Introduction
Orexin-A and orexin-B, also known as hypocretin-1
and hypocretin-2, have been implicated in a wide range of central,
as well as peripheral functions (1–4).
Orexins were initially characterized as neuropeptides restricted to
hypothalamic neurons in the brain that projected to nuclei involved
in the control of food intake, sleep-wakefulness, neuroendocrine
homeostasis and autonomic regulation (5). The functions of orexins are mediated
by two membrane bound G-protein coupled receptors, the orexin
receptor type 1 (OX1 receptor) and the orexin receptor
type 2 (OX2 receptor). While both peptides bind with
almost equal affinity to the OX2 receptor, the
OX1 receptor seems to be selective for orexin-A
(4). Further observations have
indicated that orexins and their receptors are not restricted to
the hypothalamus, but are also expressed in a few peripheral
tissues (5), including the
adrenal glands, gastrointestinal tract and the pancreas (4). To date, compelling evidence
indicates an interaction of the orexin system with the
hypothalamus-pituitary-adrenal (HPA) axis on a central, as well as
peripheral level (6). Orexins
(10−10 to 10−6 M) exert a stimulatory effect
on glucocorticoid release, adrenocortical cell growth and, in some
cases, mineralocorticoid release from the adrenal cortex of various
species (7–13). This fact seems to be related to
orexin-A (10−6 M) enhancing the expression of
3β-hydroxysteroid dehydrogenase (3β-HSD) (14). The enzymes of the 3β-HSD family
that catalyze the conversion of Δ5-3β-hydroxysteroids
into Δ4-3-ketosteroids are located in the endoplasmic
reticulum and the mitochondrial membrane (14–17). Thus, 3β-HSD plays a key role in
glucocorticoid synthesis and is expressed in a number of tissues,
including the adrenal glands, gonads and the brain (17). Wenzel et al (14) found that orexins stimulated
glucocorticoid secretion from human adrenocortical NCI-H295R cells,
and that this increase was accompanied by a simultaneous increase
in the mRNA levels of 3β-HSD. However, little is known regarding
the mechanisms involved in the effects of orexins leading to an
increased level of 3β-HSD in adrenal cells.
AKT, also known as protein kinase B (PKB), is
essential for cell survival and growth during development and
carcinogenesis. AKT is a serine-threonine kinase that is regulated
mainly following the activation of the second messenger,
phosphatidylinositol 3-kinase (PI3K). Abundant evidence indicates
that AKT is a key regulator of multiple cell survival mechanisms
(18–24). AKT was first characterized for its
function in regulating cell proliferation and survival, which may
be due to the direct or indirect effects of AKT on a number of
cellular proteins. For example, AKT phosphorylates and activates
mammalian target of rapamycin (mTOR) in response to growth factors
and oncogenes (19–21). As such, AKT plays key roles in
cell survival (22), cell
proliferation (23), cell growth
(24) and apoptosis (22). To date, certain studies have
indicated the involvement of orexins in the regulation of cell
viability, cell proliferation and cortisol production (25,26). These effects can be mediated
through multiple signaling pathways, including protein kinase A
(PKA), protein kinase C (PKC) and mitogen-activated protein kinase
(MAPK) cascade-dependent mechanisms (14,25,26). However, little is known of the
ability of orexins to activate the PKB/AKT pathway in adrenal
cells.
In the present study, human NCI-H295R cells were
used as an adrenocortical cell model (27). The cells were exposed to various
concentrations of orexin-A (10−10 to 10−6 M),
in the presence of OX1 receptor antagonist, AKT
antagonist or a combination of both, and cell proliferation assays
were then performed to assess the effects of orexin-A on
adrenocortical cell growth. Our results revealed that orexin-A
significantly enhanced the expression of 3β-HSD and the production
of cortisol, and increased the phosphorylation of AKT in the
NCI-H295R cells. Our data present evidence for a functional role of
orexin-A in human adrenocortical cells through the OX1
receptor-stimulated AKT signaling pathway.
Materials and methods
Reagents
Orexin-A was obtained from Sigma (St. Louis, MO,
USA). RPMI-1640 medium and fetal bovine serum were purchased from
Gibco (Grand Island, NY, USA). The AKT inhibitor, PF-04691502, was
purchased from Selleck Chemicals LLC (Houston, TX, USA). The
OX1 receptor-specific antagonist, SB334867, was obtained
from Tocris Bioscience (Minneapolis, MN, USA). The cell
proliferation enzyme-linked immunosorbent assay (ELISA) BrdU
colorimetric kit was purchased from Roche Diagnostics (Penzberg,
Germany). Total-AKT polyclonal antibody (ab8805), phospho-AKT
(s473) polyclonal antibody (ab8932) and OX1 receptor
antibody (ab68718) were obtained from Abcam (Cambridge, UK). The
Cortisol Express EIA kit was purchased from Alpco (Paris, France).
β-actin antibody (C4; sc-47778) and 3β-HSD antibody (37-2;
sc-100466) were obtained from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA.
Cell culture
Human NCI-H295R adrenocortical cells were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
maintained in RPMI-1640 medium supplemented with 10% (wt/vol) fetal
bovine serum, L-glutamine, penicillin (50 μg/ml) and streptomycin
(100 μg/ml). The cells were grown in a humidified atmosphere
containing 5% CO2 at 37°C. Prior to the experiments, the
cells were grown in petri dishes in serum-free medium for 24 h. The
following day, the cells (4×103 cells/well in 96-well
plates, 5×105 cells/well in 6-well plates) were treated
with various concentrations of orexin-A (0, 10−10,
10−8 and 10−6 M), or 10−6 M
orexin-A with either SB334867 or PF-04691502, or a combination of
both.
Cell proliferation assays
The adrenocortical NCI-H295R cells were seeded
(2×103 cells/well) in 96-well plates and cultured for 24
h. To synchronize the cell cycle, the cells were serum-deprived for
24 h and then treated with the test agents for a further 24 h. BrdU
incorporation into DNA was measured using the Cell Proliferation
ELISA BrdU colorimetric kit (Roche Diagnostics). The cells were
incubated with BrdU fresh medium at 37°C and 5% CO2 for
12 h and fixed with 200 μl of fixative/denaturing solution for 30
min at room temperature. Peroxidase-conjugated BrdUrd antibody was
then added to each well followed by incubation for 1 h. After
washing thoroughly, the bound peroxidase-conjugated BrdUrd antibody
was quantified with peroxidase substrate tetramethylbenzidine.
Finally the BrdUrd absorbance was measured at 440 nm using an ELISA
plate reader (BioTek Instruments, Winooski, VT, USA). A control
without cells was used to measure the background absorbance of the
medium and was subtracted from the results.
Cortisol measurements
For cortisol release experiments, the NCI-H295R
cells were cultured in 6-well plates until the cells were at
approximately 80–85% confluence. The cells were serum starved
overnight, then washed and incubated in fresh serum-free medium
containing various concentrations of orexin-A and the different
inhibitors for 24 h. At the end of the incubation period, the
supernatant was removed and snap-frozen immediately in liquid
nitrogen until the cortisol measurements were taken. Cortisol
levels were assessed using the ELISA kit according to the
manufacturer’s instructions.
Total RNA preparations and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the NCI-H295R cells
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The mRNA
expression of OX1 receptor and OX2 receptor
was detected by real-time PCR using TaqMan reagents (Takara, Otsu,
Japan). The following specific primers were used: OX1
receptor forward, 5′-TGC GGC CAA CCC TAT CAT CTA-3′ and reverse,
5′-ACC GGC TCT GCA AGG ACAA-3′; OX2 receptor forward,
5′-ATC GCA GGG TAT ATC ATC GTG TTC-3′ and reverse, 5′-TGA CTG TCC
TCA TGT GGT GGT TC-3′; 3β-HSD forward, 5′-AGC AAA AAG ATG GCC GAG
AA-3′ and reverse, 5′-GGC ACA AGT ATG CAA TGT GCC-3′. As an
internal control for reverse transcription (RT) and reaction
efficiency, the amplification of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) mRNA was carried out in parallel for each
sample. The following specific primers were used: GAPDH forward,
5′-GGC ACA GTC AAG GCT GAG AAT G-3′ and reverse, 5′-ATG GTG GTG AAG
ACG CCA GTA-3′. The PCR reactions were carried out using the
following conditions: 95°C for 30 sec, then 40 cycles of 95°C for 5
sec, 60°C for 30 sec, and 95°C for 15 sec. All primers and TaqMan
probes specific to OX1 receptor, OX2
receptor, 3β-HSD and GAPDH were designed using Primer Premier 5.0
software (Premier Biosoft International, Palo Alto, CA, USA).
Protein preparations and western blot
analysis
The NCI-H295R cells were washed with cold
phosphate-buffered saline (PBS) and harvested in RIPA buffer
containing protease inhibitors. The cell lysates were incubated on
ice for 30 min and were collected and centrifuged at 12,000 × g for
10 min at 4°C. The supernatants were collected and mixed with 5X
loading buffer, then denatured by boiling for 10 min. The samples
were separated by sodium dodecyl sulfate-polyacrylamide gel
(SDS-PAGE) and transferred onto PVDF membranes at 60 V for 2.5 h in
transfer buffer containing 20 mM Tris, 150 mM glycine and 20%
methanol. The membranes were incubated in non-fat dry milk for 120
min at room temperature, and then washed 3 times with TBST for 30
min, then incubated with primary antibody against OX1
receptor at a 1:250 dilution, phospho/total-AKT at a 1:1,000
dilution or antibody against 3β-HSD at a 1:1,000 dilution in TBST
overnight at 4°C. The membranes were washed and incubated with a
secondary antibody (source: rabbit) for 1.5 h at room temperature,
and then washed 3 times with TBST for 30 min. Protein was
visualized using the ECL method. Band densities were measured using
Quantity One software.
Statistical analysis
The results are expressed as the means ± standard
error of the mean (SEM) and differences between the means were
analyzed by one-way analysis of variance (ANOVA). A value of P≤0.05
was considered to indicate a statistically significant
difference.
Results
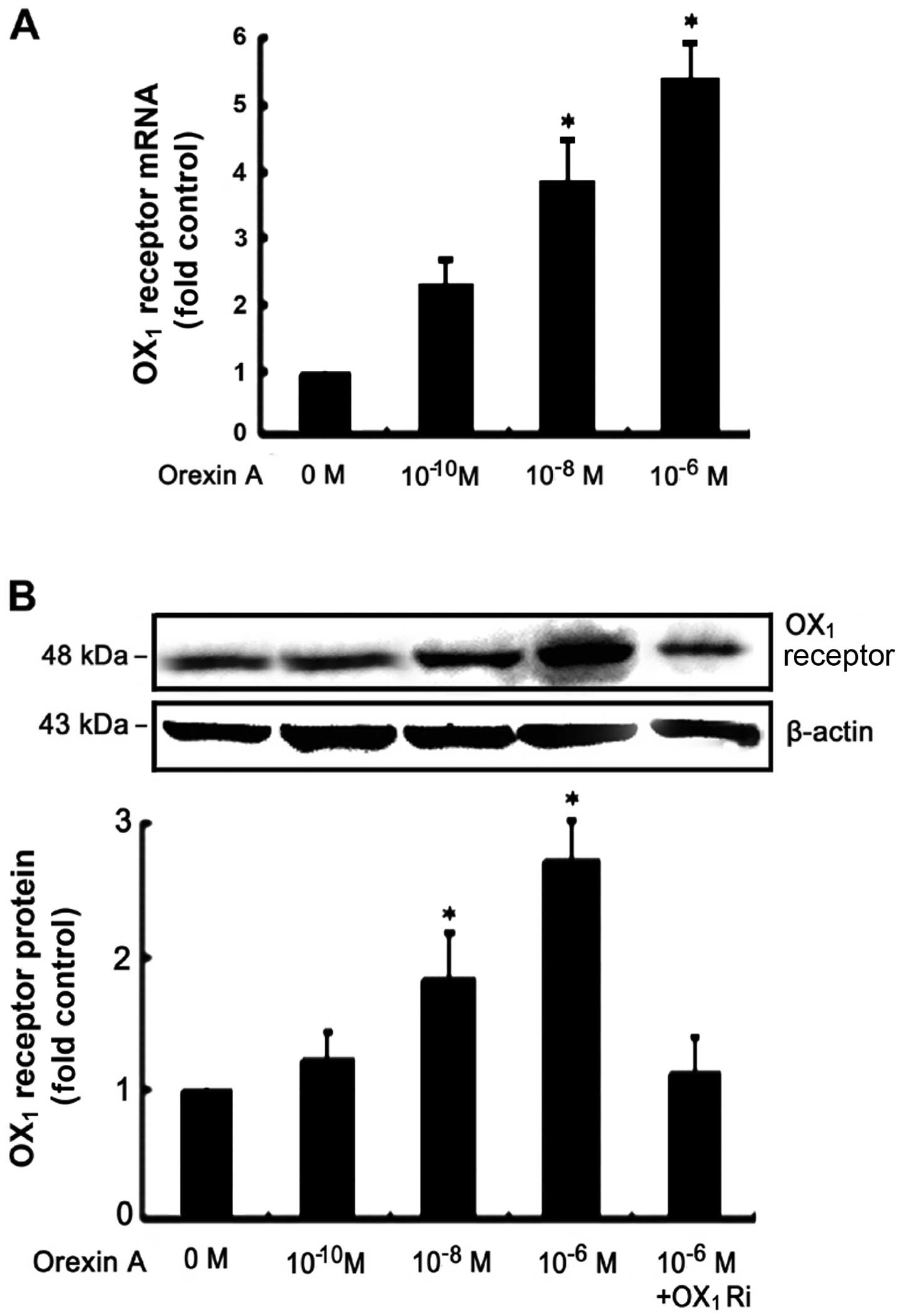
Orexin-A receptor expression in NCI-H295R
cells
RT-qPCR assays demonstrated that OX1
receptor mRNA was expressed in the NCI-H295R cells (Fig. 1A). However, OX2
receptor mRNA was not detectable under the same conditions (data
not shown). Orexin-A (10−10, 10−8 and
10−6 M) induced a significant increase in the mRNA and
protein levels of OX1 receptor in a dose-dependent
manner (Fig. 1). Treatment with
orexin-A increased OX1 receptor protein expression in
the NCI-H295R cells, an increase that was dependent upon the
concentration of orexin-A, with 10−6 M of orexin-A
exerting the most potent effect (Fig.
1B). This increase in expression was attenuated in the presence
of 10−6 M SB334867, a high-affinity, OX1
receptor-specific, non-peptide antagonist (Fig. 1B).
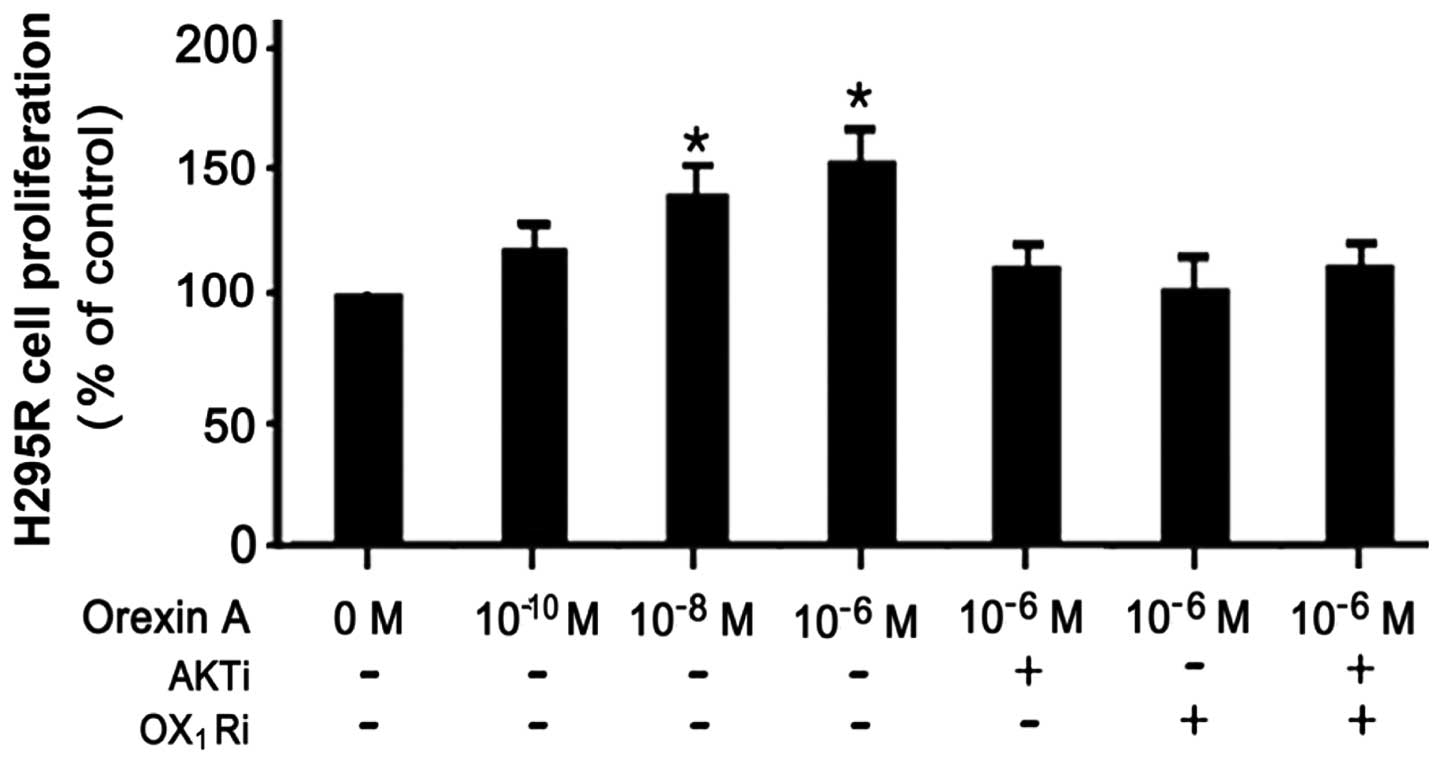
Orexin-A enhances the proliferation of
NCI-H295R cells
To determine the effects of orexin-A on cell
survival, as well as the involvement of the AKT signaling pathway
in the effects of orexin-A in the NCI-H295R cells, we employed BrdU
incorporation assay to examine cell proliferation. The NCI-H295R
cells were treated with various concentrations (0,
10−10, 10−8 and 10−6 M) of
orexin-A; the promoting effects on cell proliferation were
dose-dependent. Concentrations of 10−6 and
10−8 M of orexin-A led to a 0.8- and 0.6-fold increase
in cell proliferation, respectively. However, these effects were
partly blocked when the cells were treated with 10−6 M
orexin-A and the OX1 receptor antagonist, SB334867
(10−6 M), or the AKT antagonist, PF-04691502
(10−6 M), as well as with a combination of both
antagonists. Taken together, these data suggest that AKT
participates in the orexin-A-induced stimulation of the
proliferation of NCI-H295R cells (Fig. 2).
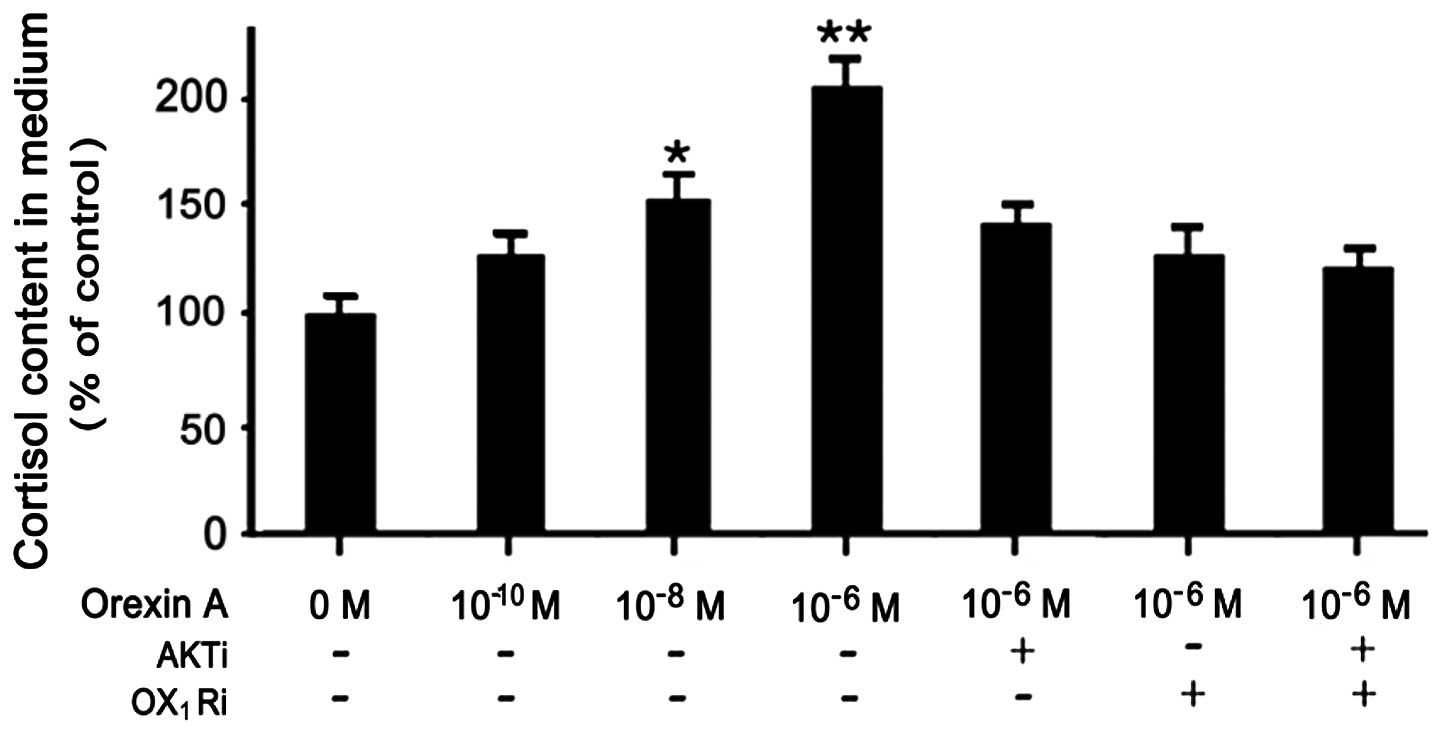
Orexin-A induces cortisol secretion from
NCI-H295R cells
To determine whether the production of the cortisol
is affected in orexin-A-stimulated NCI-H295R cells, cortisol levels
in the culture medium were assessed using an ELISA kit. The
dose-dependent effects of orexin-A on the cortisol content in the
medium were determined from the cell culture supernatants. The
effects of 10−6 and 10−8 M orexin-A reached
statistical significance, increasing cortisol secretion by 1.0- and
0.5-fold, respectively compared to the controls (untreated cells).
This affect was blocked in the presence of PF-04691502
(10−6 M), SB334867 (10−6 M) and the
combination of both antagonists (Fig.
3).
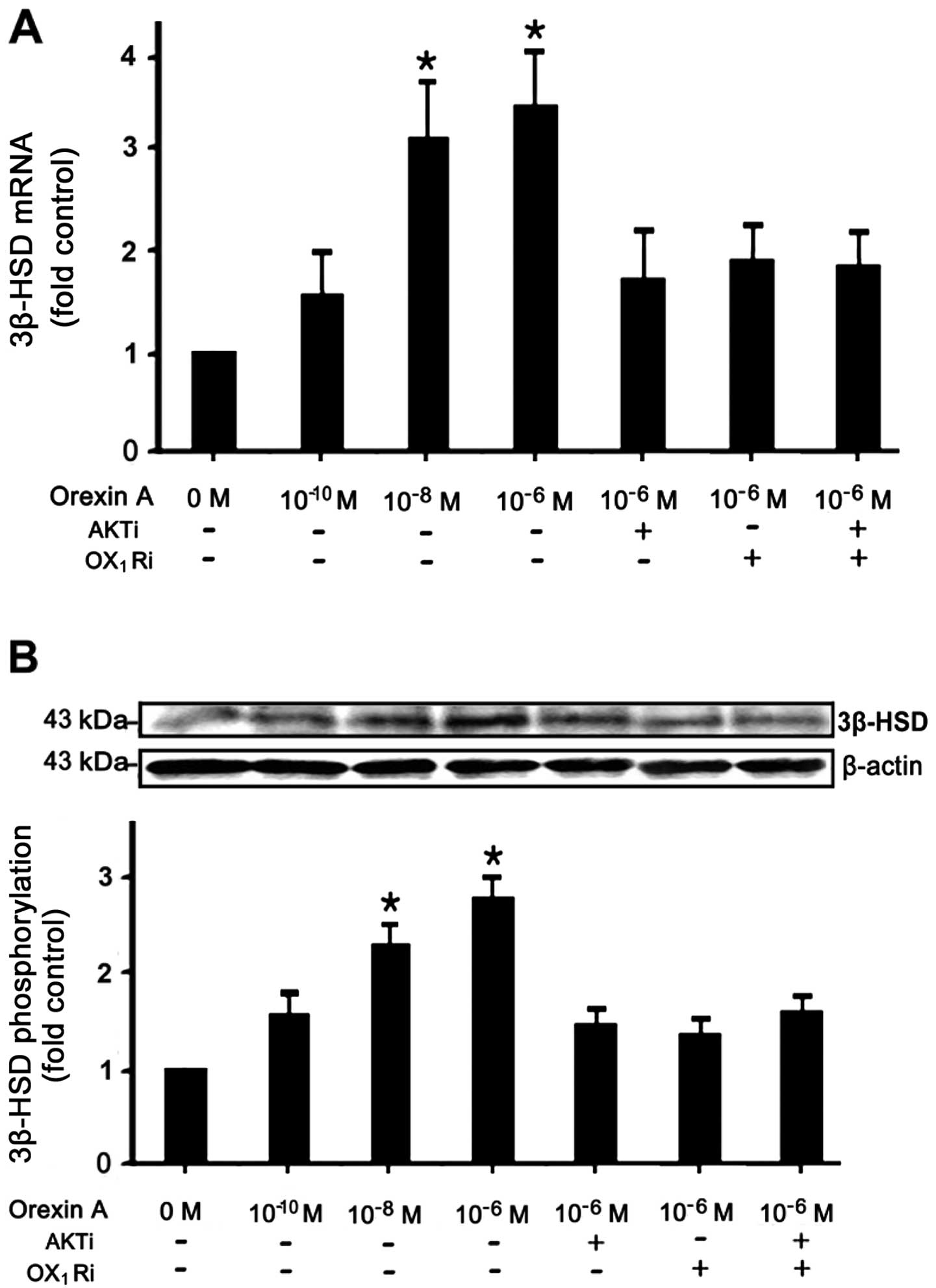
Effects of orexin-A on 3β-HSD mRNA and
protein expression
Follwoing starving overnight in serum-free medium,
the NCI-H295R cells were incubated with various concentrations (0,
10−10, 10−8 and 10−6 M) of
orexin-A, and the cells were then treated with 10−6 M
orexin-A and the OX1 receptor antagonist, SB334867
(10−6 M), or the AKT antagonist, PF-04691502
(10−6 M), or a combination of both antagonists.
Treatment with orexin-A increased the mRNA and protein expression
levels of 3β-HSD in the NCI-H295R cells, an increase that was
dependent on the concentration of orexin-A, with 10−6 M
of orexin-A exerting the most potent effect. Concentrations of
10−6 M and 10−8 M orexin-A led to a 2.1- and
2.4-fold increase in the mRNA levels, respectively (Fig. 4A). As regards protein expression,
10−6 M orexin-A and 10−8 M orexin-A induced a
1.8- and 1.2-fold increase in expression, respectively compared to
the controls (Fig. 4B). This
increase was attenuated in the presence of 10−6 M
SB334867 or 10−6 M PF-04691502, or the combination of
both antagonists.
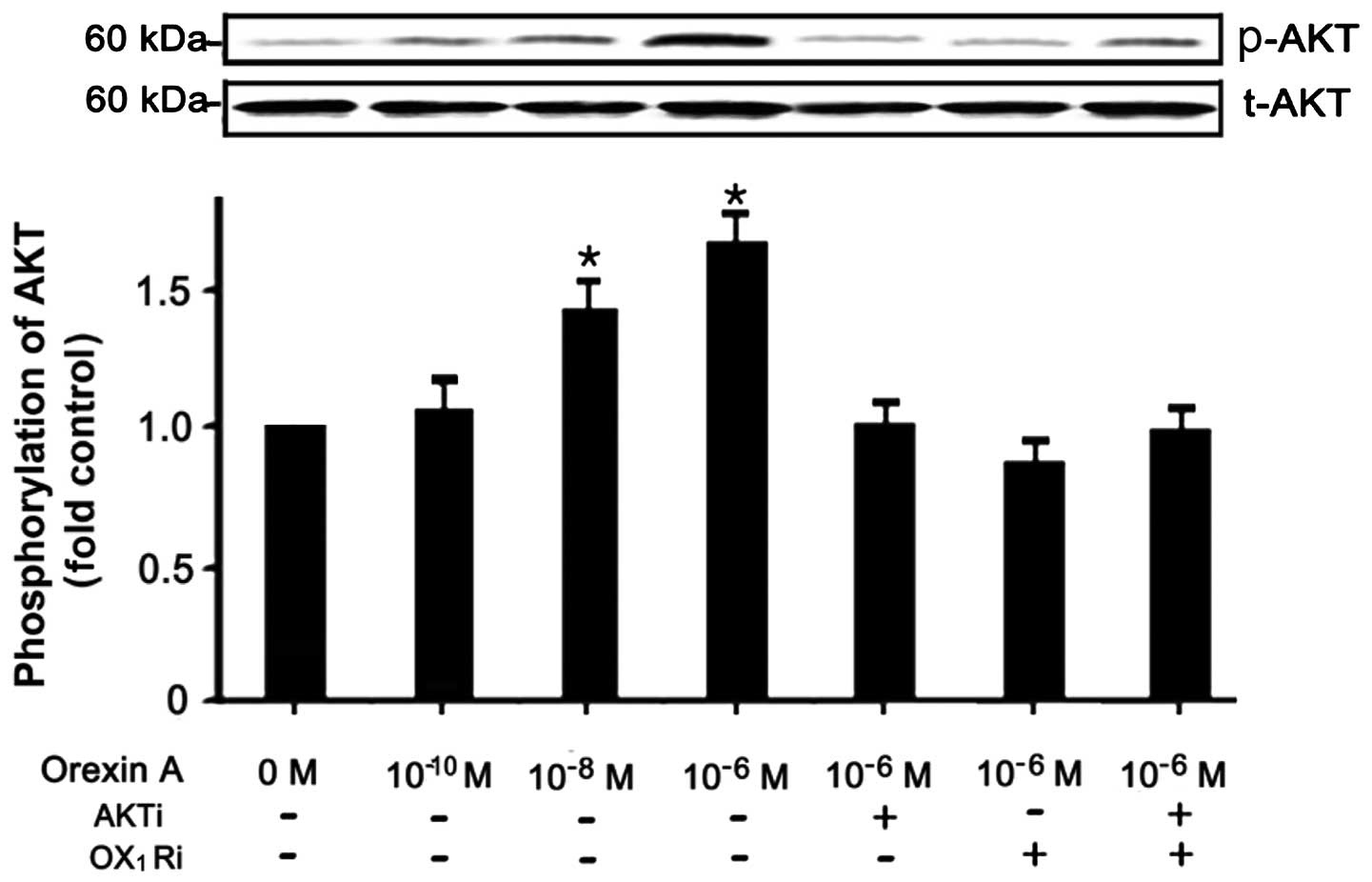
Orexin-A signals through the AKT
pathway
To determine wether the orexin-A-stimulation of
NCI-H295R cells induces the activation of AKT, the NCI-H295R cells
were stimulated with various concentrations (0, 10−6,
10−8 and 10−10 M) of orexin-A. The data
demonstrated a specific increase in the levels of phospo-AKT in the
NCI-H295R cells treated with 10−6 and 10−8 M
orexin-A, increasing by 0.7- and 0.45-fold, respectively, compared
to the untreated controls (Fig.
5). The levels of total AKT, however, remained unaltered by
treatment. In addition, the relative increase in AKT activation in
response to orexin-A was abolished by treatment with AKT antagonist
(PF-04691502, 10−6 M), OX1 receptor
antagonist (SB334867, 10−6 M), as well as the
combination of both antagonsits (Fig.
5). Above all, these data suggest that the regulation of the
AKT signaling pathway is intimately associated with the
orexin-A-stimulated NCI-H295R cell survival through the
OX1 receptor.
Discussion
Studies have indicated an association between the
hypocretin/orexin system and the HPA axis on a central, as well as
peripheral level (7). In this
study, we dertermined the effects of orexin-A on the expression of
OX1 receptor and the proliferation of human
adrenocortical cells. Consistent with the study by Blanco et
al (28), we did not observe
the expression of OX2 receptor in the NCI-H295R cells.
Furthermore, we found a marked increase in cortisol production and
3β-HSD expression following the stimulation of the NCI-H295R cells
with orexin-A, that was associated with an increased activity of
AKT. This finding indicates that another signaling pathway, the AKT
pathway, partly regulates the survival and functions of
adrenocortical cells stimulated with orexin-A through the
OX1 receptor.
Previous studies addressing the effects of orexins
on adrenal function focused on the effects of the HPA axis. One of
the first in vivo studies described an increase in both
adrenocorticotropic hormone (ACTH) and corticosterone plasma levels
1–2 h following a single systemic administration of orexin-A in
rats, while orexin-B had no stimulating effect (9). Other studies clearly demonstrated an
additional direct action of orexins on the adrenal cortex without
the involvement of the HPA axis (7,12).
Orexin-A enhances the production of mineralocorticoids,
glucocorticoids and androgens in the adrenal glands (29). Furthermore, studies have
demonstrated the important role of orexin-A in adrenocortical cell
proliferation (8,12). However, data on whether orexins
regulate the growth of carcinoma cells are inconlcusive. Certain
studies have found that orexins promote the growth of cancer cells
(8,10), athough others have shown
inhibitory effects (30,31). The different cell types used may
be the reason for these conflicting results. Consistent with
previous studies, we found that orexin-A stimulated NCI-H295R cell
proliferation (8,10) and promoted the release of cortisol
in a dose-dependent manner, while the effects were blunted by
co-treatment with the OX1 receptor antagonist, SB334867.
The synthesis of glucocorticoids in human adrenal glands is
achieved by the selective expression of some steroid-synthesizing
enzymes, such as 3β-HSD. As previously reported, the selective
upregulation of 3β-HSD mRNA expression following prolonged
stimulation with orexins indicated the existence of a specific
pathway for the transcriptional regulation of orexins (14). In this study, we found the highest
cortisol synthesis rate after 24 h of treatment with orexin-A,
possibly due to the stimulation of the expression of 3β-HSD and the
proliferation of adrenocortical cells. This was consistent with the
results of a previous study by Wenzel et al, who
demonstrated that orexins increased the expression of steroidogenic
enzymes at the transcriptional level and that orexins played a role
in the long-term regulation of adrenal steroid production (14).
As is well known, among mechanisms controlling the
cell growth and survival, the PI3K signaling pathway is often
activated. The serine/threonine kinase AKT, acting downstream of
PI3K signaling, is a key regulator of multiple survival routes
(32–37). Previous studies focused on the
effects of orexin-A on the MAPK pathway (6,25).
Göncz et al (38) found
that orexin-A modulated glucagon secretion and gene expression
through the PI3K/AKT-dependent pathway in clonal pancreatic A-cells
(InR1-G9 cells). They found an increase in the phosphorylation of
AKT and phosphoinositide-dependent kinase-1 (PDK-1) proteins in
response to treatment with orexin-A (10−5 M) in InR1-G9
cells. Our study demonstrated that orexin-A (10−10 to
10−6 M) stimulated NCI-H295R cell proliferation and
promoted the release of cortisol and increased the expression of
3β-HSD. In addition we observed a specific increase in the levels
of phospho-AKT in the NCI-H295R cells treated with 10−6
and 10−8 M orexin-A. The levels of total AKT, however,
remained unaltered by treatment. The increase in AKT
phosphorylation may be involved in the regulation of the expression
of 3β-HSD. However, these effects were partly blocked by
co-treatment with the OX1 receptor antagonist, SB334867,
or the AKT antagonist, PF-04691502, as well as with the combination
of both antagonists. With respect to the concentration of orexin-A,
it is possible that the cell type may be an important factor
contributing to the physiological effects of orexin-A. Further
studies are required in order to further determine to what extent
the cell type plays a role in the effects of orexin-A. Taken
together, these data suggest that orexin-A stimulates 3β-HSD
expression and cortisol production in human adrenocortical cells
through the OX1 receptor mediated through the AKT
pathway.
In conclusion, we demonstrate that the AKT signaling
pathway is involved in the orexin-A stimulated increase in the
synthesis of cortisol in human adrenocortical cells. Although this
interaction of orexins with adrenocortical cell functions,
particularly with glucocorticoid production, has now bee
established, more comprehensive and specific mechanisms remain to
be elucidated to clarify the nature of the signaling pathway. On
the whole, orexins, together with leptin, may comprise a
counter-regulatory system that controls body weight and energy
homeostasis through the regulation of adrenocortical steroid
production.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30872724, 81071460 and
81271996). We are thankful to the China Medical University
Affiliated Hospital Laboratory Center for kindly providing the
necessary equipment.
Abbreviations:
|
OX1 receptor
|
orexin receptor type 1
|
|
OX2 receptor
|
orexin receptor type 2
|
|
AKT/PKB
|
protein kinase B
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PKA
|
protein kinase A
|
|
PKC
|
protein kinase C
|
|
3β-HSD
|
3β-hydroxysteroid dehydrogenase
|
References
|
1
|
de Lecea L, Kilduff TS, Peyron C, Gao X,
Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT,
Bartlett FS II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM
and Sutcliffe JG: The hypocretins: hypothalamus-specific peptides
with neuroexcitatory activity. Proc Natl Acad Sci USA. 95:322–327.
1998.PubMed/NCBI
|
|
2
|
Heinonen MV, Purhonen AK, Mäkelä KA and
Herzig KH: Functions of orexins in peripheral tissues. Acta Physiol
(Oxf). 192:471–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuki T and Sakurai T: Orexins and
orexin receptors: from molecules to integrative physiology. Results
Probl Cell Differ. 46:27–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakurai T1, Amemiya A, Ishii M, Matsuzaki
I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP,
Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS,
McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ and
Yanagisawa M: Orexins and orexin receptors: a family of
hypothalamic neuropeptides and G protein-coupled eceptors that
regulate feeding behavior. Cell. 92:573–585. 1998. View Article : Google Scholar
|
|
5
|
Voisin T, Rouet-Benzineb P, Reuter N and
Laburthe M: Orexins and their receptors: structural aspects and
role in peripheral tissues. Cell Mol Life Sci. 60:72–87. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kagerer SM and Jöhren O: Interactions of
orexins/hypocretins with adrenocortical functions. Acta Physiol
(Oxf). 198:361–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malendowicz LK, Hochol A, Ziolkowska A,
Nowak M, Gottardo L and Nussdorfer GG: Prolonged orexin
administration stimulates steroid-hormone secretion, acting
directly on the rat adrenal gland. Int J Mol Med. 7:401–404.
2001.PubMed/NCBI
|
|
8
|
Malendowicz LK, Jedrzejczak N, Belloni AS,
Trejter M, Hochol A and Nussdorfer GG: Effects of orexins A and B
on the secretory and proliferative activity of immature and
regenerating rat adrenal glands. Histol Histopathol. 16:713–717.
2001.PubMed/NCBI
|
|
9
|
Malendowicz LK, Tortorella C and
Nussdorfer GG: Orexins stimulate corticosterone secretion of rat
adrenocortical cells, through the activation of the adenylate
cyclase-dependent signaling cascade. J Steroid Biochem Mol Biol.
70:185–188. 1999. View Article : Google Scholar
|
|
10
|
Mazzocchi G, Malendowicz LK, Gottardo L,
Aragona F and Nussdorfer GG: Orexin A stimulates cortisol secretion
from human adrenocortical cells through activation of the adenylate
cyclase-dependent signaling cascade. J Clin Endocrinol Metab.
86:778–782. 2001. View Article : Google Scholar
|
|
11
|
Nanmoku T, Isobe K, Sakurai T, Yamanaka A,
Takekoshi K, Kawakami Y, Goto K and Nakai T: Effects of orexin on
cultured porcine adrenal medullary and cortex cells. Regul Pept.
104:125–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spinazzi R, Rucinski M, Neri G,
Malendowicz LK and Nussdorfer GG: Preproorexin and orexin receptors
are expressed in cortisol-secreting adrenocortical adenomas, and
orexins stimulate in vitro cortisol secretion and growth of tumor
cells. J Clin Endocrinol Metab. 90:3544–3549. 2005. View Article : Google Scholar
|
|
13
|
Ziolkowska A, Spinazzi R, Albertin G,
Nowak M, Malendowicz LK, Tortorella C and Nussdorfer GG: Orexins
stimulate glucocorticoid secretion from cultured rat and human
adrenocortical cells, exclusively acting via the OX1
receptor. J Steroid Biochem Mol Biol. 96:423–429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wenzel J, Grabinski N, Knopp CA, Dendorfer
A, Ramanjaneya M, Randeva HS, Ehrhart-Bornstein M, Dominiak P and
Jöhren O: Hypocretin/orexin increases the expression of
steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J
Physiol Regul Integr Comp Physiol. 297:R1601–R1609. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayes HM Jr and Wilson GP:
Hormone-dependent neoplasms of the canine perianal gland. Cancer
Res. 37:2068–2071. 1977.PubMed/NCBI
|
|
16
|
Sauer LA, Chapman JC and Dauchy RT:
Topology of 3 beta-hydroxy-5-ene-steroid dehydrogenase/delta
5-delta 4-isomerase in adrenal cortex mitochondria and microsomes.
Endocrinology. 134:751–759. 1994.PubMed/NCBI
|
|
17
|
Zhao HF, Labrie C, Simard J, de Launoit Y,
Trudel C, Martel C, Rhéaume E, Dupont E, Luu-The V, Pelletier G and
Labriel F: Characterization of rat 3 beta-hydroxysteroid
dehydrogenase/delta 5- delta 4 isomerase c DNAs and differential
tissue specific expression of the corresponding mRNAs in
steroidogenic and peripherial tissues. J Biol Chem. 266:583–593.
1991.
|
|
18
|
van Blitterswijk WJ and Verheij M:
Anticancer mechanisms and clinical application of
alkylphospholipids. Biochim Biophys Acta. 1831:663–674.
2013.PubMed/NCBI
|
|
19
|
Fu L, Kim YA, Wang X, Wu X, Yue P, Lonial
S, Khuri FR and Sun SY: Perifosine inhibits mammalian target of
rapamycin signaling through facilitating degradation of major
components in the mTOR axis and induces autophagy. Cancer Res.
69:8967–8976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar
|
|
21
|
Richardson CJ, Schalm SS and Blenis J:
PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol.
15:147–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh AC, Truitt ML and Ruggero D:
Oncogenic AKTivation of translation as a therapeutic target. Br J
Cancer. 105:329–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sale EM and Sale GJ: Protein kinase B:
signalling roles and therapeutic targeting. Cell Mol Life Sci.
65:113–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blandino-Rosano M, Chen AY, Scheys JO,
Alejandro EU, Gould AP, Taranukha T, Elghazi L, Cras-Méneur C and
Bernal-Mizrachi E: mTORC1 signaling and regulation of pancreatic
β-cell mass. Cell Cycle. 11:1892–1902. 2012.
|
|
25
|
Ramanjaneya M, Conner AC, Chen J, Kumar P,
Brown JE, Jöhren O, Lehnert H, Stanfield PR and Randeva HS:
Orexin-stimulated MAP kinase cascades are activated through
multiple G-protein signalling pathways in human H295R
adrenocortical cells: diverse roles for orexins A and B. J
Endocrinol. 202:249–261. 2009. View Article : Google Scholar
|
|
26
|
Ramanjaneya M, Conner AC, Chen J,
Stanfield PR and Randeva HS: Orexins stimulate steroidogenic acute
regulatory protein expression through multiple signaling pathways
in human adrenal H295R cells. Endocrinology. 149:4106–4115. 2008.
View Article : Google Scholar
|
|
27
|
Rainey WE, Saner K and Schimmer BP:
Adrenocortical cell lines. Mol Cell Endocrinol. 228:23–38. 2004.
View Article : Google Scholar
|
|
28
|
Blanco M, García-Caballero T, Fraga M,
Gallego R, Cuevas J, Forteza J, Beiras A and Diéguez C: Cellular
localization of orexin receptors in human adrenal gland,
adrenocortical adenomas and pheochromocytomas. Regul Pept.
104:161–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Payne AH and Hales DB: Overview of
steroidogenic enzymes in the pathway from cholesterol to active
steroid hormones. Endocr Rev. 25:947–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rouet Benzineb P, Rouyer Fessard C, Jarry
A, Avondo V, Pouzet C, Yanagisawa M, Laboisse C, Laburthe M and
Voisin T: Orexins acting at native OX(1) receptor in colon cancer
and neuroblastoma cells or at recombinant OX(1) receptor suppress
cell growth by inducing apoptosis. J Biol Chem. 279:45875–45886.
2004.PubMed/NCBI
|
|
31
|
Biegańska K, Sokołowska P, Jöhren O and
Zawilska JB: Orexin A suppresses the growth of rat C6 glioma cells
via a caspase-dependent mechanism. J Mol Neurosci. 48:706–712.
2012.PubMed/NCBI
|
|
32
|
Alessandro R and Kohn EC: Signal
transduction targets in invasion. Clin Exp Metastasis. 19:265–273.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sachdev P, Jiang YX, Li W, Miki T, Maruta
H, Nur-E-Kamal MS and Wang LH: Differential requirement for Rho
family GTPases in an oncogenic insulin-like growth factor-I
receptor-induced cell transformation. J Biol Chem. 276:26461–26471.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen KT, Wang WJ, Chan JL and Wang LH:
Differential requirements of the MAP kinase and PI3 kinase
signaling pathways in Src- versus insulin and IGF-1
receptors-induced growth and transformation of rat intestinal
epithelial cells. Oncogene. 19:5385–5397. 2000. View Article : Google Scholar
|
|
35
|
Sachdev LP, Zeng and Wang LH: Distinct
role of phosphatidylinositol 3-kinase and Rho family GTPases in
Vav3-induced cell transformation, cell motility, and morphological
changes. J Biol Chem. 277:17638–17648. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nguyen KT, Zong CS, Uttamsingh S, Sachdev
P, Bhanot M, Le MT, Chan JL and Wang LH: The role of
phosphatidylinositol 3-kinase, rho family GTPases, and STAT3 in
Ros-induced cell transformation. J Biol Chem. 277:11107–11115.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray
MR, Snow BE, Ayala R, Danino M, Karlan BY and Slamon DJ:
Overexpression of AKT2/protein kinase Bbeta leads to up-regulation
of beta1 integrins, increased invasion, and metastasis of human
breast and ovarian cancer cells. Cancer Res. 63:196–206.
2003.PubMed/NCBI
|
|
38
|
Göncz E, Strowski MZ, Grötzinger C, Nowak
KW, Kaczmarek P, Sassek M, Mergler S, El-Zayat BF, Theodoropoulou
M, Stalla GK, Wiedenmann B and Plöckinger U: Orexin-A inhibits
glucagon secretion and gene expression through a Foxo1-dependent
pathway. Endocrinology. 149:1618–1626. 2008.PubMed/NCBI
|