Introduction
The aberrant trafficking and cellular accumulation
of cholesterol are ascribed to a number of diseases, including
atherosclerotic cardiovascular disease and cancer (1,2).
Macrophage scavenger receptors (SRs) are membrane receptors
responsible for the internalization of oxidized low-density
lipoprotein (LDL) that promotes the cellular accumulation of
cholesterol (3). Oxidized LDL is
degraded to oxysterols that are active components present in
oxidized LDL as inflammatory factors in the formation of
atherosclerotic plaque (4,5).
Cholesterol efflux from foam cells, the initial step of reverse
cholesterol transport, is the most relevant step with respect to
atherosclerosis (6). Several
ATP-binding cassette (ABC) transporters are known to play a crucial
role in cholesterol homeostasis by mediating cholesterol efflux and
translocation (7). ABCA1 and
ABCG1, membrane transporters abundant in macrophages, mediate the
efflux of cholesterol and phospholipids to lipid-free
apolipoproteins (apoA1 and apoE) to then form nascent high-density
lipoprotein (HDL) (6,8). The disruption of ABCA1 or ABCG1 in
mice has been shown to promote the accumulation of excess
cholesterol in macrophages, and the physiological manipulation of
ABCA1 expression affects atherogenesis (6). Thus, there are growing therapeutic
approaches that target ABCA1 and ABCG1 to stimulate the flux of
lipids through the reverse cholesterol transport (RCT) pathway
(9).
The ligand activation of the nuclear orphan
receptor, liver X receptor (LXR), by oxysterols from mitochondrial
cholesterol or present in oxidized LDL is pivotal in the cellular
response to an elevated sterol content, triggering cholesterol
efflux mechanisms by potently upregulating the gene expression of
ABCA1 and ABCG1 (6,10). The activation of the LXR pathway
interferes with various mechanisms underlying the development of
atherosclerotic plaque (11,12). LXR agonists have shown promise as
potential therapeutics with anti-atherogenic and anti-inflammatory
properties (12). Thus, the
function of LXR in cholesterol efflux and inflammatory signaling
make it attractive as a therapy for cardiovascular and inflammatory
diseases. Peroxisome proliferator-activated receptor γ (PPARγ) is
highly expressed in macrophages and foam cells of atherosclerotic
lesions, and has been shown to upregulate LXR expression and
elevate macrophage ABCA1 levels (8,13).
Thus, the PPAR-LXR-ABCA1 interaction may be integral to cholesterol
homeostasis and HDL metabolism. The therapeutic manipulation of ABC
transporters is thus feasible using PPAR and LXR agonists.
Previous studies have revealed the antioxidant
activities of purple perilla (Perilla frutescens) (14,15). In a recent study, it was
demonstrated that red perilla extracts possess high antioxidant
activity and prevent LDL oxidation and lipid peroxide formation
in vitro and in human subjects (14). In another study, it was also
demonstrated that an aqueous fraction of Perilla frutescens
Britton has anti-atopic dermatitis activity in an animal model of
2,4-dinitrofluorobenzene-induced atopic dermatitis (16). It has been shown that rosmarinic
acid and caffeic acid are major compounds in Perilla
frutescens (15,17). Our nuclear magnetic resonance
spectrometry study revealed that in the ethyl acetate soluble
fraction of methanol extracts of purple perilla, the main compounds
inhibiting aldose reductase were identified as chlorogenic acid,
rosmarinic acid, luteolin and methyl rosmarinic acid (18). Rosmarinic acid in purple perilla
leaves is the abundant phenolic acid with DPPH radical scavenging
activity and reducing power (15). Caffeic acid from perilla leaves
has also shown an additive hepatic protection with rosmarinic acid
against oxidative hepatic damage (17). In addition, perilla leaf extract
has been shown to ameliorate obesity and dyslipidemia induced by a
high-fat diet through the downregulation of adipogenic
transcription factor and specific target genes (19).
In the present study, we hyopthesized that purple
perilla (hexane) extracts (PPE) may improve macrophage cholesterol
efflux by activating PPARγ-LXRα-ABC transporter pathway for the
clearance of excess cholesterol induced by exposure to oxidized
LDL. To examine the hypothesis, we investigated whether PPE
stimulates ABC transporter-mediated cholesterol efflux from
lipid-laden murine macrophages. On the basis of cholesterol efflux
and HDL formation, a major PPE component was identified and
structurally characterized.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) chemicals,
fatty acid-free bovine serum albumin (BSA), TO-091317 (LXR
agonist), pioglitazone (PPAR agonist) and Oil Red O were purchased
from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), as were all
other reagents, unless stated otherwise.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Duchefa Biochemie (Haarlem, The Netherlands).
Fetal bovine serum (FBS) and penicillin-streptomycin were obtained
from Lonza (Basel, Switzerland). SR-B1 antibody (#sc67099) and
β-asarone protein were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Antibodies to ABCA1 (#NB400-105) and
ABCG1 (#NB400-132) were obtained from Novus Biologicals (Littleton,
CO, USA). PPARγ antibody (#2430) was supplied by Cell Signaling
Technology (Danvers, MA, USA). LXRα antibody (#PAI-332) was
provided by Pierce Biotechnology (Rockford, IL, USA). α-asarone
protein was purchased from Cayman Chemical Co. (Ann Arbor, MI,
USA). β-actin antibody (#A5441) was purchased from Sigma Aldrich
Chemical Co. Horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (#111-035-003) was supplied from Jackson
ImmunoResearch Laboratory (West Grove, PA, USA).
Preparation and oxidation of human plasma
LDL
Human plasma LDL was prepared by discontinuous
density gradient ultracentrifugation as previously described
(20,21). A pooled human normolipidemic
plasma LDL fraction was dialyzed and used within 4 weeks. The
present study for LDL isolation was approved by the Hallym
University Institutional Review Board (HIRB-2011-007-2). The
concentration of total cholesterol was measured using commercial
diagnostic kits (Asan Pharmaceutical, Seoul, Korea). Oxidized LDL
was prepared by incubation with 10 μM CuSO4 in
F-10 medium at 37°C for 24 h. The extent of LDL oxidative
modification was routinely determined using thiobarbituric acid
reactive substances measurements and eletrophoretic mobility
assay.
PPE preparation and identification of
α-asarone
Purple perilla was purchased from a local market in
Chuncheon, Korea. A voucher sample (RIC-2012-5) has been deposited
at the Center for Efficacy Assessment and Development of Functional
Foods and Drugs, Hallym University, Chuncheon, Korea. The specimens
were authenticated by Emeritus Professor Hyung-Joon Chi, Seoul
National University, Korea. Dried leaves of Perilla
frutescens (2 kg) were extracted 3 times with 99.5% methanol
for 5 h. The solvent was evaporated under reduced pressure below
45°C to yield a methanol extract (yield, 11.68%). The extract was
suspended in distilled water and partitioned with n-hexane (n-Hex),
methylene chloride (CH2Cl3), ethyl acetate
(EtOAc), n-butanol (n-BuOH), and H2O to yield n-Hex
(40.83 g), EtOAc (25.20 g), CH2Cl3 (22.24 g),
n-BuOH (116.88 g) and H2O fractions (27.42 g). A portion
of the n-Hex fraction (PPE) was purified by chromatography on a
silica gel eluted with chloroform and increasing proportion
methanol (10:0-9:1) to yield 11 parts (parts 1–11). The ability of
each fraction to reduce the viability of DU145 human prostate
cancer cells was evaluated by MTT assay. The part 5 (0.46 g)
revealing the most potent activity was further purified by
recrystallization to yield compound 1 (76 mg).
The 1H- and 13C-nuclear
magnetic resonance (NMR) spectra of the isolated pure compound 1
were recorded with a Bruker DPX 400 NMR spectrometer (400 MHz),
using DMSO-d6 as a solvent. Compound 1:
1H-NMR (CD3OD, 400 MHz) δ 1.82 (3H, dd,
J=6.5, 1.6 Hz, H-3′), δ 3.17 (3H, s, -OCH3), δ
3.76 (3H, s, -OCH3), δ 3.78 (3H, s, -OCH3), δ
6.11 (1H, dq, J=10.6, 6.6 Hz, H-2′), δ 6.56 (1H, dd,
J=16, 1.6 Hz, H-1′), δ 6.63 (1H, s, H-3), δ 7.25 (1H, s,
H-6); 13C-NMR (CD3OD, 100 MHz) δ 19.42
(C-3′), 56.56 (-OCH3), 57.02 (-OCH3), 57.09
(-OCH3), 99.22 (C-3), 110.91 (C-6), 118.43 (C-5), 124.12
(C-2′), 125.79 (C-1′), 143.81 (C-1), 149.65 (C-2), 151.16 (C-4);
ESI-MS (m/z) 209 [M+H]+; 193.1 [M-CH3]+,
165.1 [M-CH3CO]+; UV (MeCN, λmax nm) 212,
258, 312. The 1H-NMR, 13C-NMR, ESI-MS and UV
data confirmed compound 1 as α-asarone, according to the isolation
and identification described in a previous study (22).
Cell culture and Oil Red O staining
The mouse macrophage-like cell line, J774A.1
(#TIB-67; American Type Culture Collection, Manassas, VA, USA), was
grown in DMEM supplemented with 10% FBS at 37°C in a humidified
atmosphere of 5% CO2 in air. The viability of the
J774A.1 cells was determined using a colorimetric assay based on
the uptake of MTT by viable cells. There was no noticeable
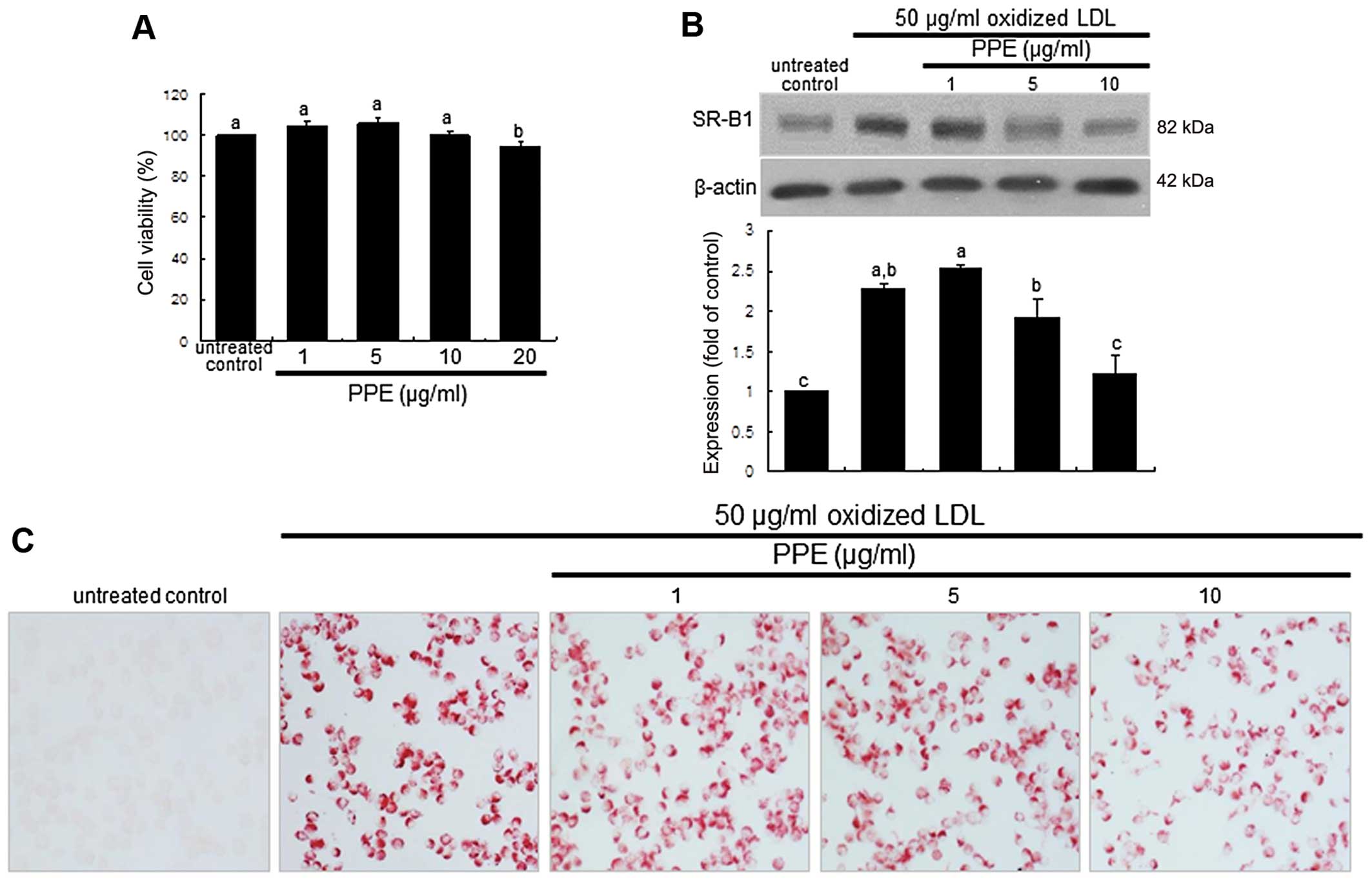
cytotoxicity observed with the dose of ≤10 μg/ml PPE
(Fig. 1A). The macrophages were
pre-treated with 1–10 μg/ml PPE and exposed to 50
μg/ml oxidized LDL or the other agonists, 10 μM
pioglitazone or 1 μM TO-091317, for various periods of time.
The J774A.1 macrophages were incubated in DMEM supplemented with
0.4% fatty acid-free BSA and treated with CuSO4-oxidized
LDL.
Oil Red O staining was used to visualize the lipid
uptake and foam cell formation in the macrophages. Oil Red O is a
fat-soluble dye used for staining lipids and some lipoproteins.
Following the culture of J774A.1 cells with 50 μg/ml
oxidized LDL in the absence or presence of PPE, the cells were
treated with 0.5% Oil Red O dissolved in 60% 2-propanol. After
mounting, images were obtained using an optical microscope (AXIO
Imager microscope; Carl Zeiss, Oberkochen, Germany).
Western blot analysis
Following the culture protocols, the cells were
lysed in lysis buffer. Equal protein amounts of cell lysates were
electrophoresed on a 6–8% sodium dodecyl sulfate-polyacrylamide gel
(SDS-PAGE) and transferred onto a nitrocellulose membrane. After
blocking with 5% skim milk, the membrane was incubated with
polyclonal rabbit antibodies to SR-B1, ABCA1, ABCG1, PPARγ and
LXRα. Following triple washes, the membrane was incubated for 1 h
with a goat anti-rabbit IgG conjugated to HRP. The individual
protein level was determined using Immobilon Western
Chemiluminescnet HRP substrate (Millipore, Billerica, MA, USA).
Incubation with mouse β-actin antibody was also performed for
comparative controls. After performing immunoblotting, the blot
bands were visualized on Agfa X-ray film (Agfa-Gevaert, Mortsel,
Belgium).
Cholesterol efflux assay
The J774A.1 macrophages were treated with 1–10
μg/ml PPE or 1–10 μM α-asarone for 12 h and then
equilibrated with 1 μg/ml 3-dodecanoyl-NBD-labeled
cholesterol (Cayman Chemical Co.) for an additional 6 h. The cells
exposed to NBD-labeled cholesterol were washed with
phosphate-buffered saline for various periods of time and freshly
incubated in DMEM for 6 h. Fluorescence-labeled cholesterol
released from the cells into the medium for 6 h was detected using
a fluorometer at ranges from λ=485 to 538 nm (Fluoroskan Ascent FL,
#5210450; Thermo Scientific, Waltham, MA, USA). Cholesterol efflux
was expressed as the percentage fluorescence in the medium relative
to the total fluorescence.
RT-PCR
Following the culture protocols, total RNA was
isolated from the J774A.1 macrophages using a commercially
available TRIzol reagent kit (Molecular Research Center,
Cincinnati, OH, USA). RNA (5 μg) was reverse transcribed
with 200 units of reverse transcriptase (Promega Corp., Madison,
WI, USA) and 0.5 mg/ml oligo(dT)15 primer (Bioneer,
Daejeon, Korea). RT-PCR was also performed for semi-quantifying the
levels of the mRNA transcripts of SR-B1 and retinoid X receptor
(RXR)α. The PCR conditions for SR-B1 [5′-ATGGGCCAGCGTGCTTTTATGA-3′
(forward) and 5′-AACCACAGCAACGGCAGAACTA-3′ (reverse) 752 bp] were
94°C (3 min), and 30 cycles at 94°C (30 sec), 60°C (45 sec) and
72°C (45 sec) and the conditions for RXRα
[5′-CAATGGCGTCCTCAAGGTTC-3′ (forward) and
5′-ACTCCACCTCGTTCTCATTC-3′ (reverse) 326 bp)] were 95°C (10 min)
and 35 cycles at 94°C (30 sec), 60°C (45 sec), and 72°C (45 sec).
The housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) [5′-AACTTTGGCATTGTGGAAGGG-3′ (forward) and
5′-GACACATTGGGGGTAGGAACAC-3′ (reverse) 224 bp], was used for
internal normalization for the co-amplification with the respective
gene.
Data analysis
The results are presented as the means ± SEM.
Statistical analyses were conducted using the SAS software package
version 6.12 (SAS Institute, Cary, NC, USA). One-way ANOVA was used
to determine the inhibitory effects of PPE on the effects of
oxidized LDL on macrophages. Differences between the treatment
groups were analyzed using Duncan’s multiple-range test and
considered statistically significant at a value of P<0.05.
Results
Suppression of SR-B1 induction and foam
cell formation by PPE
The internal uptake of oxidized LDL is known to
require SR induction in macrophages (23). Oxidized LDL rapidly increased
SR-B1 expression within 2 h, which was sustained for up to 8 h
(data not shown). When the cells were exposed to oxidized LDL for 6
h and treated with 1–10 μg/ml PPE, SR-B1 expression
decreased in a dose-dependent manner (Fig. 1B). A further experiment was
conducted to examine intracellular lipid accumulation in
macrophages, evidenced by using Oil Red O staining. There was
strong reddish staining observed in the macrophages exposed to 50
μg/ml oxidized LDL for 18 h. This indicates the cellular
accumulation of lipids through the upregulation of SR-B1. When the
macrophages were treated with 10 μg/ml PPE for 18 h, the
number of reddish lipid droplets decreased (Fig. 1C). Thus, PPE delayed foam cell
formation in the macrophages exposed to 50 μg/ml oxidized
LDL.
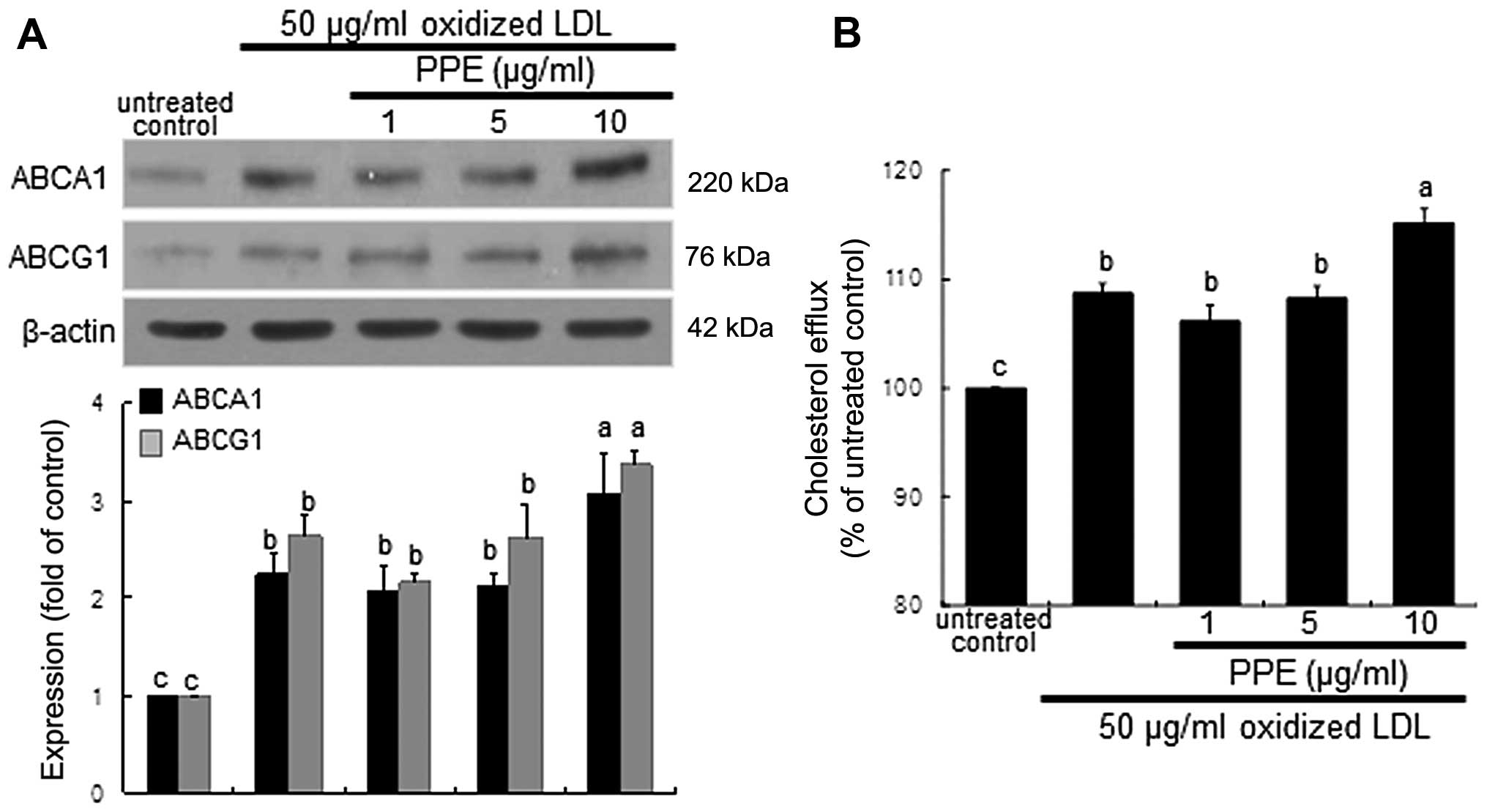
Increase in ABCA1 and ABCG1 expression
and cholesterol efflux by PPE
The membrane proteins, ABCA1 and ABCG1, play a role
in cholesterol efflux from lipid-laden macrophages (6,8).
Exposure of the macrophages for 8 h to oxidized LDL highly promoted
the induction of ABCA1 and ABCG1. When 10 μg/ml PPE was
applied to the oxidized LDL-exposed macrophages, the expression of
these proteins was further increased (Fig. 2A). Thus, PPE promotes cholesterol
efflux from lipid-laden foam cells. As expected, the cholesterol
efflux was enhanced in the macrophages exposed to 50 μg/ml
oxidized LDL and was further accelerated by treatment with 10
μg/ml PPE (Fig. 2B).
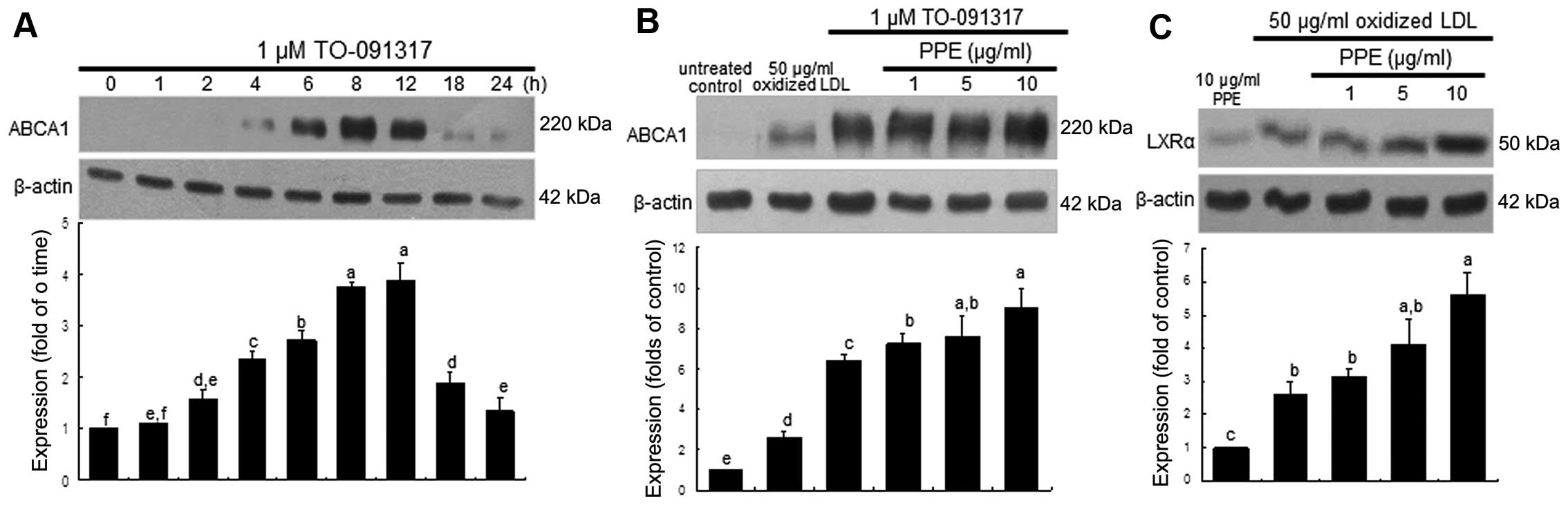
Promoting effect of PPE on ABCA1
expression induced by LXR
We wished to confirm whether the upregulation of
ABCA1 entails the nuclear induction of LXR, which was further
accelerated by the administration of PPE. ABCA1 expression was
highly induced within 4 h in the macrophages stimulated with the
LXR agonist, TO-091317, at 1 μM and was sustained at high
levels for up to 12 h (Fig. 3A).
It should be noted that the induction of ABCA1 by TO-091317 was
much more prominent than that induced by oxidized LDL (Fig. 3B). When the J774A.1 macrophages
were treated with 1 μM TO-091317 for 8 h, the induction of
ABCA1 was accelerated by treatment with 10 μg/ml PPE
(Fig. 3B). In addition, the
induction of LXRα by oxidized LDL was further enhanced in the
PPE-treated macrophages (Fig.
3C). However, PPE per se did not induce nuclear LXRα
expression (Fig. 3C). This
indicated that the nuclear induction of LXRα by PPE in the presence
of oxidized LDL appeared to be synergistic to that of TO-091317,
which led to an increase in ABCA1 expression and cholesterol
efflux.
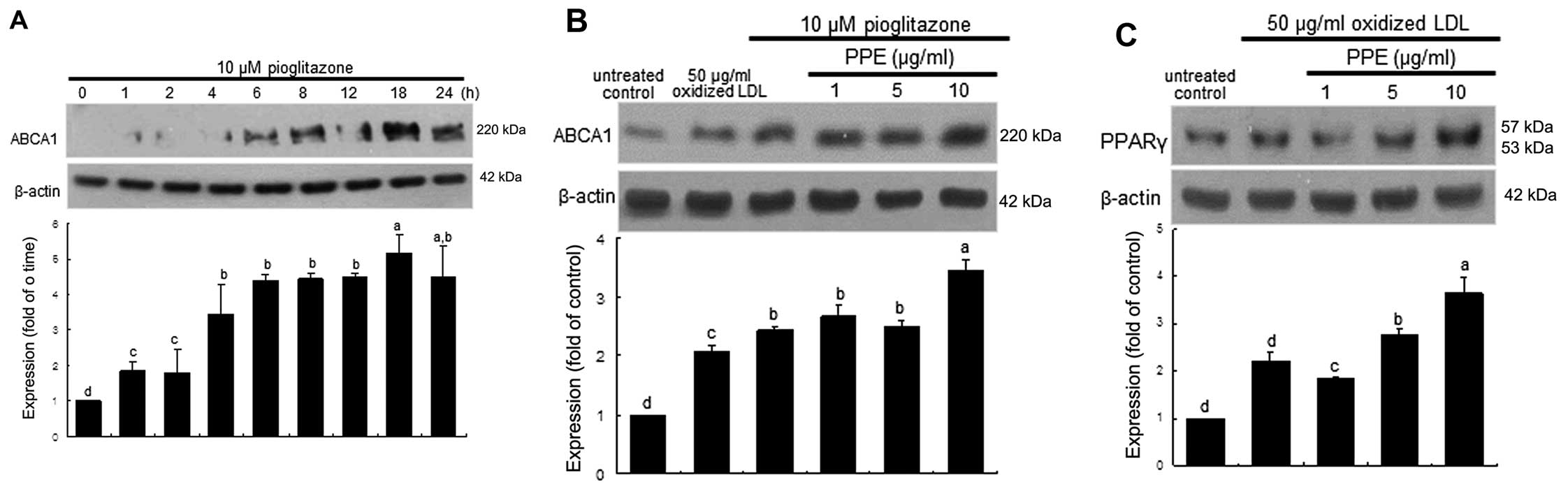
Increase in PPARγ expression by PPE
PPARγ is highly expressed in macrophages and has
been shown to elevate ABCA1 levels (8,13).
As expected, ABCA1 expression was temporally induced from 6 h in
the J774A.1 macrophages stimulated with the PPARγ agonist,
pioglitazone at 10 μM, and was sustained at high levels for
up to 24 h (Fig. 4A). Treatment
with PPE at 10 μg/ml further increased ABCA1 expression in
the macrophages following 18 h of treatment with 10 μM
pioglitazone (Fig. 4B). The
induction of PPARγ was promoted to an even greater extent in the
oxidized LDL-treated macrophages, and this increase was even
further elevated by PPE (Fig.
4C). These results suggest that PPE promotes the activation of
the PPAR-LXR-ABCA1 pathway, leading to an increase in cholesterol
efflux.
Induction of ABCA1 by PPE-α-asarone
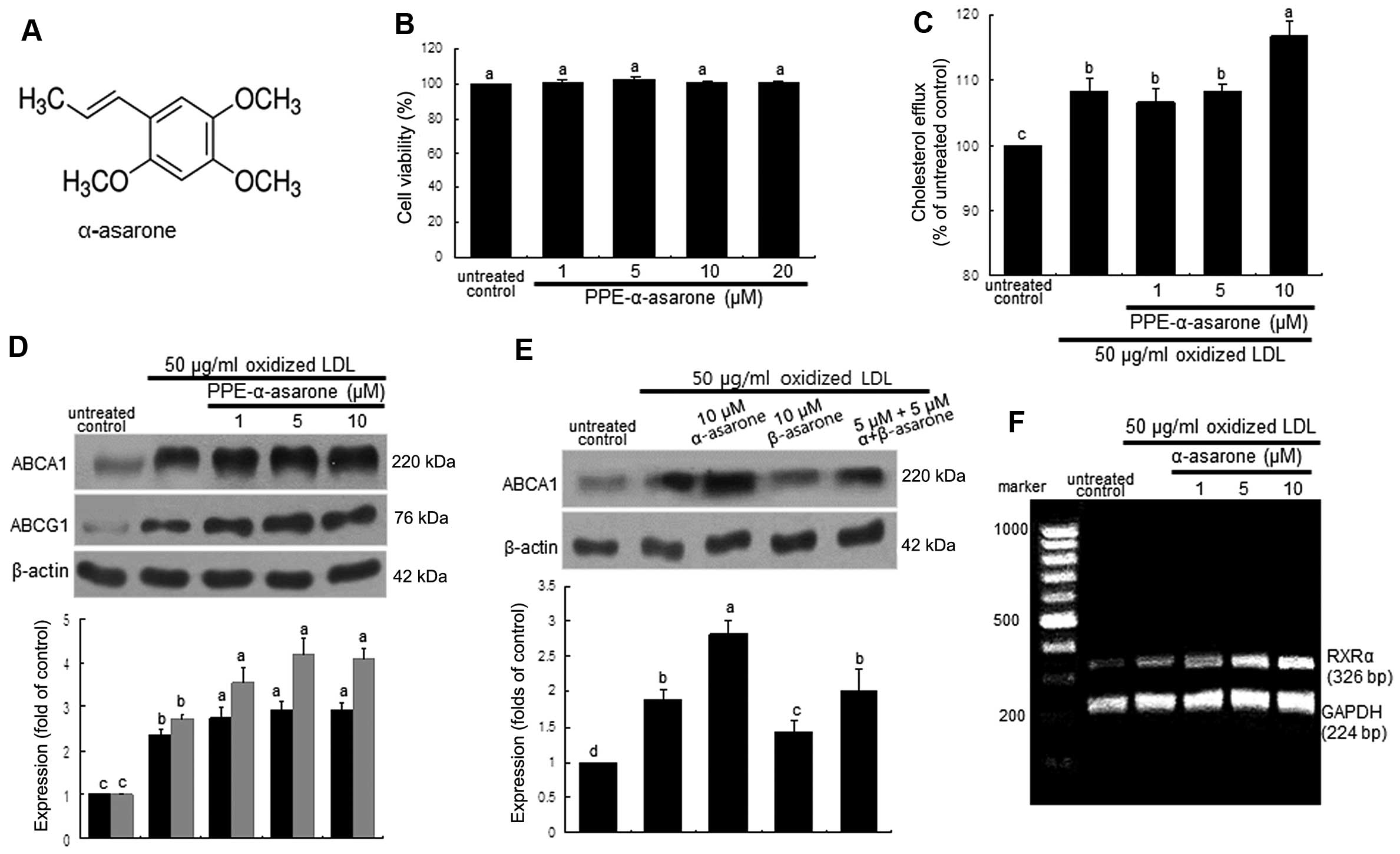
α-asarone (Fig.
5A) (PPE-α-asarone), which was isolated and identified as the
major effective component in PPE, did not exert any toxic effects
on the macrophages (Fig. 5B). The
cholesterol efflux enhanced by oxidized LDL was further enhanced in
the macrophages treated with 10 μM α-asarone (Fig. 5C). Consistently, the induction of
ABCA1 and ABCG1 by treatment with 50 μg/ml oxidized LDL for
8 h was further upregulated by treatment with ≥1 μM
α-asarone isolated from PPE (Fig.
5D). By contrast, treatment with 10 μM β-asarone did not
exert such an induction in the oxidized LDL-exposed macrophages
(Fig. 5E). Thus, the promotion of
cholesterol efflux from lipid-laden foam cells by PPE may be due to
the mechanistic action of α-asarone present in PPE. On the other
hand, the ligand activation of RXRα was achieved by exposure of the
macrophages to oxidized LDL and this effect was further enhanced by
treatment with ≥5 μM α-asarone (Fig. 5F).
Inhibition of lipid uptake by
PPE-α-asarone
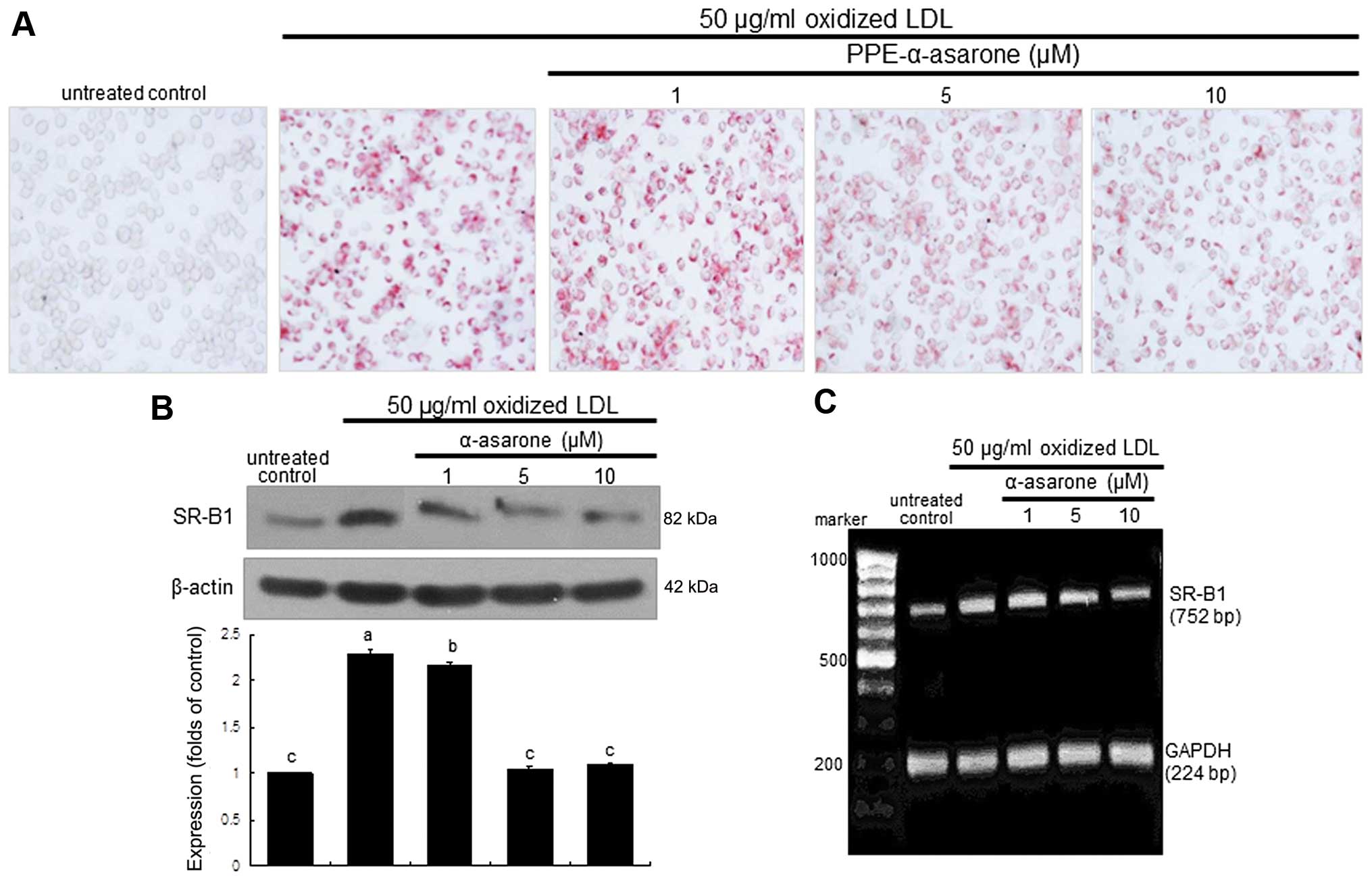
The intracellular lipid accumulation was diminished
in the macrophages exposed to 50 μg/ml oxidized LDL and
treated with 10 μM PPE-α-asarone (Fig. 6A). The expression of SR-B1 induced
by oxidized LDL was decreased in a dose-dependent manner by
treatment with 1–10 μM α-asarone isolated from PPE (Fig. 6B). Treatment with non-toxic
α-asarone at 10 μM diminished SR-B1 transcription,
indicating that the induction of SR-B1 by LDL was disrupted at the
transcriptional level (Fig. 6C).
Thus, α-asarone from PPE is effective in blocking foam cell
formation in macrophages.
Discussion
Seven major findings were observed in this study. i)
non-cytotoxic PPE at ≤10 μg/ml attenuated oxidized LDL
uptake and cellular lipid accumulation in J774A.1 murine
macrophages by reducing the induction of SR-B1; ii) treatment of
the macrophages with 10 μg/ml PPE enhanced the oxidized
LDL-induced expression of ABCA1 and ABCG1 and subsequent
cholesterol efflux; iii) ABCA1 expression was highly upregulated in
the macrophages stimulated with the LXR agonist, TO-091317, and
these effects were further enhanced by treatment with 10
μg/ml PPE; iv) the induction of LXRα by oxidized LDL was
elevated in a dose-dependent manner by PPE; v) ABCA1 expression was
further enhanced by PPE in the macrophages exposed to the PPAR
agonist, pioglitazone, and treatment with 10 μg/ml PPE
further enhanced the expression of PPAR induced by oxidized LDL;
vi) α-asarone was identified as a major compound present in the
hexane extract, PPE, enhancing cholesterol efflux from lipid-laden
foam cells; and vii) non-toxic α-asarone stimulated the protein
expression of ABCA1 and ABCG1 concomitant with the ligand
activation of RXRα, whereas SR-B1 induction and foam cell formation
were blocked by this compound at the transcriptional level. These
observations demonstrate that non-cytotoxic α-asarone (in PPE) is
effective in blocking oxidized LDL internalization by disrupting
the induction of SR-B1 and promoting the ABCA1-mediated cholesterol
efflux from lipid-laden foam cells by triggering PPARγ-LXRα
signaling.
Macrophage membrane SR is responsible for the uptake
of oxidized LDL and promotes the cellular accumulation of
cholesterol (3). The aberrant
cellular accumulation of cholesterol has been implicated in the
development of atherosclerosis (23,24). In the present study, we
demonstrated that macrophages became lipid droplet-loaded foam
cells due to oxidized LDL. Any surplus of cholesterol is released
from the cell through ABC transporters for the regulation of
cellular cholesterol homeostasis by mediating cholesterol efflux
(1,7). As expected, oxidized LDL induced the
expression of the atheroprotective membrane proteins, ABCA1 and
ABCG1, and enhanced cholesterol efflux from lipid-laden foam cells.
Ongoing research using animals and cells has produced increasing
evidence that cholesterol efflux pathways are mediated by ABC
transporters and that HDL suppresses atherosclerosis (25). In addition, the specific knockout
of macrophage transporters has confirmed their role in the
suppression of inflammatory responses in the arterial wall
(26,27). Thus, the activation of cholesterol
efflux pathways targeting ABCA1 and ABCG1 may prove to be novel
therapeutic approaches to the treatment of atherosclerosis
(9).
Phenolic compounds with high antioxidant activity,
such as rosmarinic acid and caffeic acid have been characterized in
purple perilla leaves (15,17,18). Perilla leaf extract ameliorates
high-fat diet-induced obesity and dyslipidemia by downregulating
epididymal adipose tissue genes, including CoA carboxylase and
PPARγ (19). In addition, ethyl
acetate extracts of purple perilla have been shown to inhibit the
activity of aldose reductase, which is involved in diabetic
complications (18). In this
study, we found that PPE deterred SR-B1-mediated cholesterol
internalization leading to foam cell formation, and enhanced ABC
transporter-mediated cholesterol efflux from lipid-laden murine
macrophages. This study attempted to characterize the major
components of PPE which promote cholesterol efflux from
macrophages. The active hexane fractions from PPE were isolated by
conducting bioactivity-guided fractionation. Based on NMR and mass
spectrometric analyses, the active ingredient responsible for the
induction of ABCA1 was identified as α-asarone
(2,4,5-trimethoxyphenyl-2-propene). α-asarone and β-asarone may be
potential therapeutic agents for managing cognitive impairment due
to their neuroprotective and cognitive-enhancing properties
(28,29). α-asarone has been shown to inhibit
HMG-CoA reductase, lower serum LDL cholesterol levels and to reduce
the biliary cholesterol saturation index in hypercholesterolemic
rats (30). In the present study,
non-toxic α-asarone enhanced the expression of ABCA1 and ABCG1
induced by oxidized LDL and activated the cholesterol efflux
pathway-mediated HDL formation. However, β-asarone did not show
such atheroprotective effects related to cholesterol efflux. Thus,
there was an isomeric difference in the induction of the
cholesterol efflux-associated membrane proteins, ABCA1 and
ABCG1.
The ligand activation of LXR by oxysterols from
mitochondrial cholesterol or present in oxidized LDL initiates the
cholesterol efflux pathway by enhancing ABCA1 and ABCG1 gene
expression (6,10). Since the activation of the LXR
pathway attenuates atherosclerotic plaque development, LXR agonists
have shown promise as potential therapeutics (11). This study demonstrated that the
LXR agonist, TO-091317, elevated ABCA1 expression in murine
macrophages exposed to oxidized LDL, which was further enhanced by
the presence of PPE. Thus, it can be hypothesized that PPE is an
LXR agonist. Since PPE per se did not elicit the induction
of LXRα, PPE appeared to be synergistic to TO-091317 in inducing
ABCA1 expression. Furthermore, PPAR agonists, such as glitazones
may be other therapeutic manipulators of ABC transporters. PPARγ is
known to upregulate LXR expression and elevate macrophage ABCA1
levels (8,13). In this study, PPE enhanced the
expression of PPARγ induced in the macrophages by oxidized LDL,
indicating the activation of the PPARγ-LXRα pathway to promote
ABCA1 protein expression. α-asarone also promoted the oxidized
LDL-induced ligand activation of the nuclear receptor RXRα
dimerized with LXR in facilitating the binding and transactivation
of receptors with DNA response elements. Gemfibrozil analogues
conjugated with α-asarone have been shown to activate the PPARα
receptor and enhance the activity of the PPARα-regulated carnitine
palmitoyltransferase IA gene involved in fatty acid catabolism
(31). Thus, α-asarone may
activate the PPARγ-LXRα-RXRα pathway for the induction of ABC
transporters. On the other hand, ABCA1 protein stabilization by PPE
or α-asarone may be another therapeutic mechanism. ABCA1 protein
degradation may be promoted in atherosclerotic plaque milieu, thus
compromising cholesterol efflux. Although the present findings are
promising, further studies are required to elucidate the efficacy
and side-effects of PPE and α-asarone for future use in human
subjects.
In conclusion, in the current study, we demonstrated
that α-asarone-rich PPE encumbered oxidized LDL uptake and
cholesterol influx in J774A.1 murine macrophages by reducing the
early expression of SR-B1. In addition, PPE promoted cholesterol
efflux from lipid-loaded macrophages by inducing the expression of
ABCA1 and ABCG1. The induction of ABCA1 by PPE was mediated through
the activation of the PPARγ-LXRα-responsive cellular signaling
pathway, thus transferring effluxed cholesterol onto lipid-poor
apolipoproteins, thus promoting the formation of HDL particles.
Thus, PPE functions as an agonist of LXR and PPARγ in macrophages,
and α-asarone, which is present in PPE may be effective in cellular
cholesterol handling in lipid-loaded macrophages. Although PPE may
serve as a beneficial modulator against cholesterol
efflux-associated atherogenesis in vitro, its role in
vivo remains to be elucidated.
Acknowledgments
This study was supported by the High Value-added
Food Technology Development Program, Korea Institute of Planning
and Evaluation for Technology in Food, Agriculture, Forestry and
Fisheries (112085-03-1-SB010) and by the National Research
Foundation of Korea through the Human Resource Training Project for
Regional Innovation (2012-01-A-05-003-12-010100).
References
|
1
|
Li G, Gu HM and Zhang DW: ATP-binding
cassette transporters and cholesterol translocation. IUBMB Life.
65:505–512. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cruz PM, Mo H, McConathy WJ, Sabnis N and
Lacko AG: The role of cholesterol metabolism and cholesterol
transport in carcinogenesis: A review of scientific findings,
relevant to future cancer therapeutics. Front Pharmacol. 4:1192013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yue P, Chen Z, Nassir F, Bernal-Mizrachi
C, Finck B, Azhar S and Abumrad NA: Enhanced hepatic apoA-I
secretion and peripheral efflux of cholesterol and phospholipid in
CD36 null mice. PLoS One. 5:e99062010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen Y and Leake DS: Low density
lipoprotein undergoes oxidation within lysosomes in cells. Circ
Res. 100:1337–1343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shibata N and Glass CK: Macrophages,
oxysterols and atherosclerosis. Circ J. 74:2045–2051. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye D, Lammers B, Zhao Y, Meurs I, Van
Berkel TJ and Van Eck M: ATP-binding cassette transporters A1 and
G1, HDL metabolism, cholesterol efflux, and inflammation: Important
targets for the treatment of atherosclerosis. Curr Drug Targets.
12:647–660. 2011. View Article : Google Scholar
|
|
7
|
Hafiane A and Genest J: HDL,
atherosclerosis, and emerging therapies. Cholesterol.
2013:8914032013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogata M, Tsujita M, Hossain MA, Akita N,
Gonzalez FJ, Staels B, Suzuki S, Fukutomi T, Kimura G and Yokoyama
S: On the mechanism for PPAR agonists to enhance ABCA1 gene
expression. Atherosclerosis. 205:413–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brewer HB Jr: HDL metabolism and the role
of HDL in the treatment of high-risk patients with cardiovascular
disease. Curr Cardiol Rep. 9:486–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bełtowski J: Liver X receptors (LXR) as
therapeutic targets in dyslipidemia. Cardiovasc Ther. 26:297–316.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calkin AC and Tontonoz P: Liver x receptor
signaling pathways and atherosclerosis. Arterioscler Thromb Vasc
Biol. 30:1513–1518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu R, Ou Z, Ruan X and Gong J: Role of
liver X receptors in cholesterol efflux and inflammatory signaling
(Review). Mol Med Rep. 5:895–900. 2012.PubMed/NCBI
|
|
13
|
Neve BP, Fruchart JC and Staels B: Role of
the peroxisome proliferator-activated receptors (PPAR) in
atherosclerosis. Biochem Pharmacol. 60:1245–1250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saita E, Kishimoto Y, Tani M, Iizuka M,
Toyozaki M, Sugihara N and Kondo K: Antioxidant activities of
Perilla frutescens against low-density lipoprotein oxidation in
vitro and in human subjects. J Oleo Sci. 61:113–120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jun HI, Kim BT, Song GS and Kim YS:
Structural characterization of phenolic antioxidants from purple
perilla (Perilla frutescens var. acuta) leaves. Food Chem.
148:367–372. 2014. View Article : Google Scholar
|
|
16
|
Heo JC, Nam DY, Seo MS and Lee SH:
Alleviation of atopic dermatitis-related symptoms by Perilla
frutescens Britton. Int J Mol Med. 28:733–737. 2011.PubMed/NCBI
|
|
17
|
Yang SY, Hong CO, Lee GP, Kim CT and Lee
KW: The hepato-protection of caffeic acid and rosmarinic acid,
major compounds of Perilla frutescens, against t-BHP-induced
oxidative liver damage. Food Chem Toxicol. 55:92–99. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paek JH, Shin KH, Kang YH, Lee JY and Lim
SS: Rapid identification of aldose reductase inhibitory compounds
from Perilla frutescens. Biomed Res Int. 2013:6794632013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MJ and Kim HK: Perilla leaf extract
ameliorates obesity and dyslipidemia induced by high-fat diet.
Phytother Res. 23:1685–1690. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SH, Kim JL, Lee ES, Han SY, Gong JH,
Kang MK and Kang YH: Dietary ellagic acid attenuates oxidized LDL
uptake and stimulates cholesterol efflux in murine macrophages. J
Nutr. 141:1931–1937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SH, Kim JL, Kang MK, Gong JH, Han SY,
Shim JH, Lim SS and Kang YH: Sage weed (Salvia plebeia) extract
antagonizes foam cell formation and promotes cholesterol efflux in
murine macrophages. Int J Mol Med. 30:1105–1112. 2012.PubMed/NCBI
|
|
22
|
Wang D, Geng Y, Fang L, Shu X, Liu J, Wang
X and Huang L: An efficient combination of supercritical fluid
extraction and high-speed counter-current chromatography to extract
and purify (E)- and (Z)-diastereomers of α-asarone and β-asarone
from Acorus tatarinowii Schott. J Sep Sci. 34:3339–3343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tangirala RK, Bischoff ED, Joseph SB, et
al: Identification of macrophage liver X receptors as inhibitors of
atherosclerosis. Proc Natl Acad Sci USA. 99:11896–11901. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Westerterp M, Bochem AE, Yvan-Charvet L,
Murphy AJ, Wang N and Tall AR: ATP-binding cassette transporters,
atherosclerosis, and inflammation. Circ Res. 114:157–170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calpe-Berdiel L, Zhao Y, de Graauw M, et
al: Macrophage ABCA2 deletion modulates intracellular cholesterol
deposition, affects macrophage apoptosis, and decreases early
atherosclerosis in LDL receptor knockout mice. Atherosclerosis.
223:332–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu X, Lee JY, Timmins JM, et al:
Increased cellular free cholesterol in macrophage-specific Abca1
knock-out mice enhances pro-inflammatory response of macrophages. J
Biol Chem. 283:22930–22941. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar H, Kim BW, Song SY, Kim JS, Kim IS,
Kwon YS, Koppula S and Choi DK: Cognitive enhancing effects of
alpha asarone in amnesic mice by influencing cholinergic and
antioxidant defense mechanisms. Biosci Biotechnol Biochem.
76:1518–1522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geng Y, Li C, Liu J, Xing G, Zhou L, Dong
M, Li X and Niu Y: Beta-asarone improves cognitive function by
suppressing neuronal apoptosis in the beta-amyloid hippocampus
injection rats. Biol Pharm Bull. 33:836–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodríguez-Páez L, Juárez-Sanchez M,
Antúnez-Solís J, Baeza I and Wong C: Alpha-asarone inhibits HMG-CoA
reductase, lowers serum LDL-cholesterol levels and reduces biliary
CSI in hypercholesterolemic rats. Phytomedicine. 10:397–404. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Filippis B, Giancristofaro A,
Ammazzalorso A, D’Angelo A, Fantacuzzi M, Giampietro L, Maccallini
C, Petruzzelli M and Amoroso R: Discovery of gemfibrozil analogues
that activate PPARα and enhance the expression of gene CPT1A
involved in fatty acids catabolism. Eur J Med Chem. 46:5218–5224.
2011. View Article : Google Scholar : PubMed/NCBI
|