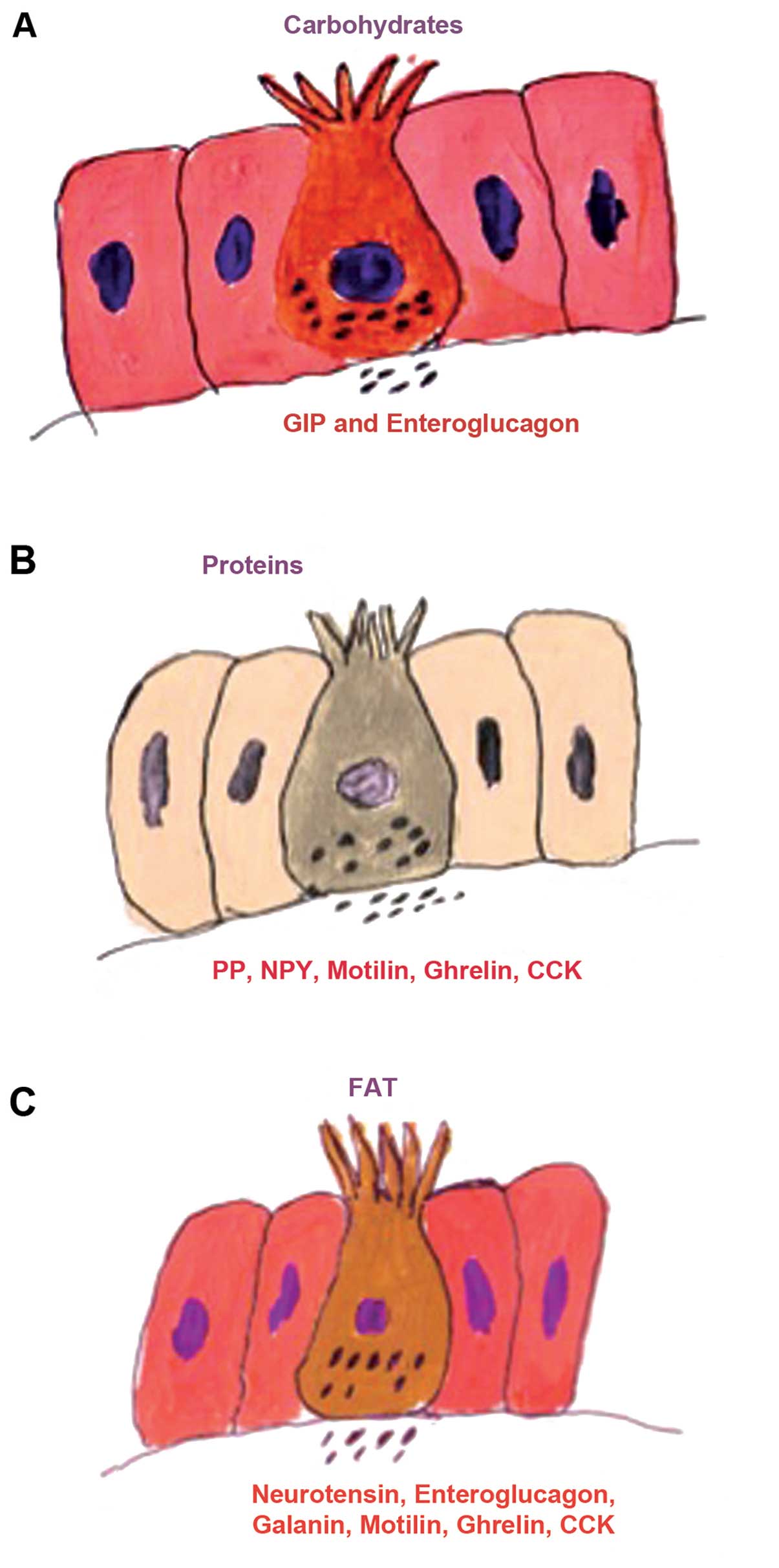
The gut endocrine cells are spread between the
epithelial cells of the mucosa facing the gut lumen (1,53).
They are present in all the segments of the gastrointestinal tract
apart from the esophagus (1).
There are several different populations of gut endocrine cells
(22,32,53–55); the distribution, functions and
modes of action of the most important types have been reported
previously (22,32,53,56–68). Some of the different endocrine
cell types are located only in specific areas of the gut, while
others are found throughout the gut (53–55). Thus, serotonin- and
somatostatin-secreting cells are found throughout the
gastrointestinal tract, while those producing ghrelin and gastrin
are found in the stomach; those producing secretin, cholecystokinin
(CCK), gastric inhibitory peptide (GIP) and motilin are found in
the upper small intestine, and those producing polypeptide YY
(PYY), pancreatic polypeptide (PP) and enteroglucagon are located
in the lower small intestine and large intestine (53–55). These cells have specialized
microvilli that project into the lumen and function as sensors for
the gut contents (mostly for nutrients), and respond to luminal
stimuli by releasing their hormones into the lamina propria
(Fig. 1) (69–81). The gut intraluminal contents of
carbohydrates, proteins and fats triggers the release of the
different signaling substances (i.e., hormones) from the gut
endocrine cells (1,53). These signaling substances may
exert their actions locally on nearby structures (paracrine mode)
or by entering the circulating blood and reaching distant targets
(endocrine mode) (82). The gut
endocrine cells interact and integrate with each other, and with
the enteric nervous system (ENS) and the afferent and efferent
nerve fibers of the autonomic nervous system and the central
nervous system (CNS) (22,53,59,83).
In doing so, they regulate several functions of the
gastrointestinal tract, including visceral sensation, motility,
secretion, absorption, local immune defense and food intake
(22,53–55,83).
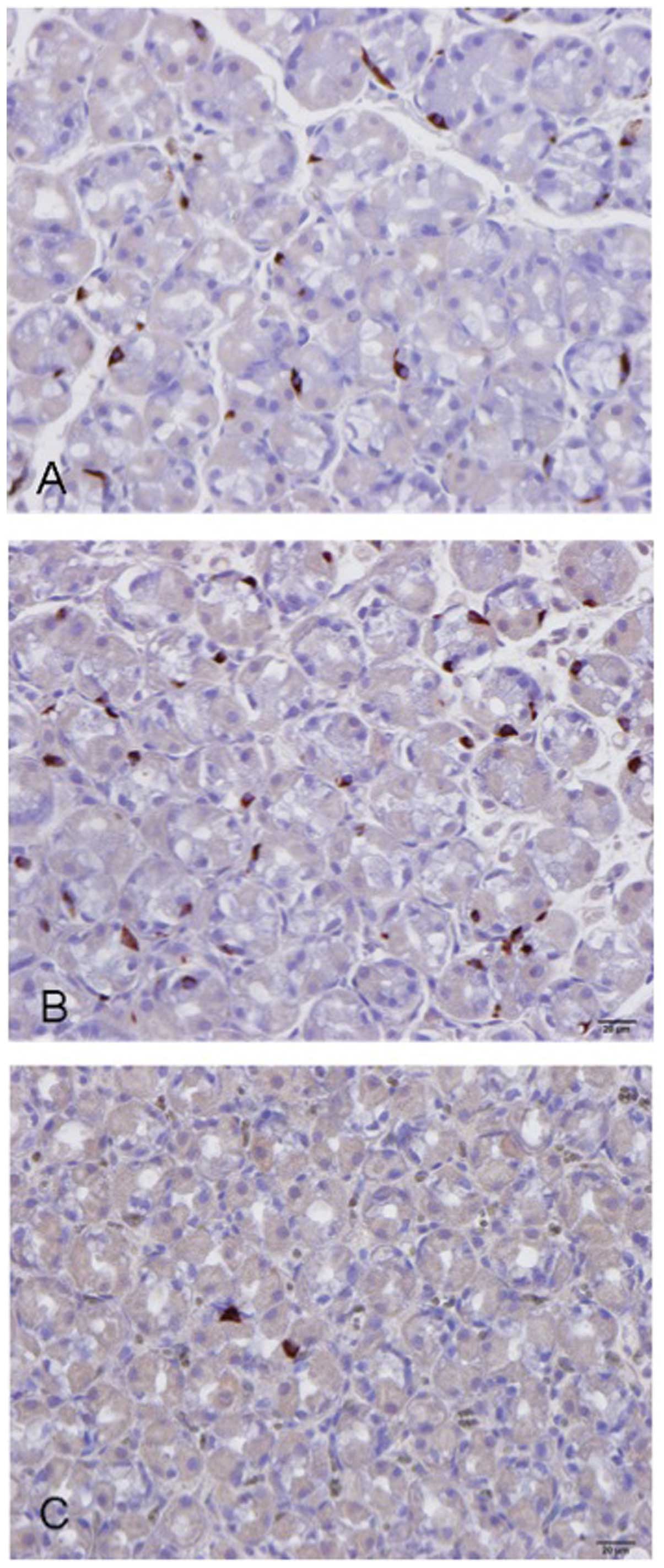
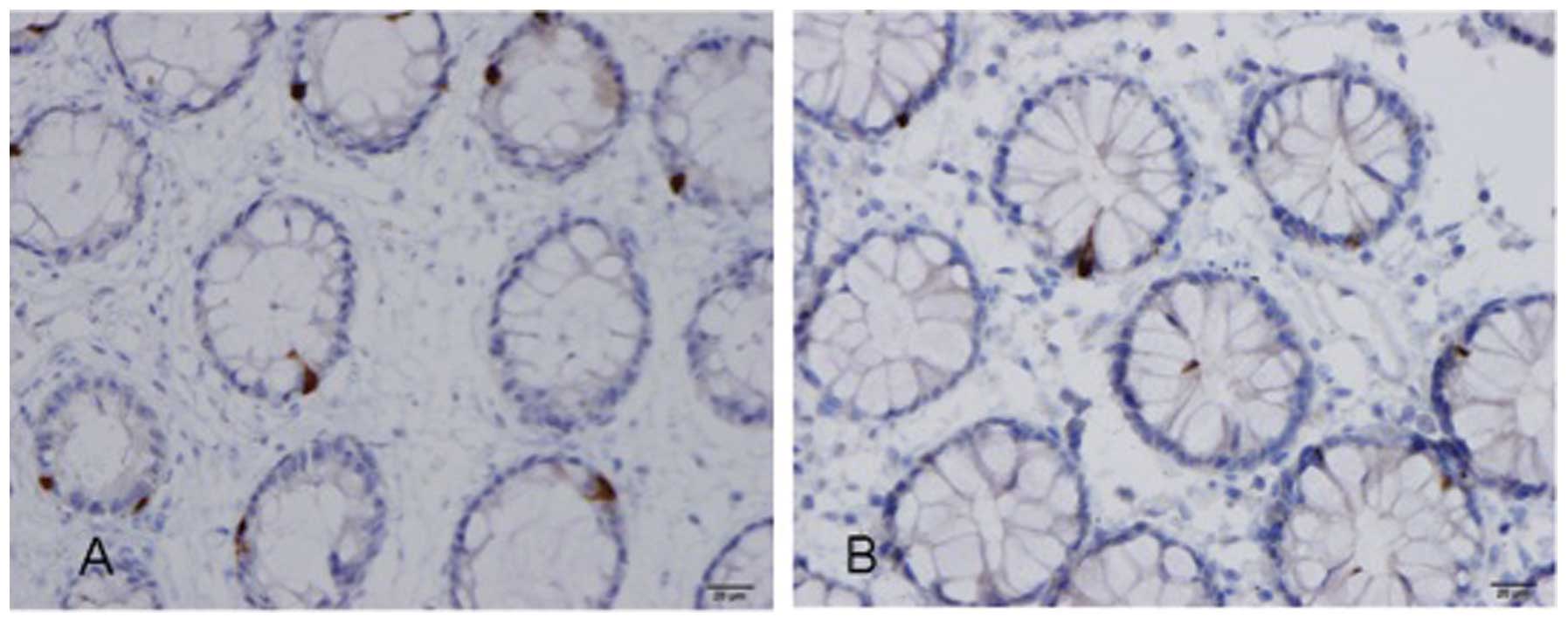
Several abnormalities in the gut endocrine cells
have been described in IBS patients (84–100), as summarized in Table I and illustrated in Figs. 2 and 3. The etiology of these abnormalities in
sporadic (non-specific) IBS patients can be genetically inherited
and/or caused by environmental factors. A genetic etiology is
supported by the familial aggregation of IBS and the results of
twin studies (101–111). Alternatively, endocrine cells
have a rapid turnover, and it is possible that factors related to
luminal content, such as diet or bacterial flora can provoke an
increase or decrease in the endocrine cell population (54,55). In post-infectious IBS (PI-IBS),
the abnormalities in gut endocrine cells may be the result of
endocrine/immune interactions (i.e., the endocrine/immune axis),
which are in turn caused by low-grade inflammation following
gastroenteritis in predisposed individuals (112,113).
In IBS patients, the gut endocrine cells may be
responsible for the abdominal pain/discomfort resulting from the
aforementioned gas production and consequent increase in
intraluminal pressure and large-intestinal distension following the
breakdown of FODMAPs and fibers by the intestinal bacterial flora.
An increase in the intraluminal pressure would possibly result in
the release of serotonin and substance P into the interstitial
fluid. Serotonin activates the submucosal sensory branch of the
ENS, which conveys the sensation to the CNS, possibly causing the
sensation of abdominal pain/discomfort (114,115). Furthermore, serotonin controls
gastrointestinal motility and chloride secretion via interneurons
and motor neurons, which may result in disturbances in both
motility and gastrointestinal secretion (114,115).
There is neither consistent evidence for an allergic
response nor documented evidence for intolerance to a specific food
in IBS (1,116–122). Although a food allergy mediated
by mucosal mechanisms has been suggested for IBS (123,124), these mechanisms may play a role
in only a subset of patients who may have atopy or PI-IBS (1,123,125). Different classes of antibodies
(IgG) have been implicated in food-related allergies in IBS
(126, 127). The results of studies on this
subject are controversial, possibly sicne the tests used are not
sufficiently sensitive or specific (31,116,117,123,124,128–133).
The association between IBS and celiac disease (CD)
has drawn much attention of late. The breadth of the spectrum of
symptoms in IBS means that there is the potential for overlap with
CD symptomatologies. Thus, patients with CD presenting with
relatively vague abdominal symptoms can be diagnosed as having IBS
(39,40). Furthermore, the symptoms of both
IBS and CD patients are triggered by the ingestion of wheat
products. The reported prevalence of CD in IBS varies between 0.04
and 4.7% (134–144). It has been suggested that IBS
patients with wheat intolerance and who carry the genotype
associated with CD (HLA DQ2 or DR3), but do not have typical
serological markers or changes in small-intestine histology exhibit
other immunological evidence of gluten reactivity and response to a
gluten-free diet (145).
The augmentation of IBS symptoms by the ingestion of
wheat products has been attributed to the content of the sugar
polymers, fructans and galactans (38,146). In clinical practice, some IBS
patients describe a reduction in symptoms upon eating a gluten-free
diet. Although this has been dismissed by clinicians as a placebo
effect, there is emerging new data regarding non-celiac gluten
sensitivity (147). The
existence of non-celiac gluten intolerance has been demonstrated by
a double-blinded, randomized, placebo-controlled rechallenge trial
(148). However, the diets of
the subjects in that study excluded wheat products, which contain
gluten, as well as fructans and galactans. A recent
placebo-controlled, cross-over study found no evidence of the
specific effects of gluten in non-celiac gluten sensitivity
(149). Thus, the role of gluten
intolerance in IBS has yet to be clarified, and further studies are
required.
It is clear that IBS patients need guidance on diet
management. Providing IBS patients with diet guidance has been
found to reduce symptoms and to improve their quality of life
(29,150). Furthermore, this guidance leads
IBS patients to consume a more adequate diet in terms of the levels
of vitamins and minerals, and makes them aware of all FODMAP-rich
foods, the consumption of which they should either avoid or reduce.
They also consume foods supplemented with Lactobacillus spp.
and Bifidobacterium spp., which increase their tolerance to
FOODMAPs (29).
Diet guidance should be individualized, since IBS
patients have different tolerances to various FODMAP-rich foods,
possibly due to differences in their intestinal flora. The aim of
diet guidance should be to provide information about FODMAPs and
their role in the symptoms of individual patients, and to instruct
them to avoid such foods. Moreover, the effects of the proportional
intakes of protein, fats and carbohydrates on their symptoms should
be examined. In clinical practice, we have found that reducing the
carbohydrate or fat intake and increasing the protein intake
improves the symptoms in certain patients. In addition, IBS
patients should be encouraged to consume foods that are
supplemented with Lactobacillus spp. and
Bifidobacterium spp. Other lifestyle factors, such as
regular exercise and regular intake of probiotics, may augment the
effect of diet management (151).
Diet triggers symptoms in IBS patients, possibly as
a result of interactions with the gut endocrine cells, which are
defective in IBS patients. The effects of the food content of
FODMAPs and fiber on IBS symptoms are possibly mediated through gut
endocrine cells. FODMAPs in the diet increase the osmotic pressure
and provide a substrate for bacteria fermentation and gas
production in the large intestine, resulting in abdominal
distension. The increase in intestinal pressure may cause the
release of serotonin and substance P, which in turn may result in
the sensation of abdominal discomfort or pain. The protein, fat and
carbohydrate content of ingested foods determine the amount and
type of gut hormones released, which will in turn regulate and
control gastrointestinal motility and sensation, that have been
reported to be abnormal in IBS patients (152–179). Although it is possible that IBS
patients suffer from gluten intolerance, further studies are
required to confirm this before any definitive conclusions can be
drawn. Guidance on diet management, including individually tailored
restrictions of FODMAP-rich foods and the testing of protein-, fat-
and carbohydrate-rich/poor diets reduce IBS symptoms and
accordingly improve the overall management of the health of IBS
patients.
|
1
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable bowel syndrome: diagnosis, pathogenesis and
treatment options. Nova Science Publishers Inc.; New York: 2012
|
|
2
|
Thompson WG: A world view of IBS.
Irritable Bowel Syndrome. Camilleri M and Spiller RC: Saunders;
Philadelphia and London: pp. 17–26. 2002
|
|
3
|
Agreus L, Svardsudd K, Nyren O and Tibblin
G: Irritable bowel syndrome and dyspepsia in the general
population: overlap and lack of stability over time.
Gastroenterology. 109:671–680. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson WG, Irvine EJ, Pare P, Ferrazzi S
and Rance L: Functional gastrointestinal disorders in Canada: first
population-based survey using Rome II criteria with suggestions for
improving the questionnaire. Dig Dis Sci. 47:225–235. 2002.
View Article : Google Scholar
|
|
5
|
Kennedy TM, Jones RH, Hungin AP,
O’Flanagan H and Kelly P: Irritable bowel syndrome,
gastro-oesophageal reflux, and bronchial hyper-responsiveness in
the general population. Gut. 43:770–774. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drossman DA, Li Z, Andruzzi E, et al: U.S.
householder survey of functional gastrointestinal disorders.
Prevalence, sociodemography, and health impact. Dig Dis Sci.
38:1569–1580. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Talley NJ, Gabriel SE, Harmsen WS,
Zinsmeister AR and Evans RW: Medical costs in community subjects
with irritable bowel syndrome. Gastroenterology. 109:1736–1741.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hungin AP, Whorwell PJ, Tack J and Mearin
F: The prevalence, patterns and impact of irritable bowel syndrome:
an international survey of 40,000 subjects. Aliment Pharmacol Ther.
17:643–650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones R and Lydeard S: Irritable bowel
syndrome in the general population. BMJ. 304:87–90. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bordie AK: Functional disorders of the
colon. J Indian Med Assoc. 58:451–456. 1972.PubMed/NCBI
|
|
11
|
O’Keefe EA, Talley NJ, Zinsmeister AR and
Jacobsen SJ: Bowel disorders impair functional status and quality
of life in the elderly: a population-based study. J Gerontol A Biol
Sci Med Sci. 50:M184–M189. 1995.PubMed/NCBI
|
|
12
|
Everhart JE and Renault PF: Irritable
bowel syndrome in office-based practice in the United States.
Gastroenterology. 100:998–1005. 1991.PubMed/NCBI
|
|
13
|
Wilson S, Roberts L, Roalfe A, Bridge P
and Singh S: Prevalence of irritable bowel syndrome: a community
survey. Br J Gen Pract. 54:495–502. 2004.PubMed/NCBI
|
|
14
|
Quigley EM, Locke GR, Mueller-Lissner S,
et al: Prevalence and management of abdominal cramping and pain: a
multinational survey. Aliment Pharmacol Ther. 24:411–419. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harvey RF, Salih SY and Read AE: Organic
and functional disorders in 2000 gastroenterology outpatients.
Lancet. 1:632–634. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spiller R, Aziz Q, Creed F, et al:
Guidelines on the irritable bowel syndrome: mechanisms and
practical management. Gut. 56:1770–1798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thompson WG and Heaton KW: Functional
bowel disorders in apparently healthy people. Gastroenterology.
79:283–288. 1980.PubMed/NCBI
|
|
19
|
Miller V, Whitaker K, Morris JA and
Whorwell PJ: Gender and irritable bowel syndrome: the male
connection. J Clin Gastroenterol. 38:558–560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whitehead WE, Burnett CK, Cook EW III and
Taub E: Impact of irritable bowel syndrome on quality of life. Dig
Dis Sci. 41:2248–2253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gralnek IM, Hays RD, Kilbourne A, Naliboff
B and Mayer EA: The impact of irritable bowel syndrome on
health-related quality of life. Gastroenterology. 119:654–660.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
El-Salhy M: Irritable bowel syndrome:
diagnosis and pathogenesis. World J Gastroenterol. 18:5151–5163.
2012.
|
|
23
|
Simren M, Mansson A, Langkilde AM, et al:
Food-related gastrointestinal symptoms in the irritable bowel
syndrome. Digestion. 63:108–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nanda R, James R, Smith H, Dudley CR and
Jewell DP: Food intolerance and the irritable bowel syndrome. Gut.
30:1099–1104. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bohn L, Storsrud S, Tornblom H, Bengtsson
U and Simren M: Self-reported food-related gastrointestinal
symptoms in IBS are common and associated with more severe symptoms
and reduced quality of life. Am J Gastroenterol. 108:634–641. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jarrett M, Heitkemper MM, Bond EF and
Georges J: Comparison of diet composition in women with and without
functional bowel disorder. Gastroenterol Nurs. 16:253–258. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saito YA, Locke GR III, Weaver AL,
Zinsmeister AR and Talley NJ: Diet and functional gastrointestinal
disorders: a population-based case-control study. Am J
Gastroenterol. 100:2743–2748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams EA, Nai X and Corfe BM: Dietary
intakes in people with irritable bowel syndrome. BMC Gastroenterol.
11:92011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ostgaard H, Hausken T, Gundersen D and
El-Salhy M: Diet and effects of diet management on quality of life
and symptoms in patients with irritable bowel syndrome. Mol Med
Rep. 5:1382–1390. 2012.PubMed/NCBI
|
|
30
|
Böhn L, Störsrud S and Simrén M: Nutrient
intake in patients with irritable bowel syndrome compared with the
general population. Neurogastroenterol Motil.
25:23-e212013.PubMed/NCBI
|
|
31
|
Ligaarden SC, Lydersen S and Farup PG:
Diet in subjects with irritable bowel syndrome: a cross-sectional
study in the general population. BMC Gastroenterol. 12:612012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
El-Salhy M, Ostgaard H, Gundersen D,
Hatlebakk JG and Hausken T: The role of diet in the pathogenesis
and management of irritable bowel syndrome (Review). Int J Mol Med.
29:723–731. 2012.PubMed/NCBI
|
|
33
|
Dizdar V, Spiller R, Singh G, et al:
Relative importance of abnormalities of CCK and 5-HT (serotonin) in
Giardia-induced post-infectious irritable bowel syndrome and
functional dyspepsia. Aliment Pharmacol Ther. 31:883–891.
2010.PubMed/NCBI
|
|
34
|
Monsbakken KW, Vandvik PO and Farup PG:
Perceived food intolerance in subjects with irritable bowel
syndrome - etiology, prevalence and consequences. Eur J Clin Nutr.
60:667–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vesa TH, Seppo LM, Marteau PR, Sahi T and
Korpela R: Role of irritable bowel syndrome in subjective lactose
intolerance. Am J Clin Nutr. 67:710–715. 1998.PubMed/NCBI
|
|
36
|
Geissler C: Human Nutrition. Geissler C
and Powers H: Elsevier; Churchill Livingstone: 2005
|
|
37
|
Wald A and Rakel D: Behavioral and
complementary approaches for the treatment of irritable bowel
syndrome. Nutr Clin Pract. 23:284–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heizer WD, Southern S and McGovern S: The
role of diet in symptoms of irritable bowel syndrome in adults: a
narrative review. J Am Diet Assoc. 109:1204–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morcos A, Dinan T and Quigley EM:
Irritable bowel syndrome: role of food in pathogenesis and
management. J Dig Dis. 10:237–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eswaran S, Tack J and Chey WD: Food: the
forgotten factor in the irritable bowel syndrome. Gastroenterol
Clin North Am. 40:141–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Austin GL, Dalton CB, Hu Y, et al: A very
low-carbohydrate diet improves symptoms and quality of life in
diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol
Hepatol. 7:706–708. e7012009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barrett JS and Gibson PR: Fermentable
oligosaccharides, disaccharides, monosaccharides and polyols
(FODMAPs) and nonallergic food intolerance: FODMAPs or food
chemicals? Therap Adv Gastroenterol. 5:261–268. 2012. View Article : Google Scholar
|
|
43
|
Barrett JS: Extending our knowledge of
fermentable, short-chain carbohydrates for managing
gastrointestinal symptoms. Nutr Clin Pract. 28:300–306. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Biesiekierski JR, Rosella O, Rose R, et
al: Quantification of fructans, galacto-oligosacharides and other
short-chain carbohydrates in processed grains and cereals. J Hum
Nutr Diet. 24:154–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Roest RH, Dobbs BR, Chapman BA, et al:
The low FODMAP diet improves gastrointestinal symptoms in patients
with irritable bowel syndrome: a prospective study. Int J Clin
Pract. 67:895–903. 2013.PubMed/NCBI
|
|
46
|
Staudacher HM, Whelan K, Irving PM and
Lomer MC: Comparison of symptom response following advice for a
diet low in fermentable carbohydrates (FODMAPs) versus standard
dietary advice in patients with irritable bowel syndrome. J Hum
Nutr Diet. 24:487–495. 2011. View Article : Google Scholar
|
|
47
|
Bijkerk CJ, Muris JW, Knottnerus JA, Hoes
AW and de Wit NJ: Systematic review: the role of different types of
fibre in the treatment of irritable bowel syndrome. Aliment
Pharmacol Ther. 19:245–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ford AC, Talley NJ, Spiegel BM, et al:
Effect of fibre, antispasmodics, and peppermint oil in the
treatment of irritable bowel syndrome: systematic review and
meta-analysis. BMJ. 337:a23132008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Francis CY and Whorwell PJ: Bran and
irritable bowel syndrome: time for reappraisal. Lancet. 344:39–40.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bijkerk CJ, de Wit NJ, Muris JW, Whorwell
PJ, Knottnerus JA and Hoes AW: Soluble or insoluble fibre in
irritable bowel syndrome in primary care? Randomised placebo
controlled trial. BMJ. 339:b31542009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kassinen A, Krogius-Kurikka L, Makivuokko
H, et al: The fecal microbiota of irritable bowel syndrome patients
differs significantly from that of healthy subjects.
Gastroenterology. 133:24–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Si JM, Yu YC, Fan YJ and Chen SJ:
Intestinal microecology and quality of life in irritable bowel
syndrome patients. World J Gastroenterol. 10:1802–1805.
2004.PubMed/NCBI
|
|
53
|
El-Salhy M, Seim I, Chopin L, Gundersen D,
Hatlebakk JG and Hausken T: Irritable bowel syndrome: the role of
gut neuroendocrine peptides. Front Biosci (Elite Ed). 4:2783–2800.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gunawardene AR, Corfe BM and Staton CA:
Classification and functions of enteroendocrine cells of the lower
gastrointestinal tract. Int J Exp Pathol. 92:219–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
May CL and Kaestner KH: Gut endocrine cell
development. Mol Cell Endocrinol. 323:70–75. 2010. View Article : Google Scholar
|
|
56
|
Mawe GM, Coates MD and Moses PL: Review
article: intestinal serotonin signalling in irritable bowel
syndrome. Aliment Pharmacol Ther. 23:1067–1076. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wade PR, Chen J, Jaffe B, Kassem IS,
Blakely RD and Gershon MD: Localization and function of a 5-HT
transporter in crypt epithelia of the gastrointestinal tract. J
Neurosci. 16:2352–2364. 1996.PubMed/NCBI
|
|
58
|
Gershon MD and Tack J: The serotonin
signaling system: from basic understanding to drug development for
functional GI disorders. Gastroenterology. 132:397–414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gershon MD: 5-Hydroxytryptamine
(serotonin) in the gastrointestinal tract. Curr Opin Endocrinol
Diabetes Obes. 20:14–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gershon MD: Serotonin is a sword and a
shield of the bowel: serotonin plays offense and defense. Trans Am
Clin Climatol Assoc. 123:268–280. 2012.PubMed/NCBI
|
|
61
|
El-Salhy M, Mazzawi T, Gundersen D,
Hatlebakk JG and Hausken T: The role of peptide YY in
gastrointestinal diseases and disorders (Review). Int J Mol Med.
31:275–282. 2013.PubMed/NCBI
|
|
62
|
Dubrasquet M, Bataille D and Gespach C:
Oxyntomodulin (glucagon-37 or bioactive enteroglucagon): a potent
inhibitor of pentagastrin-stimulated acid secretion in rats. Biosci
Rep. 2:391–395. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schjoldager BT, Baldissera FG, Mortensen
PE, Holst JJ and Christiansen J: Oxyntomodulin: a potential hormone
from the distal gut. Pharmacokinetics and effects on gastric acid
and insulin secretion in man. Eur J Clin Invest. 18:499–503. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Schjoldager B, Mortensen PE, Myhre J,
Christiansen J and Holst JJ: Oxyntomodulin from distal gut. Role in
regulation of gastric and pancreatic functions. Dig Dis Sci.
34:1411–1419. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dakin CL, Small CJ, Batterham RL, et al:
Peripheral oxyntomodulin reduces food intake and body weight gain
in rats. Endocrinology. 145:2687–2695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wynne K, Park AJ, Small CJ, et al:
Subcutaneous oxyntomodulin reduces body weight in overweight and
obese subjects: a double-blind, randomized, controlled trial.
Diabetes. 54:2390–2395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Camilleri M: Peripheral mechanisms in
irritable bowel syndrome. N Engl J Med. 367:1626–1635. 2012.
View Article : Google Scholar
|
|
68
|
Jianu CS, Fossmark R, Syversen U, Hauso O
and Waldum HL: A meal test improves the specificity of chromogranin
A as a marker of neuroendocrine neoplasia. Tumour Biol. 31:373–380.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sandstrom O and El-Salhy M: Ageing and
endocrine cells of human duodenum. Mech Ageing Dev. 108:39–48.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
El-Salhy M: Ghrelin in gastrointestinal
diseases and disorders: a possible role in the pathophysiology and
clinical implications (Review). Int J Mol Med. 24:727–732. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tolhurst G, Reimann F and Gribble FM:
Intestinal sensing of nutrients. Handb Exp Pharmacol. 309–335.
2012. View Article : Google Scholar
|
|
72
|
Lee J, Cummings BP, Martin E, et al:
Glucose sensing by gut endocrine cells and activation of the vagal
afferent pathway is impaired in a rodent model of type 2 diabetes
mellitus. Am J Physiol Regul Integr Comp Physiol. 302:R657–R666.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Parker HE, Reimann F and Gribble FM:
Molecular mechanisms underlying nutrient-stimulated incretin
secretion. Expert Rev Mol Med. 12:e12010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Raybould HE: Nutrient sensing in the
gastrointestinal tract: possible role for nutrient transporters. J
Physiol Biochem. 64:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
San Gabriel A, Nakamura E, Uneyama H and
Torii K: Taste, visceral information and exocrine reflexes with
glutamate through umami receptors. J Med Invest. 56(Suppl):
S209–S217. 2009.PubMed/NCBI
|
|
76
|
Rudholm T, Wallin B, Theodorsson E,
Naslund E and Hellstrom PM: Release of regulatory gut peptides
somatostatin, neurotensin and vasoactive intestinal peptide by acid
and hyperosmolal solutions in the intestine in conscious rats.
Regul Pept. 152:8–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sternini C, Anselmi L and Rozengurt E:
Enteroendocrine cells: a site of ‘taste’ in gastrointestinal
chemosensing. Curr Opin Endocrinol Diabetes Obes. 15:73–78.
2008.
|
|
78
|
Sternini C: Taste receptors in the
gastrointestinal tract. IV. Functional implications of bitter taste
receptors in gastrointestinal chemosensing. Am J Physiol
Gastrointest Liver Physiol. 292:G457–G461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Buchan AM: Nutrient Tasting and Signaling
Mechanisms in the Gut III. Endocrine cell recognition of luminal
nutrients. Am J Physiol. 277:G1103–G1107. 1999.PubMed/NCBI
|
|
80
|
Montero-Hadjadje M, Elias S, Chevalier L,
et al: Chromogranin A promotes peptide hormone sorting to mobile
granules in constitutively and regulated secreting cells: role of
conserved N- and C-terminal peptides. J Biol Chem. 284:12420–12431.
2009. View Article : Google Scholar
|
|
81
|
Shooshtarizadeh P, Zhang D, Chich JF, et
al: The antimicrobial peptides derived from
chromogranin/secretogranin family, new actors of innate immunity.
Regul Pept. 165:102–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rindi G, Inzani F and Solcia E: Pathology
of gastrointestinal disorders. Endocrinol Metab Clin North Am.
39:713–727. 2010. View Article : Google Scholar
|
|
83
|
Seim I, El-Salhy M, Hausken T, Gundersen D
and Chopin L: Ghrelin and the brain-gut axis as a pharmacological
target for appetite control. Curr Pharm Des. 18:768–775. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wendelbo I, Mazzawi T and El-Salhy M:
Increased serotonin transporter immunoreactivity intensity in the
ileum of patients with irritable bowel disease. Mol Med Rep.
9:180–184. 2014.PubMed/NCBI
|
|
85
|
El-Salhy M, Wendelbo IH and Gundersen D:
Reduced chromogranin A cell density in the ileum of patients with
irritable bowel syndrome. Mol Med Rep. 7:1241–1244. 2013.PubMed/NCBI
|
|
86
|
El-Salhy M, Vaali K, Dizdar V and Hausken
T: Abnormal small-intestinal endocrine cells in patients with
irritable bowel syndrome. Dig Dis Sci. 55:3508–3513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
El-Salhy M, Mazzawi T, Gundersen D and
Hausken T: Chromogranin A cell density in the rectum of patients
with irritable bowel syndrome. Mol Med Rep. 6:1223–1225. 2012.
|
|
88
|
El-Salhy M, Lomholt-Beck B and Hausken T:
Chromogranin A as a possible tool in the diagnosis of irritable
bowel syndrome. Scand J Gastroenterol. 45:1435–1439. 2010.
View Article : Google Scholar
|
|
89
|
El-Salhy M, Lillebo E, Reinemo A and
Salmelid L: Ghrelin in patients with irritable bowel syndrome. Int
J Mol Med. 23:703–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
El-Salhy M, Hatlebakk JG, Gundersen D and
Hausken T: Endocrine cells in the gastric oxyntic mucosa of
patients with irritable bowel syndrome. Dig Dis Sci. 2013.
|
|
91
|
El-Salhy M, Gundersen D, Ostgaard H,
Lomholt-Beck B, Hatlebakk JG and Hausken T: Low densities of
serotonin and peptide YY cells in the colon of patients with
irritable bowel syndrome. Dig Dis Sci. 57:873–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Chromogranin a cell density as a diagnostic marker for
lymphocytic colitis. Dig Dis Sci. 57:3154–3159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Gastric endocrine cells in the oxyntic mucosa ofpatients
with irritable bowel syndrome. Dig Dis Sci. 2013.
|
|
94
|
El-Salhy M, Gilja OH, Hatlebakk JG and
Hausken T: Gastric antral endocrine cells in patients with
irritable bowel syndrome. BMC Gastroenterol. 2013.
|
|
95
|
El-Salhy M, Gilja OH and Hausken T:
Chromogranin A cells in the stomach of patients with sporadic
irritable bowel syndrome. Histol Histopathol. 2013.
|
|
96
|
Sjolund K, Ekman R and Wierup N:
Covariation of plasma ghrelin and motilin in irritable bowel
syndrome. Peptides. 31:1109–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang SH, Dong L, Luo JY, et al: Decreased
expression of serotonin in the jejunum and increased numbers of
mast cells in the terminal ileum in patients with irritable bowel
syndrome. World J Gastroenterol. 13:6041–6047. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Park JH, Rhee PL, Kim G, et al:
Enteroendocrine cell counts correlate with visceral
hypersensitivity in patients with diarrhoea-predominant irritable
bowel syndrome. Neurogastroenterol Motil. 18:539–546. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Coates MD, Mahoney CR, Linden DR, et al:
Molecular defects in mucosal serotonin content and decreased
serotonin reuptake transporter in ulcerative colitis and irritable
bowel syndrome. Gastroenterology. 126:1657–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
El-Salhy M, Wendelbo I and Gundersen D:
Serotonin and serotonin transporter in the rectum of patients with
irritable bowel disease. Mol Med Rep. 8:451–455. 2013.PubMed/NCBI
|
|
101
|
Hotoleanu C, Popp R, Trifa AP, Nedelcu L
and Dumitrascu DL: Genetic determination of irritable bowel
syndrome. World J Gastroenterol. 14:6636–6640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Whorwell PJ, McCallum M, Creed FH and
Roberts CT: Non-colonic features of irritable bowel syndrome. Gut.
27:37–40. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Locke GR III, Zinsmeister AR, Talley NJ,
Fett SL and Melton LJ III: Familial association in adults with
functional gastrointestinal disorders. Mayo Clin Proc. 75:907–912.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kalantar JS, Locke GR III, Zinsmeister AR,
Beighley CM and Talley NJ: Familial aggregation of irritable bowel
syndrome: a prospective study. Gut. 52:1703–1707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kanazawa M, Endo Y, Whitehead WE, Kano M,
Hongo M and Fukudo S: Patients and nonconsulters with irritable
bowel syndrome reporting a parental history of bowel problems have
more impaired psychological distress. Dig Dis Sci. 49:1046–1053.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Morris-Yates A, Talley NJ, Boyce PM,
Nandurkar S and Andrews G: Evidence of a genetic contribution to
functional bowel disorder. Am J Gastroenterol. 93:1311–1317. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Levy RL, Jones KR, Whitehead WE, Feld SI,
Talley NJ and Corey LA: Irritable bowel syndrome in twins: heredity
and social learning both contribute to etiology. Gastroenterology.
121:799–804. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lembo A, Zaman M, Jones M and Talley NJ:
Influence of genetics on irritable bowel syndrome,
gastro-oesophageal reflux and dyspepsia: a twin study. Aliment
Pharmacol Ther. 25:1343–1350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wojczynski MK, North KE, Pedersen NL and
Sullivan PF: Irritable bowel syndrome: a co-twin control analysis.
Am J Gastroenterol. 102:2220–2229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bengtson MB, Ronning T, Vatn MH and Harris
JR: Irritable bowel syndrome in twins: genes and environment. Gut.
55:1754–1759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mohammed I, Cherkas LF, Riley SA, Spector
TD and Trudgill NJ: Genetic influences in irritable bowel syndrome:
a twin study. Am J Gastroenterol. 100:1340–1344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Akiho H, Ihara E and Nakamura K: Low-grade
inflammation plays a pivotal role in gastrointestinal dysfunction
in irritable bowel syndrome. World J Gastrointest Pathophysiol.
1:97–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wouters MM and Boeckxstaens GE:
Neuroimmune mechanisms in functional bowel disorders. Neth J Med.
69:55–61. 2011.PubMed/NCBI
|
|
114
|
Kuwahara A, Kuramoto H and Kadowaki M:
5-HT activates nitric oxide-generating neurons to stimulate
chloride secretion in guinea pig distal colon. Am J Physiol.
275:G829–G834. 1998.PubMed/NCBI
|
|
115
|
Gershon MD: Plasticity in serotonin
control mechanisms in the gut. Curr Opin Pharmacol. 3:600–607.
2003. View Article : Google Scholar
|
|
116
|
Zar S, Benson MJ and Kumar D:
Food-specific serum IgG4 and IgE titers to common food antigens in
irritable bowel syndrome. Am J Gastroenterol. 100:1550–1557. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zar S, Kumar D and Benson MJ: Food
hypersensitivity and irritable bowel syndrome. Aliment Pharmacol
Ther. 15:439–449. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Park MI and Camilleri M: Is there a role
of food allergy in irritable bowel syndrome and functional
dyspepsia? A systematic review. Neurogastroenterol Motil.
18:595–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Uz E, Turkay C, Aytac S and Bavbek N: Risk
factors for irritable bowel syndrome in Turkish population: role of
food allergy. J Clin Gastroenterol. 41:380–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dainese R, Galliani EA, De Lazzari F, Di
Leo V and Naccarato R: Discrepancies between reported food
intolerance and sensitization test findings in irritable bowel
syndrome patients. Am J Gastroenterol. 94:1892–1897. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Bischoff S and Crowe SE: Gastrointestinal
food allergy: new insights into pathophysiology and clinical
perspectives. Gastroenterology. 128:1089–1113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Murch S: Allergy and intestinal
dysmotility--evidence of genuine causal linkage? Curr Opin
Gastroenterol. 22:664–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gui XY: Mast cells: a possible link
between psychological stress, enteric infection, food allergy and
gut hypersensitivity in the irritable bowel syndrome. J
Gastroenterol Hepatol. 13:980–989. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Teufel M, Biedermann T, Rapps N, et al:
Psychological burden of food allergy. World J Gastroenterol.
13:3456–3465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Walker MM and Talley NJ: Functional
gastrointestinal disorders and the potential role of eosinophils.
Gastroenterol Clin North Am. 37:383–395. vi2008. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Petitpierre M, Gumowski P and Girard JP:
Irritable bowel syndrome and hypersensitivity to food. Ann Allergy.
54:538–540. 1985.PubMed/NCBI
|
|
127
|
Tobin MC, Moparty B, Farhadi A, DeMeo MT,
Bansal PJ and Keshavarzian A: Atopic irritable bowel syndrome: a
novel subgroup of irritable bowel syndrome with allergic
manifestations. Ann Allergy Asthma Immunol. 100:49–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Atkinson W, Sheldon TA, Shaath N and
Whorwell PJ: Food elimination based on IgG antibodies in irritable
bowel syndrome: a randomised controlled trial. Gut. 53:1459–1464.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Whorwell PJ: The growing case for an
immunological component to irritable bowel syndrome. Clin Exp
Allergy. 37:805–807. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hunter JO: Food elimination in IBS: the
case for IgG testing remains doubtful. Gut. 54:1203author reply
1203. 2005.PubMed/NCBI
|
|
131
|
Beyer K and Teuber SS: Food allergy
diagnostics: scientific and unproven procedures. Curr Opin Allergy
Clin Immunol. 5:261–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Choung RS, Branda ME, Chitkara D, et al:
Longitudinal direct medical costs associated with constipation in
women. Aliment Pharmacol Ther. 33:251–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ortolani C, Bruijnzeel-Koomen C, Bengtsson
U, et al: Controversial aspects of adverse reactions to food.
European Academy of Allergology and Clinical Immunology (EAACI)
Reactions to Food Subcommittee. Allergy. 54:27–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Frissora CL and Koch KL: Symptom overlap
and comorbidity of irritable bowel syndrome with other conditions.
Curr Gastroenterol Rep. 7:264–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
El-Salhy M, Lomholt-Beck B and Gundersen
D: The prevalence of celiac disease in patients with irritable
bowel syndrome. Mol Med Rep. 4:403–405. 2011.PubMed/NCBI
|
|
136
|
Catassi C, Kryszak D, Louis-Jacques O, et
al: Detection of Celiac disease in primary care: a multicenter
case-finding study in North America. Am J Gastroenterol.
102:1454–1460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Fasano A, Berti I, Gerarduzzi T, et al:
Prevalence of celiac disease in at-risk and not-at-risk groups in
the United States: a large multicenter study. Arch Intern Med.
163:286–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
van der Wouden EJ, Nelis GF and Vecht J:
Screening for coeliac disease in patients fulfilling the Rome II
criteria for irritable bowel syndrome in a secondary care hospital
in The Netherlands: a prospective observational study. Gut.
56:444–445. 2007.PubMed/NCBI
|
|
139
|
Locke GR III, Murray JA, Zinsmeister AR,
Melton LJ III and Talley NJ: Celiac disease serology in irritable
bowel syndrome and dyspepsia: a population-based case-control
study. Mayo Clin Proc. 79:476–482. 2004. View Article : Google Scholar
|
|
140
|
Hin H, Bird G, Fisher P, Mahy N and Jewell
D: Coeliac disease in primary care: case finding study. BMJ.
318:164–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Shahbazkhani B, Forootan M, Merat S, et
al: Coeliac disease presenting with symptoms of irritable bowel
syndrome. Aliment Pharmacol Ther. 18:231–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Korkut E, Bektas M, Oztas E, Kurt M,
Cetinkaya H and Ozden A: The prevalence of celiac disease in
patients fulfilling Rome III criteria for irritable bowel syndrome.
Eur J Intern Med. 21:389–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Sanders DS, Patel D, Stephenson TJ, et al:
A primary care cross-sectional study of undiagnosed adult coeliac
disease. Eur J Gastroenterol Hepatol. 15:407–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Verdu EF, Armstrong D and Murray JA:
Between celiac disease and irritable bowel syndrome: the ‘no man’s
land’ of gluten sensitivity. Am J Gastroenterol. 104:1587–1594.
2009.
|
|
145
|
Wahnschaffe U, Schulzke JD, Zeitz M and
Ullrich R: Predictors of clinical response to gluten-free diet in
patients diagnosed with diarrhea-predominant irritable bowel
syndrome. Clin Gastroenterol Hepatol. 5:844–850; quiz 769. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Carroccio A, Mansueto P, Iacono G, et al:
Non-celiac wheat sensitivity diagnosed by double-blind
placebo-controlled challenge: exploring a new clinical entity. Am J
Gastroenterol. 107:1898–1906; quiz 1907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Aziz I and Sanders DS: Emerging concepts:
from coeliac disease to non-coeliac gluten sensitivity. Proc Nutr
Soc. 71:576–580. 2012. View Article : Google Scholar
|
|
148
|
Newnham ED: Does gluten cause
gastrointestinal symptoms in subjects without coeliac disease? J
Gastroenterol Hepatol. 26(Suppl 3): S132–S134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Biesiekierski JR, Peters SL, Newnham ED,
Rosella O, Muir JG and Gibson PR: No effects of gluten in patients
with self-reported non-celiac gluten sensitivity after dietary
reduction of fermentable, poorly absorbed, short-chain
carbohydrates. Gastroenterology. 145:320–328. e3232013. View Article : Google Scholar
|
|
150
|
Mazzawi T, Hausken T, Gundersen D and
El-Salhy M: Effects of dietary guidance on the symptoms, quality of
life and habitual dietary intake of patients with irritable bowel
syndrome. Mol Med Rep. 8:845–852. 2013.PubMed/NCBI
|
|
151
|
El-Salhy M, Lilbo E, Reinemo A, Salmeøid L
and Hausken T: Effects of a health program comprising reassurance,
diet management, probiotic administration and regular exercise on
symptoms and quality of life in patients with irritable bowel
syndrome. Gastroenterol Insights. 2:21–26. 2010.
|
|
152
|
Posserud I, Syrous A, Lindstrom L, Tack J,
Abrahamsson H and Simren M: Altered rectal perception in irritable
bowel syndrome is associated with symptom severity.
Gastroenterology. 133:1113–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ritchie J: Pain from distension of the
pelvic colon by inflating a balloon in the irritable colon
syndrome. Gut. 14:125–132. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Mertz H, Naliboff B, Munakata J, Niazi N
and Mayer EA: Altered rectal perception is a biological marker of
patients with irritable bowel syndrome. Gastroenterology.
109:40–52. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Whitehead WE and Palsson OS: Is rectal
pain sensitivity a biological marker for irritable bowel syndrome:
psychological influences on pain perception. Gastroenterology.
115:1263–1271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Whitehead WE, Holtkotter B, Enck P, et al:
Tolerance for rectosigmoid distention in irritable bowel syndrome.
Gastroenterology. 98:1187–1192. 1990.PubMed/NCBI
|
|
157
|
Bouin M, Plourde V, Boivin M, et al:
Rectal distention testing in patients with irritable bowel
syndrome: sensitivity, specificity, and predictive values of pain
sensory thresholds. Gastroenterology. 122:1771–1777. 2002.
View Article : Google Scholar
|
|
158
|
Bradette M, Delvaux M, Staumont G,
Fioramonti J, Bueno L and Frexinos J: Evaluation of colonic sensory
thresholds in IBS patients using a barostat. Definition of optimal
conditions and comparison with healthy subjects. Dig Dis Sci.
39:449–457. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Camilleri M, McKinzie S, Busciglio I, et
al: Prospective study of motor, sensory, psychologic, and autonomic
functions in patients with irritable bowel syndrome. Clin
Gastroenterol Hepatol. 6:772–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Costantini M, Sturniolo GC, Zaninotto G,
et al: Altered esophageal pain threshold in irritable bowel
syndrome. Dig Dis Sci. 38:206–212. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Trimble KC, Farouk R, Pryde A, Douglas S
and Heading RC: Heightened visceral sensation in functional
gastrointestinal disease is not site-specific. Evidence for a
generalized disorder of gut sensitivity. Dig Dis Sci. 40:1607–1613.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zighelboim J, Talley NJ, Phillips SF,
Harmsen WS and Zinsmeister AR: Visceral perception in irritable
bowel syndrome. Rectal and gastric responses to distension and
serotonin type 3 antagonism. Dig Dis Sci. 40:819–827. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Accarino AM, Azpiroz F and Malagelada JR:
Selective dysfunction of mechanosensitive intestinal afferents in
irritable bowel syndrome. Gastroenterology. 108:636–643. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Kanazawa M, Hongo M and Fukudo S: Visceral
hypersensitivity in irritable bowel syndrome. J Gastroenterol
Hepatol. 26(Suppl 3): S119–S121. 2011. View Article : Google Scholar
|
|
165
|
Whorwell PJ, Clouter C and Smith CL:
Oesophageal motility in the irritable bowel syndrome. Br Med J
(Clin Res Ed). 282:1101–1102. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Clouse RE and Eckert TC: Gastrointestinal
symptoms of patients with esophageal contraction abnormalities. Dig
Dis Sci. 31:236–240. 1986. View Article : Google Scholar
|
|
167
|
Soffer EE, Scalabrini P, Pope CE II and
Wingate DL: Effect of stress on oesophageal motor function in
normal subjects and in patients with the irritable bowel syndrome.
Gut. 29:1591–1594. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Lind CD: Motility disorders in the
irritable bowel syndrome. Gastroenterol Clin North Am. 20:279–295.
1991.PubMed/NCBI
|
|
169
|
Lee OY: Asian motility studies in
irritable bowel syndrome. J Neurogastroenterol Motil. 16:120–130.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
van Wijk HJ, Smout AJ, Akkermans LM,
Roelofs JM and ten Thije OJ: Gastric emptying and dyspeptic
symptoms in the irritable bowel syndrome. Scand J Gastroenterol.
27:99–102. 1992.PubMed/NCBI
|
|
171
|
Charles F, Phillips SF, Camilleri M and
Thomforde GM: Rapid gastric emptying in patients with functional
diarrhea. Mayo Clin Proc. 72:323–328. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Caballero-Plasencia AM,
Valenzuela-Barranco M, Herrerias-Gutierrez JM and Esteban-Carretero
JM: Altered gastric emptying in patients with irritable bowel
syndrome. Eur J Nucl Med. 26:404–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Portincasa P, Moschetta A, Baldassarre G,
Altomare DF and Palasciano G: Pan-enteric dysmotility, impaired
quality of life and alexithymia in a large group of patients
meeting ROME II criteria for irritable bowel syndrome. World J
Gastroenterol. 9:2293–2299. 2003.PubMed/NCBI
|
|
174
|
Stanghellini V, Tosetti C, Barbara G, et
al: Dyspeptic symptoms and gastric emptying in the irritable bowel
syndrome. Am J Gastroenterol. 97:2738–2743. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Leahy A, Besherdas K, Clayman C, Mason I
and Epstein O: Abnormalities of the electrogastrogram in functional
gastrointestinal disorders. Am J Gastroenterol. 94:1023–1028. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Evans PR, Bak YT, Shuter B, Hoschl R and
Kellow JE: Gastroparesis and small bowel dysmotility in irritable
bowel syndrome. Dig Dis Sci. 42:2087–2093. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Nielsen OH, Gjorup T and Christensen FN:
Gastric emptying rate and small bowel transit time in patients with
irritable bowel syndrome determined with 99mTc-labeled pellets and
scintigraphy. Dig Dis Sci. 31:1287–1291. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Narducci F, Bassotti G, Granata MT, et al:
Colonic motility and gastric emptying in patients with irritable
bowel syndrome. Effect of pretreatment with octylonium bromide. Dig
Dis Sci. 31:241–246. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Acharya U, Waite N, Howlett P, Tanner AR
and Smith CL: Failure to demonstrate altered gastric emptying in
irritable bowel syndrome. Dig Dis Sci. 28:889–892. 1983. View Article : Google Scholar
|