Introduction
Sphingolipids are the main components of lipid rafts
and have critical functions as signaling molecules. Sphingosine
1-phosphate (S1P) and ceramide regulate proliferation and apoptosis
(1–4). In response to various stimuli,
ceramide mediates cell death and apoptosis, whereas S1P abrogates
apoptosis and mediates cell proliferation and migration (5). Sphingosine kinase 1 (SK1) is the key
enzyme responsible for converting sphingosine to S1P. The signaling
pathway via the S1P receptor contributes to cancer cell survival
and proliferation (6), apoptosis
reduction (7) and oncogenic
transformation (8). Various types
of cancer cells show high levels of SK1 expression/activity,
increasing their resistance to anticancer agents, such as
anthracyclines, doxorubicin and camptothecin (9,10).
Thus, SK1 may play an important role in the development and
proliferation of cancers, such as melanoma (11–13). FTY720 (fingolimod) is
phosphorylated by SK1 and SK2 (14), and acts as an S1P receptor
antagonist (14,15). FTY720 induces apoptosis in
prostate (15), liver (7), bladder cancer (12) and melanoma (16,17) by the direct inhibition of SK1
(18). In the present study, we
investigated the combined effects of FTY720 and cisplatin, a DNA
damaging agent, on cell death, SK1 expression, S1P signaling and
epidermal growth factor receptor (EGFR) expression in anticancer
drug-resistant melanoma cells.
Materials and methods
Chemical reagents
The S1P receptor antagonist, FTY720, was obtained
from Cayman Chemical Co. (Ann Arbor, MI, USA). Cisplatin,
carboplatin, paclitaxel, dacarbazine, bovine serum albumin,
Dulbecco’s modified Eagle’s medium (DMEM) and Eagle’s minimal
essential medium (EMEM) were purchased from Wako Pure Chemical
Industries (Osaka, Japan). RPMI-1640, non-essential amino acids
(NEAA) and dimethyl sulfoxide (DMSO) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal antibodies
against SK1, p53, cleaved poly(ADP-ribose) polymerase (PARP),
phosphoinositide 3-kinase (PI3K), phosphorylated (p)-PI3K, Akt,
p-Akt, mammalian target of rapamycin (mTOR), p-mTOR, EGFR and
p-EGFR were obtained from Cell Signaling Technology (Beverly, MA,
USA) and rabbit polyclonal antibody against SK2 was obtained from
Abcam (Cambridge, UK).
Cell lines and measurement of cell
viability
The human melanoma SK-Mel-28 cells were obtained
from the JCRB Cell Bank (Osaka, Japan), the human melanoma cell
lines (A375, A2058 and WM115) and the mouse melanoma B16 cells were
purchased from the European Collection of Cell Cultures (ECACC).
The SK-Mel-28 and WM115 cells were cultured in RPMI-1640 containing
10% fetal bovine serum (FBS) and 0.1% tyrosine. The A375 and B16
cells were cultured in DMEM containing 10% FBS. The A2058 cells
were grown in EMEM containing 1% of NEAA and 10% FBS. To assess
viability, melanoma cells were plated at 1×104
cells/well and 5×103 cells/well in 24- and 96-well
plates, respectively. The anticancer drugs were dissolved in DMSO.
When the determination of IC50 was performed in 96-well
plates, cisplatin, paclitaxel, carboplatin and dacarbazine were
added at doses of up to 50, 10, 350 and 750 μM, respectively. In
24-well plates, the cells were treated with up to 150 μM of
cisplatin. The cells treated with DMSO only were used as controls.
Viability was analyzed following 48 h of treatment with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
using a standard assay, and 50% inhibitory concentrations
(IC50) were calculated.
Western blot analysis
The cells were lysed by sonication in lysis buffer
(1% Nonidet P-40, 0.5% sodium cholate, 1% SDS, 1 mM EDTA, 1 mM
EGTA, 150 mM NaCl, 20 mM HEPES, 3 mM MgCl2, protease
inhibitors, 0.5 mM 4-deoxypyridoxine, 20 mM β-glycerophosphate, 1
mM sodium fluoride and 1 mM sodium orthovanadate, pH 7.4). Total
cell lysates (20 μg protein) were separated by electrophoresis on
10-7.5% SDS-polyacrylamide gels and transferred onto polyvinylidene
difluoride membranes (Millipore, Danvers, MA, USA). The membranes
were blocked with 5% bovine serum albumin. The expression of SK1,
SK2, cleaved PARP, total PI3K, p-PI3K, total Akt, p-Akt, total
mTOR, p-mTOR, total EGFR and p-EGFR was measured by western blot
analysis. Anti-β-actin antibody was used as the loading control.
Followign repeated washes, bound antibodies were detected using the
ECL Western Blotting Detection system (GE Healthcare Ltd.,
Buckinghamshire, UK). Protein band density was determined using a
densitometer (Multi Gauge, version 3.1; Fuji Film, Tokyo,
Japan).
Statistical analysis
Data are expressed as the means ± SD and compared
using the Student’s t-test. P-values <0.05 were considered to
indicate statistically significant differences.
Results
Changes in cell viability induced by
anticancer drugs
We examined the sensitivity of 5 melanoma cell lines
(i.e., SK-Mel-28, A375, WM115, A2058 and B16) to 4 anticancer drugs
(cisplatin, paclitaxel, carboplatin and dacarbazine). The
IC50 value for cisplatin was the highest in the
SK-Mel-28 cells and the lowest in the A375 cells (data not shown).
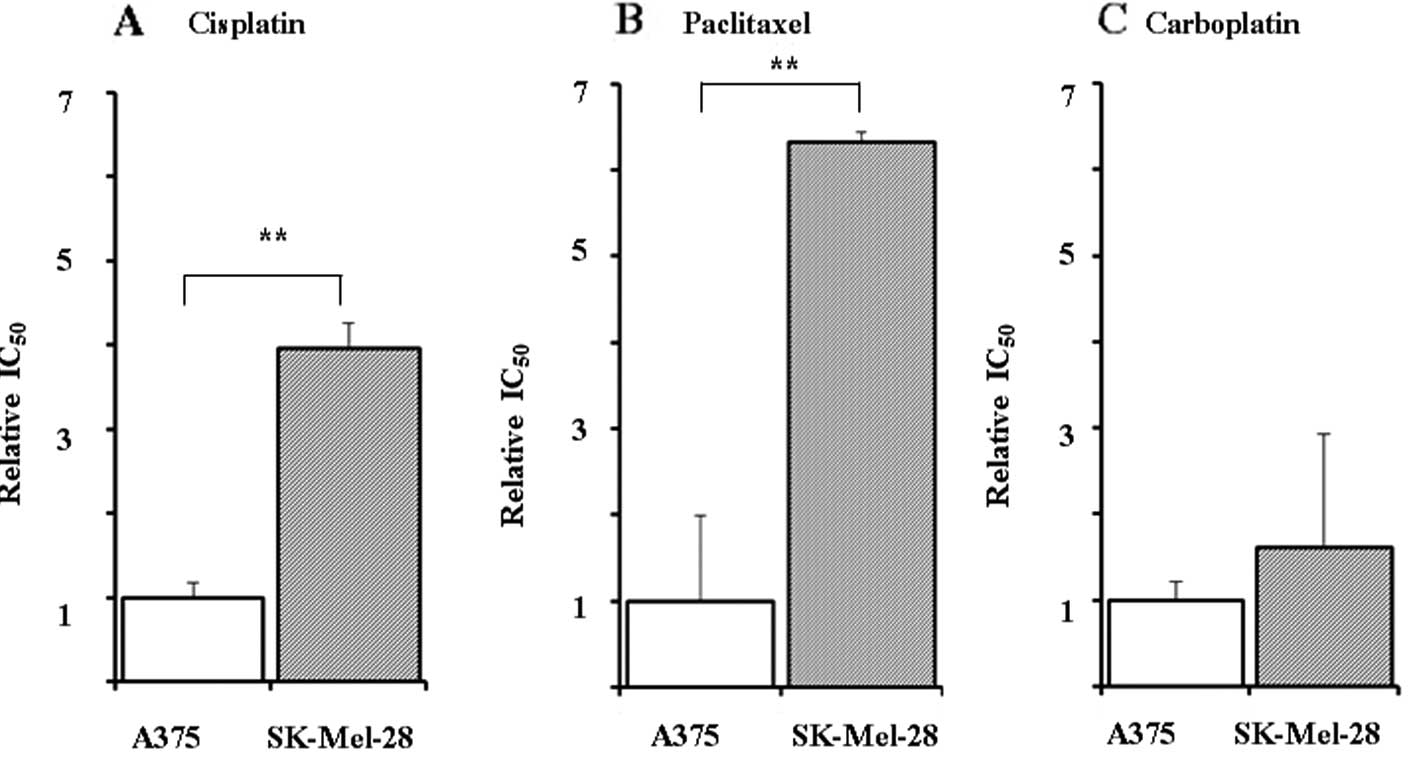
The relative IC50 values for cisplatin, paclitaxel and
carboplatin in the SK-Mel-28 cells vs. the A375 cells were 3.97-,
6.33- and 1.62-fold greater, respectively (Fig. 1). The IC50 value for
dacarbazine was >20-fold greater than that of cisplatin in the
SK-Mel-28 cells (data not shown). Therefore, for further analysis,
the SK-Mel-28 and A375 cells were used as cisplatin-resistant and
cisplatin-sensitive cell lines, respectively.
Effects of FTY720 on viability of
cisplatin-resistant cells
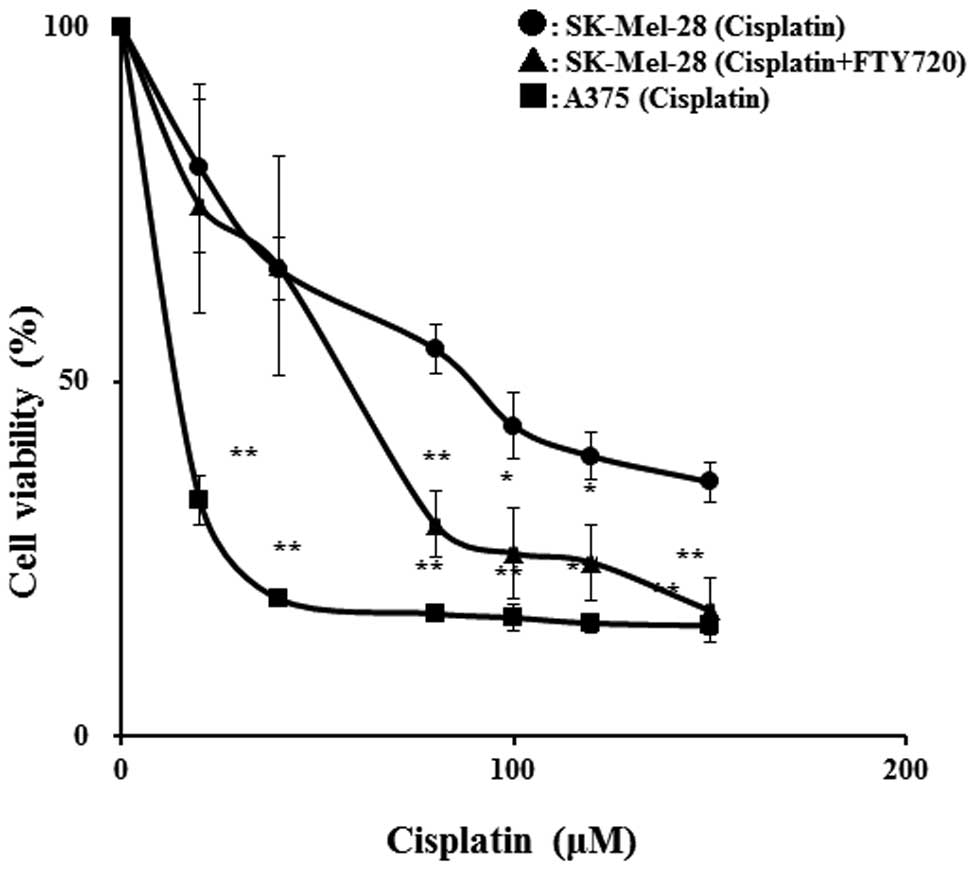
We investigated the effects of FTY720 on the
viability of the anticancer drug-resistant cell line, SK-Mel-28. In
the SK-Mel-28 cells treated with cisplatin, the IC50
value was reduced from 85.9 to 54.2 μM in the presence of 3 μM
FTY720 (an approximate 37% reduction) (Fig. 2). The IC50 value for
cisplatin in the A375 cells (1.3 μM) was lower than that in the
SK-Mel-28 cells and the combination with FTY720 had no effects on
the IC50 value for cisplatin in the A375 cells (data not
shown). Treatment with FTY720 alone at 3 μM had no significant
effect on cell viability, although treatment with 7 μM FTY720
reduced viability to 10% of the control (data not shown). These
results suggest that the combinatio of of FTY720 and cisplatin
significantly reduces viability in comparison to treatment with
cisplatin alone in SK-Mel-28 cells.
Effects of FTY720 on apoptosis
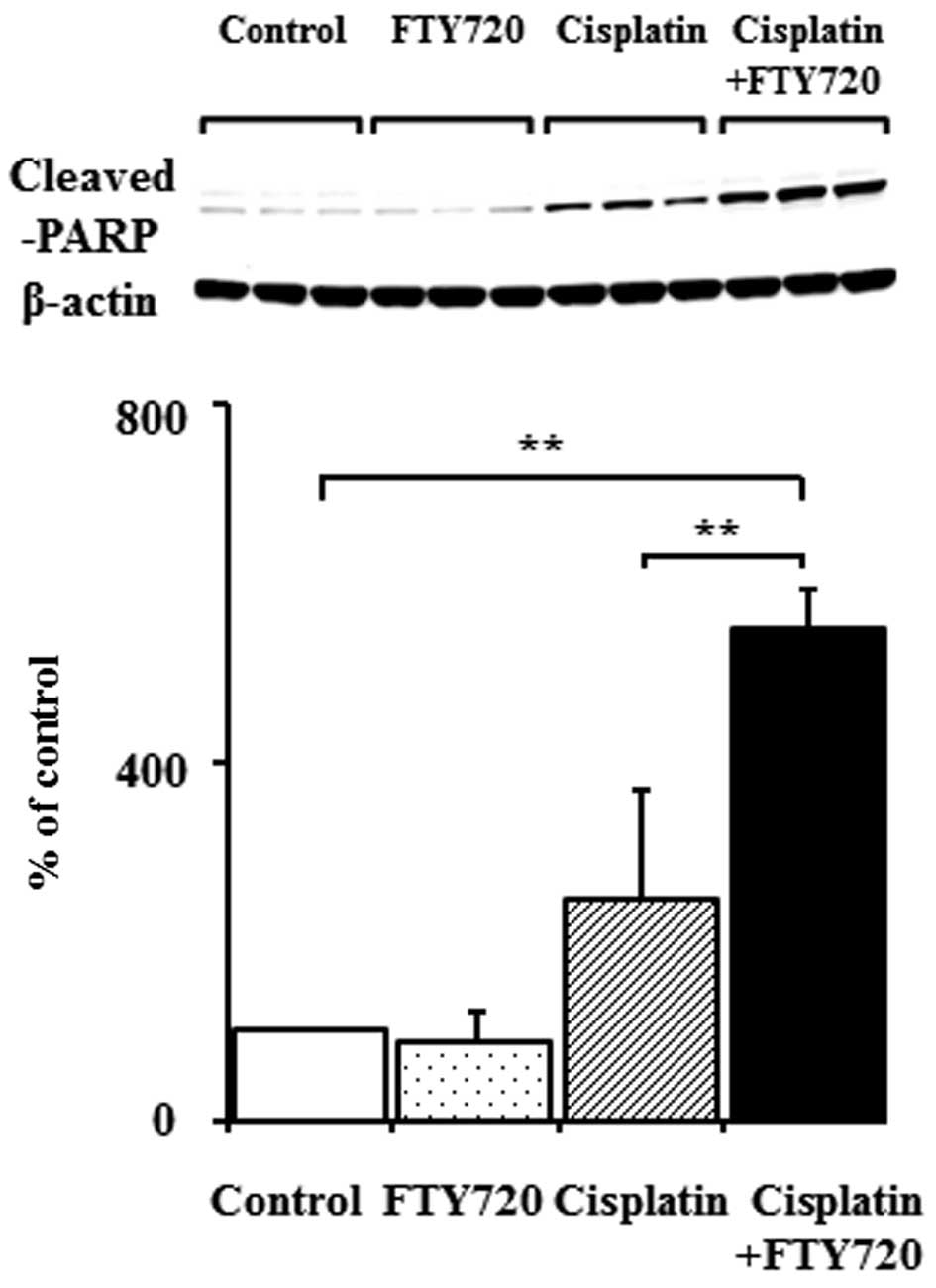
We investigated the effects of FTY720 on the
apoptosis of SK-Mel-28 and A375 cells. Cleaved PARP, a marker of
apoptosis, was examined by western blot analysis. In the A375
cells, the expression of cleaved PARP significantly increased
following treatment with cisplatin, but not following treatment
with FTY720 alone (Fig. 3).
Although PARP degradation increased in the SK-Mel-28 cells treated
with cisplatin alone, combined treatment with FTY720 induced a
synergistic increase in PARP degradation (Fig. 3). These results demonstrated that
the combination of FTY720 and cisplatin enhanced apoptosis in
comparison to treatment with cisplatin alone in the SK-Mel-28
cells, but not in the A375 cells.
Changes in SK1 and SK2 expression induced
by FTY720
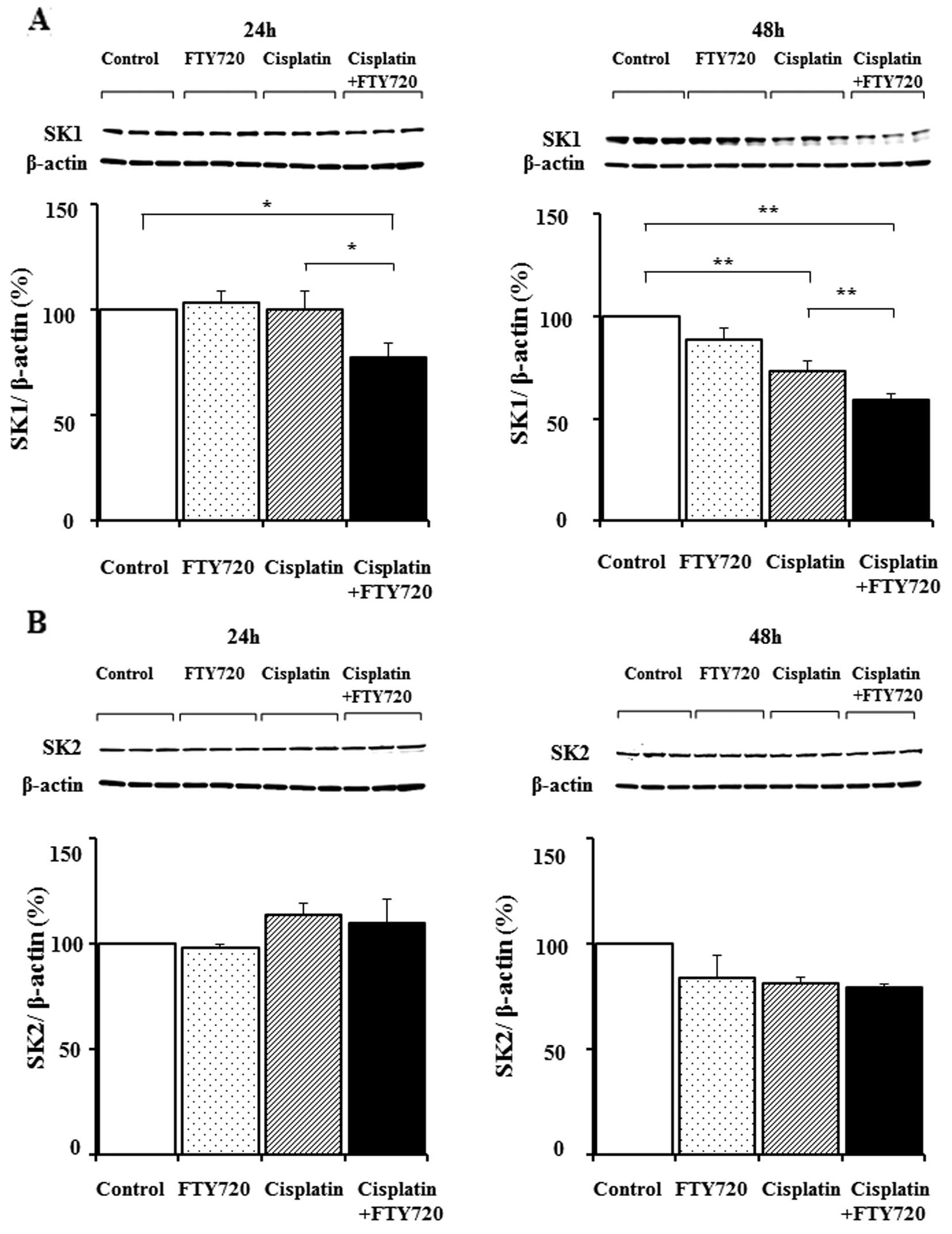
We investigated the changes in SK expression levels
in the SK-Mel-28 cells following treatment with FTY720, cisplatin
or a combination of FTY720 and cisplatin. Treatment with FTY720 or
cisplatin alone produced no change in SK1 protein expression
(Fig. 4A). Treatment with a
combination of FTY720 and cisplatin for 24 and 48 h caused a
synergistic decrease in SK1 expression, while SK2 expression
remained unaltered (Fig. 4B).
Thus, the combination of FTY720 and cisplatin mainly reduced SK1
expression in the SK-Mel-28 cells within 48 h.
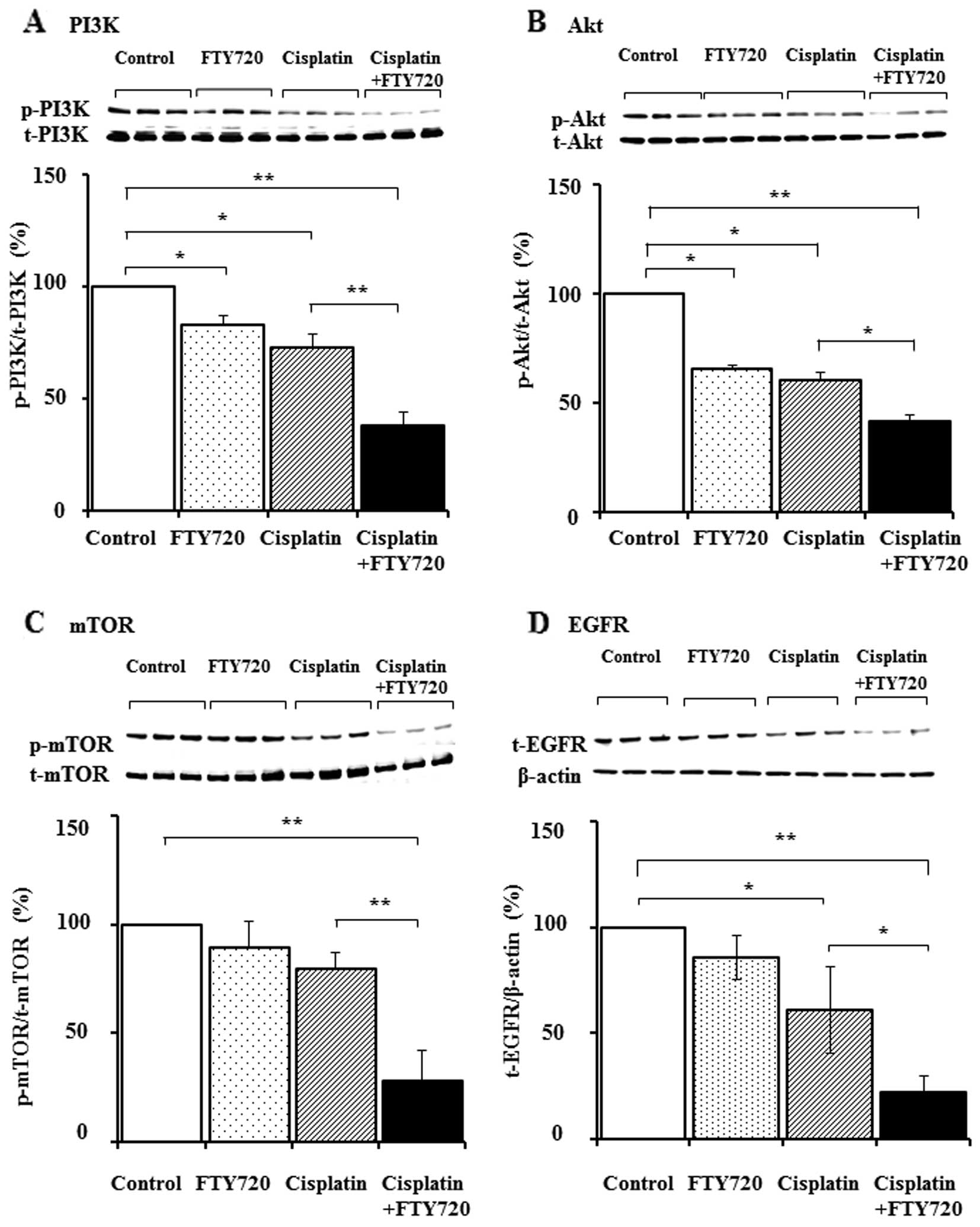
Downregulation of S1P signaling and the
decrease in EGFR expression following treatment with a combination
of FTY720 and cisplatin
To investigate the effects of FTY720 on signal
transduction via the S1P receptor, the expression levels of total
and phosphorylated forms of PI3K, Akt and mTOR were examined by
western blot analysis in the SK-Mel-28 cells following treatment
with FTY720, cisplatin alone, or a combination of FTY720 and
cisplatin. The combination of FTY720 and cisplatin reduced the
expression of the phosphorylated forms of PI3K, Akt and mTOR, but
not the total protein expression (Fig. 5A–C). Unexpectedly, the expression
of total and p-ERK remained unaltered following combination
treatment (data not shown). Cross-talk between SK1/S1P and
EGFR-dependent pathways has been previously reported (19–21). Subsequently, we examined the
effects of FTY720 on EGFR expression in the SK-Mel-28 cells. As
shown in Fig. 5D, total EGFR
expression was decreased following treatment with a combination of
FTY720 and cisplatin, whereas total EGFR expression remained
unaltered following treatment with cisplatin and FTY720 alone.
Discussion
Combination chemotherapy is an important strategy
for improving therapeutic outcomes and reducing the side-effects of
anticancer drugs. Various studies have indicated the possible
utility of combining anticancer drugs with agents associated with
the SK1/S1P pathway for cancer therapy (22–24). The combination of SK inhibitors,
including siRNA, with anticancer drugs has been shown to be
effective against drug-resistant colon cancer (22,23) and pancreatic cancer (24). The combination of doxorubicin and
SK1-locked acid-antisense oligonucleotides has also been shown to
be effective against gastric cancer (25). Recent studies have indicated that
FTY720, which is phosphorylated by SK and acts as an antagonist of
S1P, may enhance the efficacy of anticancer therapies. This
compound has been shown to induce dose-dependent cell death in
several human tumor cell lines, such as prostate and bladder
cancer, as well as hepatocellular carcinoma (7,12,15). Moreover, FTY720 has been shown to
reduce tumor growth and metastasis by increasing radiotherapeutic
sensitivity in prostate cancer (26). In murine melanoma, FTY720 reduces
the development of metastatic melanoma in vivo and apoptosis
in tumor cells (16).
Furthermore, FTY720 has been shown to inhibit S1P- and VEGF-induced
angiogenesis and tumor vascularization in a murine model of
melanoma cells (17). These
studies suggest that functional antagonism between S1P receptors
and FTY720 inhibits angiogenesis and provides a novel anticancer
drug in chemotherapy. However, the precise mechanisms underlying
the induction of cell death have not yet been elucidated. In this
study, we demonstrated that the SK-Mel-28 cell line was the most
resistant to anticancer drugs, such as cisplatin (Fig. 1), and that the combination of
FTY720 and cisplatin significantly reduced viability in this cell
line (Fig. 2). The viability of
the SK-Mel-28 cells remained unaltered by low concentrations of
FTY720 alone, but treatment with over 7 μM FTY720 induced cell
death (data not shown). Several mechanisms have been proposed to
explain the anticancer efficacy of FTY720; for instance, it induces
apoptosis in various types of cancer (9,12,16,17,25) through the direct inhibition and
proteasomal degradation of SK1 at a high concentration (10 μM) in
human prostate cancer cells (18). Other studies have indicated that
SK1 is downregulated by genotoxic stress and proteolysis of p53
induced by caspases (27,28). The resistant melanoma cell line,
SK-Mel-28, harbors a p53 mutation, and cell death induced by
anticancer drugs is independent of p53 (29). In the present study, the
expression of SK1, but not that of SK2, in the SK-Mel-28 cells was
reduced following treatment with a combination of FTY720 and
cisplatin, but not following treatment with cisplatin or FTY720
alone (Fig. 4). Combination
treatment with FTY720 and cisplatin likely induced SK1 degradation
by p53-independent caspase activation, resulting in a significant
increase in PARP cleavage and the induction of apoptosis. Further
studies required to investigate the involvement of SK2 in melanoma,
as the roles of SK2 in cancer cells have not yet been
elucidated.
Moreover, we investigated the molecular mechanisms
underlying the induction of apoptosis following treatment with a
combination of FTY720 and cisplatin in the SK-Mel-28 cells.
Previous studies have demonstrated that the interaction between
FTY720 and the S1P1 receptor blocks STAT3 signaling in
B-cell lymphoma (30) and the
FTY-720-induced apoptosis of renal cancer cells is mediated by the
inhibition of ERK activation (13). Apoptosis induced by
chemotherapeutic agents in SK-Mel-28 cells is mediated by the
activation of stress kinases, such as p38 MAP kinase and SAPK/JNK
(29), and by the increased
expression of the transactivation factor, E2F-1 (31). Furthermore, accumulating evidence
suggests that the PI3K/Akt pathway is related to chemoresistance,
mainly through escape from apoptosis, and these signaling pathways
promote cell survival and proliferation (32). The MAPK pathway via
ras-raf-MEK-ERK and the PI3K-Akt pathway are major pathways in
cancer development and progression (33), and the latter is particularly
important in cell survival in melanoma (34). Our results revealed that
combination treatment with FTY720 and cisplatin reduced the levels
of pp-PI3K and p-Akt (Fig. 5).
However, this treatment did not reduce ERK activation, suggesting
that signal transduction via the PI3K/Akt pathway through the
S1P1 receptor was suppressed by FTY720. Furthermore,
mTOR, which belongs to the PI3K family, regulates cell growth and
survival via p70S6 kinase activation in cancer cells (35,36). Therefore, studies have noted that
the inhibition of mTOR reduces the proliferation of colorectal
cancer, prostate cancer and esophageal squamous cell carcinoma
(37). In the present study, we
demonstrated that the combination of FTY720 and cisplatin, but not
cisplatin alone, suppressed p-mTOR levels (Fig. 5). Thus, FTY720 may inhibit the
PI3K/Akt/mTOR pathway in cancer therapy. We also demonstrated that
the combination of FTY720 and cisplatin reduced EGFR expression in
the SK-Mel-28 cells (Fig. 5). It
is well known that estrogen induces S1P release via SK1 activation,
and that the subsequent activation of the S1P receptor
S1P3, leads to receptor transactivation in human breast
cancer (19). Such cross-talk
between S1P/SK1 and EGFR has also been reported in gastric cancer
(20) and human glioblastoma
(21). Therefore, our results
suggest that the transactivation of EGFR by S1P was also reduced by
combination treatment with FTY720 in melanoma cells. EGFR
expression is enhanced in various types of cancer cells and
anti-EGFR drugs have been developed as anticancer agents (38). Thus, the decrease in EGFR
expression may be an important mechanism through which the
combination of FTY720 and cisplatin induces apoptosis in SK-Mel-28
cells.
In conclusion, compared to treatment with alone,
combination treatment with FTY720 and cisplatin exerted synergistic
effects on the induction of apoptosis in drug-resistant melanoma
cells by SK1 degradation, possibly due to the downregulation of the
PI3K/Akt/mTOR pathway via the S1P receptor and the reduced EGFR
expression. These results indicate that the inhibition of survival
signals via the SK/S1P pathway may be a molecular target for cancer
therapy, and FTY720 may serve as a useful agent in
chemotherapy.
Acknowledgements
The present study was supported in part by
Grants-in-Aid for Scientific Research (C) 24590185 from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan.
References
|
1
|
Taha TA, Hannun YA and Obeid LM:
Sphingosine kinase: biochemical and cellular regulation and role in
disease. J Biochem Mol Biol. 39:113–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogretmen B and Hannun YA: Biologically
active sphingolipids in cancer pathogenesis and treatment. Nat Rev
Cancer. 4:604–616. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olivera A, Kohama T, Edsall LC, et al:
Sphingosine kinase expression increases intracellular
sphingosine-1-phosphate and promotes cell growth and survival. J
Cell Biol. 147:545–558. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia P, Wang L, Gamble JR and Vadas MA:
Activation of sphingosine kinase by tumor necrosis factor-alpha
inhibits apoptosis in human endothelial cells. J Biol Chem.
274:34499–34505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spiegel S and Milstien S:
Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol
Cell Biol. 4:397–407. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia P, Gamble JR, Wang L, et al: An
oncogenic role of sphingosine kinase. Curr Biol. 10:1527–1530.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ho JW, Man K, Sun CK, Lee TK, Poon RT and
Fan ST: Effects of a novel immunomodulating agent, FTY720, on tumor
growth and angiogenesis in hepatocellular carcinoma. Mol Cancer
Ther. 4:1430–1438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pchejetski D, Doumerc N, Golzio M, et al:
Chemosensitizing effects of sphingosine kinase-1 inhibition in
prostate cancer cell and animal models. Mol Cancer Ther.
7:1836–1845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
French KJ, Schrecengost RS, Lee BD, et al:
Discovery and evaluation of inhibitors of human sphingosine kinase.
Cancer Res. 63:5962–5969. 2003.PubMed/NCBI
|
|
10
|
Shida D, Takabe K, Kapitonov D, Milstien S
and Spiegel S: Targeting SphK1 as a new strategy against cancer.
Curr Drug Targets. 9:662–673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vadas M, Xia P, McCaughan G and Gamble J:
The role of sphingosine kinase 1 in cancer: oncogene or
non-oncogene addiction? Biochim Biophys Acta. 1781:442–447. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azuma H, Takahara S, Horie S, Muto S,
Otsuki Y and Katsuoka Y: Induction of apoptosis in human bladder
cancer cells in vitro and in vivo caused by FTY720 treatment. J
Urol. 169:2372–2377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ubai T, Azuma H, Kotake Y, et al: FTY720
induced Bcl-associated and Fas-independent apoptosis in human renal
cancer cells in vitro and significantly reduced in vivo tumor
growth in mouse xenograft. Anticancer Res. 27:75–88.
2007.PubMed/NCBI
|
|
14
|
Billich A, Bornancin F, Dévay P,
Mechtcheriakova D, Urtz N and Baumruker T: Phosphorylation of the
immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem.
278:47408–47415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang JD, Takahara S, Nonomura N, Ichimaru
N, Toki K and Azuma H: Early induction of apoptosis in
androgen-independent prostate cancer cell line by FTY720 requires
caspase-3 activation. Prostate. 40:50–55. 1999. View Article : Google Scholar
|
|
16
|
Pereira FV, Arruda DC, Figueiredo CR, et
al: FTY720 induces apoptosis in B16F10-NEX2 murine melanoma cells,
limits metastatic development in vivo, and modulates the immune
system. Clinics (Sao Paulo). 68:1018–1027. 2013. View Article : Google Scholar
|
|
17
|
LaMontagne K, Littlewood-Evans A, Schnell
C, et al: Antagonism of sphingosine-1-phosphate receptors by FTY720
inhibits angiogenesis and tumor vascularization. Cancer Res.
66:221–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tonelli F, Lim KG, Loveridge C, et al:
FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1
and promote its proteasomal degradation in human pulmonary artery
smooth muscle, breast cancer and androgen-independent prostate
cancer cells. Cell Signal. 22:1536–1542. 2010. View Article : Google Scholar
|
|
19
|
Sukocheva O, Wadham C, Holmes A, et al:
Estrogen transactivates EGFR via the sphingosine 1-phosphate
receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol.
173:301–310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shida D, Fang X, Kordula T, et al:
Cross-talk between LPA1 and epidermal growth factor receptors
mediates up-regulation of sphingosine kinase 1 to promote gastric
cancer cell motility and invasion. Cancer Res. 68:6569–6577. 2008.
View Article : Google Scholar
|
|
21
|
Paugh BS, Paugh SW, Bryan L, et al: EGF
regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway
involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma
cells. FASEB J. 22:455–465. 2008. View Article : Google Scholar
|
|
22
|
Nemoto S, Nakamura M, Osawa Y, et al:
Sphingosine kinase isoforms regulate oxaliplatin sensitivity of
human colon cancer cells through ceramide accumulation and Akt
activation. J Biol Chem. 84:10422–10432. 2009. View Article : Google Scholar
|
|
23
|
Kawahara S, Otsuji Y, Nakamura M, et al:
Sphingosine kinase 1 plays a role in the upregulation of CD44
expression through extracellular signal-regulated kinase signaling
in human colon cancer cells. Anticancer Drugs. 24:473–483. 2013.
View Article : Google Scholar
|
|
24
|
Guillermet-Guibert J, Davenne L,
Pchejetski D, et al: Targeting the sphingolipid metabolism to
defeat pancreatic cancer cell resistance to the chemotherapeutic
gemcitabine drug. Mol Cancer Ther. 8:809–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuereder T, Hoeflmayer D, Jaeger-Lansky A,
et al: Sphingosine kinase 1 is a relevant molecular target in
gastric cancer. Anticancer Drugs. 22:245–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pchejetski D, Bohler T, Brizuela L, et al:
FTY720 (fingolimod) sensitizes prostate cancer cells to
radiotherapy by inhibition of sphingosine kinase-1. Cancer Res.
70:8651–8661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heffernan-Stroud LA, Helke KL, Jenkins RW,
De Costa AM, Hannun YA and Obeid LM: Defining a role for
sphingosine kinase 1 in p53-dependent tumors. Oncogene.
31:1166–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taha TA, Osta W, Kozhaya L, et al:
Down-regulation of sphingosine kinase-1 by DNA damage: dependence
on proteases and p53. J Biol Chem. 279:20546–20554. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rudolf K, Cervinka M and Rudolf E: Dual
inhibition of topoisomerases enhances apoptosis in melanoma cells.
Neoplasma. 57:316–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Deng J, Wang L, et al: S1PR1 is an
effective target to block STAT3 signaling in activated B cell-like
diffuse large B-cell lymphoma. Blood. 120:1458–1465. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong YB, Yang HL, Elliott MJ and McMasters
KM: Adenovirus-mediated E2F-1 gene transfer sensitizes melanoma
cells to apoptosis induced by topoisomerase II inhibitors. Cancer
Res. 62:1776–1783. 2002.PubMed/NCBI
|
|
32
|
Kim D, Cheng GZ, Lindsley CW, Yang H and
Cheng JQ: Targeting the phosphatidylinositol-3 kinase/Akt pathway
for the treatment of cancer. Curr Opin Investig Drugs. 6:1250–1258.
2005.PubMed/NCBI
|
|
33
|
Meier F, Schittek B, Busch S, et al: The
RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular
targets for the effective treatment of advanced melanoma. Front
Biosci. 10:2986–3001. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sinnberg T, Lasithiotakis K, Niessner H,
et al: Inhibition of PI3K-AKT-mTOR signaling sensitizes melanoma
cells to cisplatin and temozolomide. J Invest Dermatol.
129:1500–1515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Powlas J, Shi Y, et al: Rapamycin
inhibits Akt-mediated oncogenic transformation and tumor growth.
Anticancer Res. 24:2697–2704. 2004.PubMed/NCBI
|
|
36
|
Sun SY, Rosenberg LM, Wang X, et al:
Activation of Akt and eIF4E survival pathways by rapamycin-mediated
mammalian target of rapamycin inhibition. Cancer Res. 65:7052–7208.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Francipane MG and Lagasse E: mTOR pathway
in colorectal cancer: an update (Review). Oncotarget. 15:49–66.
2014.
|
|
38
|
Rosa R, Marciano R, Malapelle U, et al:
Sphingosine kinase 1 overexpression contributes to cetuximab
resistance in human colorectal cancer models. Clin Cancer Res.
19:138–147. 2013. View Article : Google Scholar : PubMed/NCBI
|