Introduction
Inflammation is an immune response to recover
injured or infected tissue. Under injury and infection, various
factors, such as cytokines and chemokines, are secreted by immune
cells and the cells are transferred to the area to resolve the
abnormal condition. The characteristics of inflammatory response
have been observed from the ancient era, and are still being
extensively studied in order to determine the correlation between
various diseases and inflammation (1). During inflammation, inducible nitric
oxide (NO) synthase (iNOS) and cyclooxygenase-2 (COX-2) play
important roles in amplifying the inflammatory response. The
enzymatic activity of iNOS converts larginine to NO (2). iNOS is expressed in response to
interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α),
interferon-γ and lipopolysaccharide (LPS). NO is beneficial in
eliminating microorganisms and improving the blood supply to
injured tissue; however, it can cause tissue damage when it forms
the highly reactive peroxynitrite by reacting with reactive oxygen
species (ROS) (3). COX-2, an
inducible form of COXs, is overexpressed in LPS-stimulated
macrophages. It carries out an enzymatic role, transforming
arachidonic acid to prostaglandin E2 (PGE2)
(4). During the inflammatory
response, NO increases COX activity, resulting in an increase in
the production of pro-inflammatory prostagalandins (PGs) including
PGE2. Consequently, the inflammatory response is
exacerbated (5).
Although inflammation is a solution to normalize
troubled tissue, immune dysfunction can convert inflammation into a
weapon which causes chronic inflammatory diseases. Rheumatoid
arthritis (RA) is a representative chronic inflammatory disease
characterized by synovial inflammation, the hyperplasia of synovial
tissues and the destruction of bone and cartilage, contributing to
joint disability. For patients with RA, TNF-α inhibitors are mainly
used to inhibit inflammatory responses, resulting in the reduction
of symptom aggravation (6,7).
In the 19th century, the mud bath, a folk remedy, was used to
ameliorate the symptoms of RA. A mud bath involves plant-derived
sediments, termed humic substances. The sediments are produced
through a humification process in the environment. The process
synthesizes and decomposes humic substances. As a result, stable
compounds remain in the sediments. Examples of plant-derived
sediments include peat, sapropel and mumie. Among the plant-derived
sediments, peat is a light brown to black organic material produced
under marshy conditions from decomposed waterlogged vegetation,
including mosses (8–10). Based on the traditional remedy,
the pharmacological effects of sediments have been demonstrated
in vivo and in vitro. In previous studies, humate, a
derivative from bituminous or brown coal, has been shown to exert
anti-inflammatory effects, including the inhibition of
hypersensitivity in rats (11),
of the granulation and adhesion of neutrophils (12), and of cytokine expression and
complement production of mononuclear lymphocytes (13).
There are several studies available demonstrating
the pharmacological effects of coal-derived sediments (11–13); however, to the best of our
knowledge, the anti-inflammatory properties of peat moss extracts
have not been investigated to date. In the present study, we aimed
to investigate the anti-inflammatory effects of peat moss aqueous
extracts (PME) on inflamed RAW 264.7 macrophages at the molecular
level, as well as to identify the signal transduction pathways
involved.
Materials and methods
Preparation of PME
Peat moss extracts were a kind gift from Green
Voltex Co. (Busan, Korea). Briefly, the peat moss was powdered, and
then filtered using 6-mesh screen. Ground peat moss (100 g) and
sodium bicarbonate (5 g) were mixed and fermented at 30°C for 5 to
7 days until reaching pH 7.0. The PME from the fermented peat moss
was prepared with hot distilled water (60°C) for 24 h, stirring 4–5
times during this period. The infusates were filtered through
Whatman No 4 paper and then centrifuged at 13,200 rpm. The total
volume was measured and the extraction yield (11.1 g) determined
with the dry weight of 1 ml duplicate samples. The extracts were
dried at 30°C before being used in the experiments.
Cell culture
The RAW 264.7 cells, murine macrophage-like cells,
were purchased from the American Tissue Culture Collection (ATCC,
Manassas, VA, USA). The cells were grown in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) and 1% (v/v) penicillin (100 U/ml)/streptomycin (100 μg/ml)
under humidified conditions of 5% CO2 at 37°C. The RAW
264.7 cells were stimulated to induce an inflammatory response
using LPS which as purchased from Sigma-Aldrich Chemical Co. (St.
Louis, MO, USA). DMEM, FBS and penicillin/streptomycin were
purchased from Cellgro Mediatech (Manassas, VA, USA).
Cell viability assay
Cell viability was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, the RAW 264.7 cells (5×105 cells/ml)
were seeded in a 96-well plate. The cells were incubated with 10,
25 and 50 μg/ml PME for 24 h or were pre-treated with 50 μg/ml PME
for 1 h and then stimulated with 500 ng/ml LPS or 1 mM
H2O2 for 24 h. Following incubation with PME,
LPS and H2O2, the cultured medium was changed
to fresh medium and the cells were incubated with 0.5 mg/ml MTT
solution (Sigma-Aldrich Chemical Co. ) for 2 h. Subsequently, the
supernatant was discarded and formazan blue, which was formed in
the cells, was dissolved with DMSO. The optical density was
measured at 540 nm using a microplate reader (Dynatech
Laboratories, Chantilly, VA, USA). The assay was performed in
triplicate.
Nitrite measurement
The RAW 264.7 cells were seeded in each well of a
96-well plate. The cells were treated solely with PME (10, 25 and
50 μg/ml) for 24 h or were pre-treated with 50 μg/ml PME for 1 h
and stimulated with 500 ng/ml LPS. Following incubation for 24 h,
the supernatant of each well was mixed with the same volume of
Griess reagent (Sigma-Aldrich Chemical Co.) for 10 min at room
temperature in the dark. The absorbance of the reacted supernatant
was measured at 540 nm using a microplate reader. All samples were
conducted in triplicate.
PGE2, TNF-α and IL-1β
measurement
To measure the inhibitory effects of PME on the
production of PGE2, TNF-α and IL-1β, enzyme-linked
immunosorbent assay (ELISA) kits were purchased from Cayman
Chemical Co. (Ann Arbor, MI, USA) for PGE2, and from
R&D Systems Inc. (Minneapolis, MN, USA) for TNF-α and IL-1β.
The cell culture conditions were same as those for the nitrite
measurement assay. Following incubation with PME and LPS for 24 h,
the concentration of PGE2, TNF-α and IL-1β in the
culture medium was determined by selective ELISA kits.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The cells were incubated with PME (10, 25 and 50
μg/ml) alone for 24 h or pre-treated with 50 μg/ml PME for 1 h
prior to LPS stimulation for 24 h. Total RNA was isolated from the
cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA). One microgram of total RNA was used for cDNA synthesis using
AccuPower® RT premix (Bioneer, Daejeon, Korea)
containing M-MLV reverse transcriptase. The iNOS, COX-2 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; used as an
internal control) genes, were amplified from the cDNA by PCR. The
PCR primers were as follows: iNOS (5′-ATG TCC GAA GCA AAC ATC AC-3′
and 5′-TAA TGT CCA GGA AGT AGG TG-3′), COX-2 (5′-ATG GTC AGT AGA
CTT TTA CGA CTA-3′ and 5′-GGA GAG ACT ATC AAG ATA GTG ATC-3′),
IL-1β (5′-GGG CTG CTT CCA AAC CTT TG-3′ and 5′-GCT TGG GAT CCA CAC
TCT CC-3′), TNF-α (5′-TCT CAT CAG TTC TAT GGC CC-3′ and 5′-GGG AGT
AGA CAA GGT ACA AC-3′), and GAPDH (5′-AGG CCG GTG CTG AGT ATG TC-3′
and 5′-TGC CTG CTT CAC CAC CTT CT-3′). The amplified DNA was
visualized on an agarose gel containing ethidium bromide (EtBr;
Sigma-Aldrich Chemical Co.).
Western blot analysis
The cells were cultured with or without PME for 1 h
prior to stimulation with 500 ng/ml LPS for 24 h. The control group
was cultured in medium without PME and were not treated with LPS.
For total protein extraction, the cells were lysed with lysis
buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM
ethylenediaminetetraacetic acid (EDTA), 1% NP-40, 1 mM
pheny-methylsulfonyl fluoride (PMSF) and 5 mM dithiothreitol (DTT)]
for 1 h. Insoluble materials were discarded by centrifugation at
14,00 rpm for 20 min at 4°C. In a parallel experiment, nuclear and
cytosloic proteins were prepared using nuclear extraction reagents
(Pierce, Rockford, IL, USA) according to the manufacturer’s
instructions. The protein concentration in the cell lysate was
determined using detergent-compatible protein assay from Bio-Rad
Laboratories (Hercules, CA, USA). Equal amounts of protein were
separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels. The
separated protein was transferred to nitrocellulose membranes
(Schleicher & Schuell, Keene, NH, USA) and subsequently blocked
with Tris-buffered saline (10 mM Tris-Cl, pH 7.4) containing 0.5%
Tween-20 and 5% non-fat dry milk for 1 h at room temperature. The
protein was probed with primary antibodies overnight at 4°C. After
probing with the primary antibodies, the membranes were incubated
with horseradish peroxidase-conjugated anti-rabbit IgG as the
secondary antibody, purchased from Amersham Corp. (Arlington
Heights, IL, USA). Using the enhanced chemiluminescence (ECL)
detection system (Amersham Corp.), immunoreactive bands were
detected and exposed to X-ray film. All primary antibodies,
including antibodies to iNOS, COX-2, nuclear factor (NF)-κB, IκBα,
p38, extracellular signal-regulated kinase (ERK), c-Jun
NH2-terminal kinase (JNK), Akt and heme oxygenase-1 (HO-1) were
purchased from Cell Singnaling Technology (Beverly, MA, USA) apart
from nuclear factor erythroid 2-related factor 2 (Nrf2) which was
from Abcam (Cambridge, UK).
Immunofluorescence staining
The RAW 264.7 cells were seeded on coverslip bottom
dishes for 24 h. The cells pre-treated with 50 μg/ml PME for 30 min
prior to LPS stimulation for 30 min. Following incubation with PME
and LPS, 4′,6-diamidino-2-phenylindole (DAPI; Roche Diagnostics
Corp., Indianapolis, IN, USA) staining was conducted for 15 min,
and 4% paraformaldehyde (Junsei Chemical Co., Ltd., Tokyo, Japan)
was used for fixing the DAPI-stained cells. The fixed cells were
blocked with 5% mouse and rabit serum (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA), and then antibodies for p65 or Nrf2 (1
μg/well) and 0.3% Triton X-100 were applied for 1 h. The cells were
incubated with Alexa Fluor 488-tagged anti-rabbit IgG (Cell
Signaling Technology, Berverly, MA, USA) for 1 h, and were embedded
with ProLong Antifade Reagent (Invitrogen, Eugene, OR, USA). The
cells were observed using a Nikon Eclipse 50i microscope equipped
with a charged-coupled device camera (Nikon, Tokyo, Japan). To
determine the subcellular regions of protein co-localization,
individual red-, blue- and green-stained images derived from the
same field were merged using with High-Content Analysis software
(Cambridge Healthtech Institute, Needham, MA, USA). Densitometric
analysis of the stained cells was evaluated using ImageJ
software.
Data analysis
Results are expressed as the means ± standard
deviation (SD). Differences in mean values between groups were
analyzed by a one-way analysis of variance followed by Dunnett’s
test. Differences were considered statistically significant with
P-values <0.05.
Results
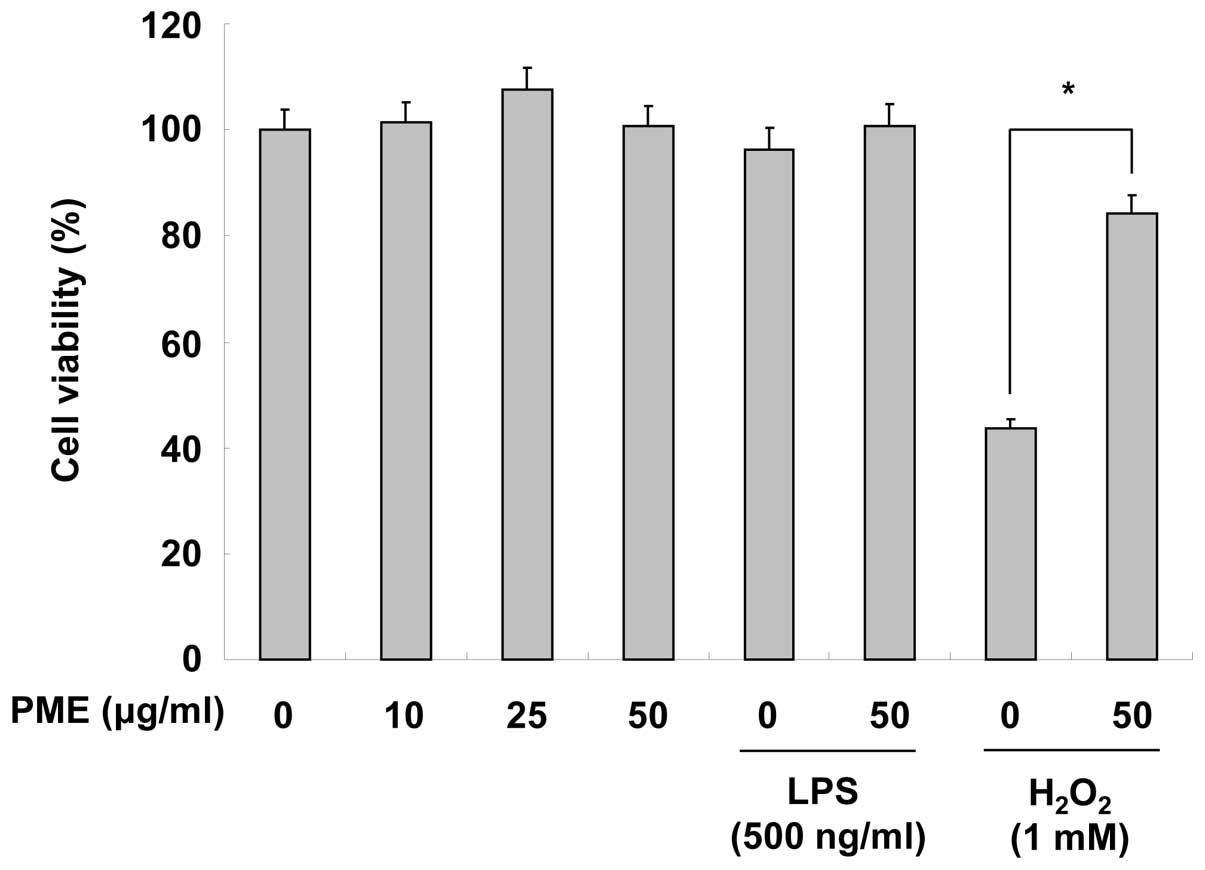
Cytotoxicity of peat moss extracts
Before analyzing the anti-inflammatory effects of
PME, we examined the cytotoxic effects of PME on RAW 264.7 cells by
MTT assay. Teatment with PME (10, 25 and 50 μg/ml) did not appear
cytotoxic to the RAW 264.7 cells. Stimulation with LPS (500 ng/ml)
also did not have any particular cytotoxic effect on the cells.
When the cells were treated with H2O2, cell
viability markedly decreased; however, pre-treatment with PME
inhibited the cytotoxic effects of H2O2 on
the RAW 264.7 cells (Fig. 1).
Hence, these results indicate that PME does not have any cytotoxic
effects and prevents oxidative stress.
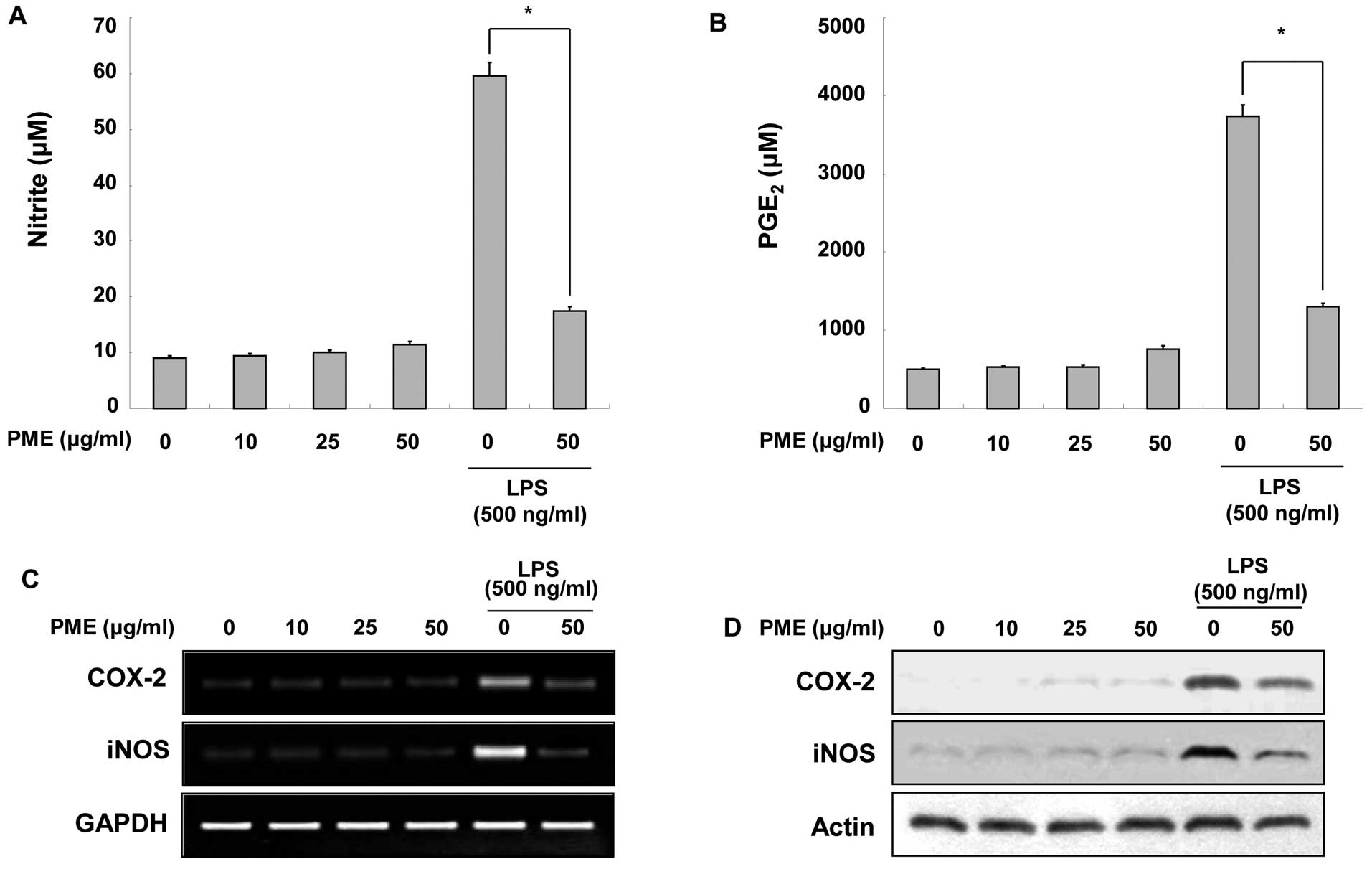
Regulatory effects of PME on the
production of NO and PGE2
To investigate the regulatory effects of PME on the
production of NO and PGE2, the RAW 264.7 cells were
treated with the indicated concentrations of PME. As shown in
Fig. 2A, the RAW 264.7 cells did
not produce NO following treatment with PME alone. LPS stimulation
increased NO production as compared to the basal levels without
LPS. Pre-treatment with 50 μg/ml PME inhibited the production of NO
in the LPS-stimulated RAW 264.7 cells. Similar to NO production,
treatment with PME did not induce a particular increase in
PGE2 levels in the intact RAW 264.7 cells (Fig. 2B). LPS stimulation increased
PGE2 production; however, treatment with PME prior to
LPS stimulation suppressed PGE2 amplification. Since the
production of NO and PGE2 was inhibited by PME, we
examined whether PME alters the expression of iNOS and COX-2 at the
mRNA and protein levels. In the RAW 264.7 cells not stimulated with
LPS, PME did not induce iNOS and COX-2 expression at the mRNA and
protein levels. The stimulation of RAW 264.7 cells with LPS
enhanced iNOS and COX-2 expression; however, the pre-treatment with
PME inhibited iNOS and COX-2 expression (Fig. 2C). These results indicate that PME
decreases NO and PGE2 production in LPS-stimulated RAW
264.7 cells by inhibiting iNOS and COX-2 expression, respectively.
Additionally, PME regulates the expression of iNOS and COX-2 at the
transcriptional level.
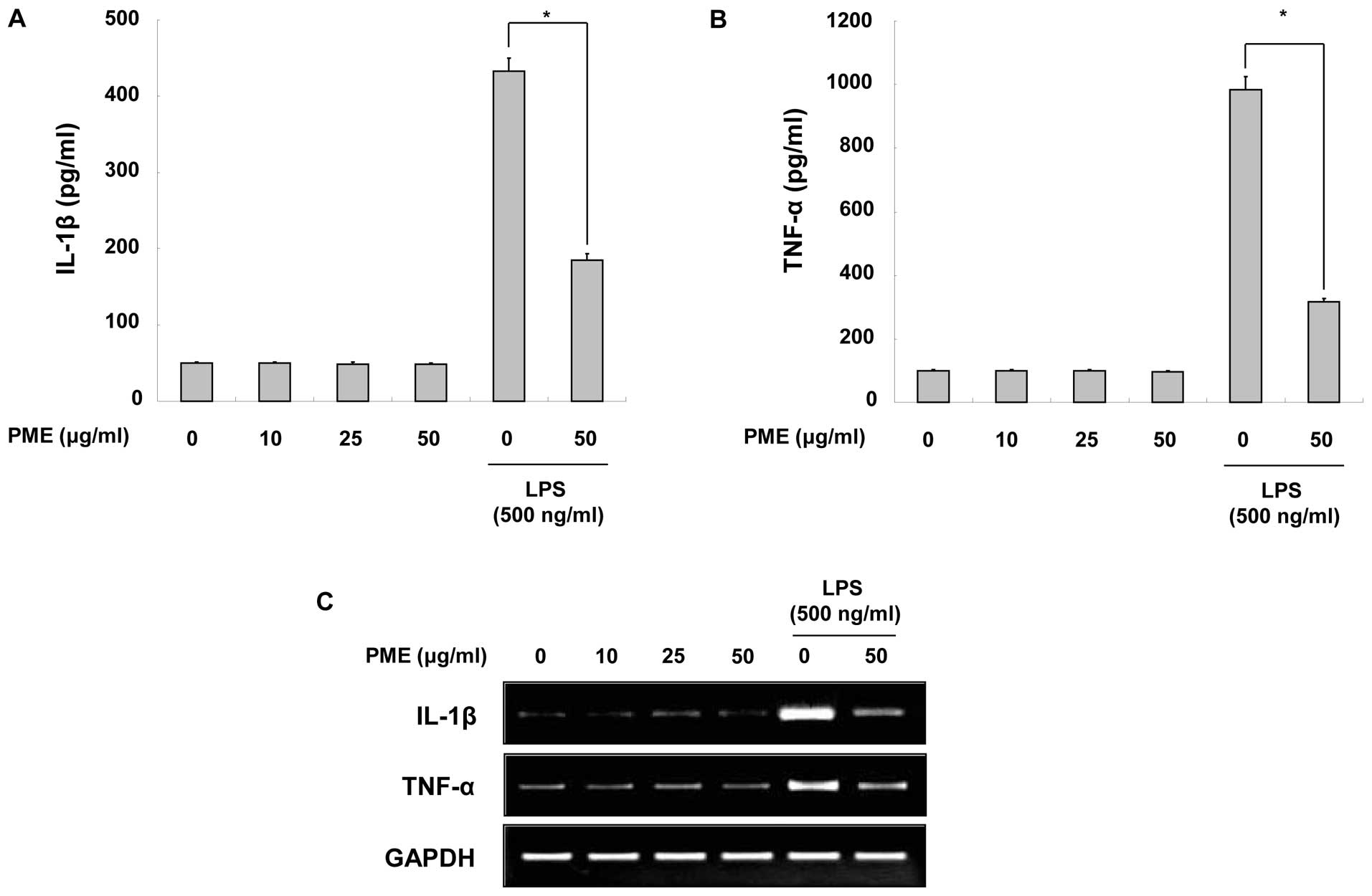
Regulatory effects of PME on the
production of pro-inflammatory cytokines
To identify the anti-inflammatory properties of PME,
we quantified the production of IL-1β and TNF-α in the cultured
medium. When the RAW 264.7 cells were treated solely with PME,
there were not particularly changes in cytokine production. The
production of IL-1β and TNF-α was increased by LPS stimulation;
however, pre-treatment with PME decreased cytokine production in
the LPS-stimulated RAW 264.7 cells (Fig. 3A and B). As cytokine secretion was
reduced by PME treatment, we evaluated whether PME negatively
regulates the mRNA synthesis of cytokines in the cells. As shown by
RT-PCR, the mRNA expression of the cytokines was downregulated
following pre-treatment with PME (Fig. 3C). Based on these results, we
identified that PME negatively regulates the production of
pro-inflammatory cytokines, including IL-1β and TNF-α, at the
transcriptional level.
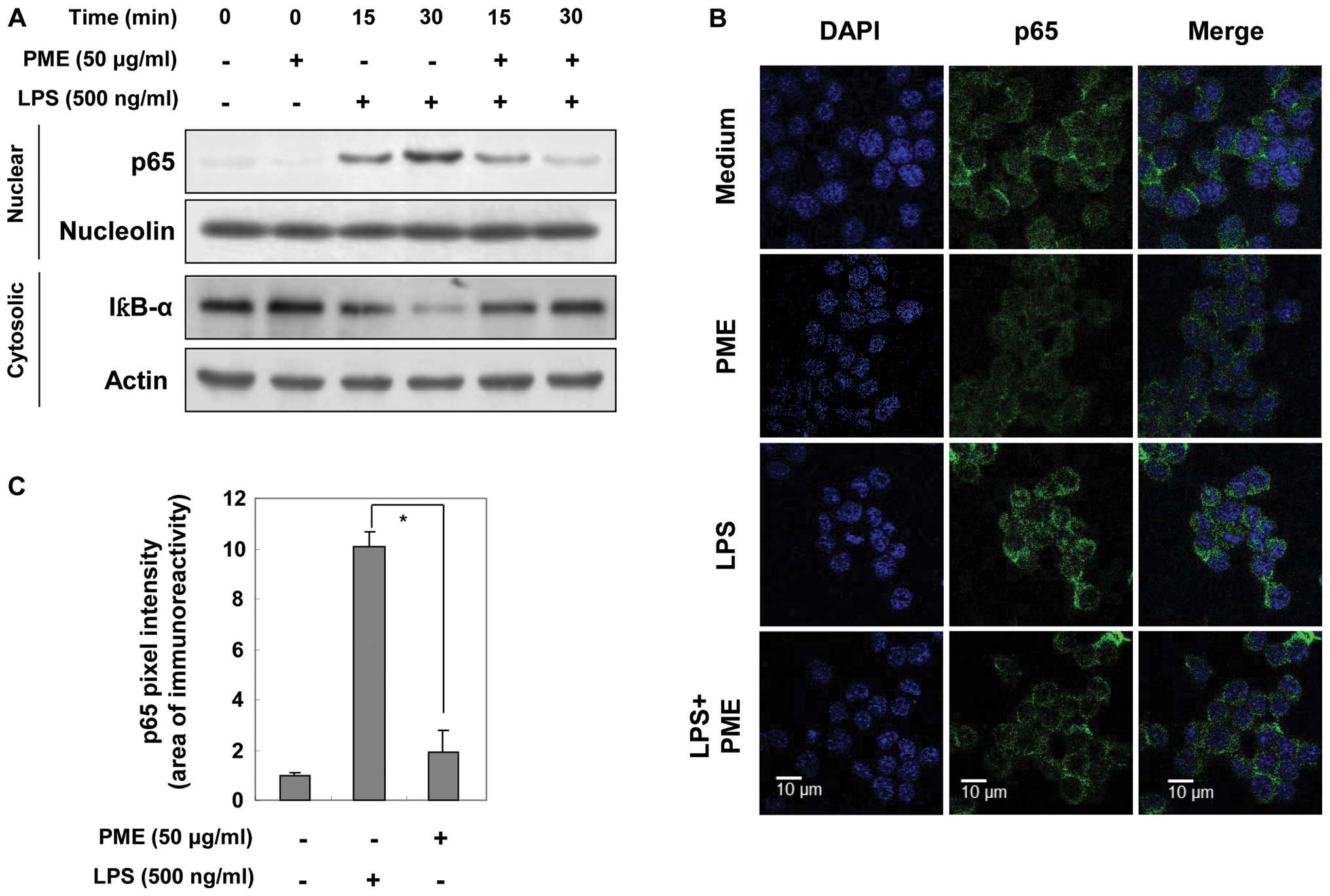
Regulatory effects of PME on NF-κB
activation
As shown by our results, the expression of iNOS,
COX-2 and pro-inflammatory cytokines, including TNF-α and IL-1β,
was regulated at the transcriptional level through PME treatment.
Based on these results, we evaluated the expression of nuclear p65,
a subunit of NF-κB and cytosolic IκBα by western blot analysis and
immunofluorescence. Before evaluating the inhibitory effects of PME
on p65 translocation, we investigated whether treatment with PME
alone induces the activation of p65. As shown in Fig. 4A, nuclear p65 expression was not
specifically amplified by PME. Stimulation with LPS gradually
increased nuclear p65 expression in a time-dependent manner.
However, pre-treatment with PME reduced the expression of nuclear
p65, which had returned to basal levels at 30 min of treatment with
PME. In the same manner, LPS stimulation decreased IκBα expression
in the cytosol in a time-dependent manner; however, pre-treatment
with PME somewhat amplified cytosolic IκBα expression at 30 min. In
order to confirm NF-κB inactivation by PME, we visualized the
location of NF-κB in the cells by immunofluorescence staining. In
the control group (medium only) and PME-treated group, NF-κB was
located in the cytosol. LPS stimulation facilitated the
translocation of NF-κB into the nucleus. In the group pre-treated
with PME and then with LPS, NF-κB was arrested in cytosolic area
(Fig. 4B). Densitometric analysis
of the nuclear area showed that nuclear p65 density was amplified
10-fold by LPS stimulation (Fig.
4C). Compared with the LPS-stimulated RAW 264.7 cells, the
PME-pre-treated cells maintained the basal levels of p65 intensity.
Thus, PME disrupts the NF-κB translocation into the nucleus by
helping to sustain IκBα expression, resulting in the
transcriptional regulation of iNOS, COX-2 and pro-inflammatory
cytokines.
Regulatory effects of PME on the
activation of mitogen-activated protein kinases (MAPKs) and
Akt
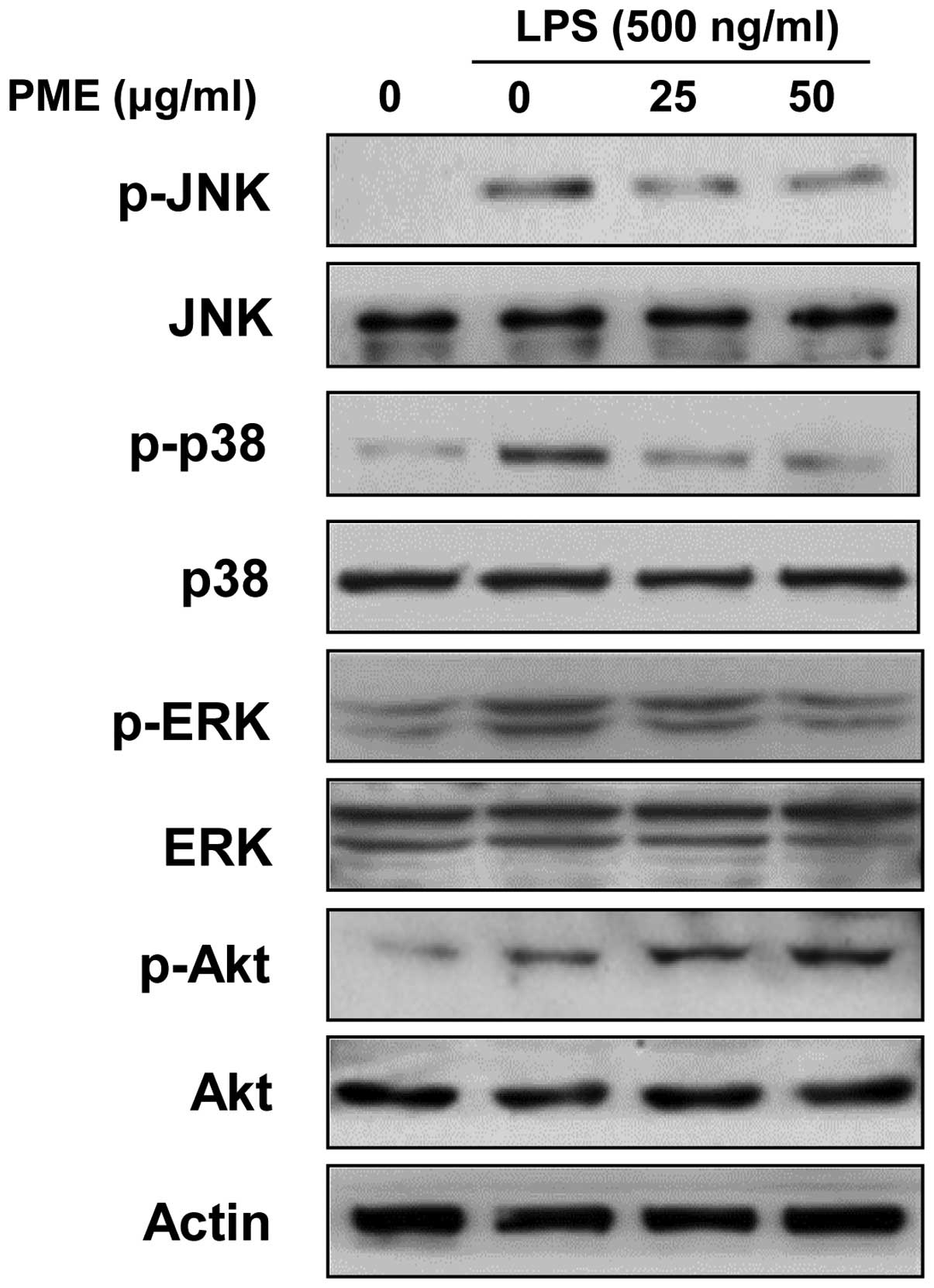
We examined whether the activation of MAPKs and Akt
in LPS-stimulated RAW 264.7 cells is regulated by PME. LPS
stimulation for 30 min activated all members of MAPKs.
Pre-treatment with PME for 1 h suppressed the phosphorylation of
MAPKs in a dose-dependent manner (Fig. 5). Although PME significantly
attenuated the activation of p38 MAPK and ERK, JNK was less
sensitively regulated than p38 MAPK and ERK. Akt activation was
increased by LPS stimulation, and treatment with PME induced the
further amplification of Akt activation in a
concentration-dependent manner. Our results suggest that the
inhibition of MAPK activation and the enhancement of Akt activation
suppresses the inflammatory response in LPS-stimulated RAW 264.7
cells.
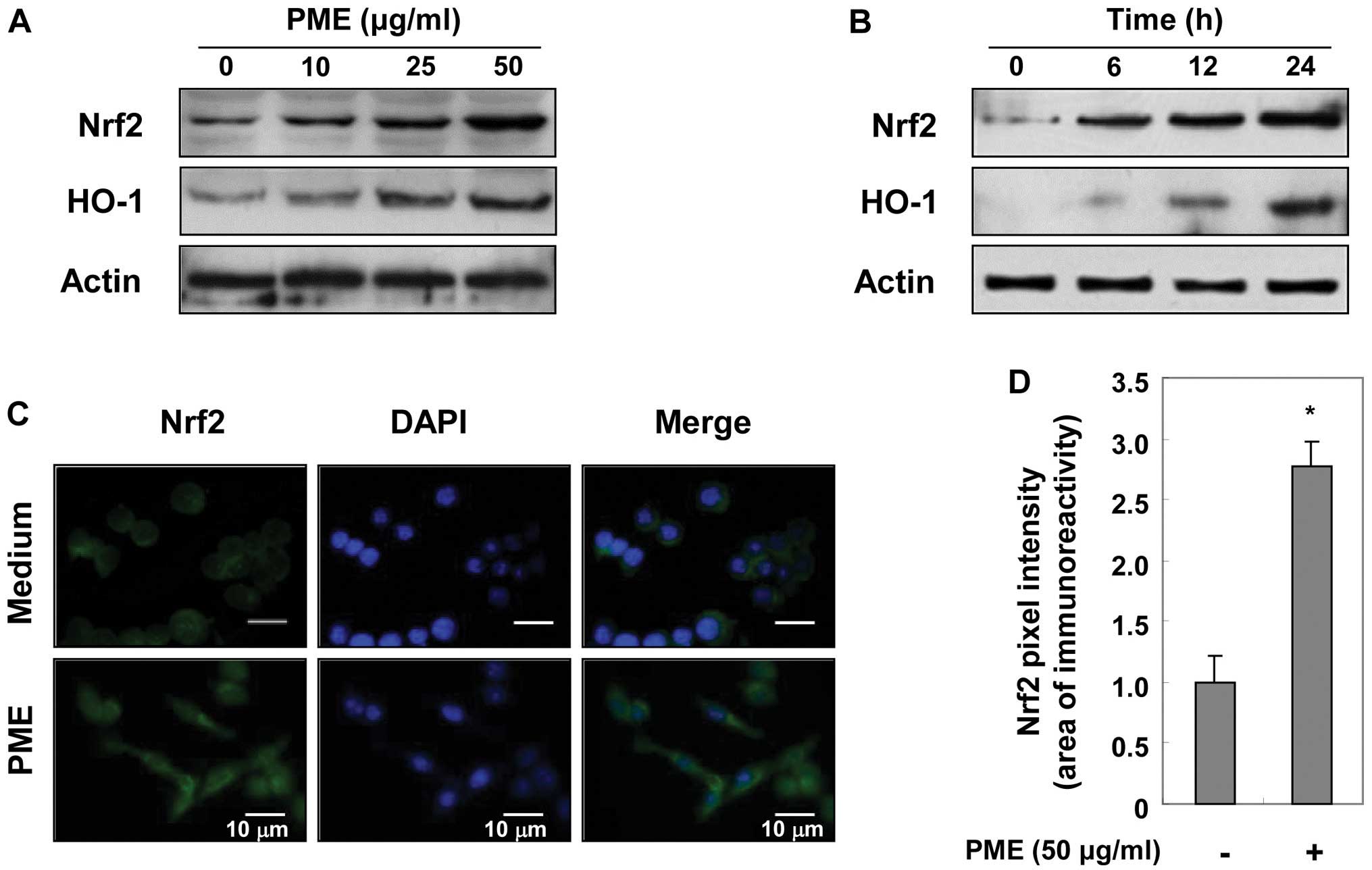
Regulatory effects of PME on Nrf2 and
HO-1 expression
As PME prevented cell death from
H2O2-induced oxidative stress (Fig. 1), we hypothesized that
pre-treatment with PME may enhance the expression of antioxidant
enzymes in RAW 264.7 cells. We examined whether the expression of
HO-1 and Nrf2 was regulated by PME. Initially, the RAW 264.7 cells
were cultured with PME at the indicated concentrations for 12 h. As
shown by western blot analysis, PME gradually enhanced Nrf2 and
HO-1 expression in a concentration-dependent manner (Fig. 6A). Using 50 μg/ml PME, protein
induction was evident at 6 h, and reached a maximum after 24 h of
treatment with PME (Fig. 6B). We
then examined the location of Nrf2 at 12 h using immunofluorescence
staining. Immunofluorescent intensity in the nucleus showed that
treatment with PME enhanced the translocation of Nrf2 into the
nucleus (Fig. 6C and D). These
results indicate that PME induces HO-1 expression by facilitating
Nrf2 activation.
Discussion
Peat moss has been used for the investigation of the
absorbance efficacy of irons, such as nickel and copper, and is
potentially used for purging hazardous irons from polluted water
(14,15). In the biomedical field,
hypersensitivity pneumonitis and chronic respiratory disorder of
peat moss processing factory workers have been investigated
(16,17). However, to the best of our
knowledge, the anti-inflammatory or antioxidant effects of PME have
not been investigated to date. In the present study, we demonstrate
the anti-inflammatory and antioxidant properties of PME in
LPS-stimulated RAW 264.7 cells.
In order to evaluate whether the inhibitory effects
of PME on LPS-induced NO production, MTT assay was conducted. PME
did not appear significantly cytotoxic to the RAW 264.7 cells,
although it protected the cells against
H2O2-induced oxidative stress. PME attenuated
the LPS-induced NO and PGE2 production, accompanied by
the downregulation of iNOS and COX-2 expression. In addition, in
RAW 264.7 cells PME negatively regulated the synthesis of
pro-inflammatory cytokines, including TNF-α and IL-1β, at the
transcriptional level. The results of the present study provide
evidence that PME induces the anti-inflammatory response in
LPS-stimulated RAW 264.7 cells. The inducible enzymes (iNOS and
COX-2) and their reaction products are associated with inflammatory
diseases. Both enzymes are upregulated in the inflammatory
response, and their reactive products, NO and PGE2,
respectively, are closely related to various chronic diseases, such
as ulcers and RA (18,19). TNF-α and IL-1β secreted from
activated monocytes and macrophages exerts a vareity of
pro-inflammatory effects on many cell types (20). These cytokines are important in
chronic inflammation, such as RA (21). Even though our experiments were
conducted under acute inflammatory conditions, our results provide
valuable information on the regulatory effects of PME on
macrophages, cells critical in the process of chronic inflammation
(22). There is evidence that a
folk remedy, a mud bath including peat, relieves the symptoms of RA
(23). Based on our results, peat
moss may have anti-inflammatory abilities in acute inflammatory
responses, which potentially ameliorates chronic inflammation,
including rheumatoid diseases.
The LPS-induced expression of the iNOS, COX-2
pro-inflammatory cytokines was regulated by PME at the
transcriptional level. In order to examine whether the inhibitory
effects of PME are mediated through the inactivation of NF-κB,
western blot analysis for nuclear p65 and cytosolic IκBα and a
microscopic observation for p65 were performed in the present
study. Pre-treatment with PME led to a significant decrease in
nuclear p65 levels and an increase in cytosolic IκBα levels. The
inhibitory effects of PME on p65 translocation into the nucleus and
p65 expression were also shown by microscopic observation (Fig. 4). NF-κB has been implicated in the
induction of the expression of iNOS and COX-2 protein. NF-κB is
primarily composed of two proteins, p65 and p50. In the resting
state, NF-κB can be found in the cytosol and is bound to the
inhibitory protein, IκB. The activation of cells with various
stimuli initiates IκB phosphorylation, which triggers proteolytic
degradation. NF-κB is then released from the inactive complex, and
translocates to the nucleus (24). Nuclear NF-κB binds to the κB
binding sites in the promoter regions of target genes. NF-κB
response elements are present on the promoters of iNOS and COX-2
(24,25). NF-κB is also involved in the
transcription of TNF-α and IL-1β (26). Our findings suggest that the
inhibition of NF-κB by PME may be due to the inhibition of IκBα
phosphorylation, thereby suppressing the translocation of p65.
Therefore, the PME-mediated regulation of the expression of iNOS,
COX-2, and pro-inflammatory cytokines was induced by the inhibition
of NF-κB activation.
LPS-stimulated signaling events in macrophages lead
to the activation of several MAPK signaling pathways. MAPKs consist
of three major members, including ERK, p38 MAPK and JNK, which are
activated by MAPK kinases (MEKs) in LPS-stimulated macrophages.
Although LPS stimulation activates all MAPK families, each kinase
can be differentially activated in response to a particular
stimuli. In the case of ERK activation, growth factors and phorbol
esters play roles as stimulators. JNK and p38 MAPK are selectively
activated by cellular stress, UV light and osmosis (27,28). LPS-induced MAPK activation leads
to the expression of iNOS, COX-2, TNF-α and IL-1β in macrophages.
The activation of ERK and p38 MAPK is related to the expression of
COX-2, TNF-α and IL-1β. ERK and p38 MAPK activates cyclic AMP
response element-binding protein (CREB) through mitogen- and
stress-activated protein kinase (MSK)-1, resulting in the
expression of COX-2 and IL-1β. In the case of IL-1β, the activation
of MAP kinase-activated protein kinase 2 (MAPKAP-K2) by p38 MAPK
mainly regulates IL-1β expression (29). iNOS expression by LPS stimulation
is augmented by JNK activation. In LPS-stimulated macrophages, p38
MAPK counteracts JNK which suppresses iNOS expression. Treatment
with SB203580, a p38 MAPK inhibitor, facilitates JNK
phosphorylation and iNOS expression (30). In the present study, the
activation of JNK, p38 MAPK and ERK was downregulated by PME in a
concentration-dependent manner, suggesting that the inactivation of
MAPKs by PME induces the downregulation of iNOS, COX-2, TNF-α and
IL-1β.
Previous studies have indicated that PI3K/Akt-linked
cascades are involved in the negative regulation of LPS-induced
inflammatory responses (31,32). The present study investigated the
effects of PME on Akt activation in LPS-stimulated RAW 264.7 cells.
Our findings showed that Akt activation was increased by LPS
stimulation, and was further enhanced by PME treatment (Fig. 5). Consistent with our results,
resveratrol has been shown to enhance Akt activation which is
mediated by the inhibition of inflammatory responses in
LPS-stimulated RAW 264.7 cells. Treatment of LPS-stimulated RAW
264.7 cells with resveratrol augmented Akt activation, resulting in
the downregulation of iNOS, COX-2, and TNF-α expression. However,
the regulatory effects of resveratrol on the expression of
inflammatory mediators were not induced under PI3K-inhibited
conditions (33). As Akt
activation is required for the inactivation of MAPKs, resveratrol
does not appear to have anti-inflammatory effects under
PI3K-inhibited conditions. Similarly, malvidin, a major red wine
polyphenol, appears to have anti-inflammatory effects in
LPS-stimulated macrophages through Akt activation. Treatment with
malvidin amplified Akt activation in LPS-stimulated macrophages,
playing a protective role in LPS-induced mitochondrial
depolarization (34). In
addition, Akt activation is closely associated with the induction
of antioxidant effects through Nrf2 activation. The inhibition of
PI3K leads to the reduction of Nrf2 activation, resulting in a
decrease in HO-1 expression (35,36). Our results are consistent with
these studies, in that the expression of Nrf2 and HO-1 was
augmented by PME in unstimulated RAW 264.7 cells. Based on previous
studies, our data suggest that the anti-inflammatory and
antioxidant effects of PME are induced through the activation of
Akt.
HO-1 belongs to a larger family of stress proteins
whose transcriptional regulation also responds to cellular injury,
including thermal or oxidant stress, and protects cells against
stress (37). HO-1 plays a role
as a rate-limiting enzyme in the production of bilirubin. In this
reaction, hemin-induced HO-1 catalyzes the conversion of heme into
biliverdin which is then changed into bilirubin, possessing
antioxidant abilities (38,39). Studies have demonstrated that
curcumin, a phenolic compound, prevents oxidative stress by
increasing HO-1 activity (40,41). Elevated HO-1 activity induces an
increase in glutathione levels in astrocytes. Furthermore, under
hypoxic conditions, curcumin significantly amplifies HO-1 activity
in vascular endothelial cells, protecting the cells against
oxidative stress. In the case of Nrf2, it is a basic leucine zipper
transcription factor activating the antioxidant response element
(ARE) in the promoters of antioxidant genes. These data suggest
that the expression of HO-1 is related to the antioxidative process
(42). Nrf2 is sequestered in the
cytosol by Keap1. Upon stimulation, Nrf2 is released from Keap1 and
translocates to the nucleus to activate ARE on the HO-1 promoter
(43). In addition, HO-1
negatively regulates iNOS expression in LPS-stimualted RAW 264.7
cells. A previous study on the anti-inflammatory effects of
genipin, an aglycon of geniposide, proved that the inhibitory
effects of genipin on iNOS expression are supressed in
HO-1-inhibited RAW 264.7 cells (44). Based on these previous studies,
HO-1 and Nrf2 participate in antioxidant and anti-inflammatory
processes. In the present study, pre-treatment with PME enhanced
the viability of H2O2-treated RAW 264.7 cells
(Fig. 1), and increased Nrf2
activation and HO-1 expression in intact RAW 264.7 cells (Fig. 6). Hence, our data indicate that
PME increases Nrf2 activation and HO-1 expression which may lead to
the prevention of oxidative stress-induced cell death and supports
the negative regulation of iNOS expression in LPS-stimulated RAW
264.7 cells.
In conclusion, PME exerts an anti-inflammatory
effects by regulating the production of pro-inflammatory mediators
through the downregulation of NF-κB phosphorylation, and the
inactivation of p38 MAPK and JNK in LPS-stimulated RAW 264.7 cells.
Under oxidative conditions, PME improved cell viability by
augmenting Nrf2 activation and HO-1 expression. These results
suggest that PME may be a promising candidate for the treatment of
inflammatory diseases by regulating inflammatory macrophages.
Acknowledgements
The present study was supported by the R&D
Program of MKE/KEIT (10040391, Development of Functional Food
Materials and Device for Prevention of Aging-associated Muscle
Function Decrease) and the Blue-Bio Industry Regional Innovation
Center (RIC08-06-07) at Dongeui University as a RIC program under
MKE and Busan, Republic of Korea. This study was also supported by
a grant from the Next Generation BioGreen 21 Program (SSAC, grant
no. PJ009615), Rural Development Administration, Republic of
Korea.
References
|
1
|
Medzhitov R: Inflammation 2010: new
adventures of an old flame. Cell. 140:771–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaffrey SR and Snyder SH: Nitric oxide: a
neural messenger. Annu Rev Cell Dev Biol. 11:417–440. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Won JS, Im YB, Singh AK and Singh I: Dual
role of cAMP in iNOS expression in glial cells and macrophages is
mediated by differential regulation of p38-MAPK/ATF-2 activation
and iNOS stability. Free Radic Biol Med. 37:1834–1844. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu C and Kitts DD: Luteolin and
luteolin-7-O-glucoside from dandelion flower suppress iNOS and
COX-2 in RAW264. 7 cells. Mol Cell Biochem. 265:107–113. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salvemini D, Misko TP, Masferrer JL,
Seibert K, Currie MG and Needleman P: Nitric oxide activates
cyclooxygenase enzymes. Proc Natl Acad Sci USA. 90:7240–7244. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tak PP: A personalized medicine approach
to biologic treatment of rheumatoid arthritis: a preliminary
treatment algorithm. Rheumatology. 51:600–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahn JK, Huang B, Bae EK, et al: The role
of α-defensin-1 and related signal transduction mechanisms in the
production of IL-6, IL-8 and MMPs in rheumatoid fibroblast-like
synoviocytes. Rheumatology. 52:1368–1376. 2013.
|
|
8
|
Cocozza C, D’orazio V, Miano T and Shotyk
W: Characterization of solid and aqueous phases of a peat bog
profile using molecular fluorescence spectroscopy, ESR and FT-IR,
and comparison with physical properties. Org Geochem. 34:49–60.
2003. View Article : Google Scholar
|
|
9
|
Bonifacio E, Falsone G and Petrillo M:
Humus forms, organic matter stocks and carbon fractions in forest
soils of northwestern Italy. Biol Fert Soils. 47:555–566. 2011.
View Article : Google Scholar
|
|
10
|
Schepetkin I, Khlebnikov A and Kwon BS:
Medical drugs from humus matter: focus on mumie. Drug Develop Res.
57:140–159. 2002. View Article : Google Scholar
|
|
11
|
Van Rensburg CE, Snyman JR, Mokoele T and
Cromarty AD: Brown coal derived humate inhibits contact
hypersensitivity; an efficacy, toxicity and teratogenicity study in
rats. Inflammation. 30:148–152. 2007.PubMed/NCBI
|
|
12
|
Jooné GK and van Rensburg CE: An in vitro
investigation of the anti-inflammatory properties of potassium
humate. Inflammation. 28:169–174. 2004.PubMed/NCBI
|
|
13
|
Van Rensburg CE and Naude PJ: Potassium
humate inhibits complement activation and the production of
inflammatory cytokines in vitro. Inflammation. 32:270–276.
2009.PubMed/NCBI
|
|
14
|
Ho Y, John Wase D and Forster C: Batch
nickel removal from aqueous solution by sphagnum moss peat. Water
Res. 29:1327–1332. 1995. View Article : Google Scholar
|
|
15
|
Gardea-Torresdey J, Tang L and Salvador J:
Copper adsorption by esterified and unesterified fractions of
Sphagnum peat moss and its different humic substances. J Hazard
Mater. 48:191–206. 1996. View Article : Google Scholar
|
|
16
|
Cormier Y, Israel-Assayag E, Bedard G and
Duchaine C: Hypersensitivity pneumonitis in peat moss processing
plant workers. Am J Respir Crit Care Med. 158:412–417. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cormier Y, Boulet LP and Bérubé-Genest F:
Effects of chronic organic dust exposure on respiratory function
and airway responsiveness in peat moss factory workers. Arch
Environ Health. 45:20–23. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakurai H, Kohsaka H, Liu MF, et al:
Nitric oxide production and inducible nitric oxide synthase
expression in inflammatory arthritides. J Clin Invest.
96:2357–2363. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abd-El-Aleem SA, Ferguson MW, Appleton I,
Bhowmick A, McCollum CN and Ireland GW: Expression of
cyclooxygenase isoforms in normal human skin and chronic venous
ulcers. J Pathol. 195:616–623. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gayathri B, Manjula N, Vinaykumar K,
Lakshmi B and Balakrishnan A: Pure compound from Boswellia
serrata extract exhibits anti-inflammatory property in human
PBMCs and mouse macrophages through inhibition of TNFα, IL-1β, NO
and MAP kinases. Int Immunopharmacol. 7:473–482. 2007.
|
|
21
|
Dayer JM: The process of identifying and
understanding cytokines: from basic studies to treating rheumatic
diseases. Best Pract Res Clin Rheumatol. 18:31–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lefkowitz DL, Mills K, Lefkowitz S, Bollen
A and Moguilevsky N: Neutrophil-macrophage interaction: a paradigm
for chronic inflammation. Med Hypotheses. 44:58–62. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naudé P, Cromarty AD and van Rensburg CE:
Potassium humate inhibits carrageenan-induced paw oedema and a
graft-versus-host reaction in rats. Inflammopharmacology. 18:33–39.
2010.PubMed/NCBI
|
|
24
|
Neurath M, Becker C and Barbulescu K: Role
of NF-κB in immune and inflammatory responses in the gut. Gut.
43:856–860. 1998.
|
|
25
|
Schmedtje JF, Ji YS, Liu WL, DuBois RN and
Runge MS: Hypoxia induces cyclooxygenase-2 via the NF-κB p65
transcription factor in human vascular endothelial cells. J Biol
Chem. 272:601–608. 1997.
|
|
26
|
O’Neill LA and Kaltschmidt C: NF-κB: a
crucial transcription factor for glial and neuronal cell function.
Trends Neurosci. 20:252–258. 1997.
|
|
27
|
Robinson MJ and Cobb MH: Mitogen-activated
protein kinase pathways. Curr Opin Cell Biol. 9:180–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cobb MH and Goldsmith EJ: How MAP kinases
are regulated. J Biol Chem. 270:14843–14846. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caivano M and Cohen P: Role of
mitogen-activated protein kinase cascades in mediating
lipopolysaccharide-stimulated induction of cyclooxygenase-2 and
IL-1β in RAW264 macrophages. J Immunol. 164:3018–3025.
2000.PubMed/NCBI
|
|
30
|
Chan ED and Riches DW: IFN-γ+
LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 mapk
in a mouse macrophage cell line. Am J Physiol Cell Physiol.
280:C441–C450. 2001.
|
|
31
|
Rajaram MV, Ganesan LP, Parsa KV, Butchar
JP, Gunn JS and Tridandapani S: Akt/protein kinase B modulates
macrophage inflammatory Response to Francisella infection
and confers a survival advantage in mice. J Immunol. 177:6317–6324.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang WJ, Wei H, Hagen T and Frei B:
α-lipoic acid attenuates LPS-induced inflammatory responses by
activating the phosphoinositide 3-kinase/Akt signaling pathway.
Proc Natl Acad Sci USA. 104:4077–4082. 2007.
|
|
33
|
Zong Y, Sun L, Liu B, Deng YS, Zhan D,
Chen YL, He Y, Liu J, Zhang ZJ, Sun J and Lu D: Resveratrol
inhibits LPS-induced MAPKs activation via activation of the
phosphatidylinositol 3-kinase pathway in murine RAW 264.7
macrophage cells. PLoS One. 7:e441072012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bognar E, Sarszegi Z, Szabo A, Debreceni
B, Kalman N, Tucsek Z, Sumegi B and Gallyas F Jr: Antioxidant and
anti-inflammatory effects in RAW 264.7 macrophages of malvidin, a
major red wine polyphenol. PLoS One. 8:e653552013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Chen Y, Sternberg P and Cai J:
Essential roles of the PI3 kinase/Akt pathway in regulating
Nrf2-dependent antioxidant functions in the RPE. Inverst Ophthalmol
Vis Sci. 49:1671–1678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zou W, Chen C, Zhong Y, An J, Zhang X, Yu
Y, Yu Z and Fu J: PI3K/Akt pathway mediates Nrf2/ARE activation in
human L02 hepatocytes exposed to low-concentration HBCDs. Environ
Sci Technol. 47:12434–12440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hoetzel A, Vagts DA, Loop T, et al: Effect
of nitric oxide on shock-induced hepatic heme oxygenase-1
expression in the rat. Hepatology. 33:925–937. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stocker R, Yamamoto Y, McDonagh AF, Glazer
AN and Ames BN: Bilirubin is an antioxidant of possible
physiological importance. Science. 235:1043–1046. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitani K, Fujita H, Fukuda Y, Kappas A and
Sassa S: The role of inorganic metals and metalloporphyrins in the
induction of haem oxygenase and heat-shock protein 70 in human
hepatoma cells. Biochem J. 290:819–825. 1993.PubMed/NCBI
|
|
40
|
Scapagnini G, Foresti R, Calabrese V,
Stella AG, Green C and Motterlini R: Caffeic acid phenethyl ester
and curcumin: a novel class of heme oxygenase-1 inducers. Mol
Pharmacol. 61:554–561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Motterlini R, Foresti R, Bassi R and Green
CJ: Curcumin, an antioxidant and anti-inflammatory agent, induces
heme oxygenase-1 and protects endothelial cells against oxidative
stress. Free Radic Biol Med. 28:1303–1312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kobayashi A, Kang MI, Okawa H, et al:
Oxidative stress sensor Keap1 functions as an adaptor for
Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2.
Mol Cell Biol. 24:7130–7139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Itoh K, Mochizuki M, Ishii Y, et al:
Transcription factor Nrf2 regulates inflammation by mediating the
effect of 15-deoxy-Δ12, 14-prostaglandin J2.
Mol Cell Biol. 24:36–45. 2004.PubMed/NCBI
|
|
44
|
Jeon WK, Hong HY and Kim BC: Genipin
up-regulates heme oxygenase-1 via PI3-kinase-JNK1/2-Nrf2 signaling
pathway to enhance the anti-inflammatory capacity in RAW264.7
macrophages. Arch Biochem Biophys. 512:119–125. 2011. View Article : Google Scholar : PubMed/NCBI
|