Introduction
DNA topoisomerases are one of the most promising
molecular targets for the development of anticancer agents
(1). They are nuclear enzymes
that transiently break one or two strands of DNA providing
solutions to various DNA topological problems associated with DNA
replication, transcription, recombination and other vital cellular
processes (2,3). Due to the crucial role of
topoisomerase in the maintenance and replication of DNA during
proliferation, cells become highly vulnerable when these functions
are lost (4). Therefore,
topoisomerases are attractive targets for designing anticancer
agents (5). DNA topoisomerases
are generally classified into topoisomerases I and II, depending on
their mechanisms of action, leading to either single or
double-strand breaks, respectively (6).
Several synthetic compounds, such as benzoxanthone
derivatives, thiosemicarbazones, benzophenanthridines, purine
analogues, anilinothiazoloquinolines, benzofuroquinolinediones,
coumarin derivatives and trisubstituted pyridines have been
reported as topoisomerase inhibitors (7). Previously, our research group
synthesized various rigid analogues of 2,4,6-trisubstituted
pyridines and evaluated thse analogues for their topoisomerase
inhibitory activity, as well as cytotoxicity, in order to determine
the effects of rigid structure on anticancer activity (8–10).
Rigid structures are commonly considered to have little
conformational entropy compared to flexible structures, and can be
more efficiently fitted into the active site of a receptor
(11). It has been reported that
planar molecules are able to intercalate into the DNA helix and
stabilize the topoisomerase-DNA covalent cleavage, converting
topoisomerase into a lethal DNA-damaging agent. Recently, we
synthesized various phenanthroline derivatives possessing a
quinoline core for the introduction of a rigid back bone bearing 5
membered aromatic rings, such as furyl or thienyl at the 4-position
and evaluated these derivatives for their topoisomerase I and II
inhibitory activity and cytotoxicity against several human cancer
cell lines. Among the tested compounds,
4-(furan-2-yl)-2-(pyridin-2-yl)-5,6-dihydro-1,10-phenanthroline
(FPDHP) was found to exhibit a significant and selective
topoisomerase I inhibitory activity of 60.9% at a dose of 100 μM
which corresponded a 1.25-fold greater activity than camptothecin,
and also to have strong cytotoxicity against several human cancer
cells [DU145 (prostate), HCT115 (colon) and T47D (breast)]
(12). However, the mechanisms
underlying the FPDHP-mediated cytotoxicity against cancer cells
remain elusive. In the present study, we investigated the
inhibitory effects of FPDHP on the growth of various types of
cancer cells, including Caki (kidney), A549 (lung), HT29 (colon)
and MDA-MB-231 (breast) camcer cells, and determined the molecular
and/or cellular mechanisms involved.
Materials and methods
Cell lines and culture
The MDA-MB-231 and A549 cells (American Type Culture
Collection, Manassas, VA, USA) were grown in RPMI-1640 medium
supplemented with 10% heated-inactivated fetal bovine serum (FBS),
2 mM L-glutamine, 100 μg/ml streptomycin and 100 μg/ml penicillin.
The Caki (American Type Culture Collection) and HT29 cells
(American Type Culture Collection) were grown in Dulbecco’s
modified Eagle’s medium, containing 10% heat-inactivated FBS, 20 mM
HEPES buffer and 100 μg/ml streptomycin and 100 μg/ml penicillin.
Caki cells overexpressing cellular FLICE-like inhibitory protein
(cFLIP) (Caki/cFLIP) or Akt (Caki/Akt) and the control cells
(Caki/vector) were kindly supplied by Dr T.K. Kwon (Keimyung
University, Daegu, Korea).
Drugs and materials
FPDHP was kindly supplied by Dr E.-S. Lee (Yeungnam
University, Daegu, Korea) (Fig.
1A). The pan-caspase inhibitor, z-VAD-FMK (z-VAD), was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Anti-cFLIP, anti-B-cell lymphoma-2 (Bcl-2),
anti-phospholipase C (PLC)-γ1 and anti-pro-caspase-3 antibodies
were purchased from Santa Cruz Biotechnology, Inc. Anti-Akt and
anti-phospho-Akt (p-Akt), anti-extracellular signal-related kinase
(ERK) and anti-cleaved caspase-3 antibodies were purchased from
Cell Signaling Technology (Beverly, MA, USA).
2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
inner salt (XTT) assay
Cell proliferation was detected by XTT assay
(WelGENE Inc., Daegu, Korea). When the cultured cells were in the
log phase, they were seeded on a 96-well plate (2×104
cells/100 μl/well) for 24 h. The cells were then treated with or
without FPDHP for 24 h. Absorbance (A) was detected using an enzyme
calibrator at 450 nm. Relative cell growth (%) = (A of study
group/A of control group) ×100.
Western blot analysis
Caki cells seeded in a 6-well plate
(4×105 cells/4 ml/well) the day before treatment were
treated with or without FPDHP in the presence or absence of the
pan-caspase inhibitor, z-VAD, for the indicated periods of time. To
prepare cellular lysates, the conditioned cells were initially
exposed to a lysis buffer (137 mM NaCl, 15 mM EGTA, 0.1 mM sodium
orthovanadate, 15 mM MgCl2, 0.1% Triton X-100, 25 mM
MOPS, 100 mM phenylmethylsulfonyl fluoride and 20 mM leupeptin,
adjusted to pH 7.2). The samples were further disrupted by
sonication and extracted at 48°C for 30 min. The lysates were
centrifuged at 10,000 × g for 15 min at 48°C, and the supernatant
fractions were collected. Approximately 50 μg of protein was
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and electrotransferred onto Immobilon-P
membranes (Millipore Corp., Billerica, MA, USA). The membranes were
incubated with blocking buffer (0.1% Triton X-100 with 5% non-fat
dry milk in TBS) for 30 min. Following 3 washes with TBST, the
membranes were incubated with primary antibody overnight. The
membranes were washed 3 times with TBST, and incubated with
HRP-conjugated secondary antibody. The detection of specific
proteins was carried out using an ECL western blotting kit
according to the manufacturer’s instructions (Millipore Corp.).
Flow cytometric analysis
Approximately 1×106 Caki cells were
suspended in 100 μl phosphate-buffered saline (PBS), and 200 μl of
95% ethanol were added while vortexing. The cells were incubated at
4°C for 1 h, washed with PBS and resuspended in 250 μl of 1.12%
sodium citrate buffer (pH 8.4) together with 12.5 μg RNase.
Incubation was continued at 37°C for 30 min. The cellular DNA was
then stained by applying 250 μl of propidium iodide (50 μg/ml) for
30 min at room temperature. The stained cells were analyzed by
fluorescence-activated cell sorting (FACS) on a FACScan flow
cytometer (Becton Dickinson and Co., Franklin Lakes, NY, USA) for
the relative DNA content based on fluorescence.
Asp-Glu-Val-Asp-ase (DEVDase) activity
assays
The cells were washed twice with PBS and incubated
in lysis buffer. Insoluble materials were removed by centrifugation
(15,115 × g for 10 min at 4°C), and protein concentrations were
quantified using the Bio-Rad protein assay (Bio-Rad, Hercules, CA,
USA). Caspase activities were determined with colorimetric assays
using caspase-3 (DEVDase) and caspase-8 activity assay kits
(Calbiochem, San Diego, CA, USA), according to the manufacturer’s
instructions. DEVDase assays were performed in 96-well microtiter
plates by incubating 20 μg of cell lysates in 100 μl of reaction
buffer (1% NP-40, 20 mm Tris-HCl, pH 7.5, 137 mm NaCl, 10%
glycerol) containing each caspase substrate (5 μm). The lysates
were incubated at 37°C for 2 h. Thereafter, absorbance at 405 nm
was measured using a spectrophotometer.
RNA isolation and reverse
transcriptase-polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted using TRIzol
reagent (Life Technologies Corp., Carlsbad, CA, USA). Single-strand
cDNA was synthesized from 2 μg of total RNA using M-MLV reverse
transcriptase (Promega, Madison, WI, USA) according to the
manufacturer’s instrucions. The cDNA for cFLIP and β-actin was
amplified using the following specific primers: cFLIP (sense)
5′-CCCAGTGGAC AGCGAGC-3′ and (antisense)
5′-ACTGCAGGCTTCCTGTGCGC-3′, and actin (sense)
5′-GGCATCGTCACCAACTGGGAC-3′ and (antisense)
5′-CGATTTCCCGCTCGGCCGTGG-3′. PCR amplification was carried out as
follows: 1 cycle (94°C, 3 min); 30 cycles (94°C, 45 sec; 59°C, 45
sec; and 72°C, 1 min); and 1 cycle (72°C, 10 min). PCR products
were analyzed by agarose gel electrophoresis and visualized by
ethidium bromide.
Results
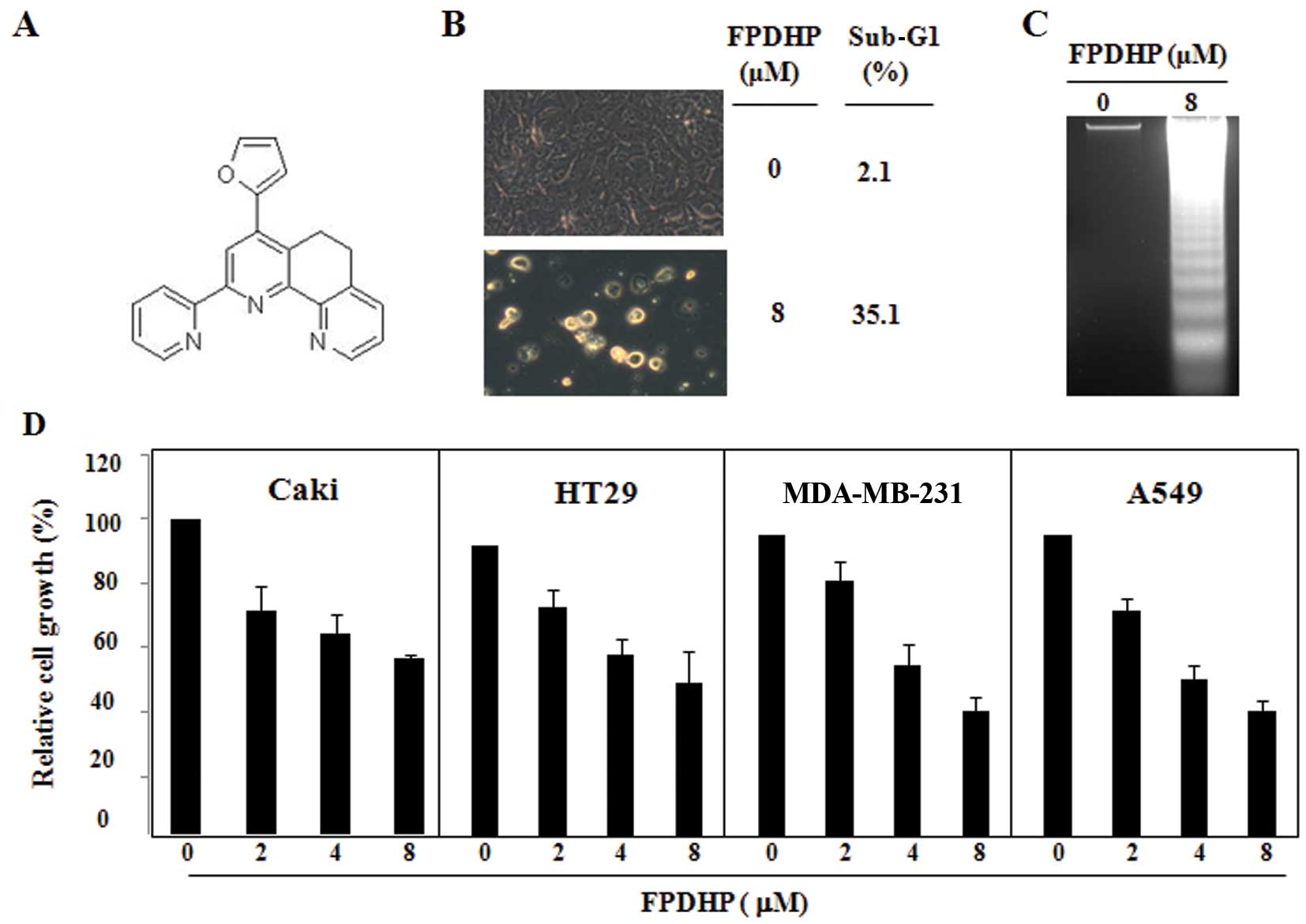
FPDHP induces apoptosis and DNA
fragmentation in Caki cells, and attenuates the growth of various
cancer cell lines
Initially, we investigated the cell death-inducing
ability of FPDHP in Caki cells. The Caki cells were treated for 24
h with the indicated concentrations of FPDHP and then evaluated for
morphological changes and DNA content following propidium iodide
staining. As shown in Fig. 1B,
compared with the control cells, 24 h of treatment with FPDHP (8
μM) induced cell detachment and morphological changes contributing
to apoptosis and increased the cell populations in the sub-G1
phase. Treatment with FPDHP (8 μM) markedly increased the amount of
DNA fragmentation (Fig. 1C). In
order to evaluate the anticancer effects of FPDHP, various types of
cancer cells, such as Caki, HT29, MDA-MB-231 and A549 cells were
treated with FPDHP for 24 h, followed by the measurement of the
respective cell growth by XTT assay. FPDHP attenuated the growth of
all the cells tested in a dose-dependent manner (Fig. 1D).
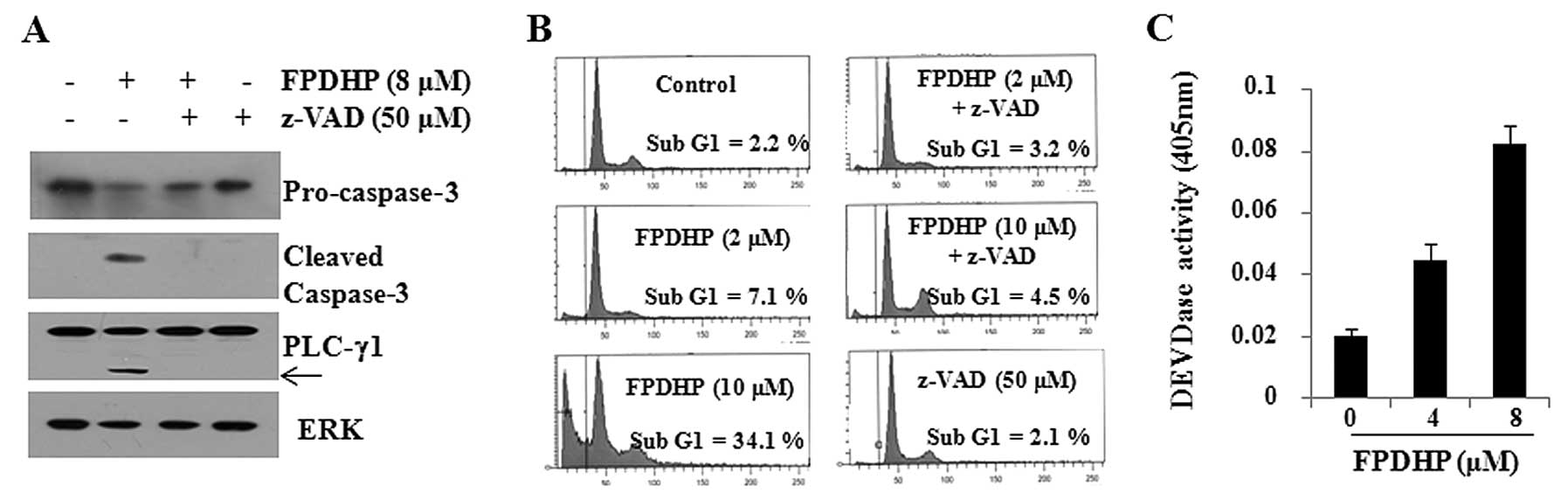
Apoptosis induced by FPDHP is dependent
on caspase activation
Considering that caspases play key roles in
apoptosis, we then examined whether FPDHP triggers the activation
of caspases in Caki cells. FPDHP increased the DEVDase activity in
Caki cells, indicating the activation of caspases by this small
molecule (Fig. 2A). We then
evaluated caspase dependency in FPDHP-mediated Caki cell death. For
this purpose, we treated the Caki cells with or without FPDHP in
the presence or absence of z-VAD for 24 h, followed by the
measurement of the cell populations in the sub-G1 phase and the
cellular levels of pro-caspase-3, PLC-γ1 and ERK in the conditioned
cells by FACS and western blot analysis. FPDHP increased the
population of Caki cells in the sub-G1 phase, which was largely
suppressed by pre-treatment with z-VAD, a pan-caspase inhibitor
(Fig. 2B). FPDHP decreased the
cellular levels of pro-caspase-3 (inactive), while it increased the
cleaved forms of caspase-3 (active) and PLC-γ1, a downstream
substrate of caspases, in the Caki cells (Fig. 2C). However, the FPDHP-mediated
decrease in the levels of pro-caspase-3 and the increase in the
levels of caspase-3 and PLC-γ1 were not observed in the cells
pre-treated with z-VAD. These results suggest that the activation
of caspase-3 is a key executioner of the apoptosis induced by
FPDHP.
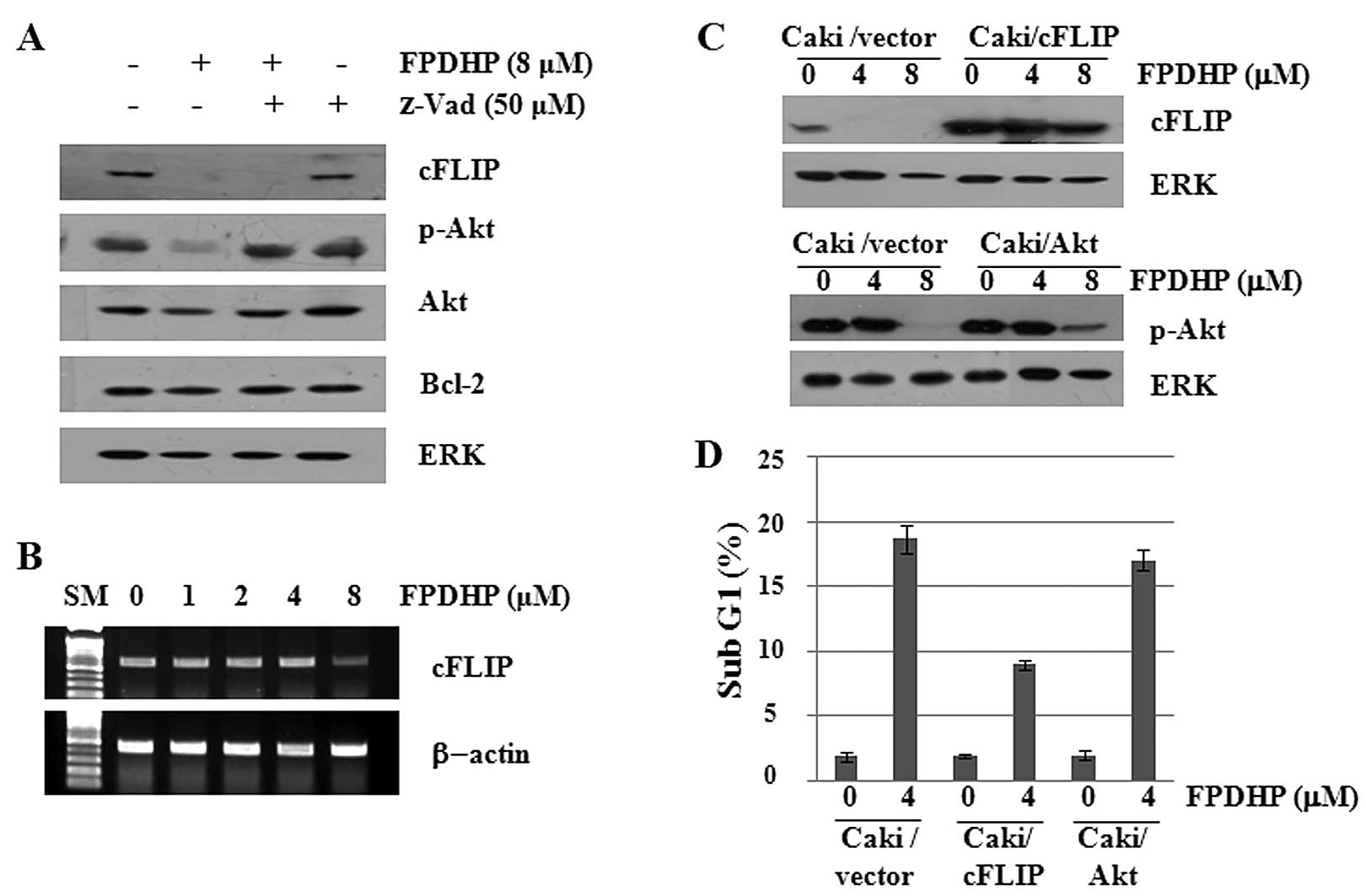
FPDHP downregulates cFLIP and p-Akt
expression in Caki cells, and overexpression of cFLIP partially
inhibits FPDHP-mediated apoptosis
To further evaluate the apoptosis-inducing
mechanisms of FPDHP, we then determined whether FPDHP affects the
expression levels of cell growth- and/or apoptosis-related
signaling proteins in the Caki cells. As shown in Fig. 3A, compared with the control
(untreated cells), FPDHP decreased the cellular levels of cFLIP,
p-Akt and total Akt. FPDHP, however, did not alter the expression
levels of Bcl-2. Distinctly, pre-treatment with z-VAD blocked the
downregulation of p-Akt, but not that of cFLIP, which was induced
by FPDHP. Furthermore, FPDHP also suppressed the mRNA expression of
cFLIP (Fig. 3B). These results
suggest that treatment with FPDHP leads to the downregulation of
cFLIP expression at the transcriptional levels. To determine
whether the downregulation of cFLIP and p-Akt contributes to
FPDHP-mediated apoptosis, we treated the control cells
(Caki/vector), cFLIP overexpressing cells (Caki/cFLIP) or the Akt
overexpressing cells (Caki/Akt) with or without FPDHP, followed by
the measurement of cFLIP or p-Akt expression levels and the
populations of cells in the sub G1 phase by western blot analysis
and flow cytometry, respectively. The overexpression of cFLIP, but
not that of p-Akt, led to a partial attenuation of FPDHP-mediated
apoptosis (Fig. 3C and D).
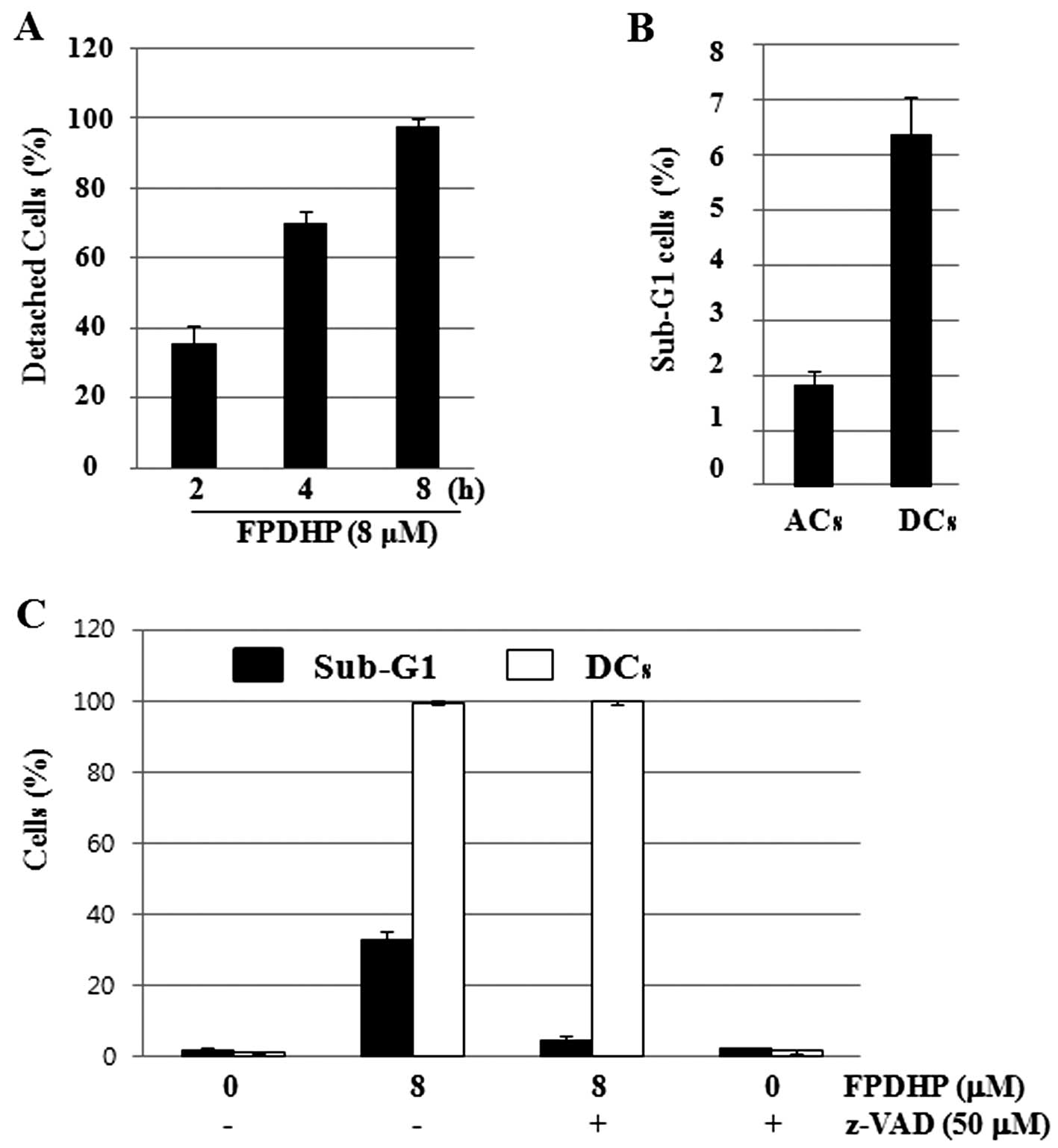
FPDHP rapidly induces cell detachment and
increases the number of apoptotic cells in the detached cells
Considering that FPDHP led to the detachment of Caki
cells from the cell culture dish, particularly during the early
treatment periods (Fig. 1B), we
then analyzed the cell detachment-inducing capacity and kinetics of
FPDHP and its association with FPDHP-mediated apoptosis. To this
end, we treated the Caki cells with or without FPDHP for the
designated periods of time, and harvested and counted the numbers
of attached cells (ACs) and detached cells (DCs) separately at each
time point. The numbers of DCs were increased in a time-dependent
manner, and most of the cells were detached within 8 h after FPDHP
treatment (Fig. 4A). To evaluate
whether cell detachment affects apoptosis, the ACs and DCs were
harvested separately from the Caki cells treated with FPDHP (8 μM)
for 4 h, and the populations of cells in the Sub-G1 phase in the
ACs and DCs were measured by flow cytometry. The Sub-G1 ratio of
the DCs was higher than that of the ACs (Fig. 4B). These results suggest that cell
detachment induced by FPDHP may affect the induction of apoptosis.
To determine whether caspase activated by FPDHP affects
FPDHP-mediated cell detachment and apoptosis, the Caki cells were
treated with or without FPDHP in the presence or absence of z-VAD
for 24 h and the numbers of DCs and the populations of cells in the
Sub-G1 phase were measured by cell counting and flow cytometry,
respectively. As shown in Figs.
1B and 4A, respectively, the
numbers of DCs increased in a time-dependent manner, and most of
the cells were detached within 8 h after FPDHP treatment,
regardless of z-VAD pre-treatment (data not shown). Twenty-four
hours later, the cells treated with both FPDHP and z-VAD were
almost detached, but apoptosis was markedly suppressed by z-VAD
(Fig. 4D).
Discussion
Increasing the understanding of the underlying
molecular events regulating several different cell death
mechanisms, such as apoptosis, necroptosis and autophagic cell
death has opened many new possibilities in the development of novel
anticancer agents (13–15). Among several death mechanisms, the
induction of apoptosis is the most important method in the
treatment of cancers, as cancer is one of the scenarios where too
little apoptosis occurs, resulting in cancer cells that will not
die, and defects at any point along the apoptosis pathways lead to
the malignant transformation of the affected cells, tumor
metastasis and resistance to anticancer drugs (16,17). Therefore, a number of
apoptosis-modulating drugs have been developed (18). Previously, we designed and
synthesized FPDHP as a phenanthroline derivative, and demonstrated
its topoisomerase I inhibitory activity and cytotoxicity against
several human cancer cell lines (12). In this study, to the best of our
knowledge, we report for the first time the anti-growth and
pro-apoptotic effects of FPDHP on Caki human renal cancer cells
through multiple mechanisms, including caspase-dependent apoptosis
and the downregulation of cFLIP and the caspase-independent cell
detachment, which may suggest that FPDHP is a novel inducer of
apoptosis.
The induction of apoptosis is associated with a
variety of proteins and/or factors. Among these, caspase-3 is one
of the most important cell death-inducing proteases that cleave a
number of proteins essential for cell survival (19,20). In this study, we demonstrate that
FPDHP induces Caki cell death and attenuates the growth of various
cancer cell lines. In particular, the present study clearly
demonstrates that FPDHP stimulates the activity of caspase-3, the
cleavage of PLC-γ1, and increases the numbers of Caki cells in the
sub-G1 phase. Importantly, we demonstrate that pre-treatment with
the pan-caspase inhibitor, z-VAD, significantly inhibits the
majority of the anticancer responses induced by FPDHP, suggesting
that caspases play critical roles in FPDHP-mediated apoptosis in
Caki cells. FPDHP also induced cell death in other cancer cells,
such as HT29 and A549 cells, and these cell deaths were also
inhibited by pre-treatment with z-VAD (data not shown). These
results suggest that FPDHP has an ability to induce
caspase-dependent apoptosis in numerous cancer cells.
To further delineate the regulatory mechanisms
underlying the killing effect on Caki cells by FPDHP, we measured
the expression levels of proteins associated with cell growth
and/or apoptosis in the Caki cells. In this study, we demonstrate
that FPDHP decreases the expression of cFLIP, known as cellular
FLICE-inhibitory protein, by transcriptional repression in Caki
cells. However, in this study, pharmacological inhibition
experiments revealed that pre-treatment with z-VAD did not
attenuate the downregulation of cFLIP in the Caki cells treated
with FPDHP. These results thus indicate that the cFLIP
downregulation is caspase-independent. Furthermore, the
overexpression of cFLIP attenuated apoptosis induced by FPDHP.
cFLIP has been identified as an inhibitor of apoptosis triggered by
the engagement of death receptors, such as Fas or TRAIL, and
abnormal cFLIP expression has been identified in various types of
cancer (21,22). Therefore, this suggests that the
transcriptional downregulation of cFLIP by FPDHP may be important
for the induction of FPDHP-mediated apoptosis.
During the observation of cellular morphological
changes under a microscope, we have found that FPDHP significantly
induced cell detachment from the early incubation time points after
FPDHP treatment. Cell detachment-induced cell death is known as
anoikis. A number of studies have shown that when cancer cells are
detached from the original cancer mass, the induction of anoikis is
important for the prevention of cancer metastasis (23,24). In this study, we demonstrated that
FPDHP induced the detachment of the majority of Caki cells within 8
h of incubation, and the sub-G1 ratio of the DCs was much higher
than that of the ACs (Fig. 4A).
Moreover, we further demonstrated that pre-treatment with z-VAD
inhibited apoptosis, but not the cell detachment induced by FPDHP.
These results thus suggest that FPDHP downregulates certain types
of cell adhesion molecules in a caspase-independent manner, and
that cell detachment induced by FPDHP is associated with the
induction of caspase-dependent apoptosis.
Taken together, the results from the present study
demonstrate that FPDHP induces apoptosis in Caki cells through the
activation of caspases, the caspase-dependent downregulation of
cFLIP and cell detachment. These novel properties of FPDHP which
functions as a topoisomerase inhibitor suggest that this compound
is worthy of being developed as a novel anticancer agent.
References
|
1
|
Pommier Y: DNA topoisomerase I inhibitors:
chemistry, biology, and interfacial inhibition. Chem Rev.
109:2894–2902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang JC: DNA topoisomerases. Annu Rev
Biochem. 65:635–692. 1996. View Article : Google Scholar
|
|
3
|
Nitiss JL: Investigating the biological
functions of DNA topoisomerases in eukaryotic cells. Biochim
Biophys Acta. 1400:63–81. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kellner U, Rudolph P, Parwaresch R, et al:
Human DNA-topoisomerases - diagnostic and therapeuticimplications
for cancer. Onkologie. 23:424–430. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh SK, Ruchelman AL, Li TK, et al:
Nitro and amino substitution in the D-ring of
5-(2-dimethylaminoethyl)-2,3-methylenedioxy-5H-dibenzo[c,h)[1,6)naphthyridin-6-ones:
effect on topoisomerase-I targeting activity and cytotoxicity. J
Med Chem. 46:2254–2257. 2003.PubMed/NCBI
|
|
6
|
Forterre P, Gribaldo S, Gadelle D and
Serre MC: Origin and evolution of DNA topoisomerases. Biochimie.
89:427–446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bailly C: Contemporary challenges in the
design of topoisomerase II inhibitors for cancer chemotherapy. Chem
Rev. 112:3611–3640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong BS, Choi H, Kwak YS and Lee ES:
Synthesis of 2,4,6-tripyridyl pyridines, and evaluation of their
antitumor cytotoxicity, topoisomerase I and II inhibitory activity,
and structure-activity relationship. Bull Korean Chem Soc.
32:3566–3570. 2011. View Article : Google Scholar
|
|
9
|
Thapa U, Thapa P, Karki R, et al:
Synthesis of 2,4-diaryl chromenopyridines and evaluation of their
topoisomerase I and II inhibitory activity, cytotoxicity, and
structure-activity relationship. Eur J Med Chem. 46:3201–3209.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thapa P, Karki R, Yoo HY, et al:
2,4-Diaryl-5,6-dihydro-1,10-phenanthroline and
2,4-diaryl-5,6-dihydrothieno[2,3-h) quinoline derivatives for
topoisomerase I and II inhibitory activity, cytotoxicity, and
structure-activity relationship study. Bioorg Chem. 40:67–78.
2012.PubMed/NCBI
|
|
11
|
Lee SH, Van HT, Yang SH, et al: Molecular
design, synthesis and docking study of benz[b)oxepines and
12-oxobenzo[c)phenanthridinones as topoisomerase 1
inhibitors. Bioorg Med Chem Lett. 19:2444–2447. 2009.PubMed/NCBI
|
|
12
|
Thapa P and Lee ES:
2,4-Diaryl-5,6-dihydro-1,10-phenanthrolines with furyl or thienyl
moiety at 4-position: synthesis, topoisomerase I and II inhibitory
activity, and cytotoxicity. Bull Korean Chem Soc. 33:1769–1772.
2012. View Article : Google Scholar
|
|
13
|
Long JS and Ryan KM: New frontiers in
promoting tumour cell death: targeting apoptosis, necroptosis and
autophagy. Oncogene. 31:5045–5060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu X, Deng Q, Bode AM, et al: The role of
necroptosis, an alternative form of cell death, in cancer therapy.
Expert Rev Anticancer Ther. 13:883–893. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reyjal J, Cormier K and Turcotte S:
Autophagy and cell death to target cancer cells: exploiting
synthetic lethality as cancer therapies. Adv Exp Med Biol.
772:167–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mashima T and Tsuruo T: Defects of the
apoptotic pathway as therapeutic target against cancer. Drug Resist
Updat. 8:339–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez-Nieto S and Zhivotovsky B: Role
of alterations in the apoptotic machinery in sensitivity of cancer
cells to treatment. Curr Pharm Des. 12:4411–4425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ocker M and Höpfner M:
Apoptosis-modulating drugs for improved cancer therapy. Eur Surg
Res. 48:111–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emoto Y, Manome Y, Meinhardt G, et al:
Proteolytic activation of protein kinase C delta by an ICE-like
protease in apoptotic cells. EMBO J. 14:6148–6156. 1995.PubMed/NCBI
|
|
20
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Micheau O: Cellular FLICE-inhibitory
protein: an attractive therapeutic target? Expert Opin Ther
Targets. 7:559–573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Pan X, Zhang H, et al:
Overexpression of cFLIP in head and neck squamous cell carcinoma
and its clinicopathologic correlations. J Cancer Res Clin Oncol.
134:609–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiarugi P and Giannoni E: Anoikis: a
necessary death program for anchorage-dependent cells. Biochem
Pharmacol. 76:1352–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|