Introduction
In the present study, we produced a mouse monoclonal
antibody, Y-49, against synthesized polypeptides with 17 amino
acids selected from the published survivin sequence (1). However, the anbitody detects
α-tubulin, with a very similar amino acid sequence, and we found
intense immunoreactivity in tumor cell nuclei to be linked with a
poor prognosis of patients with non-Hodgkin’s lymphoma (NHL).
Tubulin subunits of microtubules (MTs), i.e.,
cylindrical organelles found in almost all eukaryote cells, which
are involved in a number of cellular processes, including mitosis,
cilia and flagella motility and the intracellular transport of
vesicles and organelles (2), are
mainly composed of heterodimers of two polypeptides designated as α
and β (3). γ-tubulin is required
for the initiation of MT assembly and it is found at the minus end
of MTs (4). Over the years, four
new members of the tubulin family, δ, ɛ, ζ and η, have been
identified; however, their cellular functions are yet to be fully
established (5–9). α- and β-tubulin are encoded by a
family of genes that produces polypeptides which differ primarily
in the C-terminal variable domain, which consists of 15 amino acids
(10), and have several isotypes
(2). In mammalians, the class III
β-tubulin isotype is expressed in a differentiation-dependent
manner in human neuroblastic tumors, such as medulloblastomas
(11), retinoblastomas (12) and peripheral neuroblastomas
(13). In addition, a
corresponding epitope has been detected in cases of squamous cell
carcinoma, lymphoma and melanoma (14). As regards the specificity of
α-tubulin isotypes, six isotypes have been identified and found to
be distributed in various organs (15). However, to the best of our
knowledge, no study on the expression of α-tubulin in malignant
tumors has been published to date.
Materials and methods
Production of Y-49 antibody
Using the published survivin sequence (1), a 17-amino acid oligopeptide was
synthesized, conjugated with Freund’s incomplete adjuvant, and then
injected into female BALB/c mice intraperitoneally three times at
10-day intervals for immunization. After boosting, splenocytes were
fused with P3×63-Ag8653 mouse myeloma cells and hybridomas were
grown in RPMI-1640 medium supplemented with 10% fetal calf serum
and were then subcloned twice. The supernatants were screened for
reactivity with the original antigens and one positive line was
named Y-49.
Western blot anlaysis
Two cultured cell lines, Raji (Burkitt’s lymphoma)
and MKN28 (gastric cancer), were purchased from Riken Cell Bank,
Tsukuba, Japan. A total of 1–10×106 cultured cells and
two samples of normal lymphocytes obtained from tonsils resected
for chronic tonsillitis were homogenized in 0.01 M
phosphate-buffered saline (PBS) and centrifuged (19,000 × g, 30
min). The supernatants were mixed with 62.5 mM Tris-HCl buffer, pH
6.8, containing 2% sodium dodecyl sulfate (SDS), 5%
2-mercaptoethanol, 7% glycerol and 0.01% bromophenol blue, and then
boiled for 10 min. Proteins (10 μg aliquots) were electrophoresed
on 8% SDS-polyacrylamide gels at 30 mA for 3 h, and transferred
onto 0.45 mm polyvinylidene fluoride (PVDF) membranes (Immobilon-P,
Millipore, Bedford, MA, USA), using a semi-dry system (Biocraft,
Tokyo, Japan) at 200 mA for 30 min. The membranes were blocked with
5% skimmed milk in PBS and then incubated with Y-49 (dilution,
×200), α-tubulin (Neomarkers, Westinghouse, CA, USA; DM1A, ×200)
and HSP90 (Medical and Biological Laboratories, Nagoya, Japan)
antibodies at 4°C overnight, followed by exposure to horseradish
peroxidase-conjugated rabbit anti-mouse immunoglobulin-G (IgG;
Dako, Copenhagen, Denmark). Specific binding was determined by
enhanced chemiluminescence (Amersham, Buckinghamshire, UK) on X-ray
films (RX-U; Fuji, Tokyo, Japan). All procedures for western blot
analysis were repeated in triplicate for confirmation.
Immunoprecipitation
Immunoprecipitation was performed according to
previously described methods (16). The Raji cell samples (50 mg) were
washed twice with ice-cold PBS, homogenized in 1 ml of IP buffer
(50 mM HEPES; 150 mM NaCl, 0.1 mM phenyl-methylsulfonyl fluoride,
20 U/ml aprotinin, pH 8.0) and centrifuged at 14,000 × g for 3 min.
Protein G-Sepharose (30 μl) was added to the supernatant followed
by rotation at 4°C for 30 min. Following centrifugation at 5,000 ×
g for 1 min, 0.5 μg of antibody were added to the supernatant
followed by rotation at 4°C for 1 h. Subsequently, 30 ml of protein
G-Sepharose wee introduced with rotation at 4°C for 30 min. The
sediments were washed with ice-cold IP buffer three times under the
same conditions (3,000 × g, 5 min, 4°C). The same volume of 2× SDA
sample buffer was added to the sediment solution and the resultant
preparations were used for western blot analysis. The antibodies
employed for immunoprecipitation were the following: Y-49, DM1A
(α-tubulin), β-tubulin (Neomarkers) and HSP90.
Cell fractionation
Cell fractionation was performed as previously
described (17). A total of
4×106 NHL cells were collected after rinsing with cold
STE (100 mM NaCl, 10 mM Tris, 1 mM EDTA, pH 7.4) and centrifuged at
1,000 × g for 5 min. Following the addition of 0.8 ml hypotonic
lysis buffer (10 mM Tris, 0.2 mM Mg, pH 7.4) to the pellet and
gentle vortexing, the cells were broken open by with 30 strokes in
a Dounce homogenizer followed by the addition of 200 μl 1.25 M
sucrose and 2 μl 0.5 M EDTA. Following centrifugation at 1,000 × g
for 10 min, the supernatant (S1) was removed to an ultracentrifuge
tube and the pellet was resuspended with 1 ml solution of 0.25 M
sucrose, 10 mM Tris, 1 mM EDTA, pH 7.4, homogenized ten strokes,
and centrifuged at 1,000 × g for 10 min. The pellet, which was
designated as the P1 fraction, was enriched with nuclei. The
supernatant S1 was removed and further centrifuged at 100,000 × g
for 1 h to yield the supernatant S100 fraction, containing
cytosolic proteins. A fraction enriched in mitochondrial membranes
could be obtained by centrifuging S1 at 10,000 × g for 10 min.
Finally, western blot analysis was performed on each fraction as
described above.
Analysis of amino acid sequences
Amino acid sequence analysis was performed by the
in-gel digestion method described by Rosenfeld et al
(18). After immunoprecipitation
and western blot analysis employing Y-49, in-gel-digested protein
bands stained with Coomassie brilliant blue were excised and washed
twice with 150 μl of 50% acetonitrile in 200 mM ammonium carbonate
(pH 8.9) for 20 min at 30°C in an Eppendorf tube. The gel slices
were partially rehydrated with 5 μl of 200 mM ammonium
carbohydrate, 0.02% Tween-20 (pH 8.9). Subsequently, 2 μl of
porcine trypsin solution (250 μg/ml in ammonium carbonate, pH 8.9)
were added. After the absorption of the protease solution, 5 μl of
ammonium carbonate buffer were added together with a minimum volume
of rehydration buffer, which was added to totally immerse the gel
slices. The digestion was carried out for 4 h at 30°C and then
terminated by the addition of 1.5 μl of fluoroacetic acid. The
resulting peptides were recovered by two extractions of 20 min
each, with 100 μl of a solution of 60% acetonitrile, 0.1%
trifluoroacetic acid, at 30°C with shaking in an Ependorf
thermomixer.
The peptides eluted from the polyacrylamide matrices
were separated using C18 Brownlee reverse-phase columns (Brownlee
Labs, Santa Clara, CA, USA) and were eluted with a linear gradient
of acetonitrile, 0.1% trifluoroacetic acid at a flow rate of 0.3
ml/min with the Brownlee Labs microgradient system. Elution was
monitored at 218 nm with a Spectroflow 783 programmable absorbance
detector (Kratos Analytical Ltd., Manchester, UK), and peaks were
manually collected. N-terminal sequence analysis was performed on a
gas-phase sequencer (470A; Applied Biosystems).
Immunofluorescence double staining
Immunofluorescence double staining was employed for
the demonstration of the localization of Y-49 binding antigens.
Formalin-fixed, paraffin-embedded fibrin-clots obtained from two
NHL cell lines were prepared and cut into 4 μm-thick sections. Y-49
(dilution, ×100) and Ki-67 (Dako; dilution, ×1) were used as
primary antibodies and anti-mouse IgG conjugated with fluorescein
Texas red (FITC; Dako; dilution, ×200) and anti-rabbit IgG
conjugated with fluorescein isothiocyanate (FITC; Dako; dilution,
×200) were utilized as the respective secondary antibodies. The
sections in these cases were not treated with the microwave oven
heating method, as this often results in overstaining. All
preparations were examined under a confocal microscope (model
LSM-GB200; Olympus, Tokyo, Japan), equipped with argon and
argon-krypton lasers. Details of the staining and confocal
microscopy procedures have been described in a previous study
(19).
Flow cytometry
Normal lymphocytes obtained from healthy volunteers
were incubated at 37°C for 96 h under stimulation with
phytohemagglutinin (PHA, 5 μg/ml) and fixed for 40 min with
paraformaldehyde solution (0.5 g/ml) in PBS at 4°C and were washed
and subsequently permeabilized with 0.1% Triton X-100 in PBS.
Following incubation with Y-49, DM1A (α-tubulin) and HSP90 at 4°C
for 1 h, the lymphocytes were washed and labeled with fluorescein
isothiocyanate goat anti-mouse serum for 30 min. An IgG2b antibody
(Dako) was used as the negative control. Cytometry was performed on
a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA,
USA), DNA staining being recorded on a linear scale and antibody
staining on a log scale. A total of 1,024 channels were
analyzed.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue blocks were
available for each case. Sections cut at 4 μm thickness were
stained with the standard labeled streptavidin-biotin-peroxidase
method (LSAB kit; Dako) to investigate the localization and degree
of reactivity with Y-49 (dilution, ×100), α-tubulin (Clone DM1A,
dilution, ×100) and MIB-1 against the Ki-67 antigen (Dako;
dilution, ×100), all being mouse monoclonal antibodies. Prior to
the immunohistochemical procedures, the sections were placed in
heat-resistant plastic staining jars containing an
antigen-retrieval solution, 10 mM citric acid with pH 6.0 (Wako,
Osaka, Japan), heated in a microwave oven for 5 min at 500 W three
times, and then allowed to cool to room temperature. Finally, color
was developed at the sites of reactivity with a substrate kit
followed by counterstaining with Meyer’s hematoxylin. The
immunohistochemical control procedure, employed in conjunction with
all the methods described above, consisted of the replacement of
the primary antibodies with an equivalent amount of normal mouse
IgG (Dako). Negative results were thereby obtained in all
instances. Tonsil, esophagus and stomach tissues were also employed
for the evaluation of Y-49 and DM1A in normal tissues.
Assessment of reactivity for Y-49 and
MIB-1
In the present study, we focused on Y-49 positivity
in nuclei since weak Y-49 cytoplasmic positivity was noted in
almost all cases examined. Overexpression in nuclei was determined
by counting immunoreactive cells in >1,000 NHL cells in
high-power, microscope images of representative sections in each
case. A cut-off value was defined from the results of histograms
for positive cells among all examined cases as detailed in Results,
and the cases were divided into two groups, with high and low
labeling indices (LIs). LIs for MIB-1 staining were calculated in a
similar manner, with counts of >1,000 cells. The cases were
again subdivided into two groups with high and low indices.
Patients examined
Data from 116 patients for whom a diagnosis of NHL
was made on the basis of clinical, radiological and histological
examinations were randomly retrieved from the patient files at
Kitasato University Hospital (Sagamihara, Japan) and Toyama
University Hospital (Toyama, Japan). Reviewing the available tissue
slides in each case, the histological subtypes of NHL were
determined by two of the authors (S.H. and Y.T.), according to the
criteria established by the Revised European and American Lymphoma
(REAL) Study Group (20).
Clinical information was obtained from the patient
files. All the patients underwent appropriate chemo- and/or
radiotherapy shortly after the establishment of the final
diagnosis.
Study approval
The study complied with the Declaration of Helsinki
and the Local Ethics Committee of the University of Toyama (Toyama,
Japan) approved the research protocol. Written informed consent was
obtained from each participant. Animal care and surgical procedures
were in accordance with the ‘Principles of Laboratory Animal Care’
prepared by the National Academy of Sciences.
Statistical analysis
Statistical analyses were performed using the
program StatView J 5.0 for Windows. All values shown are the means
± standard error (SE), unless otherwise stated. Correlations of
categorical variables were analyzed using the χ2 test.
To compare the distributions of continuous variables in the two
groups, the Mann-Whitney U test was utilized. Survival analysis was
performed by the Kaplan-Meier method with the log-rank test and the
Cox multivariate method. Differences were considered statistically
significant when the p-value was <0.05.
Results
Western blot analysis,
immunoprecipitation and cell fractionation
Western blot analysis with Y-49 demonstrated one
strong band of approximately 57 kDa and one weak band of
approximately 90 kDa in the Raji and MKN28 cells and normal
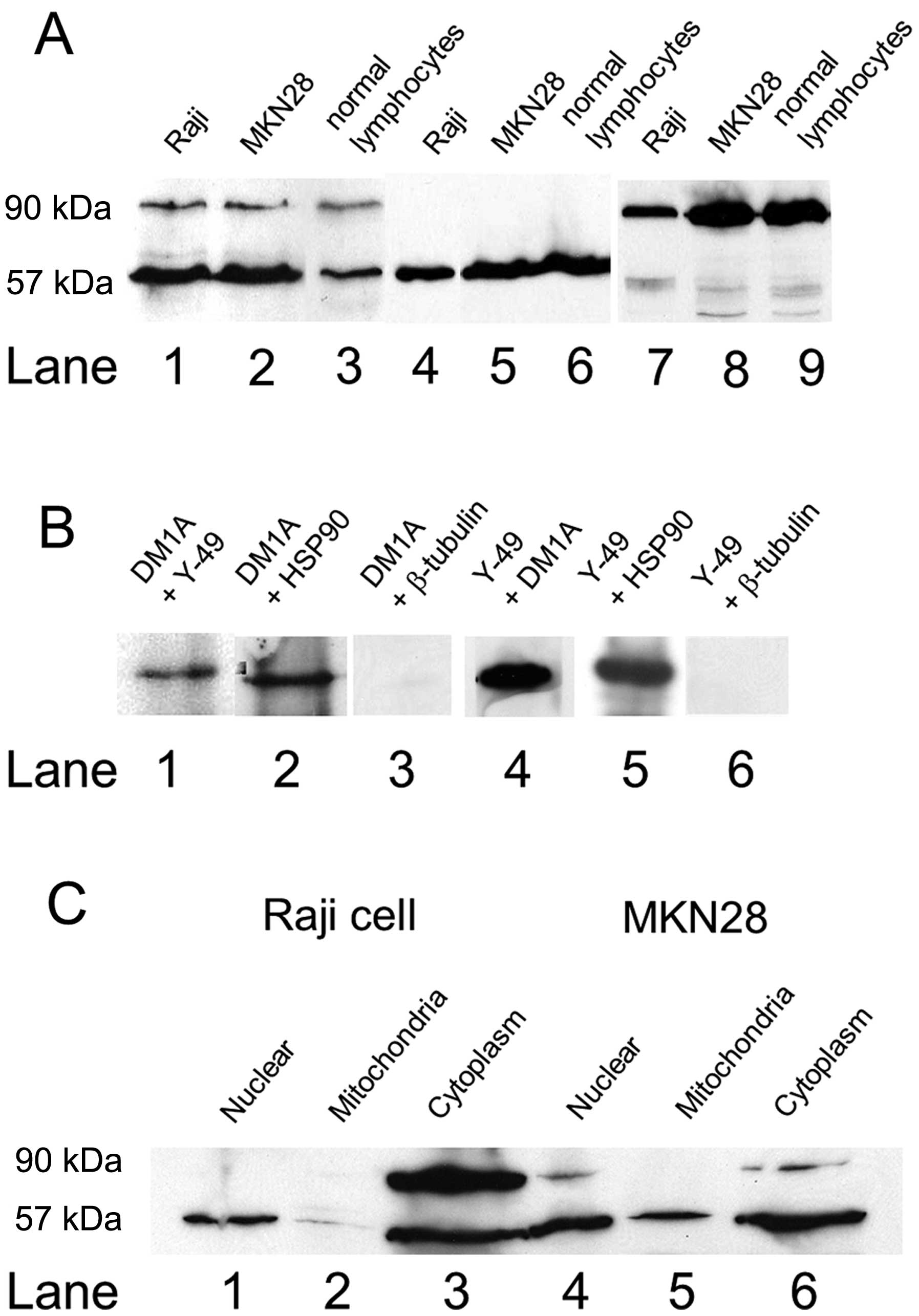
lymphocytes (Fig. 1A, lanes 1–3).
The intensity of the 57 kDa band in the normal lymphocytes was less
than that in the Raji and NKM28 cells. With DM1A, only clear single
bands of approximately 57 kDa were evident in the samples (Fig. 1A, lanes 4–6). With HSP90, clear
bands of approximately 90 kDa were observed (Fig. 1A, lanes 7–9). Western blot
analysis of the Raji cells revealed clear single bands, with DM1A
after immunoprecipitation using Y-49 or HSP90, but not with
β-tubulin (Fig. 1B, lanes 1–3).
Furthermore, the binding of Y-49 after immunoprecipitation using
DM1A and HSP90 was detected (Fig.
1B, lanes 4–6). Cell fractionation revealed a clear band of
approximately 57 kDa in the nuclear fraction (Fig. 1C, lanes 1 and 4), two bands of
approximately 57 and 90 kDa in the cytoplasmic fraction (Fig. 1C, lane 3) and a clear band of
approximately 57 kDa in the mitochondrial fraction.
Immunoprecipitation confirmed no presence of HSP90 in the nuclear
fraction.
Analysis of amino acid sequence
Amino acid sequence analysis of the 57 kDa protein
band revealed a ten polypeptide sequence from the N-terminus
‘Val-Gly-Ile-Asn-Tyr-Gln-Pro-Pro-Thr-Val’, identical to a stretch
of α-tubulin (The Swiss Institute of Bioinformatics and the EMBL
outstation - The European Bioinformatics Institute). Amino acid
sequence analysis of the 90-kDa protein demonstrated a ten
polypeptide sequence from the N-terminus
‘Ile-Arg-Tyr-Glu-Ser-Leu-Thr-Asp-Pro-Ser’, identical to part of
HSP90 (The Swiss Institute of Bioinformatics and the EMBL
outstation - The European Bioinformatics Institute).
Immunofluorescence double staining
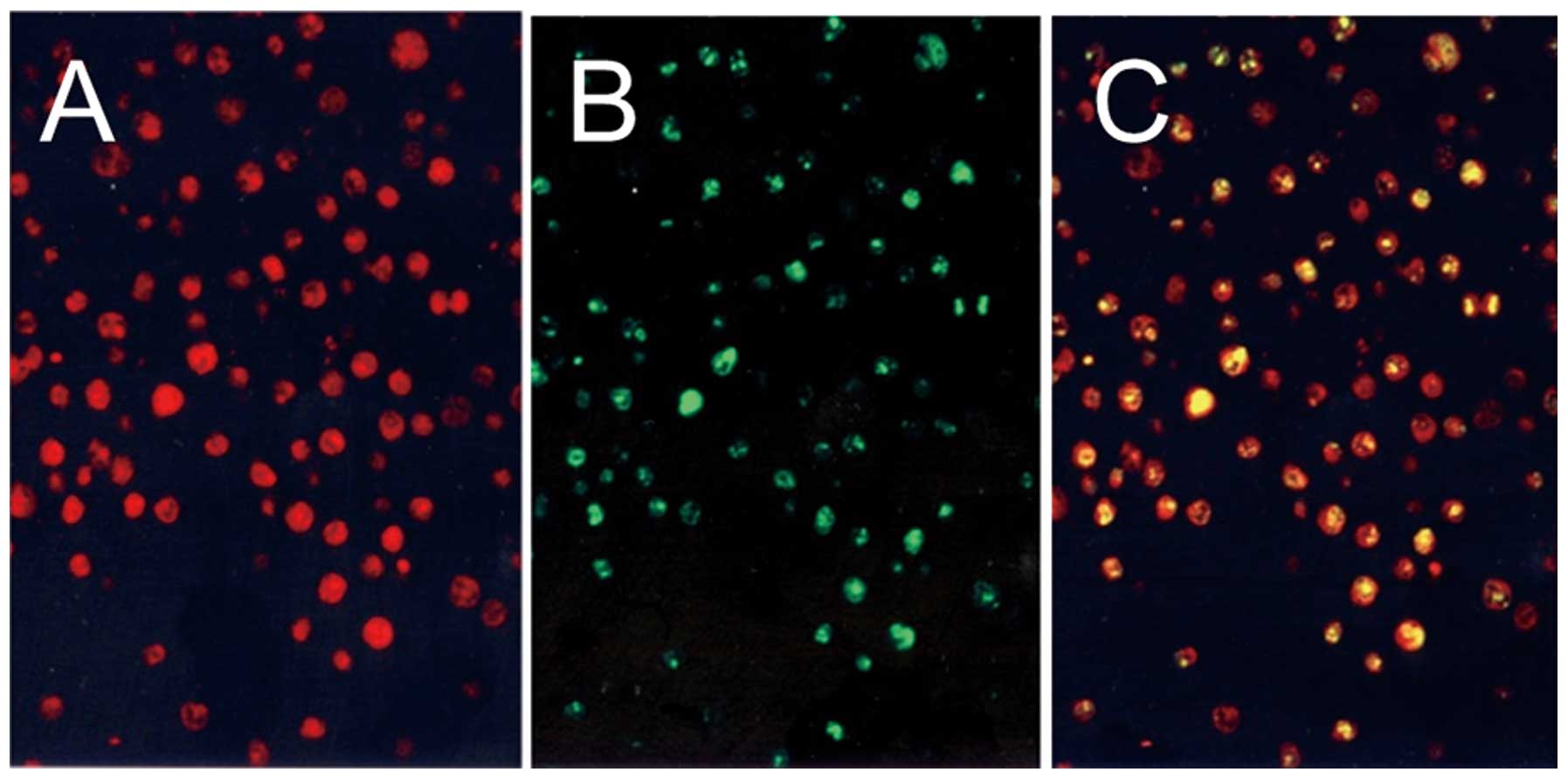
Fig. 2
demonstrates the results of the immunofluorescence double staining
of Y-49 and Ki-67. The red color, Y-49, was visible in both the
nuclei and the cytoplasm (Fig.
2A) and the green color, showing positivity of Ki-67, was found
restricted to nuclei (Fig. 2B).
The yellow color indicated the double staining of Y-49 and Ki-67.
There was a great deal of overlap as shown by double staining
(Fig. 2C), but Y-49-positive
cells were slightly more frequent than Ki-67-positive cells.
Flow cytometry
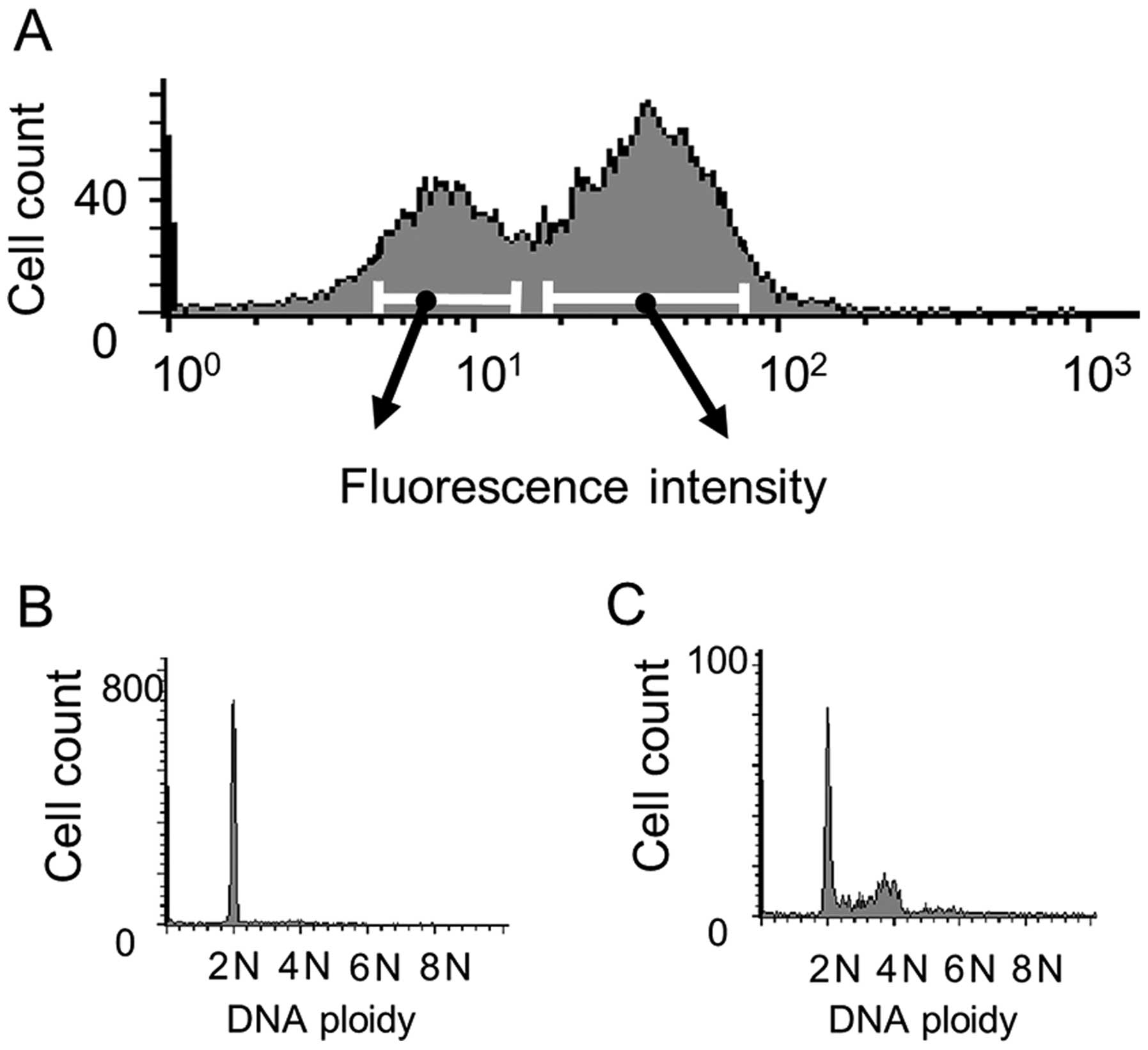
Fig. 3A shows the
results of flow cytometric with Y-49, 96 h following PHA
stimulation. The vertical axis represents the cell count and the
horizontal axis the fluorescent intensity, two major peaks being
recognized. After gating analysis, cells of the left peak were
found to be 2N in accordance with G0/G1 (Fig. 3B) and cells of the right peak
demonstrated entrance into the cell cycle (Fig. 3C). The results with DM1A and HSP90
at 96 h after stimulation revealed one peak, as shown in Fig. 3A (left), showing 2N on gating.
Immunohistochemistry of normal
tissues
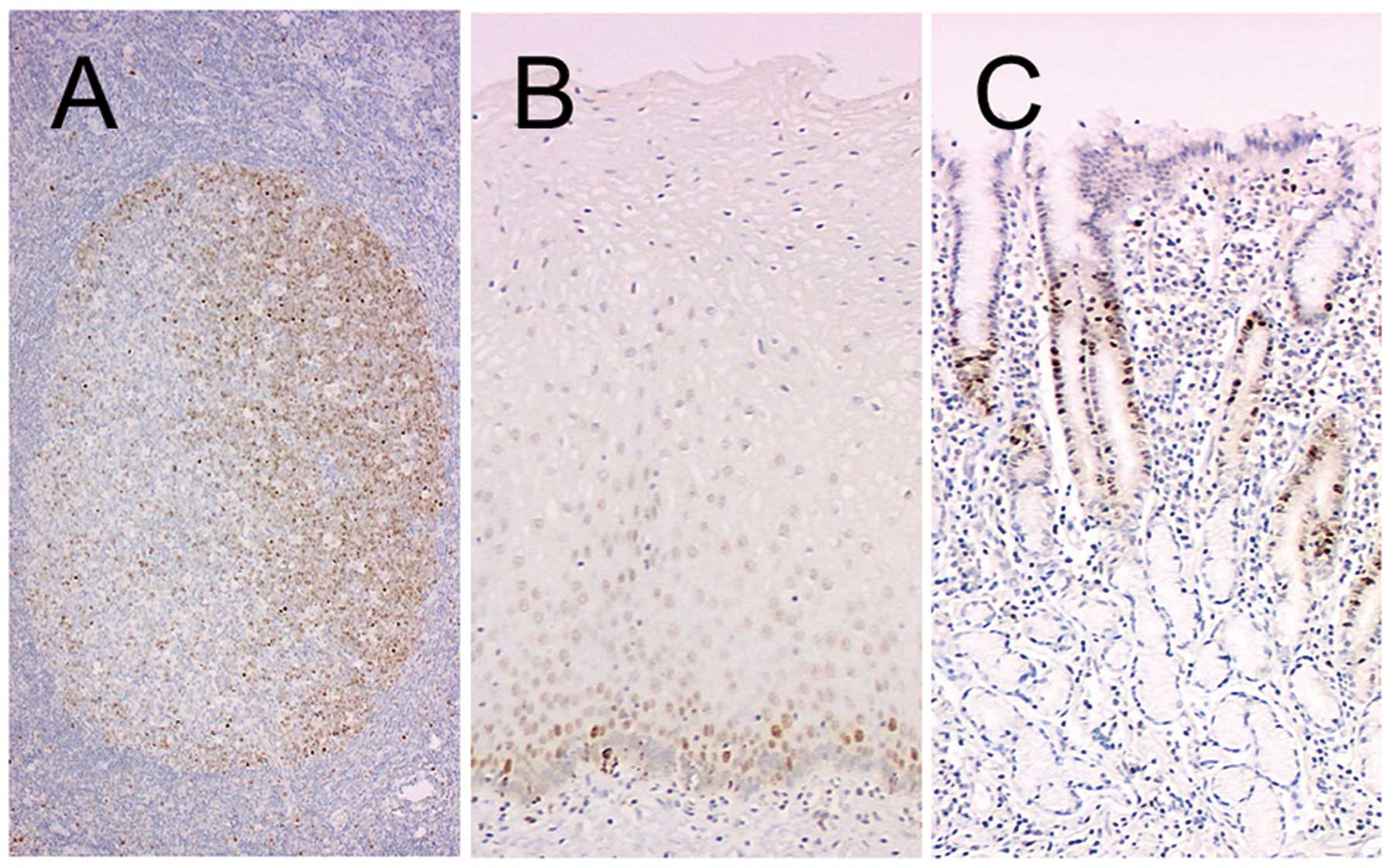
Tonsil, esophagus and stomach samples were used to
determine the distribution of Y-49-positive cells in normal
tissues. The cells in the dark zone of germinal centers, harboring
proliferating cells, were strongly positive (Fig. 4A), and suprabasal epithelial cells
of the esophageal epithelium (Fig.
4B) and the cells in the neck zone of the gastric epithelium
(Fig. 4C) also demonstrated a
positive reaction. By contrast, DM1A positivity was almost
restricted to the cytoplasm, although diffusely in almost all
cells.
Clinical characteristics
The data for clinical and histological
characteristics are summarized in Table I. The age of the patients ranged
from 9 to 90 years (median, 55 years); 67 were males and 49 were
females. Immunohistochemistry of the NHLs revealed that 30 cases
were of the T-cell lineage and 86 of the B-cell lineage. The
primary site was nodal in 81 patients and extranodal in 35
patients. The tumor stages were early (stage I/II) in 54 patients,
advanced (stage III/IV) in 60 patients and unknown in 2 patients.
According to the REAL classification, the cases were subclassified
into 60 cases of diffuse large B-cell lymphoma, 13 cases of
follicle center cell lymphoma and 30 cases of T-cell type lymphoma,
the remaining 13 being of other subtypes (Table I). Shortly after diagnosis, chemo-
and/or radiotherapy were performed in 112 patients. Such therapy
was effective in 91 cases.
 | Table IClinical and histopathological
characteristics of the 116 patients with non-Hodgkin’s
lymphoma. |
Table I
Clinical and histopathological
characteristics of the 116 patients with non-Hodgkin’s
lymphoma.
| Y-49 labeling indices
(LIs) |
|---|
|
|
|---|
| Characteristic | Total (%) | High (%) | Low (%) | p-value |
|---|
| Median age | 55 | 59 | 49 | 0.1827 |
| (years) | 9–90 | 9–90 | 10–83 | |
| Gender | | | | 0.9553 |
| Male | 67 (57.8) | 40 (59.7) | 27 (40.3) | |
| Female | 49 (42.2) | 29 (59.2) | 20 (40.8) | |
| T/B lineage | | | | 0.4256 |
| T-cell | 30 (25.9) | 16 (53.3) | 14 (46.7) | |
| B-cell | 86 (74.1) | 53 (61.6) | 33 (38.4) | |
| Primary site | | | | 0.0031 |
| Nodal | 81 (69.8) | 41 (50.6) | 40 (49.4) | |
| Extranodal | 35 (30.2) | 28 (80.0) | 7 (20.0) | |
| Histological
subtype | | | | 0.0005 |
| Diffuse large
B | 60 (51.7) | 47 (78.3) | 13 (21.7) | |
| Follicle
center | 13 (11.2) | 2 (15.4) | 11 (84.6) | |
| T-cell
lymphoma | 30 (25.9) | 16 (53.3) | 14 (46.7) | |
| Others | 13 (11.2) | 4 (30.8) | 9 (69.2) | |
| Tumor stage | | | | 0.4939 |
| I/II | 54 (46.6) | 34 (63.0) | 20 (37.0) | |
| III/IV | 60 (51.7) | 34 (56.7) | 26 (43.3) | |
| Unknown | 2 (1.7) | 1 (50.0) | 1 (50.0) | |
| Therapy |
| Chemotherapy | 109 (94.0) | | | |
| Radiation | 54 (46.6) | | | |
| No therapy | 4 (3.4) | | | |
| Response to
therapy | | | | 0.6424 |
| Complete/partial
response | 91 (81.3) | 57 (62.4) | 34 (37.6) | |
| No response | 16 (14.3) | 9 (56.3) | 7 (43.8) | |
| Unknown | 5 (4.5) | 2 (40.0) | 3 (60.0) | |
Immunohistochemistry for NHL cases
The reaction for Y-49 was strong in the nuclei in
the positive NHL cases, but present in the cytoplasm in almost all
cases. Therefore, we did not devote further attention to the
cytoplasmic positivity.
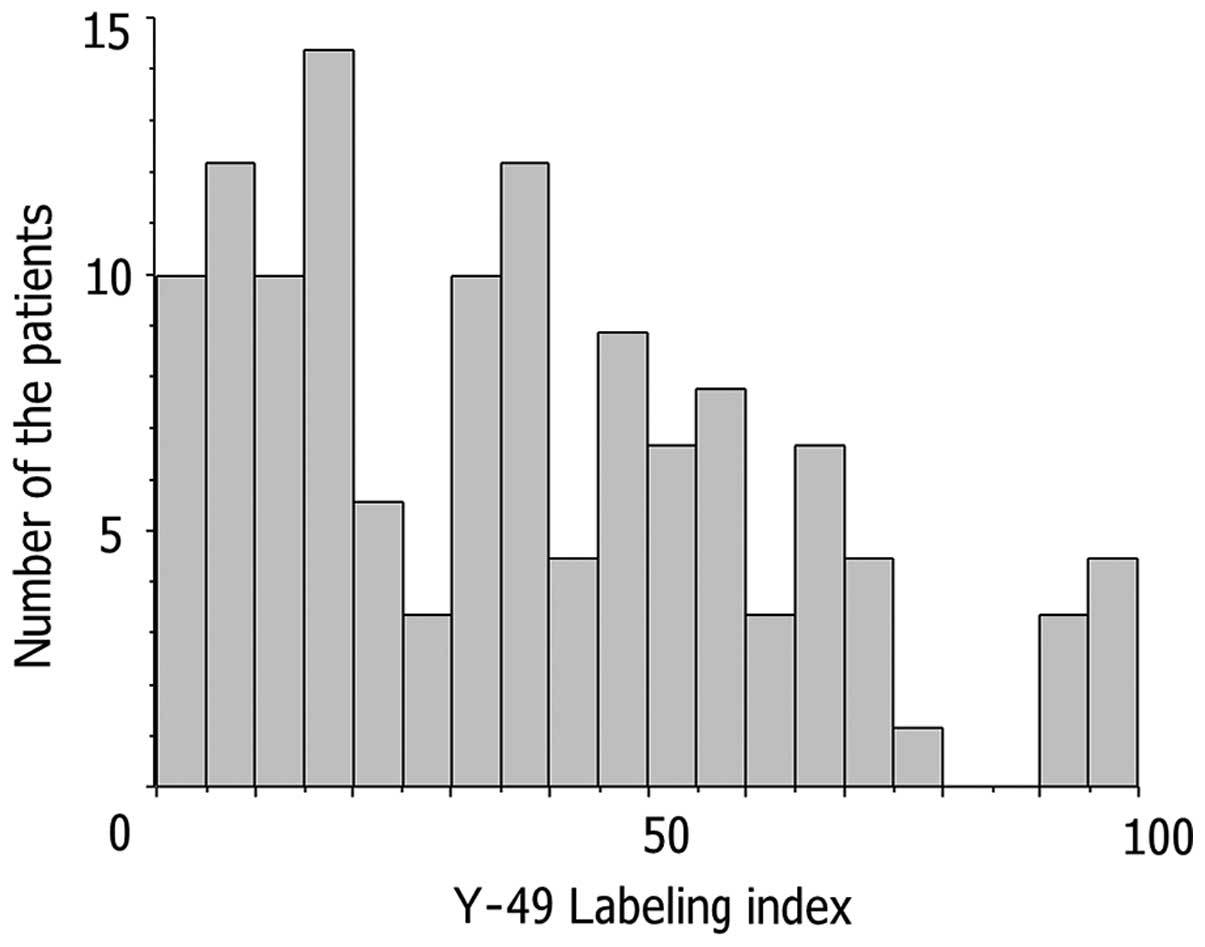
Histograms of Y-49 LIs for NHL cases, demonstrating
two major peaks, are shown in Fig.
5. The cut-off value was determined as the trough LI (25.0)
between the two peaks.
The results for Y-49 immunoreactivity in relation to
the LIs for MIB-1 were significantly correlated (p<0.0001).
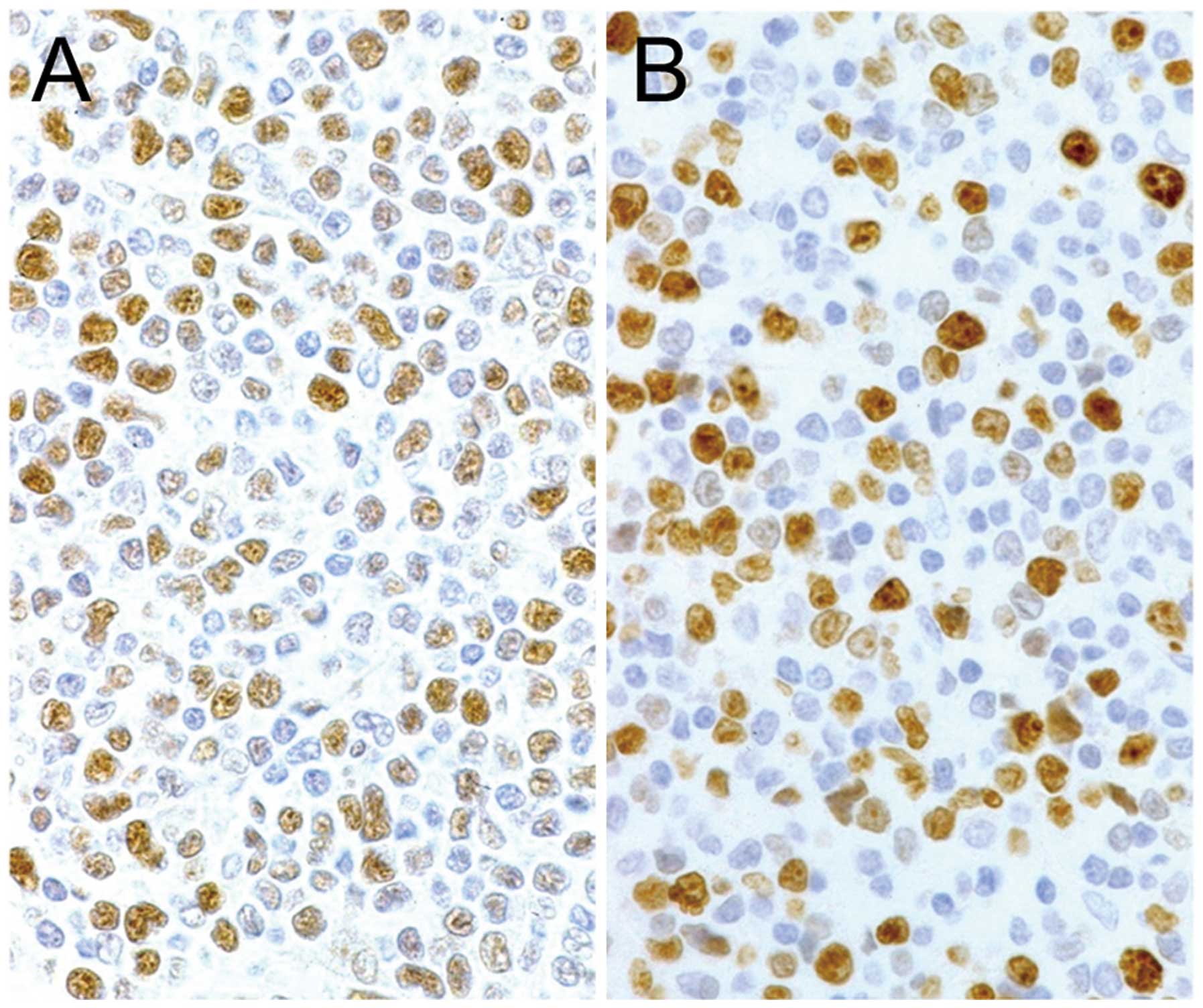
Microscopic features are shown in Fig. 6. On the basis of histogram results
of the LIs for Y-49, 47 (40.5%) and 69 (59.5%) of the 116 cases
were subdivided into groups with high and low LIs, respectively.
Cases of the diffuse large B-cell type were more likely to have
high LIs for Y-49 and MIB-1 than the other histological subtypes.
DM1A staining demonstrated no linkage to either Y-49 or MIB-1
staining.
Survival and evaluation of
clinicopathological variables in relation to reactivity for
Y-49
The overexpression of Y-49 binding proteins was
associated with older age (p=0.00348), an extranodal primary site
(p=0.0003) and the diffuse large B-cell type histological subtype
(p<0.0001). The other clinicopathologic variables did not show
any significant correlation with Y-49 (Table I). MIB-1 LIs did not correlate
with any of the variables examined in this study.
Follow-up data were available for all patients.
Overall survival ranged from 1 to 107 months, with a median of 28
months. With univariate analyses of all variables, older age
(>65 years, p=0.0460), tumor stage (I/II vs. III/IV, p=0.0006),
response to chemo- and radiotherapy (CR/PR vs. none, p<0.0001)
and reactivity for Y-49 (high vs. low, p=0.0183) showed strong
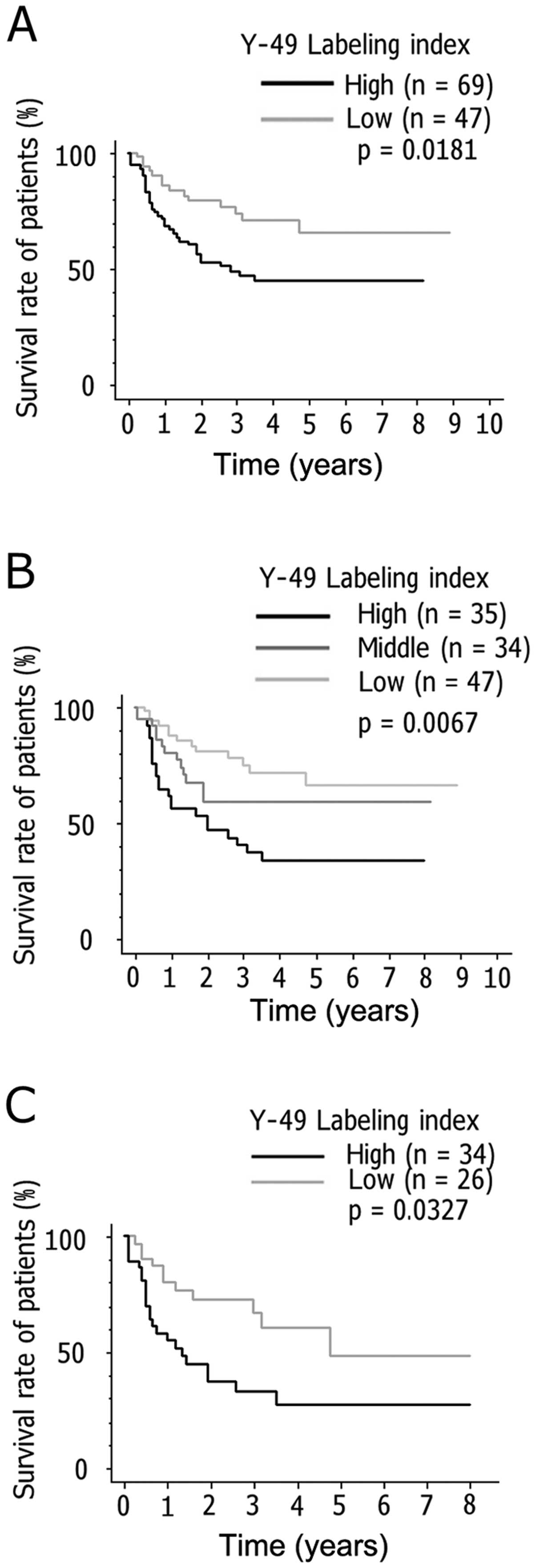
correlations with overall survival. Survival analysis by the
Kaplan-Meier method revealed significant differences in survival at
23 months between patients with and without overexpression of Y-49
binding proteins (p=0.0181; Fig.
7A). When the NHL cases were divided into three groups
according to a Y-49 LI higher than the mean + 1/2 standard
deviation (SD), within the mean ± 1/2 SD and lower than the mean -
1/2 SD, the Kaplan-Meier method also revealed significant
differences (p=0.0067; Fig. 7B).
In late-stage NHL cases, a higher Y-49 LI was also associated with
a poorer prognosis (p=0.0327; Fig.
7C). High MIB-1 LIs tended to be positively linked with
survival, although this was not statistically significant
(p=0.1605). The other clinicopathological variables did not show
any meaningful correlation with survival.
Multivariate analysis to identify prognostic factors
for survival was performed for 107 patients as follows: first, all
variables listed in Table I were
defined as baseline factors; then, Cox model forward and backward
selection procedures were applied to identify which of the four
variables described above were most strongly associated with
survival for the patients. All of the four variables proved to be
independent prognostic factors (Table II). Response to therapy and
reactivity for Y-49 demonstrated significant links with the
survival of the patients. By contrast, DM1A positivity had no
association with the survival of NHL patients.
 | Table IIResults of multivariate Cox analysis
in 107 patients with non-Hodgkin’s lymphoma. |
Table II
Results of multivariate Cox analysis
in 107 patients with non-Hodgkin’s lymphoma.
| Relative risk | 95%
CIa | p-value |
|---|
| Response to
therapy | 5.814 | 2.770–12.20 | <0.0001 |
| Overexpression of
Y-49 | 2.786 | 1.389–5.590 | 0.0039 |
| Stage of tumor | 2.530 | 1.270–5.040 | 0.0083 |
| Age (≥65
years) | 1.957 | 1.048–3.650 | 0.0349 |
Discussion
The present study indicated that α-tubulin
expression in NHL may play an important role in patient survival.
The mouse monoclonal antibody, Y-49, was newly raised against a
synthesized 17-amino acid polypeptide based on the survivin amino
acid sequence as an immunogen. When examined by FASTA of the
National Center of Biotechnology Information (21), this polypeptide showed no homology
with already known proteins apart from survivin. However, it
appears that the antibody also recognizes antigens completely
unrelated to the survivin molecule.
In the present study, a high Y-49 LI closely
correlated with the poor prognosis of patients with NHL, both for
all cases and advanced cases alone (stage III/IV). Among the
clinical and histopathological variables examined in the present
study, only old age, tumor stage, status of therapeutic response
and the overexpression of Y-49 binding proteins in the nuclei were
found to be independent prognostic factors, the latter two showing
the most significant correlations with survival. Moreover, Y-49 LIs
were strongly associated with proliferative activity, as assessed
by MIB-1 labeling. Indeed, Y-49 reacted with proliferating cells,
such as those in the dark zone of germinal centers, esophageal
supurabasal epithelial cells and gastric neck zone epithelial
cells, of normal tissues as observed in previous studies for Ki-S2
(22), Ki-67 (23) and proliferating cell nuclear
antigen (PCNA) (24). Similar
results were also obtained from flow cytometric analysis,
indicating Y-49-positive cells to have entered the cell cycle,
although the background noise of HSP90 and DM1A needs to be
carefully considered. There are many proliferation-associated
nuclear antigens, such as Ki-S2, Ki-67, PCNA and the laminin
receptor, whose overexpression is closely associated with the poor
survival of patients with malignant lymphomas and other malignant
epithelial tumors (25–29). Based on the present results, it
can be concluded that α-tubulin also plays a role in survival.
Whether this is directly linked to proliferation remains to be
determined.
While the overexpressed molecule in the nuclei
stained with Y-49 was shown to be α-tubulin by cell fractionation
and western blot analysis, a commercially available α-tubulin
monoclonal antibody (Clone DM1A) detected varying amounts in the
cytoplasm, but seldom demonstrated any nuclear binding. The
difference in reactivity from Y-49 is presumably due to the antigen
isotype, of which α-tubulin has six, differentially distributed
(15). For example, α1 is mostly
found in the brain and the lungs, less often in the testes, and
even less often in the heart, kidneys, muscle, spleen, stomach and
thymus (30). By contrast, α3/7
is expressed only in the testes at very high levels (30), whereas α2 follows a similar
pattern to α1 (31). There may be
a functional significance, but this has not been established yet
(2). Generally, tubulin molecules
are subjected to a large number of post-translational
modifications, such as phosphorylation, acetylation, tyrosination,
polyglutamylation and polyglycylation, positively or negatively
related to the regulation of MT assembly, MT stabilization and the
cell cycle (2,32). In activated human B-lymphocytes,
α-tubulin appears to be phosphorylated on a tyrosine residue near
the C terminus by the tyrosine kinase, Syk (33). Acetylation appears to occur
following incorporation into MTs, inducing an increase in their
stabilization (34). Tyrosinated
and non-tyrosinated α-tubulin often form different MTs in the same
cell; the former is common in the interphase network and in the
spindle, while the non-tyrosinated form occurs in some interphase
MTs (35). DM1A was produced
through the immunization of native chick brain microtubules and
recognizes an epitope in the parts of the C terminal region of the
α-tubulin (36). It is considered
that the differences between α-tubulin detected by Y-49 and DM1A
are derived from variations in the recognized epitopes and/or
post-translational modifications.
Y-49 also recognizes HSP90, albeit only weakly. This
may be only a cross-reaction, but it has been reported that HSP90
can bind to tubulin and inhibit its polymerization (37). In the present study,
immunoprecipitation proved the binding of HSP90 and α-tubulin.
Thus, our data suggest that Y-49 detects the epitope of α-tubulin
which binds to HSP90.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research from the Ministry of Education, Science,
Sports, Culture and Technology of Japan.
References
|
1
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luduena RF: Multiple forms of tubulin:
different gene products and covalent modifications. Int Rev Cytol.
178:207–275. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dutcher SK: The tubulin fraternity: alpha
to eta. Curr Opin Cell Biol. 13:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiebel E: γ-tubulin complexes: binding
to the centrosome, regulation and microtubule nucleation. Curr Opin
Cell Biol. 12:113–118. 2000.
|
|
5
|
Dutcher SK and Trabuco E: The UN13 gene is
required for the assembly of basal bodies in Chlamydomonas and
encodes delta tubulin, a new member of the tubulin superfamily. Mol
Biol Cell. 9:1293–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruiz F, Garreau de Lobresse N and Beisson
J: A mutation affecting basal body duplication and cell shape in
Paramecium. J Cell Biol. 104:417–430. 1987.PubMed/NCBI
|
|
7
|
Ruiz F, Krzywicka A, Klotz C, et al: The
SM19 gene, required for duplication of basal bodies in
Paramecium, encodes a novel tubulin, eta-tubulin. Curr Biol.
10:1451–1454. 2000. View Article : Google Scholar
|
|
8
|
Chang P and Stearns T: δ- and ɛ-tubulin:
two new human centrosomal tubulins reveal new aspects of centrosome
structure and function. Nat Cell Biol. 2:30–35. 2000.
|
|
9
|
Vaughn S, Attwood T, Navarro M, Scott V,
McKean P and Gull K: New tubulins in protozoal parasites. Curr
Biol. 10:R258–R259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Draberova E, Lukas Z, Ivanyi D, Viklicky V
and Draber P: Expression of class III β-tubulin in normal and
neoplastic human tissues. Histochem Cell Biol. 109:231–239.
1998.
|
|
11
|
Katsetos CD, Herman MM, Frankfurter A, et
al: Cerebellar desmoplastic medulloblastomas. A further
immunohistochemical characterization of the reticulin-free pale
islands. Arch Pathol Lab Med. 113:1019–1029. 1989.
|
|
12
|
Katsetos CD, Herman MM, Frankfurter A,
Uffer S, Perentes E and Rubinstein LJ: Neuron-associated class III
β-tubulin isotype, microtubule-associated protein 2, and
synaptophysin in human retinoblastomas in situ. Further
immunohistochemical observations on the Frlexner-Wintersteiner
rosettes. Lab Invest. 64:45–54. 1991.
|
|
13
|
Katosetos CD, Karkavelas G, Frankfurter A,
et al: The stromal Schwann cell during maturation of peripheral
neuroblastomas: immunohistochemical observations with antibodies to
the neuronal class III beta-tubulin isotype (beta III) and S-100
protein. Clin Neuropathol. 13:171–180. 1994.
|
|
14
|
Scott CA, Walker CC, Neal DA, et al:
Beta-tubulin epitope expression in normal and malignant epithelial
cells. Arch Otolaryngol Head Neck Surg. 116:583–589. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis SA and Cowan NJ: Complex regulation
and functional versatility of mammalian α- and β-tubulin isotypes
during differentiation of testis and muscle cells. J Cell Biol.
106:2023–2033. 1988.
|
|
16
|
Murai Y, Dobashi Y, Okada E, Ishizawa S,
Shiota M, Mori S and Takano Y: Study on the role of G1 cyclins in
Epstein-Barr virus-associated human lymphomas maintained in severe
combined immune deficiency (SCID) mice. Int J Cancer. 92:232–239.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okamura H, Sigal CT, Alland L and Resh MD:
Rapid high-resolution western blotting. Methods Enzymol.
254:535–550. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosenfeld J, Capdevielle J, Guillemot JC
and Ferrara P: In-gel digestion of proteins for internal sequence
analysis after one- or two- dimensional gel electrophoresis. Anal
Biochem. 203:173–179. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsui K, Jin XM, Kitagawa M and Miwa A:
Clinicopathologic features of neuroendocrine carcinoma of the
stomach: appraisal of small-cell and large-cell variants. Arch
Pathol Lab Med. 122:1010–1017. 1998.PubMed/NCBI
|
|
20
|
Harris NL, Jaffe ES, Stein H, et al: A
revised European-American classification of lymphoid neoplasms: a
proposal from the International Lymphoma Study Group. Blood.
84:1361–1392. 1994.PubMed/NCBI
|
|
21
|
Pearson WR and Lipman DJ: Imported tools
for biological sequence comparison. Proc Natl Acad Sci USA.
85:2444–2448. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heidebrecht HJ, Buck F, Steinmann J,
Sprenger R, Wacker HH and Parwaresch R: p100: a novel
proliferation-associated nuclear protein specifically restricted to
cell cycle phases S, G2 and M. Blood. 90:226–233. 1997.PubMed/NCBI
|
|
23
|
Reynolds GM, Rowlands DC and Mead GP:
Detection of Ki-67 antigen by a new sheep polyclonal antiserum. J
Clin Pathol. 48:1138–1140. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibahara K and Stillman B:
Replication-dependent marking of DNA by PCNA facilitates
CAF-1-coupled inheritance of chromatin. Cell. 96:575–585. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabattini E, Gerdes J, Gherlinzoni F, et
al: Comparison between the monoclonal antibodies Ki-67 and PC10 in
125 malignant lymphomas. J Pathol. 169:397–403. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki H: Expression of the 67-KD
laminin-binding protein in human lymphomas. Hum Pathol. 30:361–362.
1999. View Article : Google Scholar
|
|
27
|
Rudolph P, Alm P, Heidebrecht HJ, et al:
Immunologic proliferation marker Ki-S2 as prognostic indicator for
lymph node-negative breast cancer. J Natl Cancer Inst. 91:271–278.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fontanini G, Vignati S, Chiné S, et al:
67-Kilodalton laminin receptor expression correlates with worse
prognostic indicators in non-small cell carcinomas. Clin Cancer
Res. 3:227–231. 1997.PubMed/NCBI
|
|
29
|
Waltregny D, De Leval L, Ménard S, De
Leval J and Castronovo V: Independent prognostic value of the 67-kd
laminin receptor in human prostate cancer. J Natl Cancer Inst.
89:1224–1227. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewis SA and Cowan NJ: Tubulin genes:
structure, expression, and regulation. Avilla J: Microtubule
Proteins. CRC Press; Boca Raton, FL: pp. 37–66. 1990
|
|
31
|
Przyborski SA and Cambray-Deakin MA:
Developmental regulation of α-tubulin mRNAs during the
differentiation of cultured cerebellar granule cells. Brain Res Mol
Brain Res. 36:179–183. 1996.
|
|
32
|
MacRae TH: Tubulin post-translational
modifications: enzymes and their mechanisms of action. Eur J
Biochem. 244:265–278. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peters JD, Furlong MT, Asai DJ, Harrison
ML and Geahlen RL: Syk, activated by cross-linking the B-cell
antigen receptor, localizes to the cytosol where it interacts with
and phosphorylates α-tubulin on tyrosine. J Biol Chem.
271:4755–4762. 1996.PubMed/NCBI
|
|
34
|
Wilson PJ and Forer A: Acetylated
α-tubulin in spermatogenic cells of the crane fly Nephrotoma
suturalis: kinetochore microtubules are selectively acetylated.
Cell Motil Cytoskeleton. 14:237–250. 1989.
|
|
35
|
Gumdersen GG, Kalmoski MH and Bulinski JC:
Distinct populations of microtubules: tyrosinated and
nontyrosinated alpha tubulin are distributed differently in vivo.
Cell. 38:779–789. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Breitling F and Little M: Carboxy-terminal
regions on the face of tubulin and microtubules. Epitope locations
of YOL1/34, DM1A and DM1B. J Mol Biol. 189:367–370. 1986.
View Article : Google Scholar
|
|
37
|
Garnier C, Barbier P, Gilli R, Lopez C,
Peyrot V and Briand C: Heat-shock protein 90 (hsp90) binds in vitro
to tubulin dimmer and inhibits microtubule formation. Biochem
Biophys Res Commun. 250:414–419. 1998. View Article : Google Scholar : PubMed/NCBI
|