Introduction
Epithelial ovarian carcinoma (EOC) is the fifth
leading cause of cancer-related mortality and is one of the most
aggressive tumors of all gynecological malignancies in Western
countries. According to Cancer Statistics, it was estimated that
approximately 15,500 individuals succumbed to the disease in the
United States in 2012 (1). The
majority of EOC patients have advanced intraperitoneal metastatic
diseases at diagnosis, as this carcinoma frequently remains
clinically silent. Since the treatment strategy consisting of
maximum cytoreductive surgery followed by taxane plus platinum
chemotherapy was established, the short-term prognosis of patients
with EOC has improved. However, despite the high-level sensitivity
of EOC to paclitaxel, the prognosis of advanced or recurrent cases
remains poor, as the majority of mortality cases are the result of
metastasis, which is refractory to these chemotherapeutic agents.
Although various additional molecular-targeting therapies, such as
the use of anti-angiogenic agents, have been investigated in order
to overcome paclitaxel resistance, the effect of this treatment is
limited (2,3). Currently, numerous studies have
investigated new methods and targets to treat this disease
(4–6).
Recently, increasing attention has been paid to the
association between the nervous system and cancer, as increasing
evidence supports that common genetic mechanisms are involved in
cancer development and the progression of neurodegenerative disease
(7). The nervous system may exert
a potential influence on cancer development; environmental
enrichment has been shown to significantly inhibit xenograft tumor
growth, but the mechanism remains elusive (8). Members of the semaphorin (SEMA)
family, which were originally reported as axon guidance molecules
(9,10), have gained increasing attention
recently due to their roles in tumor growth and metastasis
(1–13). SEMAs can be classified into eight
classes (SEMA1–7 and viral SEMA). Class 3 SEMAs (SEMA3) are the
only secreted SEMAs in vertebrates. Several class 3 SEMAs,
including SEMA3A, SEMA3B, SEMA3E and SEMA3F, have been
characterized as anti-angiogenic agents (14–19). For example, SEMA3B, SEMA3F and
SEMA4D regulate tumor angiogenesis, growth and metastasis in
different manners (20,21). Previous studies showed that
SEMA3A, which is considered as the candidate tumor suppressor, is
often downregulated in numerous types of cancer, including prostate
cancer, breast cancer and glioma (22–24). However, whether SEMA3A is also
downregulated in epithelial ovarian carcinoma remains unclear.
Therefore, the present study focused on the expression of SEMA3A in
epithelial ovarian carcinoma and the potential contribution of
SEMA3A in the prediction of prognosis.
Materials and methods
Patient information and tissue
sampling
A total of 125 specimens of epithelial ovarian
carcinoma from patients diagnosed between 2000 and 2010 were
obtained from surgery in the Department of Obstetrics and
Gynecology and the Department of Pathology in the Affiliated
Hospital of Nantong University (Nantong, China). None of the
patients had received any form of tumor-specific therapy prior to
surgery. Samples were collected (median age, 59 years; range, 33–82
years), and according to the classification of the International
Federation of Gynecology and Obstetrics (FIGO) in 2009, there were
20 cases of stage I, 39 stage II, 35 stage III and 37 stage IV. The
histological grade of the tumor was classified as GI
(well-differentiated) in 55 cases, GII (moderately differentiated)
in 32 cases and GIII (poorly differentiated) in 38 cases. Of all
the samples, there were 72 cases with lymphatic metastasis (median
age, 55 years; range, 39–75 years), 58 with pelvic metastasis
(median age, 53 years; range, 42–79 years) and 53 with peritoneal
metastasis (median age, 56; range, 35–68 years). The follow-up
period ranged from 2 to 60 months with an average of 29.7 months
and a median of 20 months. The 15 cases of normal ovarian
epithelium specimens were obtained from preventive excision of the
uterus and accessories. All the tissues were obtained with the
consent of the patients. The study protocol followed the guidelines
in the Helsinki Declaration and was approved by the ethics
committee (Institutional Review Board) of Nantong University.
Double-labeling immunofluorescence
staining and confocal microscopy
All the specimens were embedded in optimum cutting
temperature compound and frozen in liquid nitrogen cooled
2-methylbutane. The samples were subsequently divided into
20-μm sections using a cryostat. Sections were fixed with
cold acetone, blocked with 10% bovine serum albumin (BSA) in
phosphate-buffered saline (PBS) containing 0.2% triton X-100, and
further permeabilized/blocked in the blocking solution (5% BSA in
PBS containing 0.3% Triton X-100) for 1 h at room temperature.
Sections were first blocked with 10% BSA to prevent non-specific
binding and incubated with a goat polyclonal SEMA3A primary
antibody (SC-1148, 1:100; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) overnight at 4°C, followed by rabbit anti-goat IgG
(H&L) secondary antibody (FITC; 1:100; Abnova Corporation,
Taipei, Taiwan) for 2 h at room temperature in a humidified chamber
with minimal exposure to light. 4′6-Diamidino-2-phenylindole (DAPI)
(Sigma-Aldrich, St. Louis, MO, USA) was used to visualize nuclei
and all the washes were performed in 1X PBS. The sample images were
captured using a confocal microscope. Sections were analyzed with a
Leica SP5 high-speed spectral confocal laser-scanning microscope
(Leica Microsystems, Wetzlar, Germany) or a Zeiss LSM 710 confocal
microscope (Carl Zeiss, Oberkochen, Germany).
Immunofluorescence staining for single- or
double-contractile markers was performed using randomly selected
slides (4–5 slides per each eye) containing four sections per slide
and was examined under the confocal microscope. Specific
fluorescence was captured by confocal microscopy with exposure time
kept constant across all the images. Immunoreactivity was evaluated
by the quantification and stereological counting procedure, as well
as semi-quantitative evaluation using the immunofluorescence
staining intensity score and distribution score.
From the quantification and stereological counting
procedure, 16-bit image sections were analyzed by NIH Image J
software (National Institutes of Health). Fluorescence intensity of
pixel gray values in eight separate regions of interest per region
of the normal and tumor tissues was calculated and averaged across
each tissue region. This was performed separately for each label
(SEMA3A and DAPI). The fluorescence intensity for SEMA3A in normal
and tumor tissues was subsequently compared using analysis of
variance and Tukey’s and Sidak’s comparison tests.
For the semi-quantitative evaluation method, the
immuno-reactive score was defined as the proportion score
multiplied by the intensity score, according to the way of
evaluation of immunohistochemistry (IHC). The proportion scores
were defined as 0, negative; 1, <10%; 2, 11–50%; 3, 51–80%; and
4, >80% positive cells. The intensity scores were defined as 0,
negative; 1, weak; 2, moderate; and 3, strong. The total score
ranged from 0 to 12. The immunoreactivity scores were divided into
one of the following three groups based on the final score;
negative immunoreactivity was defined as a total score of 0,
moderate expression was defined as a total score of 1–4, and strong
expression was defined as a total score of >4.
Western blot analysis
Total protein was extracted by a lysis buffer
containing protease inhibitors (Promega, Madison, WI, USA). Equal
amounts of protein were separated by 10% sulfate polyacrylamide gel
electrophoresis and subsequently transferred to a polyvinylidene
fluoride membrane. The membrane was blocked for 2 h with 5% skimmed
milk in TBS (Tris-buffered saline). After incubation with the
primary antibodies overnight at 4°C [goat polyclonal SEMA3A primary
antibody (SC-1148, 1:200) or a goat anti-β-actin as internal
reference (1:2000; Sigma-Aldrich)], membranes were washed for 5 min
with TBS containing 0.1% Tween-20 three times and subsequently
incubated with horseradish peroxidase-coupled mouse
anti-rabbit/goat IgG antibodies (1:1,000; AB Biotec, Stockholm,
Sweden) for 2 h at room temperature. Signals were detected using
electrochemiluminescence (Pierce Corp., Rockford, IL, USA) followed
by film development.
Expression analysis by reverse
transcription-quantitative polymerase chain reaction (RT–qPCR)
The mRNA expression of SEMA3A was analyzed by
RT-qPCR. Total RNA was extracted using the TRIzol reagent (Gibco
Life Technologies, Beijing, China). RT-qPCR was performed using the
HotStart-IT SYBR Green qPCR Master mix (2X; USB Corp., Cleveland,
OH, USA). According to the manufacturer’s instructions, 25
μl reactions were carried out with 25 μl of cDNA.
RT-qPCR experiments were performed in a LightCycler 480 system
(Roche Applied Sciences, Basal, Switzerland). Cycling parameters
were as follows: Hot start at 95°C for 10 min; 40 cycles of
amplification/quantification at 95°C for 10 sec, 60°C for 30 sec
and 72°C for 30 sec, during which time fluorescence was measured.
Melting curve analysis was performed using continuous fluorescence
acquisition from 65–97°C. These cycling parameters generated single
amp icons for the two primer sets used according to the presence of
a single melt peak. GAPDH was selected as the internal reference.
All RT-qPCR reactions were repeated three times for each gene and
each sample was performed in triplicate. Sequences of the primers
for SEMA3A were: Forward, 5′-ATCTGTATCAGGTGCCTCTTACC-3′; and
reverse, 5′-TCTCAACGAATCGTCTTAGGAC-3′. The relative changes in gene
expression were analyzed by the 2-∆∆CT method.
Triplicates were performed for each sample in three independent
experiments.
Clinicopathological analysis
The mRNA expression levels of SEMA3A in ovarian
tissues were used to analyze the association between SEMA3A
expression and clinicopathological characteristics, as well as the
survival time of the patients. Pathological analysis was performed
by the Departments of Pathology of Nantong University, and
validated by qualified experts. During the follow-up period,
overall survival was measured from diagnosis to fatality or to the
last follow-up (at five years). At the time of analysis, 86
patients (68.8%) succumbed, 37 patients (29.6%) were alive, and 2
patients were lost during the follow-up. The estimated median
survival time for all patients was 28 months, and the calculated
survival rates were 72.8% at 1 year, 48.0% at 2 years, and 29.6% at
5 years.
Post-operative follow-up
Following surgery, each patient was scheduled for a
follow-up examination, including physical examination, complete
blood count, tumor markers’ tests and ultrasound scan of the pelvis
every 3 months in the first year, semi-annually in the second year,
and annually after 3 years. More frequent examinations were
scheduled when clinically indicated. The cause of mortality was
registered and classified as mortality due to this cancer, other
causes or unknown causes. Fatality of a patient was ascertained by
reporting from the family and verified by a review of public
records.
Statistical analysis
Tukey’s and Sidak’s comparison tests were used to
compare the fluorescence intensity. SPSS 19.0 statistical software
(IBM Corp., Armonk, NY, USA) was adopted for data analysis.
Counting data comparisons between groups were subjected to the
χ2 test. Survival analysis was computed by means of the
Kaplan-Meier method and significant levels were assessed using the
log-rank test. The results are expressed as the means ± standard
deviation of at least three independent experiments, and for all
statistical analyses, P<0.05 was considered to indicate a
statistically significant difference.
Results
SEMA3A is detected at a lower level in
epithelial ovarian carcinoma
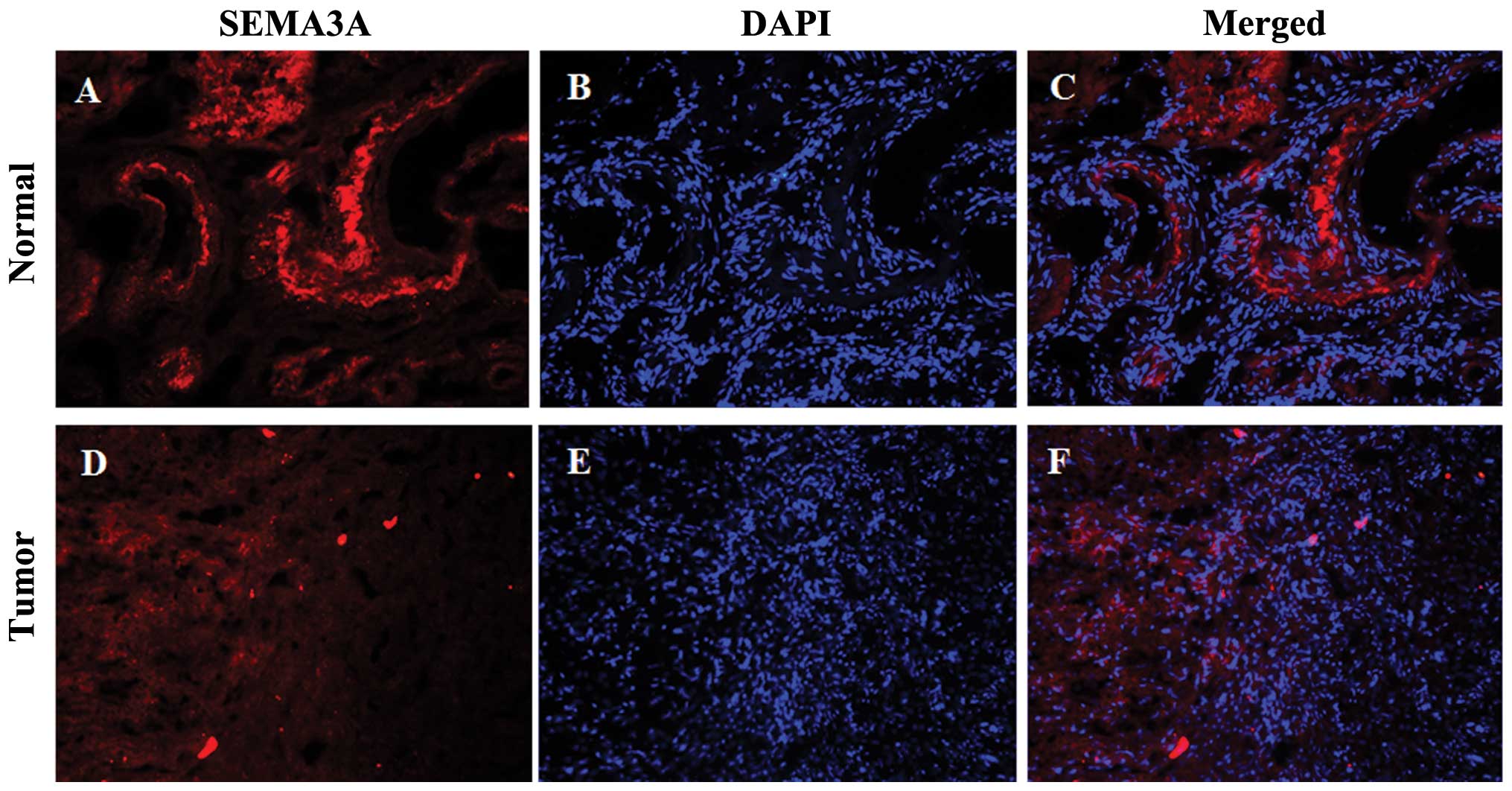
SEMA3A was detected primarily in the nucleus and
cytoplasm of the normal ovarian epithelium (Fig. 1A and C). Only 2 (13.3%) of the 15
normal ovarian epithelium showed moderate intensity, while 13
(86.7%) showed a strong intensity (Table I). However, among the 125
epithelial ovarian carcinoma cases, moderate intensity was observed
in 78 (62.4%), and a strong intensity was observed in 47 (37.6%)
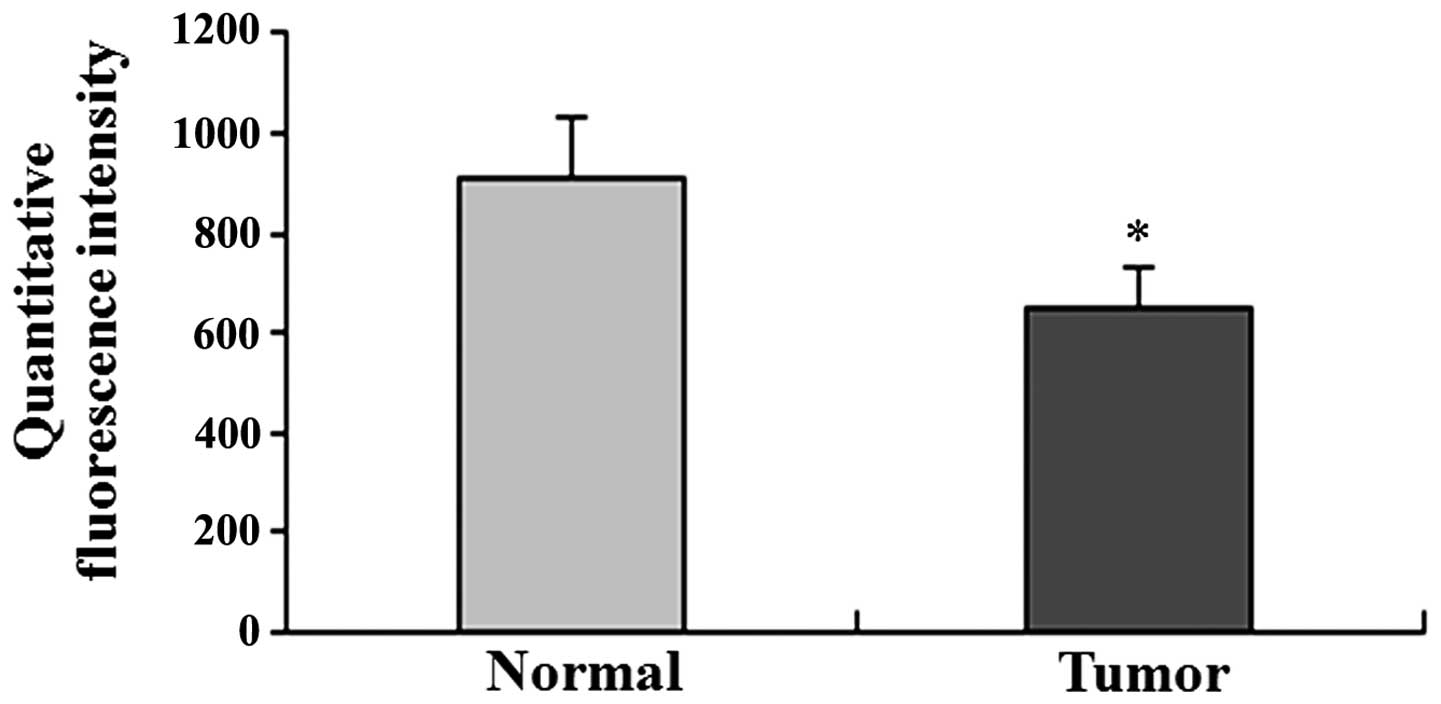
(Table I). Quantitative
fluorescence intensity of SEMA3A was lower in the tumor compared to
the normal specimens (Fig. 2).
There was a significant difference in the expression of SEMA3A
between normal and tumor tissues (P<0.001; Table I and Fig. 2).
 | Table IExpression of SEMA3A in normal ovarian
epithelum and epithelial ovarian carcinoma. |
Table I
Expression of SEMA3A in normal ovarian
epithelum and epithelial ovarian carcinoma.
| Tissue | No. | SEMA3A
|
|---|
| M | S | P-value |
|---|
| Tumor | 125 | 78 | 47 | <0.001 |
| Normal | 15 | 2 | 13 | |
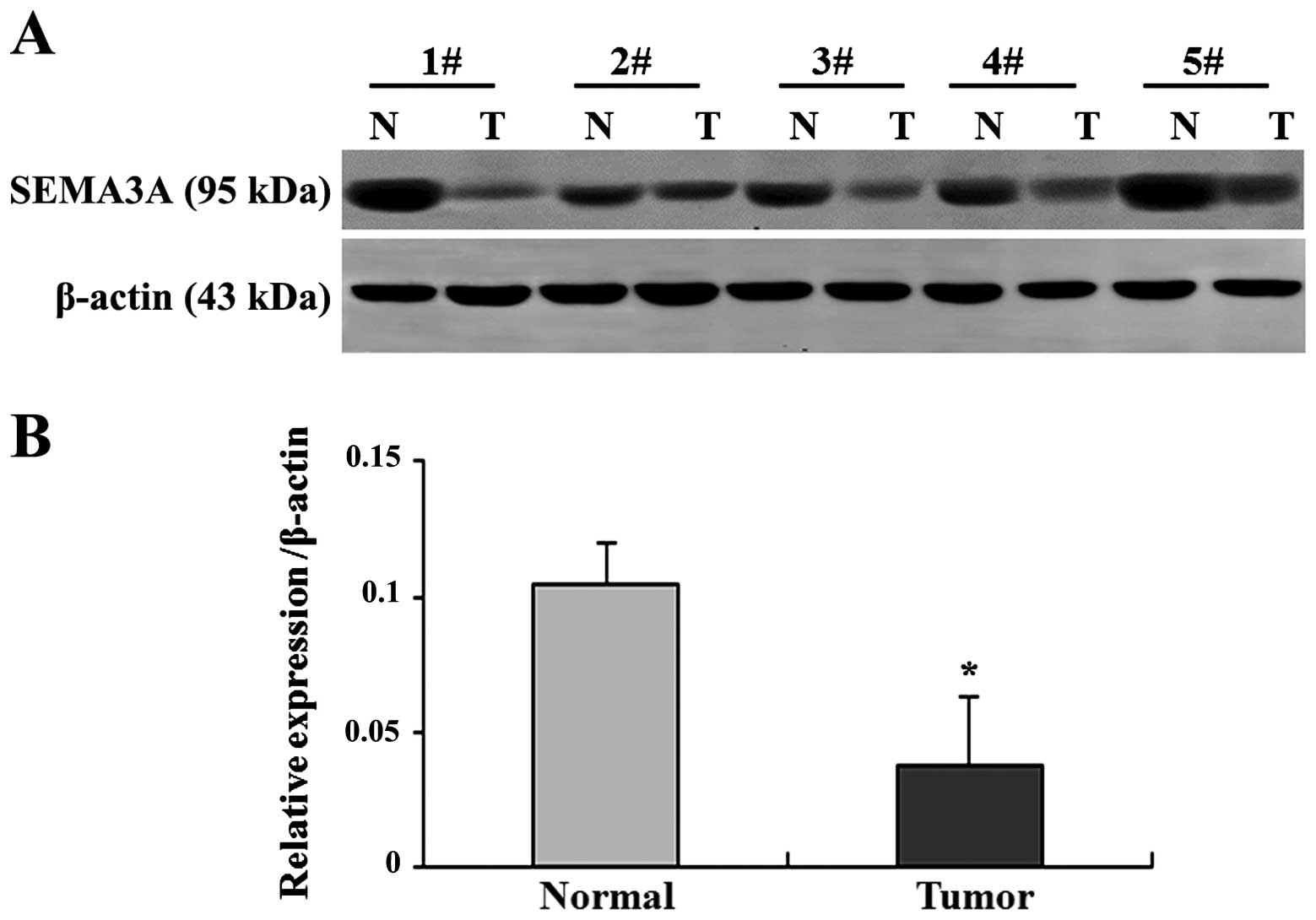
SEMA3A protein expression is
downregulated in ovarian carcinoma tissues
The protein expression of SEMA3A was examined by
western blot analysis in ovarian tumors and normal ovarian
epithelium, as performed on all the epithelial ovarian carcinoma
and the normal ovarian epithelial tissues. The relative quantity of
SEMA3A protein expression was normalized to β-actin. Five pairs of
cancerous and normal ovarian tissues were randomly selected and
presented in Fig. 3A, while
summary data are presented in Fig.
3B. The expression of the SEMA3A protein was downregulated in
the majority of the samples of ovarian tumors compared to in the
normal tissues (Fig. 3A). In
extremely few cases, such as the second pair (2#) in Fig. 3A, it appeared that the expression
of SEMA3A in the tumor was close to the normal tissue (Fig. 3A). The average SEMA3A protein
level in the epithelial ovarian carcinoma was significantly lower
than that in the normal ovarian epithelial tissues (P<0.05;
Fig. 3B).
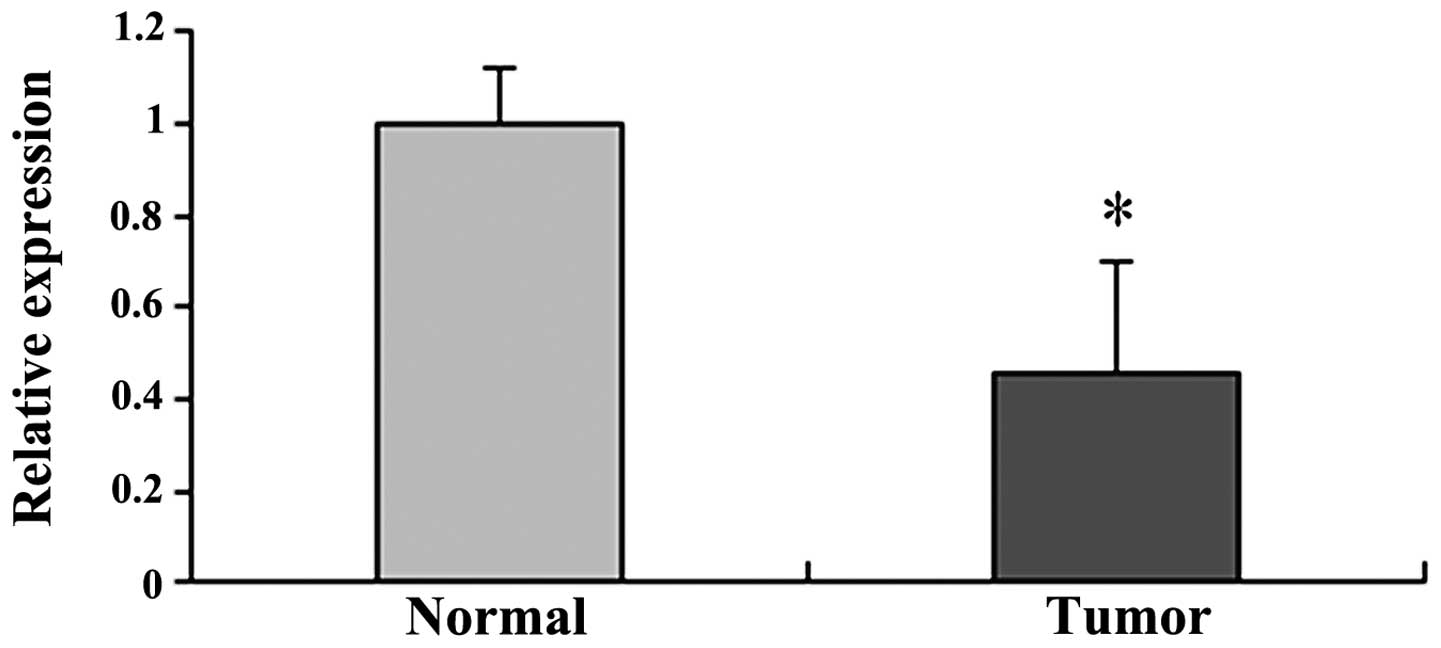
SEMA3A mRNA expression is downregulated
in ovarian carcinoma tissues
In addition to the protein expression of SEMA3A, the
mRNA expression was also detected in the epithelial ovarian
carcinoma and normal ovarian epithelial tissues. As shown in
Table II, the mRNA expression of
SEMA3A (median, 51 copies/μl; range from 5 to 112
copies/μl) was significantly reduced in ovarian carcinoma
samples compared to the normal samples (median, 171
copies/μl; range from 49 to 349 copies/μl)
(P<0.001). Quantification of SEMA3A mRNA expression revealed a
significant decrease in cancerous compared to normal tissues
(Table II and Fig. 4).
 | Table IImRNA expression of SEMA3A in normal
ovarian epithelum and epithelial ovarian carcinoma. |
Table II
mRNA expression of SEMA3A in normal
ovarian epithelum and epithelial ovarian carcinoma.
| Gene | Normal, median
copies/μl (range) | Tumor, median
copies/μl (range) | P-value |
|---|
| SEMA3A | 171 (49–349) | 51 (3–112) | <0.001 |
The association between SEMA3A mRNA expression
levels of the ovarian tumors and clinicopathological
characteristics are presented in Table III.
 | Table IIIAssociation between SEMA3A expression
levels of epithelial ovarian carcinoma and clinicopathological
factors. |
Table III
Association between SEMA3A expression
levels of epithelial ovarian carcinoma and clinicopathological
factors.
|
Characteristics | No. | SEMA3A
|
|---|
| N | P | P-value |
|---|
| Age, years |
| ≤59 | 63 | 36 | 27 | 0.070 |
| >59 | 62 | 42 | 20 | |
| Tumor size, cm |
| ≤2 | 45 | 25 | 20 | 0.076 |
| >2 | 80 | 53 | 27 | |
| FIGO stage |
| I/II | 57 | 31 | 26 | 0.035 |
| III/IV | 68 | 47 | 21 | |
| Histogical
grade |
| Well | 55 | 25 | 30 | 0.000 |
| Moderate +
poor | 70 | 53 | 17 | |
| Histotype |
| Serous | 58 | 35 | 23 | 0.496 |
| Mucinous | 48 | 33 | 15 | |
| Endometroid | 14 | 7 | 7 | |
| Clear cell | 3 | 2 | 1 | |
|
Undifferentiated | 2 | 1 | 1 | |
| Lymph node
metastasis |
| Negative | 53 | 26 | 27 | 0.004 |
| Positive | 72 | 52 | 20 | |
| Distant
metastasis |
| Negative | 67 | 47 | 20 | 0.024 |
| Positive | 58 | 31 | 27 | |
Correlations between the RT-qPCR results of SEMA3A
expression in ovarian tumor tissues and various clinicopathological
characteristics of patients were analyzed by χ2 test and
are listed in Table III. Using
the quartile limits of mRNA expression to divide patient population
into low and high producers allowed the interquartile range to be
set as a cut-off and a significant correlation between the mRNA
expression of SEMA3A and clinicopathological characteristics to be
established. Median expression of SEMA3A in cancerous tissues was
51 copies/μl, dividing the samples into two groups: The
negative (≤51 copies/μl) and positive expression groups of
SEMA3A (>51 copies/μl).
The downregulation of SEMA3A significantly
correlated with FIGO stage, histological grade, lymphatic
metastasis and distant metastasis (P<0.05). However, there was
no significant correlation between SEMA3A expression and age, tumor
size or histological type (P>0.05; Table III).
Expression of SEMA3A is associated with
the survival rate of ovarian carcinoma patient
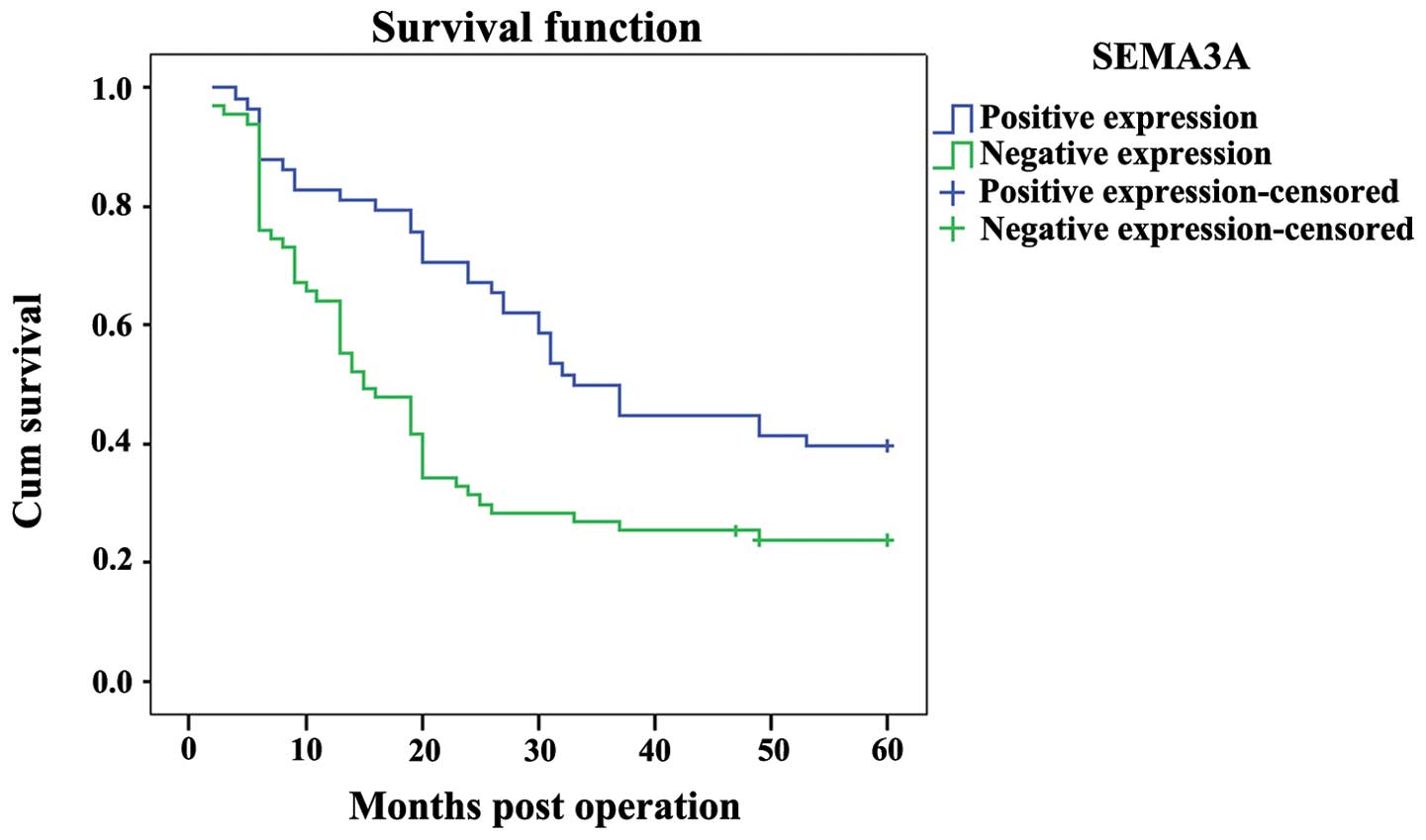
The prognostic role of SEMA3A on the overall
survival rate of ovarian carcinoma patients was investigated by
comparing the 5-year survival rate of patients with high or low
levels of SEMA3A expression in tumors using Kaplan-Meier survival
curves and the log-rank test. There were 58 cases in the positive
SEMA3A expression group (>51 copies/μl), of which 35
succumbed, and the 5-year overall survival rate was 39.7%. In the
negative SEMA3A expression group (<51 copies/μl) there
were 67 cases, of which 51 succumbed and 2 were lost during
follow-up. The 5-year overall survival rate for the negative group
was 20.9%. The overall survival rate of the high SEMA3A expression
group was significantly longer than that of the low SEMA3A
expression group (P<0.001; Fig.
5).
Discussion
SEMA is a multifunctional protein whose function
includes, but is not limited to, axonal guidance (25,26). Class 3 SEMAs, such as SEMA3A,
SEMA3B and SEMA3F, have been previously characterized as natural
tumor suppressors and there are indications that SEMA3E may also
function as a natural tumor suppressor (17,27–30). Previous studies have also shown
that SEMAs function as potent inhibitors of angiogenesis (15,19). The expression of these SEMAs in
several types of breast cancer-derived tumor cells can inhibit the
growth of tumors following the subcutaneous implantation of these
cells (13). SEMA3A is considered
to be a candidate tumor suppressor in certain types of cancer, as
it inhibits the proliferation of malignant mesothelium cells,
decreases the adhesion or migration of prostate or breast cancer
cells, and promotes apoptosis in leukemic T cells (7). However, the roles of SEMA3A in
patients with ovarian cancer have not been extensively studied.
Ovarian cancer, having the highest fatality rate of the female
reproductive diseases, is the leading type with the prominent
features of hidden onset, malignancy, easy metastasis of the normal
tissue adjacent to ovarian cancer tissue and lack of effective
early screening methods (1).
The present study focused on the expression of
SEMA3A in the most common type of ovarian cancer, epithelial
ovarian carcinoma. From the immunofluorescence staining results,
there was a significant difference in the expression of SEMA3A
between normal and cancerous tissues. The protein and mRNA
expression of SEMA3A in ovarian carcinoma tissues and in normal
tissues was also determined. As expected, the average SEMA3A
protein level in the epithelial ovarian carcinoma was significantly
lower than that in the normal ovarian epithelial tissue, while the
mRNA expression of SEMA3A was only 27% in cancerous compared to
normal tissues. The correlations between SEMA3A mRNA expression in
ovarian tumor tissues and various clinicopathological
characteristics of patients were analyzed by χ2 test.
The downregulation of SEMA3A significantly correlated with FIGO
stage, histological grade, lymphatic metastasis and distant
metastasis. However, there was no significant correlation between
SEMA3A expression and age or tumor size. The overall survival rate
of the positive SEMA3A expression group was significantly longer
than that of the negative SEMA3A expression group. The discrepant
changes for SEMA3A in ovarian cancer in the present study indicate
the important role of SEMA3A in the development of epithelial
ovarian carcinoma. Additionally, decreased expression of SEMA3A was
correlated with poor prognosis in ovarian tumor, suggesting that
SEMA3A may be an inhibitor in ovarian epithelial cancer. SEMA3A has
been indicated as a tumor suppressor in other types of cancer
(22,30–33). The mechanism of the inhibitory
role of SEMA3A may be associated with its interaction with
integrins. For example, in breast cancer, SEMA3A inhibits cell
attachment and cell migration by affecting the activation or the
stabilization of surface integrins. Inhibition of integrins by
SEMA3A resulted in a blockade of endothelial and tumor cell
migration, leading to reduced tumor angiogenesis and metastasis
(34,35). The present results may reflect the
ability of SEMA3A to block tumor cell migration and metastasis. In
addition, a more recent study on microRNA (miR) observed that the
upregulation of miR-30b/30d correlates with a higher metastatic
potential, shorter time to recurrence and reduced overall survival
time. Among the target genes of miR-30b/30d, the investigators
identified a significant downregulation of SEMA3A (36). Taken together with the present
results, these data indicate that SEMA3A may be involved in cancer
metastasis. The ability of SEMA3A to inhibit tumor angiogenesis by
competing with vascular endothelium growth factor for binding with
neuropilin 1 has been more intensively studied in colorectal
cancer. Therefore, much remains to be studied regarding the exact
role of SEMA3A in different types of cancer.
Although significant results of SEMA3A in epithelial
ovarian carcinoma were obtained, there were only 15 cases of normal
tissues. More cases are required in future studies as it is
possible that this affected the results.
Double-labeling immunofluorescence staining and
confocal microscopy were used instead of IHC, which is a common
technique used for diagnostic and research purposes. IHC is one of
the most important methods in pathology due to its central role in
the classification of diseases by the evaluation of receptors and
other cellular components in biopsies and surgical resections. IHC
involves staining a thin representative tissue section to evaluate
the intensity and localization of the staining in order to
understand antigen expression. Estimation of the distribution and
the expression is subjectively performed by trained investigators
through visual inspection using a microscope, and staining is
commonly reported as −, +, ++ and +++. The technique provides
superior spatial resolution, but is operator-dependent and further
relies on multi-layered end-point measurements that increase
inaccuracy (37). By contrast,
fluorescence objectively reflected the expression under the
conditions with the case.
In the present study, the IHC method and the
measurement of quantitative fluorescence intensity was applied,
providing a new method for the evaluation of immunoreactivity,
particularly when there were multiple targets and antibody
labeling. Quantitative mRNA expression was also used to analyze the
association of SEMA3A with clinicopathological characteristics, as
well as the overall survival rate. Using the quartile limits of
mRNA expression to divide the population of patients into low and
high groups allowed a significant correlation between mRNA
expression and clinicopathological characteristics to be
established, as well as survival rate, which more accurately
reflected the real situation.
In conclusion, SEMA3A was downregulated in human
epithelial ovarian carcinoma, and the decreased SEMA3A expression
was strongly associated with worse patient survival. Therefore,
SEMA3A could be used as a valuable prognostic marker, as well as a
potential molecular therapy target for ovarian cancer.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant no. BK20131199).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Teoh D and Secord AA: Antiangiogenic
agents in combination with chemotherapy for the treatment of
epithelial ovarian cancer. Int J Gynecol Cancer. 22:348–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Usha L, Sill MW, Darcy KM, et al: A
Gynecologic Oncology Group phase II trial of the protein kinase
C-beta inhibitor, enzastaurin and evaluation of markers with
potential predictive and prognostic value in persistent or
recurrent epithelial ovarian and primary peritoneal malignancies.
Gynecol Oncol. 121:455–461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu H, Yao L, Mei J and Li F: Development
of synthetic of peptide-functionalized liposome for enhanced
targeted ovarian carcinoma therapy. Int J Clin Exp Pathol.
8:207–216. 2015.PubMed/NCBI
|
|
5
|
Kannan K, Coarfa C, Chao PW, et al:
Recurrent BCAM-AKT2 fusion gene leads to a constitutively activated
AKT2 fusion kinase in high-grade serous ovarian carcinoma. Proc
Natl Acad Sci USA. Mar 2–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gurler H, Yu Y, Choi J, Kajdacsy-Balla AA
and Barbolina MV: Three-dimensional collagen type I matrix
up-regulates nuclear isoforms of the microtubule associated protein
tau implicated in resistance to Paclitaxel therapy in ovarian
carcinoma. Int J Mol Sci. 16:3419–3433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morris LG, Veeriah S and Chan TA: Genetic
determinants at the interface of cancer and neurodegenerative
disease. Oncogene. 29:3453–3464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao L, Liu X, Lin EJ, et al: Environmental
and genetic activation of a brain-adipocyte BDNF/leptin axis causes
cancer remission and inhibition. Cell. 142:52–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chilton JK: Molecular mechanisms of axon
guidance. Dev Biol. 292:13–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dickson BJ: Molecular mechanisms of axon
guidance. Science. 298:1959–1964. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bielenberg DR, Hida Y, Shimizu A, et al:
Semaphorin 3F, a chemorepulsant for endothelial cells, induces a
poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J
Clin Invest. 114:1260–1271. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Christensen C, Ambartsumian N, Gilestro G,
et al: Proteolytic processing converts the repelling signal Sema3E
into an inducer of invasive growth and lung metastasis. Cancer Res.
65:6167–6177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neufeld G and Kessler O: The semaphorins:
versatile regulators of tumour progression and tumour angiogenesis.
Nat Rev Cancer. 8:632–645. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kigel B, Varshavsky A, Kessler O and
Neufeld G: Successful inhibition of tumor development by specific
class-3 semaphorins is associated with expression of appropriate
semaphorin receptors by tumor cells. PLoS One. 3:e32872008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kessler O, Shraga-Heled N, Lange T, et al:
Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res.
64:1008–1015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Casazza A, Fu X, Johansson I, et al:
Systemic and targeted delivery of semaphorin 3A inhibits tumor
angiogenesis and progression in mouse tumor models. Arterioscler
Thromb Vasc Biol. 31:741–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maione F, Molla F, Meda C, et al:
Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks
tumor growth and normalizes tumor vasculature in transgenic mouse
models. J Clin Invest. 119:3356–3372. 2009.PubMed/NCBI
|
|
18
|
Sakurai A, Gavard J, Annas-Linhares Y, et
al: Semaphorin 3E initiates antiangiogenic signaling through plexin
D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 30:3086–3098. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varshavsky A, Kessler O, Abramovitch S, et
al: Semaphorin-3B is an angiogenesis inhibitor that is inactivated
by furin-like pro-protein convertases. Cancer Res. 68:6922–6931.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rolny C, Capparuccia L, Casazza A, et al:
The tumor suppressor semaphorin 3B triggers a prometastatic program
mediated by interleukin 8 and the tumor microenvironment. J Exp
Med. 205:1155–1171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng C, Zhou Q, Wu F, et al: Semaphorin3F
down-regulates the expression of integrin alpha(v)beta3 and
sensitizes multicellular tumor spheroids to chemotherapy via the
neuropilin-2 receptor in vitro. Chemotherapy. 55:344–352. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bagci T, Wu JK, Pfannl R, Ilag LL and Jay
DG: Autocrine semaphorin 3A signaling promotes glioblastoma
dispersal. Oncogene. 28:3537–3550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li K, Chen MK, Li LY, et al: The
predictive value of sema-phorins 3 expression in biopsies for
biochemical recurrence of patients with low- and intermediate-risk
prostate cancer. Neoplasma. 60:683–689. 2013. View Article : Google Scholar
|
|
24
|
Staton CA, Shaw LA, Valluru M, et al:
Expression of class 3 semaphorins and their receptors in human
breast neoplasia. Histopathology. 59:274–282. 2011.PubMed/NCBI
|
|
25
|
Neufeld G, Shraga-Heled N, Lange T,
Guttmann-Raviv N, Herzog Y and Kessler O: Semaphorins in cancer.
Front Biosci. 10:751–760. 2005. View
Article : Google Scholar
|
|
26
|
Goshima Y, Ito T, Sasaki Y and Nakamura F:
Semaphorins as signals for cell repulsion and invasion. J Clin
Invest. 109:993–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tomizawa Y, Sekido Y, Kondo M, et al:
Inhibition of lung cancer cell growth and induction of apoptosis
after reexpression of 3p21.3 candidate tumor suppressor gene
SEMA3B. Proc Natl Acad Sci USA. 98:13954–13959. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang R, Davalos AR, Hensel CH, Zhou XJ,
Tse C and Naylor SL: Semaphorin 3F gene from human 3p21.3
suppresses tumor formation in nude mice. Cancer Res. 62:2637–2643.
2002.PubMed/NCBI
|
|
29
|
Vacca A, Scavelli C, Serini G, et al: Loss
of inhibitory semaphorin 3A (SEMA3A) autocrine loops in bone marrow
endothelial cells of patients with multiple myeloma. Blood.
108:1661–1667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moriya J, Minamino T, Tateno K, et al:
Inhibition of semaphorin as a novel strategy for therapeutic
angiogenesis. Circ Res. 106:391–398. 2010. View Article : Google Scholar
|
|
31
|
Pan H, Wanami LS, Dissanayake TR and
Bachelder RE: Autocrine semaphorin3A stimulates alpha2 beta1
integrin expression/function in breast tumor cells. Breast Cancer
Res Treat. 118:197–205. 2009. View Article : Google Scholar
|
|
32
|
Herman JG and Meadows GG: Increased class
3 semaphorin expression modulates the invasive and adhesive
properties of prostate cancer cells. Int J Oncol. 30:1231–1238.
2007.PubMed/NCBI
|
|
33
|
Catalano A, Caprari P, Rodilossi S, et al:
Cross-talk between vascular endothelial growth factor and
semaphorin-3A pathway in the regulation of normal and malignant
mesothelial cell proliferation. FASEB J. 18:358–360. 2004.
|
|
34
|
Oinuma I, Ishikawa Y, Katoh H and Negishi
M: The Semaphorin 4D receptor Plexin-B1 is a GTPase activating
protein for R-Ras. Science. 305:862–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toyofuku T, Yoshida J, Sugimoto T, et al:
FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat
Neurosci. 8:1712–1719. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gaziel-Sovran A, Segura MF, Di Micco R, et
al: miR-30b/30d regulation of GalNAc transferases enhances invasion
and immunosuppression during metastasis. Cancer Cell. 20:104–118.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dubois L, Andersson K, Asplund A and
Bjorkelund H: Evaluating real-time immunohistochemistry on multiple
tissue samples, multiple targets and multiple antibody labeling
methods. BMC Res Notes. 6:5422013. View Article : Google Scholar : PubMed/NCBI
|