Introduction
Keloids are benign fibrous skin tumors which develop
due to the overproduction of extracellular matrix (ECM). Keloids
occur as a response to dermal injuries and are indicative of an
imbalance in wound healing. One of the major characteristics of
keloids is the progressive invasion into the adjacent normal skin,
exceeding the original wound margin (1–3).
The excess deposition of ECM components and the lack of growth
regression results in an ischemic and hypoxic microenvironment
surrounding the scar tissue (4).
This increases the risk of ulceration, which in turn leads to the
development of repeated infections, eventually developing into scar
carcinomas (5,6). Numerous treatment modalities for
keloids are applied alone or in combination; however, there is
insufficient evidence to prove their effectiveness.
Previous studies have found that hypoxia exists
inside of keloid tissue (7–9).
Due to exposure to constant hypoxic conditions, hypoxia-inducible
factor-1α (HIF-1α) is highly expressed in keloid tissue and is an
important transcriptional regulator which helps cells adapt to the
hypoxic microenvironment (8,10).
Extensive research has indicated that the stable accumulation of
HIF-1α-promotes fibrogenesis in a wide range of tumors through
epithelial-to-mesenchymal transition (EMT), which leads to ECM
accumulation (11–14).
EMT is a process which leads to the loss of
epithelial cell polarity and cell-cell adhesion. Through the
process of EMT, epithelial cells acquire migratory and invasive
behavior and are thus able to transform into mesenchymal cells. EMT
is necessary for several early embryonic developmental processes,
including mesoderm formation and neural tube formation (15-17). During the pathological state, EMT
is also involved in organ fibrosis, wound healing and in the
initiation of tumor metastasis (18–22).
Although the prominence of both hypoxia and the
subsequent activation of HIF-1α in the tumor EMT process are known,
their functions in regulating keloid pathological processes remain
unclear. In the current study, we hypothesized that hypoxia/HIF-1α
is a key factor in the transition of keloid keratinocytes into
mesenchymal-type cells. This transition enhances the invasive
capacity of the keloid-derived fibroblasts. To examine this
hypothesis, the expression levels of EMT markers and HIF-1α were
determined in vitro by reverse transcription- quantitative
polymerase chain reaction (RT-qPCR), western blot analysis and
fluorescence staining, as well as immunohisto-chemistry.
Furthermore, we examined the invasive ability of the keloid
keratinocytes under hypoxic conditions using a Transwell co-culture
system. Additionally, the involvement of HIF-1α in the
transformation of keloid keratinocytes was confirmed by
transfecting the cells with siRNA targeting HIF-1α under the same
conditions.
Materials and methods
Collection of keloid tissue specimens and
isolation of keloid keratinocytes
This study was reviewed and approved by the Ethics
Committee at the Chinese Academy of Medical Sciences and Peking
Union Medical College Hospital (Beijing, China). We collected
keloid scar specimens from 30 patients treated at Peking Union
Medical College Hospital (Beijing, China). All patients provided
written informed consent before participating in this study. All
keloid cases were clinically and pathologically proven.
Full-thickness skin specimens were harvested from the keloid scar
tissues. No patients had previously received any treatment for
keloids.
Cell culture
All skin specimens were incubated overnight at 4°C
in dispase II (Sigma-Aldrich, St. Louis, MO, USA) to separated the
epidermis from the dermis. Keratinocytes were extracted from the
epidermis using 0.25% trypsin (Sigma-Aldrich) at 37°C for 30 min.
All cells were filtered through a cell strainer (70 μm;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Primary
keratinocytes were incubated in Keratinocyte-SFM (1X) (Life
Technologies, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (Invitrogen, San Diego, CA, USA) and 1X
penicillin-streptomycin-fungizone (PSF; Life Technologies) for 48
or 72 h at 37°C to allow the cells to adhere to the culture dishes.
Non-adherent cells were washed out with phosphate-buffered saline
(PBS), and the remaining cells were subcultured or collected for
the following analysis at approximately 80–90% confluence to avoid
contact inhibition and differentiation. Cells at up to passage 3
were used for analyses.
In addition to the normoxic state, the influence of
the hypoxic culture condition on epithelial cells was also
determined. When the keratinocytes reached 50% confluence, they
were then moved to a hypoxic incubation chamber (New Brunswick
Scientific Co., Enfield, CT, USA). The hypoxic condition was
maintained by continuous flushing with a mixed gas (1%
O2, 5% CO2 and 94% N2). The
humidity was maintained, as well as the temperature (37°C).
Cell morphology
The morphological alterations of the keratinocytes
were observed by phase contrast microscopy. The keratinocytes were
incubated under hypoxic conditions, and compared to the cells
incubated under normoxic culture conditions for 12, 24 and 36 h.
The morphological changes were recorded by using phase contrast
microscopy and an Olympus inverted microscope (Olympus Corp.,
Tokyo, Japan).
Immunohistochemistry
Keloid biopsies were acquired and immediately fixed
in 4% formaldehyde for 30 min. The specimens were then embedded in
paraffin. Each slide was cut into 5-μm-thick sections. The
slides were stained with different primary antibodies for
immunohistochemistry, including mouse anti-E-cadherin (ab1416;
1:1,000), rabbit anti-vimentin (Ab92547; 1:1,000) (both from Abcam,
Cambridge, UK), mouse anti-fibronectin (sc-8422; 1:400; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and mouse anti-HIF-1α
antibodies (610958; 1:400; BD Biosciences, San Jose, CA, USA). All
slides were incubated in biotin-labeled goat anti-rabbit (sc-45101)
or goat anti-mouse (sc-395764) serum (1:200; Santa Cruz
Biotechnology, Inc.) for 0.5 h, and detection was carried out using
the VECTASTAIN Universal ABC-Alkaline Phosphatase kit with ImmPACT
NovaRED Peroxidase Substrate (Vector Laboratories, Inc.,
Burlingame, CA, USA).
Immunofluorescence staining
The expression of multiple secondary produced
mesenchymal proteins, and HIF-1α protein in the epithelial cells
was examined by immunofluorescence staining. We isolated primary
keratinocytes as described above, then seeded them into dishes at a
density of 1×105 cells/well, and waited until they
reached 70% confluence in each well under normoxic (21%
O2) or hypoxic (1% O2) conditions. The cells
were then fixed with 4% formaldehyde for 10 min. The cells were
washed 3 times with PBS and the fixed cells were then treated with
EMT-related and HIF-1α primary antibodies (described above) at the
appropriate dilution at 4°C overnight, followed by incubation with
FITC (sc-65218)- or TRITC (sc-2492)-conjugated secondary antibodies
(1:150; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C after
extensively rinsing with PBS. Images were captured using an Olympus
inverted fluorescence microscope (Olympus Corp.).
HIF-1α siRNA plasmid construction and
cell transfection
The siRNA expression vector for HIF-1α was
constructed. To observe the HIF-1α-mediated changes in gene and
protein expression under hypoxic conditions, we introduced the
HIF-1α siRNA vector, pSilenser 2.1/HIF-si, including a
HIF-1α-specific targeting sequence (Table I) and the control scramble siRNA
vector, pSilenser, into the keloid keratinocytes. Cell transfection
was performed using Lipofectamine 2000 (Life Technologies)
according to the manufacturer’s instructions. The transfected cells
were cultured for 48 h for further experiments under hypoxic
conditions.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Sense primers | Antisense
primers |
|---|
| β-actin |
5′-CATCACTATCGGCAATGAGC-3′ |
5′-GACAGCACTGTGTTGGCATA-3′ |
| HIF-1α |
5′-CAAAACACACAGCGAAGC-3′ |
5′-TCAACCCAGACATATCCACC-3′ |
| HIF-1α-specific
target sequence |
5′-AAAGAGGTGGATATGTCTGGG-3′ |
5′-TTTCTCCACCTATACAGACCC-3′ |
| E-cadherin |
5′-ATTCTGATTCTGCTGCTCTTG-3′ |
5′-AGTCCTGGTCCTCTTCTCC-3′ |
| ZO-1 |
5′-CAACATACAGTGACGCTTCACA-3′ |
5′-GACGTTTCCCCACTCTGAAAA-3' |
| Vimentin |
5′-AATGACCGCTTCGCCAAC-3′ |
5′-CCGCATCTCCTCCTCGTAG-3′ |
| Fibronectin |
5′-CCCCATTCCAGGACACTTCTG-3′ |
5′-GCCCACGGTAACAACCTCTT-3′ |
RT-qPCR
We used RT-qPCR to detect the expression of selected
genes. Total RNA was extracted from the cultured keratinocytes
derived from keloid scars using TRIzol reagent (Invitrogen),
followed by treatment with DNase I (Promega Corp., Madison, WI,
USA). First-stand cDNA was synthesized with 2 μg total RNA
in 30 μl of reaction buffer using a high capacity cDNA
synthesis kit (Takara Bio, Inc., Tokyo, Japan) according to the
manufacturer’s instructions. Gene expression data were detected
using the ABI StepOnePlus system (Applied Biosystems®,
Life Technologies). The thermal cycling parameters were 95°C for 1
min, followed by 40 cycles of 95°C for 10 sec and 60°C for 40 sec.
qPCR was performed to detect the mRNA levels using SYBR-Green I
(Takara Bio, Inc.). The expression levels of genes were normalized
to β-actin housekeeping gene expression. All RT-qPCR reactions were
performed in technical triplicates. The primer sequences used for
amplication are listed in Table
I.
Western blot analysis
The protein levels of epithelial and mesenchymal
markers in the keratinocytes were quantified by western blot
analysis. Total protein was extracted using RIPA lysis buffer
(Beyotime, Shanghai, China) which included complete Protease
Inhibitor Cocktail (Sigma-Aldrich) and supernatants were collected
by centrifugation at 13,000 × g at 4°C for 30 min. The protein
concentration was detected using a QuantiPro BCA assay kit
(Sigma-Aldrich) followed by separation on a 10% sodium dodecyl
sulfate polyacrylamide gel. The proteins were relocated to 0.2
μm polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). The proteins were blocked with Tris-buffered
saline (TBS) with 5% skim milk for 1 h at room temperature. The
membranes were then incubated with primary antibodies overnight at
4°C. Mouse anti-E-cadherin (ab1416; 1:1,000; Abcam), rabbit
anti-zonula occludens-1 (ZO-1; sc-10804) (1:400; Santa Cruz
Biotechnology, Inc.), rabbit anti-vimentin (Ab92547; 1:1,000;
Abcam), mouse anti-fibronectin (sc-8422; 1:400; Santa Cruz
Biotechnology, Inc.), mouse anti-HIF-1α (610958; 1:400; BD
Biosciences) and mouse anti-β-actin (1:4,000; Sigma-Aldrich) were
used as primary antibodies. Horseradish peroxidase-labeled goat
anti-mouse/rabbit IgG (Beyotime) was then added as the secondary
antibody. Signals were visualized using an Immobilon Western
Chemiluminescent HRP Substrate (WBKLS0100; Millipore) and image
detection was performed using the ImageQuant LAS 4000 mini imaging
system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). The
results were normalized to β-actin (Sigma-Aldrich) where
appropriate.
In vitro invasion assays
The invasiveness of the keloid keratinocytes under
normoxic and hypoxic conditions was measured by Transwell
co-culture assay. The keratinocytes (2.0×105) were
seeded into the top chamber of 24-well plates (pore size, 8
μm; Corning, Inc., Corning, NY, USA) covered with Matrigel
(Coating Matrix; Life Technologies) and incubated in full
supplemented medium. The lower chambers were contained with
chemoattractant (20% serum culture medium). The whole plates were
placed into either a normoxic or hypoxic chamber for incubation 12,
24 and 36 h for observation. In addition, the keratinocytes
transfected with HIF-1α siRNA, control scramble siRNA and the
untransfected keratinocytes were also analyzed in triplicate wells.
For those cells that did not go through the membranes, they were
wiped out using a cotton swap. Those traversed cells were fixed in
methanol and stained in Crystal violet. Phase contrast microscopy
was used to record digital images. Three microscopic fields were
recorded in each well. At the same time, the elution of Crystal
violet was measured at 570 nM spectrophotometer absorbance.
Statistical analysis
Continuous data are expressed as the means ±
standard deviation. For statistical analysis, the Student’s t-test
was carried out using SPSS Statistics 19.0 software (SPSS, Inc.,
Chicago, IL, USA). A value of P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of mesenchymal markers and
HIF-1α in the epithelial layer of keloid specimens
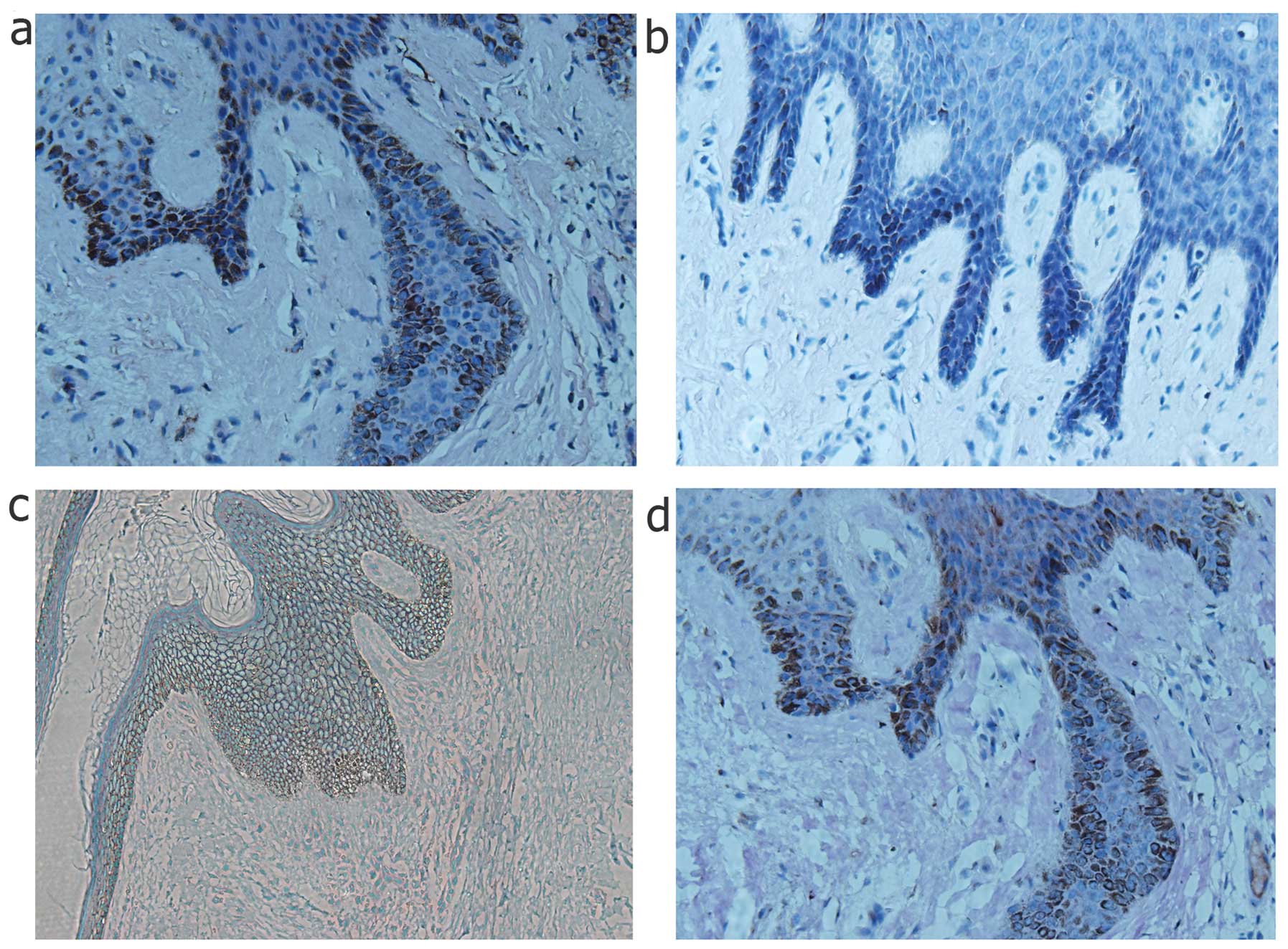
To demonstrate EMT characteristics in the keloid
scars, keloid scar biopsy specimens were examined by histological
analysis as shown in Fig. 1.
Among these specimens, the epithelial cells clearly underwent EMT,
as shown by the abnormal expression of the mesenchymal markers,
vimentin and fibronectin, at the basement membrane zone of the
epithelial layer, which is the migration zone to the dermis.
However, epithelial markers, such as E-cadherin also exhibited a
slightly decreased expression around the basement membrane area,
which confirmed the occurence of transformation. In addition,
HIF-1α was also stained in the same specimens, which coincidentally
had a high expression at the same location as the mesenchymal
markers (Fig. 1).
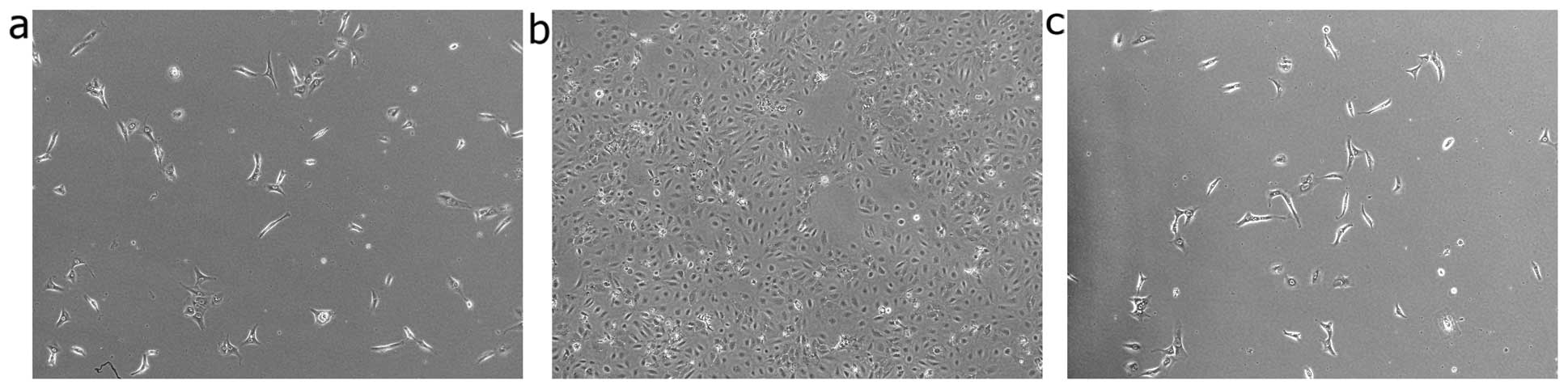
Induction of mesenchymal phenotypic
transformation in human keratinocytes under hypoxic conditions
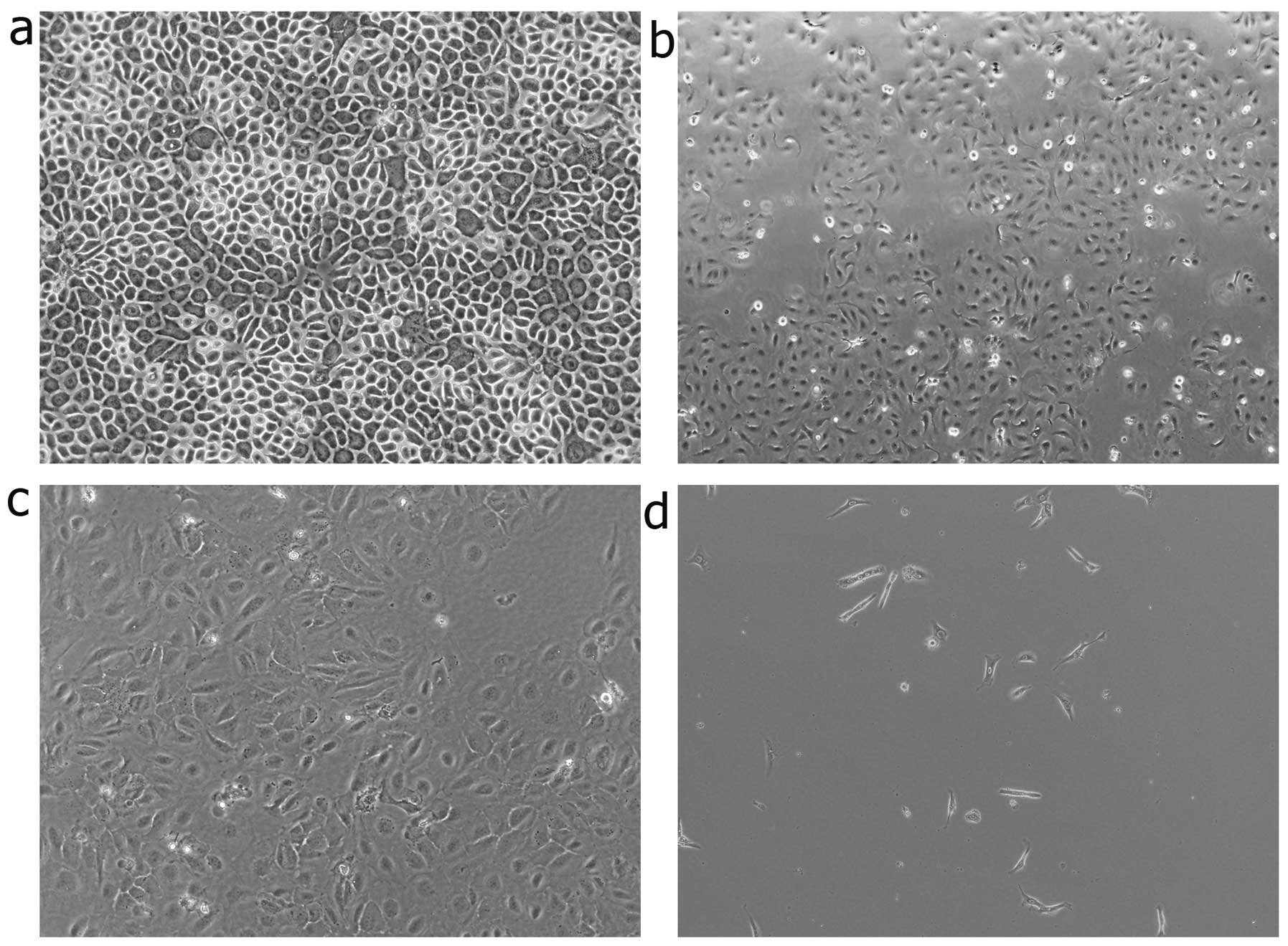
Morphometric analysis was performed to further
estimate the degree of EMT induction under hypoxic culture
conditions. The cells were observed at 12, 24 and 36 h. The
keratinocytes cultured under normoxic conditions displayed a
characteristic polygonal and cobblestone monolayer morphology.
Following culture for 12 h under hypoxic conditions, phenotypic
changes, from trivial to remarkable, were observed. Compared to the
control cells (cultured under normoxic conditions), the
hypoxia-stimulated keratinocytes displayed an elongated,
spindle-shaped similar morphology, resembling fibroblasts (Fig. 2).
Expression of genes in keratinocytes
under both hypoxic and normoxic conditions in vitro
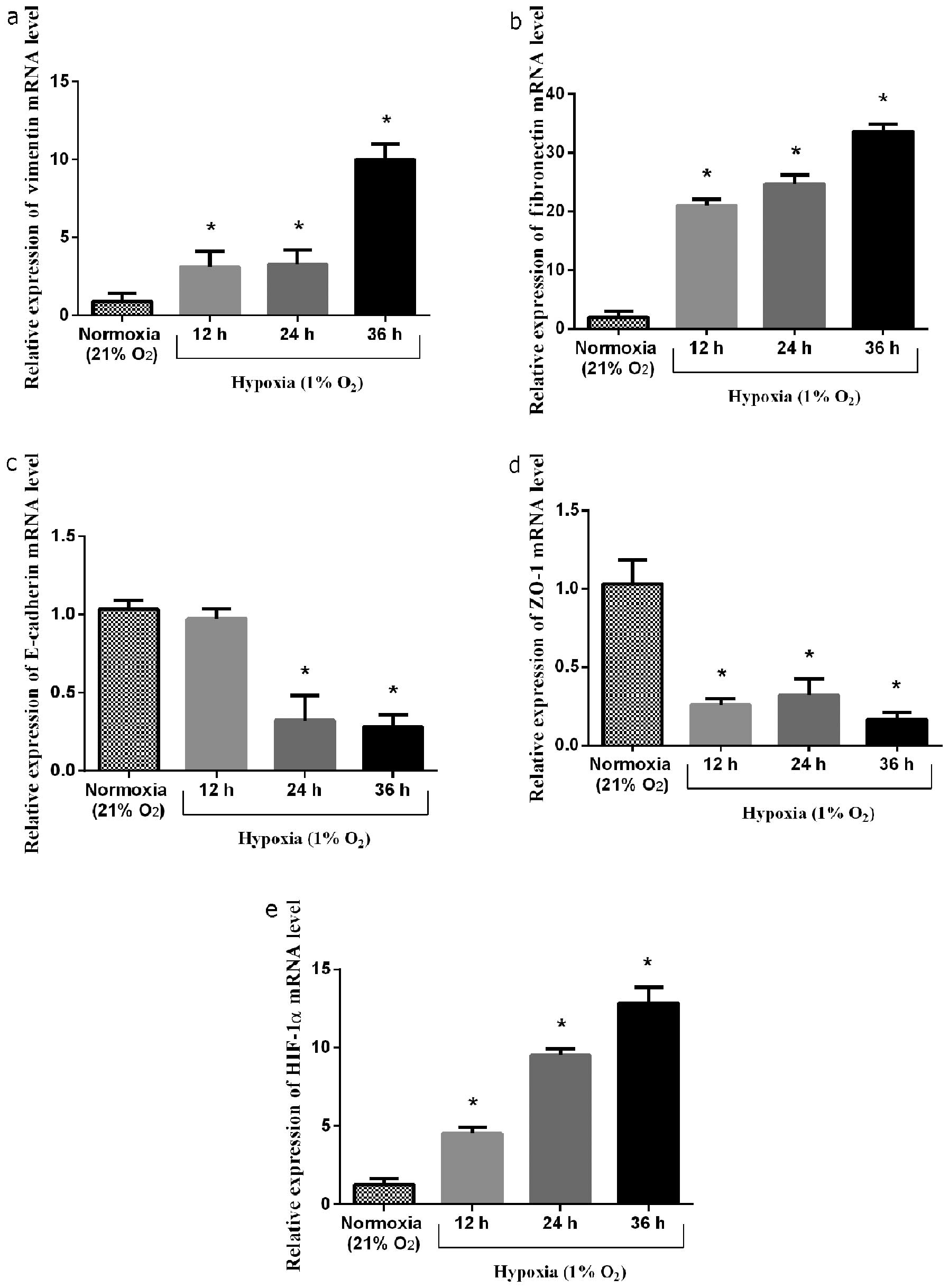
The induction of the expression of EMT marker genes
was measured under both normoxic and hypoxic conditions. Following
incubation for 12, 24 and 36 h in 1% oxygen, the mRNA level of
HIF-1α increased by 3.5-, 7.5- and 10-fold, respectively
(P<0.05, 0.05 and 0.05, respectively) compared to the controls.
Accompanied by the increase in HIF-1α mRNA expression, the mRNA
expression of vimentin increased by 3.4-, 3.6- and 11-fold,
respectively (P<0.05, 0.05 and 0.05, respectively) compared to
the controls. The mRNA expression of fibronectin increased by
10.5-, 12- and 17-fold, respectively (P<0.05, 0.05, and 0.05,
respectively) compared to the controls. However, the mRNA
expression of ZO-1 was suppressed under the hypoxic conditions by
approximately 74.8±1.7% at 12 h, 69.0±3.3% at 24 h and 84.2±2.7% at
36 h compared to the controls (P<0.05, 0.05 and 0.05,
respectively). The mRNA expression of E-cadherin in response to the
hypoxic conditions decreased by approximately 5.8±1.3% at 12 h,
68.7±5.1% at 24 h and 72.6±2.3% at 36 h compared to the controls
(P=0.30, P<0.05 and 0.05, respectively) (Fig. 3).
Expression of EMT markers in human
keratinocytes under hypoxic conditions in vitro
Due to the association between HIF-1α and EMT
markers in keloid scar specimens and hypoxia-induced phenotypic
changes in keratinocytes, HIF-1α may potentially provoke or
intensify EMT markers during human keloid scar formation. To
examine this hypothesis, we first cultured primary keloid-derived
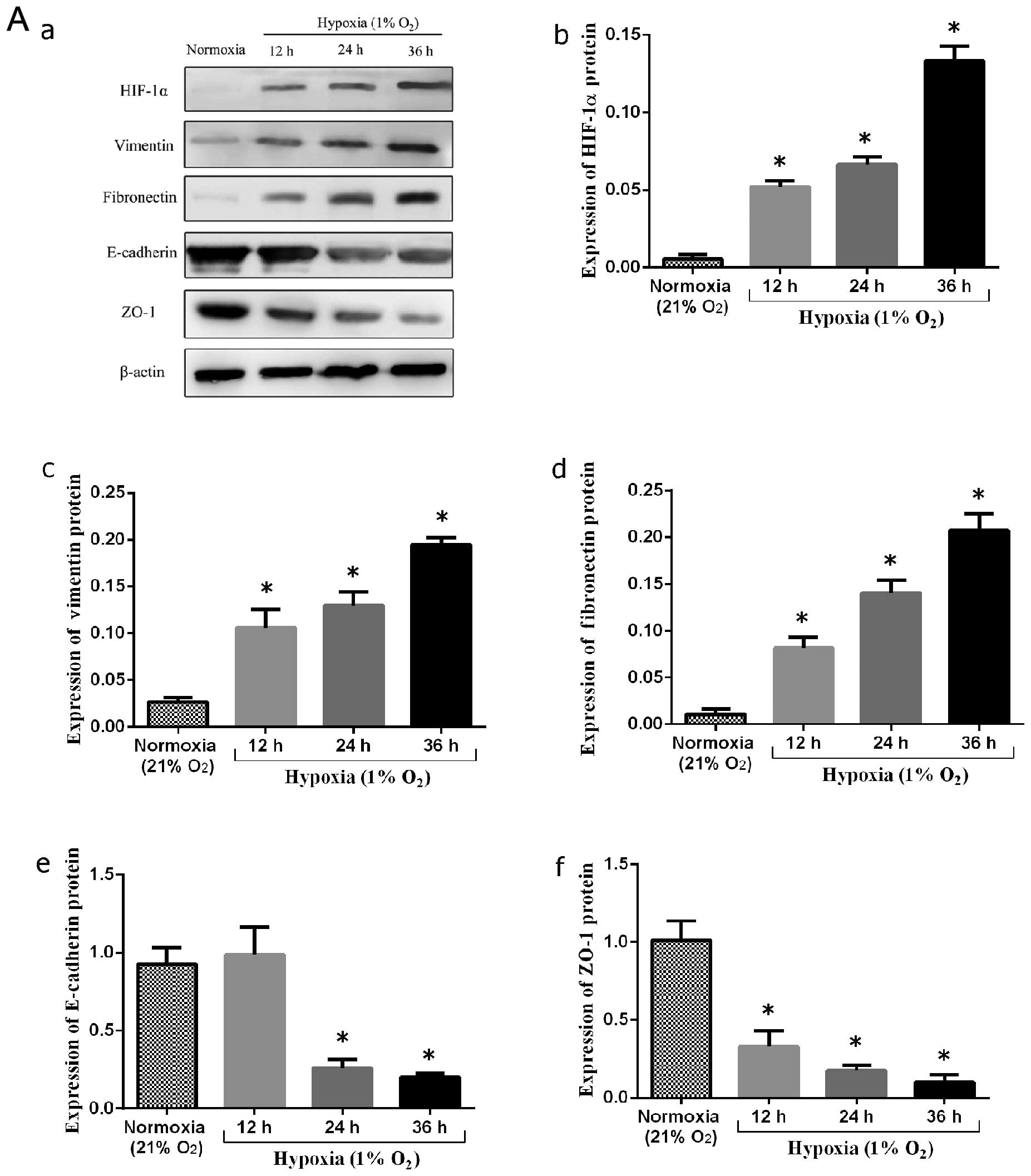
keratinocytes in a hypoxic environment (1% O2). At 12,
24 and 36 h, the keratinocytes were collected for western blot
analysis. In the normoxic control group, vimentin-and
fibronectin-positive cells were absent, as shown by western blot
analysis. Following culture under hypoxic conditions, the protein
epxression of vimentin and fibronectin increased significantly
(P<0.05 and 0.05, respectively). By contrast, the epxression of
epithelial markers, including E-cadherin and ZO-1 was significantly
decreased compared to the normoxic controls (P<0.05 and 0.05,
respectively) (Fig. 4A).
To further demonstrate the expression of EMT markers
in the cells cultured under normoxic and hypoxic conditions,
immunofluorescence staining was performed to evaluate the
expression of vimentin, fibronectin, E-cadherin, ZO-1 and HIF-1α.
E-cadherin and ZO-1 were strongly manifested in the control group;
no expression of mesenchymal markers was observed in the controls
(Fig. 4B). However, following
exposure to hypoxia, the expression of ZO-1 and E-cadherin was
significantly downregulated, and that of mesenchymal markers was
increased (Fig. 4C).
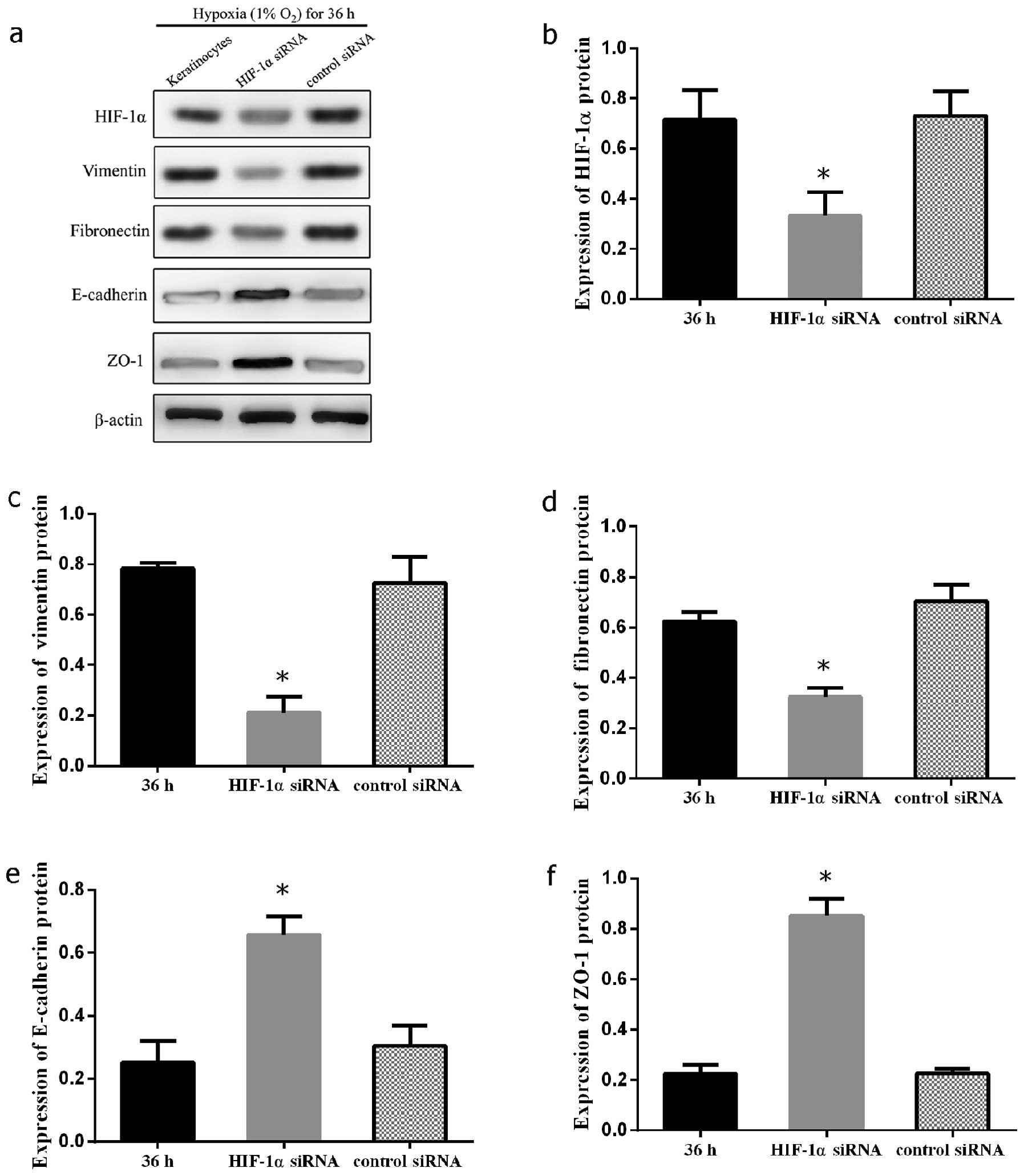
Silencing HIF-1α signaling inhibits the
hypoxia-induced mesenchymal transformation of keloid
keratinocytes
In order to confirm the mechanisms through which
HIF-1α participates in hypoxia-mediated EMT in keloid-derived
keratinocytes, the HIF-1α gene was knocked down by siRNA in
keloid-derived keratinocytes. The expression levels of E-cadherin,
ZO-1, fibronectin and vimentin were measured by western blot
analysis following the silencing of HIF-1α by introducing HIF-1α
siRNA or control scramble siRNA into the keratinocytes under
hypoxic (1% O2) culture conditions. A significant
decrease in the expression of vimentin and fibronectin was observed
(P<0.05 and 0.05, respectively). On the contrary, the marked
restoration in the expression of E-cadherin and ZO-1 suggested the
distinctive importance of HIF-1α signaling in EMT in keloid
keratinocytes (P<0.05 and 0.05, respectively) (Fig. 5). The effects of siRNA on keloid
keratinocytes under hypoxic conditions were also confirmed by phase
contrast microscopy. The cellular morphology of the keratinocytes
transfected with HIF-1α siRNA was reversed back to a typical
epithelial-like shape compared to the control cells transfected
with the scramble siRNA under hypoxic conditions (Fig. 6). This indicated that the
mesenchymal changes induced by hypoxia in the keloid keratinocytes
were regulated by HIF-1α signaling.
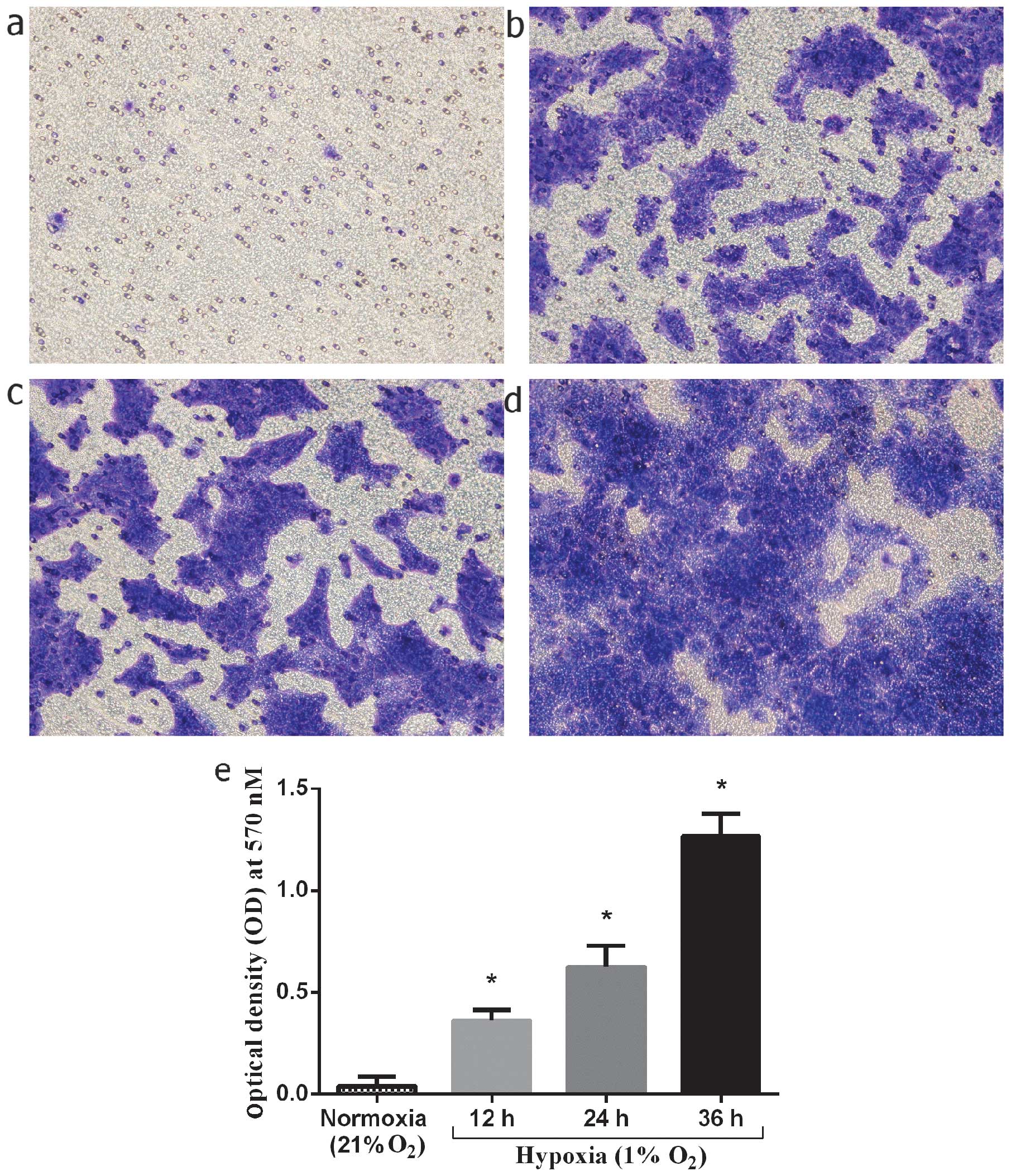
Invasiveness of normal keratinocytes in
vitro under hypoxic culture conditions
The stimulating effect of hypoxia on the invasion of
keloid-derived keratinocytes was investigated under hypoxic culture
conditions. The keratinocytes were cultured under hypoxic
conditions (1% O2) for 12, 24 and 36 h, and compared to
the cells cultured under normoxic conditions. The number of
migrated keratinocytes at 12, 24, and 36 h of culture under hypoxic
conditions markedly increased by 9-, 15.8- and 32-fold,
respectively compared to the normoxic controls (P<0.01, 0.01 and
0.01, respectively) (Fig. 7). In
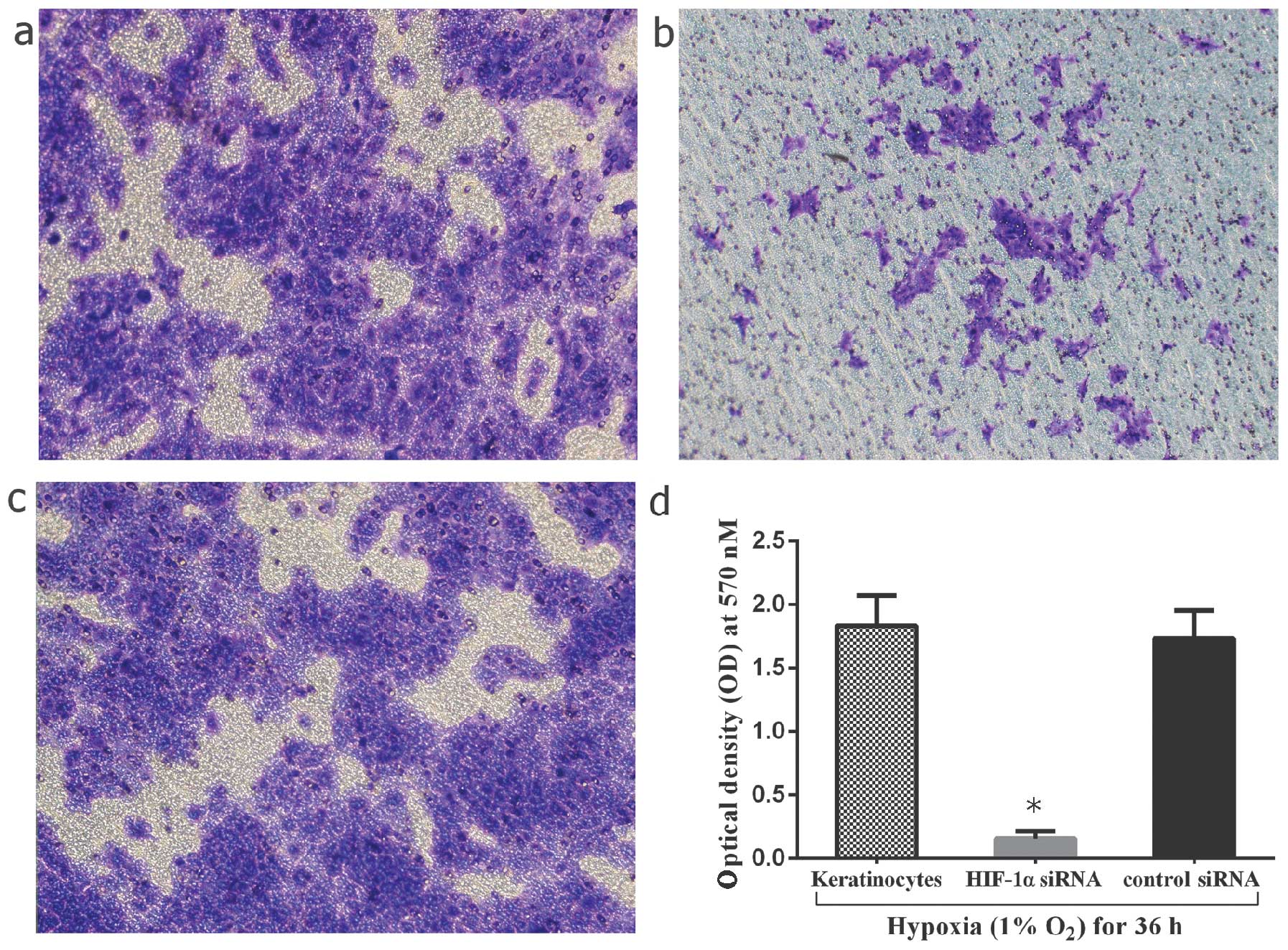
addition, the invasive capacity of the cells was compared between
the HIF-1α siRNA-transfected keratinocytes and the hypoxia-exposed
keratinocytes. The results revealed a significant decrease in the
number of infiltrated cells by 91.3±1.2% in the HIF-1α
siRNA-transfected group (P<0.05) (Fig. 8). As a result, hypoxia/HIF-1α may
enhance the invasive capacity of keloid keratinocytes.
Discussion
Keloids are benign fibrous skin tumors characterized
by fibroblast proliferation and the excessive accumulation of ECM.
Unlike typical hypertrophic scars, keloids can increase in size
indefinitely and can spread beyond the original wound margin, which
leads to body disfiguration and dysfunction (23,24).
In this study, we detected the expression of HIF-1α,
as well as that of epithelial and mesenchymal markers in keloid
tissue biopsy speciments. It was observed that HIF-1α was highly
expressed in the epithelial layer of the keloid skin specimens,
particularly along the basal layer, which was adjacent to the
dermis. Furthermore, it should be noted that mesenchymal markers
were located in the epidermal basal layer, where E-cadherin was not
detected or was expressed at low levels (Fig. 1). Inspired by the evidence in
vivo, we further confirmed that the epithelial markers,
E-cadherin and ZO-1, were downregulated in the primary keloid
keratinocytes under hypoxic culture conditions compared to the
normoxic control group. In addition, the mesenchymal markers,
fibronectin and vimentin, were overexpressed in the hypoxic group
in a time-dependent manner. Immunofluorescence staining and western
blot analysis also confirmed that HIF-1α siRNA inhibited the EMT
process in the keloid keratinocytes under hypoxic conditions. These
findings suggest that the inhibition of HIF-1α-mediated EMT
transcription factors may be crucial in the repression of keloid
development induced by a hypoxic microenvironment.
The majority of relevant studies have focused more
on keloid fibroblasts rather than on keloid keratinocytes (25-27). However, certain studies have found
that keloid keratinocytes are involved in the keloid pathological
process and have a significant impact on the biological
characteristics of fibroblasts (28-31). In this study, we confirmed that
keloid keratinocytes responded to hypoxic conditions by acquiring a
fibroblast-like appearance in vitro. This indicates that
HIF-1α is involved in the initiation and modulation of the
expression of epithelial and mesenchymal markers in keloid
keratinocytes. This suggests that keloid keratinocytes may be a
potential origin of fibroblasts. To confirm this hypothesis, we
also investigated the morphological changes by culturing primary
keloid keratinocytes under hypoxic conditions, and our results
revealed a distinct epithelial-to-mesenchymal morphological
alteration.
Hypoxia is an important characteristic in the tumor
tissue microenvironment and is involved in numerous critical
pathological developments. It is recognized that the hypoxic
microenvironment exists inside of keloids and causes the
accumulation of HIF-1α (9). A
previous study demonstrated that a high HIF-1α expression in
keloids was closely associated with the tumor bioenergetic
characteristics and tumor metastasis (3). Several studies have focused on
HIF-1α as the key promoter of ECM accumulation (32). However, the role of HIF-1α in
keloid formation and growth is still not clearly understood. Our
study demonstrated that keloid keratinocytes underwent a transition
from an epithelial to a mesenchymal phenotype in response to
HIF-1α, with a marked upregulation in the expression of vimentin
and fibronectin, and a decrease in E-cadherin and ZO-1 expression.
Notably, this HIF-1α EMT signaling was inhibited and reversed by
siRNA targeting HIF-1α, suggesting that HIF-1α may be the crucial
EMT initiator and mediator in the hypoxia-induced EMT process in
keloid scars.
Previously published studies have demonstrated that
the hypoxic microenvironment is a favorable condition for tumor
cell growth and invasion (33,34). The key characteristic of HIF-1α is
the promotion of angiogenesis, which is necessary for cancer tumor
growth and invasion. Under the EMT process, keloid keratinocytes
lose their epithelial characteristics, and acquire a mesenchymal
phenotype. By acquiring a fibroblast-like appearance, the keloids
secrete more ECM, and this causes further HIF-1α accumulation,
which may exacerbate the keloid malignant growth pattern (9). Our results revealed that the
invasive capacity of the keloid keratinocytes cultured under
hypoxic conditions (1% O2) was higher than that of those
cultured under normoxic (21% O2) conditions. Notably,
the HIF-1α knockdown in the keratinocytes weakened the invasive
capacity compared to the cells cultured under hypoxic (1%
O2) conditions. All the above findings indicate a
possible role for hypoxia/HIF-1α in the progression and development
of keloids, promoting a suitable microenvironment contributing to
invasive spreading.
In conclusion, our study demonstrates that HIF-1α
upregulates vimentin and fibronectin expression and downregulates
E-cadherin and ZO-1 expression in keloid keratinocytes under
hypoxic conditions, which promotes the EMT process and enhances the
invasive capacity of the keloid keratinocytes. These findings
provide evidence of the influence of hypoxia on EMT in keloids.
HIF-1α may thus be an attractive target for genetic and
pharmacological modulations of this process. However, the
mechanisms underlying the role of HIF-1α in these processes remain
to be determined in further research.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (NSFC) (no. 81071571).
We thank Dr Nianhua Feng and Dr Jianfeng Wei for their technical
assistance and Dr Zhidong Lv for providing precious advice.
References
|
1
|
Zhang Z, Nie F, Kang C, Chen B, Qin Z, Ma
J, Ma Y and Zhao X: Increased periostin expression affects the
proliferation, collagen synthesis, migration and invasion of keloid
fibroblasts under hypoxic conditions. Int J Mol Med. 34:253–261.
2014.PubMed/NCBI
|
|
2
|
Zhang Q, Yamaza T, Kelly AP, Shi S, Wang
S, Brown J, Wang L, French SW, Shi S and Le AD: Tumor–like stem
cells derived from human keloid are governed by the inflammatory
niche driven by IL–17/IL–6 axis. PLoS One. 4:e77982009. View Article : Google Scholar
|
|
3
|
Vincent AS, Phan TT, Mukhopadhyay A, Lim
HY, Halliwell B and Wong KP: Human skin keloid fibroblasts display
bioener–getics of cancer cells. J Invest Dermatol. 128:702–709.
2008. View Article : Google Scholar
|
|
4
|
Kischer CW and Brody GS: Structure of the
collagen nodule from hypertrophic scars and keloids. Scan Electron
Microsc. (Pt 3): 371–376. 1981.PubMed/NCBI
|
|
5
|
Balestri R, Misciali C, Zampatti C,
Odorici G and Balestri JA: Keloidal basal cell carcinoma: Should it
be considered a distinct entity? J Dtsch Dermatol Ges.
11:1196–1198. 2013.PubMed/NCBI
|
|
6
|
Lewis JE: Keloidal basal cell carcinoma.
Am J Dermatopathol. 29:4852007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steinbrech DS, Mehrara BJ, Chau D, Rowe
NM, Chin G, Lee T, Saadeh PB, Gittes GK and Longaker MT: Hypoxia
upregulates VEGF production in keloid fibroblasts. Ann Plast Surg.
42:514–519; discussion 519–520. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Wu Y, Ann DK, Messadi DV, Tuan
TL, Kelly AP, Bertolami CN and Le AD: Mechanisms of hypoxic
regulation of plasminogen activator inhibitor–1 gene expression in
keloid fibroblasts. J Invest Dermatol. 121:1005–1012. 2003.
View Article : Google Scholar
|
|
9
|
Zhang Q, Wu Y, Chau CH, Ann DK, Bertolami
CN and Le AD: Crosstalk of hypoxia–mediated signaling pathways in
upregulating plasminogen activator inhibitor–1 expression in keloid
fibroblasts. J Cell Physiol. 199:89–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Y, Zhang Q, Ann DK, Akhondzadeh A,
Duong HS, Messadi DV and Le AD: Increased vascular endothelial
growth factor may account for elevated level of plasminogen
activator inhibitor–1 via activating ERK1/2 in keloid fibroblasts.
Am J Physiol Cell Physiol. 286:C905–C912. 2004. View Article : Google Scholar
|
|
11
|
Du R, Xia L, Ning X, Liu L, Sun W, Huang
C, Wang H and Sun S: Hypoxia-induced Bmi1 promotes renal tubular
epithelial cell–mesenchymal transition and renal fibrosis via
PI3K/Akt signal. Mol Biol Cell. 25:2650–2659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han
S, Liu L, Du R, Xia L, He L and Fan D: Hypoxia–inducible
factor–1alpha induces Twist expression in tubular epithelial cells
subjected to hypoxia, leading to epithelial–to–mesenchymal
transition. Kidney Int. 75:1278–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins DF, Kimura K, Bernhardt WM,
Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler
M, Cohen CD, et al: Hypoxia promotes fibrogenesis in vivo via HIF–1
stimulation of epithelial–to–mesenchymal transition. J Clin Invest.
117:3810–3820. 2007.PubMed/NCBI
|
|
14
|
Jiang J, Tang YL and Liang XH: EMT: A new
vision of hypoxia promoting cancer progression. Cancer Biol Ther.
11:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chalamalasetty RB, Garriock RJ, Dunty WC
Jr, Kennedy MW, Jailwala P, Si H and Yamaguchi TP: Mesogenin 1 is a
master regulator of paraxial presomitic mesoderm differentiation.
Development. 141:4285–4297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bronner ME: Formation and migration of
neural crest cells in the vertebrate embryo. Histochem Cell Biol.
138:179–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rogers CD, Saxena A and Bronner ME: Sip1
mediates an E–cadherin-to-N-cadherin switch during cranial neural
crest EMT. J Cell Biol. 203:835–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin SY, Peng AP, Huang LT, Wang YT, Lan CW
and Yang NS: The phytochemical shikonin stimulates
epithelial-mesenchymal transition (EMT) in skin wound healing. Evid
Based Complement Alternat Med. 2013:2627962013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savagner P and Arnoux V:
Epithelio–mesenchymal transition and cutaneous wound healing. Bull
Acad Natl Med. 193:1981–1991; discussion 1992, 2009 (In
French).
|
|
20
|
Yan C, Grimm WA, Garner WL, Qin L, Travis
T, Tan N and Han YP: Epithelial to mesenchymal transition in human
skin wound healing is induced by tumor necrosis factor–alpha
through bone morphogenic protein-2. Am J Pathol. 176:2247–2258.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun S and Qiu XS: Cancer stem cells and
tumor metastasis. J Cancer Res Ther. 9(Suppl): S150–S152. 2013.
View Article : Google Scholar
|
|
23
|
Seifert O and Mrowietz U: Keloid scarring:
Bench and bedside. Arch Dermatol Res. 301:259–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robles DT, Moore E, Draznin M and Berg D:
Keloids: Pathophysiology and management. Dermatol Online J.
13:92007.
|
|
25
|
Ashcroft KJ, Syed F and Bayat A:
Site–specific keloid fibroblasts alter the behaviour of normal skin
and normal scar fibroblasts through paracrine signalling. PLoS One.
8:e756002013. View Article : Google Scholar
|
|
26
|
Muthusubramaniam L, Zaitseva T, Paukshto
M, Martin G and Desai T: Effect of collagen nanotopography on
keloid fibroblast proliferation and matrix synthesis: Implications
for dermal wound healing. Tissue Eng Part A. 20:2728–2736. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suarez E, Syed F, Rasgado TA, Walmsley A,
Mandal P and Bayat A: Skin equivalent tensional force alters keloid
fibroblast behavior and phenotype. Wound Repair Regen. 22:557–568.
2014.PubMed/NCBI
|
|
28
|
Xia W, Phan TT, Lim IJ, Longaker MT and
Yang GP: Complex epithelial-mesenchymal interactions modulate
transforming growth factor–beta expression in keloid-derived cells.
Wound Repair Regen. 12:546–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Do DV, Ong CT, Khoo YT, Carbone A, Lim CP,
Wang S, Mukhopadhyay A, Cao X, Cho DH, Wei XQ, et al:
Interleukin–18 system plays an important role in keloid
pathogenesis via epithelial–mesenchymal interactions. Br J
Dermatol. 166:1275–1288. 2012. View Article : Google Scholar
|
|
30
|
Ong CT, Khoo YT, Tan EK, Mukhopadhyay A,
Do DV, Han HC, Lim IJ and Phan TT: Epithelial–mesenchymal
interactions in keloid pathogenesis modulate vascular endothelial
growth factor expression and secretion. J Pathol. 211:95–108. 2007.
View Article : Google Scholar
|
|
31
|
Phan TT, Lim IJ, Bay BH, Qi R, Huynh HT,
Lee ST and Longaker MT: Differences in collagen production between
normal and keloid–derived fibroblasts in serum–media co–culture
with keloid–derived keratinocytes. J Dermatol Sci. 29:26–34. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Distler JH, Jüngel A, Pileckyte M, Zwerina
J, Michel BA, Gay RE, Kowal–Bielecka O, Matucci–Cerinic M, Schett
G, Marti HH, et al: Hypoxia–induced increase in the production of
extracellular matrix proteins in systemic sclerosis. Arthritis
Rheum. 56:4203–4215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hasmim M, Messai Y, Noman MZ and Chouaib
S: Tumor hypoxia: A key player in the regulation of stromal and
anti-tumor responses. Med Sci (Paris). 30:422–428. 2014.In French.
View Article : Google Scholar
|
|
34
|
Vaupel P and Mayer A: Hypoxia in tumors:
Pathogenesis–related classification, characterization of hypoxia
subtypes, and associated biological and clinical implications. Adv
Exp Med Biol. 812:19–24. 2014. View Article : Google Scholar
|