Introduction
Exposure to high environmental temperatures or
whilst carrying out strenuous work frequently leads to heat stroke
(HS), which is a life-threatening illness that is clinically
characterized by a high core temperature (>40°C) and dysfunction
of the central nervous system, including delirium, seizures and
coma (1). HS is considered one of
the most deadly natural hazards with a high morbidity and mortality
rate during heat waves (2,3).
Aggressive clinical therapies, including rapid cooling, fluid
resuscitation to stabilize organ function and supportive
treatments, have been applied in the treatment of HS; however,
there is still a lack of proper diagnosis and treatment approaches.
Currently, there is an urgent need to develop effective clinical
therapeutics.
To date, knowledge of the pathology of HS is still
poorly understood. The pathology of HS has been suggested to be
associated with multiple organ dysfunction syndrome and systemic
inflammatory response syndrome (4). Heat stress causes gut epithelial
barrier damage, resulting in bacteria and/or endotoxin leakage into
the circulation, which initiates excessive inflammation and
subsequent cytokine secretion (5,6).
Cytokines, key regulators of inflammation, have been suggested to
account for systemic inflammatory response syndrome in HS (4). Clinical investigations have revealed
that circulating levels of interleukin (IL)-1, tumor necrosis
factor (TNF) α, interferon (IFN) γ, IL-6, IL-10 and corresponding
receptors are elevated in patients with HS (7–10).
In an animal model of HS, plasma concentrations of IL-1, IL-6 and
IL-10 were also found to be upregulated with the increasing core
temperature (4). Multiple organ
dysfunction has been suggested to occur due to heat cytotoxicity,
coagulopathies and systemic inflammation (11). Hyperthermia has been suggested to
be associated with arterial hypotension (12). Certain studies have demonstrated
that the inhibition of the IL-1 receptor by receptor antagonists
can attenuate arterial hypotension in HS (13,14).
The renin-angiotensin system plays an important role
in regulating blood pressure (15). Heat stress can induce the
activation of the renin-angiotensin system to synthesize
angiotensin II (Ang II), a type of vasoconstrictor, which engages
Ang II type 1 (AT1) receptors to promote blood pressure (16). In some conditions such as sepsis,
although Ang II concentrations are elevated, blood pressure still
decreases due to the desensitized receptor (16,17). Receptor insensitivity may be
caused by the inhibition of receptor transcription, translation
and/or membrane trafficking (18,19). Previous studies have identified
AT1 receptor-associated protein 1 (Arap1) as the most relevant
mediator of AT1 receptor sensitivity, which binds to AT1 receptors
and regulates AT1 receptor internalization, membrane re-trafficking
and receptor sensitivity (20,21). It has also been demonstrated that
the suppression of Arap1 contributes to the development of
hypotension during sepsis (22).
In our previous study, we demonstrated that the
injection of Xuebijing (XBJ) alleviated HS-induced arterial
hypotension (23). However, the
underlying mechanisms have not been yet been fully elucidated. XBJ
injection is a traditional Chinese compound preparation combining
safflower yellow A, tetramethylpyrazine, Danshensu and ferrulic
acid, which has been shown to exert protective effects against
sepsis (24). In the current
study, we aimed to explore the underlying mechanisms of XBJ in
alleviating HS-induced arterial hypotension. Using a rat model of
HS, we found that the surface expression of AT1 receptor was
markedly decreased and that Arap1 expression was also suppressed
during HS; these effects were attenuated by pre-treatment with XBJ.
In addition, we used a cell model [rat macrophages stimulated with
lipopolysaccharide (LPS)] to mimic endotoxin leakage into the
circulation to initiate inflammation. Pre-treatment with XBJ
inhibited the release of pro-inflammatory pro-inflammatory
cytokines, including IL-1β and TNFα, in the LPS-stimulated
macrophages. Furthermore, our results revealed that XBJ was
inhibited the activation of the nuclear factor κB (NF-κB) signaling
pathway, which is responsible for promoting pro-inflammatory
cytokine expression induced by LPS. Considering the inhibitory
effect of pro-inflammatory cytokines on Arap1 expression (22), and taking our data into account,
it can be concluded that XBJ upregulates Arap1 expression by
inhibiting the activation of the NF-κB signaling pathway, which
alleviates blood pressure reduction in rats suffering from HS.
Materials and methods
Experimental animals and ethics
statement
Due to the effects of estrogen on HS (25), 72 male adult (25-week-old)
pathogen-free Wistar rats (weighing 250-220 g) purchased from the
Experimental Animal Center of the Guangzhou General Hospital of
Guangzhou Military Command (Animal Quality Certification No.
SCXK2006-0015, Guangzhou, China) were used in this study. The rats
were housed in an environment of 25±0.5°C with 35±5% humidity in
individual wire hanging cages with a normal light-dark cycle (14:10
h; 06:00-20:00 light cycle and 20:00-06:00 dark cycle) and were
allowed free access to food and water. Prior to each experiment,
the rats were allowed to adapt to the new living environment for at
least 2 weeks. This study was carried out in strict accordance with
the guidelines of the National Health and Medical Research Council
for the Care and Use of Animals for Experimental Purposes in China.
The protocol was approved by the Institutional Animal Care and Use
Committee of Guangzhou General Hospital of Guangzhou Military
Command.
Cell culture
A rat macrophage cell line (RMa-bm) obtained from
ScienCell (Carlsbad, CA, USA) was cultured in macrophage medium
(MaM) (Cat. No. 1921) according to the manufacturer’s instructions
(ScienCell) and maintained at 37°C in 5% carbon dioxide
(CO2)-enriched air in a cell incubator (HF90; Healforce,
Shanghai, China).
Study design and animal model
preparation
After the rats were allowed to acclimatize for 2
weeks at an ambient temperature (25±0.5°C) and humidity (35±5%),
the 72 rats were randomly divided into 3 groups. Prior to the
induction of HS, 2 groups of rats (n=24/group) were intravenously
injected with phosphate-buffered saline (PBS) (4 ml/kg body weight)
twice daily for 3 days through the tail vein, and another group
(n=24) was intravenously injected with XBJ (4 ml/kg body weight;
Chinese medicine accurate character Z20040033; Chase Sun
Pharmaceutical Co., Ltd., Tianjin, China) twice daily for 3 days,
as previously described (23).
For the HS modelestab, the rats were generally anesthetized with
pentobarbital sodium (50 mg/kg; Merck KGaA, Darmstadt, Germany) by
intraperitoneal injection to abolish corneal and pain reflexes and
to minimize their suffering. A trocar (24 G) was cannulated in the
right femoral artery of each rat to monitor mean arterial pressure
(MAP). The rats in the group pre-treated with PBS (n=24) and the
group pre-treated with XBJ (n=24) were placed in an incubator with
a stable temperature (40.0±0.5°C) and relative humidity (60±5%);
these groups were termed the HS group and XBJ group, respectively.
The rats in the group pre-treated with PBS (n=24) were exposed to a
temperature of 25±0.5°C with a humidity of 35±5%; this was used as
the sham-operated group. A multi-parameter Physiological Monitor
(Infinity® Delta XL; Dräger, Lübeck, Germany) was used
to monitor rectal temperature (Tc) at intervals of 10 min. When the
MAP decreased by 25 mmHg and the core temperature was >42°C, the
rats were withdrawn from the chamber, as previously described
(26).
Detection of Ang II expression in
myocardial tissue and serum
During the preparation of the model of HS, 8 rats
from each group were randomly selected at 30, 60 and 90 min for the
measurement of Ang II expression. Blood samples were collected
through the orbital vein and serum was separated by centrifugation
and stored at −80°C. Following blood collection, the chests of the
rats were opened immediately and the hearts were excised. Left
ventricular myocardial tissue was isolated and weighed. A total of
100 mg myocardial tissues was collected and boiled in 1 ml of
acetic acid (0.5 mM) for 10 min. The tissues were cut into sections
and homogenized under ice-cold conditions followed by
centrifugation (12,000 rpm) at 4°C for 20 min. The supernatants
were then collected for analysis. Ang II levels in the myocardial
tissue or serum were determined using the rat Ang II ELISA kit
(Biorbyt Ltd., Cambridge, UK) according to standard protocols.
Cell stimulation
The rat macrophages were pre-treated with XBJ at
concentrations of 10, 25, or 50 mg/ml for 24 h. Subsequently, LPS
(10 mg/ml; Sigma, St. Louis, MO, USA) was added followed by
incubation for 24 h. Total cell RNA or protein was harvested
according to standard protocols for further analysis.
Quantitative reverse transcription PCR
(RTqPCR)
Total RNA was extracted from the right common
carotid arteries of the rats or from the cells using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and 5 μg of total RNA were
reverse-transcribed into cDNA using M-MLV reverse transcriptase
(Clontech, Palo Alto, CA, USA). cDNA was used as the template for
RT-qPCR. For rat Arap1, the sense primer was
5′-ccagaaagcgagtactataagctgc-3′ and the antisense primer was
5′-cttaatggaaagtgttggggttgg-3′. Rat GAPDH primers (sense,
5′-acagcaacagggtggtggac-3′ and antisense,
5′-tttgagggtgcagcgaactt-3′) were used as the controls. The RT-qPCR
mixture system contained 5 μl SsoFast™ EvaGreen Supermix (Bio-Rad,
Hercules, CA, USA), 1 μl of cDNA (diluted in 1:50) and 2 μl of each
of the forward and reverse primers (1 μM) to a final volume of 10
μl. The PCR procedure was as follows: 94°C for 4 min; 94°C for 20
sec, 55°C for 30 sec and 72°C for 20 sec; 2 sec for plate reading
for 35 cycles; and melting curve from 65 to 95°C. GAPDH was used as
the control for normalizing gene expression. Three independent
experiments were performed. The data obtained were calculated using
the 2−ΔΔCt method and statistical analysis was carried
out as previously described (27), followed by an unpaired sample
t-test.
Membrane protein and nuclear protein
isolation
Membrane protein was isolated using the Membrane
Protein Extraction kit (Sangon, Shanghai, China) as per the
manufacturer’s instructions. Briefly, the cells or tissues (200 mg,
cut into small sections) were washed with wash buffer at least 3
times. Subsequently, 1 ml of extract buffer containing DTT (1
μg/ml) was added and homogenized under ice-cold conditions followed
by centrifugation (14,000 rpm) at 4°C for 10 min. The supernatants
were collected, bathed at 37°C for 10 min, and then centrifuged
(13,000 rpm) at room temperature for 5 min. The samples were
divided into 2 layers: the upper layer containing cytoplasmic
proteins was collected and the bottom layer containing membrane
proteins was dissolved in 500 μl of ice-cold sterile water for 5
min at 4°C followed by incubation in a water bath at 37°C for 5
min. Following centrifugation (13,000 rpm) at room temperature for
5 min, the bottom layer was collected and dissolved in ice-cold
sterile water again following the above steps. Finally, the
membrane extracts were collected and the protein concentration was
measured using the BCA kit (Pierce Biotechnology, Inc., Rockford,
IL, USA). For SDS-PAGE, a total of 100 μl membrane protein was
mixed with 0.9 ml acetone, and incubated under ice-cold conditions
for 20 min followed by centrifugation (10,000 rpm) for 20 min. The
supernatants were removed, and 100 μl of loading buffer and 2 μl of
β-mercaptoethanol were added to dissolve the sediments for
SDS-PAGE. Nuclear proteins were extracted using an extraction kit
(Sangon) according to the manufacturer’s instructions. Briefly, the
cells were lysed in cytoplasmic buffer containing protease
inhibitors, mixed and incubated for 15 min at 4°C, followed by
centrifugation at 12,000 rpm for 20 min at 4°C. Cell sediments were
collected and were resuspended in nuclear buffer for 10 min at 4°C.
The sample was then centrifuged at 12,000 rpm for 10 min at 4°C.
The supernatant containing nuclear proteins was collected for
analysis.
Western blot analysis
Proteins separated by 12% SDS-PAGE were
electrotransferred onto a nitrocellulose membrane (Amersham, Little
Chalfont, UK). The membrane was then incubated with 2% non-fat dry
milk in Tris-buffered saline (TBS) to block non-specific binding at
room temperature for 1 h. Subsequently, the membrane was incubated
with the following primary antibodies: rabbit anti-rat AT1 receptor
polyclonal IgG (1:1,000) antibody, goat anti-rat Arap1 polyclonal
IgG (1:1,000; both from Abcam, Cambridge, UK), goat anti-rat
Histone polyclonal IgG (1:1,000; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) rabbit anti-rat NF-κB p65 polyclonal IgG
(1:1,000; Santa Cruz Biotechnology, Inc.), goat anti-rat IκBα and
p-IκBα monoclonal IgG (1:2,000; Cell Signaling Technology, Inc.,
Boston, MA, USA), rabbit anti-rat GAPDH polyclonal IgG and
caveolin-1 (1:1,000; Abcam) antibody diluted in blocking buffer
overnight at 4°C. Subsequently, the membrane was incubated in
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG or
rabbit anti-goat IgG (Boster Biological Technology Co., Ltd, Wuhan,
China) diluted in blocking buffer according to the corresponding
primary antibodies for 1 h. Finally, 4-chloro-1-naphthol (4-CN) as
an HRP substrate was used for protein visualization. The protein
gray intensity was detected by using BandScan 0.5 software
(ProZyme, San Leandro, CA, USA).
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). The statistical significance of differences between 2 groups
was determined by the Student’s t-test, and differences among
multiple groups were determined by one-way ANOVA. A value of
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
version 11.5 software (SPSS Inc., Chicago, IL, USA).
Results
XBJ has no effect on Ang II levels
following during HS
A previous study (12) revealed that arterial hypotension
occurs during HS. In our previous data (23), we demonstrated that XBJ alleviated
the decrease in MAP during HS in a rat model. Ang II, a type of
vasoconstrictor, increases blood pressure (16). In this study, in order to
investigate the levels of Ang II in rats during HS and the effects
of XBJ on Ang II, we evaluated Ang II levels in serum or myocardial
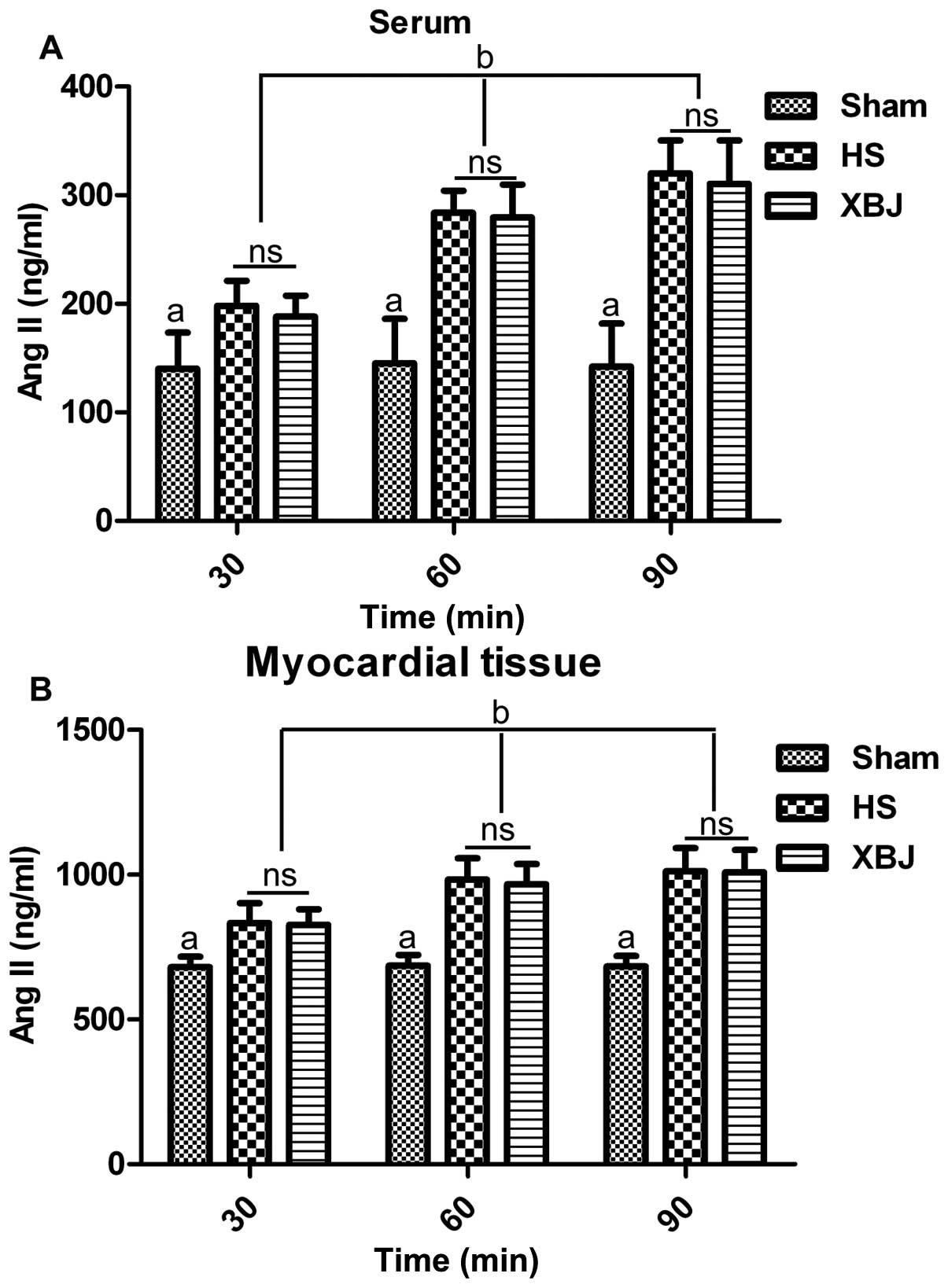
tissue. The results revealed that the serum levels of Ang II in the
HS or XBJ groups were markedly increased compared with those in the
sham-operated group at 30, 60 and 90 min (p<0.05) (Fig. 1A). However, there was no
significant difference in the serum levels of Ang II between the HS
and XBJ groups. Furthermore, the Ang II serum levels increased from
30 to 90 min in the HS and XBJ groups in a time-dependent manner
(p<0.05), while the Ang II levels remained stable in the
sham-operated group (Fig. 1A).
Similar results were obtained in the myocardial tissue (Fig. 1B). These data indicated that the
Ang II levels were upregulated during HS, and that pre-treatment
with XBJ had no obvious effect on the Ang II levels in the rats
suffering from HS. Therefore, it is intriguing that the arterial
pressure still decreased as shown in our previous study (23) during HS, while the Ang II levels
increased. However, our previous data (23) demonstrated that pre-treatment with
XBJ still attenuated hypotension. Collectively, our data indicated
that XBJ alleviated the decrease in blood pressure and that this
decrease was independent of the Ang II levels in the rats suffering
from HS.
XBJ accelerates AT1 receptor trafficking
to the membrane
The Ang II levels were increased during HS; however,
blood pressure was still downregulated. We hypothesized that Ang II
signal transduction is inhibited during HS. In order to examine our
hypothesis, we analyzed the Ang II corresponding receptor, AT1
receptor, which has been suggested to be desensitized by elevated
levels of Ang II (16,17,28). Hence, we detected the membrane and
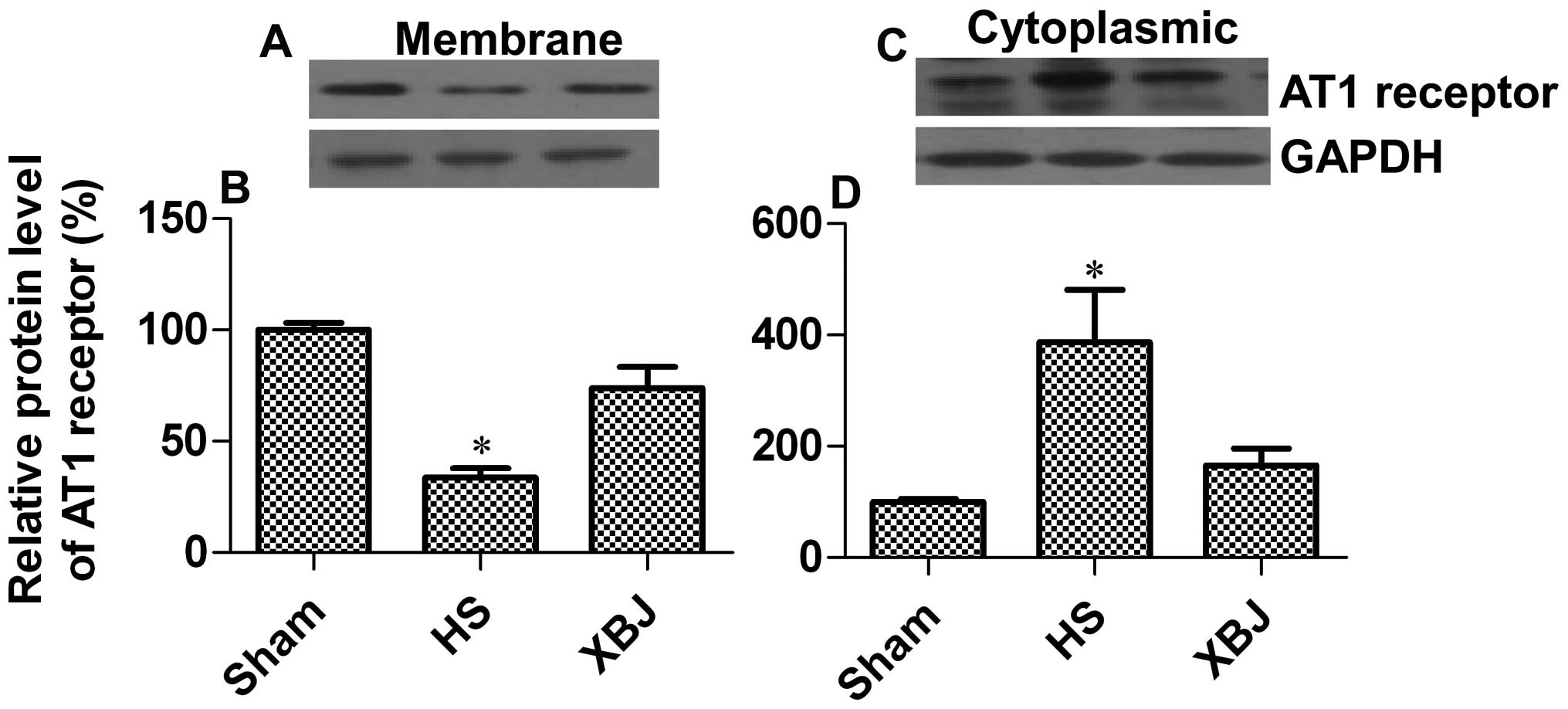
cytoplasmic levels of the AT1 receptor in arterial tissue. The
results revealed that AT1 receptor membrane expression was
decreased by 66.4% in the HS group in comparison with the
sham-operated group, while pre-treatment with XBJ increased AT1
receptor membrane expression by 40.3% when compared with the HS
group (p=0.0437) (Fig. 2A and B).
There was a significant accumulation of cytoplasmic AT1 receptor
(3.9-fold increase) in the HS group as compared with the
sham-operated group, while pre-treatment with XBJ markedly
attentuated this effect (57.4% decrease; p=0.0202) (Fig. 2C and D). These data suggest that
pre-treatment with XBJ promotes AT1 receptor surface
expression.
XBJ increases AT1 receptor surface
expression through the upregulation of Arap1
Arap1 has been suggested as the most relevant
mediator of AT1 receptor trafficking to the cell membrane (20,21); therefore, we wished to investigate
whether Arap1 expression is altered during HS and the effects of
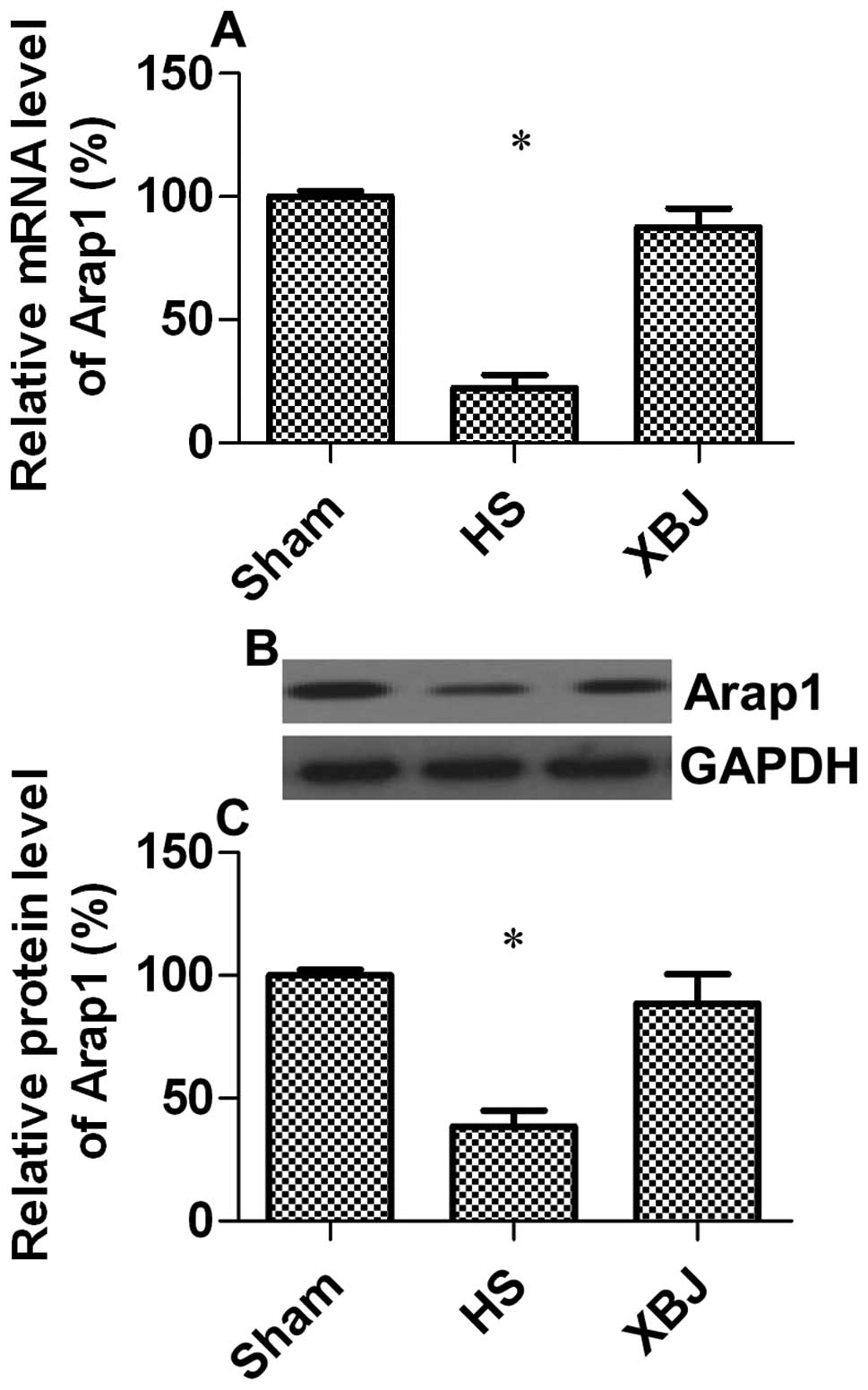
XBJ on Arap1 expression. The results from RT-qPCR indicated that
the Arap1 transcription levels in the HS group were significantly
decreased by 77.6% compared with the sham-operated group (Fig. 3A). These results were confirmed by
western blot analysis (decrease of 61.4%; p=0.0132) (Fig. 3B and C). This may explain why
hypotension still occurs while Ang II levels increase during HS. We
also hypothesized that XBJ positively regulates Arap1 expression.
As expected, pre-treatment with XBJ attenuated the effects of HS on
Arap1 expression, indicating that XBJ accelerated Ang II signal
transduction through the upregulation of Arap1.
XBJ inhibits the secretion of
pro-inflammatory cytokines in macrophages in vitro
The main hazard of heat stress is dysregulated
intestinal permeability leading to the translocation of bacteria
and endotoxins into the circulation, which then results in a
dramatic inflammatory response and cytokine secretion (5,6).
Pro-inflammatory cytokines have been shown to have an inhibitory
effect on Arap1 expression (22).
Our previous study demonstrated that pre-treatment with XBJ has an
inhibitory effect on pro-inflammatory cytokine secretion in a rat
model of HS (23). In this study,
we wished to investigate whether XBJ inhibits cytokine expression
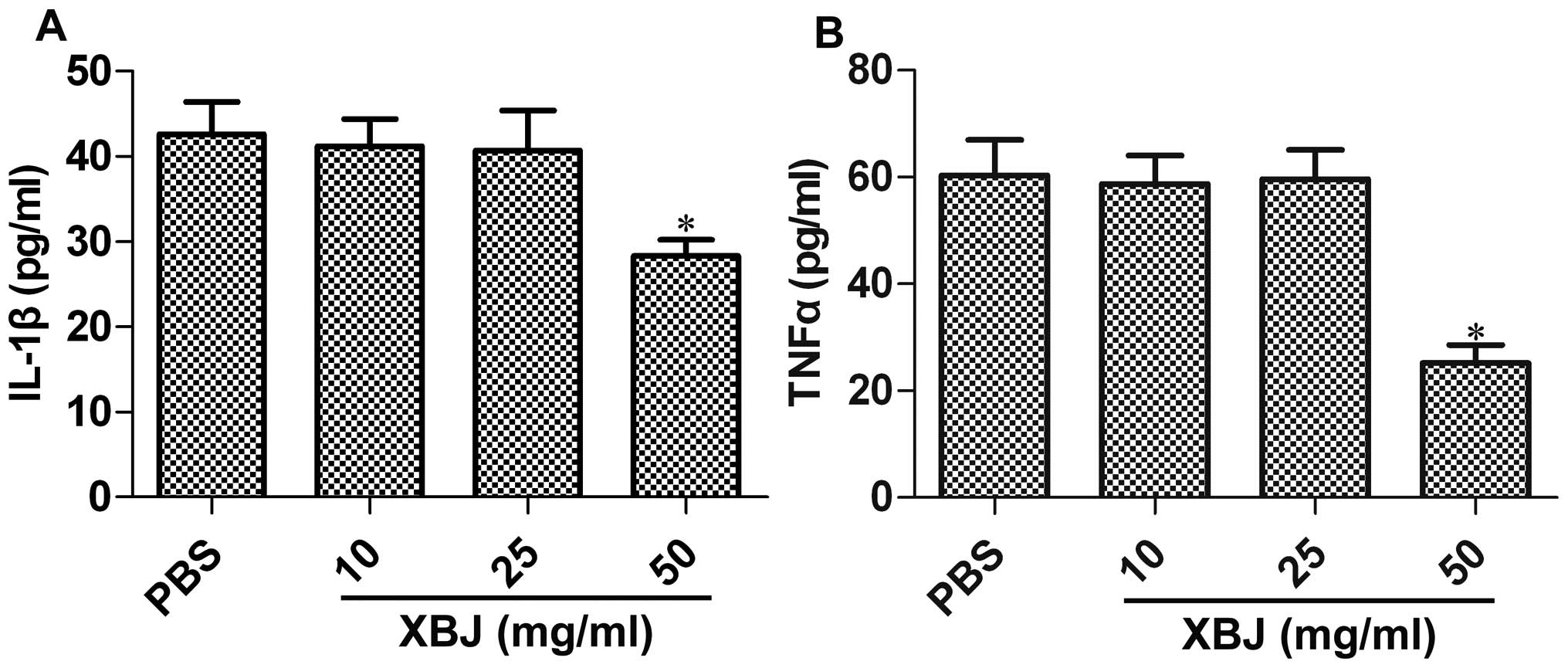
in an in vitro cell model. Rat macrophages were pre-treated
with XBJ at concentrations of 10, 25 and 50 mg/ml, and then
stimulated with LPS; the levels of IL-1β or TNFα were then
determined. The results revealed that the XBJ concentration of 50
mg/ml resulted in a significant decrease in the IL-1β levels by
33.6% (p=0.0308; Fig. 4A) and in
the TNFα levels by 58.2% (p=0.0237; Fig. 4B). However, XBJ at concentrations
of 10 and 25 mg/ml had no effect on the release of pro-inflammatory
cytokines (p>0.05; Fig.
4).
XBJ inhibits the activation of the NF-κB
signaling pathway in macrophages in vitro
It has been shown that bacterial LPS regulates the
expression of pro-inflammatory cytokines through the NF-κB
signaling pathway (29). Thus, we
hypothesized that XBJ may modify pro-inflammatory cytokine
secretion through the NF-κB signaling pathway. As the concentration
of 50 mg/ml of XBJ effectively suppressed the secretion of IL-1β or
TNFα, we used this sample to analyze the activation status of the
NF-κB signaling pathway. As is already known, NF-κB dimers are
inhibited by IκB (α, β or ɛ) in the cytoplasm, while the
phosphorylation of IκB results in NF-κB nuclear translocation and
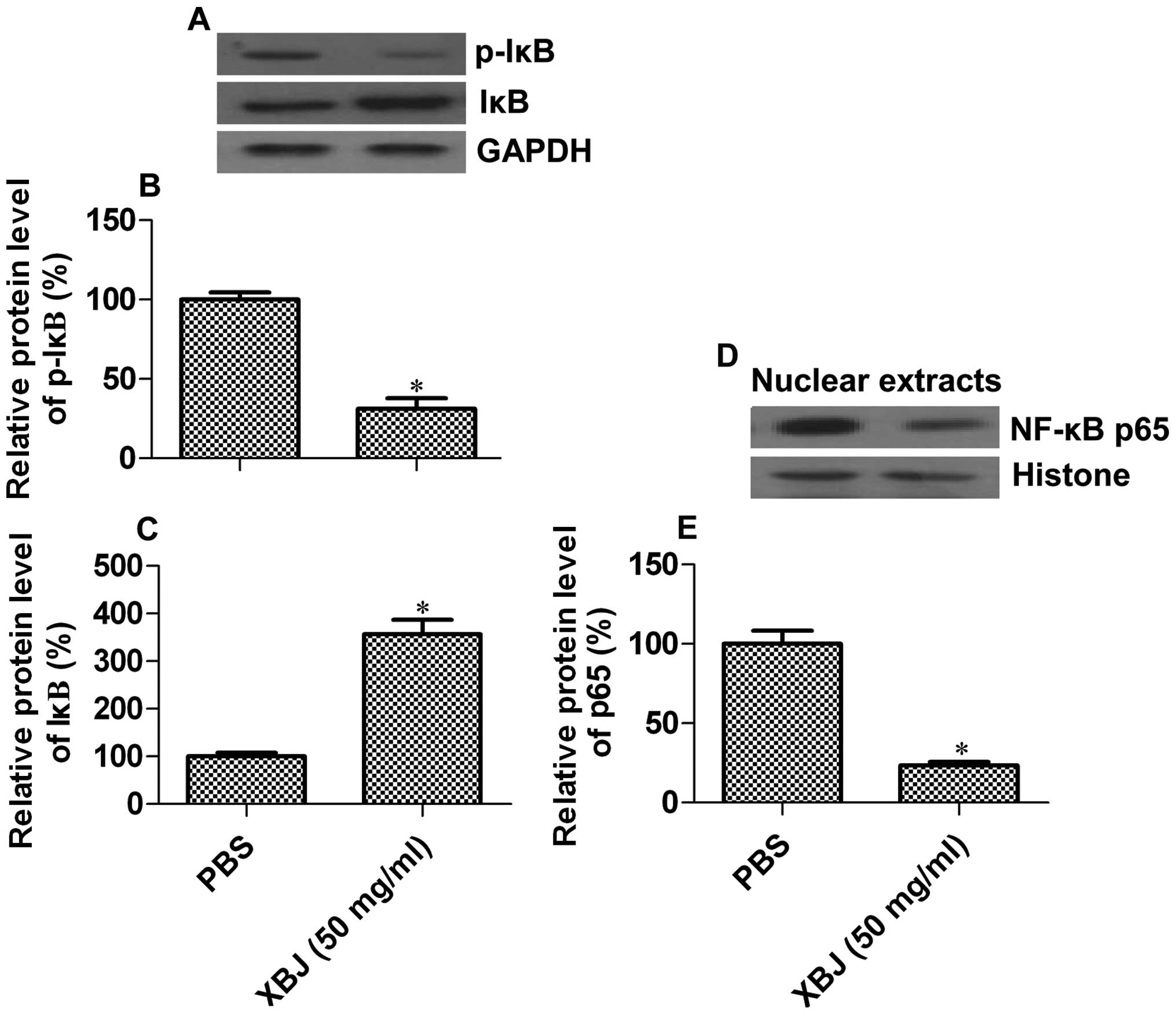
activation (30). We detected the
phosphorylation status of IκB, and found that pre-treatment with
XBJ decreased the phosphorylation levels of IκBα by 69.8%
(p=0.0297) [Fig. 5A (top panel)
and B]. By contrast, the levels of unphosphorylated IκBα were
increased by 2.56-fold (p=0.0142) [Fig. 5A (middle panel) and C].
Additionally, the protein levels of p65, a subunit of NF-κB, were
decreased by 76.6% in the nuclear extracts of the XBJ-pre-treated
cells upon LPS stimulation (Fig. 5D
and E). These results suggest that XBJ inhibits NF-κB signaling
pathway transduction induced by LPS.
Discussion
In the present study, we demonstrated that XBJ
inhibits the activation of the NF-κB signaling pathway induced by
LPS, leading to a decrease in the release of pro-inflammatory
cytokines. Considering the inhibitory effect of pro-inflammatory
cytokines on Arap1 expression (22), our data may partly explain why
pre-treatment with XBJ promotes Arap1 expression in rats during HS
and thus attenuates hypotension.
It has been established that Ang II levels are
increased upon heat stress (31);
Ang II is associated with cardiovascular diseases, such as
hypertension (32). In the
current study, we found that Ang II levels in the serum and
myocardial tissue were increased during HS. Considering the
vasoconstrictor effect of Ang II, blood pressure should be
elevated. However, arterial pressure was markedly decreased during
HS. Although pre-treatment with XBJ had no obvious effect on Ang II
levels, XBJ alleviated the decrease in blood pressure. These data
indicated that HS reduced Ang II signal transduction following the
elevation of blood pressure. Ang II, the major determinant of
arterial pressure and volume homeostasis in mammals, mainly exerts
its function through action on AT1 receptor (33,34). We therefore examined the AT1
receptor, and the results revealed that AT1 surface expression was
markedly inhibited during HS, which may explain why Ang II signal
transduction was blocked. Furthermore, XBJ greatly improved AT1
receptor membrane trafficking.
Following the binding of ligands, the AT1 receptor
is internalized through caveolae and clathrin-coated vesicles,
which are then trafficked to endosomes (35–38). An increasing number of studies has
revealed that the carboxyl terminal region of the AT1 receptor is
tightly associated with receptor phosphorylation (39,40), internalization (41,42) and desensitization (43). Subsequently, a novel protein
termed Arap1 was found to specifically bind the carboxyl terminal
region of the AT1 receptor using the yeast two-hybrid system, and
was shown to play an essential role in receptor trafficking and
recycling (20). Given that Arap1
accelerates receptor trafficking and recycling following
ligand-induced receptor internalization, Arap1 may be significantly
involved in the regulation of Ang II-mediated processes. In this
study, we found that Arap1 expression was significantly inhibited
druing HS; however, pre-treatment with XBJ attenuated this effect.
These data suggest that pre-treatment with XBJ promotes Arap1
expression, which increases AT1 surface expression and
re-sensitizes the cells (following exposure to Ang II), thus
leading to enhanced Ang II signal transduction.
In rats with sepsis induced by LPS injection, plasma
Ang II concentrations have been shown to be markedly increased
(44); however, Arap1 expression
was significantly inhibited, which was suggested to account for the
hyporesponsiveness to Ang II (22). The inhibition of the IL-1 receptor
has been shown to attenuate arterial hypotension in rats suffering
from HS (14). Our previous study
established that pre-treatment with XBJ markedly decreased the
secretion of pro-inflammatory cytokines, including IL-1β, TNFα and
IL-6 in rats suffering from HS (23). In this study, we also observed
similar inhibitory effects of XBJ on cytokine expression in
LPS-stimulated macrophages in vitro. Recently, a study
demonstrated that Arap1 expression was markedly downregulated in
cultured cells in the presence of pro-inflammatory cytokines
(22). As a result, we
hypothesized that XBJ may promote Arap1 expression by inhibiting
the secretion of pro-inflammatory cytokines. Heat stress can
increase intestinal permeability, leading to the translocation of
bacteria and/or endotoxins into the circulation, which initiates
inflammation and subsequent pro-inflammatory cytokine release
(5,6). LPS binds to its pattern recognition
receptor, Toll like receptor 4 (TLR4), which then activates the
NF-κB signaling pathway through a series of cascade events, leading
to pro-inflammatory cytokine expression (29). NF-κB is considered to be an
important mediator of inducible transcription in the immune system
(45). Excessive stimulation of
the immune system with LPS results in hypotension and septic shock
(46). In this study, using an
in vitro cell model, we observed that XBJ inhibited NF-κB
activation in LPS-stimulated macrophages. Our results were
consistent with those of a recent study which demonstrated that the
components of XBJ, including senkyunolide I, paeoniflorin,
Danshensu, safflower yellow A, oxypaeoniflorin and
benzoylpaeoniflorin, had an inhibitory effect against NF-κB and
cytokine expression (24).
Therefore, the anti-inflammatory effects of XBJ mainly depend on
the inhibitory effects on NF-κB.
Taken together, we provide evidence that XBJ
attenuates hypotension during HS through the upregulation of Arap1.
The mechanisms underlying these effects may be due to the ability
of XBJ to inhibit cytokine expression through the NF-κB signaling
pathway. However, XBJ is a complex mixture of which complex drug
composition causes adverse drug reactions (47). Hence, the effective components
should be purified and screened to further study their synergistic
mechanisms by network pharmacology.
Abbreviations:
|
HS
|
heat stroke
|
|
XBJ
|
Xuebijing
|
|
Ang II
|
angiotensin II
|
|
AT1
|
angiotensin II type 1
|
|
Arap1
|
angiotensin II type 1
receptor-associated protein 1
|
|
NF-κB
|
nuclear factor κB
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
IFN
|
interferon
|
|
LPS
|
lipopolysaccharide
|
References
|
1
|
Knochel JP: Disorders due to heat and
cold. Textbook of Medicine. Wyngaarden JB and Smith LH: Saunders;
Philadelphia: pp. 2304–2306. 1985
|
|
2
|
Semenza JC, Rubin CH, Falter KH, et al:
Heat-related deaths during the July 1995 heat wave in Chicago. N
Engl J Med. 335:84–90. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robine JM, Cheung SL, Le Roy S, et al:
Death toll exceeded 70,000 in Europe during the summer of 2003. C R
Biol. 331:171–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leon LR, Blaha MD and DuBose DA: Time
course of cytokine, corticosterone, and tissue injury responses in
mice during heat strain recovery. J Appl Physiol (1985).
100:1400–1409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu KC, Wang JY, Lin SH, Chu P and Lin YF:
Role of circulating cytokines and chemokines in exertional
heatstroke. Crit Care Med. 32:399–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bouchama A, Ollivier V, Roberts G, et al:
Experimental heatstroke in baboon: analysis of the systemic
inflammatory response. Shock. 24:332–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bouchama A, Hammami MM, Al Shail E and De
Vol E: Differential effects of in vitro and in vivo hyperthermia on
the production of interleukin-10. Intensive Care Med. 26:1646–1651.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bouchama A, al-Sedairy S, Siddiqui S,
Shail E and Rezeig M: Elevated pyrogenic cytokines in heatstroke.
Chest. 104:1498–1502. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hammami MM, Bouchama A, Al-Sedairy S, et
al: Concentrations of soluble tumor necrosis factor and
interleukin-6 receptors in heatstroke and heatstress. Crit Care
Med. 25:1314–1319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashim IA, Al-Zeer A, Al-Shohaib S,
Al-Ahwal M and Shenkin A: Cytokine changes in patients with
heatstroke during pilgrimage to Makkah. Mediators Inflamm.
6:135–139. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bouchama A and Knochel JP: Heat stroke. N
Engl J Med. 346:1978–1988. 2002. View Article : Google Scholar
|
|
12
|
Bouchama A, Roberts G, Al Mohanna F, et
al: Inflammatory, hemostatic, and clinical changes in a baboon
experimental model for heatstroke. J Appl Physiol (1985).
98:697–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin MT, Kao TY, Jin YT and Chen CF:
Interleukin-1 receptor antagonist attenuates the heat
stroke-induced neuronal damage by reducing the cerebral ischemia in
rats. Brain Res Bull. 37:595–598. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin MT, Liu HH and Yang YL: Involvement of
interleukin-1 receptor mechanisms in development of arterial
hypotension in rat heatstroke. Am J Physiol. 273:H2072–H2077.
1997.PubMed/NCBI
|
|
15
|
Cumming AD, Driedger AA, McDonald JW, et
al: Vasoactive hormones in the renal response to systemic sepsis.
Am J Kidney Dis. 11:23–32. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hilgenfeldt U, Kienapfel G, Kellermann W,
Schott R and Schmidt M: Renin-angiotensin system in sepsis. Clin
Exp Hypertens A. 9:1493–1504. 1987. View Article : Google Scholar
|
|
17
|
Benedict CR and Rose JA: Arterial
norepinephrine changes in patients with septic shock. Circ Shock.
38:165–172. 1992.PubMed/NCBI
|
|
18
|
Castrop H: Angiotensin receptor-associated
proteins: local modulators of the renin-angiotensin system.
Pflugers Arch. 465:111–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kai H, Griendling KK, Lassègue B, et al:
Agonist-induced phosphorylation of the vascular type 1 angiotensin
II receptor. Hypertension. 24:523–527. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo DF, Chenier I, Tardif V, Orlov SN and
Inagami T: Type 1 angiotensin II receptor-associated protein ARAP1
binds and recycles the receptor to the plasma membrane. Biochem
Biophys Res Commun. 310:1254–1265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Doblinger E, Höcherl K, Mederle K, et al:
Angiotensin AT1 receptor-associated protein Arap1 in the kidney
vasculature is suppressed by angiotensin II. Am J Physiol Renal
Physiol. 302:F1313–F1324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mederle K, Schweda F, Kattler V, et al:
The angiotensin II AT1 receptor-associated protein Arap1 is
involved in sepsis-induced hypotension. Crit Care. 17:R1302013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Tong H, Zhang X, et al: Xuebijing
injection alleviates liver injury by inhibiting secretory function
of Kupffer cells in heat stroke rats. J Tradit Chin Med.
33:243–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang M, Zhou M, Han Y, et al:
Identification of NF-κB inhibitors in Xuebijing injection for
sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J
Ethnopharmacol. 147:426–433. 2013.
|
|
25
|
Chen SH, Chang FM, Niu KC, Lin MY and Lin
MT: Resuscitation from experimental heatstroke by estrogen therapy.
Crit Care Med. 34:1113–1118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen CM, Hou CC, Cheng KC, et al:
Activated protein C therapy in a rat heat stroke model. Crit Care
Med. 34:1960–1966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta (CT)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohtani R, Ohashi Y, Muranaga K, Itoh N and
Okamoto H: Changes in activity of the renin-angiotensin system of
the rat by induction of acute inflammation. Life Sci. 44:237–241.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodriguez-Fernandez M, Grosman B,
Yuraszeck TM, et al: Modeling the intra- and extracellular cytokine
signaling pathway under heat stroke in the liver. PLoS One.
8:e733932013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gilmore TD: Introduction to NF-kappaB:
players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe T, Miyoshi M and Imoto T:
Angiotensin II: its effects on fever and hypothermia in systemic
inflammation. Front Biosci. 9:438–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paradis P, Dali-Youcef N, Paradis FW,
Thibault G and Nemer M: Overexpression of angiotensin II type I
receptor in cardiomyocytes induces cardiac hypertrophy and
remodeling. Proc Natl Acad Sci USA. 97:931–936. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hall JE: Control of sodium excretion by
angiotensin II: intrarenal mechanisms and blood pressure
regulation. Am J Physiol. 250:R960–R972. 1986.PubMed/NCBI
|
|
34
|
Hall JE and Granger JP: Adenosine alters
glomerular filtration control by angiotensin II. Am J Physiol.
250:F917–F923. 1986.PubMed/NCBI
|
|
35
|
Hunyady L, Catt KJ, Clark AJ and Gáborik
Z: Mechanisms and functions of AT(1) angiotensin receptor
internalization. Regul Pept. 91:29–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gaborik Z, Szaszák M, Szidonya L, et al:
Beta-arrestin- and dynamin-dependent endocytosis of the AT1
angiotensin receptor. Mol Pharmacol. 59:239–247. 2001.PubMed/NCBI
|
|
37
|
Perry SJ and Lefkowitz RJ: Arresting
developments in heptahelical receptor signaling and regulation.
Trends Cell Biol. 12:130–138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ
and Luttrell LM: beta-Arrestin scaffolding of the ERK cascade
enhances cytosolic ERK activity but inhibits ERK-mediated
transcription following angiotensin AT1a receptor stimulation. J
Biol Chem. 277:9429–9436. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smith RD, Baukal AJ, Zolyomi A, et al:
Agonist-induced phosphorylation of the endogenous AT1 angiotensin
receptor in bovine adrenal glomerulosa cells. Mol Endocrinol.
12:634–644. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smith RD, Hunyady L, Olivares-Reyes JA, et
al: Agonist-induced phosphorylation of the angiotensin AT1a
receptor is localized to a serine/threonine-rich region of its
cytoplasmic tail. Mol Pharmacol. 54:935–941. 1998.PubMed/NCBI
|
|
41
|
Conchon S, Peltier N, Corvol P and Clauser
E: A noninternalized nondesensitized truncated AT1A receptor
transduces an amplified ANG II signal. Am J Physiol. 274:E336–E345.
1998.PubMed/NCBI
|
|
42
|
Hunyady L, Bor M, Balla T and Catt KJ:
Identification of a cytoplasmic Ser-Thr-Leu motif that determines
agonist-induced internalization of the AT1 angiotensin receptor. J
Biol Chem. 269:31378–31382. 1994.PubMed/NCBI
|
|
43
|
Tang H, Guo DF, Porter JP, Wanaka Y and
Inagami T: Role of cytoplasmic tail of the type 1A angiotensin II
receptor in agonist- and phorbol ester-induced desensitization.
Circ Res. 82:523–531. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hagiwara S, Iwasaka H, Matumoto S, Hidaka
S and Noguchi T: Effects of an angiotensin-converting enzyme
inhibitor on the inflammatory response in in vivo and in vitro
models. Crit Care Med. 37:626–633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghosh S and Hayden MS: New regulators of
NF-kappaB in inflammation. Nat Rev Immunol. 8:837–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Watters TM, Kenny EF and O’Neill LA:
Structure, function and regulation of the Toll/IL-1 receptor
adaptor proteins. Immunol Cell Biol. 85:411–419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li T: Avoiding adverse drug reactions to
Chinese medicine injections. J Evid Based Med. 3:44–49. 2010.
View Article : Google Scholar
|