Introduction
Allergic responses frequently occur in developed
nations, with anaphylactic shock being particularly fatal (1). Anaphylactic shock is a rapidly
occurring, severe allergic response that may cause mortality
(2). Anaphylaxis can occur in
response to any foreign substance, including venom from insects,
foods and medications (3–5). Globally, 0.05–2% of people are
estimated to suffer anaphylaxis during their lifetime and this rate
appears to be increasing (6).
Avoiding the specific triggers, such as foods and medications, is
critically imporant to prevent anaphylaxis (7). However, this is not always possible.
Thus, development of immunoadjuvants is essential for preventing
anaphylaxis and allergic reactions.
Mast cells derived from hematopoietic cells are
associated with tissue immunity and innate immunity and play an
significant role in allergic and anaphylactic reactions (1). Mast cells can secrete pre-formed
mediators, including histamine, heparin and inflammatory cytokines,
by degranulation (8,9). Antigen immunoglobulin E
(IgE)-dependent-activated mast cells induce degranulation to
secrete three types of mediators. Several inflammatory and
chemotactic cytokines, such as interleukin (IL)-1β, IL-6 and tumor
necrosis factor (TNF)-α, are produced from activated mast cells
(10). Additionally, these
pro-inflammatory cytokines support the well-recognized role of mast
cells in allergic inflammation and hypersensitivity (10). Histamine release from mast cells
stimulates cardiac contraction, vascular permeability and
anaphylaxis; the IgE-dependent pathway, which results in histamine
release, is part of the mechanism associated with anaphylaxis
(1).
The production of these cytokines is transmitted
through signal molecules, such as the transcription factors
mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB
(11,12). MAPKs are expressed and activated
in a systemic inflammatory disorder and play an important role in
the control of cytokines, chemokines and cell proliferation
(13). As transcriptional
factors, MAPK and NF-κB play a pivotal role in inflammation by
virtue of their ability to induce transcription of an array of
inflammatory genes, particularly the regulation of pro-inflammatory
molecules, including IL-1β, IL-6 and TNF-α (14,15).
The phytoestrogen genistein
(4′,5,7-trihydroxyisoflavone) is an isoflavonoid compound
containing soy beans. Genistein has a variety of biological
effects, including anti-inflammatory and antioxidant properties,
and inhibits protein tyrosine kinases (PTK) and influences immune
responses (16,17). Genistein also modulates the
activation of NF-κB and Akt during inflammation (18). Immortalized human mammary
epithelial cells demonstrated decreased extracellular
signal-regulated kinase (ERK)1/ERK2 phosphorylation when treated
with genistein, specifically inhibiting cytokine-induced ERK
phosphorylation (19). Several
animal studies indicate that genistein reduces production of
pro-inflammatory molecules, such as IL-6 and TNF-α, in rat plasma.
In addition, in an acute liver inflammation model, oral
administration of soy-derived genistein suppresses IL-6, IL-1β and
TNF-α in RAW264.7 macrophages (20,21). However, the signaling pathways
involved in the anti-inflammatory effect of genistein on human mast
cell activation remain unknown.
The aim of the present study was to investigate the
anti-inflammatory effects of genistein on phorbol 12-myristate
13-acetate (PMA)- and A23187-, a calcium ionophore, induced
expression of pro-inflammatory cytokines and histamine release, as
well as their associated regulatory signaling pathways.
Materials and methods
Cell culture and genistein treatment
The human leukemic mast cell (HMC)-1 line was
obtained from Dr Dae Ki Kim at Chunbuk National University
(Jeonju-si, Korea). HMC-1 were cultured in Iscove’s modified
Dulbecco’s medium (IMDM) supplemented with 10% heat-inactivated
fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml
streptomycin (PAA Laboratories Inc., Piscataway, NJ, USA) at 37°C
and 5% CO2. Genistein was dissolved in dimethyl
sulfoxide (DMSO) (Sigma, St. Louis, MO, USA) and diluted to the
desired concentration in IMDM (final DMSO concentration 0.1% v/v).
An equal amount of DMSO was added to the control samples (medium
only).
Cell viability
Cell viability was measured by the
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT)
assay. HMC-1 (2×104 cells/well) were seeded in 96-well
U-bottom culture plates with IMDM and incubated at 37°C and 5%
CO2. Cells were treated with various concentrations of
genistein (12.5–50 μmol/ml) and incubated at 37°C for 24 h.
Following treatment, MTT (0.5 mg/ml) in medium was added to each
well and cells were incubated at 37°C for 4 h. Following
incubation, the MTT solution was removed and the formazan product
was dissolved in a solvent (DMSO:ethanol = 1:1) generating a
colored solution. Absorbance was measured by an enzyme-linked
immunosorbent assay (ELISA) microplate reader at a wavelength of
570 nm (BioTek, Winooski, VT, USA).
RNA isolation and reverse transcription
PCR (RT-PCR)
Total RNA was isolated from genistein-treated cells
using TRI reagent (Sigma) according to the manufacturer’s
instructions. To synthesize cDNA, 0.5 μg of total RNA was primed
with oligo(dT) and reacted with a mixture of Moloney murine
leukemia virus reverse transcriptase (M-MLV reverse transcriptase),
dNTP and reaction buffer (Promega, Madison, WI, USA). The mRNA
levels of inflammatory cytokines were measured using synthetic cDNA
and selective primers for PCR: IL-6 forward, GAG GCA CTG GCA
GAA AAC AA; and reverse, TTG GGT CAG GGG TGG TTA TT; IL-1β
forward, GTA CCT GAG CTC GCC AGT GA; and reverse, TGA AGC CCT TGC
TGT AGT GG; TNF-α forward, CCA TCA GAG GGC CTG TAC CT; and
reverse, CAG ACT CGG CAA AGT CGA GA; GAPDH forward, AAG GGT
CAT CAT CTC TGC CC; and reverse, GTG ATG GCA TGG ACT GTG GT. The
PCR products were stained with Loading Star (Dynebio co., Ltd.,
Seongnam-si, Korea) and electrophoresed on a 1% agarose gel. The
bands were detected by a UV transilluminator (Core Bio, La Jolla,
CA, USA).
Western blot analysis
HMC-1 (2×106 cells/well) were seeded in a
60Φ cell culture dish and starved with serum-free IMDM for 6 h.
After starvation, cells were pretreated with genistein for 30 min,
and stimulated with 20 nmol/l PMA and 1 μmol/l of the calcium
ionophore, A23187, for 15, 30 and 60 min. Treated cells were washed
with cold phosphate-buffered saline and lysed with modified
radioimmunoprecipitation assay buffer [50 mmol/l Tris-HCl, 0.1%
sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 1% NP-40
and 150 mmol/l sodium chloride (pH 8.0)] at 4°C for 30 min. The
lysates were centrifuged at 13,000 × g for 15 min and the
supernatant was used as protein samples. Protein concentration was
measured according to the manufacturer’s instructions by
colorimetric bicinchoninic acid kit (Thermo Scientific, Pittsburgh,
PA, USA). Equivalent amounts of protein were separated by 10%
SDS-polyacrylamide gel electrophoresis and electrophoretically
transferred to polyvinylidene fluoride membrane (Millipore,
Billerica, MA, USA). The membranes were incubated with blocking
solution (5% skimmed milk in Tris-buffered saline TBS) for 1 h.
Following blocking, membranes were probed with anti-ERK
(sc-292838), anti-p-ERK (sc-7383), anti-p38 (sc-535) and anti-p-p38
(sc-7973) primary antibodies (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), and with horseradish peroxidase (HRP)-conjugated
anti-rabbit or anti-mouse secondary antibodies (Santa Cruz
Biotechnology) for 2 h. Bands were visualized using an enhanced
chemiluminescence (Bio-Rad Laboratories, Hercules, CA, USA)
detection system and exposed to radiographic film.
ELISA
HMC-1 (2×104 cells/well) were seeded in
96-well U-bottom culture plates. Cells were pretreated with various
concentrations of genistein (12.5–50 μmol/l) for 30 min, and
stimulated with PMA/A23187 for 48 h. Cultured cells were separated
by microcentrifugation and the supernatant was used for samples.
IL-6 release was measured by Human IL-6 ELISA MAX™ Deluxe Sets
(BioLegend, San Diego, CA, USA), according to the manufacturer’s
instructions. Briefly, standards and samples were incubated on a
capture antibody-coated plate overnight at 4°C. The detection
antibody was added and samples were incubated for 1 h and
avidin-HRP bound to the detection antibody. Substrate solution was
added to each well, and the reaction was stopped by addition of a
stop solution (2N H2SO4). Absorbance was
measured by an ELISA microplate reader at a wavelength of 405
nm.
β-hexogeminidase assay
HMC-1 (5×105 cells/well) were seeded in
24-well plates with IMDM. After a 10-min incubation at 37°C, cells
were pretreated with genistein for 30 min followed by stimulation
with PMA/A23187 for 1 h. Cultured cells were collected by
centrifugation (1,500 × g) for 5 min. The pellet and supernatant
were transferred to separate microtubes. The pellet was lysed with
1% Triton X-100 in Tris-HCl (pH 8.0) for 20 min at 4°C. The lysate
(50 μl) was combined with 50 μl 2 mM
p-nitrophenyl-N-acetyl-β-D-glucosaminide (Calbiochem, Canada)
dissolved in 0.1 mol/l citrate buffer (pH 4.5) for 1 h at 37°C. The
reaction was ended with 100 μl of a stop solution (0.1 mol/l
NaHCO3/Na2CO3), and the absorbance
was measured at 405 nm using an ELISA microplate reader.
Statistical analysis
All the results are expressed as the mean ± standard
deviation of the indicated number of the experiments. Statistical
significance was estimated using a Student’s t-test for unpaired
observations, and the differences were compared with regard to
statistical significance by one-way analysis of variance, followed
by Bonferroni’s post-hoc test. The categorical data from the
fertility test were subjected to statistical analysis via the
χ2 test.
Results
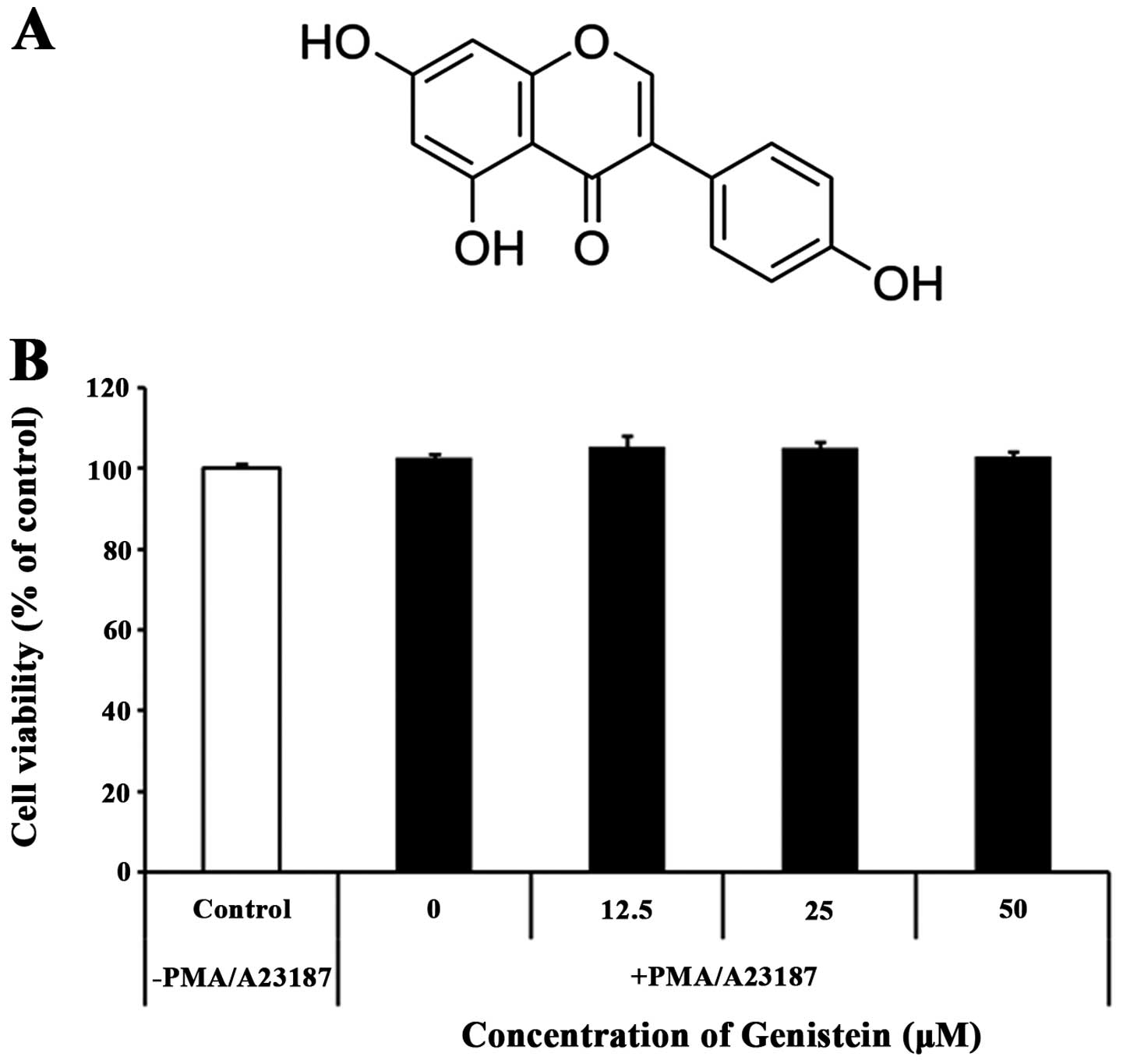
Genistein had no effect on cytotoxicity
in a human mast cell line (HMC-1)
In an initial series of experiments, whether
genistein influenced the cytotoxicity of a human mast cell line
(HMC-1) was investigated. HMC-1 cells were treated with various
concentrations of genistein, ranging from 0 to 50 μM, for 24 h and
subjected to an MTT assay. As shown in Fig. 1B, the addition of 50 μM genistein
did not alter HMC-1 numbers when compared to the control or cells
treated with only medium [DMSO (0.02%) in IMDM]. Therefore, this
range of genistein concentrations was used to determine the
anti-inflammatory effects of genistein during mast cell
activation.
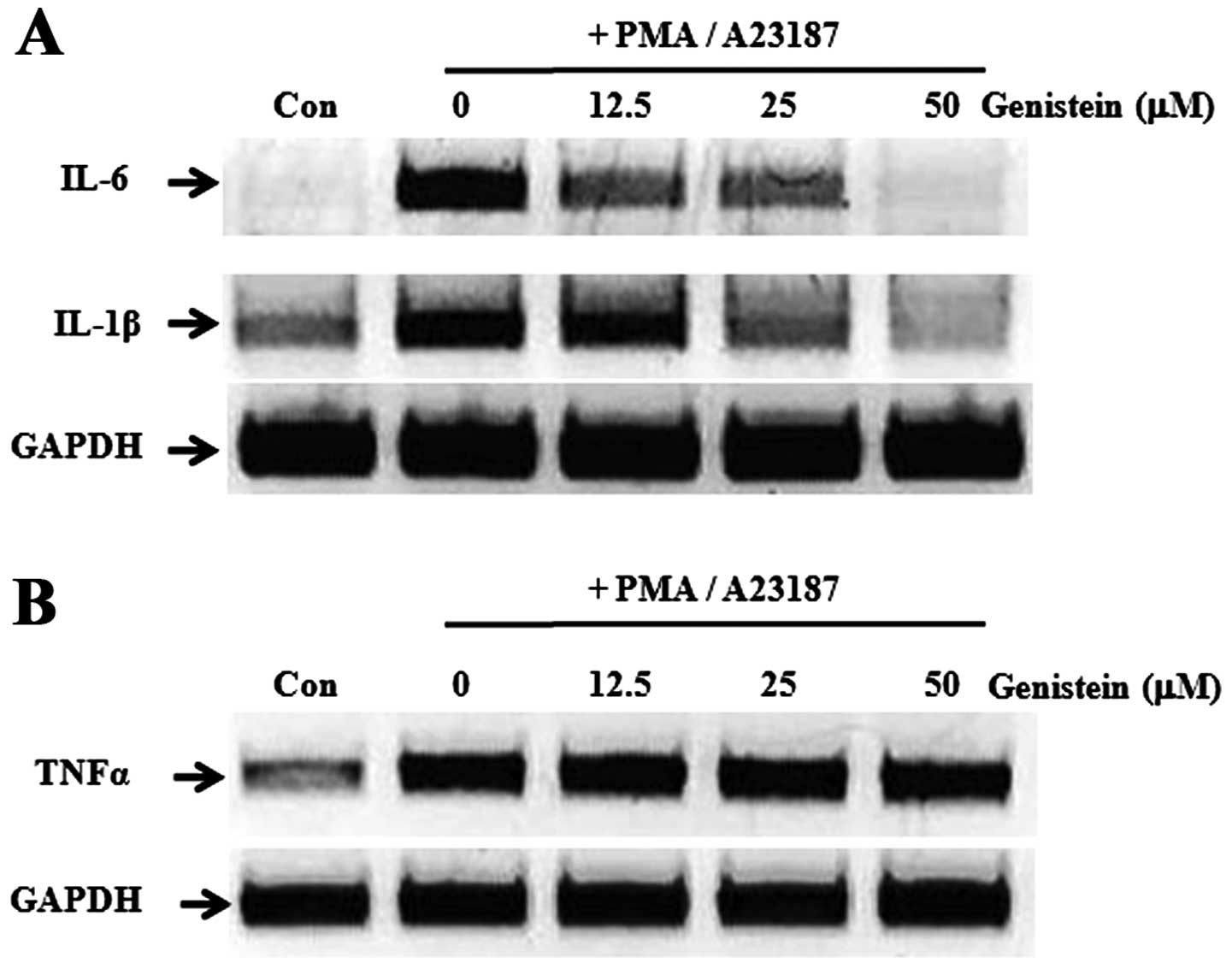
Genistein suppresses the expression
levels of pro-inflammatory cytokines in PMA/A23187-induced
HMC-1
Pro-inflammatory cytokines are important mediators
of inflammation, cell recruitment and allergenic responses
(22). To evaluate the effect of
genistein on the gene expression of pro-inflammatory cytokines,
HMC-1 was initially treated with genistein and the cells were
stimulated with PMA (20 nM) and A23187 (1 μM), prior to analyzing
the gene expression of the pro-inflammatory cytokines using RT-PCR.
As shown in Fig. 2A, a high level
of genistein (50 μM) significantly suppressed the gene expression
of IL-1β and IL-6. However, TNF-α gene expression remained
unaltered (Fig. 2B).
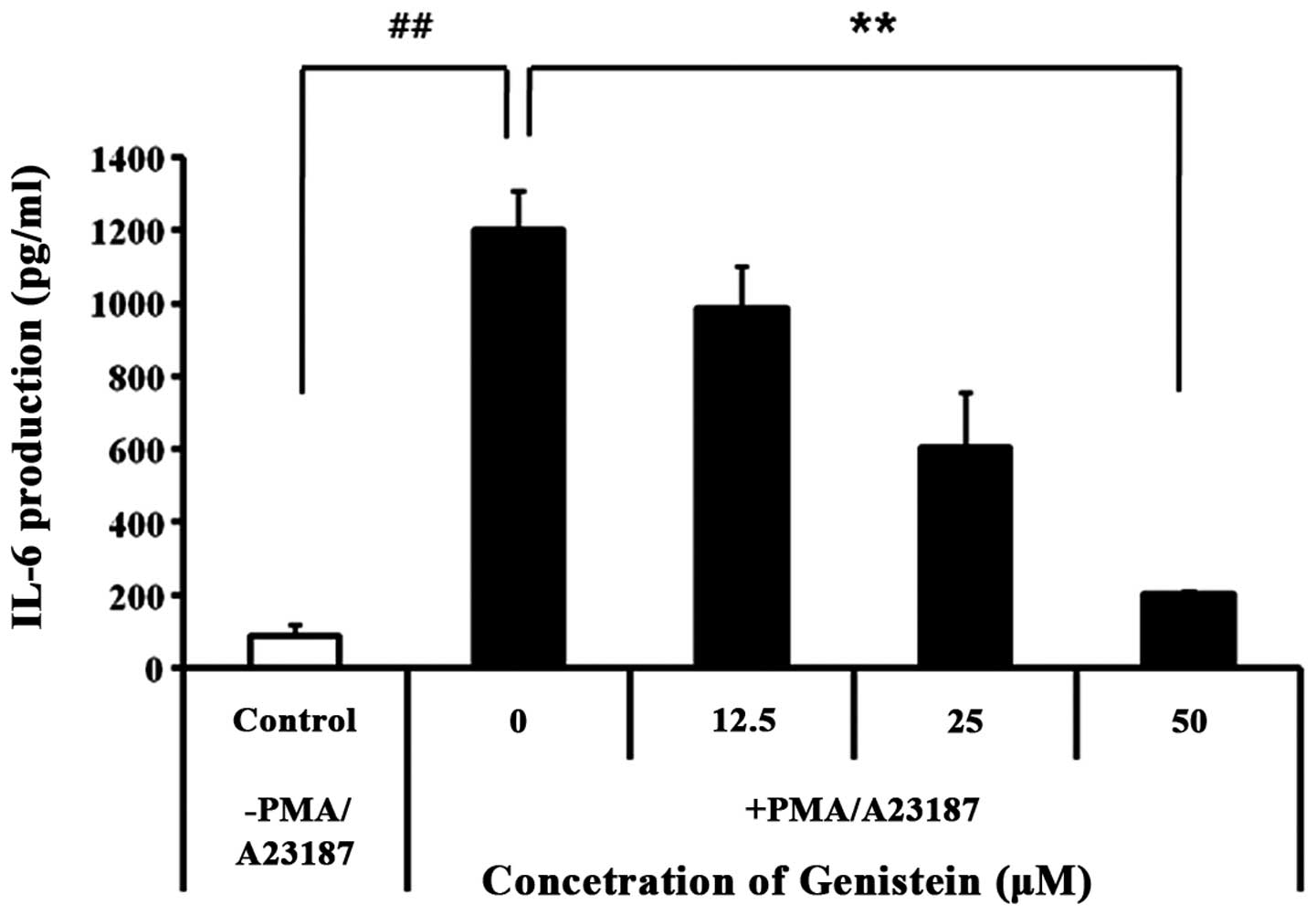
Genistein significantly inhibits IL-6
production in PMA/A23187-induced HMC-1
IL-6 is crucial for mast cell maturation; activated
mast cells increase IL-6 mRNA associated with protein kinase C
(PKC) activity and also upregulate histamine production (23). We found that genistein suppressed
gene expression of pro-inflammatory cytokines IL-1β and IL-6
(Fig. 2A). To confirm the effect
of genistein on the gene expression of pro-inflammatory cytokines,
culture supernatants were assayed for cytokine levels by ELISA.
HMC-1 cells were pretreated with genistein (12.5–50 μM) for 30 min,
and subsequently stimulated with PMA and A23187 for 48 h. As shown
in Fig. 3, genistein strongly
decreased the production of IL-6 in PMA/A23187-induced HMC-1. These
results indicate that genistein inhibits pro-inflammatory
cytokines, such as IL-1β and IL-6 in PMA/A23187-activated
HMC-1.
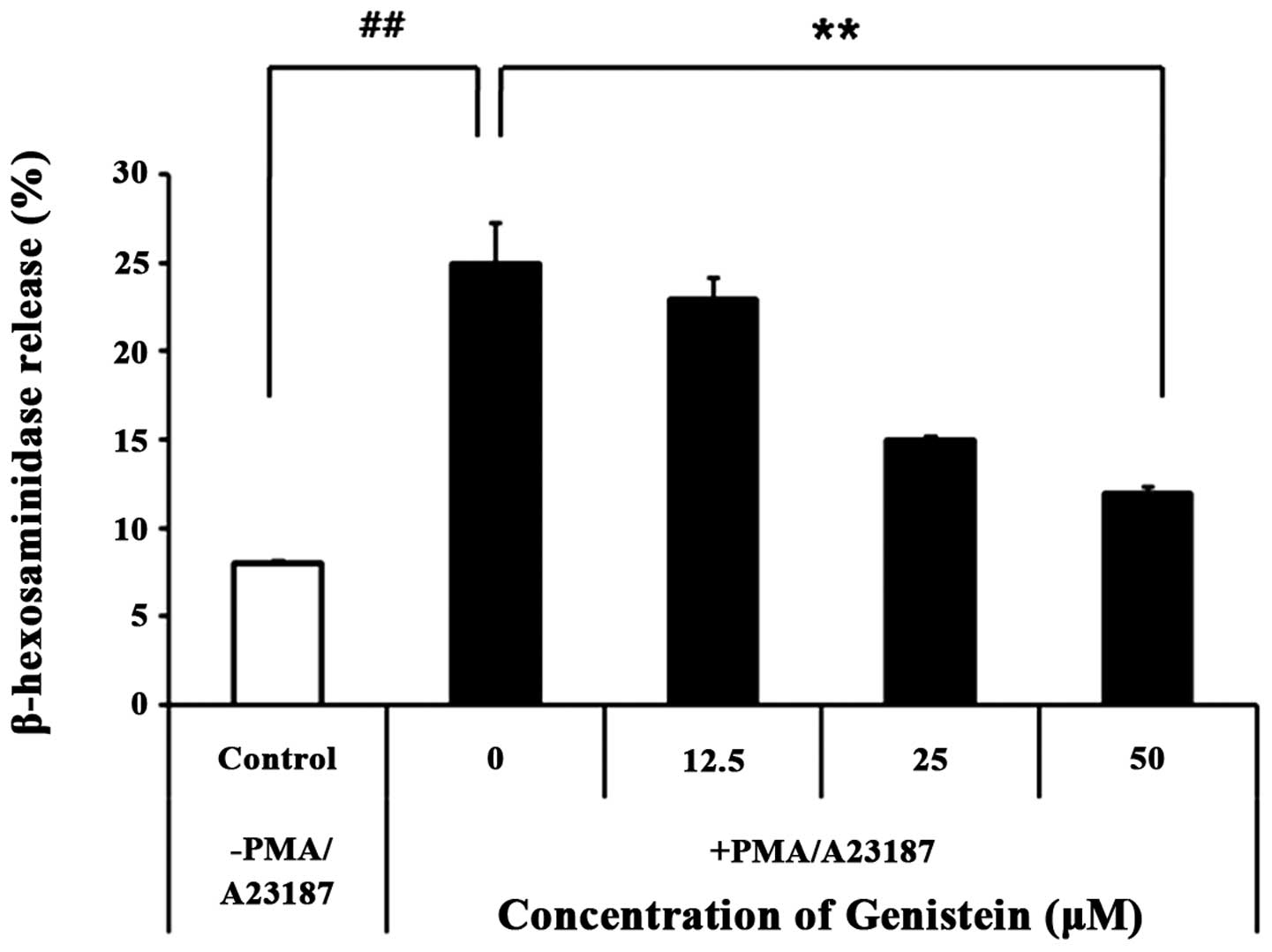
Genistein inhibits histamine release in
PMA/A23187-induced HMC-1
In order to determine the effect of genistein on
mast cells degranulation, the effect of genistein was investigated
on the release of β-hexosaminidase, a secretory granule marker that
is released in parallel with histamine. As shown in Fig. 4, 50 μM genistein reduced
β-hexosaminidase release by 2-fold, indicating that genistein
inhibits mast cell degranulation.
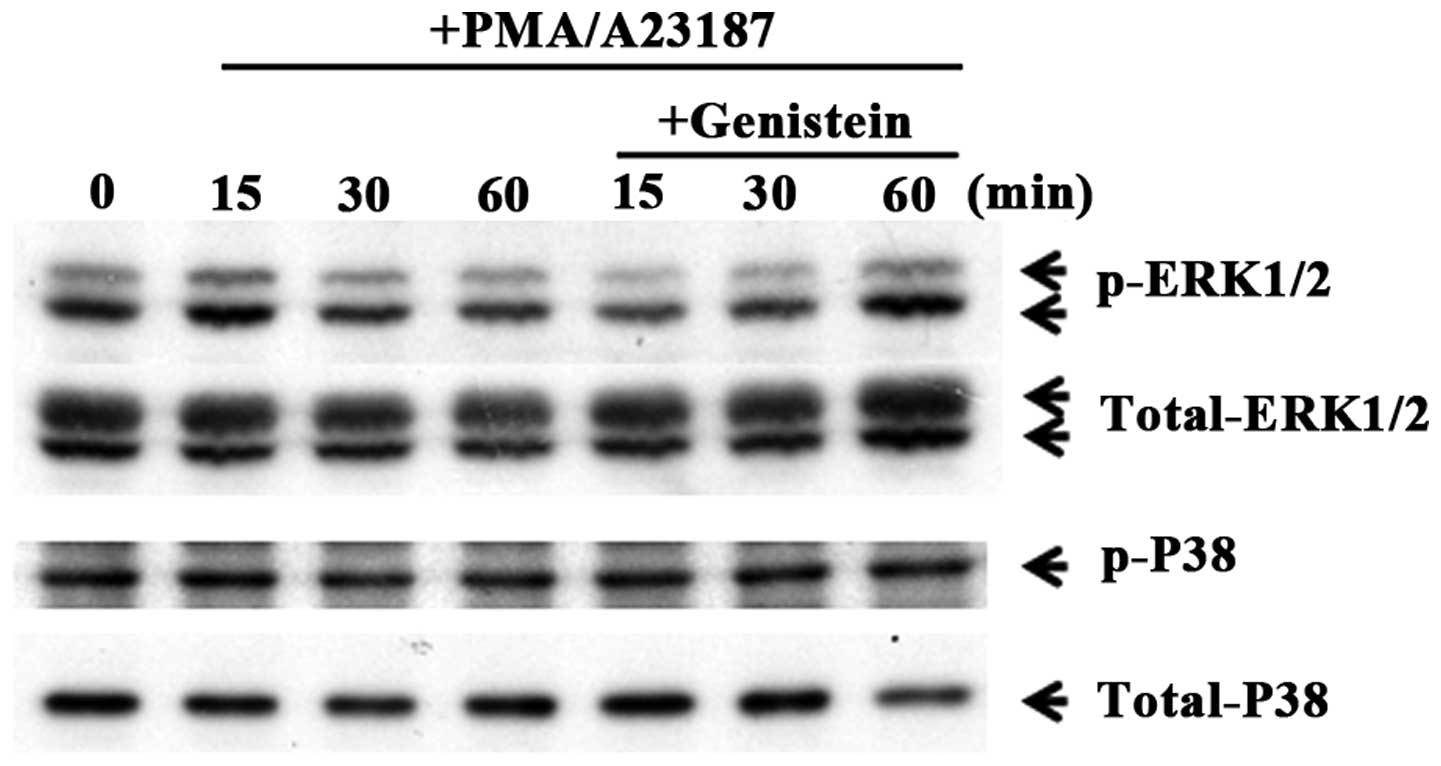
Genistein regulates phosphorylation of
ERK
To evaluate the mechanism of the effect of genistein
on gene expression of pro-inflammatory cytokines, the influence of
genistein on MAPK phosphorylation was investigated. IL-1β and IL-6
expression is regulated by a transcription factor, NF-κB, and
activated by MAPK pathways (24).
In order to investigate the effects of genistein on the MAPK
signaling pathways, HMC-1 cells were pretreated with 50 μM
genistein and kinase activation was assessed. Phosphorylation of
p38 MAPK and ERK1/2 were measured by phosphor-specific western
blotting. As shown in Fig. 5,
genistein inhibited the phosphorylation of ERK1/2 in
PMA/A23187-induced mast cells. These results indicate that
genistein inhibits pro-inflammatory cytokine production via
regulation of the ERK pathways.
Discussion
Allergic diseases, including asthma and anaphylaxis,
are a severe health burden for a number of nations. In the United
States, an estimated 20 million patients are treated for allergic
diseases, at an annual cost exceeding $15 billion (25). Anaphylaxis is a type I
hypersensitivity reaction mediated by IgE-activated mast cells and
occurs locally and systemically; it is caused by various
inflammatory mediators, such as histamine, tryptase and several
cytokines from activated mast cells. These inflammatory mediators
affect leukocyte recruitment, and cause vasodilation, increased
vascular permeability and bronchial-constriction (26). Therefore, numerous investigators
have sought to develop novel therapeutic adjuvants for allergenic
inflammation via regulation of mast cells. To address this, the
anti-inflammatory effects of genistein, a phytoestrogen, were
investigated on mast cell activation.
In general, PMA activates PKC, resulting in
activation of mast cells. In addition, the calcium ionophore,
A23187, increases the permeability of the cell membrane to
Ca2+ and can selectively activate gene expression of
calcium-regulated genes (27). In
addition, A23187 alone has been shown to induce granule release in
mast cells (8). The present study
assessed HMC-1, an immature human mast cell line derived from the
peripheral blood of a patient suffering from mast cell leukemia.
HMC-1 lacks FcɛRI, making it difficult to activate the mast cells
through IgE-mediated responses. In addition, co-treatment with PMA
and A23187 was also used to activate mast cells.
Genistein is a phytoestrogen isolated from
Genista tinctoria; the chemical name is derived from the
generic name. Kim et al (28) reported that
genistein-4′-O-α-L-rhamnopyranosyl-(1→2)- β-D-glucopyranoside from
Sophora japonica (Leguminosae) ameliorates mast
cell-mediated allergic inflammation in vivo and in
vitro. In addition, the study by Kim et al indicates
that the anti-inflammatory effect of the genistein compound is
involved in the regulation of inflammatory cytokines, including
IL-8 and TNFα. A recent study found that genistein inhibited
transcription factors, including GATA-binding protein-3 and signal
transducer and activator of transcription-6, which control the
Th1/Th2 response in an asthma mouse model. Specifically, genistein
decreased Th2-type cytokine levels and attenuated ovalbumin-induced
airway inflammation (29).
However, the underlying mechanisms by which genistein inhibits
inflammatory mediators in mast cell activation remain unknown.
To the best of our knowledge, this is the first
study showing that genistein inhibits pro-inflammatory cytokines in
activated mast cells. Genistein was found to significantly suppress
IL-1β and IL-6 expression in PMA/A23187-induced mast cells
(Fig. 2A). In general, mast cells
release an array of mediators with the potential to cause allergic
inflammation, such as the pro-inflammatory cytokines, IL-1β, IL-6
and TNF-α. Specifically, IL-1β plays an important role in allergic
response; the local accumulation of IL-6 is associated with a local
allergic reaction, and IL-1β and IL-6 promote inflammation and a
mast cell-mediated immune response (30–32). These studies indicate that
reduction of pro-inflammatory cytokines from mast cells is one of
the keys to reducing inflammatory symptoms. The results of the
present study, showing inhibition of IL-1β and IL-6 expression by
genistein, support the idea that genistein has an anti-inflammatory
effect resulting from the reduction of these mediators in mast
cells. Additionally, the induction of these cytokines was involved
in the activation of the MAPK and NF-κB pathway. The study by
Blackwell et al (33)
reported on the role of NF-κB activation in the regulation of
cytokine production in allergic inflammation, and showed that NF-κB
activation is also associated with MAPK activation. To address
this, the inhibitory effect of genistein was investigated on
pro-inflammatory cytokines via regulation of MAPK activation.
Genistein inhibited the phosphorylation of ERK. However, p38 MAPK
levels were not altered by pretreatment with genistein in activated
mast cells (Fig. 5). These
results indicate that the inhibitory effect of genistein on
pro-inflammatory cytokine production is associated with ERK
signaling pathways.
In conclusion, genistein inhibits the gene
expression and production of pro-inflammatory cytokines IL-1β and
IL-6, but does not alter TNF-α levels. Additionally, genistein
attenuates activation of the ERK signaling pathway. The present
study also indicates that genistein has potential for use as a
treatment for allergic inflammation and anaphylactic shock.
Acknowledgements
The present study was financially supported by the
Ministry of Education, Science Technology (MEST) and National
Research Foundation of Korea (NRF) through the Human Resource
Training Project for Regional Innovation (2011-04-Dae-05-016) and
the Leading Foreign Research Institute Recruitment Program through
the National Research Foundation of Korea (NRF) funded by the
Ministry of Education, Science and Technology (MEST)
(2011-0030034).
References
|
1
|
Metcalfe DD, Peavy RD and Gilfillan AM:
Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin
Immunol. 124:639–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oskeritzian CA, Price MM, Hait NC, et al:
Essential roles of sphingosine-1-phosphate receptor 2 in human mast
cell activation, anaphylaxis, and pulmonary edema. J Exp Med.
207:465–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Worm M: Epidemiology of anaphylaxis. Chem
Immunol Allergy. 95:12–21. 2010. View Article : Google Scholar
|
|
4
|
Boden SR and Wesley Burks A: Anaphylaxis:
a history with emphasis on food allergy. Immunol Rev. 242:247–257.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JK and Vadas P: Anaphylaxis:
mechanisms and management. Clin Exp Allergy. 41:923–938. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simons FE: World Allergy Organization
survey on global availability of essentials for the assessment and
management of anaphylaxis by allergy-immunology specialists in
health care settings. Ann Allergy Asthma Immunol. 104:405–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simons FE: Anaphylaxis: recent advances in
assessment and treatment. J Allergy Clin Immunol. 124:625–636.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Alysandratos KD, Angelidou A, et
al: Human mast cell degranulation and preformed TNF secretion
require mitochondrial translocation to exocytosis sites: relevance
to atopic dermatitis. J Allergy Clin Immunol. 127:1522–1531. 2011.
View Article : Google Scholar
|
|
9
|
Tchougounova E, Pejler G and Abrink M: The
chymase, mouse mast cell protease 4, constitutes the major
chymotrypsin-like activity in peritoneum and ear tissue. A role for
mouse mast cell protease 4 in thrombin regulation and fibronectin
turnover. J Exp Med. 198:423–431. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee S, Park HH, Son HY, et al: DA-9601
inhibits activation of the human mast cell line HMC-1 through
inhibition of NF-κB. Cell Biol Toxicol. 23:105–112. 2007.PubMed/NCBI
|
|
11
|
Shin J, Pan H and Zhong XP: Regulation of
mast cell survival and function by tuberous sclerosis complex 1.
Blood. 119:3306–3314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalesnikoff J and Galli SJ: New
developments in mast cell biology. Nat Immunol. 9:1215–1223. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guma M, Kashiwakura J, Crain B, et al:
JNK1 controls mast cell degranulation and IL-1β production in
inflammatory arthritis. Proc Natl Acad Sci USA. 107:22122–22127.
2010.PubMed/NCBI
|
|
14
|
Lu Y, Piao D, Zhang H, et al: Saucerneol F
inhibits tumor necrosis factor-α and IL-6 production by suppressing
Fyn-mediated pathways in FcɛRI-mediated mast cells. Food Chem
Toxicol. 59:696–702. 2013.PubMed/NCBI
|
|
15
|
Lee J and Lim KT: Expression of TNF-α and
IL-6 in HMC-1 cells treated with bisphenol A is attenuated by
plant-originating glycoprotein (75 kDa) by blocking p38 MAPK.
Naunyn Schmiedebergs Arch Pharmacol. 382:51–61. 2010.
|
|
16
|
Masilamani M, Wei J, Bhatt S, Paul M,
Yakir S and Sampson HA: Soybean isoflavones regulate dendritic cell
function and suppress allergic sensitization to peanut. J Allergy
Clin Immunol. 128:1242–1250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dixon RA and Ferreira D: Genistein.
Phytochemistry. 60:205–211. 2002. View Article : Google Scholar
|
|
18
|
Kitaura J, Asai K, Maeda-Yamamoto M,
Kawakami Y, Kikkawa U and Kawakami T: Akt-dependent cytokine
production in mast cells. J Exp Med. 192:729–740. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frey RS and Singletary KW: Genistein
activates p38 mitogen-activated protein kinase, inactivates
ERK1/ERK2 and decreases Cdc25C expression in immortalized human
mammary epithelial cells. J Nutr. 133:226–231. 2003.
|
|
20
|
Zhao JH, Arao Y, Sun SJ, Kikuchi A and
Kayama F: Oral administration of soy-derived genistin suppresses
lipopolysaccharide-induced acute liver inflammation but does not
induce thymic atrophy in the rat. Life Sci. 78:812–819. 2006.
View Article : Google Scholar
|
|
21
|
Palanisamy N, Kannappan S and Anuradha CV:
Genistein modulates NF-κB-associated renal inflammation, fibrosis
and podocyte abnormalities in fructose-fed rats. Eur J Pharmacol.
667:355–364. 2011.
|
|
22
|
Woolley DE and Tetlow LC: Mast cell
activation and its relation to proinflammatory cytokine production
in the rheumatoid lesion. Arthritis Res. 2:65–74. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conti P, Kempuraj D, Di Gioacchino M, et
al: Interleukin-6 and mast cells. Allergy Asthma Proc. 23:331–335.
2002.
|
|
24
|
de Bittencourt Pasquali MA, Gelain DP,
Zeidan-Chulia F, et al: Vitamin A (retinol) downregulates the
receptor for advanced glycation endproducts (RAGE) by
oxidant-dependent activation of p38 MAPK and NF-κB in human lung
cancer A549 cells. Cell Signal. 25:939–954. 2013.PubMed/NCBI
|
|
25
|
Pullen NA, Barnstein BO, Falanga YT, et
al: Novel mechanism for FcɛRI-mediated signal transducer and
activator of transcription 5 (STAT5) tyrosine phosphorylation and
the selective influence of STAT5B over mast cell cytokine
production. J Biol Chem. 287:2045–2054. 2012.
|
|
26
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Resendez E Jr, Ting J, Kim KS, Wooden SK
and Lee AS: Calcium ionophore A23187 as a regulator of gene
expression in mammalian cells. J Cell Biol. 103:2145–2152. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SJ, Kim YJ, Lee JH, et al:
Genistein-4′-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside
from Sophora japonica (Leguminosae) ameliorates mast
cell-mediated allergic inflammation in vivo and in vitro. Orient
Pharm Exp Med. 11:207–213. 2011.
|
|
29
|
Gao F, Wei D, Bian T, et al: Genistein
attenuated allergic airway inflammation by modulating the
transcription factors T-bet, GATA-3 and STAT-6 in a murine model of
asthma. Pharmacology. 89:229–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu SC and Li XY: Effect of ginsenoside on
IL-1β and IL-6 mRNA expression in hippocampal neurons in chronic
inflammation model of aged rats. Acta Pharmacol Sin. 21:915–918.
2000.
|
|
31
|
Ganeshan K, Johnston LK and Bryce PJ:
TGF-β1 limits the onset of innate lung inflammation by promoting
mast cell-derived IL-6. J Immunol. 190:5731–5738. 2013.
|
|
32
|
Ganeshan K and Bryce PJ: Regulatory T
cells enhance mast cell production of IL-6 via surface-bound TGF-β.
J Immunol. 188:594–603. 2012.PubMed/NCBI
|
|
33
|
Blackwell TS, Blackwell TR and Christman
JW: Impaired activation of nuclear factor-kappaB in
endotoxin-tolerant rats is associated with down-regulation of
chemokine gene expression and inhibition of neutrophilic lung
inflammation. J Immunol. 158:5934–5940. 1997.PubMed/NCBI
|